Abstract
The aim of the current study was to develop and validate a nomogram based on a large population to estimate the 3- and 5-year survival rates of patients with malignant melanoma (MM). Patients were selected from the Surveillance, Epidemiology and End Results database and randomly divided into the training and validation cohorts. A nomogram was developed, and was used to assess the accuracy of the model. Independent prognostic factors associated with overall survival (OS) rate were identified through multivariate analysis, and were included in the internal validation of the nomogram. The nomogram provided high C-indexes for the training cohort [area under the time-dependent receiver operating characteristic curve (AUC) of 0.877 for 3-year OS rate and 0.872 for 5-year OS rate] and the validation cohort (AUC of 0.880 for 3-year OS rate and 0.874 for 5-year OS rate), indicating that the model had good discrimination ability. Calibration plots showed that the predicted 3- and 5-year OS rates probabilities for the training and validation groups were almost identical to the actual observations. The 3- and 5-year decision curves indicated net benefits for both the training and validation cohorts. The nomogram may aid clinicians to provide more accurate prognosis prediction in patient consultations and more personalized postoperative management plans.
Keywords: malignant melanoma, nomogram, risk factors, survival rate, Surveillance, Epidemiology and End Results program
Introduction
The incidence of melanoma is increasing worldwide (1–3). A statistical fact sheet from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) stated that the estimated number of new melanoma cases in 2019 in the USA was 96,480, accounting for 5.5% of all new cancer cases, and that the estimated death toll was 7,230 (https://seer.cancer.gov/statfacts/html/melan.html). Malignant melanoma (MM) is the most aggressive and deadly type of skin cancer (4). MM has a rapid development, high metastasis rate and poor prognosis; therefore, its early diagnosis and treatment are particularly important (5,6).
The SEER program of the NCI is a coordinated system of population-based state cancer registries collecting demographic, clinical and outcome information on all cancer cases diagnosed in representative geographic regions and subpopulations (7,8). It includes 18 registries that cover 30% of the population in the USA (9).
Nomograms incorporating and demonstrating important prognostic factors have been widely used in cancer prognosis predictions, and have become reliable and convenient tools for quantifying risk associated with diseases (10,11). They can reduce statistical predictive models to a single numerical estimate of the probability (PI) of an event (12,13). Several authors have reported that nomograms are more accurate in prognosis prediction than the traditional staging systems for patients with prostate, colon, breast and stomach cancer (14–18). Previous studies have reported a nomogram of melanoma at a site, or a nomogram of lymph node status in patients with melanoma (19,20). Our previous study also only reported a nomogram of nodular melanoma (21). However, there is no nomogram for predicting survival in patients with MM the from SEER database.
The aim of the current study was to develop and validate a nomogram based on a large population that could be used to estimate the 3- and 5-year survival rates of patients with MM. In addition to the American Joint Committee on Cancer (AJCC) staging, predictors such as age, sex and insurance status were included to provide a personalized and accurate assessment of patient survival.
Materials and methods
Patient selection
A retrospective search of the SEER (seer.cancer.gov) database was performed for cases of MM diagnosed between January 2007 and December 2015. The SEER database was accessed using the SEER*Stat software (version 8.3.4, http://seer.cancer.gov/seerstat/). Patients were identified using the International Classification of Diseases for Oncology-O-3 (22) histological type codes of 8720/3-8723/3, 8726/3, 8727/3, 8730/3, 8740/3-8746/3, 8761/3, 8770-8773/3, 8780/3 and 8790/3, and primary site codes of C44, C51.0, C60.9 and C63.2, and were used as the inclusion criteria. Cases that were not confirmed by microscopy or only by autopsy were excluded, as were those with unknown or incomplete variables. Additionally, patients <18 years were excluded. The selection criteria applied resulted in a total of 110,727 eligible patients. As data on cancer is reported by the SEER database each year, informed patient consent was not required to utilize the data released by the SEER database. The present study was exempted from the requirement for ethical approval and patient consent by the Institutional Research Committee of The First Affiliated Hospital of Xi'an Jiaotong University.
Nomogram construction
For the construction and validation of the nomogram, 70% (n=77,508) of the patients were randomly assigned to the training cohort and 30% (n=33,219) were assigned to the validation cohort. The following variables were assessed: Age at diagnosis, ethnicity, sex, marital status, tumor primary site, AJCC and SEER stage, insurance status and median family income.
Validation of the nomogram
The nomogram was validated using discrimination and calibration with the validation cohort. The predictive accuracy of the nomogram was evaluated by the area under the time-dependent receiver operating characteristic (ROC) curve. Concordance index (C-index), proposed by Frank E. Harrell Jr, Professor of biostatistics at Vanderbilt University in 1996 (23), is used to calculate the discrimination between the predicted and real values of Cox models in survival analysis. In general, a C-index >0.75 is considered to represent relatively good discrimination (24). The agreement between the predicted PI and the actual outcome was evaluated by calibration plotting. Calibration represents the ability of a model to provide unbiased estimates of outcome, and a perfectly accurate nomogram would result in a plot on which predictions fall along a 45° diagonal line. Discrimination and calibration were evaluated using bootstrapping with 500 resamples. Bootstrapping is a random sampling method and the sampling was repeated 500 times using R software (https://www.r-project.org/; version 3.6.1), increasing the accuracy.
The specificity and sensitivity may be determined from the ROC curve when evaluating a diagnostic method. Vickers and Elkin (25) developed a method of evaluation termed decision curve analysis (DCA), which was used to test the clinical value of the predictive models in the present study, in terms of the net benefit. DCA is a simple way to evaluate clinical predictive models. Traditional models, such as ROC curves, only measure the accuracy of predictive model diagnosis, in order to consider the clinical utility of the model, while the DCA curve considers clinical utility (26).
Statistical analysis
Cox regression, nomogram, C-index, AUC, calibration plotting and DCA were performed using SPSS (version 24.0; IBM Corp.) and R software. P<0.05 was considered to indicate a statistically significant difference. The continuous variable, age, was assessed for normal distribution using Shapiro-Wilk test. Kaplan-Meier curves were generated using R software. Clinical pathological characteristics of the training and validation cohorts were selected using the backward stepwise selection method in the Cox regression model. Only variables that were statistically significant in the univariate Cox regression models were analyzed in multivariate Coxregression models.
Results
Patient characteristics
The present study included 110,727 patients with MM. The median age (25th-75th percentile: 52–74) at the time of diagnosis of was 62 years in the two cohorts. The majority of the patients were male (59.7% for the training cohort; 60.1% for the validation cohort), white (98.5%) and married (68.5%). The most common primary tumor site was the trunk (31.1%). The majority of the patients were in AJCC stage I (69.7%). In both cohorts, 82.3% patients had a localized tumor, 11.7% patients had regional metastasis and 6.0% patients had distant metastasis. The majority of the patients were insured (93.8%) and had a median family income of USD 50,060-98,030 (82.2%; Table I).
Table I.
Clinical characteristics of patients with malignant melanoma in the present study.
| Characteristic | Training cohort | Validation cohort |
|---|---|---|
| Median age at diagnosis, (25th-75th percentile) | 62 (52–74) | 62 (52–74) |
| Ethnicity, n (%) | ||
| White | 76,377 (98.5) | 32,735 (98.5) |
| Black | 421 (0.5) | 163 (0.5) |
| Other | 710 (1.0) | 321 (1.0) |
| Sex, (%) | ||
| Male | 46,286 (59.7) | 19,949 (60.1) |
| Female | 31,222 (40.3) | 13,270 (39.9) |
| Marital status, n (%) | ||
| Married | 52,970 (68.5) | 22,748 (68.5) |
| Single | 11,739 (15.0) | 4,982 (15.0) |
| Divorced, separated or widowed | 12,799 (16.5) | 5,489 (16.5) |
| Site, n (%) | ||
| Head and neck | 16,870 (21.8) | 7,202 (21.8) |
| Trunk | 24,075 (31.1) | 10,407 (31.1) |
| Upper Limbs | 19,472 (25.1) | 8,326 (25.1) |
| Lower Limbs | 13,775 (17.8) | 5,905 (17.8) |
| Other | 3,316 (4.2) | 1,379 (4.2) |
| AJCC stage, n (%) | ||
| I | 53,935 (69.7) | 23,143 (69.7) |
| II | 12,163 (15.6) | 5,177 (15.6) |
| III | 7,222 (9.3) | 3,070 (9.2) |
| IV | 4,188 (5.4) | 1,829 (5.5) |
| SEER stage, n (%) | ||
| Localized | 63,803 (82.3) | 27,355 (82.3) |
| Regional | 9,076 (11.7) | 3,872 (11.7) |
| Distant | 4,629 (6.0) | 1,992 (6.0) |
| Insurance status, n (%) | ||
| Any medicaid | 3,209 (4.1) | 1,413 (4.2) |
| Insured | 72,713 (93.8) | 31,132 (93.8) |
| Uninsured | 1,586 (2.1) | 674 (2.0) |
| Median family income, USD, n (%) | ||
| 24,880-50,000 | 5,461 (7.2) | 2,385 (7.2) |
| 50,060-98,030 | 63,817 (82.2) | 27,311 (82.2) |
| 100,190-125,990 | 8,230 (10.6) | 3,523 (10.6) |
SEER, Surveillance, Epidemiology, and End Results; AJCC, American Joint Committee on Cancer; USD, United states dollar.
Cox regression analysis of the training cohort
Following univariate Cox regression analysis, data on age at diagnosis, ethnicity, sex, marital status, tumor primary site, AJCC and SEER stage, insurance status and median family income were entered into the multivariate Cox regression analysis. The multivariate Cox regression analysis revealed that age [hazard ratio (HR)=1.015; P<0.001], being single (HR=1.095 vs. married; P=0.011), being divorced, separated or widowed (DSW) (HR=1.184 vs. married; P<0.001), AJCC stage II (HR=5.759 vs. AJCC stage I; P<0.001), AJCC stage III (HR=9.320 vs. AJCC stage I; P<0.001), AJCC stage IV (HR=19.148 vs. AJCC stage I; P<0.001), having regional metastasis (HR=1.592 vs. localized; P<0.001), having distant metastasis (HR=1.858 vs. localized; P<0.001), and not being insured (HR=1.201 vs. any medicaid insurance; P=0.013) were significant risk factors for the overall survival (OS) rate. Locations in the trunk (HR=0.893 vs. head and neck; P=0.001), upper limbs (HR=0.753 vs. head and neck; P<0.001) and lower limbs (HR=0.866 vs. head and neck; P<0.001) were protective factors. Ethnicity was not statistically significant in the multivariate Cox regression (P>0.05). (Table II).
Table II.
Univariate and multivariate Cox regression analysis (training cohort).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at diagnosis | 1.020 | 1.019–1.022 | <0.001 | 1.015 | 1.013–1.016 | <0.001 |
| Ethnicity | 1.477 | 1.357–1.609 | <0.001 | |||
| White | ||||||
| Black | ||||||
| Other | ||||||
| Sex | 0.612 | 0.582–0.645 | <0.001 | |||
| Male | Reference | |||||
| Female | 0.806 | 0.763–0.852 | <0.001 | |||
| Marital status | 1.260 | 1.225–1.297 | <0.001 | |||
| Married | Reference | |||||
| Single | 1.095 | 1.021–1.174 | 0.011 | |||
| DSW | 1.184 | 1.114–1.259 | <0.001 | |||
| Site | 1.393 | 1.365–1.423 | <0.001 | |||
| Head and neck | Reference | |||||
| Trunk | 0.893 | 0.832–0.958 | 0.001 | |||
| Upper limbs | 0.753 | 0.695–0.816 | <0.001 | |||
| Lower limbs | 0.866 | 0.797–0.941 | <0.001 | |||
| Other | 1.011 | 0.930–1.099 | 0.800 | |||
| AJCC stage | 3.249 | 3.183–3.316 | <0.001 | |||
| I | Reference | |||||
| II | 5.759 | 5.300–6.258 | <0.001 | |||
| III | 9.320 | 8.132–10.682 | <0.001 | |||
| IV | 19.148 | 15.089–24.299 | <0.001 | |||
| SEER stage | 4.719 | 4.594–4.847 | <0.001 | |||
| Localized | Reference | |||||
| Regional | 1.592 | 1.420–1.785 | <0.001 | |||
| Distant | 1.858 | 1.487–2.321 | <0.001 | |||
| Insurance status | 0.612 | 0.560–0.670 | <0.001 | |||
| Any medicaid | Reference | |||||
| Insured | 0.762 | 0.698–0.832 | <0.001 | |||
| Uninsured | 1.201 | 1.039–1.388 | 0.013 | |||
| Median family income, USD | 0.746 | 0.705–0.790 | <0.001 | |||
| 24,880-50,000 | Reference | |||||
| 50,060-98,030 | 0.916 | 0.843–0.994 | 0.035 | |||
| 100,190-125,990 | 0.800 | 0.713–0.896 | <0.001 | |||
HR, hazard ratio; CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results; AJCC, American Joint Committee on Cancer; USD, United states dollar.
Nomogram validation
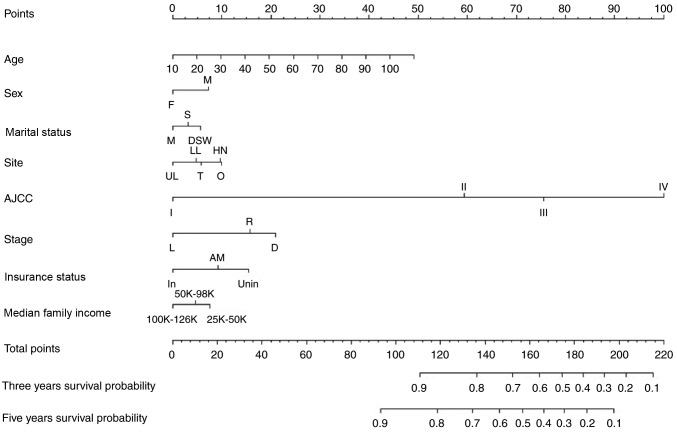
Independent prognostic factors associated with the OS rate identified in the multivariate analysis were incorporated into the nomogram internal validation. The nomogram was used by first drawing a vertical line up to the points row to obtain the points for each variable, adding up the points for all the variables to obtain the total points, and drawing a vertical line down from the total points row to obtain the 3- and 5-year OS rates (Fig. 1).
Figure 1.
Nomogram predicting 3- and 5-year survival. F, female; M, male; M, married; S, single; DSW, divorced, separated or widowed; UL, upper limbs; LL, lower limbs; T, trunk; HN, head and neck; O, other; SEER, Surveillance, Epidemiology, and End Results; L, localized; R, regional; D, distant. status: In, insured; AM, any medicaid (Indian/Public Health Service; Medicaid, Medicaid-Administered through a managed care plan; Medicare with Medicaid eligibility); Unin, uninsured; AJCC, American Joint Committee on Cancer. The nomogram is used by first giving each variable a score on its points scale. The scores for all variables are then added to obtain the total score and a vertical line is drawn from the total-points row to estimate the 3-year and 5-year survival rates.
Performance of the nomogram
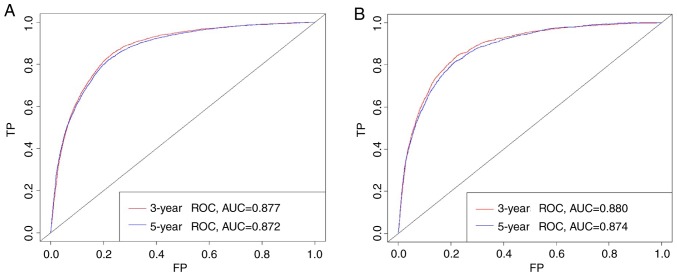
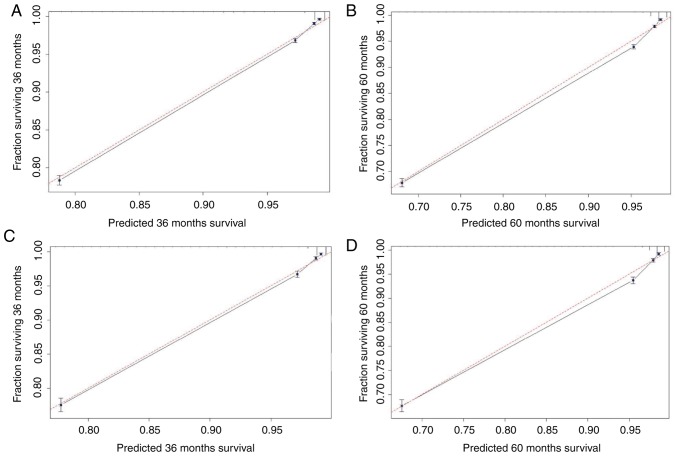
Based on the C-index analysis of the SEER training cohort, the nomogram provided high C-indexes for the 3- and 5-year OS rates, with AUCs of 0.877 and 0.872, respectively (Fig. 2A). Similarly, the corresponding values for the validation cohort were high, 0.880 and 0.874, respectively, indicating that the model had good discrimination ability (Fig. 2B). As presented in Fig. 3, the predicted 3- and 5-year OS rate probabilities for the SEER training and validation groups were almost identical to the actual observations.
Figure 2.
AUCs of the nomogram. (A) Training and (B) validation cohorts. AUC, area under the curve; ROC, receiver operating characteristic; TP, true positive rate; FP, false positive rate.
Figure 3.
Calibration curves for the nomogram. The x-axis is the predicted survival calculated by the nomogram, and the y-axis is the actual survival estimated by the Kaplan-Meier method. The 95% confidence intervals of the Kaplan-Meier estimates are indicated by black vertical lines at each point. The red dashed line presents the reference line and is a 45° diagonal. The closer the black line drawn by the model is to the red dashed line, the better the model. (A) Calibration plot of the 3-year OS for the training cohort. (B) Calibration plot of the 5-year OS for the training cohort. (C) Calibration plot of the 3-year OS for the validation cohort. (D) Calibration plot of the 5-year OS for the validation cohort. OS, overall survival.
DCA
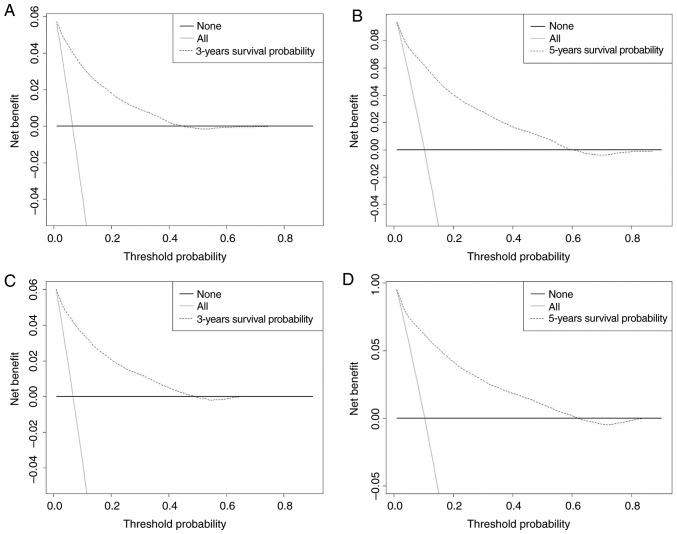
The abscissa of a decision curve is the threshold PI and the ordinate is the net benefit rate. A horizontal line indicates that all samples are negative and not treated (with a net benefit of zero), i.e. these patients are disease-free and do not require treatment. An oblique line, with a positive slope, indicates that all samples are positive and the patients had received treatment, and the net benefit is indicated by an oblique line with a negative slope. In our model, the 3- and 5-year DCA curves yielded net benefits for both the training and validation cohorts (Fig. 4).
Figure 4.
DCA of the 3- and 5-year OS for the training and validation cohorts. The abscissa represents the threshold probability and the ordinate represents the net benefit rate. The horizontal line indicates that all samples are negative and all are not treated, with a net benefit of zero. The oblique line indicates that all samples are positive. The net benefit is represented by a negative slope. The dashed line does not coincide with the other two lines, and when it is in the upper right corner, it means that the model is valuable. (A) DCA of the 3-year OS for the training cohort. (B) DCA of the 5-year OS for training cohort, (C) DCA of 3-year OS for the validation cohort. (D) DCA of the 5-year OS for the validation cohort. DCA, decision curve analysis; OS, overall survival.
Discussion
The AJCC staging manual has become the benchmark for classifying patients with cancer, predicting their prognosis and selecting appropriate treatment approaches (27). However, the prognosis may differ in patients at the same pathological stage due to other influencing factors such as age, sex, histological type and adjuvant chemotherapy, all of which may affect the OS rate (16). MM is one of the most aggressive forms of cutaneous neoplasm, and its incidence is increasing (28). Therefore, a more individualized approach for predicting the survival of patients with MM is required. Nomograms may provide an accurate and informative prognosis for individual patients (12,29,30), and have already been applied for prognosis prediction of various tumor types. For example, Cai et al (31) developed a nomogram for hepatic functional reserve and tumor characteristics of hepatocellular carcinoma following therapeutic hepatectomy. Zhang et al (32) established a gallbladder cancer-specific survival model to predict the prognosis of patients with non-metastatic gallbladder cancer following non-surgical treatment. Pietrantonio et al (33) established a nomogram for predicting the survival PI of patients with advanced gastric cancer. The SEER database provides a large number of samples for exploring risk factors and developing accurate predictive models. The analysis of data collected by the SEER database renders the results obtained in the current study more generalizable than those from single-center studies. Our group has recently published an article on nodular melanoma, which is limited to nodular tumors (21). However, the present study investigated the prognosis of all malignant melanomas and includes more cases and is more comprehensive/accurate. To the best of our knowledge, the present study is the first to use a nomogram to develop a predictive model for predicting 3- and 5-year survival rates in patients with MM.
The present study included 110,727 cases from the SEER database, and 70 and 30% of the patients were randomly assigned to the training and validation cohorts, respectively. Factors that affect patient survival were assessed using the Cox proportional-hazards model. Variables that were significant in the univariate analysis were included in the final multivariate model. The multivariate Cox regression analysis revealed that the age at diagnosis was a risk factor for survival in patients with MM. As for all types of cancer, the incidence of melanoma increases with age (34), which is consistent with the results obtained in the current study. Compared with being married, being single and DSW were risk factors for survival, which has also been observed in previous studies (35,36). Being female was a protective factor, as was having localized disease. McLaughlin et al (37) found that among married, single, divorced or separated patients, the risk of a late diagnosis was >50% higher in males than in females. A previous study investigating head and neck MM found that the prognosis was better in female patients compared with male patients (38).
Ethnicity was not statistically significant in the multivariate Cox regression. Compared with the head and neck, locations in the trunk, upper and lower limbs were protective factors. The results of ethnicity and site remain to be interpreted, but may be due to the use of different data and the specific factors selected in the predictive model.
The present study included DCA, which is a recently described analytical technique. Although novel, a previous study has recommended the use of DCA (26). The present study revealed that the 3- and 5-year DCA curves yielded net benefits for both the training and validation cohorts, indicating that the model provides certain clinical benefits. For example, in a validation set of three years of survival, the PI of a patient diagnosed with MM is recorded as PI, and when the threshold of PI reaches 20%, it is defined as positive and treatment is received. At this point, there will be patients benefiting, and there will be non-patients receiving treatment, causing damage, and patients not receiving treatment, which is a loss. The ordinate is the net benefit, and two of the 100 people would have a net benefit.
The present study was subject to some limitations. Firstly, only patients for whom complete information was available were included, which may have introduced selection bias. Secondly, common variables associated with prognosis, such as smoking history and genetic mutations (39), were not included in the model as no relevant data was available in the SEER database. Thirdly, an internal validation approach was used to evaluate the model performance. Although the model exhibited good performance, external verification is required to estimate its accuracy and verify its utility for decision-making. Fourthly, the values predicted by the nomogram should be considered as reference values, rather than representing certain prognoses. Finally, the current study was based on retrospective data, and so inherent bias was inevitable.
The present study developed and internally validated a nomogram for predicting the 3- and 5-year survival rates in patients with MM. This nomogram predicted the survival rate of individual patients with a high C-index, and was found to be well calibrated and the DCA curves yielded net benefits. The results obtained in the current study may aid clinicians to provide more accurate prognosis predictions in patients with MM.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Social Science Foundation of China (grant no. 16BGL183) and the Research Fund of Health Bureau of Xi'an (grant no. QFO1330).
Availability of data and materials
The datasets analyzed during the current study are publicly available in the Surveillance, Epidemiology, and End Results repository (https://seer.cancer.gov/).
Authors' contributions
JL conceived and designed the study. JY and QL screened data from the database. JY, ZP and QZ analyzed the data. QL, FZ, XF and JL participated in data interpretation. JY wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bhandaru M, Ardekani GS, Zhang G, Martinka M, McElwee KJ, Li G, Rotte A. A combination of p300 and Braf expression in the diagnosis and prognosis of melanoma. BMC Cancer. 2014;14:398. doi: 10.1186/1471-2407-14-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrocal A, Cabañas L, Espinosa E, Fernández-de-Misa R, Martín-Algarra S, Martínez-Cedres JC, Ríos-Buceta L, Rodríguez-Peralto JL. Melanoma: Diagnosis, staging, and treatment. consensus group recommendations. Adv Ther. 2014;31:945–960. doi: 10.1007/s12325-014-0148-2. [DOI] [PubMed] [Google Scholar]
- 3.Mishra H, Mishra PK, Ekielski A, Jaggi M, Iqbal Z, Talegaonkar S. Melanoma treatment: From conventional to nanotechnology. J Cancer Res Clin Oncol. 2018;144:2283–2302. doi: 10.1007/s00432-018-2726-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Zhang N. Clinical and prognostic factors in 98 patients with malignant melanoma in China. J Int Med Res. 2017;45:1369–1377. doi: 10.1177/0300060517708922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan X, Zhu Y, Zhang L, Hou W. Gefitinib inhibits malignant melanoma cells through the VEGF/AKT signaling pathway. Mol Med Rep. 2018;17:7351–7355. doi: 10.3892/mmr.2018.8728. [DOI] [PubMed] [Google Scholar]
- 6.Kang X, Zeng Y, Liang J, Li J, Ren D, Chai L, Sun Z, Yu S, Wu X, Han W, Wang W. Aberrations and clinical significance of BRAF in malignant melanoma. Medicine. 2018;97:e9509. doi: 10.1097/MD.0000000000009509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You H, Yang J, Liu Q, Tang L, Bu Q, Pan Z, Lyu J. The impact of the lymph node density on overall survival in patients with Wilms' tumor: A SEER analysis. Cancer Manag Res. 2018;10:671–677. doi: 10.2147/CMAR.S163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and end results (SEER) program and pathology. Am J Surg Pathol. 2016;40:e94–e102. doi: 10.1097/PAS.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healy MA, Morris AM, Abrahamse P, Ward KC, Kato I, Veenstra CM. The accuracy of chemotherapy ascertainment among colorectal cancer patients in the surveillance, epidemiology, and end results registry program. BMC Cancer. 2018;18:481. doi: 10.1186/s12885-018-4405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Yuan P, Wang L, Wang Y, Ma H, Yuan X, Lv W, Hu J. Clinical nomogram for predicting survival of esophageal cancer patients after esophagectomy. Sci Rep. 2016;6:26684. doi: 10.1038/srep26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun W, Jiang YZ, Liu YR, Ma D, Shao ZM. Nomograms to estimate long-term overall survival and breast cancer-specific survival of patients with luminal breast cancer. Oncotarget. 2016;7:20496–20506. doi: 10.18632/oncotarget.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, Levine DA, Chi DS, Barakat RR, Iasonos A. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: Toward improving individualized cancer care. Gynecol Oncol. 2010;116:399–403. doi: 10.1016/j.ygyno.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 14.Weiser MR, Landmann RG, Kattan MW, Gonen M, Shia J, Chou J, Paty PB, Guillem JG, Temple LK, Schrag D, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–385. doi: 10.1200/JCO.2007.14.1291. [DOI] [PubMed] [Google Scholar]
- 15.Ohori Tatsuo Gondo, Riu Hamada M, Gondo T, Hamada R. Nomogram as predictive model in clinical practice. Gan To Kagaku Ryoho. 2009;6:901–906. (In Japanese) [PubMed] [Google Scholar]
- 16.Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, Sano T, Park BJ, Kim WH, Yang HK. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834–3840. doi: 10.1200/JCO.2012.41.8343. [DOI] [PubMed] [Google Scholar]
- 17.Albert JM, Liu DD, Shen Y, Pan IW, Shih YC, Hoffman KE, Buchholz TA, Giordano SH, Smith BD. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30:2837–2843. doi: 10.1200/JCO.2011.41.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 19.Mariani P, Dureau S, Savignoni A, Rouic LL, Levy-Gabriel C, Piperno-Neumann S, Rodrigues MJ, Desjardins L, Cassoux N, Servois V. Development of a prognostic nomogram for liver metastasis of uveal melanoma patients selected by liver MRI. Cancers (Basel) 2019;11(pii):E863. doi: 10.3390/cancers11060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods JF, De Marchi JA, Lowery AJ, Hill AD. Validation of a nomogram predicting sentinel lymph node status in melanoma in an Irish population. Ir J Med Sci. 2015;184:769–773. doi: 10.1007/s11845-014-1166-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Pan Z, Zhao F, Feng X, Liu Q, Li Y, Lyu J. A nomogram for predicting survival in patients with nodular melanoma: A population-based study. Medicine (Baltimore) 2019;98:e16059. doi: 10.1097/MD.0000000000016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavuluru R, Hands I, Durbin EB, Witt L. Automatic extraction of ICD-O-3 primary sites from cancer pathology reports. AMIA Jt Summits Transl Sci Proc 2013. 2013:112–116. [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, You S, Yang Z, Cheng S. Nomogram predicting survival of hepatocellular carcinoma with portal vein tumour thrombus after curative resection. ANZ J Surg. 2019;89:E20–E25. doi: 10.1111/ans.14708. [DOI] [PubMed] [Google Scholar]
- 25.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rousson V, Zumbrunn T. Decision curve analysis revisited: Overall net benefit, relationships to ROC curve analysis, and application to case-control studies. BMC Med Inform Decis Mak. 2011;11:45. doi: 10.1186/1472-6947-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 28.Mou K, Ding M, Han D, Zhou Y, Mu X, Liu W, Wang L. miR-590-5p inhibits tumor growth in malignant melanoma by suppressing YAP1 expression. Oncol Rep. 2018;40:2056–2066. doi: 10.3892/or.2018.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W, Sakamoto N, Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: A population-based analysis. BMC Cancer. 2016;16:413. doi: 10.1186/s12885-016-2438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu XL, Cheng H, Tang MS, Zhang HL, Wu RY, Yu Y, Li X, Wang XM, Mai J, Yang CL, et al. A novel nomogram based on LODDS to predict the prognosis of epithelial ovarian cancer. Oncotarget. 2017;8:8120–8130. doi: 10.18632/oncotarget.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai BB, Shi KQ, Li P, Chen BC, Shi L, Johnson PJ, Lai P, Toyoda H, Zhou MT. A nomogram integrating hepatic reserve and tumor characteristics for hepatocellular carcinoma following curative liver resection. Clin Chim Acta. 2018;485:187–194. doi: 10.1016/j.cca.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Hong HJ, Chen YL. Establishment of a gallbladder cancer-specific survival model to predict prognosis in non-metastatic gallbladder cancer patients after surgical resection. Dig Dis Sci. 2018;63:2251–2258. doi: 10.1007/s10620-018-5103-7. [DOI] [PubMed] [Google Scholar]
- 33.Pietrantonio F, Barretta F, Fanotto V, Park SH, Morano F, Fucà G, Niger M, Prisciandaro M, Silvestris N, Bergamo F, et al. Estimating survival probabilities of advanced gastric cancer patients in the second-line setting: The gastric life nomogram. Oncology. 2018;95:344–352. doi: 10.1159/000491753. [DOI] [PubMed] [Google Scholar]
- 34.Ribero S, Stucci LS, Marra E, Marconcini R, Spagnolo F, Orgiano L, Picasso V, Queirolo P, Palmieri G, Quaglino P, Bataille V. Effect of age on melanoma risk, prognosis and treatment response. Acta Derm Venereol. 2018;98:624–629. doi: 10.2340/00015555-2944. [DOI] [PubMed] [Google Scholar]
- 35.Reyes Ortiz CA, Freeman JL, Kuo YF, Goodwin JS. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. J Gerontol A Biol Sci Med Sci. 2007;62:892–898. doi: 10.1093/gerona/62.8.892. [DOI] [PubMed] [Google Scholar]
- 36.Jiang AJ, Rambhatla PV, Eide MJ. A systematic review of socioeconomic and lifestyle factors and melanoma. Br J Derm. 2014;74:885–915. doi: 10.1111/bjd.13500. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin JM, Fisher JL, Paskett ED. Marital status and stage at diagnosis of cutaneous melanoma: Results from the surveillance epidemiology and end results (SEER) program, 1973–2006. Cancer. 2011;117:1984–1993. doi: 10.1002/cncr.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arce PM, Camilon PR, Stokes WA, Nguyen SA, Lentsch EJ. Is sex an independent prognostic factor in cutaneous head and neck melanoma? Laryngoscope. 2014;124:1363–1367. doi: 10.1002/lary.24439. [DOI] [PubMed] [Google Scholar]
- 39.Vaithinathan AG, Asokan V. Public health and precision medicine share a goal. J Evid Based Med. 2017;10:76–80. doi: 10.1111/jebm.12239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are publicly available in the Surveillance, Epidemiology, and End Results repository (https://seer.cancer.gov/).