Abstract
Type 1 diabetes (T1D) results from the immune-mediated destruction of insulin-producing β cells located within the pancreatic islets of Langerhans. The autoimmune process leads to a deficiency in insulin production and resultant hyperglycemia requiring lifelong treatment with insulin administration. T1D continues to dramatically increase in incidence, especially in young children. Substantial knowledge surrounding human disease pathogenesis exists, such that T1D is now predictable with the measurement of antibodies in the peripheral blood directed against insulin and other β cell proteins. With the ability to predict, it naturally follows that T1D should be preventable. As such, over the last two decades, numerous well-controlled clinical trials have been completed attempting to prevent diabetes onset or maintain residual β cell function after clinical onset, all providing relatively disappointing results. Here, we review the T1D prevention efforts, the current landscape of clinical therapies, and end with a discussion regarding the future outlook for preventing T1D.
Keywords: Diabetes, Type 1, Autoimmunity, Prevention, Intervention, Clinical trials
Introduction
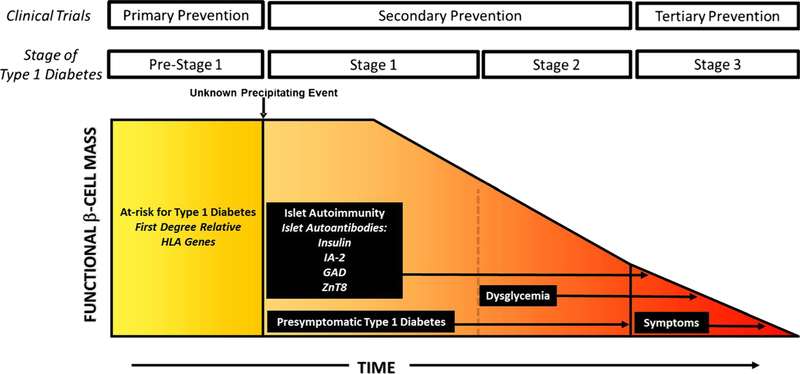
Type 1 diabetes (T1D), the immune-mediated form of diabetes, continues to dramatically increase in incidence and is one of the most common chronic diseases in children [1••]. Although treatment with intensive insulin therapy and blood glucose monitoring has improved dramatically in the last two decades, the majority of T1D patients do not meet recommended glycemic goals [2]. These individuals are at an increased risk of developing microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular complications associated with diabetes, severe hypoglycemia, and depression [3–6]. In order to decrease T1D burden, preventing or delaying T1D onset is critical such that patients can live with the disease for fewer years and more easily meet glycemic goals with residual β cell function. Developing safe, specific, and effective treatments and optimally timing those therapies during T1D development requires that the natural history and pathogenesis of T1D be well defined. It is well appreciated that T1D is a chronic T lymphocyte-mediated autoimmune disorder leading to specific pancreatic β cell destruction that evolves over time defined by stages (Fig. 1) [7]. Several prospective cohort studies following at-risk children have aided understanding the natural history of T1D. The Diabetes Autoimmunity Study in the Young (DAISY) in Colorado, The Environmental Determinants of Diabetes in the Young (TEDDY) multicenter international collaboration, the Type 1 Diabetes Prediction and Prevention Study (DIPP) in Finland, and BABYDIAB studies in Germany have followed genetically at-risk children from birth and have been instrumental in outlining the development of islet autoimmunity (determined by measuring antibodies in the peripheral blood directed against insulin, glutamic acid decarboxylase, islet antigen 2, and zinc transporter 8) and progression to clinical T1D, which is marked by hyperglycemia [8–12]. Importantly, once an individual develops two or more islet autoantibodies (iAb), the risk of developing T1D is >85 % within 15 years and nearly 100 % over time [13••]. DIPP also determined that of the children who developed T1D before puberty, 65 % had measureable iAb before the age of 2 years and 95 % before the age of 5 years [14]. Therefore, T1D is not only predictable but can also be identified at a very young age.
Fig. 1.
Stages in the development of type 1 diabetes as functional β cell mass declines over time. Genes and currently unknown environmental factors initiate β cell autoimmunity. In stage 1, islet autoimmunity, marked by the presence of serum islet autoantibodies (insulin, GAD, IA-2, ZnT8), develops. Glucose homeostasis is normal, and patients are asymptomatic. As β cell function wanes, dysglycemia occurs, but not overt hyperglycemia (stage 2). Finally, overt type 1 diabetes (stage 3) develops with loss of significant insulin release and resultant hyperglycemia with clinical symptoms (polyuria, polydipsia, weight loss). Staging type 1 diabetes allows for prevention strategies in an attempt to preserve residual β cell function based upon the stage of disease such that primary prevention occurs in pre-stage 1, secondary prevention in stages 1 and 2, and tertiary prevention (or new-onset intervention) at stage 3
A recent scientific statement from the Juvenile Diabetes Research Foundation, Endocrine Society, and American Diabetes Association divides T1D into three distinct stages (Fig. 1) [15•]. Stage 1 is defined by the presence of islet autoimmunity with normal blood glucose and no symptoms. In stage 2, islet autoimmunity and dysglycemia are present, but there are still no clinical symptoms. Stage 3 is clinically diagnosable T1D with iAb, hyperglycemia, and the presence of polyuria, polydipsia, and weight loss. Being genetically at risk, which is predominantly conferred by human leukocyte antigen (HLA) genes, is classified as pre-stage 1. As a person progresses through the stages of T1D, it is believed that there is a steady decline in functional β cells. Intervening early in the disease course when a substantial amount of β cell mass exists is important as preserving β cell function improves metabolic control, decreases severe hypoglycemia, and protects from long-term microvascular changes seen in T1D [16]. Now that T1D is predictable, prevention trials should routinely be offered earlier in the disease course. Intervening early has many potential benefits including increased efficacy and the ability to use fewer therapies, thereby reducing drug side effects. We provide an overview of completed prevention trials moving through the stages of disease development, with a particular focus on prevention trials before clinical diagnosis (stages 1 and 2). We will also discuss those agents that showed mild efficacy in new-onset cohorts (stage 3) and the future challenges and outlook for making T1D a preventable disease.
Primary Prevention Trials in Genetically At-Risk Individuals (Pre-Stage 1)
Islet autoantibodies are used to establish T1D risk; however, antibodies can develop at any age, as evidenced from studying T1D development in identical twins [17], which necessitates repeated iAb measurements over time. Prior to the presence of iAb, family history with a first-degree relative and genes provides risk assessment. Genes within the HLA complex confer over 50 % of the genetic risk for T1D development. Specifically, HLA class II genes account for most of this risk [18], and these genes are expressed on antigen-presenting cells (B cells, dendritic cells, macrophages) functioning to present processed protein fragments to CD4 T cells, also referred to as helper T cells [19]. Importantly, approximately 90 % of all individuals with T1D have specific HLA genes termed DR4-DQ8 and/or DR3-DQ2 [20]. However, the vast majority of individuals with diabetes-susceptible HLA class II genes do not develop the disease such that only 5–10 % of those with high-risk HLA genes develop T1D [21, 22]. These observations strongly implicate environmental factors in the presence of disease-susceptible HLA genes leading to the development of islet autoimmunity and eventual clinical T1D.
Most primary prevention trials have focused on very safe interventions, such as diet and supplements, as these studies are conducted in very young children (Table 1). One well-studied intervention is the composition of infant milk formulas. The hypothesis tested was that formulas composed of digested milk proteins (i.e., Nutramigen) may provide less of an immune stimulus than conventional cow milk formula (CMF). The Trial to Reduce Incidence of Diabetes in Genetically at Risk (TRIGR) in Finland randomized 230 infants (high-risk HLA and first-degree relatives) to either CMF or a casein hydrosylate formula (Nutramigen) within the first 8 months of life at the time the infants began to require breastfeeding supplementation [23]. Infants who received the casein hydrosylate formula had a decreased incidence of iAb development over the subsequent 10 years. A larger confirmatory trial enrolled 2159 infants from 78 centers in 15 countries within the first week of life. Infants were randomized to casein hydrosylate formula (n = 1078) or CMF (n = 1081) in addition to breastfeeding as often as desired. Unfortunately, the trial did not decrease the risk for developing multiple iAb, and the children are being followed to clinical T1D development [24].
Table 1.
Primary prevention trials in type 1 diabetes (pre-stage 1)
| Intervention (trial) | Number | Age (years) | Primary endpoint | Status | Clinicaltrials.gov identifier |
|---|---|---|---|---|---|
| Antigen-specific therapies Oral insulin (Pre-POINT) | 25 | 2–7 | Hypoglycemia | Completed, safe and signals of efficacy for protective immune response to insulin | |
| Dietary exposure therapies Casein hydrosylate vs. cow’s milk formula | 230 | Newborn infants | Decreased development of islet autoantibodies | Completed | |
| Casein hydrosylate formula vs. cow’s milk formula (TRIGR) | 2159 | Newborn infants | Decreased development of islet autoantibodies | Completed enrollment, in follow-up | |
| Cow’s milk formula vs. whey-based hydrosylate vs. whey-based hydrosylate free from bovine insulin (FINDIA) | 1133 | Newborn infants | Decreased development of islet autoantibodies | Completed enrollment, in follow-up | |
| Omega-3 and docosahexaenoic acid (DHA) (NIP) | 98 | Prenatal and newborn infants | Decreased inflammatory cytokines in peripheral blood | Completed |
In the Finnish Dietary Intervention Trial for the Prevention of Type 1 Diabetes (FINDIA) trial, 1133 infants with high-risk HLA were randomized to CMF, whey hydrosylate formula, or a whey-based formula free of bovine insulin in the first 6 months of life. Within the first 3 years of the study, infants receiving the insulin-free formula were less likely to develop islet autoimmunity than those receiving CMF [25]. These patients need to be followed over a longer time period to determine the effect of formula composition on the development of islet autoimmunity and clinical T1D.
Dietary omega-3 fatty acids are thought to have anti-inflammatory properties and have retrospectively been shown to reduce the risk of islet autoimmunity in genetically at-risk children [26]. Based on this premise, the TrialNet Nutritional Intervention to Prevent T1D (NIP) study was a randomized, double-blinded pilot trial that supplemented infants who had high genetic risk DR3 and/or DR4 genes with docosahexaenoic acid (DHA). The two arms of the study consisted of mothers supplemented with DHA in the third trimester (n = 41) and supplementation beginning in early infancy (n = 57). After following the infants for 3 years, the levels of DHA increased significantly in red blood cells, but peripheral blood inflammatory cytokines (IL-1β, TNFα, and IL-12p40) were not reduced [27].
Looking beyond dietary interventions, Pre-POINT is a recent pilot study completed in children aged 2–7 years (n = 25) determined to be at high risk for T1D development by HLA genes and family history of T1D [28]. Oral insulin, given in escalating doses up to 67.5 mg daily, resulted in protective immune responses to insulin. The ability of oral insulin to induce an immune response in pre-stage 1 of diabetes argues for a larger clinical trial to assess the progression to islet autoimmunity and dysglycemia in these patients. Oral insulin has also been used extensively in T1D secondary prevention trials as outlined in following section.
Secondary Prevention Trials in Autoantibody-Positive Individuals (Stage 1 and 2 T1D)
Many well-controlled randomized clinical trials have been conducted in individuals with iAb but no overt hyperglycemia. Most of these trials have utilized antigen-specific therapies with a focus on insulin preparations administered subcutaneously, orally, or intranasally (Table 2). Antigen-specific therapy aims to restore self-tolerance to insulin and β cell proteins without having to administer immunosuppressive agents [29]. Insulin is an important autoantigen for both T and B cells in T1D as it is often the first iAb to appear in young children, a higher titer correlates with a faster time to onset of T1D, and Tcell responses to insulin can be detected in the peripheral blood [30, 31••]. The goal of insulin administration in iAb individuals is to enhance regulatory T cell function that will then traffic to pancreatic islets and ideally help to prevent immune-mediated destruction of insulin-producing β cells by dampening the immune responses to insulin and other β cell proteins [32].
Table 2.
Secondary prevention trials in type 1 diabetes (stages 1 and 2)
| Intervention (trial) | Age (years) | Primary endpoint | Status | Clinicaltrials.gov identifier |
|---|---|---|---|---|
| Antigen-specific therapies | ||||
| Parenteral insulin (DPT-1) | 4–45 | T1D onset | Completed | |
| Parenteral insulin (Belgian Diabetes Registry) | 10–23 | T1D onset | Completed | |
| Oral insulin (DPT-1) | 3–45 | T1D onset | Completed; post hoc analysis showed delay in progression to T1D in relatives with insulin autoantibodies ≥80 U/ml | |
| Oral insulin | 3–45 | T1D onset | Follow-up | TrialNet |
| Oral insulin | 3–45 | Change in insulin immune function | Recruiting; open-label dosing and immune mechanism of action study | TrialNet |
| Intranasal insulin (DIPP birth cohort) | 1–5 | T1D onset | Completed; trial stopped after interim analysis showed no effect on T1D progression | |
| Intranasal insulin (DIPP sibling cohort) | 4–11 | T1D onset | Completed; trial stopped after interim analysis showed no effect on T1D progression | |
| Intranasal insulin (INIT-2) | 4–20 | T1D onset | Completed; data collection and analysis | |
| GAD-alum (DiAPREV-IT) | 4–18 | T1D onset | Follow-up | |
| GAD-alum and vitamin D3 (DiAPREV-IT2) | 4–18 | T1D onset | Recruiting | |
| Anti-inflammatory therapies | ||||
| Nicotinamide (DENIS) | 3–12 | T1D onset | Completed | DENIS [42] |
| Nicotinamide (ENDIT) | 5–40 | T1D onset | Completed | ENDIT [41] |
| Immune-modulating therapies | ||||
| CTLA-4 Ig, abatacept | 6–45 | Abnormal glucose tolerance by OGTT | Enrolling | TrialNet |
| Anti-CD3 mAb, teplizumab | 8–45 | T1D onset | Enrolling | TrialNet |
To date, β cell-specific or cell-based therapies have not been used in secondary type 1 diabetes clinical prevention trials
In the Diabetes Prevention Trial, Type 1 (DPT-1), sponsored by the National Institutes of Health, two insulin therapies were evaluated simultaneously to prevent T1D in at-risk relatives [33]. Relatives with iAb positivity and dysglycemia, thereby having a higher risk of progression to T1D, were randomized to placebo or low doses of ultralente insulin daily along with continuous insulin infusions for 4 days at the beginning of the study and annually thereafter (n = 339). The second cohort enrolled relatives with a lower T1D risk having iAb positivity (insulin autoantibodies present) and no metabolic abnormalities to daily oral insulin or placebo (n = 372). Unfortunately, after 6 years of follow-up, neither treatment delayed progression to T1D. Notably, a post hoc analysis of the oral insulin participants showed that patients with high insulin autoantibody levels (≥80 U/ml) had a delayed onset to T1D of approximately 5 years (n = 263) [34]. This finding prompted a repeat trial through TrialNet (), which is set to report results in 2017. In addition to these studies and the recent Pre-POINTstudy in primary prevention, TrialNet is currently conducting a secondary prevention study administering higher doses of oral insulin, to individuals with the same two iAb criteria for the current oral insulin trial, to help elucidate the mechanism of oral insulin’s immunologic effect ().
The Belgian T1D Registry also conducted a prospective, nonrandomized study to determine if parenteral insulin could delay progression to T1D. Patients with IA-2 autoantibodies were given two injections of short-acting insulin for 36 months. Similar to the DPT-1 trial, the treated patients developed T1D at the same rate as the control population [35].
Intranasal insulin has also been used to prevent T1D and induce tolerance. The Intranasal Insulin Trial (INIT)-1 was a randomized, double-blinded, crossover pilot study conducted in Australia (n = 38) that indicated that intranasal insulin was safe, tolerable, and decreased T cell responses to insulin. These results prompted a larger study, INIT-2, which has completed enrollment and is following patients for 10 years to evaluate for T1D development () [36]. Another study utilized the DIPP cohort in Finland and randomized iAb-positive individuals to intranasal insulin or placebo (n = 264). An interim analysis showed no benefit of intranasal insulin in delaying progression to T1D, which resulted in stopping the trial [37].
Other therapies that have been used for secondary prevention include glutamic acid decarboxylase 65 formulated with the adjuvant aluminum hydroxide (GAD-alum) and nicotinamide (vitamin B6). The DIA-PREVIT trial is an ongoing study administering a GAD-alum vaccine to children with one or more iAb, one of which is an autoantibody to GAD. Participants are being followed for T1D development over 5 years. Nicotinamide increases nicotinamide adenine dinucleotide (NAD) and can potentially inhibit free radical formation, resulting in reduced β cell inflammation. Pilot studies had promising results [38–40]; however, two large randomized, double-blinded, placebo-controlled trials failed to delay progression to T1D [41, 42]. Other agents have been utilized in pilot trials including bacillus Calmette-Guerin (BCG), ketotifen (histamine antagonist), and cyclosporine [43–45]. Oral cyclosporine administration resulted in a slight delay in the time to T1D onset but was not able to prevent T1D onset.
The National Institutes of Health (NIH)-sponsored DPT-1 has continued as Type 1 Diabetes TrialNet with the goals of understanding disease pathogenesis, screening at-risk relatives for iAb and conducting clinical intervention trials to prevent T1D [46, 47]. Two therapies currently being used in TrialNet studies to delay T1D onset include an anti-CD3 monoclonal antibody (teplizumab) and CTLA-4 Ig (abatacept), which both had a mild effect on preserving β cell function (i.e., extending the “honeymoon period”) in new-onset T1D patients. TrialNet is currently enrolling children and adults with multiple iAb positivity into trials utilizing these agents to assess if there is a change from normal to abnormal glucose tolerance with the use of abatacept (). Teplizumab is being used in iAb-positive individuals with impaired glucose tolerance (stage 2 in Fig. 1), which represents a period closer to clinical T1D, to delay T1D onset ().
New-Onset T1D Trials (Stage 3 T1D)
A large number of clinical trials have been completed in new-onset T1D, which is a population with limited β cell function. New-onset or tertiary prevention trials have aimed to preserve residual β cell function by targeting inflammation, modulating the adaptive immune response, inducing tolerance with antigen-specific therapies, and infusing cell-based therapies. The most widely accepted method to assess efficacy in these trials is to monitor β cell function by measuring stimulated C-peptide, which is cleaved 1:1 from insulin, over a 2–4-h period in response to a mixed meal tolerance test (MMTT) [48]. The most successful trial to date required an aggressive course of therapies including autologous nonmyeloblative stem cell transplantation (AHSCT) with granulocyte colony stimulation factor (G-CSF) for cell mobilization, and cyclophosphamide plus anti-thymocyte globulin (ATG) for induction therapy to remove activated immune cells [49–52]. The protocol aimed to induce peripheral tolerance through hematopoietic stem cell immune regulation. In the initial trial, 20 of 23 patients were free from insulin use (12 continuously, 8 transiently) for a mean of 31 months [51, 52]. Of the three patients who did not have a time of insulin independence, they either had diabetic ketoacidosis or received glucocorticoids prior to the treatment regimen. It is likely that approaches requiring this degree of medical intervention provide more risk than benefit, especially in a pediatric population, given that good glycemic control is possible with current medical therapy. However, the results do provide a proof of concept that the autoimmune destruction of pancreatic β cells can be halted for a period of time late in the disease course. In fact, a recent randomized, single-blinded, placebo-controlled pilot trial using ATG and G-CSF preserved residual β cell function in recent-onset T1D patients for the first year after therapy [53]. A larger trial is now enrolling new-onset adolescents and adults (). Interestingly, ATG alone [54, 55] and G-CSF [56] alone do not maintain endogenous insulin production, indicating that certain combination therapies are capable of providing synergy to improve efficacy.
Although no new-onset study to date has permanently stopped β cell destruction, several studies have shown transient preservation of β cell function in phase 2/3 efficacy studies. A number of studies utilized a humanized monoclonal antibody to CD3 (teplizumab or otelixizumab), a cell surface marker found on mature T cells, in an attempt to target pathogenic immune cells [57–62]. With initial signals of efficacy in phase 2 trials, a large phase 3 clinical trial using teplizumab did not meet its primary endpoint to reduce daily insulin use and improve hemoglobin A1c values compared to placebo-treated controls. A post hoc analysis identified a distinct subset of responders that were young (ages 8–17 years), received drug within 6 weeks of diagnosis, and started treatment with good metabolic control (A1C <7.5 %, C-peptide mean AUC >0.2 nmol/l) [57, 61].
Other immune-modulating agents that have shown variable degrees of success include rituximab (anti-CD20) [63, 64], abatacept (CTLA-4 Ig) [65, 66], and alefacept (anti-CD2) [67]. Binding of rituximab to the CD20 transmembrane protein on B cells essentially eliminates mature B cells, which may be important antigen-presenting cells to activate self-reactive T cells. New-onset T1D patients treated with a course of rituximab had an approximate 8-month delay in C-peptide decline compared to placebo-treated controls. Similarly, patients receiving abatacept, which blocks T cell costimulatory receptor CTLA4, had an approximate 10-month delay in C-peptide decline in response to a 2-h MMTT and also had a lower HbA1c than the control group. Another recent multicenter, randomized, double-blinded, placebo-controlled trial (TIDAL) administered alefacept (n = 33) or placebo (n = 16) to new-onset T1D patients in two 12-week courses over 9 months. Alefacept targets memory T cells by binding to CD2, which is predominately expressed on the CD4+ and CD8+ effector T cells that are thought to play a key role in β cell destruction. Administration of alefacept maintained endogenous β cell function after 1 year, and treated patients also had lower daily insulin requirements and less hypoglycemic events [67].
Other agents that can modulate the immune response have been investigated and have not preserved residual β cell function. Two randomized, multicenter, placebo-controlled trials administered agents directed against interleukin-1, an inflammatory cytokine [68]. In the first trial, patients with new-onset T1D were given subcutaneous injections of human monoclonal anti-interleukin-1 antibody, canakinumab, monthly for a year. The second trial gave anakinra, a human interleukin-1 receptor antagonist, by mouth daily for 9 months. Using an intention to treat analysis, neither agent delayed the loss of C-peptide in response to a MMTT. Several new-onset clinical trials utilizing antigen-specific therapies have been completed with the administration of GAD-alum, various forms of insulin and proinsulin, or heat-shock protein peptide (DiaPep277) [69–74]. Unfortunately, none of these trials preserved residual β cell function. As antigen-specific therapies move forward, the peptide, dose, route, and timing within T1D development need to be optimized [75].
Finally, cell-based therapies, including dendritic cells and regulatory T cells, have been safely administered in phase 1 trials [76, 77]. A recent trial isolated and expanded regulatory T cells from patients and then re-infused them into a total of 14 adult patients with new-onset T1D divided into four dosing cohorts. There were no infusion reactions or severe adverse events. Interestingly, there were several patients who had persistence of C-peptide in response to a MMTT at 1 year, and a phase 2 trial is planned to examine the effect of this therapy on maintaining β cell function after T1D diagnosis. Although not cell-based, low-dose interleukin-2 is currently being evaluated to specifically induce regulatory T cells to preserve β cell function [78, 79].
At the current time, despite T1D being a well-studied autoimmune disorder, only insulin preparations and amylin are approved for use by the US Food and Drug Administration to treat the disorder. No therapies to modulate or stop the immune destruction of β cells are used to treat T1D in clinical practice.
Challenges and Future Outlook
Identify Individuals at Risk for T1D
In order to eventually prevent T1D, two avenues need to be simultaneously pursued: (1) At-risk individuals need to be identified and (2) safe, specific, and personalized therapies to interdict the autoimmune response need to be evaluated in the preclinical T1D setting. First, the vast majority of screening efforts have focused on at-risk relatives of individuals with T1D. The American Diabetes Association currently recommends screening for iAb in relatives of patients with T1D through available clinical research studies, which is most often the Type 1 Diabetes TrialNet Pathway to Prevention Study. However, over 85 % of individuals who develop T1D do not have a family history [80]. With the incidence of diabetes increasing and diabetic ketoacidosis (DKA) being a significant comorbidity, it is our view that the general population should be screened for T1D risk by measuring iAb, especially in children. This would allow earlier diagnosis and opportunities for clinical prevention trials and lessen the morbidity and mortality of DKA [81, 82]. Screening for iAb currently requires venipuncture, which can be difficult in young children. Therefore, the need exists for a more widely adaptable and cost-effective method for screening iAb in the general population [83]. One such method is to measure iAb from dried blood spots (DBS) on filter paper. TrialNet recently conducted a study that showed decent correlation between serum and DBS measurements for GAD, IA-2, and ZnT8 [84]. Insulin autoantibodies have historically been difficult to measure from DBS, but they are often the first iAb to develop and levels correlate with time to disease onset [9, 30]. We recently developed a method for measuring all four conventional iAb, including insulin autoantibodies, from DBS which may help facilitate large-scale general population screening (unpublished data).
Personalized Therapies
Over the last two decades, many well-controlled clinical trials have been enrolled and completed in an attempt to prevent T1D. Despite the disappointing results, T1D is now predictable and the infrastructure is in place to evaluate therapies. The development of more personalized therapies with specific mechanisms of action holds the promise to preserve β cell function. As previously mentioned, T1D genetic risk is predominantly conferred through specific HLA class II genes, such that ∼60 % of all T1D patients have the DQ8 and DR4 genes, which are closely linked on chromosome 6. Studies using small “drug-like” molecules targeting the HLA-DQ8 molecule on antigen-presenting cells can block resultant peptide presentation to T cells [85]. One such molecule, methyldopa (Aldomet), is being evaluated in a phase 1b proof of concept trial in adults with recently diagnosed T1D having the DQ8 gene (). Methyldopa is a clinically well-established anti-hypertensive medication being used for more than 50 years; it is currently used clinically for the treatment of pregnancy-induced hypertension. With such a safe and well-studied medication, methyldopa provides safety, specificity, and personalization (targeting HLA-DQ8) for use in delaying or preventing T1D.
Antigen-specific therapy is also being directed to specific HLA genes. A peptide of proinsulin (C19-A3) is able to elicit T cell responses in T1D patients having HLA-DR4. A phase 1 trial treated T1D patients with intradermal injection of the peptide, showing safety and tolerability along with signals of immune efficacy. The low-dose peptide group had the induction of C19-A3-specific T cells producing interleukin-10, an anti-inflammatory cytokine associated with regulatory T cells [86]. Peptide immunotherapy personalized to a specific HLA gene is currently being evaluated in a recent-onset T1D trial ().
The combination of HLA class II molecules, self-peptide (insulin peptide) and T cell receptor form a trimolecular complex to active self-reactive T cells. The anti-insulin trimolecular complex (HLA-peptide-T cell receptor) provides components to target with specific therapies [87]. As such, there are therapies under preclinical development in animal models of autoimmune diabetes with monoclonal antibodies that can specifically block insulin/HLA complexes, thus inhibiting T cell activation [88]. There are also antibodies directed against a component of a disease-relevant T cell receptor to specifically deplete T cells involved in the autoimmune process [89]. Finally, a self-peptide that activates T cells toward islet autoimmunity has been well studied in the mouse model of spontaneous autoimmune diabetes. A specific fragment of insulin, B chain amino acids 9–23 (B:9–23), is an essential autoantigen driving T1D development [90], which may very well be the case in human disease [31••, 91]. How this peptide is presented by specific HLA-DQ molecules appears to be important in directing T cell responses [92, 93]. Attempts at mutating this peptide to improve insulin antigen-specific therapy by inducing protective T cell responses (i.e., insulin-specific regulatory T cells) have been encouraging in animal models, including humanized mice with human HLA-DQ8 [94, 95]. All of these approaches represent specific immunologic therapies with a defined target. Islet autoimmunity is extremely specific and directed toward insulin-producing β cells, and as such, we believe that therapies having these same qualities will lead to improved T1D prevention efforts.
Conclusions
Type 1 diabetes is a chronic T cell-mediated autoimmune disorder with the natural history divided into stages. These stages allow for understanding disease pathogenesis as well as providing a blueprint for clinical prevention trials. Numerous primary, secondary, and new-onset clinical trials have been completed; however, β cell function cannot be sustainably preserved in those at risk or with newly diagnosed T1D. On the horizon, there are therapies with defined molecular targets involved in disease pathogenesis. Once these therapies are validated in clinical trials and combined based upon mechanism of action, we believe that T1D will be prevented and a foundation will be in place to cure the disease.
Acknowledgments
The study was supported by grants from the National Institute of Diabetes and Digestive Kidney Diseases (K08 DK095995), Juvenile Diabetes Research Foundation (2-SRA-2016–202-S-B), and the Children’s Diabetes Foundation.
Footnotes
Conflict of Interest Dr. Simmons has nothing to disclose.
Dr. Gottlieb has a pending patent on Insulin Mimotopes and Methods of Using the Same, and a patent on Methods of Preventing and Treating Autoimmunity licensed to ImmunoMolecular Therapeutics.
Dr. Michels has two patents, Compounds That Modulate Autoimmunity and Methods of Using the Same and Methods of Preventing and Treating Autoimmunity, licensed to ImmunoMolecular Therapeutics, and a pending patent on Insulin Mimotopes and Methods of Using the Same.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383(9911):69–82.Useful review on all aspects of type 1 diabetes over the last 10 years including eitology, pathogeneses, prediction, prevention and treatment.
- 2.American Diabetes A. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes 2016;34(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329(14):977–86 [DOI] [PubMed] [Google Scholar]
- 4.Writing Team for the Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Research G. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290(16):2159–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes-specific characteristics. Diabetes Care 2006;29(6):1389–91. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth GS, Type I. Diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986;314(21):1360–8. [DOI] [PubMed] [Google Scholar]
- 8.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89(8):3896–902. [DOI] [PubMed] [Google Scholar]
- 9.Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, et al. Predictors of Progression From the Appearance of Islet Autoantibodies to Early Childhood Diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38(5):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark A, Hagopian WA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58(5):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999;48(3):460–8. [DOI] [PubMed] [Google Scholar]
- 12.Kimpimaki T, Kupila A, Hamalainen AM, Kukko M, Kulmala P, Savola K, et al. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab 2001;86(10):4782–8. [DOI] [PubMed] [Google Scholar]
- 13.••.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309(23):2473–9.This study indicates that 2 or more serum islet autoantibodies leads to clinical T1D development in children followed from birth in the United States and Europe, making type 1 diabetes a predic disease.
- 14.Parikka V, Nanto-Salonen K, Saarinen M, Simell T, Ilonen J, Hyoty H, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012;55(7):1926–36. [DOI] [PubMed] [Google Scholar]
- 15.•.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38(10):1964–74.This paper describes a new staging method for type 1 diabetes, with the first two stages defined without the presence of clinical symptoms.
- 16.Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Annals Intern Med 1998;128(7):517–23 [DOI] [PubMed] [Google Scholar]
- 17.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008;359(26):2849–50. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM, et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet 2015;47(8):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama M, Simmons KM, Michels AW. Molecular interactions governing autoantigen presentation in type 1 diabetes. Curr Diab Rep 2015;15(12):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harbor Perspect Med 2012;2(1):a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57(4):1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360(16):1646–54. [DOI] [PubMed] [Google Scholar]
- 23.Knip M, Virtanen SM, Seppa K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med 2010;363(20):1900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knip M, Akerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, et al. Hydrolyzed infant formula and early beta-cell autoimmunity: a randomized clinical trial. JAMA 2014;311(22):2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaarala O, Ilonen J, Ruohtula T, Pesola J, Virtanen SM, Harkonen T, et al. Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch Pediatr Adolesc Med 2012;166(7):608–14. [DOI] [PubMed] [Google Scholar]
- 26.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007;298(12):1420–8. [DOI] [PubMed] [Google Scholar]
- 27.Chase HP, Boulware D, Rodriguez H, Donaldson D, Chritton S, Rafkin-Mervis L, et al. Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes. Pediatr Diabetes 2015;16(4):271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonifacio E, Ziegler AG, Klingensmith G, Schober E, Bingley PJ, Rottenkolber M, et al. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA 2015;313(15):1541–9. [DOI] [PubMed] [Google Scholar]
- 29.Michels AW, von Herrath M. 2011 Update: antigen-specific therapy in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2011;18(4):235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 2011;34(6):1397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.••.Nakayama M, McDaniel K, Fitzgerald-Miller L, Kiekhaefer C, Snell-Bergeon JK, Davidson HW, et al. Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proc Natl Acad Sci U S A 2015;112(14):4429–34. doi: 10.1073/pnas.1502967112.Original research showing the ability to measure inflammatory and regulatory T cell responses in the peripheral blood to a novel insulin B chain peptide in new-onset T1D patients and healthy controls.
- 32.Michels AW, Eisenbarth GS. Immune intervention in type 1 diabetes. Semin Immunol 2011;23(3):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diabetes Prevention Trial–Type 1 Diabetes Study G. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346(22):1685–91. [DOI] [PubMed] [Google Scholar]
- 34.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28(5):1068–76. [DOI] [PubMed] [Google Scholar]
- 35.Vandemeulebroucke E, Gorus FK, Decochez K, Weets I, Keymeulen B, De Block C, et al. Insulin treatment in IA-2A-positive relatives of type 1 diabetic patients. Diabetes Metab 2009;35(4):319–27. [DOI] [PubMed] [Google Scholar]
- 36.Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care 2004;27(10): 2348–55. [DOI] [PubMed] [Google Scholar]
- 37.Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372(9651):1746–55. [DOI] [PubMed] [Google Scholar]
- 38.Herskowitz RD, Jackson RA, Soeldner JS, Eisenbarth GS. Pilot trial to prevent type I diabetes: progression to overt IDDM despite oral nicotinamide. J Autoimmun 1989;2(5):733–7. [DOI] [PubMed] [Google Scholar]
- 39.Elliott RB, Chase HP. Prevention or delay of type 1 (insulin-dependent) diabetes mellitus in children using nicotinamide. Diabetologia 1991;34(5):362–5. [DOI] [PubMed] [Google Scholar]
- 40.Manna R, Migliore A, Martin LS, Ferrara E, Ponte E, Marietti G, et al. Nicotinamide treatment in subjects at high risk of developing IDDM improves insulin secretion. Br J Clin Pract 1992;46(3):177–9. [PubMed] [Google Scholar]
- 41.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial G. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363(9413):925–31. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 42.Lampeter EFKA, Scherbaum WA, Henize E, Haastert B, Giani G, et al. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes Care 1998;47:980–4. [DOI] [PubMed] [Google Scholar]
- 43.Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal Bacille Calmette-Guerin vaccination and type 1 diabetes. Diabetes Care 2005;28(5):1204–6. [DOI] [PubMed] [Google Scholar]
- 44.Bohmer KP, Kolb H, Kuglin B, Zielasek J, Hubinger A, Lampeter EF, et al. Linear loss of insulin secretory capacity during the last six months preceding IDDM. No effect of antiedematous therapy with ketotifen. Diabetes Care 1994;17(2):138–41. [DOI] [PubMed] [Google Scholar]
- 45.Carel JC, Boltard C, Eisenbarth G, Bach J-F, Bougneres P-F. Cyclosporine delays but does not prevent clinical onset in glucose intolerant pre-type 1 diabetic children. J Autoimmun 1996;9:739–45. [DOI] [PubMed] [Google Scholar]
- 46.Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, et al. Type 1 Diabetes TrialNet—an international collaborative clinical trials network. Ann N Y Acad Sci 2008;1150: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10(2):97–104. [DOI] [PubMed] [Google Scholar]
- 48.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61(8):2066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snarski E, Milczarczyk A, Torosian T, Paluszewska M, Urbanowska E, Krol M, et al. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant 2011;46(4):562–6. [DOI] [PubMed] [Google Scholar]
- 50.Snarski E, Milczarczyk A, Halaburda K, Torosian T, Paluszewska M, Urbanowska E, et al. Immunoablation and autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: long-term observations. Bone Marrow Transplant 2016;51(3):398–402. [DOI] [PubMed] [Google Scholar]
- 51.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297(14):1568–76. [DOI] [PubMed] [Google Scholar]
- 52.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301(15):1573–9. [DOI] [PubMed] [Google Scholar]
- 53.Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, et al. Anti-thymocyte globulin/G-CSF treatment preserves beta cell function in patients with established type 1 diabetes. J Clin Invest 2015;125(1):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gitelman SE, Gottlieb PA, Rigby MR, Felner EI, Willi SM, Fisher LK, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diab Endocrinol 2013;1(4):306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia 2016;59(6):1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haller MJ, Atkinson MA, Wasserfall CH, Brusko TM, Mathews CE, Hulme M, et al. Mobilization without immune depletion fails to restore immunological tolerance or preserve beta cell function in recent onset type 1 diabetes. Clin Exp Immunol 2016;183(3):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes 2013;62(11):3901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia 2013;56(2):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009;132(2):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013;62(11):3766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378(9790):487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346(22):1692–8. [DOI] [PubMed] [Google Scholar]
- 63.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361(22):2143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, et al. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care 2014;37(2):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378(9789):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 2014;37(4):1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 2015;125(8):3285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 2013;381(9881):1905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, et al. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev 2007;23(4):292–8. [DOI] [PubMed] [Google Scholar]
- 70.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 2001;358(9295):1749–53. [DOI] [PubMed] [Google Scholar]
- 71.Raz I, Ziegler AG, Linn T, Schernthaner G, Bonnici F, Distiller LA, et al. Treatment of recent-onset type 1 diabetic patients with DiaPep277: results of a double-blind, placebo-controlled, randomized phase 3 trial. Diabetes Care 2014;37(5):1392–400. [DOI] [PubMed] [Google Scholar]
- 72.Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359(18):1909–20. [DOI] [PubMed] [Google Scholar]
- 73.Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 2012;366(5):433–42. [DOI] [PubMed] [Google Scholar]
- 74.Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011;378(9788):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peakman M, von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes 2010;59(9): 2087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011;34(9):2026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015;7(315):315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Truman LA, Pekalski ML, Kareclas P, Evangelou M, Walker NM, Howlett J, et al. Protocol of the adaptive study of IL-2 dose frequency on regulatory T cells in type 1 diabetes (DILfrequency): a mechanistic, non-randomised, repeat dose, open-label, response-adaptive study. BMJ Open 2015;5(12):e009799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 2015;64(6):2172–83. [DOI] [PubMed] [Google Scholar]
- 80.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res 2001;56:69–89. [DOI] [PubMed] [Google Scholar]
- 81.Elding Larsson H, Vehik K, Bell R, Dabelea D, Dolan L, Pihoker C, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34(11):2347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 2012;13(4):308–13. [DOI] [PubMed] [Google Scholar]
- 83.Simmons K, Michels A. Is it time to screen the general population for type 1 diabetes? US Endocrinol 2015;11(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bingley PJ, Rafkin LE, Matheson D, Steck AK, Yu L, Henderson C, et al. Use of dried capillary blood sampling for islet autoantibody screening in relatives: a feasibility study. Diabetes Technol Ther 2015;17(12):867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michels AW, Ostrov DA, Zhang L, Nakayama M, Fuse M, McDaniel K, et al. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol 2011;187(11):5921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol 2009;155(2):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michels AW. Targeting the trimolecular complex. Clin Immunol 2013;149(3):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Crawford F, Yu L, Michels A, Nakayama M, Davidson HW, et al. Monoclonal antibody blocking the recognition of an insulin peptide-MHC complex modulates type 1 diabetes. Proc Natl Acad Sci U S A 2014;111(7):2656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Cort L, Eberwine R, Herrmann T, Leif JH, Greiner DL, et al. Prevention of type 1 diabetes in the rat with an allele-specific anti-T-cell receptor antibody: Vbeta13 as a therapeutic target and biomarker. Diabetes 2012;61(5):1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435(7039):220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest 2001;107(2):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci U S A 2010;107(24):10978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci U S A 2011;108(40):16729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med 2011;208(7):1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Serr I, Furst RW, Achenbach P, Scherm MG, Gokmen F, Haupt F, et al. Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nat Commun 2016;7:10991. [DOI] [PMC free article] [PubMed] [Google Scholar]