Abstract
Background
High-mobility group box1 (HMGB1) is a cytokine that has been demonstrated to have an important role in inducing migration and homing of endothelial progenitor cells (EPCs) in the process of neovascularization during wound healing, but its specific mechanism remains elusive. The aim of this study was to investigate the effects of the HMGB-RAGE axis in EPC migration, as well as the underlying molecular mechanism responsible for these effects.
Material/Methods
EPCs were isolated from the mice and identified using flow cytometry and fluorescence staining. The effect of HMGB1 on the activity of EPCs was detected using the Cell Counting Kit-8 (CCK-8). Then, the migration of EPCs was detected by scratch wound-healing and cell migration assay. NO levels were analyzed by ELISA. The expression of p-PI3K, p-Akt, and p-eNOS was determined by Western blot analysis. RAGE expression was measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot analysis. F-actin was assessed by fluorescent staining.
Results
The results showed that HMGB1 induced a concentration-dependent migration of EPCs, and the migration was RAGE-dependent. The migration could be almost completely blocked by PI3K inhibitors and eNOS inhibitor. HMGB1-RAGE upregulated the expression of p-Akt, p-eNOS, and p-ERK. We also demonstrated that the MEK/ERK signaling pathway is not involved in the EPC migration induced by HMGB1-RAGE.
Conclusions
These data demonstrate that HMGB1 activates RAGE and induces PI3K/Akt/eNOS signaling transduction pathway activation to promote EPC migration. Therefore, the HMGB1-RAGE axis plays an important role in the EPC migration process and may become a potential target in wound healing.
MeSH Keywords: Cell Migration Assays, Endothelial Cells, HMGB1 Protein, Phosphatidylinositol 3-Kinases, Rage, Signal Transduction
Background
High-mobility group box1 (HMGB1), also known as amphoterin, is a member of the highly preserved nonhistone DAN-banding protein family, was isolated from calf thymus in 1973 by Goodwin et al. [1]. This is a special intracellular protein. When it exists in an extracellular environment, it acts as a necrosis marker selected by the innate immune system to distinguish damage of tissue and responds to repair [2]. It has been identified as a transcription factor [3] and growth factor [4] and as a late inflammatory mediator [5]. Acting as a cytokine, HMGB1 must be released into the extracellular environment, either actively secreted by immune cells such as coenocytes, macrophages, and dendritic cells, or passively delivered by dead, dying, and damaged cells [6]. HMGB1 can shuttle freely between the nucleus and cytoplasm in all type cells, while normal HMGB1 aggregates in the nucleus and binds to chromatin [7]. HMGB1 is more active in myeloid cells than in lymphoid cells [8]. Once released extracellularly, HMGB1 can bind to cell surface receptors, including advanced glycation end product receptors (RAGE) and Toll-like receptors 2 and 4 (TLR2 and TLR4) [9]. RAGE, which is cell surface signaling molecule of the immunoglobulin superfamily, is a natural receptor of HMGB1 [10]. The ligation of extracellular HMGB1 with RAGE leads to sensitization of various intracellular signaling pathways, including the activation of nuclear factor-kappa B (NF-κB), MAPKs [11], PI3K/Akt [10], and Src family kinases [12]. Activated RAGE regulates multiple cellular responses, and HMGB1-RAGE interaction is deeply involved in inflammation, immunity, cellular adhesion, proliferation, and migration in some types of cells. The PI3K/Akt signaling pathway is involved in many biological processes, including cell metabolism, cell cycle regulation, cell growth, and apoptosis. Recent studies have shown that the PI3K/Akt/eNOS signaling pathway play an essential role in chemokine-induced EPC migration [13].
Endothelial progenitor cells (EPCs) are important in endothelial repair and can migrate from bone marrow to peripheral circulation, as in ischemia [14]. In 1997, Asahara et al. [15] reported for the first time that EPC was obtained from peripheral blood and identified circulating EPC that promoted neovascularization. Since then, more and more evidence has indicated that bone marrow-derived EPC has the function of promoting neovascularization during wound healing [16]. It is estimated that endothelial cells account for 25% of neovascularization in animal models [17]. Angiogenesis begins with the mobilization of EPCs, which proliferate and form new blood vessels [18]. EPCs are the key cellular effectors of postnatal neovascularization and play a significant role in wound healing, which, when placed in the exfoliated endothelium, migrated to the location of neovascularization to restore ischemic organs [19]. The number of EPCs in wounds was reported to be decreased in diabetic mice, suggesting an abnormality in mobilization and homing mechanisms of EPC [20].
Cell proliferation and migration induced by cytokines are the basic factors of tissue repair. Some researchers have demonstrated that HMGB1 can act as a cytokine to regulate EC migration and serve as a signal of tissue damage [21]. Meanwhile, HMGB1 plays an important role in the regulation of EPCs, such as cell migration, generation, and survival [22]. Although HMGB1-RAGE is conducive to EPC migration, proliferation, and angiogenesis, the signaling transduction pathways that regulate these effects are unknown.
Therefore, the purpose of this study was to determine whether the HMGB1 induces PI3K/Akt/eNOS signaling pathways activation via interaction with RAGE, and assess its involvement in the proliferation and migration of EPCs.
Material and Methods
Isolation of EPCs from bone marrow and their characterization
EPCs were isolated as described previously [23]. Briefly, mice (Balb/c, male, 22–25 g) were sacrificed. The bone marrow of the femurs and tibias were immediately harvested with PBS. The mononuclear cells (MNCs) were isolated by density centrifugation and resuspended in BEM2 medium (Lonza, Walkersville, MD, USA) plus EGM®-2 Bullet Kit (Lonza, USA), which is composed of 2% fetal bovine serum (FBS), and growth factors containing vascular endothelial growth factor (VEGF), basic fibroblast growth factor, epidermal growth factor, insulin-like factor-1, GA-1000, hydrocortisone, heparin, and ascorbic acid. These cells were transferred to 6-well plates coated with fibronectin (Sigma, St Louis, MO, USA) and cultured in 5% carbon dioxide (CO2) at 37°C. After incubation for 4 days, we removed non-adherent cells and the medium was replaced every day. The culture was maintained for 7 days. To confirm the EPC phenotype, these cells were incubated on cover slips coated by fibronectin and incubated with 10 μg/ml acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine (Dil-ac-LDL, Molecular Probes) for 4 h at 37°C. Then, they were fixed with 4% paraformaldehyde solution for 15 min. Cells were then incubated with 10 μg/ml fluorescein isothiocyanate-conjugated Ulex europaeus agglutinin (FITC-UEA-1, Sigma, USA) for 30 min at 37°C, adding DAPI (Sangon Biotech, Shanghai, China) at room temperature in the dark for 5 min. Double-staining positive cells were observed by the fluorescence microscope and defined as EPCs. To further identify EPCs, the expression of endothelial marker proteins, including CD133, CD34 and VEGF-receptor 2 (VEGFR2) were conjugated anti-mouse CD34, CD133 (Thermo Fisher Scientific, USA) and anti-mouse VEGFR2 antibodies (Abcam, USA). The isotype anti-mouse IgG (Cell Signaling Technology, Beverly, MA, USA) was used as a negative control. After 1 h, all samples were tested into a cytoFLEX flow cytometer (BECKMAN, USA).
Ethics statement
All mice were housed in the Laboratorial Animal Center of the Institute of Burn Research, in accordance with the International Guiding Principles for Biomedical Research involving Animals (1985) and the study was approved by the Third Military Medicine University (Army Medical University) Administrative Panel on Laboratory Animal Care.
CCK-8 assay
Cell viability was assessed using CCK-8 (Dojindo, Japan) strictly following the protocols. Cells were inoculated into 96-well plates with 5×103 cells/wells, replaced with serum-free medium 24 h later. Thereafter, cells were exposed to different concentrations (0–100 ng/ml) of HMGB1 for 24 and 48 h (Sigma, USA). We added 10 μl CCK-8 solution to each well and gently vibrated it for 30 s, then the plate was incubated for 30 min at 37°C. A microplate reader was used to determine the optical density (OD) values at 450 nm. Cell viability was calculated according to the manufacturer’s directions.
Scratched wound healing assay
EPCs were cultured in 12-well plates. A wound was scratched with a sterile 200-μl pipette tip to leave a separation between the 2 parts of the monolayer of cells. The plate was washed repeatedly to remove the resulting debris. The cells were cultured in serum-free medium and stimulated with HMGB1 (0–100 ng/ml) for 12 h. To assess the amount of wound closure, cell-covered septal area was calculated by Image J software. The experiments were repeated 3 times.
Cell migration assay
Cell migration was assessed in 24-well plates using Costar Transwell permeable support (Corning, USA) [24] and the membrane was coated on both sides with fibronectin (2.5 μg/ml) overnight at 4°C. We seeded 1×105 cells/ml into the upper chambers, while the lower chamber contained different concentrations (0–100 ng/ml) of HMGB1 in serum-free BEM2 medium, incubated at 37°C in 5% CO2 for 12 h. Cells remaining on the upper surface of the membrane were taken out with a cotton swab and the cells that migrated to the lower surface of the membrane were fixed with 4% paraformaldehyde (Sangon Biotech) for 20 min, then stained with 0.1% crystal violet (Sangon Biotech). Migrating cells were observed under a phase-contrast microscope and counted from 3 random regions using Image J software. The experiment was repeated 3 times.
Analysis of NO levels
The EPCs cultured for 7 days were stimulated with HMGB1 or different signaling pathway inhibitors, as described previously. Culture supernatant was extracted and total levels of nitric oxide (NO) were quantified using Total Nitric Oxide and Nitrate/Nitrite Parameter assay kits (R&D Systems, Minneapolis, MN) following the manufacturer’s directions. OD values were measured at 450 nm, and NO concentration were calculated from the standard curve. The experiments were repeated 3 times.
Quantitative real-time polymerase chain reaction (QRT -PCR)
Total RNA was extracted by Trizol (Sangon Biotech), and reverse transcribed into cDNA using reverse transcription of DNA (Thermo Fisher Scientific, USA). To quantify the transcriptional degrees of RAGE and eNOS, qRT-PCT was performed on the Prism 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA), as described previously [25]. The primers sequences were as following:
RAGE forward 5′-AAACATCACAGCCCGGATTG-3′ and
reverse 5′-TCCGGCCTGTGTTCAGTTTC-3′;
GAPDH forward 5′-CATTCAAGACCGGACAGAGG-3′ and
reverse 5′-ACATACTCAGCACCAGCATCACC-3′.
Gene expression was computed by the comparative cycle threshold method.
Protein extraction and Western blotting
The total cell proteins were extracted using lysis buffer (Sangon Biotech) with a Protein kit (Sangon Biotech). The protein concentration was estimated by a Pierce BCA-200 Protein Assay Kit (Thermo Fisher Scientific, USA). We analyzed 50-μg protein samples extracted from different groups using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred them onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% milk and incubated overnight at 4°C with primary antibodies for PI3K (1: 1000; CST), p-PI3K (1: 1000; CST), Akt (1: 1000; CST), p-Akt (p-s473, 1: 2000; CST), ERK1/2 (1: 1500, CST), p-ERK1/2 (1: 1500, CST), eNOS (1: 1000, CST), p-eNOS (1: 1000, CST), and GAPDH (1: 5000, Protech, WuHan, China). On the second day, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1: 5000, Protech) for 1 h at 37°C. Then, the membranes were rinsed with TBST. Images were taken using the Gel Imaging System. All experiments were repeated 3 times.
Fluorescent staining of cytoskeleton
EPCs were grown to circle slides fibronectin-coated before being treated, fixed with 3.7% cold paraformaldehyde (Sangon Biotech) for 30 min, then permeabilized using 0.1% Triton X-100 for 5 min. F-actin of EPCs was probed with Texas Red-phalloidin (BD Bioscience, Lexington, KY) at 1: 200 dilution for 45 min. Stained cells were visualized by an inverted microscope (Leica, Japan) and the images were taken with a laser confocal microscope (Zeiss, Germany). The fluorescent intensity of F-actin was analyzed by Image J software.
Statistical analysis
All data were obtained through at least 3 individual separate experiments and the data are expressed as (mean ±SEM). SPSS version 16.0 was used to assess the differences of statistical significance. The unpaired t test was used to compare 2 groups and one-way ANOVA was used to compare differences among multiple groups. P<0.05 was defined as statistically significant.
Results
Characterization of bone marrow-derived EPCs
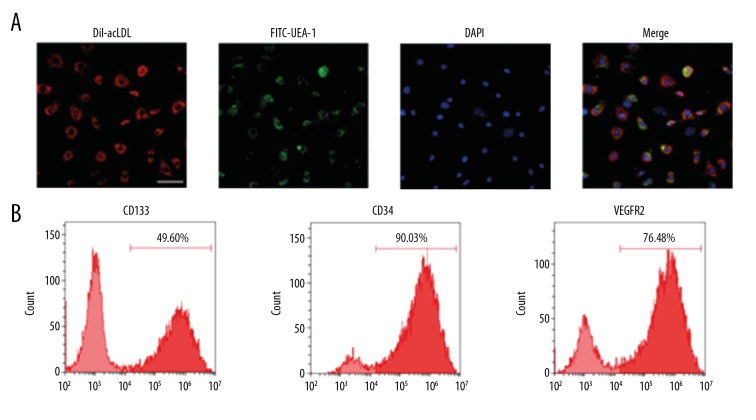
EPCs were characterized as adherent cells with double-positive staining for Dil-ac-LDL and FITC-UEA-1, Dil-ac-LDL uptake (red), and lectin binding (green) observed using a laser confocal microscope (ZEISS, LSMG800, Germany). The results demonstrated that the number of double-positive adherent cells was 85% higher (Figure 1A). Flow cytometry was used to detect EPCs among the attached cells that were cultured at 7 days and to assess expression rates of cell surface markers CD34 (90.3±3.8%), CD133 (49.6±9.5%), and VEGFR2 (76.48±4.3%). The results showed that EPCs exhibited high expression of VEGFR2 (Figure 1B).
Figure 1.
Characterization of EPCs. (A) To confirm the EPC phenotype, the adherent cells were incubated with Dil-acLDL (10 μg/ml) for 4 h and FITC-UEA-1 (10 μg/ml) for 1 h at 37°C. Then, the Dil-acLDL uptake (red) and lectin binding (green) of cells were assessed using a confocal microscope (scale bar=50 μm). Double-positive cells were recognized as the EPCs. The results demonstrated that the number of double-positive adherent cells increased by 85%. (B) To further identify EPCs, the expression levels of CD34, CD133, and VEGFR2 were detected by flow cytometry, showing 90.03% CD34, 49.6% CD133, and 76.48% VEGFR2.
HMGB1 upregulates expression of RAGE protein in EPCs and promotes proliferation of EPCs
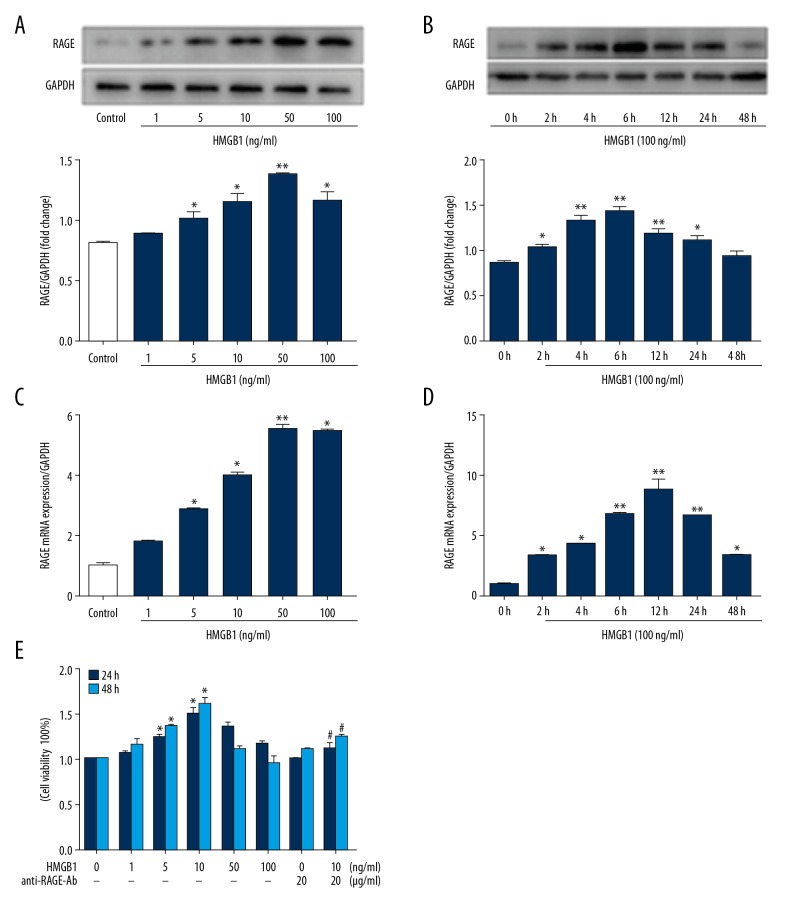
To determine the RAGE response to HMGB1 in EPCs, the cells were cultured in 6-well plates and treated with various concentrations of HMGB1 (0–100 ng/ml) for 24 h or treated with HMGB1 (100 ng/ml) for various times. The protein and mRNA expression levels of RAGE were detected by Western blot and qRT-PCR. The results of Western blot indicated the RAGE was expressed in a dose- and time-dependent manner when EPCs were stimulated by HMGB1 (Figure 2A, 2B), as well as the expression level of RAGE mRNA (Figure 2C, 2D).
Figure 2.
Effects of HMGB1 on RAGE expression and cell viability in EPCs. EPCs were treated with HMGB1 (0–100 ng/ml) for different times (0–48 h). (A, B) Western blot was utilized to measure expression of RAGE. (C, D) mRNA expression level of RAGE was measured by qRT-PCR in EPCs. (E) The viability of EPCs was analyzed by CCK-8. The data are expressed as the means ±SEM. * P<0.05, ** P<0.01, versus the control group. # P<0.05, versus the HMGB1 (10 ng/ml) group.
Previous studies have shown HMGB1 promotes cell proliferation via RAGE in gingival epithelial cells [26] and 3T3 mouse fibroblasts [27]. To investigate the role of HMGB1-RAGE in EPC proliferation, EPCs were treated with 0–100 ng/ml HMGB1 for 24 and 48 h (Figure 2E). When exogenous HMGB1 was added, the proliferation rate of cultured EPC was enhanced, but a biphasic pattern was observed. Low concentrations (1–10 ng/ml) increased viability, but higher concentrations (50–100 ng/ml) appeared to have no effect. When anti-RAGE antibody (20 μg/ml) (Abcam, USA) was added, the proliferation rate of EPC was significantly reduced. These data indicate that RAGE is involved in the proliferation of EPCs induced by HMGB1.
HMGB1 induces EPC migration in a concentration-dependent manner and activates the PI3K/Akt/eNOS signaling pathway
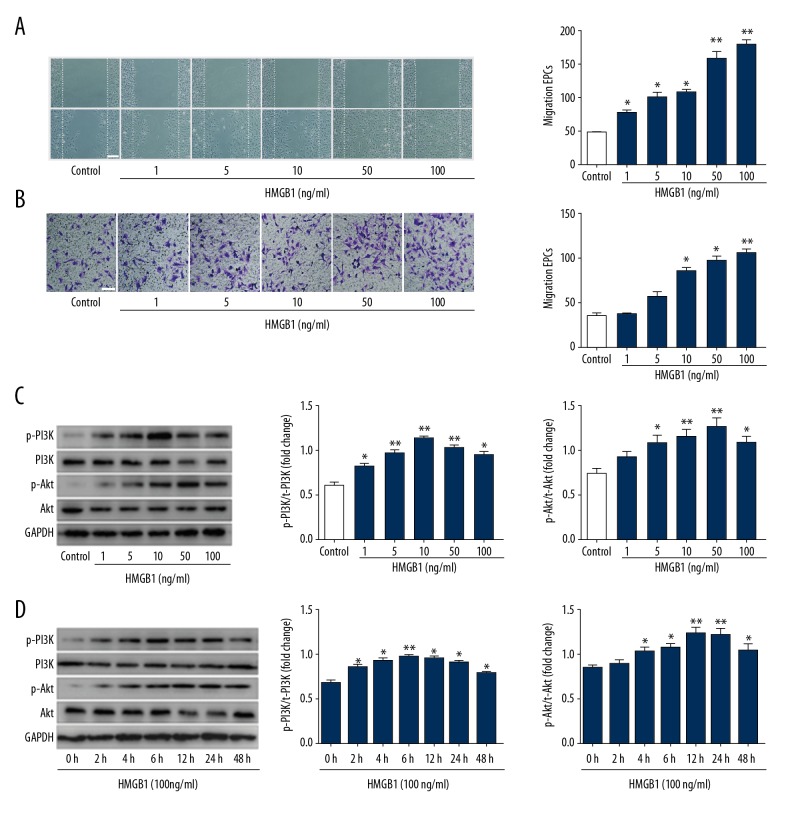
HMGB1 directly stimulates the migration of various cell types. We explored whether HMGB1 affects the angiogenesis-related functions of EPCs. The repair of the scratch depends not only on the migration of cells, but also on the proliferation. To investigate the effects of HMGB1 on EPCs motility and migration, EPCs were treated with 0–100 ng/ml HMGB1 for 12 h. The spontaneous migration of EPCs was determined by scratch wound-healing assay (Figure 3A). Chemotactic migration of EPCs was assessed by Transwell assay (Figure 3B). The data indicated that HMGB1 increased the chemotactic migration of EPCs in a concentration-dependent manner. According to these results and previous research, we chose 100 ng/ml HMGB1 as the optimal concentration for use in subsequent experiments.
Figure 3.
HMGB1 induced EPCs migration and activated PI3K/Akt/eNOS signal transduction pathways. (A) Wound-healing assay was performed on confluent monolayer EPCs. EPCs were treated with 0–100 ng/ml HMGB1 for 12 h. The wound was monitored and photographed (scale bar=200 μm) 12 h after treatment with HMGB1. Number of EPCs growing into the scratch area was analyzed using Image J software. HMGB1-induced EPC wound healing was concentration-dependent. (B) The migrated EPCs in cell migration assay were stained by crystal violet and photographed 12 h after treatment with HMGB1 (scale bar=100 μm). (C) Western blot was used to measure the interaction of Akt and PI3K protein. (D) EPCs were stimulated with 100 ng/ml HMGB1 for 0–48 h. Expression of p-PI3K and p-Akt was detected using Western blot. The results of Western blot represented as a bar graph. * P<0.05, ** P<0.01, versus control group; # P<0.05, ## P<0.01, versus HMGB1 (100 ng/ml) group.
The phosphorylation of ERK, Akt, and eNOS are critically involved in signaling of the proliferation and migration induced by angiogenic factors. Because HMGB1-induced EPC migration occurs via the PI3K/Akt/eNOS signaling pathway, our study investigated whether HMGB1-RAGE regulates the phosphorylation of PI3K, Akt, and eNOS in EPCs. Western blot analysis was used to confirm specific antibodies against their phosphorylated forms. HMGB1 treatment also increased the phosphorylation of PI3K and Akt in a time- and concentration-dependent manner, but the expression of total Akt and PI3K was not significantly different (Figure 3C, 3D).
PI3K/Akt/eNOS signaling pathway participates in HMGB1-mediated and RAGE-dependent migration of EPCs
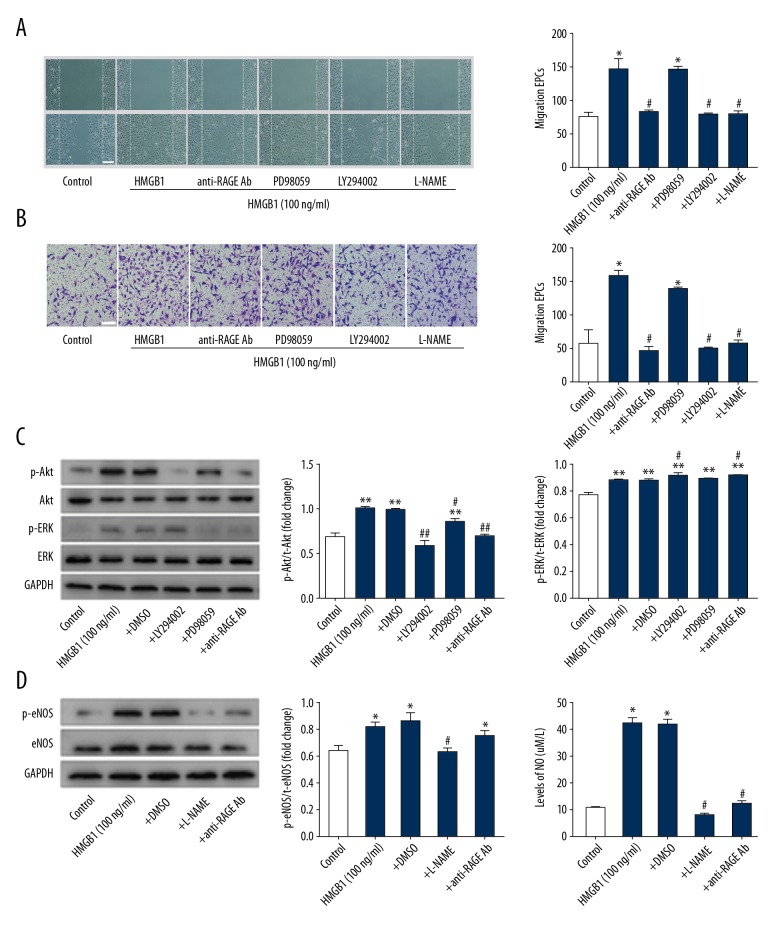
We assessed the role of PI3K/Akt and MEK/ERK signaling pathway in HMGB1-RAGE-mediated EPC migration. EPCs were cultured for 7 days with 20 μM LY294002 (PI3K inhibitor, MedChemExpress, USA), 20 μM PD98059 (MEK inhibitor, MedChemExpress, USA), 1 mM L-NAME (eNOS inhibitor, MedChemExpress, USA), and 20 μg/ml anti-RAGE antibody prior to exposure to HMGB1 (100 ng/ml) for 30 min. Cells migration was assessed at 12 h. Cell motility was detected by wound-healing assay (Figure 4A). Cell migration assay was used to assess cell migration (Figure 4B). The data showed anti-RAGE antibodies (20 μg/ml) significantly reduced the HMGB1-induced EPC migration, suggesting that the migratory effect of HMGB1 on EPCs is predominantly mediated by RAGE. Meanwhile, the HMGB1-induced migration of EPCs pretreated with 20 μM LY294002 were blocked significantly, but PD98059 did not affect HMGB1-induced migration, indicating that the PI3K/Akt signaling pathway is necessary for HMGB1-induced EPCs migration, but not the MEK/ERK signaling pathway. EPCs pretreated with 1 mM L-NAME also had less HMGB1-induced migration. A previous study demonstrated that Akt-induced endothelial cell migration depends on eNOS phosphorylation and NO production [28]. These data suggest that the PI3K/Akt/eNOS signaling pathway plays an essential role in HMGB1-induced EPCs migration.
Figure 4.
The PI3K/Akt/eNOS signaling pathway participates in HMGB1-induced cells migration and RAGE-dependent EPCs. (A) The specific inhibitor of PI3K (20 μM LY294002), eNOS (1 mM L-NAME), ERK (20 μM PD98059), and 20 μg/ml anti-RAGE antibody were used to treat EPCs. Wound-healing assay was performed on confluent monolayer EPCs. The wound was monitored and photographed (scale bar=200 μm) after 12 h. Number of EPCs growing into the scratch area was analyzed using Image J software. The data showed anti-RAGE antibody, LY294002, and L-NAME significantly abolished the HMGB1-induced cell migration, but PD98059 had not notable effect in this process. (B) The migrated EPCs in cell migration assay were stained by crystal violet and photographed 12 h after treatment with HMGB1 (scale bar=100μm), and the results are consistent with the scratch wound-healing assay. (C) Expression of p-Akt and p-ERK protein was examined by Western blot. The results showed the anti-RAGE antibody and LY294002 significantly inhibited phosphorylation of Akt induced by HMGB1, and PD98059 inhibited phosphorylation of ERK1/2. (D) EPCs were pretreated with HMGB1 (100 ng/ml), anti-RAGE antibody (20 μg/ml), and L-NAME (1 mM), and expression of eNOS phosphorylation was assessed by Western blot. Compared with other groups, the expression of eNOS phosphorylation in EPCs treated with HMGB1 increased remarkably. This effect was suppressed by L-NAME and anti-RAGE antibody. (E) The levels of NO measured by ELISA. The results of Western blot represented as a bar graph. * P<0.05, ** P<0.01, versus control group; # P<0.05, ## P<0.01, versus HMGB1 (100 ng/ml) group. All experiments were performed 3 times.
To further clarify the relationship between RAGE and the PI3K/Akt signaling pathway in mediating HMGB1-induced migration in EPCs, Western blot analysis was performed, showing that stimulated with HMGB1 significantly increased phosphorylation of Akt, whereas the HMGB1-induced phosphorylation of Akt was abolished by LY294002 and anti-RAGE Ab. PD98059 had no effect on the expression of p-Akt, but downregulated the expression of ERK1/2 (Figure 4C).
Angiogenesis factors include the activation of Akt-dependent eNOS phosphorylation by VEGF, which leads to an increase in NO production, thereby enhancing angiogenesis. NO is a key indicator for the function of EPCs and can promote cell proliferation and migration in endothelial cells. To further explore the molecular mechanisms by which HMGB1-RAGE induces EPC migration, EPCs were pre-stimulated with HMGB1 (100 ng/ml), anti-RAGE antibody (20 μg/ml), and L-NAME (1 mM), and we collected the protein and medium. The expression of p-eNOS and NO was detected by Western blot and ELISA, respectively. As showed in Figure 4D, compared with others, expression of p-eNOS was significantly increased in EPCs treated with HMGB1, and there was also increased NO production. This effect was suppressed by L-NAME and anti-RAGE antibodies (Figure 4E). These results showed that activation of PI3K and Akt participates in the angiogenesis of EPC mediated by HMGB1-RAGE.
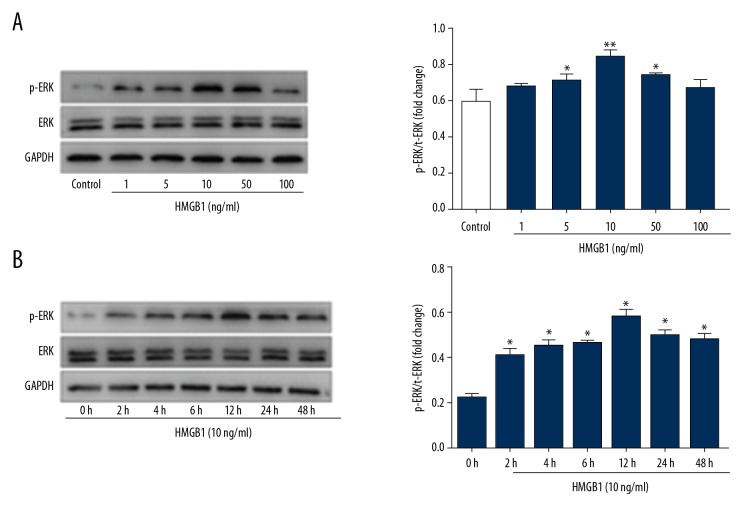
HMGB1 activates the MAPK/ERK signaling pathway
HMGB1 induced proliferation, migration, and wound closure of HaCat keratinocytes and gingival epitheal cells via RAGE and MAPK/ERK signal cascade [29,30]. To further study HMGB1-mediated cell signaling, Western blot analysis was used to investigate whether HMGB1 activates the MAPK/ERK signal pathway in EPCs. The results showed that HMGB1 activated ERK1 and ERK2, and the level of p-ERK1/2 expression peaked at 12 h after 100 ng/ml HMGB1 stimulation and phosphorylation of ERK1/2 in a dose- (Figure 5A) and time-dependent manner (Figure 5B), but expression of total ERK1/2 was not significantly changed. Moreover, our cell migration results showed that PD98059 does not affect HMGB1-induced EPC migration (Figure 4B), and the MAPK/ERK signaling pathway participates in ERK1/2 phosphorylation induced by HMGB1 in EPCs.
Figure 5.
HMGB1 increased expression of p-ERK1/2 in EPCs. (A) The EPCs treated with HMGB1 at different concentrations (0–100 ng/ml) for 12h. Expression of ERK1/2 and p-ERK1/2 protein was examined by Western blot. The results showed HMGB1 activated ERK1/2, expression level of p-ERK1/2 protein peaked after 10 ng/ml HMGB1 treatment and phosphorylation of ERK1/2 in a dose-dependent manner. (B) The EPCs were stimulated with HMGB1 (10 ng/ml) at several time points (0–48 h). Expression of ERK1/2 and p-ERK1/2 protein in the EPCs was analyzed by Western blot. The results of Western blot represented as a bar graph (* P<0.05, ** P<0.01).
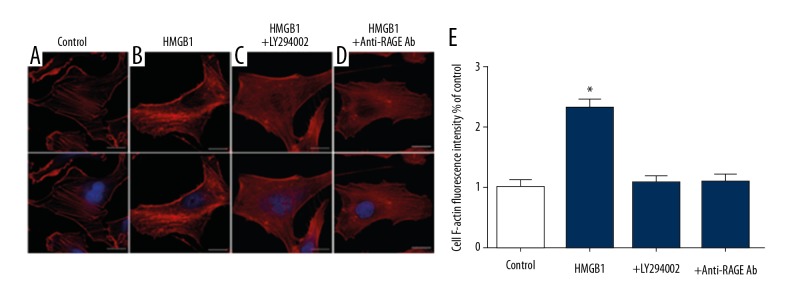
Activation of PI3K/Akt signaling pathway in EPCs enhances F-actin level
In the control group, F-actins of EPC had well-developed, thick, and long fibers (Figure 6A). F-actin was orderly and dense (Figure 6B). As expected, this effect of HMGB1 was blocked by LY294002 (20 μM) or anti-RAGE antibody (20 μg/ml) (Figure 6C, 6D). The fluorescence intensity of F-actin in the HMGB1 group was significantly greater than other groups (Figure 6E) These results indicate that PI3K/Akt mediates the HMGB1-RGAE-dependent cytoskeleton rearrangement.
Figure 6.
PI3K/Akt signaling pathway activated by HMGB1 regulated F-actin level in EPCs. EPCs were untreated (A) or were treated with 100 ng/ml HMGB1 alone (B). Plus 20 μM LY294002 (C), or plus 20 μg/ml anti-RAGE-antibody (D) to 12 h. The EPCs were treated by HMGB1, which led to increased fluorescent intensity of F-actin (E), but this process was blocked by LY294002 and anti-RAGE-antibody. (scale bar=20 μm) (* P<0.05).
Discussion
Wound healing is an intertwined dynamic process, which includes local inflammatory response, cell proliferation and migration, extracellular matrix deposition, angiogenesis, and re-epithelialization [31]. Angiogenesis is one of the key steps in wound healing. In the past several years, some studies have reported the potential therapeutic role of EPCs in endothelium injury repair of wound healing and vasculogenesis [32]. EPCs are embedded in the bone marrow microenvironment and can be moved into the injury site. Normally, the number of circulating EPCs is relatively low, but increases due to trauma or ischemia. EPCs leave the bone marrow and enter the circulation, proliferating, migrating, and homing to the damaged site, promoting the formation of neovascularization. The effect of bone marrow EPCs on neovascularization in wound repair is caused by a multistep process, which includes sensing ischemic signals from injured tissues, releasing EPCs from bone marrow niches into circulation, moving circulating EPC into target tissues, integrating EPC into blood vessels, and in situ differentiation/maturation of EPC into mature functional EC [33]. Chemokines play a pivotal role in regulating circulating endothelial cells from blood flow to ischemic tissue, such as stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) axis and VEGF [34]. HMGB1 is a nucleoprotein that can be activated by inflammatory cytokines when it is released outside the cell and serves as a chemical attractant for inflammatory cells, stem cells and EPCs, both in vitro and in vivo, during cell necrosis [35]. Hayakawa et al. [36] demonstrated that reactive astrocytes secrete HMGB1 and can bind to RAGE receptors and may attract circulating EPCs to damaged tissue in white matter injury. Recent studies have shown that HMGB1 induces migration of mesenchymal stem cells and promotes differentiation into endothelial cells via RAGE [37]. In this study, we confirm that RAGE may be the more potent receptor in HMGB1-induced EPCs migration via PI3K/Akt signaling transduction pathways.
A variety of studies have shown that extracellular HMGB1 participates in many biological processes, such as sepsis, noninfectious inflammation, angiogenesis, wound healing, and tumorigenesis [38]. In previous studies, we found that HMGB1 mutant has a specific antagonistic effect on the pro-inflammatory activity of HMGB1 [39]. It was also reported that HMGB1 has no effect on proliferation, apoptosis, and differentiation of EPCs [35]. However, in the present study, consistent with Kazuhide et al. [40], we observed that higher levels of HMGB1 had no effect on EPC proliferation, and cell viability decreased at higher concentrations, which may explain why progenitor cell or stem cell populations from different sources may show individual responses to stimulation with cytokines. HMGB1 can also attract stem cells from bone marrow and is a major participant in cell migration [22]. Cell migration occurs in many physiological and pathological processes and is the main step in multicellular biological development. Chavakis et al. [35] found that HMGB1 regulates EPC migration through RAGE, which is currently the most widely discussed HMGB1 cell surface receptor. HMGB1 induces these events via ligation of the RAGE receptor and transcription of RAGE mRNA [41]. Consistent with these reports, the present study indicates that HMGB1 induces the chemotactic migration of bone marrow-derived EPCs. These results demonstrate that RAGE is the major receptor of HMGB1 and mediates the chemotaxis of HMGB1 in EPCs.
At present, the precise mechanism by which EPCs are recruited to the injury site and participate in process of wound healing has not been fully elucidated. Activation of the PI3K signaling pathway was involved in the management of diverse cellular processes, such as proliferation, survival, cytoskeletal organization, and motility, as well as angiogenesis [42]. Moreover, we demonstrated that the expression level of p-PI3K, p-Akt, and F-actin is decreased by inhibition of the HMGB1-RAGE axis. MAPKs are composed of ERKs, p38s, and JNKs. Generally, ERKs participate in cell growth and survival, while p38 and JNK are involved in cell death or apoptosis [43]. PI3K/Akt and MAPK/ERK signaling pathways have been shown to regulate the cell migration mediated by cytokines and chemokines in various cell types. HMGB1 also stimulates ERK activation in ECs, and ERK is an important signal transduction pathway in EC angiogenic behavior. To further determine whether PI3K/Akt and MEK/ERK signaling pathways are involved in EPCs migration induced by HMGB1-RAGE axis, we used LY294002, PI3K-specific inhibitor, or PD98059, MEK-specific inhibitor and anti-RAGE antibody. The results showed that LY294002 and anti-RAGE antibody blocked HMGB1-induced EPCs migration, whereas PD98059 had no significant effect. In addition, we explored the function of PI3K/Akt and MEK/ERK signaling pathway in HMGB1-induced EPCs migration, as results indicated that the MEK/ERK signaling pathway does not significantly influence in HMGB1-stimulated EPC migration, although HMGB1 activates phosphorylation of ERK1/2. Consequently, it appears that the signal pathways involved in HMGB1-RAGE-mediated cell migration either depend on the type of cell or MAPK/ERK participating in angiogenic behavior through other methods, but these underlying mechanisms need to confirmed in further experiments.
The numbers of EPCs in circulation and wounds in animals and patients with diabetes suggests that the migration and homing of EPC is abnormal [44]. Defects of EPC migration may damage eNOS and NO cascading reactions in bone marrow. As an upstream molecule of NO, eNOS is selectively expressed in vascular endothelial cells and induces production of NO, so it is an important player in proliferation of endothelial cells [45]. Our data showed that HMGB1 significantly increased protein expression of p-eNOS. In contrast, anti-RAGE antibody suppressed p-eNOS protein expression induced by HMGB1.
The essential process of cell migration is the polarization of cells caused by external signaling, which leads to the formation of synapses or pseudopods. The cytoskeleton is a protein system involved in many important activities such as cell movement and migration. Extracellular HMGB1 may induce cell morphological changes and recombination of cytoskeleton through RAGE, thus causing cell migration. Therefore, we suggest that the PI3K/Akt signaling pathway may be involved in the migration of EPCs induced by HMGB1-RAGE, and then participates in the migration of EPC via changes in cytoskeleton structure.
Conclusions
Collectively, our data show that RAGE may be the more potent receptor in HMGB1-induced EPCs migration via the PI3K/Akt signaling pathway but not the MAPK/ERK pathway. The activation of the signaling cascade of RAGE by HMGB1 binding finally resulted in the activation of PI3K/Akt/eNOS, consequently, migration events occurred in EPCs. These results help elucidate the molecular mechanism of HMGB1-induced EPCs migration and suggest that the HMGB1-RAGE-dependent PI3K/Akt/eNOS pathway is a potential therapeutic target for promoting wound healing.
Footnotes
Source of support: This work was supported by the Natural Science Foundation of Chongqing, China (No. CSTC2015jcyjA10115)
Conflict of interests
None.
References
- 1.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 2.Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landsman D, Bustin M. Assessment of the transcriptional activation potential of the HMG chromosomal proteins. Mol Cell Biol. 1991;11:4483–89. doi: 10.1128/mcb.11.9.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura H, Izumoto Y, Kambe H, et al. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269:25143–49. [PubMed] [Google Scholar]
- 5.Huebener P, Pradere JP, Hernandez C, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125:539–50. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–95. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 7.Isackson PJ, Bidney DL, Reeck GR, et al. High mobility group chromosomal proteins isolated from muclei and cytosol of cultured hepatoma cells are similar. Biochemistry. 1980;19:4466–71. doi: 10.1021/bi00560a013. [DOI] [PubMed] [Google Scholar]
- 8.Cabart P, Kalousek I, Jandova D, et al. Differential expression of nuclear HMG1, HMG2 proteins and H1(zero) histone in various blood cells. Cell Biochem Funct. 1995;13:125–33. doi: 10.1002/cbf.290130209. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Yang H, Czura CJ, et al. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–73. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 10.Toure F, Zahm JM, Garnotel R, et al. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycosylated extracellular matrix. Biochem J. 2008;416:255–61. doi: 10.1042/BJ20080054. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Park JC, Lee MH, et al. High-mobility group box 1 mediates fibroblast activity via RAGE-MAPK and NF-kappaB Ssignaling in keloid scar formation. Int J Mol Sci. 2017;19(1) doi: 10.3390/ijms19010076. pii: E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy MA, Li SL, Sahar S, et al. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–93. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 13.Zuccolo E, Di BC, Lodola F, et al. Stromal cell-derived factor-1alpha promotes endothelial colony-forming cell migration through the Ca(2+)-dependent activation of the extracellular signal-regulated kinase 1/2 and phosphoinositide 3-kinase/AKT pathways. Stem Cells Dev. 2018;27(1):23–34. doi: 10.1089/scd.2017.0114. [DOI] [PubMed] [Google Scholar]
- 14.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 15.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–67. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 16.Banyard DA, Adnani BO, Melkumyan S, et al. Endothelial progenitor cells and burn injury – exploring the relationship. Burns Trauma. 2016;4:4. doi: 10.1186/s41038-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–86. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 18.Potente M, Gerthard H, Carmelit P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Balaji S, King A, Crombleholme TM, et al. The role of endothelial progenitor cells in postnatal vasculogenesis: Implications for therapeutic neovascularization and wound healing. Adv Wound Care (New Rochelle) 2013;2:283–95. doi: 10.1089/wound.2012.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng J, Wang L, Qu M, et al. Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1α. Stem Cell Res Ther. 2017;8(1):163. doi: 10.1186/s13287-017-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura Y, Suzuki S, Shimizu T, et al. High mobility group box 1 promotes angiogenesis from bone marrow-derived endothelial progenitor cells after myocardial infarction. J Atheroscler Thromb. 2015;22:570–81. doi: 10.5551/jat.27235. [DOI] [PubMed] [Google Scholar]
- 22.Khoo CP, Roubelakis MG, Schrader JB, et al. miR-193a-3p interaction with HMGB1 downregulates human endothelial cell proliferation and migration. Sci Rep. 2017;7:44137. doi: 10.1038/srep44137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng J, Wang L, Qu M, et al. Alterations of bone marrow-derived endothelial progenitor cells following acute pulmonary embolism in mice. Exp Biol Med (Maywood) 2010;235:989–98. doi: 10.1258/ebm.2010.010057. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini L, Xue J, Larson D, et al. HMGB1 targeting by ethyl pyruvate suppresses malignant phenotype of human mesothelioma. Oncotarget. 2017;8:22649–61. doi: 10.18632/oncotarget.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen RC, Yi PP, Zhou RR, et al. The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines. Mol Cell Biochem. 2014;390:271–80. doi: 10.1007/s11010-014-1978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Chang Y, Ye N, et al. Advanced glycation end products inhibit the proliferation of human umbilical vein endothelial cells by inhibiting cathepsin D. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020436. pii: E436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranzato E, Patrone M, Pedrazzi M, et al. Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys. 2010;57:9–17. doi: 10.1007/s12013-010-9077-0. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Wu R, Yang B, et al. Human urine-derived stem cell differentiation to endothelial cells with barrier function and nitric oxide production. Stem Cells Transl Med. 2018;7:686–98. doi: 10.1002/sctm.18-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mou K, Liu W, Han D, et al. HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J Dermatol Sci. 2017;85:162–69. doi: 10.1016/j.jdermsci.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Bhawal UK, Sasahira T, et al. Involvement of HMGB1 and RAGE in IL-1 induced gingival inflammation. Arch Oral Biol. 2012;57:73–80. doi: 10.1016/j.archoralbio.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications. 2016;30:986–92. doi: 10.1016/j.jdiacomp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869–82. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng M, Huang K, Zhou J, et al. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol. 2015;81:49–53. doi: 10.1016/j.yjmcc.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavakis E, Hain A, Vinci M, et al. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–12. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa K, Miyamoto N, Seo JH, et al. High-mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J Neurochem. 2013;125:273–80. doi: 10.1111/jnc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng X, Chen M, Su W, et al. The differentiation of mesenchymal stem cells to vascular cells regulated by the HMGB1/RAGE axis: Its application in cell therapy for transplant arteriosclerosis. Stem Cell Res Ther. 2018;9:85. doi: 10.1186/s13287-018-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinotti S, Patrone M, Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015;4:101–9. doi: 10.2147/ITT.S58064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Z, Chen J, Zhang Y, et al. Construction and characterization of the HMGB1 mutant as a competitive antagonist to HMGB1 induced cytokines release. Biochem Biophys Res Commun. 2008;372:703–7. doi: 10.1016/j.bbrc.2008.05.115. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa K, Pham LD, Katusic ZS, et al. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci USA. 2012;109:7505–10. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfson RK, Chiang ET, Garcia JG. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81:189–97. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong K, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Genetic and cellular mechanisms of oncogenesis. Current Opinion in Genetics & Development. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–12. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 44.Um J, Yu J, Park KS. Substance P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol Med Rep. 2017;15:3035–40. doi: 10.3892/mmr.2017.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katkam SK, Indumathi B, Tasneem FSD, et al. Impact of eNOS 27-bp VNTR (4b/a) gene polymorphism with the risk of Systemic Lupus Erythematosus in south Indian subjects. Gene. 2018;658:105–12. doi: 10.1016/j.gene.2018.03.021. [DOI] [PubMed] [Google Scholar]