Abstract
Immunosuppressive myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) are associated with immunologic tolerance and poor prognosis in ovarian cancer (OvCa). We hypothesized that women with germline BRCA1 and BRCA2 mutation-associated (gBRCAm) OvCa would have fewer circulating immunosuppressive immune cells compared to those with BRCA wild-type (BRCAwt) disease during their early disease course (<5 years post-diagnosis) where gBRCAm is a favorable prognostic factor. We collected and viably froze peripheral blood mononuclear cells (PBMCs) from patients with recurrent OvCa olaparib clinical trials (NCT01445418/NCT01237067). Immune subset analyses were performed using flow cytometry for Tregs, exhausted CD8+ T cells, monocytes and MDSCs. Functional marker expression, including cytotoxic T lymphocyte-associated protein 4 (CTLA-4), T cell immunoglobulin and mucin domain 3 (TIM-3) and programmed cell death protein 1 (PD-1) was evaluated. Data were analyzed using FlowJo. Pretreatment PBMCs were collected from 41 patients (16 gBRCAm/25 BRCAwt). The percentage of MDSCs among viable CD45+ PBMC was lower in gBRCAm OvCa compared with BRCAwt OvCa (median 0.565 vs. 0.93%, P=0.0086) but this difference was not seen in those women >5 years post-diagnosis. CD8+ T cells among viable CD45+ PBMCs and CTLA-4+/CD8+ T cells were higher in gBRCAm carriers than patients with BRCAwt, in particular for those <5 years post-diagnosis (median 20.4 vs. 9.78%, P=0.031 and median MFI 0.19 vs. 0.22, P=0.0074, respectively). TIM-3 expression on Tregs was associated with poor progression-free survival, independent of gBRCAm status (P<0.001). Our pilot data suggested that patients with gBRCAm OvCa may have fewer circulating MDSCs but higher CD8+ T cells in PBMCs during their early disease course. This may contribute to the observed survival benefit for these women in their first post-diagnosis decade.
Keywords: ovarian cancer, BRCA, immune system, immune cells, survival benefit
Introduction
The development of immune checkpoint inhibition has led to important clinical advances in the treatment of advanced solid tumors (1). However, patients with recurrent ovarian cancer have responded poorly to single-agent immune checkpoint blockade to date (2). Challenges to this strategy include the distinct immune microenvironment that each ovarian cancer patient may have and the lack of reliable biomarkers (1).
Tumors with DNA repair deficiency, e.g., mismatch repair defects, are recognized to have high mutational load, express more neoantigens, and are potentially susceptible to immune checkpoint inhibitors (3). Tumor mutational burden and associated neoantigen expression correlate with the clinical activity of immune checkpoint blockade in lung cancer and melanoma (4,5). In ovarian cancer, germline BRCA mutation (gBRCAm)-related high-grade serous ovarian cancer (HGSOC) has higher mutational load and associated neoantigen expression compared with BRCA wild-type (BRCAwt) disease (6), which may lead to recruitment of tumor-infiltrating lymphocytes (TILs) and host immune response (6). In support of this, increased intratumoral CD3+ TILs are present in gBRCAm HGSOC but not in BRCAwt tumors (7).
HGSOC is an immunogenic tumor (8). The presence of T cells in the tumor microenvironment is associated with improved survival (8). Immunosuppressive pathways, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), are prominent in HGSOC; these can be barriers to antitumor immunity and adversely affect clinical outcomes (9). Studies also suggest that a high density of Tregs is associated with a poor prognosis, possibly due to their suppressive effects on antitumor cytotoxic T cells (10,11). Additionally, MDSCs play a key immunosuppressive role in various types of cancer, including ovarian cancer. Wu et al reported ovarian cancer patients had significantly higher numbers of MDSCs in both peripheral blood and ascites compared to healthy donors, and ovarian cancer patients with higher levels of monocytic MDSC had a shorter relapse-free survival (12). Thus, the characterization of MDSC phenotypes and their generation in blood and/or ascites from recurrent ovarian cancer remains to be elucidated.
gBRCAm status is a favorable prognostic factor in HGSOC due to platinum-based chemosensitivity within the first decade after diagnosis. However, more recent data suggest that gBRCAm status may have a negative prognostic impact on disease-specific and all cause-survival beyond a decade post-diagnosis (13). DNA repair deficiency in tumors is found to be predictive of higher 5-year survival probability, but at 10 years post-diagnosis, the benefit appears to be lost (6). It is possible that changes in the immune milieu may partly contribute to the lack of long-term survival benefit in these patients. We therefore hypothesize that the gBRCAm HGSOC is associated with a more robust circulating immune response during their early disease course, compared to BRCAwt disease.
The immune system responds dynamically to variations in the tumor microenvironment (14). Many studies indicate that altered compositions of peripheral immune cells, e.g., lymphocyte proportion, neutrophil proportion, and neutrophil-to-lymphocyte ratios in the peripheral blood, are potential markers for survival in cancer patients (15,16). Thus, monitoring these varying immune responses over time and treatment may uncover new vulnerabilities. In this pilot study, our aim was to quantify the immune subsets and functional markers using blood samples from HGSOC patients.
Materials and methods
Patients and isolation of PBMCs
This study was approved by the Institutional Review Boards of the Center for Cancer Research, National Cancer Institute and Dana-Farber Cancer Institute (DFCI). Peripheral blood mononuclear cells (PBMC) were collected before treatment from recurrent HGSOC patients enrolled on one of the two phase I PARP inhibitor olaparib trials at the Clinical Center of National Cancer Institute (NCT01445418 and NCT01237067) for Cohort 1 (17,18). All patients had at least a 4 weeks wash-out period from previous therapy before enrollment. BRCA mutation status was confirmed by a commercial BRCA testing (Myriad Genetic Laboratories) prior to enrollement on study.
To examine the findings from Cohort 1 in a broad patient population, PBMC samples were also obtained from unselected advanced stage or recurrent ovarian cancer patients enrolled on the blood collection protocols at DFCI or National Cancer Institute (Cohort 2). Blood samples were collected in cell preparation tubes with sodium citrate (BD Vacutainer CPT Tubes; BD Biosciences). PBMCs were isolated and viably frozen within 2 h from collection at National Cancer Institute and within 24 h for the samples collected at the DFCI; a minimum of 1×105 cells were acquired for each analysis. All patients reviewed and signed an informed consent form approved by the National Cancer Institute or DFCI Institutional Review Board for collection of blood samples.
Flow cytometric analysis
Multiparameter flow cytometric analysis was performed as described previously (19). Briefly, cells were incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (1:100 dilution) (Thermo Fisher Scientific, Inc.) for 15 min at 4°C, then incubated with Fc receptor blocking agent (1:10 dilution) (Miltenyi Biotec) and stained for 20 min at 4°C in a dark room with the monoclonal antibodies listed in Table SI. For intracellular staining for Foxp3 expression, cells were fixed and permeabilized using a Fix/Perm buffer (eBiosciences) according to the manufacturer's instructions, then stained with anti-Foxp3 antibody. The immunophenotypic markers used to define immune cell subsets are listed in Table SII. All antibodies were purchased from BioLegend. Live cells were discriminated by means of the LIVE/DEAD stain, and dead cells were excluded from all analyses. All flow cytometric analyses were performed using a MACSQuant Analyzer (Miltenyi Biotec). As indicated, flow cytometric data were quantified either as a percentage of cells or as the median fluorescence intensity (MFI). Data were analyzed using FlowJo software (FlowJo LLC.).
Statistical analysis
An exact Wilcoxon rank sum test was used to compare differences in marker values between gBRCAm and BRCAwt patients. All statistical tests were two-tailed and the reported P-values are calculated using a formal correction for multiple comparisons (i.e., the Holm-Bonferroni method). This method sorts P-values in ascending order and compares them to a corresponding pre-defined value. Since there were 13 distinct hypotheses to be tested within each immune subset group, statistically significant P-values in our case were considered to be Pi<0.05/i, where i ranges from 13 to 1 in descending order. Thus, in order to declare statistical significance, the following significance thresholds were calculated: P1<0.003846, P2<0.004167, P3<0.004545, …, P13<0.05, for the first, second, third …, and thirteenth tested hypothesis, respectively.
The Kaplan-Meier method was used to obtain estimates of progression-free survival (PFS). PFS curves were compared with a two-tailed log-rank test (α=0.05). We separated marker values at their corresponding observed baseline median values. An unstratified Cox regression model was used to estimate the hazard ratio (HR) for the high marker group relative to the low marker group. All analyses were performed using GraphPad Prism software version 6.0 (GraphPad Software Inc.).
Results
Patients
Patient characteristics are detailed in Tables I and II. Cohort 1 contains pretreatment samples from 41 heavily pretreated recurrent HGSOC patients (16 gBRCAm [39%]; 25 BRCAwt [61%]). The median time from initial diagnosis was 4.27 years (<5 years [54% (22/41)] vs. >5 years post-diagnosis [46% (19/41)]) upon enrollment. Of the 19 patients who had more than 5 years from diagnosis, 17 patients relapsed within 5 years. Cohort 2 represents samples from unselected either primary or recurrent ovarian cancer patients in whom approximately 25% were gBRCAm carriers. The median time from initial diagnosis for all patients was 3.2 years (Table II). For Cohort 2, most patients (75% [55/73]) were <5 years post-diagnosis. Of the 18 patients who had more than 5 years from diagnosis, 17 patients relapsed within 5 years. We therefore chose a 5-year cut-off as a surrogate of the first decade after diagnosis given all except one patient had less than 10 years of follow-up.
Table I.
Cohort 1 patient characteristics.
| Characteristics | gBRCAm (n=16) | BRCAwt (n=25) | All (n=41) |
|---|---|---|---|
| Age, median (range), years | 52 (28–71) | 65 (49–73) | 65 (28–73) |
| Tumor status | |||
| Primary stage III/IV | 0 | 0 | 0 |
| Recurrent | 16 (100%) | 25 (100%) | 41 (100%) |
| Histology | |||
| HGSOC | 16 (100%) | 25 (100%) | 41 (100%) |
| Clear cell | 0 | 0 | 0 |
| Years from initial diagnosis | |||
| <5 years | 10 (63%) | 12 (48%) | 22 (54%) |
| ≥5 years | 6 (37%) | 13 (52%) | 19 (46%) |
| Number of previous lines of therapy, median (range) | 5 (2–8) | 7 (3–14) | 6 (2–14) |
| Platinum sensitivity | |||
| Platinum-sensitive recurrent disease | 5 (31%) | 8 (32%) | 13 (32%) |
| Platinum-resistant recurrent disease | 11 (69%) | 17 (68%) | 28 (68%) |
| Prior bevacizumab | |||
| Yes | 5 (31%) | 19 (76%) | 24 (58%) |
| No | 11 (69%) | 6 (24%) | 17 (42%) |
| Prior immune checkpoint inhibitors | 0 | 0 | 0 |
gBRCAm, germline BRCA mutation; BRCAwt, germline BRCA wild-type; HGSOC, high grade serous ovarian carcinoma.
Table II.
Cohort 2 patient characteristics.
| Characteristics | gBRCAm (n=18) | BRCAwt (n=55) | All (n=73) |
|---|---|---|---|
| Age, median (range), years | 57.5 (36–78) | 65 (30–84) | 64 (30–84) |
| Tumor status | |||
| Primary stage III/IV | 1 (6%) | 8 (15%) | 9 (12%) |
| Recurrent | 17 (94%) | 47 (85%) | 64 (88%) |
| Histology | |||
| HGSOC | 18 (100%) | 52 (95%) | 70 (96%) |
| Clear cell | 0 | 3 (5%) | 3 (4%) |
| Years from initial diagnosis | |||
| <5 years | 13 (72%) | 42 (76%) | 55 (75%) |
| ≥5 years | 5 (28%) | 13 (24%) | 18 (25%) |
| Number of previous lines of therapy, median (range) | 3 (1–11) | 5 (1–14) | 4 (1–14) |
| Platinum sensitivity | |||
| On active treatment with first line carboplatin/taxol vs. | 0 (0%) | 8 (15%) | 8 (10%) |
| Platinum-sensitive recurrent disease vs. | 9 (50%) | 7 (13%) | 16 (22%) |
| Platinum-resistant recurrent disease | 9 (50%) | 40 (72%) | 49 (68%) |
| Prior bevacizumab | |||
| Yes | 7 (64%) | 28 (51%) | 35 (48%) |
| No | 11 (36%) | 27 (49%) | 38 (52%) |
| Prior CTLA-4 inhibitor, prior vaccine and/or PD-1/PDL-1 blockade | 1 (5.6%) | 2 (3.6%) | 3 (4.1%) |
gBRCAm, germline BRCA mutation; BRCAwt: germline BRCA wild-type; HGSOC, high grade serous ovarian carcinoma; CTLA-4, cytotoxic T lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PDL-1, programmed death ligand-1.
Peripheral immune characteristics of recurrent HGSOC patients (Cohort 1)
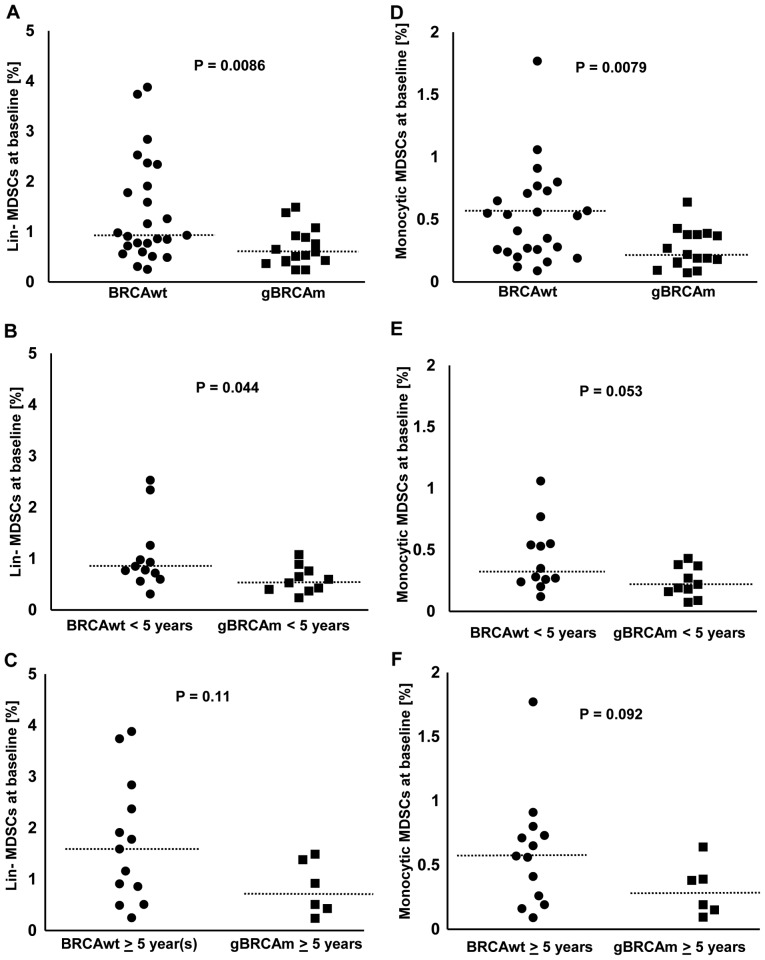
The percentage of MDSCs among viable CD45+ PBMCs was lower in gBRCAm HGSOC compared to BRCAwt disease (Fig. 1A-C). The percentage of lineage (lin)-MDSCs was overall lower in gBRCAm carriers (BRCAwt vs. gBRCAm, median 0.93 vs. 0.565%, p=0.0086; Fig. 1A). This difference was observed in gBRCAm patients <5 years post-diagnosis (BRCAwt vs. gBRCAm, median 0.815 vs. 0.565%, p=0.044; Fig. 1B) but not at >5 years post-diagnosis (Fig. 1C). Similarly, the percentage of monocytic MDSCs was overall lower in gBRCAm carriers (BRCAwt vs. gBRCAm, median 0.53 vs. 0.205%, p=0.0079; Fig. 1D). There was a trend of difference observed in gBRCAm patients <5 years post-diagnosis (Fig. 1E) but not at >5 years post-diagnosis (Fig. 1F). Additionally, gBRCAm HGSOC patients had fewer circulating lin-MDSCs and monocytic MDSCs independent of platinum-sensitivity and prior exposure to bevacizumab (Fig. S1A-D).
Figure 1.
The percentage of circulating MDSCs in gBRCAm and BRCAwt HGSOC (Cohort 1). (A) The percentage of lin-MDSCs was lower in gBRCAm HGSOC (n=25) compared with BRCAwt HGSOC (n=16; P=0.0086). (B and C) The difference of lin-MDSCs between gBRCAm (n=12) and BRCAwt (n=10) samples was seen in those <5 years from initial diagnosis (P=0.044) which did not remain in those [gBRCAm (n=13) and BRCAwt (n=6)] ≥5 years from initial diagnosis (P=0.11). (D) The percentage of monocytic MDSCs was lower in gBRCAm HGSOC (n=25) compared with BRCAwt HGSOC (n=16; P=0.0079). (E and F) There was a trend toward a lower percentage of monocytic MDSCs in gBRCAm compared with patients with BRCAwt, seen only in those <5 years from initial diagnosis (P=0.053), but not in those ≥5 years from initial diagnosis (P=0.092). The dotted lines represent the median values. gBRCAm, germline BRCA mutation; BRCAwt, germline BRCA wild-type; MDSCs, myeloid-derived suppressor cells; HGSOC, high-grade serous ovarian cancer.
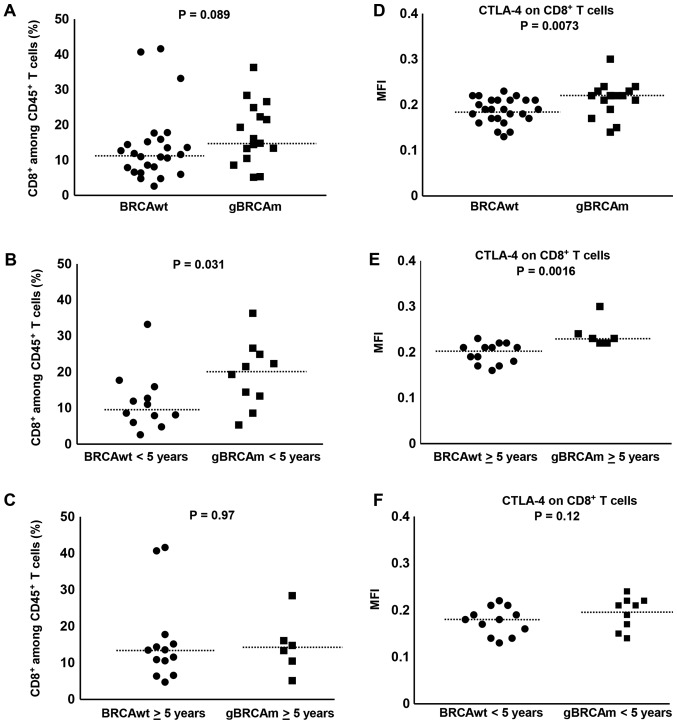
Overall, there was a trend of difference observed in gBRCAm patients in the percentage of circulating CD8+ T cells among viable CD45+ T cells (Fig. 2A) independent of platinum-sensitivity and prior exposure to bevacizumab (Fig. S1E-H). The percentage of circulating CD8+ T cells among viable CD45+ T cells was higher in gBRCAm carriers <5 years post-diagnosis over BRCAwt (BRCAwt vs. gBRCAm, median 9.78 vs. 20.4%, p=0.031; Fig. 2B). This difference was lost in survivors >5 years post-diagnosis (Fig. 2C). Further, there was significantly higher expression of CTLA-4 on CD8+ T cells in gBRCAm carriers (Fig. 2D) and this difference remained in gBRCAm carriers >5 years post-diagnosis compared to BRCAwt patients (BRCAwt vs. gBRCAm, median fluorescence intensity (MFI) 0.21 vs. 0.23, p=0.0016; Fig. 2E). This difference was lost in survivors <5 years post-diagnosis (Fig. 2F). Additionally, gBRCAm HGSOC patients had higher CTLA-4+ CD8+ T cells independent of platinum-resistant disease and prior exposure to bevacizumab (Fig. S1I-L).
Figure 2.
The percentage of CD8+ T cells and CTLA-4 expression on CD8+ T cells in gBRCAm and BRCAwt HGSOC (Cohort 1). (A) There was no difference between the percentage of CD8+ T cells in gBRCAm carriers compared to BRCAwt (P=0.089). (B and C) The percentage of CD8+ T cells was higher in gBRCAm carriers <5 years from initial diagnosis (P=0.031) but this difference did not persist in gBRCAm carriers ≥5 years from initial diagnosis (P=0.97). (D) CTLA-4 expression among CD8+ T cells was significantly higher in patients with HGSOC with gBRCAm compared with patients with BRCAwt (P=0.0073). (E and F) This difference was sustained in gBRCAm carriers ≥5 years from initial diagnosis (P=0.0016), but not in gBRCAm carriers <5 years from initial diagnosis (P=0.12). The dotted lines represent the median values. CTLA-4, cytotoxic T lymphocyte-associated protein 4; gBRCAm, germline BRCA mutation; BRCAwt, germline BRCA wild-type; HGSOC, high grade serous ovarian carcinoma; MFI, median fluorescence intensity.
We next evaluated the expression of other immunosuppressive markers, e.g., TIM-3 and PD-1 on CD8+ T cells. No significant differences were observed between gBRCAm and BRCAwt patients in expression of TIM-3 and PD-1 on CD8+ T cells (Fig. S2A-F).
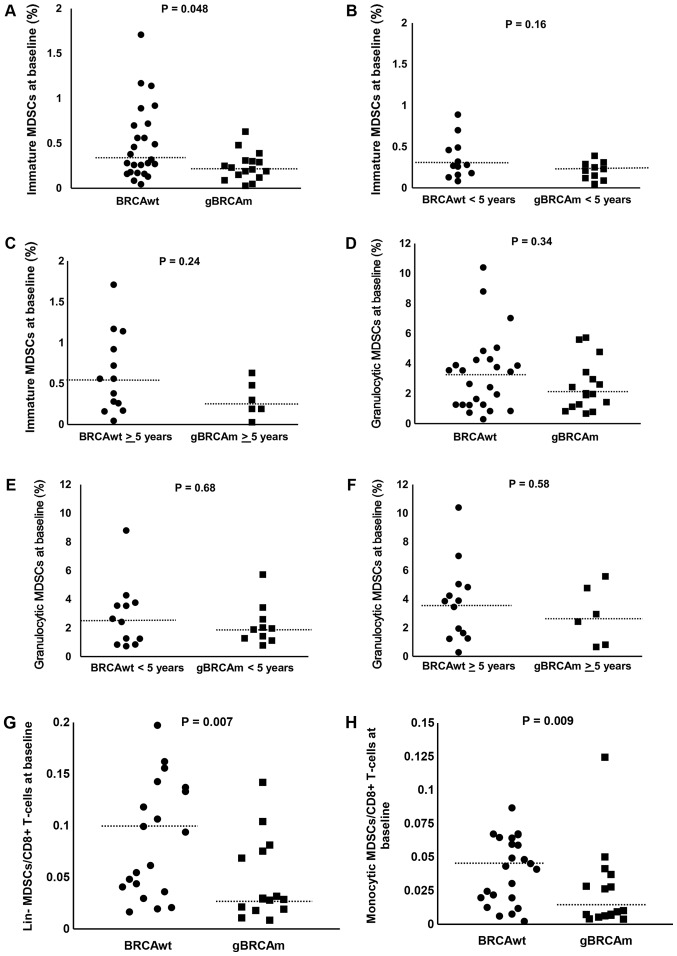
No significant differences were observed between gBRCAm and BRCAwt patients in the percentage of immature MDSCs (Fig. 3A-C). No significant differences were observed between gBRCAm and BRCAwt patients in the percentage of granulocytic MDSCs (Fig. 3D-F). We also evaluated the ratio of each subtype of MDSCs to circulating CD8+ T cells. There was a trend of difference observed in gBRCAm patients in the ratio of lin- and monocytic MDSCs to CD8+ T cells (Fig. 3G and H) but no other differences in proportion of MDSCs were found (data not shown). No difference in the percent/proportion of Tregs was found between gBRCAm and BRCAwt patients (data not shown).
Figure 3.
The percentage of circulating immature and granulocytic MDSCs in gBRCAm and BRCAwt HGSOC (Cohort 1). (A) The percentage of immature MDSCs was lower in gBRCAm HGSOC (n=25) compared with BRCAwt HGSOC (n=16; P=0.048). (B and C) The difference of immature MDSCs between gBRCAm (n=12) and BRCAwt (n=10) samples was not seen in those <5 years from initial diagnosis (P=0.16), or in those ≥5 years from initial diagnosis (P=0.24). Percentage of granulocytic MDSCs was not different between (D) gBRCAm and BRCAwt HGSOC (P=0.34), or (E) in those <5 years from initial diagnosis (P=0.68), or (F) in those ≥5 years from initial diagnosis (P=0.58). The ratio of (G) lin- and (H) monocytic MDSCs to CD8+ T cells were lower in gBRCAm (P=0.007 and P=0.009, respectively). The dotted lines represent the median values. MDSCs, myeloid-derived suppressor cells; gBRCAm, germline BRCA mutation; BRCAwt, germline BRCA wild-type; HGSOC, high grade serous ovarian carcinoma.
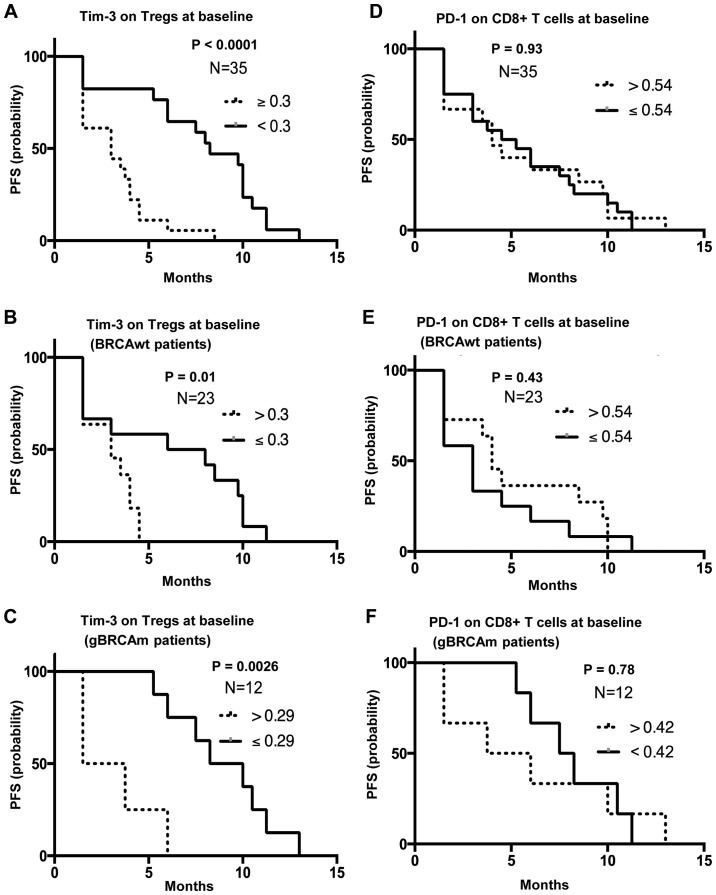
High baseline TIM-3 expression on Tregs is associated with poor PFS, independent of gBRCAm status
PFS analysis was performed using survival and progression data from Cohort 1. Patients with greater than and equal to the median MFI of TIM-3 on Tregs (n=18) were associated with poor survival compared to those with lower than the median MFI [n=17; median PFS 3 mo (1.5–8.5 mo) vs. 8.25 mo (1.5–13 mo); P<0.0001; HR, 0.2; 95% CI, 0.09–0.46; P<0.001, Fig. 4A]. This difference was observed in both BRCAwt and gBRCAm patients (HR, 0.3; 95% CI, 0.1–0.88; P=0.021; Fig. 4B, and HR, 0.09; 95% CI, 0.015–0.55; P=0.007; Fig. 4C, respectively). Other immune subsets did not differ between the two groups. Specifically, no significant differences were observed in PD-1 on CD8+ T cells expression, CTLA-4 on Tregs expression or percentage of granulocytic MDSCs between gBRCAm and BRCAwt patients (Fig. 4D-F and Fig. S3A-F).
Figure 4.
TIM-3 expression on Tregs and PD-1 on CD8+ T cells. PFS analysis was performed based on the progression/survival data of 35 patients in the Cohort 1 who were treated with at least one cycle of olaparib and carboplatin. Six patients were not available for PFS analysis because they were taken off study due to intercurrent illness (n=1), patient withdrawal (n=2) and clinical progression (n=3) within one month. (A) Women with greater than and equal to the median MFI of TIM-3 on Tregs (n=18) were associated with poor survival compared with those with lower than the median MFI [n=17; median PFS 3 months (1.5–8.5 months) vs. median PFS 8.25 months (1.5–13 months), P<0.0001]. This difference was observed in both patients with (B) BRCAwt and (C) patients with gBRCAm. (D-F) PD-1 expression was not associated with PFS. TIM-3, T cell immunoglobulin and mucin domain 3; Tregs, T regulatory cells; gBRCAm, germline BRCA mutation; BRCAwt, germline BRCA wild-type; MFI, Median Fluorescence Intensity; PFS, progression-free survival; PD-1, programmed cell death protein 1.
Peripheral immune characteristics of Cohort 2 unselected primary or recurrent HGSOC patients
We next examined our findings from Cohort 1 in the unselected Cohort 2 ovarian cancer patient population who enrolled on the blood collection protocols (Table II). Approximately 88% (64 of 73) had recurrent HGSOC and 25% (18 of 73) were gBRCAm carriers. The percentage of MDSCs among single CD45+ viable cells was not significantly different (data not shown). CTLA-4, PD-1 or TIM-3 expression on CD8+ T cells did not show any differences in gBRCAm HGSOC patients compared to BRCAwt HGSOC regardless of platinum-sensitivity, prior exposure to bevacizumab or years from initial diagnosis (data not shown). Survival and progression data were not available for PFS analysis.
Discussion
Patients with gBRCAm ovarian cancer have increased therapeutic susceptibility to platinum agents and have a longer median survival time compared to those without gBRCAm. This earlier chemosensitivity does not appear to persist into long-term survival beyond a decade from diagnosis (20). Our findings suggest that gBRCAm recurrent HGSOC patients may have fewer circulating immunosuppressive MDSCs and more CD8+ T cells early in their disease course. This is the description of a potentially active immune microenvironment that could enhance response to therapy. The loss of this benefit and/or equivocation over time could presage immune exhaustion and reduced treatment susceptibility. It is possible that the loss of this differential coincides with the progressive loss of platinum sensitivity and multiplicity of treatment regimens, and may be partly associated with immune tolerance and loss of long-term survival advantage.
There is correlative evidence that native host anti-tumor immune mechanisms play a role in clinical outcome of epithelial ovarian cancer; the presence of greater intra-tumoral CD3+ T cell infiltrates is shown to prognosticate improved outcome in advanced ovarian cancer (8). Ovarian tumors with dense CD3+ CD8+ T cell infiltrates are strongly associated with favorable clinical outcomes; the five-year overall survival rate was 38% among patients whose tumors contained CD3+ T cells and 4.5% among patients whose tumors contained no T cells (P<0.001) (21). Also, gBRCAm HGSOC is shown to have a better prognosis when there is increased immune cell infiltrate in tumors (7,22). Hwang et al reported that a lack of intraepithelial TILs was associated with a worse survival outcome among ovarian cancer patients (pooled HR: 2.24, 95% CI; 1.71–2.91) (23). In support, increased Treg infiltration is related to poor prognosis in ovarian cancer (24). These data suggest that host immunity may play a role in delaying or preventing tumor recurrence after standard treatment and that immunosuppressive cells suppress the host anti-tumor immunity, leading to poor outcomes.
In our pilot study, gBRCAm HGSOC patients had fewer circulating MDSCs and a concomitant increase in circulating CD8+ T cells during their early disease course. The low numbers of MDSCs in gBRCAm patients may indicate a lack of widespread immunosuppression. MDSCs are immunosuppressive cells, known to down-regulate anti-tumor immunity (25). MDSCs represent a heterogeneous family of myeloid cells that suppress T cell immunity in tumor-bearing hosts and promote cancer cell proliferation, epithelial-mesenchymal transition, and tumor dissemination (26), and that promote immune suppression in epithelial ovarian cancer mouse models (27). Furthermore, MDSCs suppress the antigen-specific T cell response induced by both CD4+ and CD8+ T cells, and elevated concentrations of MDSCs are detected in the peripheral blood of cancer patients when compared with normal controls (28,29). Also, it has been proposed that MDSCs may enhance the ovarian cancer stem cell pool and thus increase the risk of relapse (30).
Additionally, our findings showed a higher expression of CTLA-4 on CD8+ T cells in gBRCAm carriers. Higuchi et al reported a CTLA-4 antibody, but not PD-1/PD-L1 blockade, synergized therapeutically with a PARP inhibitor, resulting in immune-mediated tumor clearance and improved survival in immunocompetent BRCA1-deficient murine ovarian cancer models (31). In this report, authors demonstrated the survival benefit of this combination was likely T-cell mediated and dependent on increases in local interferon (IFN)-γ production in the peritoneal tumor microenvironment. BRCA1 regulates IFN-γ signaling, upregulating the signal transducers and activators of transcription (STAT)1 and STAT2, involved in type I IFN signaling and activation of innate immune responses (32). Xu and colleagues also reported loss of BRCA2 upregulates a subset of IFN-related genes, e.g., APOBEC3F and APOBEC3G in BRCA2 knockout HCT116 colorectal carcinoma cells (33). The role of BRCA1 and BRCA2 in regulating IFN-γ signaling and immunosuppressive cells, along with associated clinical outcomes remains to be further elucidated.
There are preclinical data suggesting that DNA damages induced by platinum agents or PARP inhibition activate cGAS/STING pathway, resulting in activation of type I IFN and immune responses (34,35). Ding et al (34) reported PARP inhibition induces both adaptive and innate immune responses through a STING-dependent antitumor immune response in BRCA1-deficient ovarian mouse models. gBRCAm HGSOC is shown to have a higher tumor mutational burden and neoantigen expression compared with BRCAwt disease (6), which may further induce T cell activation and anti-tumor immunity. These findings suggest the PARPi and/or carboplatin in combination with immune checkpoint blockade may be a therapeutic opportunity for subsets of ovarian cancer patients. Further preclinical and clinical studies are warranted to explore this possibility.
Shifting the immune balance by the interruption of pro-tumor immunosuppression can enhance anti-tumor immunity, as shown in melanoma (36). The PD-1/PD-L1 interaction promotes this imbalance favoring tumor-mediated immunosuppression (37). Although we did not find any differences in peripheral PD-L1 expression on immune cells in our study, Strickland et al reported a significantly higher expression of PD-1 and PD-L1 on intraepithelial and peritumoral immune cells in gBRCAm ovarian cancer compared with HR-proficient tumors (7). PD-1/PD-L1 axis upregulation causes ‘exhaustion’ of T cells allowing cancer growth and disruption of CTL-mediated tumor killing, which may partly contribute to a poor prognosis in advanced ovarian cancers (38–43). However, our post-hoc correlative findings should be interpreted with caution and viewed as hypothesis generating because of the small number of samples we assessed.
Recent studies demonstrated an important role of TIM-3 T cell exhaustion in cancer (44–46). Wu and colleagues investigated the expression of TIM-3 on peripheral CD4+ T and CD8+ T cells in ovarian cancer and showed elevated expression of TIM-3 in T cells were associated with advanced stage and a higher tumor grade (poorly differentiated) (44). Kuchroo et al reported TIM-3 and PD-1 were coexpressed on CD8+ TILs in mice bearing transplanted tumors as well as on NY-ESO-1-specific CD8+ T cells in patients with advanced melanoma (46). Consistent with these reports, our current study showed higher TIM-3 was associated with poor prognosis in recurrent ovarian cancer patients, suggesting TIM-3 negative regulation on various T cell subsets. Blockade of TIM-3 pathways therefore may be an effective strategy in controlling tumor growth.
Our study has some limitations. First, our small sample size and less than a decade follow-up in most cases may introduce biases in estimating clinical benefit and our correlative endpoints were exploratory. Also, we were not able to complete the subgroup analysis e.g., gBRCA1m vs. gBRCA2m due to the small sample size at this time; future studies on this topic are warranted given possible survival differences between gBRCA1m and gBRCA2m carriers (47). We note that the small sample sizes in the present study prevent the statistical analysis from being extrapolated to the overall gBRCAm and BRCAwt patient populations. Therefore, it is possible that this limitation may affect the clinical and statistical significance of our findings. Secondly, we did not assess tissue immune subsets due to limited or unavailable tissue samples, and thus we cannot address how many of our patients may have both tumoral and peripheral immune exhaustion characteristics. Further, we did not perform transcriptome on clinical samples to evaluate gene signatures for T cell activation or exhaustion status (48). Lastly, we did not observe similar findings across immune subsets in Cohort 2, an unselected ovarian cancer sample set, most likely reflecting the more substantial heterogeneity of that population as well as the dynamic changes of peripheral immune microenvironment between newly diagnosed and progressively treated patients. Also, Cohort 2 patients did not have a 4-week washout from previous or active treatment prior to blood sample collection that may have made findings difficult to interpret. Examination of these parameters has been prospectively planned into our ongoing phase 2 clinical trial of the PD-L1 inhibitor, durvalumab, with the PARP inhibitor, olaparib, and/or a VEGFR inhibitor, cediranib (NCT02484404), in which we collect baseline and on-treatment tissues and blood samples from recurrent ovarian cancer patients.
Overall, disease outcome is influenced by both patient host and tumor characteristics (20). Among many characteristics, our pilot data suggest that fewer circulating MDSCs and higher CD8+ T cells among total PBMCs may be associated with favorable early clinical outcome of gBRCAm HGSOC patients. It is possible that changes in the immune milieu over the course of the disease contribute to the lack of long-term survival benefit. Further studies focusing on HGSOC are needed to further elucidate the long-term impact of immune factors on survival of gBRCAm HGSOC patients.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Seth Steinberg (Biostatistics and Data Management Section, Office of the Clinical Director, Center for Cancer Research) for his statistical input.
Funding
The present study was funded by the Intramural Program of the Center for Cancer Research, NCI, NIH (JML; grant no. ZIA BC011525) and the Division of Gynecologic Oncology at the Dana-Farber Cancer Institute (UM, LAM and KMM).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JML, DAB, YT, AY, MJL, ECK, CMA, UM, LAM, JRN, KMM and JBT contributed to the conception and design of the present study, and to the collection and assembly of data. JML, DAB, YT, AY, MJL, ECK, CMA, UM, LAM, JRN, KMM and JBT performed the data analysis and interpretation, and contributed to writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Boards of the Center for Cancer Research, National Cancer Institute and Dana-Farber Cancer Institute (DFCI). All patients reviewed and signed an informed consent form approved by the National Cancer Institute or DFCI Institutional Review Board for collection of blood samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lee JM, Ivy SP, Kohn EC. Challenges and opportunities for immunotherapies in gynecologic cancers. Oncology (Williston Park) 2016;30:67–69. [PubMed] [Google Scholar]
- 2.Bourla AB, Zamarin D. Immunotherapy: New strategies for the treatment of gynecologic malignancies. Oncology (Williston Park) 2016;30:59–66. 69. [PMC free article] [PubMed] [Google Scholar]
- 3.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 6.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 7.Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, Garber JE, Chowdhury D, Wu CJ, D'Andrea AD, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Sucheston-Campbell LE, Cannioto R, Clay AI, Etter JL, Eng KH, Liu S, Battaglia S, Hu Q, Szender JB, Minlikeeva A, et al. No evidence that genetic variation in the myeloid-derived suppressor cell pathway influences ovarian cancer survival. Cancer Epidemiol Biomarkers Prev. 2017;26:420–424. doi: 10.1158/1055-9965.EPI-16-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 11.Barnett BG, Rüter J, Kryczek I, Brumlik MJ, Cheng PJ, Daniel BJ, Coukos G, Zou W, Curiel TJ. Regulatory T cells: A new frontier in cancer immunotherapy. Adv Exp Med Biol. 2008;622:255–260. doi: 10.1007/978-0-387-68969-2_20. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Deng Z, Peng Y, Han L, Liu J, Wang L, Li B, Zhao J, Jiao S, Wei H. Ascites-derived IL-6 and IL-10 synergistically expand CD14+HLA-DR-/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget. 2017;8:76843–76856. doi: 10.18632/oncotarget.20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candido-dos-Reis FJ, Song H, Goode EL, Cunningham JM, Fridley BL, Larson MC, Alsop K, Dicks E, Harrington P, Ramus SJ, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–657. doi: 10.1158/1078-0432.CCR-14-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi L, Li B, Dong Y, Xu H, Chen L, Wang H, Li P, Zhao W, Gu Y, Wang C, Guo Z. Deconvolution of the gene expression profiles of valuable banked blood specimens for studying the prognostic values of altered peripheral immune cell proportions in cancer patients. PLoS One. 2014;9:e100934. doi: 10.1371/journal.pone.0100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Showe MK, Kossenkov AV, Showe LC. The peripheral immune response and lung cancer prognosis. OncoImmunology. 2012;1:1414–1416. doi: 10.4161/onci.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, Yu M, Gordon N, Ji J, Sissung TM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106:dju089. doi: 10.1093/jnci/dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Peer CJ, Yu M, Amable L, Gordon N, Annunziata CM, Houston N, Goey AK, Sissung TM, Parker B, et al. Sequence-specific pharmacokinetic and pharmacodynamic phase I/Ib study of olaparib tablets and carboplatin in women's cancer. Clin Cancer Res. 2017;23:1397–1406. doi: 10.1158/1078-0432.CCR-16-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas A, Rajan A, Szabo E, Tomita Y, Carter CA, Scepura B, Lopez-Chavez A, Lee MJ, Redon CE, Frosch A, et al. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: A clinical and translational study. Clin Cancer Res. 2014;20:5392–5402. doi: 10.1158/1078-0432.CCR-14-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoppenot C, Eckert MA, Tienda SM, Lengyel E. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol Oncol. 2018;148:204–212. doi: 10.1016/j.ygyno.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 22.McAlpine JN, Porter H, Kobel M, Nelson BH, Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25:740–750. doi: 10.1038/modpathol.2011.211. [DOI] [PubMed] [Google Scholar]
- 23.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–372. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Facciabene A, Motz GT, Coukos G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Cai Z, Zhang Y, Yutzy WH IV, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 28.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Ye Y, Liu P, Yu W, Wei F, Li H, Yu J. Suppression of T cells by myeloid-derived suppressor cells in cancer. Hum Immunol. 2017;78:113–119. doi: 10.1016/j.humimm.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, Adams SF. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckley NE, Hosey AM, Gorski JJ, Purcell JW, Mulligan JM, Harkin DP, Mullan PB. BRCA1 regulates IFN-gamma signaling through a mechanism involving the type I IFNs. Mol Cancer Res. 2007;5:261–270. doi: 10.1158/1541-7786.MCR-06-0250. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Xian J, Vire E, McKinney S, Wei V, Wong J, Tong R, Kouzarides T, Caldas C, Aparicio S. Up-regulation of the interferon-related genes in BRCA2 knockout epithelial cells. J Pathol. 2014;234:386–397. doi: 10.1002/path.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, Li BB, Xie S, Liu JF, Stover EH, et al. PARP inhibition elicits STING-dependent antitumor immunity in brca1-deficient ovarian cancer. Cell Rep. 2018;25:2972–2980.e5. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaffari A, Peterson N, Khalaj K, Vitkin N, Robinson A, Francis JA, Koti M. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer. 2018;119:440–449. doi: 10.1038/s41416-018-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 38.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 42.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Liu C, Qian S, Hou H. The expression of Tim-3 in peripheral blood of ovarian cancer. DNA Cell Biol. 2013;32:648–653. doi: 10.1089/dna.2013.2116. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Ma Y, Xu Y, Maerkeya K. TIM-3 expression identifies a distinctive PD-1+follicular helper T cell subset, with reduced interleukin 21 production and B cell help function in ovarian cancer patients. Int Immunopharmacol. 2018;57:139–146. doi: 10.1016/j.intimp.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: Emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 47.Ovarian tumor tissue analysis (OTTA) consortium. Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, Fogarty ZC, Gentry-Maharaj A, Tołoczko A, Hein A, et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3:e173290. doi: 10.1001/jamaoncol.2017.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalachand RD, Ruscito I, Dimitrova D, Panici PB, Sehouli J, Olek S, Braicu EI, Lu L, Katsaros D, Yu H, et al. Clinical characteristics and survival outcomes in BRCA1-methylated epithelial ovarian cancer (Bmeth-OC): A pooled analysis of data for 1,278 patients across five studies. J Clin Oncol 33: 2018;(Suppl 15):S5526. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.