Abstract
The antipyretic potential of viscosine, a natural product isolated from the medicinal plant Dodonaea viscosa, was investigated using yeast-induced pyrexia rat model, and its structure–activity relationship was investigated through molecular docking analyses with the target enzymes cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), and microsomal prostaglandin E synthase-1 (mPGES-1). The in vivo antipyretic experiments showed a progressive dose-dependent reduction in body temperatures of the hyperthermic test animals when injected with viscosine. Comparison of docking analyses with target enzymes showed strongest bonding interactions (binding energy −17.34 kcal/mol) of viscosine with the active-site pocket of mPGES-1. These findings suggest that viscosine shows antipyretic properties by reducing the concentration of prostaglandin E2 in brain through its mPGES-1 inhibitory action and make it a potential lead compound for developing effective and safer antipyretic drugs for treating fever and related pathological conditions.
Introduction
Pyrexia (fever) is associated with many diseases1 and is an expression of the body’s complex immunophysiologic response to infectious or inflammatory stimuli that trigger a cascade of biochemical reactions ultimately producing various endogenous pyrogens.2,3 Of these pyrogens, prostaglandin E2 (PGE2) is the principal fever mediator in mammals.4,5 Although it is body’s natural immune response and host-defense mechanism, pyrexia results in general body discomfort and adversely affects the normal functions of various body organs.6 Excessive rise of body temperature is controlled by endogenous antipyretics liberated within the brain during fever.7 However, the use of antipyretic drugs is many times indispensable.
The commonly used antipyretic and anti-inflammatory agents (i.e., salicylates and nonsteroidal anti-inflammatory drugs (NSAIDs)) stop or lower the formation of the principal fever mediator PEG2 by inhibiting the cyclooxygenase enzymes cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) selectively or nonselectively.8 However, COX inhibition can lead to various health complications. Nonselective COX inhibitors are harmful to the gastrointestinal tract causing gastric distress,9 ulceration, and other bleeding disorders.10 Their prolonged use in children and adolescents may lead to Reye’s syndrome (liver damage).11 Prolonged use of selective COX-2 inhibitors, particularly NSAIDs, is linked with higher risks of cardiac attack12,13 and stroke, and patients with kidney, heart, or liver conditions are at risk of developing kidney damage.14 The long-term use of cyclooxygenase inhibitors as antipyretic agents is associated with serious side effects. An alternative antipyretic strategy is the inhibition of prostaglandin E2 synthases (PGES),15,16 especially the microsomal prostaglandin E synthase-1 (mPGES-1). The mPGES-1 catalyzes transformation of PGH2 to PGE2 in the last biosynthetic step and is a promisingly safe target17,18 of antipyretic agents because its inhibition is free of the side effects associated with the COX inhibition.19,20 Numerous efforts are being made for the development of next-generation anti-inflammatory and antipyretic drugs based on mPGES-1 inhibitors.21−23 Usually for optimized search of lead compounds, structure-based strategy employing ligand–receptor molecular docking24 is adopted for predicting interaction affinities and binding modes of the drug molecules with a particular target receptor.25
We have previously isolated viscosine (Figure 1) from the medicinal plant Dodonaea viscosa, which is reported to possess antinociceptive,26 neuropharmacological,27 lipoxygenase inhibitory,28 hepatoprotective,29 anticholinesterase, and antioxidant properties.30 Since compounds with analgesic and/or anti-inflammatory properties often possess antipyretic properties, we investigated the in vivo antipyretic potential of viscosine and its possible mode of action using an in silico approach. In this paper, we present the antipyretic potential of viscosine against a yeast-induced pyrexia model in rats. We also report the in silico findings showing viscosine as an efficient mPGES-1 inhibitor, suggesting it as a lead compound for developing effective and safe antipyretic drugs.
Figure 1.
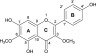
Molecular structure of viscosine.
Results
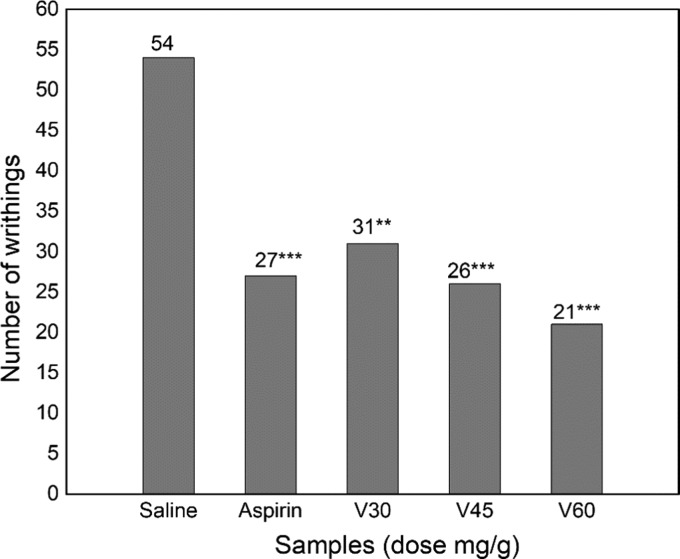
Yeast induces a biochemical cascade that ultimately leads to the release of biochemical mediators, especially prostaglandin E2 and interleukins, which in turn upregulate the thermoregulatory center, leading to hyperthermia and pyrexia.31 Any compound capable of inhibiting the cascade of pyrexia-inducing mediators will manifest antipyretic activity32 such as paracetamol and other NSAIDs. The antipyretic effects of viscosine on pyrexia are shown in Table 1. Yeast-induced pyrexia model was used to investigate the therapeutic effect of viscosine in relieving fever, which is often associated with inflammation.33 Upon treatment with viscosine, body temperatures of the test animals were observed to decrease progressively in a dose-dependent manner. Viscosine showed significant antipyretic potential (P < 0.01–0.001). In addition to the currently explored antipyretic potential, our previous studies26 also showed significant analgesic potential of viscosine compared to control, as depicted in its analgesic profile (Figure 2). To investigate the possible mechanism behind its antipyretic action, viscosine was docked with the possible target enzymes COX-1, COX-2, and mPGES-1, and their structure–activity relationships were investigated. The docking results are presented in Figures 3–5.
Table 1. Antipyretic Action of Viscosine against Yeast-Induced Pyrexia in Micea.
| dosage (mg/kg) |
||||
|---|---|---|---|---|
| time (h) | saline | V30 | V60 | aspirin (100) |
| body temperature | ||||
| 0 | 35.03 ± 0.26 | 34.77 ± 0.13 | 34.78 ± 0.12 | 35.02 ± 0.09 |
| 0.5 | 36.84 ± 0.19 | 37.11 ± 0.18 | 36.85 ± 0.14 | 36.07 ± 0.14 |
| 1 | 36.96 ± 0.12 | 36.83 ± 0.14 | 36.65 ± 0.17 | 36.59 ± 0.11 |
| 2 | 36.89 ± 0.09 | 36.54 ± 0.19 | 36.33 ± 0.09** | 36.26 ± 0.29** |
| 3 | 36.72 ± 0.11 | 36.04 ± 0.16** | 36.12 ± 0.07** | 35.81 ± 0.04*** |
| 4 | 36.59 ± 0.09 | 35.59 ± 0.12*** | 35.67 ± 0.09*** | 35.45 ± 0.02*** |
| 5 | 36.57 ± 0.06 | 35.26 ± 0.19*** | 35.28 ± 0.11*** | 35.24 ± 0.08*** |
Data are expressed as mean ± standard error of the mean. Significant at **P < 0.01, ***P < 0.001 compared to control.
Figure 2.
Analgesic profile of viscosine on acetic acid-induced writhing model: Saline, 10 mL/kg; aspirin, 150 mg/kg; V30, viscosine 30 mg/kg; V45, viscosine 45 mg/kg; V60, viscosine 60 mg/kg.
Figure 3.
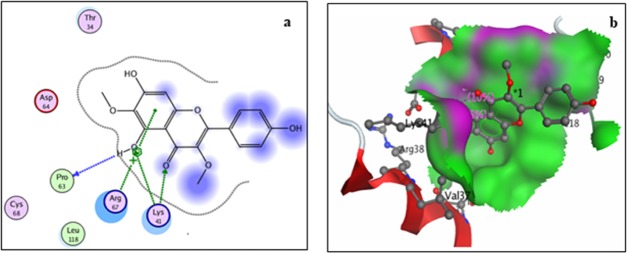
Two-dimensional (a) and three-dimensional (3D) (b) binding-site interaction models of COX-1 with viscosine: The blue highlight represents ligand exposure; H-bond lengths, 2.77–3.0 Å.
Figure 5.
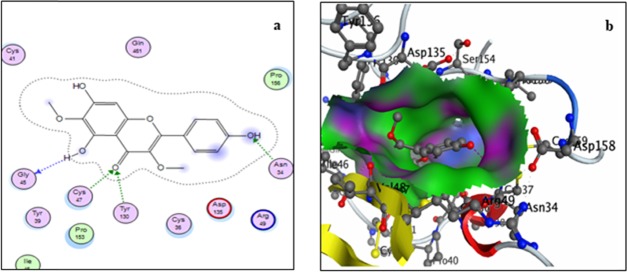
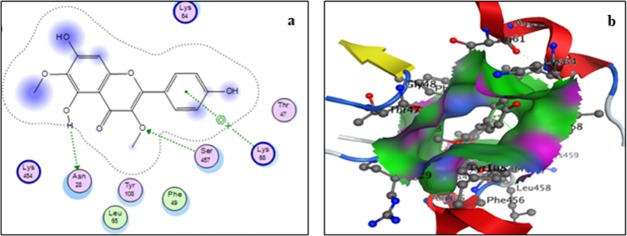
Two-dimensional (a) and three-dimensional (b) binding-site interaction models of mPGES-1 with viscosine: The blue highlight represents ligand exposure; H-bond lengths, 2.77–3.0 Å.
Discussion
In antipyretic studies, both viscosine (V60) and aspirin showed significant (P < 0.01) antipyretic activity after 2 h of their injection. The antipyretic potential of viscosine increased with passage of time. Both the low dosages of viscosine (V30 and V60) showed highly significant antipyretic potential at the later stages comparable to the reference drug, which showed significant effect at much higher dosage.
Results from the analgesic activities showed that viscosine as well as aspirin significantly suppressed the writhing response in a dose-dependent manner. It is apparent, however, that viscosine is significantly effective at lower doses compared to aspirin. In conclusion, viscosine revealed promising antipyretic potential during in vivo analyses and deserved further exploration of the molecular mechanisms involved behind its significant analgesic and antipyretic effects.
Docking analysis with COX-1 showed (Figure 3) a total of four interactions: a single interaction between hydroxyl group at position 5 of ring A with residue Gly45, two interactions involving the carbonyl group at position 4 of the ring C with residues Cys47 and Tyr130, and a single interaction between the hydroxyl group at position 4′ of the ring B with residue Asn34 of the receptor. Both the methoxy and aromatic rings showed no interactions with the receptor. All of the interactions are through hydrogen bonding and, thermodynamically, the inhibition is moderate with a binding energy of −13.34 kcal/mol.
Docking studies of viscosine with COX-2 also showed moderate ligand–receptor interactions (Figure 4). Overall, three interactions with an overall binding energy of −10.46 kcal/mol were observed, including an arene–cation interaction between ring B and residue Lys68 and two hydrogen-bonding interactions between hydroxyl moiety at position 5 with residue Asn28 and between 3-methoxy group and residue Ser457.
Figure 4.
Two-dimensional (a) and three-dimensional (b) binding-site interaction models of COX-2 with viscosine: The blue highlight represents ligand exposure; H-bond lengths, 2.77–3.0 Å.
Docking with mPGES-1 receptor also showed four interactions (Figure 5). Of these, ring A showed two arene–cation-type interactions with residues Lys41 and Arg67, while the 4-carbonyl and 5-hydroxyl groups of the ligand showed hydrogen-bonding interactions with Lys41 and Pro63, respectively. Residue Lys41 of the receptor showed a hydrogen-bonding interaction with carbonyl group of ring C and an arene–cation interaction with ring A of the ligand. It is evident from the data that viscosine interacts strongly with mPGES-1 with a predicted overall binding energy of −17.34 kcal/mol.
Based on the strongly supportive values of the binding energies obtained in our in silico investigations, it can be safely deduced that viscosine possesses favorable structural features to effectively interact and inhibit mPGES-1. This suggests viscosine to be a safe antipyretic agent and should be considered as a lead compound with promising potential for developing safe antipyretic drugs.
Conclusions
The in vivo studies performed on pyrexia-induced rats showed that viscosine possesses strong antipyretic actions. Low dosages of viscosine showed high antipyretic potential at later phases compared to aspirin. Viscosine also significantly suppressed the writhing response in a dose-dependent manner. Molecular docking studies suggest that viscosine showed stronger interactions with microsomal prostaglandin E synthase-1 than the cyclooxygenases and support the hypothesis that febrile response is reduced through mPGES-1 inhibition. Comparison of the binding energies of viscosine to that of other reported compounds32 suggests the need for further studies to confirm that viscosine strongly inhibits mPGES-1 prior to its consideration for developing safe antipyretic drugs.
Experimental Section
Materials and Methods
Experiments were conducted with adult wistar rats (weighing 180–260 g) and Swiss albino mice (weighing 18–25 g). All of the animals were housed individually at 24 ± 1 °C with abundant access to water and food until the experiment day, when only water was made available. Animal experiments performed in the manuscript were conducted in compliance with institutional guidelines,33 and permission for the experiments was previously granted by the local ethics committee for research on laboratory animals.
Yeast-Induced Pyrexia Model
The yeast-induced pyrexia model, as reported by Al-Ghamdi,34 was selected for evaluating the antipyretic properties of viscosine. Pyrexia was induced into the test animals by subcutaneously injecting a 15% aqueous yeast solution at a dosage of 10 mL/kg. Rectal temperatures of test animals were recorded 24 h before and after injecting yeast through a digital thermometer (Hartmann, Germany). The rats that did not show a minimum elevation of 0.5 °C in rectal temperatures after the yeast administration were excluded from experiment. The selected animals were divided into five groups (each group comprising six animals) and were separately treated with normal saline water, viscosine, and the reference aspirin. Rectal temperature of each animal was subsequently recorded for up to 5 h initially at 30 min and afterward at 1 h time intervals.
Molecular Docking Analysis
Viscosine was taken as a ligand for molecular docking. The ligand file was designed and optimized using the ChemBioDraw software (v. 14) and then converted to a 3D format through the “translate molecular files” tools available in the SYBYL-X 2.0 platform. Crystallographic structures of the targets COX-1 (PDB ID: 3KK6), COX-2 (PDB ID: 3LN1), and mPGES-1 (PDB ID: 3DWW) were taken from the archive of Protein Data Bank (RSCB PDB). The ligand was docked with targets using the MOE software.
Glossary
Abbreviations Used
- COX-1
cyclooxygenase 1
- COX-2
cyclooxygenase 2
- mPGES-1
microsomal prostaglandin E synthase-1
- NSAIDs
nonsteroidal anti-inflammatory drugs
- SAR
structure–activity relationship
- V30
viscosine 30 mg/kg
- V45
viscosine 45 mg/kg
- V60
viscosine 60 mg/kg
Accession Codes
Chemical Purity: Viscosine was purified through reverse-phase high-performance liquid chromatography, recrystallized, and characterized through single X-ray diffraction and NMR spectroscopy. The crystallographic data are available at the Cambridge Crystallographic Data Centre (CCDC 1041249).
The authors declare no competing financial interest.
References
- Fletcher T. E.; Bleeker-Rovers C. P.; Beeching N. J. Fever. Medicine 2017, 45, 177–183. 10.1016/j.mpmed.2016.12.013. [DOI] [Google Scholar]
- Young P. J.; Saxena M. Fever management in intensive care patients with infections. Crit. Care 2014, 18, 206. 10.1186/cc13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampronio A. R.; Hoadley M. E.; Luheshi G.; Rothwell N. J.; de Souza G. E.; Hopkins S. J. Interleukin (IL)-6 release and fever induced by a pre-formed pyrogenic factor (PFPF) derived from LPS-stimulated macrophages. Eur. Cytokine Network 2000, 11, 589–596. [PubMed] [Google Scholar]
- Romanovsky A. A.; Almeida M. C.; Aronoff D. M.; Ivanov A. I.; Konsman J. P.; Steiner A. A.; Turek V. F. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front. Biosci. 2005, 10, 2193–2216. 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Ivanov A. I.; Romanovsky A. A. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front. Biosci. 2004, 9, 1977–1993. 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Blatteis C. M. Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol. Ther. 2006, 111, 194–223. 10.1016/j.pharmthera.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Roth J. Endogenous antipyretics. Clin. Chim. Acta 2006, 371, 13–24. 10.1016/j.cca.2006.02.013. [DOI] [PubMed] [Google Scholar]
- De Souza G. E.; Cardoso R. A.; Melo M. C.; Fabricio A. S.; Silva V. M.; Lora M.; De Brum-Fernandes A. J.; Rae G. A.; Ferreira S. H.; Zampronio A. R. A comparative study of the antipyretic effects of indomethacin and dipyrone in rats. Inflammation Res. 2002, 51, 24–32. 10.1007/PL00000278. [DOI] [PubMed] [Google Scholar]
- Bombardier C. An evidence-based evaluation of the gastrointestinal safety of coxibs. Am. J. Cardiol. 2002, 89, 3–9. 10.1016/S0002-9149(02)02231-2. [DOI] [PubMed] [Google Scholar]
- Peura D. A. Gastrointestinal safety and tolerability of nonselective nonsteroidal anti-inflammatory agents and cyclooxygenase-2-selective inhibitors. Cleveland Clin. J. Med. 2002, 69, Si31–Si39. 10.3949/ccjm.69.Suppl_1.SI31. [DOI] [PubMed] [Google Scholar]
- Bailey J. M.; Low C. E.; Pupillo M. B. Reye’s syndrome and aspirin use: A possible imuunological relationship. Prostaglandins, Leukotrienes Med. 1982, 8, 211–218. 10.1016/0262-1746(82)90044-0. [DOI] [PubMed] [Google Scholar]
- Solomon D. H.; Avorn J.; Sturmer T.; Glynn R. J.; Mogun H.; Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006, 54, 1378–1389. 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G. A. Prostaglandins: modulators of inflammation and cardiovascular risk. J. Clin. Rheumatol. 2004, 10, S12–S17. 10.1097/01.rhu.0000130685.73681.8b. [DOI] [PubMed] [Google Scholar]
- Sandhu G. K.; Heyneman C. A. Nephrotoxic potential of selective cyclooxygenase-2 inhibitors. Annu. Pharmacother. 2004, 38, 700–704. 10.1345/aph.1D296. [DOI] [PubMed] [Google Scholar]
- O’Banion M. K. Prostaglandin E2 synthases in neurologic homeostasis and disease. Prostaglandins Other Lipid Mediators 2010, 91, 113–117. 10.1016/j.prostaglandins.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I.; Murakami M. Regulatory functions of prostaglandin E2 synthases. Adv. Exp. Med. Biol. 2003, 525, 103–106. 10.1007/978-1-4419-9194-2_20. [DOI] [PubMed] [Google Scholar]
- Park J. Y.; Pillinger M. H.; Abramson S. B. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–40. 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Prage E. B.; Morgenstern R.; Jakobsson P. J.; Stec D. F.; Voehler M. W.; Armstrong R. N. Observation of two modes of inhibition of human microsomal prostaglandin E synthase 1 by the cyclopentenone 15-deoxy-Delta(12,14)-prostaglandin J(2). Biochemistry 2012, 51, 2348–56. 10.1021/bi2019332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle A.; Werz O. Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders. Biochem. Pharmacol. 2015, 98, 1–15. 10.1016/j.bcp.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Lauro G.; Manfra M.; Pedatella S.; Fischer K.; Cantone V.; Terracciano S.; Bertamino A.; Ostacolo C.; Gomez-Monterrey I.; De Nisco M.; Riccio R.; Novellino E.; Werz O.; Campiglia P.; Bifulco G. Identification of novel microsomal prostaglandin E2 synthase-1 (mPGES-1) lead inhibitors from Fragment Virtual Screening. Eur. J. Med. Chem. 2017, 125, 278–287. 10.1016/j.ejmech.2016.09.042. [DOI] [PubMed] [Google Scholar]
- Rörsch F.; Buscató E. l.; Deckmann K.; Schneider G.; Schubert-Zsilavecz M.; Geisslinger G.; Proschak E.; Grösch S. Structure–Activity Relationship of Nonacidic Quinazolinone Inhibitors of Human Microsomal Prostaglandin Synthase 1 (mPGES 1). J. Med. Chem. 2012, 55, 3792–3803. 10.1021/jm201687d. [DOI] [PubMed] [Google Scholar]
- Luz J. G.; Antonysamy S.; Kuklish S. L.; Condon B.; Lee M. R.; Allison D.; Yu X.-P.; Chandrasekhar S.; Backer R.; Zhang A.; Russell M.; Chang S. S.; Harvey A.; Sloan A. V.; Fisher M. J. Crystal Structures of mPGES-1 Inhibitor Complexes Form a Basis for the Rational Design of Potent Analgesic and Anti-Inflammatory Therapeutics. J. Med. Chem. 2015, 58, 4727–4737. 10.1021/acs.jmedchem.5b00330. [DOI] [PubMed] [Google Scholar]
- Uramaru N.; Shigematsu H.; Toda A.; Eyanagi R.; Kitamura S.; Ohta S. Design, Synthesis, and Pharmacological Activity of Nonallergenic Pyrazolone-Type Antipyretic Analgesics. J. Med. Chem. 2010, 53, 8727–8733. 10.1021/jm101208x. [DOI] [PubMed] [Google Scholar]
- Guedes I. A.; de Magalhães C. S.; Dardenne L. E. Receptor–ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. 10.1007/s12551-013-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavatte P.; Yous S.; Marot C.; Baurin N.; Lesieur D. Three-Dimensional Quantitative Structure–Activity Relationships of Cyclo-oxygenase-2 (COX-2) Inhibitors: A Comparative Molecular Field Analysis. J. Med. Chem. 2001, 44, 3223–3230. 10.1021/jm0101343. [DOI] [PubMed] [Google Scholar]
- Khan A. Z. Antinociceptive potential of viscosine isolated form dodonaea viscosa in animal models. J. Chem. Soc. Pak. 2014, 36, 1150–1152. [Google Scholar]
- Karim N.; Irshad S.; Khan I.; Mohammad A.; Anis I.; Shah M. R.; Khan I.; Chebib M. GABAA receptor modulation and neuropharmacological activities of viscosine isolated from Dodonaea viscosa (Linn). Pharmacol., Biochem. Behav. 2015, 136, 64–72. 10.1016/j.pbb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Khan A. Z.; Mohammad A.; Iqbal Z.; Anis I.; Shah M. R.; Nadeem S.; Rabnawaz M.; Shahidullah A.; Khan H.; Khan I. Molecular docking of viscosine as a new lipoxygenase inhibitor isolated from Dodonaea viscosa. Bangla. J. of Pharmacol. 2013, 8, 36. 10.3329/bjp.v8i1.13088. [DOI] [Google Scholar]
- Ali H.; Kabir N.; Shah M. R.; Muhammad A.; Ali S.; Mehmood S.; Ali A.; Ali A.; Jahan A. Hepatoprotective activity of viscosine is mediated by attenuation of hepatic macrophages and iNOS expression in CCl4-intoxicated rats. Toxicol. Res. 2016, 5, 1688–1698. 10.1039/C6TX00165C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A.; Tel-Cayan G.; Öztürk M.; Nadeem S.; Duru M. E.; Anis I.; Ng S. W.; Shah M. R. Biologically active flavonoids from Dodonaea viscosa and their structure–activity relationships. Ind. Crops Prod. 2015, 78, 66–72. 10.1016/j.indcrop.2015.10.011. [DOI] [Google Scholar]
- Cranston W. I. Prostaglandins as mediators of pyrexia. Agents Actions Suppl. 1979, 79–82. [DOI] [PubMed] [Google Scholar]
- Khan I.; Nisar M.; Ebad F.; Nadeem S.; Saeed M.; Khan H.; Samiullah; Khuda F.; Karim N.; Ahmad Z. Anti-inflammatory activities of Sieboldogenin from Smilax china Linn.: experimental and computational studies. J. Ethnopharmacol. 2009, 121, 175–177. 10.1016/j.jep.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Nisar M.; Khan I.; Simjee S. U.; Gilani A. H.; Obaidullah; Perveen H. Anticonvulsant, analgesic and antipyretic activities of Taxus wallichiana Zucc. J. Ethnopharmacol. 2008, 116, 490–494. 10.1016/j.jep.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Al-Ghamdi M. S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. 10.1016/S0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]