Abstract
Background
Depression is one of the most common mental health disorders and severely impacts one’s physical, psychological, and social functioning. To address access barriers to care, we developed Ascend—a smartphone-delivered, therapist-supported, 8-week intervention based on several evidence-based psychological treatments for depression and anxiety. A previous feasibility study with 102 adults with elevated depression reported that Ascend is associated with a postintervention reduction in depression symptoms.
Objective
We aimed to examine whether Ascend is associated with a reduction in symptoms of anxiety, and importantly, whether reductions in symptoms of depression and anxiety are maintained up to 12-months postintervention.
Methods
We assessed whether the previously reported, end-of-treatment improvements seen in the 102 adults with elevated symptoms of depression extended up to 12 months posttreatment for depression symptoms (measured by the Patient Health Questionnaire-9 [PHQ-9]) and up to 6 months posttreatment for anxiety symptoms (added to the intervention later and measured using the Generalized Anxiety Disorder-7 [GAD-7] scale). We used linear mixed effects models with Tukey contrasts to compare time points and reported intention-to-treat statistics with a sensitivity analysis.
Results
The intervention was associated with reductions in symptoms of depression that were maintained 12 months after the program (6.67-point reduction in PHQ-9 score, 95% CI 5.59-7.75; P<.001; Hedges g=1.14, 95% CI 0.78-1.49). A total of 60% of the participants with PHQ-9 scores above the cutoff for major depression at baseline (PHQ≥10) reported clinically significant improvement at the 12-month follow-up (at least 50% reduction in PHQ-9 score and postprogram score <10). Participants also reported reductions in symptoms of anxiety that were maintained for at least 6 months after the program (4.26-point reduction in GAD-7 score, 95% CI 3.14-5.38; P<.001; Hedges g=0.91, 95% CI 0.54-1.28).
Conclusions
There is limited evidence on whether outcomes associated with smartphone-based interventions for common mental health problems are maintained posttreatment. Participants who enrolled in Ascend experienced clinically significant reductions in symptoms of depression and anxiety that were maintained for up to 1 year and 6 months after the intervention, respectively. Future randomized trials are warranted to test Ascend as a scalable solution to the treatment of depression and anxiety.
Keywords: digital health, depression, anxiety, mindfulness, CBT, online intervention, smartphone intervention
Introduction
Depression is a common mental health disorder and one of the leading causes of disease burden and disability worldwide [1-3]. Individuals with depression have a reduced capacity to work and function in daily life, causing major economic and societal costs [4-6]. By 2030, depression is predicted to pose the largest burden of disease in high-income countries, surpassing heart disease, dementia, and alcohol-related disorders [1].
Although there are several effective pharmacological [7] and psychological [8,9] treatments for depression, less than half of all individuals who require treatment actually receive it [10,11]. Barriers to treatment include financial and time constraints, long wait periods, a shortage of trained professionals, and fear of stigmatization [12,13]. When treated with antidepressants, an estimated 30%-50% of patients do not experience significant symptom reduction [14], up to 80% of patients report at least one mild-to-severe side effect [15], and few patients maintain a state of long-term remission [16]. Estimates suggest that up to 70% of individuals with depressive disorders have a comorbid anxiety disorder, which renders treatment even more challenging [17]. Further, up to 75% of patients referred to in-person psychotherapy either do not enter treatment or discontinue treatment prematurely [18,19]. Thus, there is an urgent need for novel, evidence-based treatments for depression and anxiety to overcome these barriers.
Recently, digital interventions delivered via smartphone apps have been developed as a means to address this need [20]. Smartphone ownership has seen rapid worldwide growth [21], and survey data suggest that smartphone-based interventions may be preferred over other online formats by health care consumers [22,23]. Smartphone-based interventions offer several advantages over traditional treatment modalities including large-scale accessibility and scalability; low costs; patient anonymity and privacy; standardized content that is less dependent on therapist skills; flexible usage at a self-determined time and pace, which is thought to enhance self-efficacy [24]; monitoring of activity, symptoms, and progression in real time; provision of personalized feedback, motivational support, and targeted care; and potential to improve adherence to treatment [25]. Preliminary evidence suggests that smartphone-based interventions are a promising means to treat depression and anxiety, with recent meta-analyses reporting small-to-moderate reductions in clinical symptoms across 27 studies [26,27].
Despite these promising results, several important questions regarding the design, efficacy, and implementation of smartphone-based interventions for common mental health disorders remain unanswered. First, few authors report on the outcomes of commercially developed smartphone-based interventions for depression and anxiety, making it difficult to evaluate their utility [25,28]. Second, despite being generally effective for the short-term treatment of symptoms of depression and anxiety, there is a dearth of evidence regarding how long the beneficial effects of smartphone-based interventions and other online interventions are maintained posttreatment [29]. This is important because an estimated 50% or more of patients with major depression or generalized anxiety disorder will relapse within 6 to 12 months after the end of an initially effective treatment [30-32]. Thus, investigation of the long-term outcomes associated with smartphone-based interventions for depression and anxiety is an important step toward understanding their true real-world effectiveness. Lastly, despite an increased interest in transdiagnostic interventions that target depression and anxiety concurrently [33], most smartphone-based interventions for mental health are disorder-specific, and it remains unknown whether smartphone-based interventions can address symptoms of depression and anxiety when they are comorbid.
We recently evaluated the feasibility of the Meru Health Ascend intervention, a novel, 8-week smartphone-based intervention for elevated symptoms of depression and anxiety, assisted by a remote therapist [34]. The intervention was found to be feasible and was associated with a postintervention reduction in depression. In this study, we extend these findings by investigating whether the previously reported postintervention reductions in depression are maintained at a 1-year follow-up, whether Ascend is associated with reductions in comorbid symptoms of anxiety, and whether any postintervention reductions in symptoms of anxiety are maintained at a 6-month follow-up. Thus, this study aims to lend important insights into the real-world, long-term impact of smartphone-based interventions for the treatment of common mental health problems. We hypothesized that postintervention reductions in symptoms of depression would be maintained at the 1-year follow-up and that Ascend would be associated with reductions in the symptoms of anxiety, which would persist at the 6-month follow-up. Lastly, as a secondary objective, we aimed to examine whether participant demographics or intervention engagement were predictive of symptom change or attrition at follow-up.
Methods
Research Design
We used a quasiexperimental research design that included a single-arm, pre- and postintervention assessment of outcomes. Symptoms of depression were measured before the intervention (“baseline”); at the end of the 8-week intervention; and at 1, 3, 6, and 12 months postintervention. Symptoms of anxiety were measured at baseline; week 4 of the intervention; the end of the 8-week intervention; and 1, 3, and 6 months postintervention.
Participants
This study included adult patients treated at the Meru Health Online Clinic, a national remote health care provider that currently operates in the United States and Finland. The clinic has had a rolling enrolment since March 2017. At the time of this study, 197 enrollees had passed the 6-month postintervention outcome window (recruited between March 2017 and June 2018), and 102 passed the 12-month postintervention outcome window (recruited between March and December 2017). Our primary analysis includes the latter group, although we also report the Patient Health Questionnaire (PHQ-9) results from the former group with 6-month postintervention outcomes only. The Ascend intervention was primarily intended to treat symptoms of depression; however, many individuals with depression also have comorbid symptoms of anxiety [17]. Thus, to evaluate whether Ascend is also associated with reductions in symptoms of anxiety, Generalized Anxiety Disorder-7 (GAD-7) scale measures were added to the intervention assessment in December 2017. At the time of this study, 102 of the original 197 enrollees with 6-month postintervention PHQ-9 outcomes also had GAD-7 (anxiety) data (recruited between January and June 2018), while 12-month postintervention anxiety data were not yet available for any participants. Note that the 102 participants with 6-month postintervention GAD-7 data are different from the 102 participants with 12-month postintervention PHQ-9 data.
Participants were recruited via online Facebook advertisements that sought participants for a smartphone-based intervention for depression that included self-guided smartphone-delivered content, private access to a therapist via messaging, and an anonymous group-chat feature with other participants. Prior to the intervention, participants were given free access to the app and trained on how to use the group-chat feature and communicate with their assigned therapist. Participant demographics (age, gender, and antidepressant medication status) were acquired at the start of the intervention via an intake questionnaire administered online. Since medication status was introduced in July 2017, these data are absent for the first 33 participants. Outcome measures were administered via the app for all within-intervention time points (including immediately postintervention) and via email for all follow-up time points.
For inclusion, participants had to provide informed consent via the Meru Health app, own a smartphone, have at least mild symptoms of depression (a score≥5 on the PHQ-9 at baseline), and acknowledge/demonstrate the ability to commit to a minimum of 20 minutes of practice per day for 6 days per week across the 8-week intervention (as judged by both the participant and their assigned therapist). Exclusion criteria included a previous suicide attempt, severe active suicidal ideation with a specific plan, severe self-harm, active substance abuse, and a history of psychosis. Inclusion/exclusion criteria were assessed prior to enrolment via phone interviews between study participants and intervention therapists, as per the standard treatment procedure at the Meru Health Online Clinic. Participants were not compensated for their time but could participate in the intervention for free. All participants provided informed consent for their anonymized data to be used for research purposes prior to engaging with the intervention. All procedures were reviewed by the Pearl Institutional Review Board, which granted exemption for analyses of previously collected and deidentified data. All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
An a priori sample size calculation was performed for comparing patient-reported outcome measures (PHQ-9 and GAD-7 scores) at baseline and follow-up time points. Using an alpha level of .05, a power of 0.8, and a medium effect size of 0.5, 33 subjects were needed. Thus, this study was sufficiently powered to detect a medium effect, even after accounting for substantial dropout over the course of the 1-year follow-up period.
Intervention
The Meru Health Ascend intervention has been described in detail previously [34]. Briefly, the intervention consists of 8 modules delivered sequentially over an 8-week period, which include content derived from evidence-based practices such as mindfulness-based stress reduction [35], mindfulness-based cognitive therapy [36], cognitive-behavioral therapy [37], and behavioral activation therapy [38]. The content includes text; video; audio-guided mindfulness meditation exercises; infographics that illustrate cognitive-behavioral therapy principles; and journal prompts. Daily content and practices range from 10 to 30 minutes, except for the first day of each week, in which a series of introductory videos extend the content to a maximum of 45 minutes. The Meru Health app can be used on both Android and iOS and is designed to be platform-agnostic and thus equivalent across different operating systems.
A licensed therapist (employed by Meru Health) provides support to participants via messaging (and less frequently, phone calls), throughout the intervention. As part of this support, therapists review practice logs using a provider “dashboard” and electronic medical records (that detail participant engagement and patient-reported outcomes to date) to monitor individual participant progress. Therapists aim to spend approximately 20 minutes (on average) supporting each participant per week of the intervention (including initiating contact at least 2-3 times per week), but are at liberty to adjust the levels of support in accordance with each participant’s individual progress. In addition, participants are free (and encouraged) to contact their allocated therapist when they require additional support. Such two-way interaction is designed to create a system of support that is structured while being tailored to each participant’s personal preference and needs. Further, therapists are instructed to conduct a phone-based assessment for any participants that show signs of mental deterioration during or immediately after the intervention. In case of an emergency, such as severe suicidality, the intervention includes a written security plan, which all participants are required to review with their therapist before engaging with the intervention.
Participants are enrolled in groups of 10-15 individuals that work through the intervention at the same time and can provide anonymous support to one another via a discussion board within the app. Specifically, participants can post anonymous reflections on practices and lessons to the discussion board, to which their therapist can respond freely, and to which other group members can respond with prewritten empathy statements and emoticons. Free cross-talk between participants is not allowed.
In Finland, Meru Health is approved by the Finnish National Supervisory Authority for Welfare and Health (Valvira approval number V/25535/2017) and is compliant with the European Union General Data Protection Regulation. In the United States, Meru Health is compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) legislation. All protected health information is kept in a HIPAA-compliant electronic medical record, which is housed in cloud-based storage systems hosted by a company named Datica [39]. All data are encrypted in transit, end-to-end, and at rest.
Measures
Patient Health Questionnaire
The PHQ-9 is a 9-item depression scale, derived from the full PHQ and is one of the most widely used instruments to screen for the presence and severity of depression in primary care [40]. Participants rate each item on a Likert scale from 0 (not at all) to 3 (nearly every day), with total scores ranging from 0 to 27. In general, a score of 10 or above suggests the presence of major depression, and scores of 5, 10, 15, and 20 are taken as cut-off points for mild, moderate, moderately severe, and severe depression, respectively. The PHQ-9 has excellent internal consistency (Cronbach α of 0.89 in primary care settings) and excellent test-retest reliability [41]. In their original validation study, Kroenke and colleagues [40] defined a clinically significant improvement in depression as a 50% reduction in the PHQ-9 score combined with a postintervention score of <10 (for participants with baseline scores≥10), and this definition has been further validated in a comparison study [42]. A similar yet more liberal definition for clinically significant change was also proposed by Löwe and colleagues [43] as a PHQ-9 score reduction of ≥5.
Generalized Anxiety Questionnaire
The GAD-7 is a 7-item scale used extensively in outpatient and primary care settings to screen for the presence and severity of an anxiety disorder [44]. Participants rate each item on a Likert scale from 0 (not at all) to 3 (nearly every day), with total scores ranging from 0 to 21. In general, a score of 10 or above is suggestive of the presence of anxiety to the extent that further evaluation is warranted, and scores of 5, 10, and 15 are taken as cut-off points for mild, moderate, and severe anxiety, respectively. The GAD-7 has excellent reliability and internal consistency (Cronbach α of 0.89) and has been validated in both the general population and primary care settings [44,45]. A clinically significant improvement in anxiety symptoms has previously been defined as a GAD-7 score reduction of ≥3 [46].
Statistical Analysis
Measures of Engagement
Descriptive statistics were calculated for participant demographics and week-by-week engagement metrics. For each participant, we calculated a measure of intervention engagement, defined as total days in which >3 minutes of app-based meditation was completed. We used a threshold of 3 minutes, as this corresponds to the shortest meditation session available in the intervention. We used logistic regression to explore whether baseline characteristics were predictive of the completion of outcome measures at 6 and 12 months postintervention. Explanatory variables included age, gender, country, intervention engagement (as defined above), and baseline PHQ-9 or GAD-7 scores.
Patient-Reported Outcomes
Outcome measures were analyzed using an intention-to-treat analysis in which all participants with outcome measures at baseline were included, regardless of intervention engagement or attrition. We used linear mixed effects models (LMMs) implemented through LME4 [47] and Tukey contrasts to compare between time points (using the “multcomp” package [48]) in the statistical computing software R [computer software] (version 3.5.2. Vienna, Austria: R Foundation for Statistical Computing). LMMs are capable of handling missing data and are considered superior to other ITT approaches such as the last observation carried forward [49]. We modeled a single within-subject factor “time” (as a fixed effect) and a separate baseline for each participant (random-intercept model). Time was modeled as a categorical predictor, and we therefore did not enforce a linear relationship between time and outcome measures. We also ran the model while controlling for participant age, gender, country, total intervention engagement (total active days), and total therapist contact, both with and without two-way interactions with time, which produced equivalent results. We report the contrast estimate, 95% CI of the estimate, and P value. P values<.05 were considered significant. The estimated marginal mean and standard error for each time point was calculated using the “emmeans” package in R.
Sensitivity Analysis
Since LMMs rely on data being “missing at random,” an assumption that is difficult to verify in clinical research, we also implemented an LMM-based pattern-mixture model (PMM) for the analysis of PHQ-9 (depression) scores. PMMs are based on a joint modeling of outcomes and missingness and can account for data that is “missing not at random” [50]. We modified the estimated marginal mean (EMM) from the previously described LMM at the 6- and 12-month time points based on specific clinical assumptions about participants with missing data. We identified three patterns of data: (1) participants with complete outcome data at baseline and 12-month follow-up (n=52); (2) participants who were lost to follow-up during or immediately postintervention (n=21); and (3) participants who were lost to follow-up at the 1-, 3-, 6-, or 12-month follow-up time points (n=29). We used the conservative approach of assuming that participants belonging to pattern 2 did not benefit from the intervention, and we set the EMM at 6 and 12 months of follow-up equal to the EMM at baseline for these participants. For group 3, we estimated the EMM at the 6- and 12-month follow-ups as being equal to the EMMs across the first three time points of the intervention (baseline to week 4). The overall treatment effect at 6 or 12 months of follow-up was then defined as a linear combination of EMMs for the three different patterns, weighted by the proportion of participants belonging to each pattern. The standard error for this estimate was calculated using the delta method (via the “car” package in R [51]), and a test of the null hypothesis of no treatment effect was performed using a Wald statistic. A full description of the LMM-based PMM procedure is provided in a previous paper [52].
Effect Size Calculation
For comparisons with previous literature, we calculated effect size (Hedges g) immediately postintervention and at 6 and 12 months after the intervention as the difference in outcome scores from baseline divided by the pooled weighted SD (based on observed outcome scores only). For the PMM, effect size (Cohen d) was calculated as the estimated reduction in outcome score (from the LMM-based PMM) divided by the SD of observed outcome scores at baseline. We also calculated the percentage of participants who met the definition for clinically significant improvement in PHQ-9 symptoms postintervention and at the 12-month follow-up, defined as either a 5-point reduction in PHQ-9 score from baseline or a 50% reduction in PHQ-9 score combined with a postintervention PHQ-9 score<10 (for participants with baseline scores ≥10).
Predictors of Outcome Change
Lastly, we performed exploratory multiple regressions to test whether participant demographics and engagement metrics predicted outcome change from baseline to 6 and 12 months postintervention with regard to PHQ-9 scores and from baseline to 6 months postintervention with regard to GAD-7 scores. In each case, we excluded participants who did not have an available change score and modeled the following predictor variables: age, gender, baseline PHQ-9 or GAD-7 score, total active days, and total days with therapist contact. Since antidepressant status was only available for a subset of participants, we repeated each analysis including and excluding antidepressant status. In addition, we excluded meditation minutes from all models, as this was highly correlated with active days. Predictors associated with a P value<.05 were considered significant.
Results
Demographics and Participation
Participant demographics for the primary group of participants with 12-month PHQ-9 data are presented in Table 1. On an average, participants were 33 years of age, had baseline symptoms above the cutoff for major depression (mean PHQ-9 score=12.8), and were predominantly female. In addition, 80 participants were based in Finland, while 22 were based in the United States. Participants based in the United States had significantly less PHQ-9 symptoms at baseline than those in Finland (United States: mean 8.33, SD 4.40; Finland: mean 13.9, SD 4.82; t101=4.82; P<.001). Approximately one-third of the participants were taking antidepressant medication at the start of the intervention, and 20 participants (19.6%) dropped out of the intervention, where dropout was defined as less than 4 weeks of active participation during the 8-week intervention combined with incomplete PHQ-9 (depression) scores immediately postintervention.
Table 1.
Participant demographics and relevant baseline data for the primary study cohort (with Patient Health Questionnaire-9 outcomes available up to 12-months postintervention).
| Demographics and baseline data | All participants | Completed 8-week outcomes | Completed 6-month outcomes | Completed 12-month outcomes | Did not complete 8-week outcomes | Did not complete 6-month outcomes | Did not complete 12-month outcomes | |
| Total participants, n (%) | 102 (100) | 83 (81.4) | 44 (43.1) | 52 (51) | 19 (18.6) | 58 (56.9) | 50 (49) | |
| Age in years, mean (SD) | 32.9 (10.3) | 33.2 (10.4) | 33.5 (11.0) | 31.3 (11.0) | 31.9 (10.0) | 32.5 (9.9) | 34.6 (9.4) | |
| Gender, n (%) | ||||||||
| Male | 23 (22.5) | 19 (22.9) | 11 (25) | 10 (19.2) | 4 (21.0) | 12 (20.7) | 13 (26) | |
| Female | 79 (77.5) | 64 (77.1) | 33 (75) | 42 (80.8) | 15 (79.0) | 46 (79.3) | 37 (74) | |
| Antidepressants, n (%) | ||||||||
| Yes | 25 (24.5) | 18 (21.7) | 7 (15.9) | 7 (13.5) | 7 (36.8) | 18 (31.0) | 18 (36) | |
| No | 44 (43.1) | 37(44.6) | 24 (54.6) | 22 (42.3) | 7 (36.8) | 20 (34.5) | 22 (44) | |
| Unknown | 33 (32.4) | 28 (33.7) | 13 (29.6) | 23 (44.2) | 5 (26.3) | 20 (34.5) | 10 (20) | |
| Country, n (%) | ||||||||
| Finland | 80 (78.4) | 67 (80.7) | 36 (81.8) | 48 (92.0) | 13 (68.0) | 44 (75.9) | 32 (64.0) | |
| Unites States | 22 (21.6) | 16 (19.3) | 8 (18.2) | 4 (8.0) | 6 (32.0) | 14 (24.1) | 18 (36.0) | |
| Baseline PHQ-9a score, mean (SD)b | 12.8 (5.2) | 13.0 (5.4) | 13.2 (5.8) | 14.3 (5.0) | 11.6 (4.6) | 12.5 (4.8) | 11.2 (5.0) | |
aPHQ-9: Patient Health Questionnaire-9.
bSignificantly associated with the presence of PHQ-9 scores at the 12-month follow-up.
Just over half (51%) of all participants reported a PHQ-9 outcome at 12 months postintervention (Table 1). Logistic regression revealed that participants with higher depression scores at baseline (B=–0.11, P=.02) and those who engaged with the intervention on more days (B=–0.05, P=.009) were more likely to complete the PHQ-9 at 12 months postintervention. When including country as a covariate, participants in the United States were less likely to complete PHQ-9 data at 12 months postintervention than participants in Finland (B=1.89, P=.01), although this may have been driven by differences in baseline PHQ-9 severity.
We also report participant demographics for a separate group of patients with anxiety outcomes available at the 6-month follow-up in Table 2. On an average, baseline GAD-7 scores suggest that participants had symptoms above the cutoff for moderate anxiety (mean GAD-7 score=10.7), despite being recruited on the basis of symptoms of depression. Age, gender, country, and baseline PHQ-9 symptoms were similar to those of the primary study cohort presented in Table 1. Intervention engagement positively predicted completion of the GAD-7 scale at 6 months postintervention (B=–0.07, P<.001), but baseline GAD-7 scores did not (P=.99).
Table 2.
Participant demographics and relevant baseline data for patients with Generalized Anxiety Disorder-7 outcomes available at 6-months postintervention.
| Demographics and baseline data | All participants | Completed 8-week outcomes | Completed 6-month outcomes | Did not complete 8-week outcomes | Did not complete 6-month outcomes | |
| Total participants, n (%) | 102 (100) | 74 (72.6) | 45 (44.1) | 28 (27.5) | 57 (55.9) | |
| Age in years, mean (SD) | 31.7 (11.4) | 31.5 (11.7) | 31.0 (12.0) | 32.1 (10.8) | 32.2 (10.9) | |
| Gender, n (%) | ||||||
| Male | 16 (15.7) | 9 (12.2) | 4 (8.9) | 7 (25.0) | 12 (21.1) | |
| Female | 86 (84.3) | 65 (87.8) | 41 (91.9) | 21 (75.0) | 45 (78.9) | |
| Antidepressants, n (%) | ||||||
| Yes | 47 (46.1) | 37 (50.0) | 22 (48.9) | 10 (35.7) | 25 (43.9) | |
| No | 53 (52.0) | 36 (48.7) | 23 (51.1) | 17 (60.1) | 30 (52.6) | |
| Unknown | 2 (1.9) | 1 (1.4) | 0 (0) | 1 (3.6) | 2 (3.5) | |
| Country, n (%) | ||||||
| Finland | 90 (88.2) | 71 (95.9) | 45 (100) | 19 (67.9) | 12 (21.1) | |
| United States | 12 (11.8) | 3 (4.1) | 0 (0) | 9 (32.1) | 45 (78.9) | |
| Baseline GAD-7a score, mean (SD) | 10.7 (4.9) | 11.0 (4.8) | 10.8 (4.8) | 9.85 (4.9) | 10.5 (4.9) | |
| Baseline PHQ-9b score, mean (SD) | 13.4 (4.9) | 13.6 (4.7) | 13.1 (4.4) | 12.9 (5.5) | 13.7 (5.4) | |
aGAD-7: Generalized Anxiety Disorder-7.
bPHQ-9: Patient Health Questionnaire-9.
Engagement
Week-by-week intervention engagement rates are summarized in Table 3. On an average, participants engaged with the intervention on 31.3 days (SD 13.5, min=3, max=56), which corresponds to 55.9% of intervention days (where engagement is defined as >3 minutes of app-based mindfulness meditation on a given day). Participants completed an average of 9.79 hours of mindfulness-based exercises (SD 5.01, min=0.79, max=24.7) and had contact with their therapist on 13.1 days (SD 8.34, min=0, max=35) or 23.4% of intervention days. In addition, 68 participants (66.7%) completed at least one app-based meditation practice on each of the 8 weeks of the intervention. As reported in a previous publication [34], the mean number of days of intervention engagement (F7,707=42.4, P<.001) and the mean number of days of therapist contact (F7,707=5.37, P<.001) decreased from week 1 to week 8 (Table 3). Of the 102 participants, 81.4% completed the PHQ-9 immediately postintervention, while 43.1% and 51% completed the PHQ-9 at 6 and 12 months postintervention, respectively.
Table 3.
Week-by-week intervention engagement across all participants. “Active days” corresponds to >3 minutes of app-based mindfulness practice on a given day.
| Metric | Week | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Active days, mean (SD) | 5.02 (1.81) | 4.65 (1.89) | 4.54 (1.95) | 4.13 (1.95) | 3.76 (2.19) | 3.59 (2.34) | 2.95 (2.21) | 2.67 (2.20) |
| Meditation minutes, mean (SD) | 93.9 (39.0) | 105.9 (52.6) | 80.6 (40.0) | 76.1 (47.6) | 82.6 (60.5) | 67.7 (55.2) | 39.0 (39.8) | 41.5 (39.5) |
| Days with therapist contact, mean (SD) | 2.02 (1.27) | 1.90 (1.53) | 1.65 (1.40) | 1.75 (1.40) | 1.47 (1.42) | 1.46 (1.45) | 1.43 (1.43) | 1.42 (1.37) |
Patient-Reported Outcomes
Depression Symptoms
Participants reported clinically significant improvements in depression symptoms (PHQ-9 scores) from baseline to postintervention (5.50-point reduction, 95% CI 4.58-6.42; P<.001, Hedges g=1.02, 95% CI 0.71-1.32; Figure 1, panel A). This improvement was maintained at 6 months (6.76-point reduction, 95% CI 5.61-7.90; P<.001; Hedges g=1.30, 95% CI 0.91-1.68) and 12 months (6.67-point reduction; 95% CI 5.59-7.75; P<.001; Hedges g=1.14, 95% CI 0.78-1.49) postintervention. The improvement at 6 months remained robust when including an additional 95 participants (total n=197) with outcomes available at 6 months postintervention only (6.53-point reduction, 95% CI 5.73-7.33; P<.001; Hedges g=1.28, 95% CI 1.00-1.55).
Figure 1.
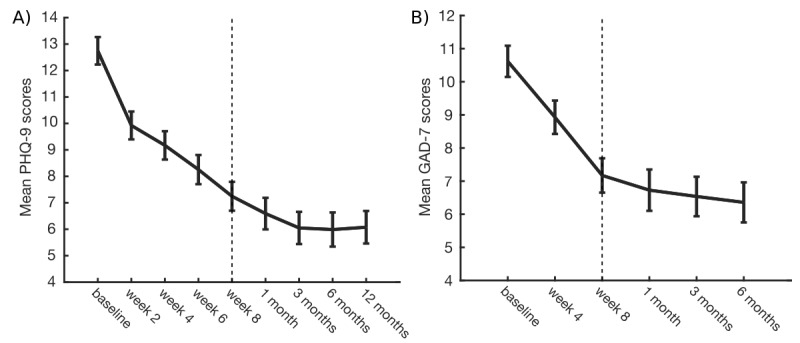
Estimated marginal means for PHQ-9 (A) and GAD-7 (B) scores across all available timepoints. The dotted line indicates the last week of the 8-week intervention. Error bars represent standard error of the mean. PHQ-9: Patient Health Questionnaire (9-item version); GAD-7: Generalized Anxiety Disorder (7-item version).
When considering participants with PHQ-9 scores≥10 at baseline (n=83), 48% reported a clinically significant improvement immediately postintervention, which increased to 60% at the 12-month follow-up (defined as postintervention score<10 combined with ≥50% symptom reduction). When including all participants and using the more liberal definition of a 5-point reduction in PHQ-9 symptoms, 56% reported clinically significant improvements immediately postintervention, which increased to 77% at the 12-month follow-up.
Sensitivity Analysis
We also estimated PHQ-9 scores at 6 and 12 months postintervention under the conservative assumption that participants with missing data did not experience any long-term benefit from the intervention or benefitted only marginally (see Methods). This approach revealed more modest yet significant reductions in PHQ-9 scores relative to baseline at both 6 months (4.35-point reduction, 95% CI 3.65-5.06; P<.001; Cohen d=0.83, 95% CI 0.43-1.24) and 12 months (4.31-point reduction, 95% CI 3.63-4.99; P<.001; Cohen d=0.82, 95% CI 0.42-1.23) postintervention.
Anxiety Symptoms
Participants reported clinically significant improvements in anxiety (GAD-7 scores) from baseline to postintervention (3.45-point reduction, 95% CI 2.51-4.38; P<.001; Hedges g=0.69, 95% CI 0.38-1.00; Figure 1, panel B). This improvement was maintained at 6 months postintervention (4.26-point reduction, 95% CI 3.14-5.38; P<.001; Hedge g=0.91, 95% CI 0.54-1.28).
Predictors of Outcome Change
Individuals with higher PHQ-9 symptoms (b=0.54, P<0.001) or higher GAD-7 symptoms (b=0.63, P<.001) at baseline were likely to experience larger reductions in depression (at the 12-month follow-up) and anxiety (at the 6-month follow-up), respectively. However, none of the reported engagement metrics or participant demographics were predictive of score change from baseline to 6- and 12-month follow-ups, for either PHQ-9 or GAD-7 (all P>.05).
Discussion
Principal Findings
The Meru Health Ascend intervention is a newly developed, smartphone-based, therapist-supported intervention for depression and anxiety, designed to overcome common barriers to treatment. A recent feasibility study reported that the intervention is feasible and associated with reduced depression symptoms immediately after the intervention [34]. This follow-up study extends these findings by demonstrating that the intervention is associated with clinically significant reductions in symptoms of both depression and anxiety and that these reductions are maintained up to 1 year and 6 months after intervention completion, respectively.
Understanding whether improvements in symptoms associated with Ascend are maintained long-term is important, as evidence suggests that a large proportion of patients are likely to relapse following treatment for depression or anxiety [30,32]. Although relapse rates associated with other smartphone-based interventions for depression and anxiety are largely unknown, previous research suggests that the risk of relapse is substantially lower following psychotherapy than following pharmacotherapy, where as many as 50%-75% of patients are likely to experience significant return of symptoms within 12 months of withdrawal from the latter [53,54]. Our results suggest that, similar to in-person psychotherapy, Ascend may be associated with enduring effects that extend for up to 12 months beyond the end of treatment. However, further work is needed to understand the long-term effectiveness of Ascend and other smartphone-based interventions for depression and anxiety under controlled conditions and relative to conventional treatment methods.
Our analysis revealed a larger effect size for the reduction in depression symptoms 12 months postintervention (g=1.14) than was reported by a recent meta-analysis [26] comparing smartphone-based interventions for depression to inactive control groups (g=0.56). However, this study was uncontrolled, and we therefore caution the reader not to overinterpret this difference. Indeed, the large effect reported here is consistent with other within-group (uncontrolled) effect sizes for app- and online-based depression interventions [55-57]. Further, a recent meta-analysis reported a similar uncontrolled effect size of g=1.29 for the reduction in depression symptoms associated with transdiagnostic, internet-delivered cognitive-behavioral therapy for depression and anxiety at follow-up [33]. Together, this suggests that the aforementioned discrepancy may stem from the lack of a comparison group in our study. We observed that the reduction in depression symptoms remained significant when making a conservative assumption that participants with missing data did not experience any long-term benefit from the intervention. However, the average reduction in depression under this assumption was marginally below the threshold for clinical significance (5-point change in PHQ-9 symptoms).
Similarly, our study revealed a slightly higher effect size for the reduction in anxiety symptoms immediately after the intervention (g=0.69) and 6 months postintervention (g=0.91) than was recently reported in a meta-analysis [27] comparing smartphone-based interventions for anxiety symptoms to inactive or waitlist control groups (g=0.45) [27]. This discrepancy may also be explained by the fact that this study was uncontrolled, and the effect size reported here may thus be an overestimate. In addition, the interventions included in the aforementioned meta-analysis were approximately 2 weeks shorter (on average) than the current 8-week intervention. Further, this study recruited participants on the basis of elevated symptoms of depression (as opposed to anxiety), making it difficult to make direct comparisons. Interestingly, the slightly higher effect size reported here is consistent with the results from online (as opposed to app-based) psychological interventions for anxiety disorders [58,59] and is, in fact, slightly lower than the effect size reported by a recent meta-analysis [33] of transdiagnostic, internet-delivered cognitive-behavioral therapy for depression and anxiety (uncontrolled pretest to follow-up, g=1.29).
The finding that Ascend was associated with reductions in symptoms of anxiety as well as depression is unsurprising, given that the intervention comprises evidence-based practices that have previously been shown to be effective at treating both conditions [60]. In addition, depression and anxiety are highly comorbid, suggesting the presence of shared underlying mechanisms that may respond to similar treatment methods [61]. Indeed, baseline scores suggest that a large proportion of participants were experiencing anxiety symptoms before the intervention, despite being recruited on the basis of having symptoms of depression. However, the magnitude of symptom reduction associated with Ascend was slightly larger for depression than for anxiety, likely reflecting the fact that the intervention was designed to address depression as the primary condition. Although previous online psychological interventions have often separated the treatment of depression and anxiety symptoms, there has been a recent trend toward the development of transdiagnostic interventions that target mechanisms common to multiple psychiatric disorders. These results add to a growing body of evidence highlighting the feasibility of such interventions [33] and suggest that smartphone apps may have the potential to address symptoms of depression and anxiety concurrently.
Two important but largely unanswered questions are who is likely to benefit the most from app-based psychological interventions and what factors are predictive of the degree to which individuals benefit over time. Our previous feasibility study suggested that a greater volume of app-based practice predicted the occurrence of fewer depressive symptoms 4 weeks after completing the Ascend intervention [34]. In this study, the degree of engagement with Ascend did not impact depression or anxiety scores at 6 or 12 months postintervention, although this analysis was likely underpowered due to a large proportion of missing data at these time points. It is also likely that a portion of the variance in scores at these time points was driven by factors not captured by our study. Further research is needed to understand the factors and components of the intervention that are predictive of long-term benefits.
Limitations
As with the former feasibility study [34], this study used a nonrandomized, uncontrolled design, which precludes causal inferences regarding the intervention and patient-reported outcome changes. In addition, the effect sizes reported in this study are likely an overestimate of the true treatment effect relative to an active control or treatment as usual.
Further, approximately one-third of the participants were taking antidepressants during (and presumably after) the intervention, making it difficult to preclude the possibility that the reduction in depression symptoms was caused or maintained by antidepressants [62] or by the tendency for a proportion of depressed patients to naturally recover over a 12-month period [63] and not by this intervention. Future randomized controlled trials that compare Ascend to treatment as usual over an extended period are required to fully address these limitations. The Meru Health Online Clinic could also consider collecting information on the antidepressant status at follow-up (and not just at baseline) to better tease apart the long-term effects associated with the current intervention versus antidepressants.
Further, although engagement with the intervention was high, approximately half of all participants did not complete questionnaires at 6 and 12 months postintervention. Thus, our estimates of the percentage of participants that experienced clinically significant improvements in depression symptoms was based on the participants with complete data only. Further, participants with fewer symptoms of depression postintervention may have been more likely to engage with questionnaires, biasing estimates of the long-term treatment effect. This was addressed by using a robust pattern-mixture model approach and applying the most conservative clinical assumptions about the treatment effect for participants who dropped out or who were lost to follow-up. Nevertheless, such assumptions are inherently unverifiable, and recent guidelines highlight the importance of minimizing the likelihood of incomplete outcome data [64].
Although this study suggests that the intervention is associated with reductions in depression and anxiety symptoms, it does not address the underlying mechanisms or mediators of outcome change. Since the intervention encompasses techniques and practices from multiple approaches (cognitive behavioral therapy, mindfulness meditation, and behavioral activation therapy) as well as remote therapist and peer support, it is difficult to differentiate which components of the intervention are the most effective. Such information could assist with the design and optimization of future iterations of Ascend and other app-based interventions for mental health [65].
Finally, the study included self-selected participants who may have shown a higher degree of motivation to engage with the intervention. Further, the majority of participants were female, and although the study included individuals from both Finland and the United States, the majority of participants were based in Finland. Moreover, the Meru Health Clinic does not currently track patient race and ethnicity, making it difficult to assess the generalizability of the findings. Together, this suggests that this study sample may not be representative of the wider population of individuals with elevated symptoms of depression or anxiety, and future studies should address this issue by using more robust recruitment strategies and more representative study samples.
Conclusions
Depression is a serious and growing problem that causes individual suffering and huge economic and societal costs worldwide. Many individuals with depression are unable to access appropriate treatment, with high costs and a lack of trained professionals being major barriers. Scalable, low-cost, app-based interventions such as Ascend are designed to overcome these barriers and may help to significantly reduce the burden of anxiety and depression. Further research is needed to investigate the efficacy of Ascend in comparison to control groups and other established treatments for depression and anxiety.
Acknowledgments
We wish to express our gratitude to all participants for their efforts and to the staff and therapists at Meru Health who were involved in developing, implementing, and supporting the study intervention.
Abbreviations
- EMM
estimated marginal mean
- GAD-7
Generalized Anxiety Disorder (7-item version)
- HIPAA
Health Insurance Portability and Accountability Act
- LMM
linear mixed effects model
- PHQ-9
Patient Health Questionnaire (9-item version)
- PMM
pattern mixture model
Footnotes
Authors' Contributions: ME, KR, AR, and VF-H conceived and designed the study. ME performed statistical analysis, interpreted the data, and drafted the manuscript. KR, AN, OH, and AR oversaw the design and creation of the study intervention. AN and OH oversaw data collection and archiving. VF-H contributed to interpretation of the data. All authors revised the manuscript and approved the final content.
Conflicts of Interest: All authors are employed, receive a salary, and/or hold equity at Meru Health Inc. KR serves as the chief executive officer of Meru Health Inc and owns a large share of the company’s stocks. AN serves as the chief technology officer of Meru Health Inc and owns a large share of the company’s stocks.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006 Nov;3(11):e442. doi: 10.1371/journal.pmed.0030442. http://dx.plos.org/10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. 2011 Nov;31(7):1117–25. doi: 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007 Sep 8;370(9590):851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013 Nov;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. http://dx.plos.org/10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papakostas GI, Ionescu DF. Updates and trends in the treatment of major depressive disorder. J Clin Psychiatry. 2014 Dec;75(12):1419–21. doi: 10.4088/JCP.14ac09610. [DOI] [PubMed] [Google Scholar]
- 6.Wade AG, Häring J. A review of the costs associated with depression and treatment noncompliance: the potential benefits of online support. Int Clin Psychopharmacol. 2010 Sep;25(5):288–96. doi: 10.1097/yic.0b013e328339fbcf. [DOI] [PubMed] [Google Scholar]
- 7.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018 Dec 07;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008 Dec;76(6):909–22. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010 Apr;78(2):169–83. doi: 10.1037/a0018555. http://europepmc.org/abstract/MED/20350028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade LH, Alonso J, Mneimneh Z, Wells JE, Al-Hamzawi A, Borges G, Bromet E, Bruffaerts R, de Girolamo G, de Graaf R, Florescu S, Gureje O, Hinkov HR, Hu C, Huang Y, Hwang I, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Matschinger H, O'Neill S, Posada-Villa J, Sagar R, Sampson NA, Sasu C, Stein DJ, Takeshima T, Viana MC, Xavier M, Kessler RC. Barriers to mental health treatment: results from the WHO World Mental Health surveys. Psychol Med. 2014 Apr;44(6):1303–17. doi: 10.1017/S0033291713001943. http://europepmc.org/abstract/MED/23931656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SAMHSA: Substance Abuse and Mental Health Services Administration. 2017. Sep 01, [2019-02-01]. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health https://www.samhsa.gov/data/report/key-substance-use-and-mental-health-indicators-united-states-results-2016-national-survey. [PubMed]
- 12.Mohr DC, Ho J, Duffecy J, Baron KG, Lehman KA, Jin L, Reifler D. Perceived barriers to psychological treatments and their relationship to depression. J Clin Psychol. 2010 Apr;66(4):394–409. doi: 10.1002/jclp.20659. http://europepmc.org/abstract/MED/20127795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weil TP. Insufficient dollars and qualified personnel to meet United States mental health needs. J Nerv Ment Dis. 2015 Apr;203(4):233–40. doi: 10.1097/NMD.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 14.El-Hage W, Leman S, Camus V, Belzung C. Mechanisms of antidepressant resistance. Front Pharmacol. 2013 Nov 22;4:146. doi: 10.3389/fphar.2013.00146. doi: 10.3389/fphar.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Han C, Bahk W, Lee S, Patkar AA, Masand PS, Pae C. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med J. 2018 May;54(2):101–112. doi: 10.4068/cmj.2018.54.2.101. https://cmj.ac.kr/DOIx.php?id=10.4068/cmj.2018.54.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pigott HE. STAR*D: A Tale and Trail of Bias. Ethical Human Psychology and Psychiatry. 2011;13(1):6–28. [Google Scholar]
- 17.Coplan JD, Aaronson CJ, Panthangi V, Kim Y. Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World J Psychiatry. 2015 Dec 22;5(4):366–78. doi: 10.5498/wjp.v5.i4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swift JK, Greenberg RP. Premature discontinuation in adult psychotherapy: a meta-analysis. J Consult Clin Psychol. 2012 Aug;80(4):547–59. doi: 10.1037/a0028226. [DOI] [PubMed] [Google Scholar]
- 19.Grant K, McMeekin E, Jamieson R, Fairfull A, Miller C, White J. Individual therapy attrition rates in a low-intensity service: a comparison of cognitive behavioural and person-centred therapies and the impact of deprivation. Behav Cogn Psychother. 2012 Mar;40(2):245–9. doi: 10.1017/S1352465811000476. [DOI] [PubMed] [Google Scholar]
- 20.Bakker D, Kazantzis N, Rickwood D, Rickard N. Mental Health Smartphone Apps: Review and Evidence-Based Recommendations for Future Developments. JMIR Ment Health. 2016;3(1):e7. doi: 10.2196/mental.4984. http://mental.jmir.org/2016/1/e7/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poushter J. Pew Research Center: Global Attitudes & Trends. 2016. Feb, Smartphone ownership and internet usage continues to climb in emerging economies https://www.pewresearch.org/global/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economies/
- 22.Proudfoot J, Parker G, Hadzi PD, Manicavasagar V, Adler E, Whitton A. Community attitudes to the appropriation of mobile phones for monitoring and managing depression, anxiety, and stress. J Med Internet Res. 2010;12(5):e64. doi: 10.2196/jmir.1475. http://www.jmir.org/2010/5/e64/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torous J, Chan SR, Yee-Marie TS, Behrens J, Mathew I, Conrad EJ, Hinton L, Yellowlees P, Keshavan M. Patient Smartphone Ownership and Interest in Mobile Apps to Monitor Symptoms of Mental Health Conditions: A Survey in Four Geographically Distinct Psychiatric Clinics. JMIR Ment Health. 2014;1(1):e5. doi: 10.2196/mental.4004. http://mental.jmir.org/2014/1/e5/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelmsen M, Lillevoll K, Risør MB, Høifødt R, Johansen M, Waterloo K, Eisemann M, Kolstrup N. Motivation to persist with internet-based cognitive behavioural treatment using blended care: a qualitative study. BMC Psychiatry. 2013;13:296. doi: 10.1186/1471-244X-13-296. http://www.biomedcentral.com/1471-244X/13/296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donker T, Petrie K, Proudfoot J, Clarke J, Birch M, Christensen H. Smartphones for smarter delivery of mental health programs: a systematic review. J Med Internet Res. 2013;15(11):e247. doi: 10.2196/jmir.2791. http://www.jmir.org/2013/11/e247/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firth J, Torous J, Nicholas J, Carney R, Pratap A, Rosenbaum S, Sarris J. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry. 2017 Oct;16(3):287–298. doi: 10.1002/wps.20472. doi: 10.1002/wps.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firth J, Torous J, Nicholas J, Carney R, Rosenbaum S, Sarris J. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017 Aug 15;218:15–22. doi: 10.1016/j.jad.2017.04.046. https://linkinghub.elsevier.com/retrieve/pii/S0165-0327(17)30015-0. [DOI] [PubMed] [Google Scholar]
- 28.Huguet A, Rao S, McGrath PJ, Wozney L, Wheaton M, Conrod J, Rozario S. A Systematic Review of Cognitive Behavioral Therapy and Behavioral Activation Apps for Depression. PLoS One. 2016;11(5):e0154248. doi: 10.1371/journal.pone.0154248. http://dx.plos.org/10.1371/journal.pone.0154248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012 Jun;32(4):329–42. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Rickels K, Etemad B, Khalid-Khan S, Lohoff FW, Rynn MA, Gallop RJ. Time to relapse after 6 and 12 months' treatment of generalized anxiety disorder with venlafaxine extended release. Arch Gen Psychiatry. 2010 Dec;67(12):1274–81. doi: 10.1001/archgenpsychiatry.2010.170. [DOI] [PubMed] [Google Scholar]
- 31.Johansson O, Lundh L, Bjärehed J. 12-Month Outcome and Predictors of Recurrence in Psychiatric Treatment of Depression: A Retrospective Study. Psychiatr Q. 2015 Sep;86(3):407–17. doi: 10.1007/s11126-015-9341-y. [DOI] [PubMed] [Google Scholar]
- 32.Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of Relapse and Recurrence in Adults with Major Depressive Disorder: Systematic Review and Meta-Analyses of Controlled Trials. Int J Neuropsychopharmacol. 2015 Jul 07;19(2) doi: 10.1093/ijnp/pyv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasarelu CR, Andersson G, Bergman NL, Dobrean A. Internet-delivered transdiagnostic and tailored cognitive behavioral therapy for anxiety and depression: a systematic review and meta-analysis of randomized controlled trials. Cogn Behav Ther. 2017 Jan;46(1):1–28. doi: 10.1080/16506073.2016.1231219. [DOI] [PubMed] [Google Scholar]
- 34.Goldin Philippe R, Lindholm Riku, Ranta Kristian, Hilgert Outi, Helteenvuori Tiia, Raevuori Anu. Feasibility of a Therapist-Supported, Mobile Phone-Delivered Online Intervention for Depression: Longitudinal Observational Study. JMIR Form Res. 2019 Jan 22;3(1):e11509. doi: 10.2196/11509. https://formative.jmir.org/2019/1/e11509/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness meditation for everyday life. New York City: Hachette Books; 1994. [Google Scholar]
- 36.Morgan D. Mindfulness-based cognitive therapy for depression: a new approach to preventing relapse. Psychother Res. 2003 Mar;13(1):123–5. doi: 10.1080/713869628. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT. Cognitive Therapy and the Emotional Disorders. London, UK: Penguin; 1979. [Google Scholar]
- 38.Jacobson NS, Martell CR, Dimidjian S. Behavioral Activation Treatment for Depression: Returning to Contextual Roots. Clinical Psychology: Science and Practice. 2001;8(3):255–270. doi: 10.1093/clipsy.8.3.255. [DOI] [Google Scholar]
- 39.Datica. https://datica.com/
- 40.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. http://europepmc.org/abstract/MED/11556941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, Falloon K, Hatcher S. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348–53. doi: 10.1370/afm.1139. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=20644190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord. 2010 Dec;127(1-3):122–9. doi: 10.1016/j.jad.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 43.Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004 Jul;81(1):61–6. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 44.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 45.Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, Herzberg PY. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008 Mar;46(3):266–74. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 46.Kroenke K, Wu J, Yu Z, Bair MJ, Kean J, Stump T, Monahan Patrick O. Patient Health Questionnaire Anxiety and Depression Scale: Initial Validation in Three Clinical Trials. Psychosom Med. 2016;78(6):716–27. doi: 10.1097/PSY.0000000000000322. http://europepmc.org/abstract/MED/27187854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 48.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008 Jun;50(3):346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 49.Baraldi AN, Enders CK. An introduction to modern missing data analyses. J Sch Psychol. 2010 Feb;48(1):5–37. doi: 10.1016/j.jsp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Ratitch B, O'Kelly M, Tosiello R. Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models. Pharm Stat. 2013;12(6):337–47. doi: 10.1002/pst.1549. [DOI] [PubMed] [Google Scholar]
- 51.Fox J, Weisberg S. An R Companion to Applied Regression. Thousand Oaks, CA: SAGE Publications; 2018. [Google Scholar]
- 52.Ratitch B, OKelly M. Pattern-Mixture Models as Linear Combinations of Least Squares Means from MMRM with Delta Method Variance Estimation. PharmaSUG 2012; May 13-16, 2012; San Francisco, CA. 2012. [Google Scholar]
- 53.Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O'Reardon JP, Lovett Margaret L, Young Paula R, Haman Kirsten L, Freeman Brent B, Gallop Robert. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005 Apr;62(4):417–22. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 54.Evans MD, Hollon SD, DeRubeis RJ, Piasecki JM, Grove WM, Garvey MJ, Tuason V B. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992 Oct;49(10):802–8. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- 55.Schlosser DA, Campellone TR, Truong B, Anguera JA, Vergani S, Vinogradov S, Arean P. The feasibility, acceptability, and outcomes of PRIME-D: A novel mobile intervention treatment for depression. Depress Anxiety. 2017 Jun;34(6):546–554. doi: 10.1002/da.22624. http://europepmc.org/abstract/MED/28419621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer B, Bierbrodt J, Schröder J, Berger T, Beevers CG, Weiss M, Jacob G, Späth C, Andersson G, Lutz W, Hautzinger M, Löwe B, Rose M, Hohagen F, Caspar F, Greiner W, Moritz S, Klein JP. Effects of an Internet intervention (Deprexis) on severe depression symptoms: Randomized controlled trial. Internet Interventions. 2015 Mar;2(1):48–59. doi: 10.1016/j.invent.2014.12.003. [DOI] [Google Scholar]
- 57.Watts S, Mackenzie A, Thomas C, Griskaitis A, Mewton L, Williams A, Andrews G. CBT for depression: a pilot RCT comparing mobile phone vs. computer. BMC Psychiatry. 2013 Feb 07;13(1):49. doi: 10.1186/1471-244X-13-49. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/1471-244X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008 Apr;69(4):621–32. doi: 10.4088/jcp.v69n0415. http://europepmc.org/abstract/MED/18363421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reger MA, Gahm GA. A meta-analysis of the effects of internet- and computer-based cognitive-behavioral treatments for anxiety. J Clin Psychol. 2009 Jan;65(1):53–75. doi: 10.1002/jclp.20536. [DOI] [PubMed] [Google Scholar]
- 60.Saddichha S, Al-Desouki M, Lamia A, Linden IA, Krausz M. Online interventions for depression and anxiety - a systematic review. Health Psychol Behav Med. 2014 Jan 01;2(1):841–881. doi: 10.1080/21642850.2014.945934. http://europepmc.org/abstract/MED/25750823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zbozinek TD, Rose RD, Wolitzky-Taylor KB, Sherbourne C, Sullivan G, Stein MB, Roy-Byrne PP, Craske MG. Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depress Anxiety. 2012 Dec;29(12):1065–71. doi: 10.1002/da.22026. http://europepmc.org/abstract/MED/23184657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon G E. Long-term prognosis of depression in primary care. Bull World Health Organ. 2000;78(4):439–45. http://europepmc.org/abstract/MED/10885162. [PMC free article] [PubMed] [Google Scholar]
- 63.Spijker J, de Graaf Ron, Bijl RV, Beekman ATF, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Br J Psychiatry. 2002 Sep;181:208–13. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- 64.Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, Frangakis C, Hogan JW, Molenberghs G, Murphy SA, Neaton JD, Rotnitzky A, Scharfstein D, Shih WJ, Siegel JP, Stern H. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012 Oct 4;367(14):1355–60. doi: 10.1056/NEJMsr1203730. http://europepmc.org/abstract/MED/23034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Hollon SD. Component studies of psychological treatments of adult depression: A systematic review and meta-analysis. Psychother Res. 2019 Dec;29(1):15–29. doi: 10.1080/10503307.2017.1395922. [DOI] [PubMed] [Google Scholar]


