Abstract
Background
The objective of this study was to assess the diagnostic value of platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), and neutrophil/lymphocyte ratio (NLR) as biomarkers in patients with rheumatoid arthritis (RA) and rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Material/Methods
Demographic and laboratory data were acquired for 198 RA and 103 RA-ILD patients and 290 healthy controls. The subjects were categorized into female and male groups and further subcategorized based on age into <60 years and ≥60 years subgroups. One-way analysis of variance (ANOVA), receiver operating characteristics (ROC), Pearson analysis, multiple linear regression analysis, and logistic regression analysis were performed to analyze the association of PLR, NLR, and LMR with RA and RA-ILD.
Results
Mean PLR and NLR were lowest in the control group, followed by the RA and RA-ILD groups (p<0.05). Mean LMR was lowest in the RA-ILD group, followed by the RA and control groups (p<0.05). The area under the ROC (AUROC) values of the PLR to distinguish between RA and controls, RA-ILD and controls, and RA-ILD and RA were 0.676, 0.776, and 0.650, respectively (p<0.001). Multiple linear regression analysis suggested a significantly positive association between the level of PLR and the level of DAS28 (p<0.001). The odds ratio of PLR was 1.101 for RA (p=0.023) and 1.217 for RA-ILD (p<0.001) when compared to the controls.
Conclusions
PLR may be applied as a new biomarker for predicting and diagnosing RA and RA-ILD and for distinguishing RA-ILD patients from RA patients and healthy subjects.
MeSH Keywords: Arthritis, Juvenile; Biological Markers; Inflammation
Background
Rheumatoid arthritis (RA) is a systemic autoimmune rheumatic disease that negatively affects the quality of life of patients. The characteristic features of RA include progressive and erosive polyarthritis that leads to joint destruction and associated significant pain and functional disability [1]. Progressive and erosive polyarthritis is a result of the actions of co-operative cytokines, reactive oxygen species, and proteolytic enzymes, together with other proinflammatory mediators [1]. The prevalence of RA in China is approximately 0.28% among the adult population [2]. Extra-articular manifestations of RA include vasculitis, interstitial lung disease, cardiovascular diseases, fragile fracture, and lymphoma [3]. Rheumatoid arthritis-interstitial lung disease (RA-ILD) is one of the most familiar clinical features in patients with RA [4], with a prevalence of approximately 39.8% among RA patients in China [5]. Bongartz et al. [6] found that RA-ILD patients had a 3 times higher risk of death than RA patients without interstitial lung disease, and the median survival after RA-ILD diagnosis was only 2.6 years. RA is associated with great personal and societal medical costs and risks for patients, especially in developing countries. The precise mechanisms involved in RA and RA-ILD are yet to be determined; however, studies suggest that inflammation plays an important role in the development and progression of RA [7].
As a simple, credible, and inexpensive laboratory biomarker of systemic inflammation, the lymphocyte/monocyte ratio (LMR), neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) have been introduced as prognostic or diagnosis markers in various cancers [8,9] and cardiovascular diseases [10,11]. Recently, several studies have suggested that PLR, LMR, and NLR may be applied as biomarkers for diagnosis [12,13] and monitoring of RA [14,15]. Peng et al. [16] reported that RA patients have significantly higher PLR and NLR levels than healthy controls and suggested that PLR may be an indicator of chronic subclinical inflammation in patients with RA. Chandrashekara et al. [17] found that C-reactive protein levels could be used as a significant baseline predictor of NLR, while NLR may serve as an effective measure of inflammation in RA. Moreover, Du et al. [18] and Fu et al. [19] reported a significantly positive correlation between NLR, PLR, and LMR and disease activity score in 28 joints (DAS28) in RA patients.
From these studies, it may be inferred that PLR, NLR, and LMR could be applied as biomarkers for diagnosis and monitoring of RA [20]. However, it is not clear whether these may be also applied as biomarkers for diagnosing and monitoring RA-ILD, and for distinguishing RA-ILD from RA. Therefore, we conducted a large-sample, cross-sectional investigation to assess the predictive and diagnostic value of PLR, LMR, and NLR as biomarkers in RA and RA-ILD patients.
Material and Methods
Patients
This study was approved by the Ethics Committee of Taian City Central Hospital, Shandong, China, and was performed according to the tenets of the Declaration of Helsinki. Moreover, informed consent was obtained from the subjects. RA patients were recruited consecutively from Taian City Central Hospital between January 2016 and October 2018. Additionally, individuals were recruited from the physical examination center of Taian City Central Hospital as healthy controls. All subjects involved in this study had undergone a standardized medical examination, including the assessment of liver function, renal function, blood pressure, heart rate, and body temperature. Data on age, sex, comorbid diabetes mellitus and hypertension, erythrocyte sedimentation rate (ESR), body mass index (BMI), and DAS28 was also collected.
Diagnostic criteria
RA was diagnosed when the patients satisfied the 1987 American College of Rheumatology criteria or the 2010 American College of Rheumatology or European League Against Rheumatism (EULAR) criteria for RA [21,22]. Newly diagnosed and referred RA subjects were included as well. The disease activity of patients was evaluated by the DAS28 system [22]. The diagnostic criterion for interstitial lung disease was based on high-resolution CT.
Inclusion and exclusion criteria
Overall, 378 subjects were enrolled as RA and RA-ILD subjects for this study, 77 of which were later excluded due to cancer (n=9), severe infections (n=28), cardiovascular disease (n=24), systemic blood disease (n=4), or other diseases (n=12). Finally, 198 RA and 103 RA-ILD subjects were included in the study. Three hundred and forty individuals were recruited as healthy controls, 50 of which were later excluded due to cancer (n=6), severe infections (n=10), cardiovascular disease (n=22), systemic blood disease (n=2), or other diseases (n=10). Ultimately, 290 healthy controls were included in the study.
Laboratory measurements
Blood samples were collected from each subject using ethylenediaminetetraacetic acid (EDTA) tubes (Chengwu, Shandong, China) to prevent blood coagulation. We used 2 mL of blood to perform a complete blood count, including the platelet, lymphocyte, neutrophil, and monocyte counts, for each subject using an automatic blood counting system (Sysmex, Tokyo, Japan).
Subgroup analysis
RA may cause a variety of extra-articular manifestations, including RA-associated ILD (RA-ILD), which typically manifests as diffuse parenchymal fibrosis [23]. Thus, RA subjects were further divided into an RA-ILD group and a group without ILD (‘RA group’ henceforth). As the literature indicated that there was a difference in RA prevalence depending on sex [24], the RA, RA-ILD, and control subjects were categorized into female and male subgroups, followed by subcategorization based on age (<60, and ≥60).
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (IBM-SPSS, Chicago, IL, USA) software. Results were presented as mean values ± standard deviations. Chi-squared test was used for categorical variables, and one-way analysis of variance (ANOVA) was used for comparing the participants’ characteristics. Receiver operating characteristics (ROC) analysis was performed to assess the best cutoff value for predicting RA and RA-ILD. Pearson analysis was performed to calculate the association between blood factors and DAS28. After Pearson analysis, multiple linear regression analysis was used to identify the statistically significant associations between laboratory parameters and DAS28. Furthermore, logistic regression analyses were performed to identify the relationship between PLR and RA or RA-ILD risk. Two-sided p-values of <0.05 were considered statistically significant.
Results
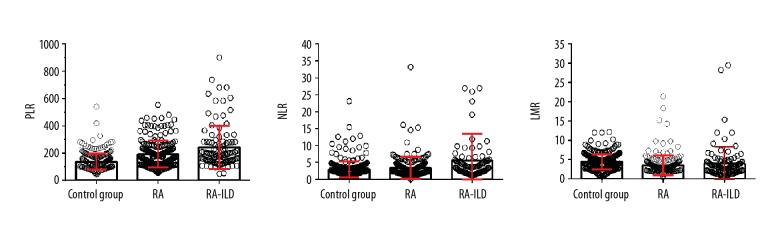
There were no significant differences between the RA, RA-ILD, and control groups (p>0.05) in terms of mean age, sex, BMI, diabetes, and hypertension. The baseline demographics of the subjects are shown in Table 1. Table 1 and Figure 1 also show the ESR, DAS28, the platelet, lymphocyte, neutrophil, and monocyte levels, and the PLRs, NLRs, and LMRs in the RA, RA-ILD, and control groups. The mean ESR, platelet, neutrophil, and monocyte levels, PLR, and NLR were lowest in the control group, followed by the RA and RA-ILD groups (p<0.05), while the mean lymphocyte levels and LMR were lowest in the RA-ILD group, followed by the RA and control groups (p<0.05).
Table 1.
Comparison of characteristics of subjects and other blood parameters among the 3 groups.
| Control group (n=290) | RA (n=198) | RA-ILD (n=103) | F value | p value | |
|---|---|---|---|---|---|
| Age (years) | 61.24±6.01 | 59.80±11.21 | 60.94±10.35 | 1.595 | 0.204 |
| Sex (Male/Female) | 62/228 | 39/159 | 24/79 | 0.546 | 0.761 |
| BMI (Kg/m2) | 22.50±3.66 | 23.22±4.31 | 22.64±3.15 | 1.495 | 0.225 |
| Hypertension (yes/no) | 86/204 | 68/130 | 27/76 | 2.360 | 0.307 |
| Diabetes (yes/no) | 25/265 | 30/168 | 10/93 | 5.339 | 0.069 |
| ESR (mm/h) | 12.29±5.78 | 42.06±26.09 | 49.31±23.36 | 43.410 | <0.001a,b,c |
| DAS28 | – | 4.63±0.81 | 5.08±1.05 | 3.701 | <0.001 |
| Platelet (109/l) | 205.65±60.25 | 270.70±92.56 | 276.91±107.62 | 50.211 | <0.001a,b |
| Lymphocyte (109/l) | 1.66±0.60 | 1.63±0.67 | 1.41±0.75 | 6.041 | 0.003b,c |
| Neutrophil (109/l) | 4.30±2.54 | 4.82±4.06 | 5.50±4.73 | 4.603 | 0.010a |
| Monocytes (109/l) | 0.43±0.27 | 0.56±0.26 | 0.54±0.32 | 14.618 | <0.001a,b |
| PLR | 136.00±58.69 | 190.69±98.75 | 241.83±158.74 | 50.732 | <0.001a,b,c |
| NLR | 2.94±2.37 | 3.40±3.17 | 5.55±7.88 | 15.609 | <0.001a,c |
| LMR | 4.51±1.95 | 3.66±3.85 | 3.96±4.35 | 4.355 | 0.013a |
Data are expressed as mean ± standard deviation (SD). One-way ANOVA, independent t test, and chi-square test were used. BMI – body mass index; PLR – platelet/lymphocyte ratio; NLR – neutrophil/lymphocyte ratio; LMR – lymphocyte/monocytes ratio; RA-ILD – rheumatoid arthritis-associated interstitial lung disease.
P<0.05 for the difference between control group and RA group (one-way ANOVA with the LSD post hoc test);
P<0.05 for the difference between control group and RA-ILD group (one-way ANOVA with the LSD post hoc test);
P<0.05 for the difference between RA group and RA-ILD group (one-way ANOVA with the LSD post hoc test).
Figure 1.
Comparison of levels of platelet/lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR), and lymphocyte/monocyte ratio (LMR) among the rheumatoid arthritis (RA), control, and rheumatoid arthritis-associated interstitial lung disease (RA-ILD) groups.
In both the sex and age subgroups of RA, RA-ILD, and control groups, the mean PLR was significantly lower than that in the control group, followed the by RA and RA-ILD groups (p<0.05) (Table 2). However, the NLRs and LMRs between the 3 groups differed from PLR results with respect to sex and age (Table 2).
Table 2.
Comparison of PLR, NLR, and LMR among the 3 groups, stratified according to age and sex.
| Control group (n=290) | RA (n=198) | RA-ILD (n=103) | F value | p value | |
|---|---|---|---|---|---|
| PLR | |||||
| Male | 130.36±47.12 | 168.91±99.29 | 254.24±187.27 | 12.322 | <0.001b,c |
| <60 | 114.97±42.19 | 127.91±44.24 | 194.13±47.88 | 7.741 | 0.002b,c |
| ≥60 | 137.68±48.05 | 181.21±108.17 | 274.28±212.31 | 8.526 | <0.001b,c |
| Female | 137.54±61.46 | 196.04±98.20 | 238.06±150.16 | 39.385 | <0.001a,b,c |
| <60 | 139.05±61.68 | 187.55±95.39 | 218.22±127.29 | 10.556 | <0.001a,b |
| ≥60 | 136.85±61.55 | 205.07±100.94 | 257.64±169.93 | 30.899 | <0.001a,b,c |
| NLR | |||||
| Male | 3.04±2.12 | 4.52±3.26 | 5.33±4.43 | 3.380 | 0.037b |
| <60 | 3.10±2.73 | 3.17±2.10 | 3.65±1.25 | 0.125 | 0.883 |
| ≥60 | 3.01±1.79 | 4.93±5.85 | 5.90±6.17 | 3.134 | 0.048b |
| Female | 2.91±2.43 | 3.12±2.34 | 5.61±8.51 | 13.398 | <0.001b,c |
| <60 | 3.13±3.21 | 2.71±1.91 | 3.75±2.31 | 2.319 | 0.033a |
| ≥60 | 2.81±1.99 | 3.56±2.67 | 7.53±11.66 | 14.901 | <0.001b,c |
| LMR | |||||
| Male | 3.73±1.67 | 2.66±1.22 | 2.77±1.36 | 5.780 | 0.004a,b |
| <60 | 4.07±1.63 | 3.21±1.69 | 2.59±0.72 | 2.511 | 0.047b |
| ≥60 | 3.56±1.68 | 2.50±1.03 | 2.82±2.71 | 3.412 | 0.037a |
| Female | 4.72±1.97 | 3.90±4.22 | 4.32±4.74 | 2.633 | 0.023a |
| <60 | 4.99±2.23 | 3.87±2.98 | 4.75±4.76 | 2.496 | 0.033a |
| ≥60 | 4.60±1.84 | 3.95±5.26 | 3.88±4.74 | 1.192 | 0.305 |
Data are expressed as mean ± standard deviation (SD). One-way ANOVA was used. PLR – platelet/lymphocyte ratio; NLR – neutrophil/lymphocyte ratio; LMR – lymphocyte/monocytes ratio; RA-ILD – rheumatoid arthritis-associated interstitial lung disease.
P<0.05 for the difference between control group and RA group (one-way ANOVA with the LSD post hoc test);
P<0.05 for the difference between control group and RA-ILD group (one-way ANOVA with the LSD post hoc test)
P<0.05 for the difference between RA group and RA-ILD group (one-way ANOVA with the LSD post hoc test).
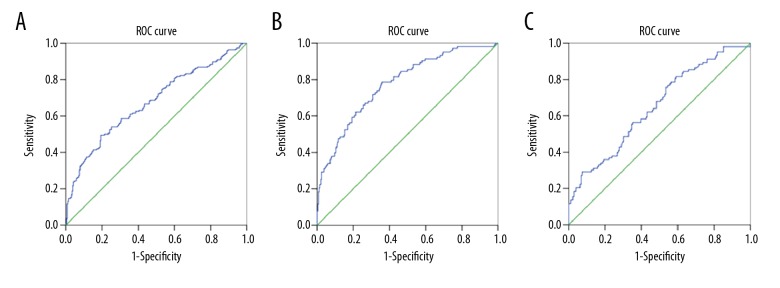
The ROC analyses of the PLRs are shown in Table 3 and Figure 2. The area under the ROC (AUROC) value of the PLR for distinguishing RA patients from control subjects was 0.676, and the best cutoff value was 171.92 (p<0.001). The AUROC value of the PLR for distinguishing RA-ILD patients from control subjects was 0.776, and the best cutoff value was 140.57 (p<0.001). Moreover, the AUROC value of the PLR for distinguishing RA-ILD from RA patients was 0.650, and the best cutoff value was 144.625 (p<0.001).
Table 3.
Receiver operating characteristic curve analysis to distinguish disease.
| AUROC | Best cutoff value | Sensitivity | Specificity | P value | |
|---|---|---|---|---|---|
| Control vs. RA | 0.676 | 171.92 | 61.28% | 81.68% | <0.001 |
| Control vs. RA-ILD | 0.776 | 140.57 | 78.64% | 65.14% | <0.001 |
| RA vs. RA-ILD | 0.650 | 144.625 | 75.73% | 53.98% | <0.001 |
RA-ILD – rheumatoid arthritis-associated interstitial lung disease, RA – rheumatoid arthritis; AUROC – area under the receiver operating characteristic curve.
Figure 2.
Receiver operating characteristic curve (ROC) analysis for distinguishing between diseases. (A) Control vs. rheumatoid arthritis. (B) Control vs. rheumatoid arthritis-associated interstitial lung disease. (C) Rheumatoid arthritis vs. rheumatoid arthritis-associated interstitial lung disease.
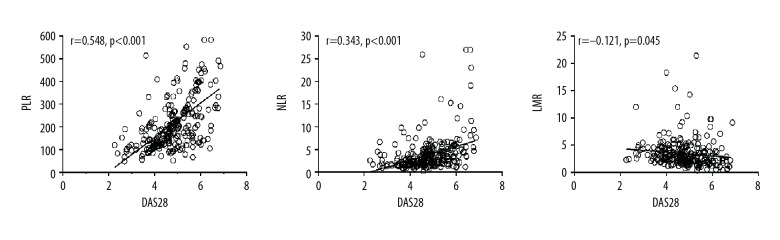
Pearson correlation analysis showed that platelet (r=0.303, p<0.001) and neutrophil (r=0.119, p=0.001) levels, PLR (r=0.548, p<0.001), and NLR (r=0.343, p<0.001) showed a statistically significant positive correlation with DAS28 (Table 4, Figure 3). Meanwhile, lymphocyte levels (r=−0.360, p<0.001) and LMR (r=−0.121, p=0.045) showed a statistically significant negative correlation with DAS28 (Table 4, Figure 3).
Table 4.
Association between DAS28 and laboratory parameters.
| Pearson analysis | Multiple linear regressions analysis | ||||
|---|---|---|---|---|---|
| r | P | B | P | (95% CI) | |
| Age | 0.070 | 0.244 | 0.026 | 0.711 | −0.015 to 0.011 |
| BMI | −0.150 | 0.055 | 0.093 | 0.153 | −0.053 to 0.008 |
| Platelet | 0.303 | <0.001 | 0.231 | 0.113 | 0.000 to 0.005 |
| Lymphocyte | −0.360 | <0.001 | 0.097 | 0.472 | −0.481 to 0.223 |
| Neutrophil | 0.199 | 0.001 | 0.155 | 0.398 | −0.094 to 0.037 |
| Monocytes | −0.006 | 0.926 | 0.062 | 0.487 | −0.800 to 0.383 |
| PLR | 0.548 | <0.001 | 0.374 | <0.001 | 0.003 to 0.005 |
| NLR | 0.343 | <0.001 | 0.203 | 0.357 | −0.034 to 0.094 |
| LMR | −0.121 | 0.045 | 0.148 | 0.059 | −0.064 to 0.001 |
BMI – body mass index; PLR – platelet/lymphocyte ratio; NLR – neutrophil/lymphocyte ratio; LMR – lymphocyte/monocytes ratio; DAS28 – 28-joint count disease activity score.
Figure 3.
Association of DAS28 with platelet/lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR), and lymphocyte/monocyte ratio (LMR).
After Pearson analysis, multiple linear regression analysis was performed to evaluate the association between blood parameters and DAS28 (Table 4). After adjustment for age, sex, BMI, NLR, LMR, and platelet, lymphocyte, neutrophil, and monocyte counts, there was a significant correlation between PLR and DAS28 (B=0.374, p<0.001, 95% CI=0.003–0.005).
Finally, logistic regression analyses were performed to identify the association of PLR, NLR, and LMR with the risk of RA and RA-ILD (Table 5). The odds ratios, after being adjusted for age, sex, BMI, and platelet, lymphocyte, neutrophil, and monocyte levels, were 1.101 for RA (p=0.023, 95% CI=1.001–1.019) and 1.217 for RA-ILD (p<0.001, 95% CI=1.012–1.022) when compared to the control subjects.
Table 5.
Logistic regression analysis of the association between PLR, NLR, and LMR with RA and RA-ILD.
| Severity | OR | P (95%CI) | |
|---|---|---|---|
| PLR | Control (reference) | 1.0 | |
| RA | 1.101 | 0.023 (1.001 to 1.019) | |
| RA-ILD | 1.217 | <0.001 (1.012 to 1.022) | |
| NLR | Control (reference) | 1.0 | |
| RA | 0.850 | 0.264 (0.639 to 1.130) | |
| RA-ILD | 0.891 | 0.226 (0.739 to 1.074) | |
| LMR | Control (reference) | 1.0 | |
| RA | 0.823 | 0.001 (0.734 to 0.923) | |
| RA-ILD | 0.910 | 0.086 (0.818 to 1.013) |
PLR – platelet/lymphocyte ratio; NLR – neutrophil/lymphocyte ratio; LMR – lymphocyte/monocyte ratio; RA-ILD – rheumatoid arthritis-associated interstitial lung disease; RA – rheumatoid arthritis.
Discussion
Accurate and early diagnosis and distinguishing RA-ILD from RA as early as possible are becoming increasingly important because of the increased risk of death and lower survival in RA-ILD patients [25,26]. Therefore, we performed a large-sample study to assess the diagnostic value of PLR, NLR, and LMR in patients with RA and RA-ILD. We showed that the mean ESR, PLR, NLR, and platelet, neutrophil, and monocyte levels were lowest in the control group, followed by the RA group and RA-ILD group, while the mean lymphocyte levels and LMR were lowest in the RA-ILD group, followed by the RA group and control group. Furthermore, logistic regression analysis confirmed an association between elevated PLR levels and an increased risk of RA and RA-ILD, which was confirmed in the sex and age subgroups.
Many efforts have been made to investigate the relationship of PLR, NLR, and LMR with RA and disease activity. An early study conducted by Peng et al. [16] reported that RA patients have significantly higher PLR (192.85±101.78 vs. 103.49±28.68) and NLR (3.20±2.06 vs. 1.56±0.47) levels than healthy controls, and that PLR had diagnostic value in patients with RA. Uslu et al. [15] also found a statistically significant difference in NLR (2.12±0.83 vs. 1.58±0.57) and PLR (136.50±53.52 vs. 114.84±29.41) between RA patients and the control group. In a larger study including 317 patients with RA, Zengin et al. [27] reported a significant difference in both NLR and PLR between the RA group and control group (both, p<0.01). In our study, which is the largest reported yet, we found that NLR (3.40±3.17 vs. 2.94±2.37) and PLR (190.69±98.75 vs. 136.00±58.69) were significantly higher in patients with RA than in healthy controls. Thus, our results of normal or RA PLRs and NLRs are comparable with those of previous studies, indicating that these parameters may be reliably used.
The multiple linear regression analysis results in our study suggested a significant correlation between PLR and DAS28, which is in line with the existing literature, in which 1 study with 128 RA patients and 78 healthy individuals reported a significantly positive correlation between NLR and PLR and DAS28 in RA patients [19], and a smaller study reported a significant correlation between DAS28 and NLR and DAS28 and PLR before and after 6 months of rituximab treatment [14].
The precise mechanisms by which PLR and NLR are involved in RA are yet to be determined [28]. Different factors might explain the PLR and NLR in patients with RA. First, given that inflammation plays a vital role in the activity and progression of RA, and monocytes, platelets, neutrophils, and lymphocytes play a vital role in the immune response, higher RA activity can lead to a greater extent of systemic inflammation by secretion of cytokines, which in turn increase the PLR and NLR, and decrease the LMR. Thus, PLR, LMR, and NLR may affect the development, activity, and progression of RA by activating the immune response. Second, the accumulation and persistence of lymphocyte infiltrate in the rheumatoid synovium are characteristic features of RA [28]. In the inflammatory response in RA, lymphocytes are eliminated by initiating the apoptotic cascade [29], resulting in a decreased level of lymphocytes, which in turn increases the PLR and NLR and decreases the LMR. This corresponds to the results of the present study. Regarding disease activity in RA, there is compelling evidence to show that reduced lymphocyte apoptosis contributes to the persistence of inflammatory response at the joints [30]. In the present study, we also found a significantly positive association between PLR and DAS28, indicating that PLR and NLR may affect the development, activity, and progression of RA.
We found that the PLR and NLR were lowest in the control group, followed by the RA and RA-ILD groups, and the LMR was lowest in the RA-ILD group, followed by the RA group and control group. As female sex and older age are reportedly associated with an increased risk of RA-ILD [31], we performed sex and age subgroup analysis and found similar results. Moreover, the AUROC value of the PLR for distinguishing RA-ILD from RA patients was 0.650, and the cutoff value was 144.625 (p<0.001). The reason for these relationships may be as follows. Nalls et al. [32] identified that neutrophil and lymphocyte counts were associated with the loci 17q21 and 6p21 in the HLA region, while the monocyte count was associated with the locus 2q31 in ITGA4. Thus, PLR, NLR, and LMR could be affected by gene variants. Interestingly, certain polymorphisms of the human leukocyte antigen (HLA) DRB and ITGA4 shared epitope were reported to be associated with an increased risk of ILD [33–35]. Furthermore, studies suggest that the severity of RA may be related to the development of RA-ILD [31]. As discussed above, since PLR was associated with disease activity in RA and was significantly different between RA and RA-ILD patients, PLR may be used as a biomarker to distinguish RA-ILD from RA [36,37].
The present study has several limitations. We used a case-control design; thus, we cannot directly conclude that PLR is a risk factor for the development of RA. Therefore, future longitudinal studies should also evaluate the value of PLR in RA and RA-ILD. Additionally, PLR, NLR, and LMR may have been affected by therapy, and therapy information was missing.
Conclusions
We found that an increased PLR level was associated with an increased risk of RA and RA-ILD. Moreover, a similar result was observed in both the sex and age subgroups. Being a simple, rapid, inexpensive, and reliable parameter, PLR might be an important tool for diagnosing RA and distinguishing it from RA-ILD. A growing body of evidence points to an important role for and utility of PLR in predictive, diagnostic, and personalized clinical medicine.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Bellucci E, Terenzi R, La Paglia GMC, et al. One year in review 2016: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(5):793–801. [PubMed] [Google Scholar]
- 2.Li R, Sun J, Ren LM, et al. Epidemiology of eight common rheumatic diseases in China: A large-scale cross-sectional survey in Beijing. Rheumatology (Oxford) 2012;51:721–29. doi: 10.1093/rheumatology/ker370. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet Lond Engl. 2016;388(10055):2023–38. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 4.Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann Rheum Dis. 2017;76(10):1700–6. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Li H, Wu N, et al. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2017;36(4):817–23. doi: 10.1007/s10067-017-3561-5. [DOI] [PubMed] [Google Scholar]
- 6.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chimenti MS, Triggianese P, Conigliaro P, et al. The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis. 2015;6:e1887. doi: 10.1038/cddis.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo A, Franchina T, Ricciardi GRR, et al. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. J Cell Physiol. 2018;233(10):6337–43. doi: 10.1002/jcp.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu X, Sun S, Gao X-S, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep. 2016;6:23893. doi: 10.1038/srep23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massiot N, Lareyre F, Voury-Pons A, et al. High neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with symptomatic internal carotid artery stenosis. J Stroke Cerebrovasc Dis. 2019;28(1):76–83. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee YSG, Baradi A, Peverelle M, et al. Usefulness of platelet-to-lymphocyte ratio to predict long-term all-cause mortality in patients at high risk of coronary artery disease who underwent coronary angiography. Am J Cardiol. 2018;121(9):1021–26. doi: 10.1016/j.amjcard.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Erre GL, Paliogiannis P, Castagna F, et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest. 2019;49(1):e13037. doi: 10.1111/eci.13037. [DOI] [PubMed] [Google Scholar]
- 13.Hao X, Li D, Wu D, Zhang N. The relationship between hematological indices and autoimmune rheumatic diseases (ARDs), a meta-analysis. Sci Rep. 2017;7(1):10833. doi: 10.1038/s41598-017-11398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargin G, Senturk T, Yavasoglu I, Kose R. Relationship between neutrophil-lymphocyte, platelet-lymphocyte ratio and disease activity in rheumatoid arthritis treated with rituximab. Int J Rheum Dis. 2018;21(12):2122–27. doi: 10.1111/1756-185X.13400. [DOI] [PubMed] [Google Scholar]
- 15.Uslu AU, Küçük A, Şahin A, et al. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis. 2015;18(7):731–35. doi: 10.1111/1756-185X.12582. [DOI] [PubMed] [Google Scholar]
- 16.Peng Y-F, Cao L, Zeng Y-H, et al. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in patients with rheumatoid arthritis. Open Med Wars Pol. 2015;10(1):249–53. doi: 10.1515/med-2015-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekara S, Mukhtar Ahmad M, Renuka P, et al. Characterization of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int J Rheum Dis. 2017;20(10):1457–67. doi: 10.1111/1756-185X.13157. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol. 2017;36(12):2689–95. doi: 10.1007/s10067-017-3815-2. [DOI] [PubMed] [Google Scholar]
- 19.Fu H, Qin B, Hu Z, et al. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin Lab. 2015;61(3–4):269–73. doi: 10.7754/clin.lab.2014.140927. [DOI] [PubMed] [Google Scholar]
- 20.Kinkorová J, Topolčan O. Biobanks in Horizon 2020: Sustainability and attractive perspectives. EPMA J. 2018;9(4):345–53. doi: 10.1007/s13167-018-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–88. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Raimundo K, Solomon JJ, Olson AL, et al. Rheumatoid arthritis-interstitial lung disease in the united states: Prevalence, incidence, and healthcare costs and mortality. J Rheumatol. 2019;46(4):360–69. doi: 10.3899/jrheum.171315. [DOI] [PubMed] [Google Scholar]
- 25.Golubnitschaja O, Costigliola V EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: White paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. doi: 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausweger C, Burgschwaiger E, Kugler A, et al. Economic concerns about global healthcare in lung, head and neck cancer: Meeting the economic challenge of predictive, preventive and personalized medicine. EPMA J. 2010;1(4):627–31. doi: 10.1007/s13167-010-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zengin O, Onder ME, Kalem A, et al. New inflammatory markers in early rheumatoid arthritis. Z Rheumatol. 2018;77(2):144–50. doi: 10.1007/s00393-016-0187-y. [DOI] [PubMed] [Google Scholar]
- 28.Sağ S, Sağ MS, Tekeoğlu I, et al. Relationship of hematologic markers with IL-17 and IL-1 beta in patients with rheumatoid arthritis. J Back Musculoskelet Rehabil. 2018;31(4):703–7. doi: 10.3233/BMR-170903. [DOI] [PubMed] [Google Scholar]
- 29.Firestein GS. The immunopathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 1991;3(3):398–406. doi: 10.1097/00002281-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6(12):1191–97. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 31.Pap T, Müller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2(5):361–67. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assayag D, Lee JS, King TE. Rheumatoid arthritis associated interstitial lung disease: A review. Medicina (Mex) 2014;74(2):158–65. [PubMed] [Google Scholar]
- 33.Nalls MA, Couper DJ, Tanaka T, et al. Multiple loci are associated with white blood cell phenotypes. PLoS Genet. 2011;7(6):e1002113. doi: 10.1371/journal.pgen.1002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez I, Collado J, Daura X, et al. The rheumatoid arthritis-associated allele HLA-DR10 (DRB1*1001) shares part of its repertoire with HLA-DR1 (DRB1*0101) and HLA-DR4 (DRB*0401) Arthritis Rheum. 2008;58(6):1630–39. doi: 10.1002/art.23503. [DOI] [PubMed] [Google Scholar]
- 35.Burkhardt J, Blume M, Petit-Teixeira E, et al. Cellular adhesion gene SELP is associated with rheumatoid arthritis and displays differential allelic expression. PloS One. 2014;9(8):e103872. doi: 10.1371/journal.pone.0103872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinkorová J. Biobanks in the era of personalized medicine: Objectives, challenges, and innovation: Overview. EPMA J. 2015;7:4. doi: 10.1186/s13167-016-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhmetov I, Bubnov RV. Assessing value of innovative molecular diagnostic tests in the concept of predictive, preventive, and personalized medicine. EPMA J. 2015;6:19. doi: 10.1186/s13167-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]