Abstract
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder that affects an estimated 30 million people worldwide. It is characterized by the destruction of pancreatic β cells by the immune system, which leads to lifelong dependency on exogenous insulin and imposes an enormous burden on patients and health-care resources. T1DM is also associated with an increased risk of comorbidities, such as cardiovascular disease, retinopathy, and diabetic kidney disease (DKD), further contributing to the burden of this disease. Although T cells are largely considered to be responsible for β-cell destruction in T1DM, increasing evidence points towards a role for B cells in disease pathogenesis. B cell-depletion, for example, delays disease progression in patients with newly diagnosed T1DM. Loss of tolerance of islet antigen-reactive B cells occurs early in disease and numbers of pancreatic CD20+ B cells correlate with β-cell loss. Although the importance of B cells in T1DM is increasingly apparent, exactly how these cells contribute to disease and its comorbidities, such as DKD, is not well understood. Here we discuss the role of B cells in the pathogenesis of T1DM and how these cells are activated during disease development. Finally, we speculate on how B cells might contribute to the development of DKD.
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder characterized by the self-reactive lymphocyte-mediated destruction of insulin-producing pancreatic β cells. In healthy individuals, self-reactive lymphocytes are tightly regulated through central and peripheral tolerance mechanisms that prevent their accumulation and activation, and the consequent development of autoimmunity1,2. In patients with T1DM, however, a combination of genetic risk alleles and environmental risk factors — such as infection or injury — allows self-reactive lymphocytes to escape these tolerance checkpoints, leading to their activation and the development of autoimmunity2. Specifically, T1DM is associated with the infiltration of self-reactive lymphocytes into the pancreas, where they destroy insulin-producing β cells. Over time the capacity of these cells to secrete insulin in response to a glucose load declines, leading to an overt elevation in serum glucose levels and a clinical diagnosis of T1DM. Despite the development of approaches to improve glycaemic control, patients with T1DM remain at increased risk of macrovascular and microvascular complications, including cardiovascular disease, neuropathy, retinopathy and nephropathy. Diabetic kidney disease (DKD) affects up to 30% of patients with T1DM and is associated with poor outcomes, being the leading cause of end-stage renal disease (ESRD) in the USA3. Early signs of DKD, including glomerular hyperfiltration and inflammation, are often already present at the time of T1DM diagnosis4,5. These initial changes in the kidney are followed by further pathologic changes in the glomerulus, including podocyte apoptosis and an accumulation of extracellular matrix, leading to thickening of the glomerular basement membrane and mesangium6. The earliest clinical evidence of DKD is persistent microalbuminuria, defined as an albumin excretion rate of 30–300 mg per day. Macroalbuminura, defined as an albumin excretion rate >300 mg per day, is indicative of a disease state that is no longer reversible, after which glomerular filtration rate will continue to decline, eventually leading to the development of ESRD. Traditional risk factors for the development of DKD include hyperglycaemia, hypertension and hypercholesterolaemia. However, good glycaemic, lipid and blood pressure control does not eliminate the risk of DKD in patients with T1DM, indicating that other factors such as immune dysfunction have a role in the pathogenesis of renal complications.
Although islet antigen-reactive T cells are generally considered to be the main pathogenic effectors of pancreatic β-cell destruction in T1DM, an increasing number of studies have demonstrated the importance of islet-reactive B cells in the pathogenesis of this disease. Such B cells have key roles in presenting antigen to T cells and in the production of cytokines and autoantibodies in humans and mice7–10. These functions of B cells might also contribute to the development of DKD in patients with T1DM. Moreover, although cell-targeted therapies for T1DM have mostly focused on eliminating T cells, targeting B cells also has beneficial effects. In this Review we discuss the role of B cells in the pathogenesis of T1DM, as well as their likely role in DKD, and describe potential therapeutic strategies to target B cells in these patients.
B cells in the development of T1DM
The emergence of self-reactive B cells
As many as 70% of newly produced B cells in the bone marrow are able to bind self-antigens, such as DNA or insulin, via their B-cell receptor (BCR), suggesting that these cells have the capacity to be highly self-reactive and destructive11. In order to prevent the development of autoimmunity, these self-reactive B cells must be culled, rehabilitated so that they no longer bind to highly avid self-antigens, or suppressed. In the bone marrow, B cells that bind self-antigen with high avidity undergo a process called receptor editing wherein the genes that encode the BCR immunoglobulin light chains are rearranged by silencing one allele and expressing a second to produce a BCR with altered specificity12,13. One study demonstrated that at least 25% of B cells present in the periphery have undergone receptor editing14. Hence, the process eliminates autoreactivity in many cells. When receptor editing fails to eliminate strong BCR signals from self-antigens, the B cell undergoes death by apoptosis (FIG. 1). These rehabilitation and culling processes are considered to be central tolerance mechanisms because they occur in the bone marrow where B cells are produced. On the opposite end of the spectrum, newly produced B cells that lack autoreactivity migrate from the bone marrow to peripheral tissues where they become mature naive B cells that function in protective immunity. Their inability to bind autoantigens makes these cells ‘ignorant’ of self-antigen; therefore, they do not pose any danger unless they acquire a high affinity for an autoantigen as a consequence of somatic hypermutation of immunoglobulin variable region genes in the germinal centre reaction.
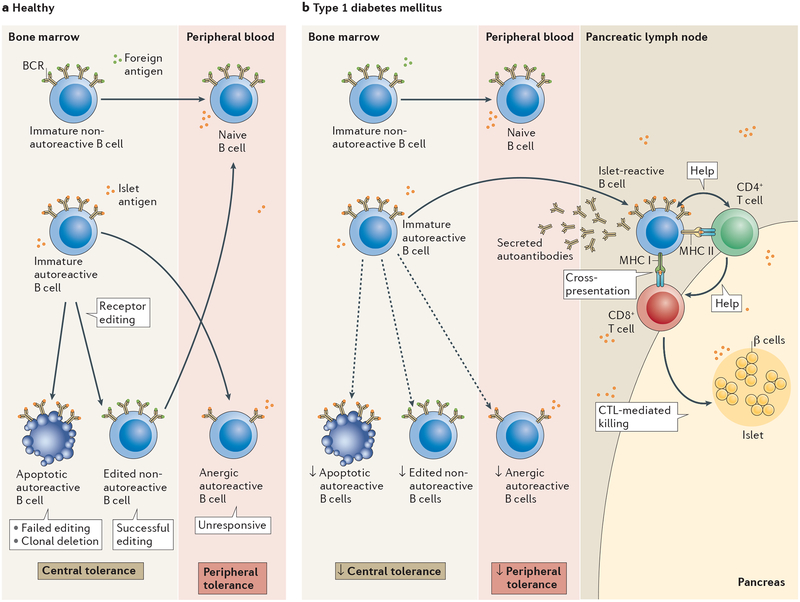
Figure 1 |. Mechanisms of autoreactive B‑cell tolerance and dysfunction in type 1 diabetes mellitus.
a | In healthy individuals non-autoreactive B cells that react to foreign antigen via their B-cell receptors (BCRs) migrate into the periphery where they become mature naive B cells. Autoreactive B cells with a high avidity for self-antigen, such as islet antigen, undergo receptor editing in the bone marrow (a process considered to be a central tolerance mechanism). Cells that are successfully edited (thus losing autoreactivity) migrate to the periphery, whereas those that fail to undergo successful receptor editing are destroyed. Autoreactive B cells with moderate avidity for self-antigens can enter the periphery and become tolerized through peripheral tolerance mechanisms by a process termed anergy, whereupon cells are unable to become activated, proliferate and produce antibody in response to self-antigen. b | In type 1 diabetes, central and peripheral tolerance mechanisms are impaired, enabling an accumulation of autoreactive B cells in the periphery. These cells participate in disease by entering the pancreas or pancreatic lymph node where they present antigen to islet-reactive CD4+ and CD8+ T cells, leading to the destruction of β cells via cytotoxic T lymphocyte (CTL)-mediated killing.
In addition to B cells that have undergone receptor editing and B cells that are ‘ignorant’ of self-antigen, B cells that bind to self-antigen with moderate avidity can enter the periphery where they are silenced via a peripheral tolerance mechanism termed anergy (FIG. 1a). Anergic B cells are characterized by their inability to become activated, proliferate, and produce antibody15–19 as a consequence of chronic BCR binding to self-antigen. Anergic B cells continue to express the BCR, but at lower levels than other B cells, and the output of BCR signalling changes. Compared to non-autoreactive B cells, BCR signalling in anergic B cells is biased towards activation of negative regulatory pathways involving phosphatases such as PTPN22, PTEN and SHIP1, which prevent anergic B cells from responding to self and cross-reactive exogenous antigens by inhibiting their activation20–22. Anergy can be undermined by genetic polymorphisms that compromise these regulatory pathways23. Importantly, the anergic status of autoreactive B cells is reversed by dissociation of the antigen from the BCR, raising the possibility that environmental influences, such as inflammatory mediators, might adversely affect anergy. Hence, breakdown in tolerance mechanisms — such as anergy and receptor editing — that normally prevent the accumulation and activation of self-reactive B cells, is likely to contribute to the development of autoimmunity1.
B cells in the pathogenesis of T1DM
Although T cells are generally considered to be the major effectors of pancreatic β-cell destruction in patients with T1DM, an emerging body of literature has highlighted an important role for B cells in this process. In non-obese diabetic (NOD) mice, B cells are required for the development of diabetes24, which can be prevented by depletion of B cells using anti-CD20 or anti-CD22 monoclonal antibodies25,26. Moreover, use of the anti-CD20 monoclonal antibody, rituximab, to deplete B cells in patients with newly diagnosed T1DM preserved β cell function and delayed the requirement for insulin administration following 1 year of treatment27,28. Although the effects of rituximab were minimal following 2 years of treatment, the findings from this study support a role for B cells in this disease. Given that at the time of T1DM diagnosis β cell mass has declined to a point at which insulin production is inadequate, it is likely that in this study the B cells had already exerted their negative effects (through autoantigen presentation, for example) at the time of rituximab administration. Hence, B cell depletion might be most beneficial in individuals at very high risk of T1DM, for example those who express multiple anti-islet antibodies, who have not yet developed disease symptoms.
A study often cited as evidence that B cells are not important in T1DM is a 2001 report of an individual who developed T1DM despite being deficient in B cells29. However, other studies have shown that conditions of lymphopenia — induced through genetic defects or by pharmacologic means — can support the accumulation of self-reactive T cells through homeostatic expansion, without the need for B cells30,31. We suspect that such an event occurred in this individual.
B cells as antigen presenters
The exact pathogenic role of B cells in T1DM is still a subject of active investigation, but studies suggest that B cells probably exert their effects by presenting islet autoantigens to diabetogenic T cells7,8, as well as through the production of cytokines32,33 and autoantibodies to islet antigens10,34,35. In the NOD mouse model, inhibition of the ability of B cells to present antigen by either MHC class I or class II molecules prevents the development of T1DM7,36, demonstrating the importance of B cells in presenting antigen to CD4+ T cells and in cross-presenting to CD8+ T cells. In addition, restricting the BCR repertoire to an irrelevant islet antigen, which prevents presentation of key islet antigens to T cells, also prevents diabetes9. By contrast, skewing BCR reactivity towards insulin increases disease incidence and accelerates disease onset37. Hence, these studies demonstrate that islet-reactive B cells are necessary for the development of T1DM, most likely by presenting self-antigens to diabetogenic T cells. Anergic B cells are not able to present antigen (M.J.S. and J.C.C., unpublished work); thus, the ability of islet-reactive B cells to act as antigen presenting cells likely results from a compromise of anergy. Loss of anergy could result from genetic and/or environmental factors. The activation and expansion of T cells as a consequence of antigen presentation by B cells likely results in the destruction of pancreatic β cells, leading to the development of T1DM.
In addition, the post-translational modification of islet cell antigens, resulting, for example, from the fusion of insulin and chromogranin A, leads to the creation of hybrid antigenic peptides. As these hybrid peptides are seen as foreign, CD4+ T cells are readily activated by antigen presenting cells such as activated islet antigen-reactive B cells38. These hybrid peptides are present in mouse models and in patients with T1DM, suggesting that they might have an important role in the development of disease38–40. B cells that react with native islet antigens could potentially bind, process and present molecules containing these hybrid peptides to T cells, facilitating their activation. The proposal that hybrid peptide-reactive T cells are the major diabetogenic force in the development of T1DM is attractive because such T cells would not normally be identified as autoreactive and would therefore not be deleted in the thymus.
B cells as producers of autoantibody
Available evidence shows that the presence of islet autoantibodies is one of the best predictors of T1DM development10. Islet autoantibodies that are assayed routinely include those against insulin, insulinoma-associated protein 2, glutamic acid decarboxylase 65, and zinc transporter 834. Individuals with high-affinity anti-insulin antibodies or antibodies to more than one islet antigen will almost certainly develop T1DM during their lifetime41–43. Although the presence of autoantibodies supports a pathogenic role for B cells in T1DM and is a harbinger of disease development, the pathogenicity of these antibodies is ambiguous. In NOD mice, the production of islet autoantibodies is dispensable for disease development44. Moreover, given the therapeutic effect of anti-CD20 antibodies (which do not target CD20− antibody-producing plasma cells45) in mice and humans25–27, autoantibodies probably do not have a pathogenic role in T1DM. We suggest that autoantibody production is essentially an epiphenomenon that occurs as a byproduct of anergic B cell activation.
Phenotype of B cells in T1DM
Given the evidence for a role of B cells in T1DM, studies over the past few years have sought to characterize changes that occur in the B cell compartment during disease development. One study suggested that autoreactive B cells might be more prevalent in the blood of patients with T1DM than in healthy individuals46. As noted above, autoreactive B cells are normally silenced at two distinct checkpoints during their development: by receptor editing or deletion of immature B cells in the bone marrow and anergy in in the periphery11 (FIG. 1a). Patients with T1DM, however, have increased numbers of autoreactive B cells at both the new emigrant and/or transitional stage and mature naive B cell stage, suggesting an impairment of central and peripheral B cell tolerance mechanisms46 (FIG. 1b). These cells are polyreactive and can bind to single-stranded DNA, double-stranded DNA, insulin, and lipopolysaccharide46. Not surprisingly, a later study found that treatment with rituximab failed to reset these impaired tolerance checkpoints47; the B cell repertoire generated after rituximab treatment was as autoreactive and polyreactive as that present before treatment, suggesting rituximab probably exerts its effects in ways other than altering the distribution of specificities in the repertoire.
To determine whether B cells in patients with T1DM have a decreased ability to undergo receptor editing, a study assessed the frequency of recombining sequence rearrangements in λ immunoglobulin light chain-positive B cells from patients with T1DM and controls. Recombining sequences accumulate in, and can be used to identify, cells that have undergone receptor editing. B cells from patients with T1DM had a reduced frequency of recombining sequence rearrangements compared to those from healthy individuals, suggesting that patients with T1DM might have a higher autoreactivity threshold for initiation of receptor editing or a defect in the receptor editing process, which could allow autoreactive B cells to enter the periphery48 (FIG. 1b).
Our analysis of autoreactive anergic B cells (identified as CD19+CD27−IgMlo/−IgD+ B cells that bind insulin via their BCR) in peripheral blood16, showed that patients with islet autoantibodies but without clinical evidence of diabetes, and those who had been diagnosed with T1DM within the past year, have significantly lower levels of insulin-binding anergic B cells in blood than healthy controls49. Of note, some of the first-degree relatives of patients with T1DM also had decreased frequencies of these cells, suggesting that loss of anergy precedes the production of autoantibodies by B cells. Interestingly, however, the proportion of insulin-binding anergic B cells in patients who had been living with T1DM for >1 year was similar to that of healthy controls, suggesting that loss of B cell anergy is transient, consistent with an environmental trigger. Moreover, insulin-binding anergic B cells found in healthy individuals were polyreactive, binding also to chromatin and lipopolysaccha-ride49. Hence, loss of B cell anergy could potentially be instigated through initial binding to a non-islet antigen, such as DNA. Consistent with this hypothesis, and as mentioned above, patients with T1DM have increased antibodies against double-stranded DNA50,51. Together, these findings demonstrate that central and peripheral B cell tolerance mechanisms are impaired in patients with T1DM and that loss of anergy might be an early initiating step in disease development (FIG. 1b).
As T1DM is an organ-specific autoimmune disorder, studies of B cell function should ideally focus on cells localized to target tissues, namely the pancreas and pancreatic lymph node. One of the earliest studies of infiltrating cell populations in cadaveric pancreatic tissue found that B cells appear in the pancreas late during the development of insulitis, together with the appearance of CD8+ T cells52. In that study, the progression of insulitis was defined based on the number of remaining insulin-containing β cells and the number of infiltrating inflammatory cells present in the tissue52. On the basis of these findings, one could speculate that B cells present antigen to CD8+ T cells. Indeed, and as described above, studies in NOD mice have demonstrated the essential role of antigen cross-presentation by B cells in the development of T1DM36. More recent studies have extended these findings by analysing B cells in the pancreas and pancreatic lymph node at various stages of disease. A 2016 study found two distinct patterns of insulitis, which the researchers classified as CD20hi (in which many B cells were present) and CD20lo (in which few B cells were present)53. Pancreatic tissue from patients who were diagnosed before 7 years of age was always CD20hi, whereas tissue from individuals diagnosed after 13 years of age always had the CD20lo phenotype. Individuals diagnosed between 7 and 13 years of age fell into either group. These findings suggest that the two patterns of disease are differentially aggressive. In line with this hypothesis, patients with the CD20hi profile exhibited more rapid loss of β-cell mass than those with the CD20lo phenotype53. The findings also suggest that patients with the CD20hi phenotype might be more likely than those with the CD20lo phenotype to respond to B cell depletion, and might help to explain why rituximab therapy was more efficacious in younger patients in a clinical trial27. We have identified seemingly activated high-affinity insulin-binding B cells in the pancreas and pancreatic lymph node in NOD mice54 and in patients with new-onset T1DM (M.J.S. and J.C.C., unpublished work). Hence, islet-reactive B cells that escape anergy are likely to migrate from the peripheral blood into the pancreas and pancreatic lymph node, where they participate in disease pathogenic processes through antigen presentation (FIG. 1b).
Interestingly, a 2017 study found that patients with newly diagnosed T1DM had lower numbers of germinal centres and fewer follicular dendritic cell networks in their pancreatic lymph nodes than patients with long-standing T1DM and healthy controls55. The researchers hypothesize that maturing self-reactive B cells could differente into antibody-secreting plasma cells more rapidly than non-autoreactive B cells, causing a disruption in normal follicular development. As CD20 was used to identify B cells in this study, enumeration of plasma cells was not possible. Future studies using additional B cell markers could help to further elucidate the role of B cells in the pancreatic lymph nodes early in disease development.
Effects of genetic risk alleles on B cells
The development of T1DM is driven by genetic and environmental risk factors, suggesting that the effects of these factors might be mediated through the loss of central and peripheral B cell tolerance. The major genetic determinant of T1DM is HLA Class II, which accounts for up to half of the genetic risk56. The susceptibility haplotypes, HLA-DR4-DQ8 and HLA-DR3-DQ2 confer the highest risk57,58. Given that autoreactive B cells require T cell help to become activated, activation of CD4+ T cells in response to HLA-DR4-DQ8 expressed by insulin-reactive B cells could evoke loss of B cell tolerance. The genetic determinant that confers the next highest risk of T1DM is a polymorphism in the VNTR region of the INS gene, which encodes insulin58. This polymorphism is thought to increase the number of insulin-reactive T cells in the periphery owing to impaired induction of T cell tolerance to insulin in the thymus59. An increased number of insulin-specific T cells can promote the activation of insulin-reactive B cells, driving them to participate in disease, likely through antigen presentation. Our studies have shown that the loss of anergic insulin-binding B cells in first-degree relatives of patients with T1DM is associated with the presence of the HLA-DR4-DQ8 haplotype and INS polymorphism, suggesting that T cells probably drive loss of B cell anergy (M.J.S. and J.C.C., unpublished work).
We have also shown that polymorphisms in the PTPN22 and PTPN2 genes are associated with loss of B cell anergy in first-degree relatives of patients with T1DM (M.J.S. and J.C.C., unpublished work). PTPN2 and PTPN22 are negative regulators of B cell signalling, suggesting that these polymorphisms might impair signalling pathways that maintain the unresponsiveness of anergic cells60. Genome-wide association studies have shown that the PTPN22 C1858T polymorphism is the third highest contributor to risk of T1DM, after the HLA and INS genes58. PTPN22 encodes a protein tyrosine phosphatase that is expressed in T cells and B cells. PTPN22 functions to dampen B cell signalling, and the expression of a risk-conferring allele is associated with an increased frequency of autoreactive B cells in the peripheral blood of patients with T1DM46,61. Interestingly, however, one study found that patients with T1DM who carry the PTPN22 risk allele had increased numbers of anergic B cells in peripheral blood61. These findings contradict those from our own study, likely due to differences in the study populations, as this study presumably assessed patients with long-standing T1DM and we analysed first-degree relatives. The discrepancy could also be due to differences in gating strategies to identify anergic B cells. As targeted ectopic expression of the PTPN22 risk allele in B cells leads to autoimmunity in vivo62, we hypothesize that this risk allele promotes the loss of anergy, leading to the development of autoimmunity.
The PTPN2 gene also encodes a protein tyrosine phosphatase that is expressed in B cells and T cells, as well as in pancreatic β cells. PTPN2 has a range of functions, including as a negative regulator of JAK/STAT signalling63 and in the dephosphorylation of the Srcfamily kinases, Lck and Fyn, which are involved in activation of T cells following stimulation through the T cell receptor64. The PTPN2 single nucleotide polymorphism rs1803217 is associated with a 40% reduction in PTPN2 mRNA expression in primary human regulatory T cells and CD4+ memory T cells65. Although this study did not examine PTPN2 expression in B cells, one would expect a similar reduction in mRNA level in these cells. Deletion of PTPN2 in the haematopoietic cells of adult mice led to the development of autoimmunity, characterized by an increase in the number of B cells, including germinal centre B cells, and in the production of anti-nuclear auto-antibodies66. Thus, polymorphisms in genes that function as negative regulators of BCR signalling could confer increased risk of T1DM by impairing peripheral tolerance. Other allelic variants of genes that are expressed in B cells and are associated with T1DM, such as BACH2, SH2B3, and IKZB3, might also impair B cell tolerance, and this hypothesis is an area of active investigation.
B cells in diabetic kidney disease
The role of B cells in the pathogenesis of DKD is not well understood, especially in patients with T1DM. However, IgG+ B cells have been shown to infiltrate the glomeruli of diabetic NOD mice67. In addition, a small study of B cells in the peripheral blood of patients with DKD showed that levels of CD19lo/+CD38+ plasma cells were increased relative to those in healthy controls. CD19lo/+CD38+ B cell counts correlated positively with albumin excretion rate and serum IgG levels, and negatively with estimated glomerular filtration rate, implying that higher frequencies of plasma cells are associated with worsening DKD68.
Mechanism of B cell involvement
B cells could contribute to the pathogenesis of DKD through their roles in antigen presentation, antibody production, immune-complex generation and/or cytokine production. The most probable mechanism by which B cells contribute to DKD is through the production of antibodies, leading to the formation of immune complexes that can be deposited in the kidney and trigger inflammation and glomerulonephritis through activation of complement. Diabetic NOD mice have increased renal deposition of IgG+ immune complexes compared to nondiabetic controls67. In patients with T1DM, microalbuminuria is associated with the presence of circulating serum immunoglobulins, including antibodies directed toward proteins modified by the oxidation of glucose and lipids69. A study of children with T1DM showed that circulating IgG immune complexes are present at the early stages of DKD70. In addition, immune complexes have been identified in the kidneys of patients with T1DM and DKD71, where the presence of immune complexes containing oxidized LDL is thought to promote mesangial expansion by stimulating the production of collagen IV (REF. 72). Thus, B cells contribute to DKD through the production of antibodies and the formation of immune complexes that deposit in the kidney (FIG. 2).
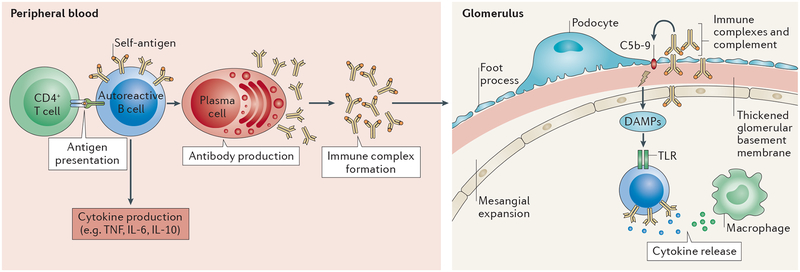
Figure 2 |. Potential roles of autoreactive B cells in diabetic kidney disease.
Autoreactive B cells in the blood and peripheral lymphoid organs can lose anergy and become activated through a variety of potential mechanisms. Collaboration with CD4+ helper T cells leads to the proliferation and differentiation of B cells that secrete inflammatory cytokines and the development of plasma cells that secrete autoantibodies, which can form immune complexes. The localization of this response is not yet fully understood; however, we hypothesize that these immune complexes, together with complement, can infiltrate the glomeruli, leading to mesangial expansion and thickening of the glomerular basement membrane. The presence of immune complexes in the glomerulus also induces macrophage accrual, promoting inflammation. The release of damage-associated molecular patterns (DAMPs) following damage to the extracellular matrix, can lead to further B cell activation, leading to further cytokine production.
The presence of immune complexes in the glomerulus can induce macrophage accrual73 and promote inflammation through the release of cytokines and the activation of complement74. The deposition of IgG in glomeruli correlates positively with levels of the complement component C3 in the glomeruli of NOD mice67. Interestingly, levels of the complement component C4, which is needed to clear immune complexes from the circulation, are low in patients with T1DM75,76. Thus, the deposition of immune complexes in the kidney might have a larger role in the pathogenesis of DKD than previously thought, through activation of inflammatory and immune regulatory pathways.
The chronic inflammation associated with DKD can damage the extracellular maxtrix77, stimulating the release of damage-associated molecular patters (DAMPs), such as DNA and RNA. B cells could become activated following the recognition of DAMPs by Toll-like receptors, leading to cytokine production. Effector B cells produce IL-6 and TNF, which have been implicated in the initiation and progression of DKD78,79. Renal biopsy samples from patients with DKD showed sclerotic glomeruli surrounded by mononuclear cells that were positive for IL-6 mRNA80. Although clinical evidence for a role of IL-6 production by B cells in T1DM does not exist, a small study in patients with type 2 diabetes mellitus (T2DM) and DKD showed that IL-6 serum levels correlate with albuminuria and thickening of the glomerular basement membrane81. The role of TNF in DKD has been studied in animal models and in the context of T2DM, in which the presence of TNF correlates with reduced glomerular filtration rate, albuminuria and increased cellular permeability and cytotoxicity82,83.
A further role for B cells in DKD could involve the production of IL-10 by regulatory B cells84. Although these cells suppress immune responses and have a documented role in the maintenance of tolerance in autoimmunity, IL-10 might have a pathogenic role in DKD. In individuals with long-standing T1DM and DKD, IL-10 levels are a better predictor of albuminuria than are glycaemic control, blood pressure control or diabetes duration85. Although findings are inconsistent, polymorphisms in IL-10 genes might confer susceptibility to DKD86.
Finally, poor glycaemic control is a well-established risk factor for DKD. In the presence of hyperglycaemia, advanced glycation end products stimulate NF-κB signalling, which has a role in the development and function of B cells. Thus, the hyperglycaemic environment might directly increase the production of both antibody-producing and cytokine-producing B cells, contributing to the development of DKD87.
B cell-targeted therapies for T1DM
Given the role of B cells in the pathogenesis of T1DM and their poorly understood, but likely important role in DKD, the potential use of B cell-targeted therapy is of great interest. To date, B cell therapy in autoimmune disease has focused on depleting B cells without consideration of antigen specificity, given the challenges of isolating and characterizing autoreactive B cell subsets. However, pan-depletion of B cells carries a considerable risk of hypogammaglobulinemia and infection, due to decreased antibody production by B cells, which must be weighed against the potential benefits of treatment.
Therapeutic interventions aimed at depleting B cells in animals have used monoclonal antibodies to directly target the B cell surface molecules CD20 and CD22 (REFS 25,26,88). By contrast, indirect approaches have targeted B lymphocyte stimulator (BLyS; also known as B cell activating factor, BAFF) and A proliferation-inducing ligand (APRIL), which are both molecules of the TNF family that are produced by the innate immune system. BLyS and APRIL bind receptors on B cells and have important roles in the regulation of B cell development, function and survival89,90.
As mentioned above, the only B cell-targeted therapy that has been used to date in patients with T1DM is rituximab, which binds the CD20 transmembrane protein expressed on the surface of pre-B cells and mature B cells27,28. Rituximab targets B cells for lysis by inducing antibody-dependent cell-mediated cytotoxicity, potentially reducing antigen presention and activation of self-reactive T cells. In a phase II clinical trial, patients with new-onset T1DM who received four infusions of rituximab had higher mean serum C-peptide levels — a measure of endogenous insulin production — in response to a mixed meal tolerance test 1 year after treatment than did non-treated patients28. Patients who received rituximab also had lower levels of glycated haemo globin and required less insulin than patients who received placebo. After a further 8 months, the rate of C-peptide decline was equivalent between rituximab and placebo groups, and after 30 months of treatment the difference between levels of stimulated C-peptide, glycated haemoglobin, and insulin use was abolished, following a recovery of B cells to baseline levels within 18 months of treatment27,28. These findings presumably reflect a return of pathogenic B cell function along with recovery of B cells as a whole. Assuming that B cells are equally important in the pathogenesis of DKD and T1DM, rituximab might also transiently delay the progression of DKD.
Other therapeutic targets to inhibit B cell function include molecules that function uniquely in B cells and are involved in BCR-mediated cell activation such as PI3 kinase δ; Toll-like receptors that are involved in activation of B cells by DAMPs; B-cell surface molecules other than CD20, such as CD79 and CD22; and co-stimulatory receptors or ligands such as CD80 or CD86, which are involved in antigen presentation to T cells. Although not yet investigated in patients with T1DM, therapeutic agents that target these molecules are under investigation for other clinical indications and should be investigated in the context of T1DM and DKD (TABLE 1).
Table 1 |.
Therapeutic agents that target B cells
| Agent | Mechanism of action | Approved indications |
|---|---|---|
| Anti-CD20 mAb (rituximab)* | B cell lysis | Non-Hodgkin lymphoma, chronic lymphocytic leukaemia, rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis |
| Anti-CD20 mAb (obinutuzumab) | B cell lysis; greater antibody-dependent cellular cytotoxicity and direct cell death than with rituximab | Chronic lymphocytic leukaemia, follicular lymphoma |
| Anti-CD20 mAb (ofatumumab) | B cell lysis; binds the extracellular (large and small) loops of the CD20 molecule | Untreated or refractory chronic lymphocytic leukaemia |
| Anti-CD20 mAb (ocrelizumab) | B cell lysis | Multiple sclerosis |
| Anti-CD22 mAb (epratuzumab) | B cell lysis | No approved indications to date |
| Human anti-BLyS mAb (belimumab) | Blocks binding of soluble BLyS to B cell receptors, thereby inhibiting the survival of B cells and their differentiation into plasma cells | Active, autoantibody positive systemic lupus erythematous |
| TACI-Ig (atacicept) | blocks BLyS and APRIL thereby inhibiting B cell survival | No approved indications to date |
| Human anti-BLyS mAb (tabalumab) | Neutralizes the soluble form and the membrane form of BLyS | No approved indications to date |
Rituximab is the only B cell-targeted agent that has been tested in patients with type 1 diabetes mellitus. APRIL, A proliferation-inducing ligand; BLyS, B lymphocyte stimulator (also known as BAFF); mAb, monoclonal antibody.
Conclusions
Although the role of B cells in the pathogenesis of DKD is not well understood, conclusive evidence supports a key role for B cells in the development of T1DM. Future research focusing on the contribution of B cells to DKD and the underlying mechanisms by which B cells induce pancreatic β-cell, and potentially renal cell damage, should lead to the identification of new treatment targets for both disorders. Therapeutic trials of B cell depletion in T1DM have so far been limited to rituximab, with limited success, suggesting that alternative strategies are needed. Strategies that inhibit B cell function by targeting molecules other than CD20 and approaches aimed at targeting islet-specific B cells, for example insulin-reactive B cells, while preserving non-islet specific B cells, require further study and might prove to be effective therapeutic approaches in the future.
Key points.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by the destruction of pancreatic β cells
An accumulating body of evidence suggests that T1DM is associated with loss of tolerance by autoreactive B cells
Although islet antigen-reactive B cells give rise to autoantibody secreting cells, their most important contribution to pathology in T1DM seems to be presentation of self-antigens to T cells
Loss of tolerance of islet-reactive B cells is associated with certain genetic polymorphisms
B cells contribute to diabetic kidney disease (DKD) through the production of antibodies that lead to the formation and deposition of immune complexes in the kidney
In a clinical trial, B cell-depletion therapy showed some efficacy in patients with T1DM; the development of non-cell depleting therapies might benefit patients with T1DM and DKD
Glossary
- Germinal centre reaction
The anatomical site in which B cells and T cells respond collaboratively to immunogen, leading to B cell proliferation, somatic Ig gene mutation, affinity maturation and immunoglobulin class switch recombination.
- Anergy
A mode of B cell tolerance characterized by unresponsiveness to antigenic stimulation, including the inability to become activated, proliferate and secrete antibody.
- Diabetogenic T cells
T cells that can cause diabetes, such as insulin-reactive T cells.
- Cross-presenting
The process by which antigen presenting cells process and present extracellular antigens to CD8+ T cells.
- High-affinity anti-insulin antibodies
Antibodies with an affinity for insulin >10−9mol/l.
- Plasma cells
Terminally differentiated B cells that secrete antibody.
- Recombining sequence rearrangements
DNA rearrangements that delete one or both Ig κ genes, leading to expression of Ig λ light chains.
- λ-Immunoglobulin light chain-positive B cells
B cells that express λ light chains as a component of their B cell antigen receptor; high levels of these cells is indicative of increased receptor editing.
- Insulitis
Inflammation of the pancreas due to infiltration of lymphocytes.
- Haplotypes
A set of genes inherited from a single parent.
- Antibody-dependent cell-mediated cytotoxicity
The process by which an effector cell of the immune system, such as a natural killer cell, targets a cell for lysis based on the presence of antibodies that are bound to surface antigens on the target cell.
- Mixed meal tolerance test
An assay to determine the amount of insulin an individual produces; the individual consumes a drink containing a mixture of protein, fat, and carbohydrates that stimulates the release of insulin from pancreatic β cells; blood is drawn several times over a period of hours and assayed for C-peptide, which reflects endogenous insulin production.
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jeker LT, Bour-Jordan H & Bluestone JA Breakdown in peripheral tolerance in type 1 diabetes in mice and humans. Cold Spring Harb. Perspect. Med 2, a007807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K & Eisenbarth G Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer IH et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med 171, 412–420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine DZ Can rodent models of diabetic kidney disease clarify the significance of early hyperfiltration?: recognizing clinical and experimental uncertainties. Clin. Sci 114, 109–118 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Bank N Mechanisms of diabetic hyperfiltration. Kidney Int 40, 792–807 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Ponchiardi C, Mauer M & Najafian B Temporal profile of diabetic nephropathy pathologic changes. Curr. Diab. Rep 13, 592–599 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Noorchashm H et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J. Immunol 163, 743–750 (1999). [PubMed] [Google Scholar]
- 8.Serreze DV et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol 161, 3912–3918 (1998). [PubMed] [Google Scholar]
- 9.Silveira PA et al. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur. J. Immunol 32, 3657–3666 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Orban T et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 32, 2269–2274 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardemann H et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Halverson R, Torres RM & Pelanda R Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol 5, 645–650 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Meffre E & Wardemann H B-Cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol 20, 632–638 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Casellas R et al. Contribution of receptor editing to the antibody repertoire. Science 291, 1541–1544 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Cambier JC, Gauld SB, Merrell KT & Vilen BJ B-Cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol 7, 633–643 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duty JA et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med 206, 139–151 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauld SB, Benschop RJ, Merrell KT & Cambier JC Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat. Immunol 6, 1160–1167 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Gauld SB, Merrell KT & Cambier JC Silencing of autoreactive B cells by anergy: a fresh perspective. Curr. Opin. Immunol 18, 292–297 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Merrell KT et al. Identification of anergic B cells within a wild-type repertoire. Immunity 25, 953–962 (2006). [DOI] [PubMed] [Google Scholar]
- 20.O’Neill SK et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity 35, 746–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Getahun A, Beavers NA, Larson SR, Shlomchik MJ & Cambier JC Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J. Exp. Med 213, 751–769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getahun A et al. Impaired B cell function during viral infections due to PTEN-mediated inhibition of the PI3K pathway. J. Exp. Med 214, 931–941 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cambier JC Autoimmunity risk alleles: hotspots in B cell regulatory signaling pathways. J. Clin. Invest 123, 1928–1931 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akashi T et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int. Immunol 9, 1159–1164 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Xiu Y et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in FcγR effector functions. J. Immunol 180, 2863–2875 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Hu CY et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest 117, 3857–3867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pescovitz MD et al. B-Lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care 37, 453–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pescovitz MD et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med 361, 2143–2152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin S et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N. Engl. J. Med 345, 1036–1040 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Jones JL et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc. Natl Acad. Sci. USA 110, 20200–20205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merayo-Chalico J et al. Lymphopenia and autoimmunity: a double-edged sword. Hum. Immunol 77, 921–929 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Tian J et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol 167, 1081–1089 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Harris DP et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol 1, 475–482 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Vehik K et al. Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 34, 1897–1901 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verge CF et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 47, 1857–1866 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Marino E, Tan B, Binge L, Mackay CR & Grey ST B-Cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 61, 2893–2905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hulbert C, Riseili B, Rojas M & Thomas JW B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J. Immunol 167, 5535–5538 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Delong T et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babon JA et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med 22, 1482–1487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiles TA et al. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J. Autoimmun 78, 11–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker JM et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J. Clin. Endocrinol. Metab 89, 3896–3902 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Sosenko JM et al. The use of electrochemiluminescence assays to predict autoantibody and glycemic progression toward type 1 diabetes in individuals with single autoantibodies. Diabetes Technol. Ther 19, 183–187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steck AK et al. ECL-IAA and ECL-GADA can identify high-risk single autoantibody-positive relatives in the TrialNet Pathway to Prevention study. Diabetes Technol. Ther 18, 410–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong FS et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 53, 2581–2587 (2004). [DOI] [PubMed] [Google Scholar]
- 45.DiLillo DJ et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol 180, 361–371 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Menard L et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest 121, 3635–3644 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chamberlain N et al. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest 126, 282–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panigrahi AK et al. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J. Exp. Med 205, 2985–2994 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith MJ et al. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes 64, 1703–1712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang SW, Haedt LH, Rich S & Barbosa J Prevalence of antibodies to nucleic acids in insulin-dependent diabetics and their relatives. Diabetes 30, 873–874 (1981). [DOI] [PubMed] [Google Scholar]
- 51.Triolo G et al. Cross-reactivity of anti-ssDNA antibodies with heparan sulfate in patients with type I diabetes mellitus. Diabetes 38, 718–722 (1989). [DOI] [PubMed] [Google Scholar]
- 52.Willcox A, Richardson SJ, Bone AJ, Foulis AK & Morgan NG Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol 155, 173–181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leete P et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 65, 1362–1369 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Packard TA et al. B cell receptor affinity for insulin dictates autoantigen acquisition and B cell functionality in autoimmune diabetes. J. Clin. Med 5, 98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willcox A et al. Germinal centre frequency is decreased in pancreatic lymph nodes from individuals with recent-onset type 1 diabetes. Diabetologia 60, 1294–1303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambert AP et al. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J. Clin. Endocrinol. Metab 89, 4037–4043 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Erlich H et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 57, 1084–1092 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Concannon P, Rich SS & Nepom GT Genetics of type 1A diabetes. N. Engl. J. Med 360, 1646–1654 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Pugliese A et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet 15, 293–297 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Cerosaletti K & Buckner JH Protein tyrosine phosphatases and type 1 diabetes: genetic and functional implications of PTPN2 and PTPN22. Rev. Diabet. Stud 9, 188–200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habib T et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J. Immunol 188, 487–496 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai X et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J. Clin. Invest 123, 2024–2036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML & McGlade CJ The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr. Biol 12, 446–453 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Wiede F et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J. Clin. Invest 121, 4758–4774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Long SA et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4+ T cells. Genes Immun 12, 116–125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiede F, Sacirbegovic F, Leong YA, Yu D & Tiganis T PTPN2-deficiency exacerbates T follicular helper cell and B cell responses and promotes the development of autoimmunity. J. Autoimmun 76, 85–100 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Xiao X et al. Cellular and humoral immune responses in the early stages of diabetic nephropathy in NOD mice. J. Autoimmun 32, 85–93 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Zhang N et al. Increased CD4+CXCR5+T follicular helper cells in diabetic nephropathy. Autoimmunity 49, 405–413 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Atchley DH, Lopes-Virella MF, Zheng D, Kenny D & Virella G Oxidized LDL-anti-oxidized LDL immune complexes and diabetic nephropathy. Diabetologia 45, 1562–1571 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Nicoloff G, Blazhev A, Petrova C & Christova P Circulating immune complexes among diabetic children. Clin. Dev. Immunol 11, 61–66 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ainsworth SK et al. Diabetic glomerulonephropathy: histopathologic, immunofluorescent, and ultrastructural studies of 16 cases. Hum. Pathol 13, 470–478 (1982). [DOI] [PubMed] [Google Scholar]
- 72.Abdelsamie SA et al. Oxidized LDL immune complexes stimulate collagen IV production in mesangial cells via Fc gamma receptors I and III. Clin. Immunol 139, 258–266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imig JD & Ryan MJ Immune and inflammatory role in renal disease. Compr. Physiol 3, 957–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saad AF, Virella G, Chassereau C, Boackle RJ & Lopes-Virella MF OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J. Lipid Res 47, 1975–1983 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Vergani D, Johnston C, N. BA & Barnett AH Low serum C4 concentrations: an inherited predisposition to insulin dependent diabetes? Br. Med. J 286, 926–928 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnett AH et al. Low plasma C4 concentrations: association with microangiopathy in insulin dependent diabetes. Br. Med. J 289, 943–945 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duran-Salgado MB & Rubio-Guerra AF Diabetic nephropathy and inflammation. World J. Diabetes 5, 393–398 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agrawal S & Gupta S TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J. Clin. Immunol 31, 89–98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M & Garcia-Perez J Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol 7, 327–340 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Suzuki Y et al. Histopathological assessment of renal biopsy specimens of subjects with urine abnormality [Japanese]. Nihon Jinzo Gakkai Shi 37, 284–290 (1995). [PubMed] [Google Scholar]
- 81.Choudhary N & Ahlawat RS Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran. J. Kidney Dis 2, 72–79 (2008). [PubMed] [Google Scholar]
- 82.Moriwaki Y et al. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52, 605–608 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Kalantarinia K, Awad AS & Siragy HM Urinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic rats. Kidney Int 64, 1208–1213 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Lund FE Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol 20, 332–338 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mysliwska J et al. High levels of circulating interleukin-10 in diabetic nephropathy patients. Eur. Cytokine Netw 16, 117–122 (2005). [PubMed] [Google Scholar]
- 86.Peng X, Xu J, Wang P, Zhou J & Guo H Interleukin-10–1082A/G polymorphism and diabetic nephropathy: a meta-analysis. Med. Sci. Monit 21, 890–894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerondakis S & Siebenlist U Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb. Perspect. Biol 2, a000182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fiorina P et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes 57, 3013–3024 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marino E et al. CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes 58, 1568–1577 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zekavat G et al. In vivo BLyS/BAFF neutralization ameliorates islet-directed autoimmunity in nonobese diabetic mice. J. Immunol 181, 8133–8144 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]