Abstract
Purpose:
The objective of our study was to incorporate stricter probable nonfatal opioid overdose case criteria, and advanced epidemiologic approaches to more reliably detect local clustering in nonfatal opioid overdose activity in EMS runs data.
Methods:
Data were obtained using emsCharts for our study area in southwestern Pennsylvania from 2007 to 2018. Cases were identified as emergency medical service (EMS) responses where naloxone was administered, and improvement was noted in patient records between initial and final Glasgow Coma Score. A subsample of all-cause EMS responses sites were used as controls and exact matched to cases on sex and 10-year-age category. Clustering was assessed using difference in Ripley’s K function for cases and controls and Kulldorff scan statistics.
Results:
Difference in K functions indicated no significant difference in probable nonfatal overdose EMS runs across the study area compared to all-cause EMS runs. However, scan statistics did identify significant local clustering of probable nonfatal overdose EMS runs (maximum likelihood = 16.40, P = 0.0003).
Conclusions:
Results highlight relevance of EMS data to detect community-level overdose activity and promote reliable use through stricter case definition criteria and advanced methodological approaches. Techniques examined have the potential to improve targeted delivery of neighborhood-level public health response activities using a near real-time data source.
Keywords: EMS runs, Cluster analysis, Nonfatal overdose
Introduction
Fatal opioid overdoses are a persistent and increasing problem in the United States, with almost 400,000 deaths reported between 1999 and 2017 [1]. Although fatal overdoses are concerning, the large majority of overdoses are nonfatal [2–4]. Nonfatal overdoses are a significant source of public health concern as they are a significant risk factor for fatal subsequent overdose and have been linked to higher occurrence of morbidities such as falls, burns, seizures, physical assault [5,6]. Measuring occurrence of nonfatal overdose is often difficult and conducted using emergency department or emergency medical service (EMS) data. Although both data sources are potentially incomplete, emergency department data do not capture patients refusing transport and are often limited to poorly defined service areas [7,8]. In addition, EMSs are the primary source for prehospital naloxone administration, high-lighting the importance of using EMS data to identify local trends in overdose [7,9–12].
The usefulness of EMS naloxone administration for describing trends in overdose has been described in past research with particular attention on the setting, patient demographics, and frequency of administration [8,13–16]. Overall, findings suggest EMS naloxone administration rates parallel overdose mortality data [8], with age and repeat nonfatal overdose being significant predictors of fatal overdose [7,8,10,13]. Other studies place specific emphasis on geographic trends in EMS data, noting agreement in county-level patterns in ambulance runs and nonfatal overdose [14], trends in EMS naloxone use in rural localities [15], and significant differences in local clustering of suspect overdoses [3].
Although these studies establish a foundation for use of EMS data and integration of spatial analysis approaches, they lack strict case definitions, and inclusion of EMS run controls in spatial analyses. These limitations can potentially result in overestimation of overdose encounters, which potentially translate to higher number of high-risk areas spuriously identified [17,18]. Variables in EMS run data like, Glasgow Coma Score (GCS), allow for identification of probable overdose encounters with greater certainty [19]. More specifically, GCS codes the level of consciousness of the patient and ranges from 3 to 15 [19,20]. An improvement in the GCS score after administration of naloxone to an initially unresponsive patient provides greater indication of opioid overdose [19]. Consequently, the objectives of this study were to incorporate a stricter probable overdose case definition and use controls in spatial cluster analysis to improve accuracy of high-risk overdose area detection.
Methods
Study area
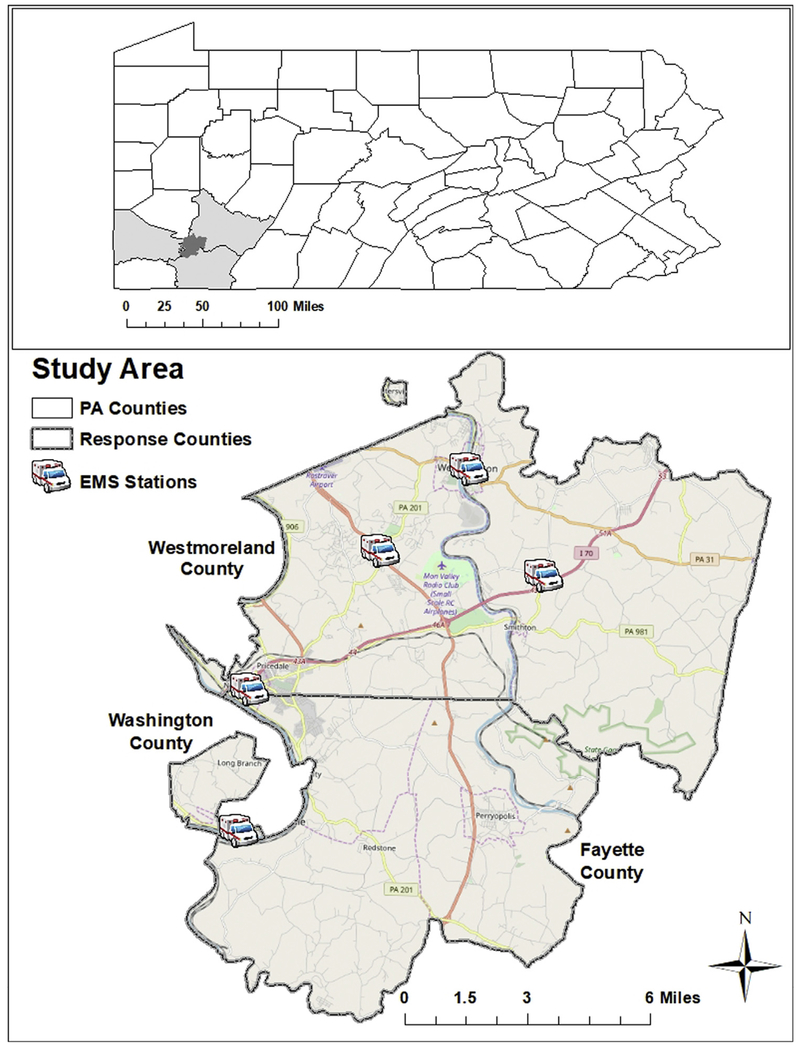
Pennsylvania has the third highest opioid overdose death rate in the nation [21]. In particular, the areas of Westmoreland, Fayette, and Washington counties in southwestern Pennsylvania had overdose death rates ranging from 47 to 57 per 100,000 persons, which were higher than the state rate of 43 deaths per 100,000 persons [22]. These areas are served by Rostraver/West Newton Emergency Services (RWNES) agency covering 137 square miles and servicing approximately 35,695 residents in a mix of rural and urban centers (Fig. 1).
Fig. 1.
Rostraver/West Newton Emergency Services (RWNES) area in southwestern Pennsylvania.
Data management
Address-level EMS response data were obtained from emsCharts software for 2007 to 2018. Cases were defined as a probable overdose if naloxone had been administered, and GCS improved (e.g., increased) after naloxone administration. Records indicating naloxone administration, but missing initial or final GCS score (n = 91, 11.3%), were individually reviewed by trained EMS personnel from the service area and added to the case group if GCS information could be obtained from a secondary source, such as field notes (n = 29, 31.8%). In total, 62 cases (12%) were excluded because of missing initial and final GCS scores. Controls included all-cause EMS records excluding patient responses, which involved naloxone administration or involving traffic crashes. Traffic crashes were identified using the medical category variable which subgroups EMS responses into general themes. Traffic crashes were excluded from potential controls to limit introduction of spatial bias due to inherent clustering of traffic crashes near major roadways. Controls were matched 5:1 to cases based on patient sex (male, female, unreported) and 10-year-age category (<10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, >70). Matching was conducted using the MatchIt package in R, to increase comparability in demographics among cases and controls [23,24]. EMS responses were geocoded using the address of the response site indicated for each patient in ArcMap 10.5 (ESRI Redlands, CA) using the ArcGIS Online World Geocoding Service. Approval to conduct the study was obtained from the West Virginia University Institutional Review Board (protocol #1901434557).
Analysis
Overall (global) clustering within the study area was evaluated using Ripley’s K function in R using the spatstat and smacpod packages [25,26]. Ripley’s K is a method for describing whether points are dispersed, are clustered, or occur randomly within an area of emphasis. In the context of this study, K functions were used to examine point patterns for cases and controls separately first to identify relevant trends in data. Next, the difference between K functions was estimated to identify clustering of probable overdose in excess of what would be expected given the distribution of all-cause EMS run data [27,28]. Monte Carlo simulations (n = 999) were incorporated to estimate 95% confidence intervals to assess statistical significance at the 0.05 alpha level.
Kulldorff spatial scan statistics were used to detect local clustering using the purely spatial Bernoulli model in SaTScan 9.1 [29,30]. The Bernoulli model is ideal for discrete analyses, such as in our case-control study, where the location of events is fixed, and the patient records represent event data (0/1) [29]. Spatial scan windows for the analysis were set to include 50% of the population at risk per the software developer recommendations [31]. As an additional measure of sensitivity, the window was set to vary from 50% to 25% to identify small and large clusters and adjust for multiple testing using the advanced analyses options [32].
The null hypothesis for the scan statistics is equivalent risk of probable overdose inside and outside the spatial scanning window. Most likely clusters identified during the analysis were those with the highest maximum likelihood values [31]. Statistical significance for each local cluster identified was accomplished through use of estimate P-values and Gini coefficients. Use of Gini coefficients in conjunction with maximum likelihood/P-values to improve robustness of scan statistic results has been highlighted in past research [33,34]. More specifically, Gini coefficients improve robustness of scan statistics by optimizing the maximum reported cluster size, enabling identification of smaller or irregular shaped clusters that would otherwise be masked by larger imprecise areas detected in the scan statistic alone [34]. Monte Carlo simulations (n = 999) were used to obtain relevant P-values and 95% confidence intervals. Statistical significance was evaluated at the 0.05 alpha level.
Results
Overall, there were 56,225 patient records during the study period, of which 10,204 were removed due to calls outside the service area, standby events, cancellations, and interfacility transport. From the 46,021 patients, 805 (1.74%) were suspect overdose responses with documented naloxone administration. Of these 805, only 432 (53.7%) met our study’s probable overdose case criteria requiring documented improvement between initial and final GCS score. Improvements in GCS score among probable overdose ranged from 1 to 12, with 277 (64%) patients improving by 6–12 points between initial and final GCS readings. Patient records with suspect or probable overdose (n = 805) or who were involved in a traffic accident (n = 4895) were removed from the available 46,021 records, after which 2160 controls were randomly selected from age- and sex-specific strata, obtained through matching. All 2592 patient records were geocoded successfully.
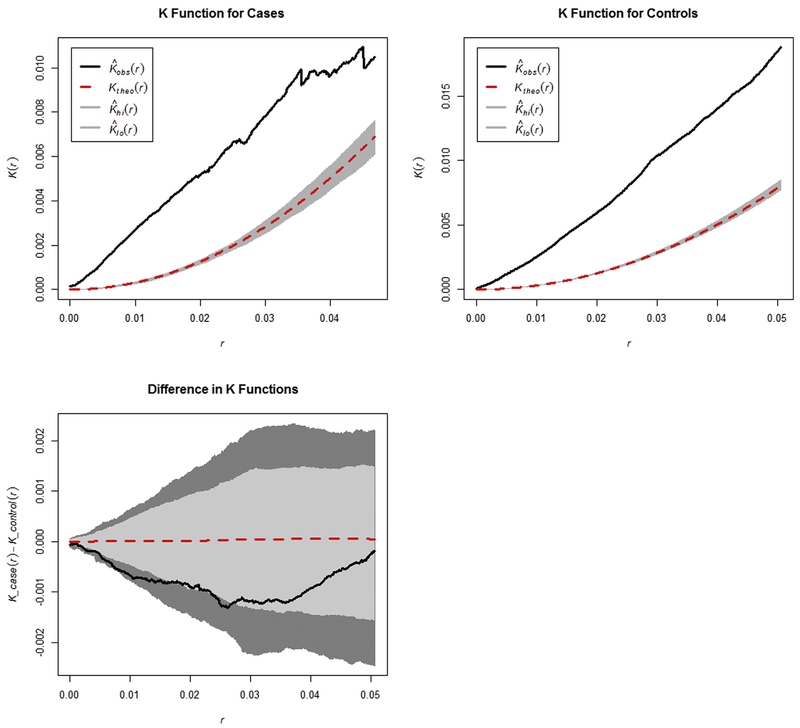
Overall, K functions displayed statistically significant overall clustering for cases and controls separately, but not for the difference (Fig. 2). Statistical significance is indicated in the graphs when the observed values (solid black lines) continue beyond the 95% confidence intervals (gray envelopes). Lack of significance in the difference between K functions suggests similarity in distribution of patients with probable overdose and all-cause EMS runs across the overall study area.
Fig. 2.
Ripley’s K functions measuring overall spatial clustering across the entire study area for cases, controls, and the difference between K functions for cases and controls. Black lines indicated the observed values, dotted red lines represent null hypothesis of no clustering, and dark gray envelopes indicate the 95% confidence interval. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
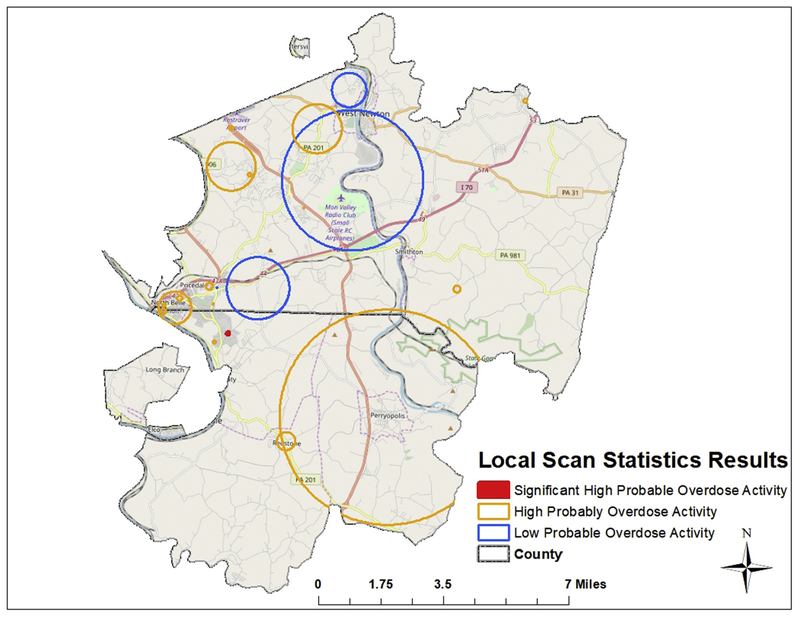
Bernoulli purely spatial scan statistics identified local-level trends in clustering for patients with probable overdose (Fig. 3). Resulting analyses identified 21 local clusters of varying size (radii ranged from 0.02 to 4.87 km) with maximum likelihood values and relative risks from 5.12 to 16.40 and 0.09 to 5.90, respectively. Areas are identified as an area of low or high probable overdose risk based on whether the relative risk is below (low) or higher (high) than one. Of the 21 clusters identified, 4 (19%) were low, and 17 (81%) were high based on observed versus expected cases designated by size of spatial scanning window. Of the 17 high-risk clusters identified, one (5.88%) was designated as a statistically significant nonoverlapping Gini cluster (maximum likelihood = 16.40, relative risk = 4.39, P = .0003).
Fig. 3.
Purely spatial Bernoulli model scan statistic results for local clustering. Orange circles indicate areas of high risk, and blue circles display areas of low risk for probable overdose. The red circle represents the statistically significant cluster of high probable overdose risk. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Discussion
Use of spatial analysis to monitor trends and identify hotspots of drug overdose activity in the United States and counties elsewhere is widely used in the literature [11,35–37]. Incorporating address level data in spatial analyses to examine small area-level trends in overdose is more sparsely described, particularly in relation to use of EMS run data [3]. To our knowledge, this study is the first to apply more stringent case definition criteria and controls to more precisely investigate local clustering of nonfatal overdose. Overall, clustering was found in multiple areas with mixed population densities, reflecting the rural to urban gradient within the EMS service area. Clusters in the western region of the service area were small relative to the cluster identified over Perryopolis (PA). This was not surprising as the clusters in the western part of the area are more densely populated contributing to the smaller radius of clusters identified. Alternatively, Perryopolis is rural and more sparsely populated contributing to the larger radius of the cluster detected. Use of satellite imagery within the single statistically significant cluster revealed that the area was a moderately populated residential area with two on-premises alcohol retailers, and several baseball fields and an elementary school within a half mile. This cluster was not located over a major roadway, but adjacent to PA State Route 201.
Strengths to the study include leveraging controls from the same EMS database as the cases, and use of matching on sex and age groups. In addition, we were able to use other raw data elements such as medical category of the EMS run to remove subgroups, such as traffic crashes, which were likely to cluster at known locations along major roadways. Incorporating two fundamentally different cluster analyses gave both a global and local perspective on nonfatal opioid overdose activity within the EMS service area. Results from the Ripley’s K analyses indicate that EMS runs aggregate within the region, but nonfatal opioid overdose EMS runs do not cluster any more than all-cause EMS runs. Findings from the spatial scan statistics identified local areas where nonfatal opioid overdose activity was highest. In both analyses, additional measures were used to ensure a higher degree of sensitivity [23,25,26,31,32].
Despite these strengths, limitations to our approach exist. Most prominently, EMS run data were not linked with medical records to confirm diagnosis or determine whether an event was ultimately fatal or nonfatal (for cases or controls) after being transported to a medical facility. Here we assume that improvement in GCS score resulted in a nonfatal opioid overdose event but have limited to no knowledge regarding the outcome of each case. In regards to controls, we assume they contain a combination of fatal and nonfatal events. This is a potential limitation as fatal EMS runs are potentially different concerning spatial clustering. We attempt to mitigate this bias where possible by excluding controls involved in traffic crashes. In addition, although improvement of GCS after administration of naloxone is indicative of narcotic overdose, the reciprocal does not always hold true; for instance, a patient with other comorbidities secondary to a narcotic overdose may not improve after administration of naloxone despite having been a narcotic overdose. Similarly, some cases may have been missed in instances of polypharmacy; mainly, a person could have been given naloxone, but other pharmacologic agents muted its effect. Finally, case acquisition was limited to circumstances where EMSs were involved, which exclude cases where naloxone was administered by a family or community member or where no-EMS contact was involved.
Despite these limitations, studies using address-level overdose data are critical in guiding targeted public health intervention. However, previous studies have noted lack of coordination between EMS and other health agencies limits utility of the highly granular overdose data and their impact on prevention [36]. This finding is consistent with lack of evidence of prevention activities, to our knowledge, occurring within our EMS area during the study period 2008–2017. However, as of 2018, the Rostraver/West Newton Emergency Services agency has been taking part in a naloxone leave behind program and entered into a partnership with the Pennsylvania Drug and Alcohol Commission in which they are trying to identify opioid and other drug- and alcohol-related patients through data collection. As part of this program, patients identified as part of a drug and alcohol EMS run are provided with an opportunity to be linked to care. Results from our study provide a sophisticated approach to visualizing overdose activity within small areas, to identify nearby resources, and support local opioid overdose prevention efforts.
Acknowledgments
The authors would like to thank Rostraver/West Newton Emergency Services for access to the data, and Dr. Casey Jelsema from the West Virginia University Department of Biostatistics for technical assistance in R. This work was supported through National Institute of General Medical Sciences, 2U54GM104942-02, and National Institute of Drug Abuse (R21DA040187 and UG3DA044825). The authors have no conflicts of interest to report.
References
- [1].Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep 2018;67(5152):1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Madah-Amiri D, Clausen T, Lobmaier P. Rapid widespread distribution of intranasal naloxone for overdose prevention. Drug Alcohol Depend 2017;173: 17–23. [DOI] [PubMed] [Google Scholar]
- [3].Dworkis DA, Weiner SG, Liao VT, Rabickow D, Goldberg SA. Geospatial clustering of opioid-related emergency medical services runs for public deployment of naloxone. West J Emerg Med 2018;19(4):641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].DEA Philadelphia Division and University of Pittsburgh. Analysis of Overdose Deaths in Pennsylvania, 2016. 2017. https://www.overdosefreepa.pitt.edu/wp-content/uploads/2017/07/DEA-Analysis-of-Overdose-Deaths-in-Pennsylvania-2016.pd_-1.pdf. [Accessed 14 April 2019].
- [5].Warner-Smith M, Darke S, Day C. Morbidity associated with non-fatal heroin overdose. Addiction 2002;97(8):963–7. [DOI] [PubMed] [Google Scholar]
- [6].Stoove MA, Dietze PM, Jolley D. Overdose deaths following previous non-fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev 2009;28(4):347–52. [DOI] [PubMed] [Google Scholar]
- [7].Knowlton A, Weir BW, Hazzard F, Olsen Y, McWilliams J, Fields J, et al. EMS runs for suspected opioid overdose: implications for surveillance and prevention. Prehosp Emerg Care 2013;17(3):317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lasher L, Rhodes J, Viner-Brown S. Identification and description of non-fatal opioid overdoses using Rhode Island EMS Data, 2016-2018. R I Med J (2013) 2019;102(2):41–5. [PubMed] [Google Scholar]
- [9].Dodson ZM, Enki Yoo EH, Martin-Gill C, Roth R. Spatial methods to enhance public health surveillance and resource deployment in the opioid epidemic. Am J Public Health 2018;108(9):1191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ray BR, Lowder EM, Kivisto AJ, Phalen P, Gil H. EMS naloxone administration as non-fatal opioid overdose surveillance: 6-year outcomes in Marion County, Indiana. Addiction 2018;113(12):2271–9. [DOI] [PubMed] [Google Scholar]
- [11].Rowe C, Santos GM, Vittinghoff E, Wheeler E, Davidson P, Coffin PO. Neighborhood-level and spatial characteristics associated with lay naloxone reversal events and opioid overdose deaths. J Urban Health 2016;93(1): 117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rowe C, Wheeler E, Jones TS, Yeh C, Coffin PO. Community-based response to fentanyl overdose outbreak, San Francisco, 2015. J Urban Health 2019;96: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cash RE, Kinsman J, Crowe RP, Rivard MK, Faul M, Panchal AR. Naloxone administration frequency during emergency medical service events - United States, 2012-2016. MMWR Morb Mortal Wkly Rep 2018;67(31):850–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Merchant RC, Schwartzapfel BL, Wolf FA, Li W, Carlson L, Rich JD. Demographic, geographic, and temporal patterns of ambulance runs for suspected opiate overdose in Rhode Island, 1997-20021. Subst Use Misuse 2006;41(9):1209–26. [DOI] [PubMed] [Google Scholar]
- [15].Faul M, Dailey MW, Sugerman DE, Sasser SM, Levy B, Paulozzi LJ. Disparity in naloxone administration by emergency medical service providers and the burden of drug overdose in US rural communities. Am J Public Health 2015;105(Suppl 3):e26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dietze P, Jolley D, Cvetkovski S. Patterns and characteristics of ambulance attendance at heroin overdose at a local-area level in Melbourne, Australia: implications for service provision. J Urban Health 2003;80(2):248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grover JM, Alabdrabalnabi T, Patel MD, Bachman MW, Platts-Mills TF, Cabanas JG, et al. Measuring a crisis: questioning the use of naloxone administrations as a marker for opioid overdoses in a large U.S. EMS system. Prehosp Emerg Care 2018;22(3):281–9. [DOI] [PubMed] [Google Scholar]
- [18].Lindstrom HA, Clemency BM, Snyder R, Consiglio JD, May PR, Moscati RM. Prehospital naloxone administration as a public health surveillance tool: a retrospective validation study. Prehosp Disaster Med 2015;30(4):385–9. [DOI] [PubMed] [Google Scholar]
- [19].Gulec N, Lahey J, Suozzi JC, Sholl M, MacLean CD, Wolfson DL. Basic and advanced ems providers are equally effective in naloxone administration for opioid overdose in Northern New England. Prehosp Emerg Care 2018;22(2):163–9. [DOI] [PubMed] [Google Scholar]
- [20].Clemency BM, Eggleston W, Shaw EW, Cheung M, Pokoj NS, Manka MA, et al. Hospital observation upon reversal (hour) with naloxone: a prospective clinical prediction rule validation study. Acad Emerg Med 2019;26(1):7–15. [DOI] [PubMed] [Google Scholar]
- [21].Centers for Disease Control and Prevention. Drug overdose deaths. 2018. https://www.cdc.gov/drugoverdose/data/statedeaths.html. [Accessed 19 December 2019].
- [22].Pennsylvania Overdose Prevention Technical Assistance Center (OverdoseFreePA), Death Data Overview. https://www.overdosefreepa.pitt.edu/know-the-facts/death-data-overview/. [Accessed 12 April 2019].
- [23].Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42(8):1–28. [Google Scholar]
- [24].Randolph JJ, Falbe K. A Step-by-Step guide to propensity score matching in R. Prac Assess Res Eval 2014;19(18):1–6. [Google Scholar]
- [25].French J smacpod: Statistical Methods for the Analysis of Case-Control Point Data 2018. Rpackage version 2.0.4. https://CRAN.Rproject.org/package=smacpod. [Google Scholar]
- [26].Baddeley A, Rubak E, Turner R. Spatial Point Patterns: Methodology and Applications with R. 2015. http://www.crcpress.com/Spatial-Point-Patterns-Methodology-and-Applications-with-R/Baddeley-Rubak-Turner/9781482210200/. [Accessed 5 April 2019].
- [27].Ramis R, Gómez-Barroso D, Tamayo I, García-Pérez J, Morales A, Romaguera EP, et al. Spatial analysis of childhood cancer: a case/control study. PLoS One 2015;10(5):e0127273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rowlingson BS, Diggle PJ. Splancs: spatial point pattern analysis code in S-Plus. Comput Geosci 1993;19:627–55. [Google Scholar]
- [29].Kulldorff M A spatial scan statistic. Commun Stat Theory Methods 1997;26(6):1481–96. [Google Scholar]
- [30].Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med 1995;14:799–810. [DOI] [PubMed] [Google Scholar]
- [31].Kulldorff M. User Guide v 9.6–Software for the spatial, temporal, and space-time scan Statistics. https://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download. [Accessed 7 April 2019].
- [32].Talbot T, Kumar S, Kulldorff M. The Bernoulli Scan Statistic for Birth Defect Data. 2015.
- [33].Han J, Zhu L, Kulldorff M, Hostovich S, Stinchcomb DG, Tatalovich Z, et al. Using Gini coefficient to determining optimal cluster reporting sizes for spatial scan statistics. Int J Health Geogr 2016;15(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim J, Jung I. Evaluation of the gini coefficient in spatial scan statistics for detecting irregularly shaped clusters. PLoS One 2017;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Goedel WC, Green TC, Viner-Brown S, Rich JD, Marshall BD. Increased overdose mortality during the first week of the month: Revisiting the “check effect” through a spatial lens. Drug Alcohol Depend 2019;197:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heavey SC, Delmerico AM, Burstein G, Moore C, Wieczorek WF, Collins RL, et al. Descriptive epidemiology for community-wide naloxone administration by police officers and firefighters responding to opioid overdose. J Community Health 2018;43(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wilt GE, Lewis BE, Adams EE. A spatial exploration of changes in drug overdose mortality in the United States, 2000-2016. Prev Chronic Dis 2019;16:E33. [DOI] [PMC free article] [PubMed] [Google Scholar]