Abstract
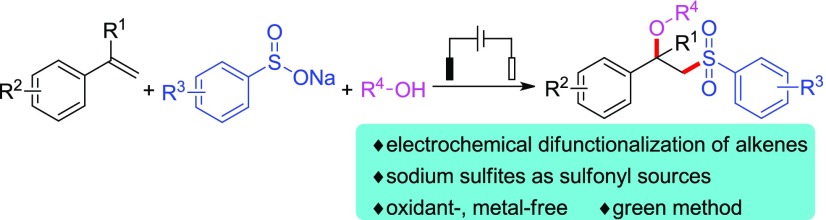
An eco-friendly method for the synthesis of β-alkoxy sulfones via electrochemical alkoxysulfonylation reaction of styrenes with sodium sulfinates as sulfonyl sources has been established. The reaction is conducted in an undivided cell at room temperature and tolerates a wide scope of styrenes, sodium sulfinates, and alcohols. The reaction does not need any chemical oxidants and transition-metal catalysts, which provides a new and green access to β-alkoxy sulfones.
Introduction
Sulfones and their derivatives belong to an important type of organic compounds,1 as well as key functional units found in variety of pharmaceuticals, materials, and natural products. Furthermore, they are also very useful building blocks in organic transformations.2 At the same time, the introduction of additional groups, such as amino, keto, hydroxyl, and alkoxyl, along with sulfonyl group into a molecule usually leads to compounds bearing good bioactivities.3 Therefore, development of the related efficient synthetic methodologies has gained many interests in this research area.4 Among them, difunctionalization of alkenes turned out to be an efficient synthetic strategy, as an additional functional group along with sulfonyl group could be introduced at the same time in one reaction.5 However, the traditional difunctionalizaton reactions usually needed chemical oxidants, transition-metal catalysts and even additives. Thus, the development of green difunctionalizaton reactions becomes very urgent.
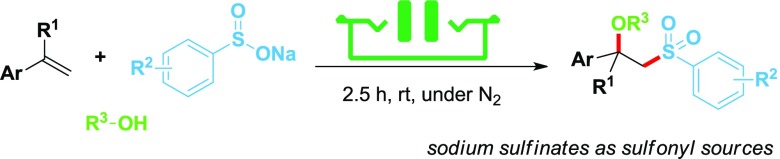
Currently, electrochemical difunctionalization6 of alkenes has become one of the hottest research areas in organic synthetic chemistry. It has been proved to be a powerful and green tool for the construction of complex organic compounds, as no chemical oxidant is needed, thus avoiding generation of reagent wastes and use of harsh reaction conditions.7 All these promising features result in many unprecedented developments on the difunctionalization of the C–C unsaturated bond to be possible for the synthesis of polyfunctionalized molecules in a very simple manner. In the past years, Moeller,8 Ackermann,9 Lei,10 Lin,11 Xu,12 and other groups13 have independently reported their elegant works in this area, such as electrochemical difunctionalization of alkenes, intermolecular annulation, and intramolecular cyclization. Recently, Lei’s group reported an electrochemical oxidative difunctionalization of alkenes for the synthesis of β-alkoxy sulfones with sulfonyl hydrazides as the sulfonyl sources.14 Very recently, the Sun group developed sulfinic acids as sulfonyl precursors for the electrochemical alkoxysulfonylation reaction of alkenes.15 However, only two examples were presented in this reaction with moderate chemical yields. Considering sodium sulfinate16 as an available, easy-to-handle and stable sulfonyl source compared with sulfonyl azide, sulfonyl hydrazide, sulfonyl cyanide, sulfonyl halide, and sulfinic acid,16,17 we envision that sodium sulfinate can be used as the substrate for the synthesis of β-alkoxy sulfones via electrochemical difunctionalization reaction. Thus, we herein reported an electrochemical procedure to synthesize the β-alkoxy sulfones with sodium sulfinates as sulfonyl precursors via alkoxysulfonylation reaction with aryl alkenes and alcohols (Scheme 1).
Scheme 1. Electrochemical Alkoxysulfonylation of Styrenes, Sodium Sulfinates, and Alcohols.
Results and Discussion
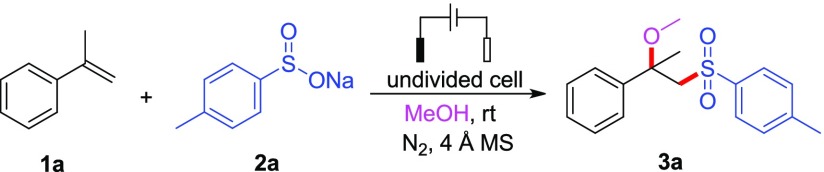
We carried out our initial studies on this electrochemical difunctionalization reaction using α-methyl styrene 1a, sodium 4-methylbenzenesulfinate 2a, and methanol as model substrates (Table 1). When the reaction was conducted in an undivided cell at a constant current of 10 mA with a carbon anode and a carbon plate cathode using LiClO4 as an electrolyte, the desired alkoxysulfonylation product 3a was obtained in 60% yield after 3 h at room temperature under a nitrogen atmosphere (entry 1). The addition of 4 Å MS was essential for this system, as a lower yield was observed when the reaction was conducted under air without using 4 Å MS (52%, entry 2), and some α-methyl styrene remained. To improve the reaction outcome, we tried to add some additives into the system; the results of entries 3 and 4 indicated that p-toluenesulfonic acid was a good choice leading to an increased yield (77%). Variations of reaction time and electric current showed no obvious effects on the reaction (entries 5–7); however, prolonging the reaction time to 4.5 h at 10 mA resulted in a slightly lower yield (entry 5). Further screening of the electrode was carried out, which was found to show a significant influence on the reaction (entries 10–13). Changing the carbon plate cathode into Pt, Ni, Cu, or Fe resulted in noticeably decreased yields (31–66%). Surprisingly, further exploration of electrolyte indicated that this reaction could proceed smoothly and result in the highest yield without the addition of additional electrolyte (82%, entry 15). It worth mentioning that TsOH played a crucial role in this transformation. In the absence of TsOH, a significant decline of yield was observed (53%, entry 16). It is mainly because of the in situ generation of sulfinic acid,15 which makes the reduction of proton easier at the cathode to release hydrogen gas. Changing the loading amount of sodium 4-methylbenzenesulfinate 2a from 2.0 to 1.5 or 3.0 equiv did not provide any improvement in yield (entries 17 and 18). Finally, the control experiment was performed without electricity, and no conversion occurred with all the starting materials remaining (entry 19).
Table 1. Optimization of the Reaction Conditionsa.
| entry | electrode | electrolyte | current (mA) | additive | time (h) | yield (%)b |
|---|---|---|---|---|---|---|
| 1 | C/C | LiClO4 | 10 | 3 | 60 | |
| 2c | C/C | LiClO4 | 10 | 3 | 52 | |
| 3 | C/C | LiClO4 | 10 | AcOH | 3 | 43 |
| 4 | C/C | LiClO4 | 10 | TsOH | 3 | 77 |
| 5 | C/C | LiClO4 | 10 | TsOH | 4.5 | 72 |
| 6 | C/C | LiClO4 | 10 | TsOH | 2.5 | 79 |
| 7 | C/C | LiClO4 | 10 | TsOH | 2 | 74 |
| 8 | C/C | LiClO4 | 5 | TsOH | 4.5 | 78 |
| 9 | C/C | LiClO4 | 15 | TsOH | 2 | 74 |
| 10 | C/Pt | LiClO4 | 10 | TsOH | 2.5 | 66 |
| 11 | C/Ni | LiClO4 | 10 | TsOH | 2.5 | 51 |
| 12 | C/Cu | LiClO4 | 10 | TsOH | 2.5 | 32 |
| 13 | C/Fe | LiClO4 | 10 | TsOH | 2.5 | 31 |
| 14 | C/C | Bu4NBF4 | 10 | TsOH | 2.5 | 63 |
| 15 | C/C | 10 | TsOH | 2.5 | 82 | |
| 16 | C/C | 10 | 2.5 | 53 | ||
| 17d | C/C | 10 | TsOH | 2.5 | 67 | |
| 18e | C/C | 10 | TsOH | 2.5 | 68 | |
| 19f | C/C | 10 | TsOH | 2.5 | 0 |
Reaction conditions: 1a (0.5 mmol), 2a (2.0 equiv), MeOH (8 mL), electrolyte (0.5 mmol), additive (0.5 mmol), 4 Å MS (200 mg), undivided cell, room temperature, and nitrogen atmosphere.
Yields based on 1a.
Under air without 4 Å MS.
2a (1.5 equiv).
2a (3.0 equiv).
Without electric current.
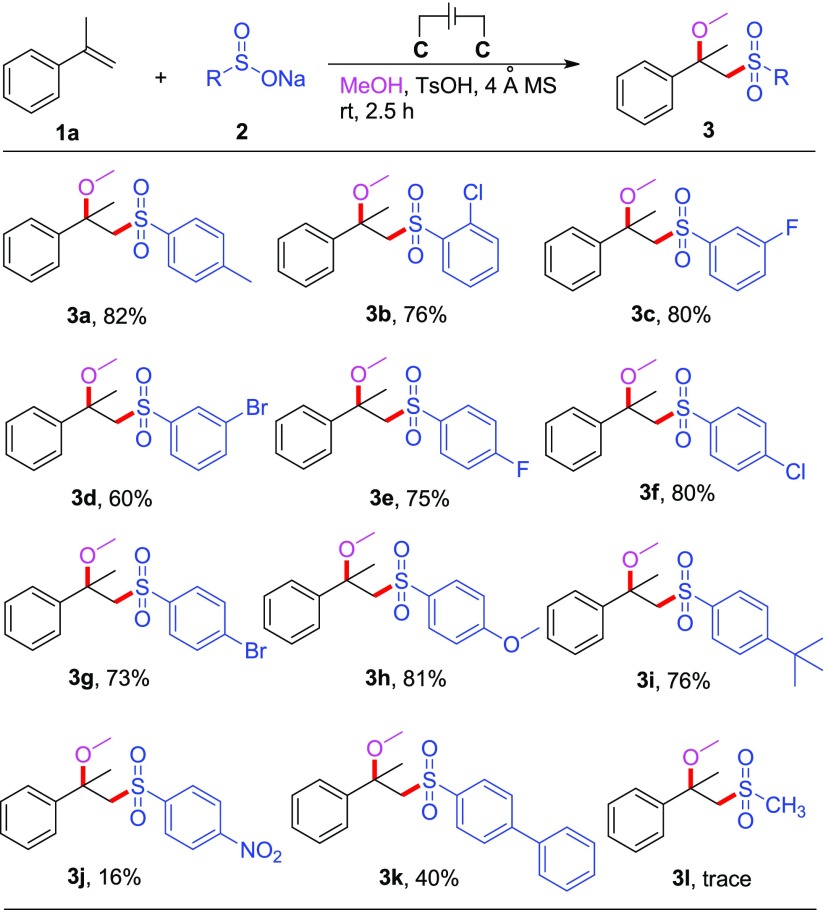
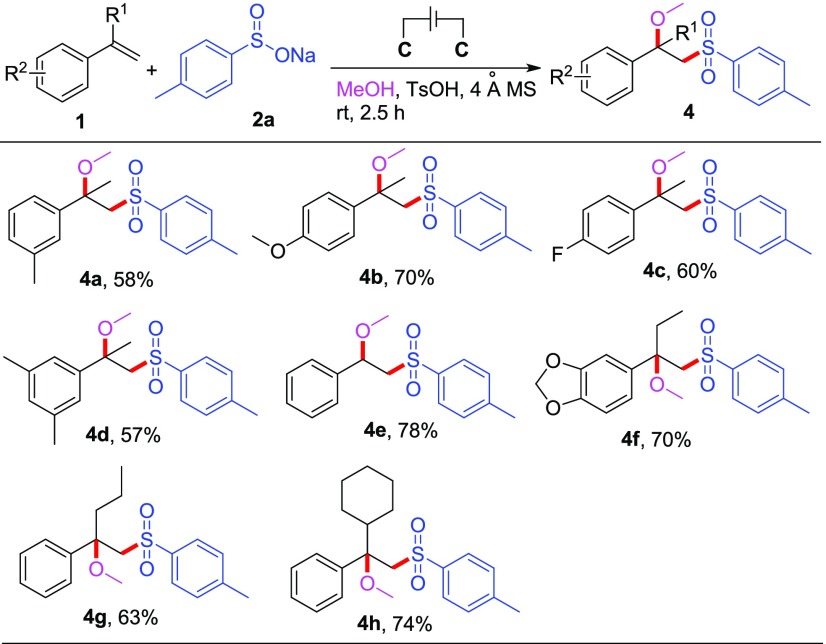
Under optimized reaction conditions, we turned our attention to investigate the substrate structural generalities of this electrochemical alkoxysulfonylation system. First, we carried out the reactions of α-methyl styrene 1a in methanol using varieties of sodium sulfinates, and the results are shown in Scheme 2. All the examined arylsulfinates worked very well in this reaction, providing the target products 3a–i in good yields (60–82%). In particular, sodium arylsulfinates bearing strong electron-donating (methoxyl, 3h) and strong electron-withdrawing (fluoro, 3c, e) substituents on the aromatic ring were all tolerated. The position of the substituents on phenyl had almost no influence on this transformation, as the ortho- and para-substituted sulfinates provided a similar outcome (76 and 80% for 3b and 3f, respectively). The reaction of the sodium arylsulfinates with nitro on the para-position was complex, and the corresponding product was isolated with poor yield (16%, 3j), with almost all the olefin starting material consumed. This is mainly because the nitro group might change the basicity of the sulfinate and influence its equilibrium with the TsOH present. One aliphatic sodium sulfinate 2l was also tried in this reaction; unfortunately, only a trace of the desired 3l was observed.
Scheme 2. Substrate Scope Study of Substituted Sodium Sulfinates,
Reaction conditions: 1a (0.5 mmol), 2 (1.0 mmol), MeOH (8 mL), TsOH (0.5 mmol), 4 Å MS (200 mg), and constant current = 10 mA, in undivided cell at room temperature under nitrogen atmosphere for 2.5 h.
Yields.
Subsequently, a variety of styrene derivatives were investigated under optimized reaction conditions. The reactions of the alkenes bearing different substituents on the aromatic ring proceeded smoothly, giving rise to the corresponding product in 58–78% yields (Scheme 3). For example, α-methyl styrene bearing 4-methyl, 4-methoxyl, 4-fluoro, and 3,5-dimethyl groups on a phenyl ring worked very well to afford 4a–d in good yields. It is worth mentioning that styrene 1e was also a suitable substrate for this reaction and was converted into the corresponding product 4e in 78% chemical yield. Then, a series of different α-substituted styrene derivatives were employed in this reaction for the investigation of steric hindrance effect. Replacing the α-substituted group from methyl by ethyl (1f) or propyl (1g), no obvious effect was found and the desired products were obtained in the yields of 70 and 63%, respectively. Even the cyclohexyl-substituted styrene (1h) could well participate in this reaction, affording the expected product 4h in 74% yield.
Scheme 3. Substrate Scope Study of Substituted Styrenes,
Reaction conditions: 1 (0.5 mmol), 2a (1.0 mmol), MeOH (8 mL), TsOH (0.5 mmol), 4 Å MS (200 mg), constant current = 10 mA, 2.5 h, undivided cell, and at room temperature under a nitrogen atmosphere.
Yields.
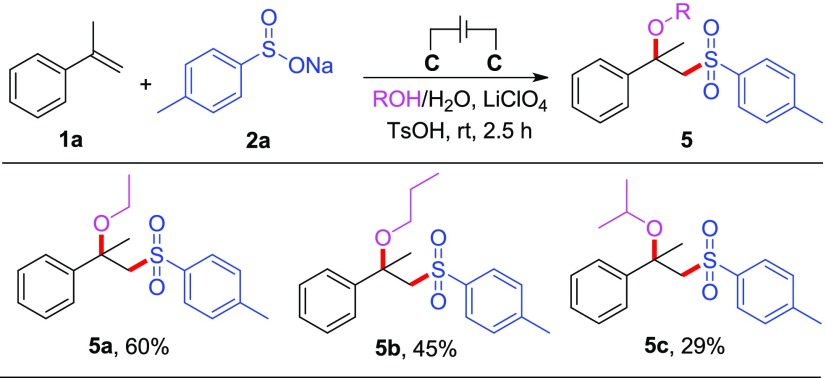
As the final objective of the substrate scope study, we tried to extend methanol to other aliphatic alcohols for this reaction (Scheme 4). Owing to the very low solubility of sodium 4-methylbenzenesulfinate 2a in these aliphatic alcohols, water was used as a co-solvent in these reactions. We were pleased to find that the reactions of ethanol and propanol could proceed smoothly to afford the corresponding alkoxysulfonylation products (5a–b) in 60 and 45% yields, respectively. Unfortunately, the reaction with isopropanol only provided 29% yield of the desired product (5c), and the competing reaction, hydroxysulfonylation, with water as a coupling partner was observed.
Scheme 4. Substrate Scope Study of Different Alcohols,
Reaction conditions: 1a (0.5 mmol), 2a (1.0 mmol), alcohol (8 mL), H2O (1 mL), TsOH (0.5 mmol), LiClO4 (1.5 mmol), constant current = 10 mA, in undivided cell at room temperature under anitrogen atmosphere for 2.5 h.
Yields.
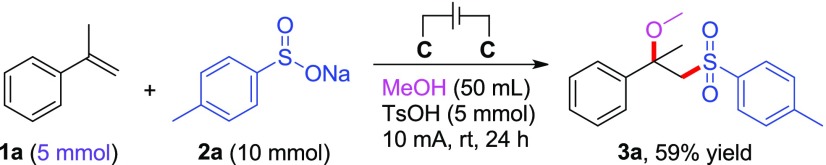
To demonstrate the practical application of this electrochemical difunctinalization system, we then examined the gram-scale preparation about this electrochemical reaction (Scheme 5). The reaction was carried out using 5 mmol of α-methyl styrene 1a under the standard reaction conditions. The transformation proceeded smoothly to afford the target compound 3a with 59% yield after 24 h. The result underscores that the current electrochemical system is a practical and eco-friendly way for the synthesis of functionalized β-alkoxysulfones.
Scheme 5. Large-Scale Synthesis.
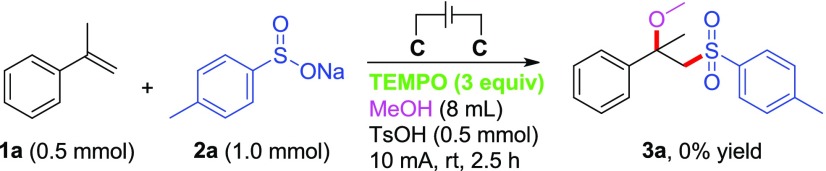
Finally, a control experiment was performed to get an insight into the mechanism of this transformation (Scheme 6). After adding a radical inhibitor TEMPO (3 equiv) to the reaction under optimized reaction conditions, the reaction was totally inhibited and no desired product 3a was obtained at all, with almost all the starting material α-methyl styrene 1a remaining. This result clearly indicates that a radical pathway may be involved in the electrochemical transformation.
Scheme 6. Control Experiment.
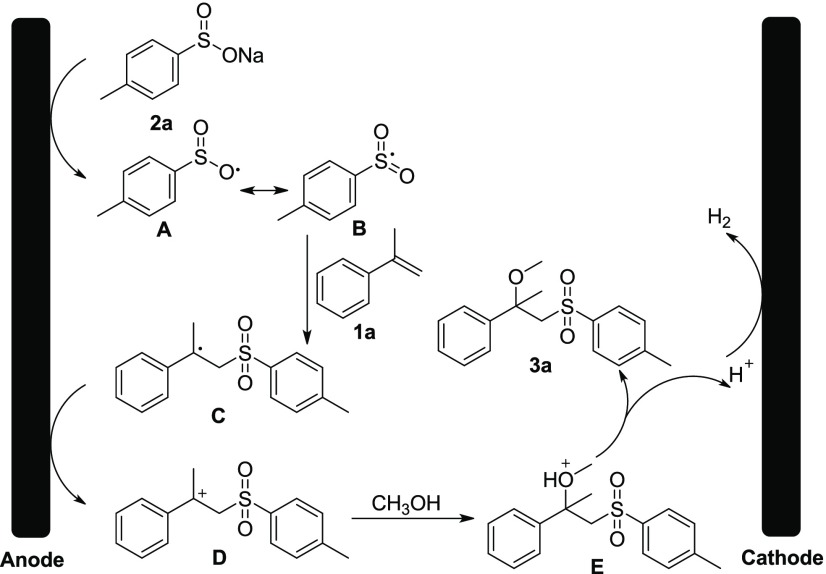
According to the above experimental results and the previous literature reports,13b,14,15 a plausible mechanism was proposed in Scheme 7 for the electrochemical transformation. Initially, sodium 4-methylbenzenesulfinate 2a is oxidized at the graphite anode to generate radical A, which easily tautomerizes to sulfonyl radical B.13b Then, sulfonyl radical B adds to the C–C double bond of α-methyl styrene 1a to afford the alkyl radical C. Subsequently, anodic oxidation of radical intermediate C generates alkyl cation D, which undergoes a nucleophilic attack reaction by methanol to produce intermediate E.13a Finally, deprotonation of E provides the target product 3a, as well as the release of H2 at the cathode.
Scheme 7. Proposed Mechanism.
Conclusions
In summary, we developed an efficient electrochemical oxidative alkoxysulfonylation of aryl alkenes with alcohols and sodium sulfinates. This environmentally benign alkoxysulfonylation of alkenes used sodium sulfinate as a new sulfonyl precursor and could easily afford a series of β-alkoxy sulfones in good yields without the use of any metal catalysts or chemical oxidants. This reaction enriches the contents of electrochemical difunctionalization of olefins and provides a new way for the synthesis of β-alkoxy sulfones.
Experimental Section
Reaction of α-Methyl Styrene with Methanol and Various Sodium Sulfinates
An undivided cell was equipped with a graphite anode and a graphite cathode and connected to a DC power supply. Into the cell flushed with nitrogen were taken α-methyl styrene 1a (0.5 mmol, 59 mg), sodium sulfinate 2 (1.0 mmol), TsOH (0.5 mmol, 86 mg), 4 Å MS (200 mg), and MeOH (8 mL). The mixture was electrolyzed under constant current (10 mA) at room temperature for 2.5 h. Then, the reaction was diluted with H2O (20 mL) and extracted with EtOAc (20 mL × 3). The combined organic layers were dried with anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography using hexane/EtOAc (5:1, v/v) as an eluent to afford the desired product 3.
1-((2-Methoxy-2-phenylpropyl)sulfonyl)-4-methylbenzene (3a)14
White solid, 82% yield, mp 94–95 °C. 1H NMR (600 MHz, CDCl3): δ = 7.65 (d, J = 8.2 Hz, 2H), 7.31–7.27 (m, 4H), 7.26–7.23 (m, 3H), 3.63 (d, J = 14.7 Hz, 1H), 3.49 (d, J = 14.6 Hz, 1H), 2.98 (s, 3H), 2.42 (s, 3H), 1.98 (s, 3H).
1-Chloro-2-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3b)
White solid, 76% yield, mp 113–115 °C. 1H NMR (600 MHz, CDCl3): δ = 7.84–7.82 (m, 1H), 7.47–7.43 (m, 2H), 7.32–7.29 (m, 3H), 7.24–7.22 (m, 2H), 7.20–7.17 (m, 1H), 3.97 (d, J = 14.9 Hz, 1H), 3.86 (d, J = 14.9 Hz, 1H), 2.91 (s, 3H), 1.95 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 141.5, 138.4, 134.0, 132.3, 131.4, 131.2, 128.4, 128.0, 127.1, 126.3, 77.2, 65.2, 49.9, 21.5. HRMS (ESI): calculated for C16H18ClO3S+ [M + H]+ 325.0660, found 325.0656.
1-Fluoro-3-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3c)
White solid, 80% yield, mp 81–82 °C. 1H NMR (600 MHz, CDCl3): δ = 7.56–7.55 (m, 1H), 7.46–7.42 (m, 1H), 7.41–7.39 (m, 1H), 7.29–7.24 (m, 6H), 3.66 (d, J = 14.8 Hz, 1H), 3.54 (d, J = 14.8 Hz, 1H), 2.97 (s, 3H), 1.98 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 162.9 (d, J = 249.8 Hz), 143.2 (d, J = 6.5 Hz), 141.7, 130.6 (d, J = 7.5 Hz), 128.5, 128.0, 126.3, 123.6 (d, J = 3.3 Hz), 120.3 (d, J = 21.1 Hz), 115.4 (d, J = 24.3 Hz), 77.3, 67.7, 50.0, 21.3. 19F NMR (565 MHz, CDCl3): δ = −110.2. HRMS (ESI): calculated for C16H18FO3S+ [M + H]+ 309.0955, found 309.0957.
1-Bromo-3-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3d)
White solid, 60% yield, mp 95–97 °C. 1H NMR (600 MHz, CDCl3): δ = 7.80 (s, 1H), 7.69–7.66 (m, 2H), 7.33–7.31 (m, 1H), 7.28–7.25 (m, 5H), 3.65 (d, J = 14.9 Hz, 1H), 3.55 (d, J = 14.9 Hz, 1H), 2.98 (s, 3H), 1.98 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 142.9, 141.4, 136.0, 131.0, 130.2, 128.4, 128.2, 126.4, 126.3, 122.7, 77.3, 67.8, 50.0, 21.3. HRMS (ESI): calculated for C16H18BrO3S+ [M + H]+ 369.0155, found 369.00151.
1-Fluoro-4-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3e)
White solid, 75% yield, mp 58–60 °C. 1H NMR (600 MHz, CDCl3): δ = 7.76–7.73 (m, 2H), 7.28–7.25 (m, 5H), 7.12–7.09 (m, 2H), 3.65 (d, J = 14.9 Hz, 1H), 3.52 (d, J = 14.8 Hz, 1H), 2.96 (s, 3H), 1.97 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 166.2 (d, J = 253.8 Hz), 141.9, 137.3 (d, J = 3.2 Hz), 130.8 (d, J = 9.5 Hz), 128.5, 127.9, 126.3, 116.0 (d, J = 7.5 Hz), 77.4, 67.8, 50.0, 21.4. 19F NMR (565 MHz, CDCl3): δ = −104.7. HRMS (ESI): calculated for C16H18FO3S+ [M + H]+ 309.0955, found 309.0952.
1-Chloro-4-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3f)
White solid, 80% yield, mp 94–95 °C. 1H NMR (600 MHz, CDCl3): δ = 7.67–7.65 (m, 2H), 7.41–7.39 (m, 2H), 7.28–7.25 (m, 5H), 3.65 (d, J = 14.9 Hz, 1H), 3.52 (d, J = 14.8 Hz, 1H), 2.95 (s, 3H), 1.96 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 141.8, 139.7, 139.6, 129.4, 129.0, 128.5, 127.9, 126.3, 77.3, 67.7, 50.0, 21.3. HRMS (ESI): calculated for C16H18ClO3S+ [M + H]+ 325.0660, found 325.0657.
1-Bromo-4-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3g)
White solid, 73% yield, mp 111–113 °C. 1H NMR (600 MHz, CDCl3): δ = 7.60–7.56 (m, 4H), 7.30–7.26 (m, 5H), 3.65 (d, J = 14.8 Hz, 1H), 3.52 (d, J = 14.9 Hz, 1H), 2.96 (s, 3H), 1.97 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 141.8, 140.2, 132.0, 129.5, 128.5, 128.3, 127.9, 126.3, 77.3, 67.8, 50.0, 21.3. HRMS (ESI): calculated for C16H18BrO3S+ [M + H]+ 369.0155, found 369.0155.
1-Methoxy-4-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3h)
White solid, 81% yield, mp 83–85 °C. 1H NMR (600 MHz, CDCl3): δ = 7.69 (d, J = 8.9 Hz, 2H), 7.31–7.24 (m, 5H), 6.91 (d, J = 8.9 Hz, 2H), 3.87 (s, 3H), 3.63 (d, J = 14.7 Hz, 1H), 3.50 (d, J = 14.6 Hz, 1H), 2.98 (s, 3H), 1.97 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 163.2, 142.3, 133.0, 130.1, 128.4, 127.8, 126.3, 114.0, 77.5, 67.7, 55.6, 50.1, 21.5. HRMS (ESI): calculated for C17H20NaO4S+ [M + Na]+ 343.0975, found 343.0971.
1-(tert-Butyl)-4-((2-methoxy-2-phenylpropyl)sulfonyl)benzene (3i)
White solid, 76% yield, mp 103–104 °C. 1H NMR (600 MHz, CDCl3): δ = 7.64 (d, J = 8.5 Hz, 2H), 7.43 (d, J = 8.6 Hz, 2H), 7.30–7.21 (m, 5H), 3.64 (d, J = 14.6 Hz, 1H), 3.53 (d, J = 14.6 Hz, 1H), 2.98 (s, 3H), 1.99 (s, 3H), 1.34 (s, 9H). 13C{1H}NMR (150 MHz, CDCl3): δ = 156.8, 142.0, 138.1, 128.4, 127.8, 127.6, 126.3, 125.8, 77.5, 67.4, 50.0, 35.1, 31.1, 21.5. HRMS (ESI): calculated for C20H27O3S+ [M + H]+ 347.1675, found 347.1672.
1-((2-Methoxy-2-phenylpropyl)sulfonyl)-4-nitrobenzene (3j)
White solid, 16% yield, mp 123–125 °C. 1H NMR (600 MHz, CDCl3): δ = 8.28 (d, J = 8.8 Hz, 2H), 7.92 (d, J = 8.8 Hz, 2H), 7.27–7.24 (m, 5H), 3.71 (d, J = 15.1 Hz, 1H), 3.58 (d, J = 15.1 Hz, 1H), 2.93 (s, 3H), 1.98 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 150.2, 146.8, 141.5, 129.4, 128.6, 128.2, 126.2, 123.7, 77.2, 68.0, 49.9, 21.1. HRMS (ESI): calculated for C16H17NNaO5S+ [M + Na]+ 358.0720, found 358.0725.
4-((2-Methoxy-2-phenylpropyl)sulfonyl)-1,1′-biphenyl (3k)
White solid, 40% yield, mp 101–103 °C. 1H NMR (600 MHz, CDCl3): δ = 7.80–7.78 (m, 2H), 7.64–7.60 (m, 4H), 7.52–7.50 (m, 2H), 7.46–7.44 (m, 1H), 7.32–7.30 (m, 2H), 7.28–7.24 (m, 3H), 3.70 (d, J = 14.8 Hz, 1H), 3.58 (d, J = 14.8 Hz, 1H), 2.99 (s, 3H), 2.01 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 145.9, 142.0, 139.7, 139.4, 129.0, 128.5, 128.4, 128.3, 127.8, 127.4, 127.3, 126.3, 77.5, 67.7, 50.0, 21.5. HRMS (ESI): calculated for C22H23O3S+ [M + H]+ 367.1362, found 367.1355.
Reaction of Styrene Derivatives with Sodium 4-Methylbenzenesulfinate and Methanol
An undivided cell was equipped with a graphite anode and a graphite cathode and connected to a DC power supply. Into the cell flushed with nitrogen were taken styrene derivatives 1 (0.5 mmol), sodium 4-methylbenzenesulfinate 2a (1.0 mmol, 178 mg), TsOH (0.5 mmol, 86 mg), 4 Å MS (200 mg), and MeOH (8 mL). The mixture was electrolyzed under constant current (10 mA) at room temperature for 2.5 h. Then, the reaction was diluted with H2O (20 mL) and extracted with EtOAc (20 mL × 3). The combined organic layers were dried with anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography using hexane/EtOAc (5:1, v/v) as an eluent to afford the desired product 4.
1-(2-Methoxy-1-tosylpropan-2-yl)-3-methylbenzene (4a)
Colorless oil, 58% yield. 1H NMR (600 MHz, CDCl3): δ = 7.62 (d, J = 8.3 Hz, 2H), 7.23 (d, J = 8.4 Hz, 2H), 7.18–7.15 (m, 1H), 7.11 (d, J = 7.9 Hz, 1H), 7.05–7.03 (m, 2H), 3.63 (d, J = 14.7 Hz, 1H), 3.50 (d, J = 14.7 Hz, 1H), 2.97 (s, 3H), 2.42 (s, 3H), 2.28 (s, 3H), 1.96 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 143.8, 142.0, 138.3, 138.0, 129.3, 128.5, 128.3, 127.9, 126.9, 123.4, 77.4, 67.5, 50.0, 21.6, 21.5, 21.4. HRMS (ESI): calculated for C18H23O3S+ [M + H]+ 319.1362, found 319.1362.
1-Methoxy-4-(2-methoxy-1-tosylpropan-2-yl)benzene (4b)
White solid, 70% yield, mp 85–87 °C. 1H NMR (600 MHz, CDCl3): δ = 7.61 (d, J = 8.3 Hz, 2H), 7.23–7.21 (m, 2H), 7.20–7.18 (m, 2H), 6.78–6.76 (m, 2H), 3.80 (s, 3H), 3.62 (d, J = 14.7 Hz, 1H), 3.50 (d, J = 14.7 Hz, 1H), 2.94 (s, 3H), 2.42 (s, 3H), 1.95 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 159.2, 143.7, 138.4, 133.8, 129.3, 127.9, 127.7, 113.6, 77.1, 67.7, 55.2, 49.7, 21.5, 21.4. HRMS (ESI): calculated for C18H22NaO4S+ [M + Na]+ 357.1131, found 357.1128.
1-Fluoro-4-(2-methoxy-1-tosylpropan-2-yl)benzene (4c)
White solid, 60% yield, mp 101–103 °C. 1H NMR (600 MHz, CDCl3): δ = 7.60 (d, J = 8.2 Hz, 2H), 7.27–7.23 (m, 4H), 6.95–6.92 (m, 2H), 3.61 (d, J = 14.7 Hz, 1H), 3.52 (d, J = 14.7 Hz, 1H), 2.97 (s, 3H), 2.43 (s, 3H), 1.95 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 163.1 (d, J = 245.6 Hz), 144.0, 138.2, 137.7 (d, J = 3.3 Hz), 129.4, 128.3 (d, J = 8.0 Hz), 127.8, 115.2 (d, J = 21.2 Hz), 77.0, 67.6, 49.8, 21.6, 21.5. 19F NMR (565 MHz, CDCl3): δ = −114.6. HRMS (ESI): calculated for C17H20FO3S+ [M + H]+ 323.1112, found 323.1108.
1-(2-Methoxy-1-tosylpropan-2-yl)-3,5-dimethylbenzene (4d)
White solid, 57% yield, mp 119–121 °C. 1H NMR (600 MHz, CDCl3): δ = 7.60 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 8.3 Hz, 2H), 6.85 (s, 2H), 6.83 (s, 1H), 3.62 (d, J = 14.7 Hz, 1H), 3.50 (d, J = 14.8 Hz, 1H), 2.97 (s, 3H), 2.41 (s, 3H), 2.24 (s, 6H), 1.94 (s, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 143.7, 141.8, 138.2, 137.8, 129.3, 129.2, 127.9, 124.1, 77.4, 67.5, 50.0, 21.5, 21.4, 21.3. HRMS (ESI): calculated for C19H25O3S+ [M + H]+ 333.1519, found 333.1515.
1-((2-Methoxy-2-phenylethyl)sulfonyl)-4-methylbenzene (4e)14
White solid, 78% yield, mp 104–106 °C. 1H NMR (600 MHz, CDCl3): δ = 7.83 (d, J = 8.3 Hz, 2H), 7.36–7.33 (m, 4H), 7.32–7.29 (m, 1H), 7.28–7.26 (m, 2H), 4.77 (dd, J = 2.8, 9.4 Hz, 1H), 3.66 (dd, J = 9.5, 14.8 Hz, 1H), 3.30 (dd, J = 2.8, 14.7 Hz, 1H), 3.13 (s, 3H), 2.45 (s, 3H).
5-(2-Methoxy-1-tosylbutan-2-yl)benzo[d][1,3]dioxole (4f)
White solid, 70% yield, mp 114–116 °C. 1H NMR (600 MHz, CDCl3): δ = 7.52 (d, J = 8.1 Hz, 2H), 7.19 (d, J = 8.0 Hz, 2H), 6.73–6.71 (m, 1H), 6.66–6.64 (m, 2H), 5.93–5.90 (m, 2H), 3.75 (d, J = 15.0 Hz, 1H), 3.65 (d, J = 15.0 Hz, 1H), 2.93 (s, 3H), 2.41 (s, 3H), 2.39–2.34 (m, 1H), 2.12–2.06 (m, 1H), 0.91 (t, J = 7.3 Hz, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 147.6, 146.8, 143.8, 137.7, 134.8, 129.2, 127.9, 120.4, 107.6, 107.3, 101.0, 79.0, 61.6, 49.2, 27.0, 21.5, 7.3. HRMS (ESI): calculated for C19H22NaO5S+ [M + Na]+ 385.1080, found 385.1076.
1-((2-Methoxy-2-phenylpentyl)sulfonyl)-4-methylbenzene (4g)
White solid, 63% yield, mp 56–58 °C. 1H NMR (600 MHz, CDCl3): δ = 7.56 (d, J = 8.2 Hz, 2H), 7.25–7.20 (m, 5H), 7.18 (d, J = 7.9 Hz, 2H), 3.79 (d, J = 14.9 Hz, 1H), 3.74 (d, J = 14.9 Hz, 1H), 2.94 (s, 3H), 2.40 (s, 3H), 2.30–2.25 (m, 1H), 2.11–2.06 (m, 1H), 1.29–1.24 (m, 2H), 0.94 (t, J = 7.3 Hz, 3H). 13C{1H}NMR NMR (150 MHz, CDCl3): δ = 143.9, 141.2, 138.0, 129.4, 128.1, 127.9, 127.3, 126.5, 79.3, 61.7, 49.6, 37.6, 21.5, 16.4, 14.2. HRMS (ESI): calculated for C19H25O3S+ [M + H]+ 333.1519, found 333.1520.
1-((2-Cyclohexyl-2-methoxy-2-phenylethyl)sulfonyl)-4-methylbenzene (4h)
White solid, 74% yield, mp 120–121 °C. 1H NMR (600 MHz, CDCl3): δ = 7.78 (d, J = 8.2 Hz, 2H), 7.28–7.23 (m, 5H), 7.14–7.12 (m, 2H), 4.03 (d, J = 15.1 Hz, 1H), 3.81 (d, J = 15.1 Hz, 1H), 3.20 (s, 3H), 2.44 (s, 3H), 2.33–2.29 (m, 1H), 2.01 (d, J = 12.3 Hz, 1H), 1.73 (d, J = 12.1 Hz, 1H), 1.68–1.66 (m, 2H), 1.57 (d, J = 12.9 Hz, 1H), 1.29–1.19 (m, 2H), 0.89–0.82 (m, 1H), 0.71–0.64 (m, 1H), 0.45–0.38 (m, 1H). 13C{1H}NMR (150 MHz, CDCl3): δ = 144.3, 138.5, 129.5, 128.2, 127.4, 127.2, 127.0, 126.0, 83.2, 57.8, 50.9, 44.8, 28.7, 26.5, 26.4, 26.2, 26.1, 21.6. HRMS (ESI): calculated for C22H28NaO3S+ [M + Na]+ 395.1651, found 395.1653.
Reaction of α-Methyl Styrene with Sodium 4-Methylbenzenesulfinate and Different Alcohols
An undivided cell was equipped with a graphite anode and a graphite cathode and connected to a DC power supply. Into the cell flushed with nitrogen were taken α-methyl styrene 1a (0.5 mmol), sodium 4-methylbenzenesulfinate 2a (1.0 mmol, 178 mg), TsOH (0.5 mmol, 86 mg), LiClO4 (1.5 mmol), alcohol (8 mL), and H2O (1 mL). The mixture was electrolyzed under constant current (10 mA) at room temperature for 2.5 h. Then, the reaction was diluted with H2O (20 mL) and extracted with EtOAc (20 mL × 3). The combined organic layers were dried with anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography using hexane/EtOAc (5:1, v/v) as an eluent to afford the desired product 5.
1-((2-Ethoxy-2-phenylpropyl)sulfonyl)-4-methylbenzene (5a)
White solid, 60% yield, mp 40–41 °C. 1H NMR (600 MHz, CDCl3): δ = 7.67 (d, J = 8.2 Hz, 2H), 7.31–7.27 (m, 4H), 7.26–7.23 (m, 3H), 3.65 (d, J = 14.8 Hz, 1H), 3.49 (d, J = 14.8 Hz, 1H), 3.27–3.22 (m, 1H), 2.98–2.93 (m, 1H), 2.43 (s, 3H), 1.96 (s, 3H), 0.93 (t, J = 7.0 Hz, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 143.8, 143.0, 138.5, 129.3, 128.4, 128.0, 127.7, 126.1, 77.0, 68.0, 57.6, 21.9, 21.5, 15.1. HRMS (ESI): calculated for C18H23O3S+ [M + H]+ 319.1362, found 319.1359.
1-Methyl-4-((2-phenyl-2-propoxypropyl)sulfonyl)benzene (5b)
Colorless oil, 45% yield. 1H NMR (600 MHz, CDCl3): δ = 7.66 (d, J = 8.3 Hz, 2H), 7.31–7.23 (m, 7H), 3.67 (d, J = 14.8 Hz, 1H), 3.49 (d, J = 14.8 Hz, 1H), 3.15–3.12 (m, 1H), 2.86–2.82 (m, 1H), 2.43 (s, 3H), 1.97 (s, 3H), 1.34–1.31 (m, 2H), 0.75 (t, J = 7.4 Hz, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 143.8, 142.8, 138.5, 129.3, 128.3, 128.0, 127.7, 126.2, 76.8, 68.0, 63.7, 23.0, 21.8, 21.5, 10.5. HRMS (ESI): calculated for C19H25O3S+ [M + H]+ 333.1519, found 333.1520.
1-((2-Isopropoxy-2-phenylpropyl)sulfonyl)-4-methylbenzene (5c)
White solid, 29% yield, mp 87–88 °C. 1H NMR (600 MHz, CDCl3): δ = 7.61 (d, J = 8.3 Hz, 2H), 7.35–7.33 (m, 2H), 7.26–7.22 (m, 5H), 3.69 (d, J = 14.8 Hz, 1H), 3.48 (d, J = 14.8 Hz, 1H), 3.43 (hept, J = 6.1 Hz, 1H), 2.43 (s, 3H), 2.02 (s, 3H), 1.04 (d, J = 6.0 Hz, 3H), 0.77 (d, J = 6.1 Hz, 3H). 13C{1H}NMR (150 MHz, CDCl3): δ = 143.6, 143.0, 138.5, 129.2, 128.0, 127.9, 127.8, 126.9, 77.2, 68.6, 65.5, 24.7, 24.1, 22.3, 21.5. HRMS (ESI): calculated for C19H25O3S+ [M + H]+ 333.1519, found 333.1518.
Large-Scale Synthesis
An undivided cell was equipped with a graphite anode and a graphite cathode and connected to a DC power supply. Into the cell flushed with nitrogen were taken α-methyl styrene 1a (5 mmol), sodium 4-methylbenzenesulfinate 2a (10 mmol), TsOH (5 mmol), 4 Å MS (1 g), and MeOH (50 mL). The mixture was electrolyzed under constant current (10 mA) at room temperature for 24 h. Then, the reaction was diluted with H2O (100 mL) and extracted with EtOAc (50 mL × 3). The combined organic layers were dried with anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography using hexane/EtOAc (5:1, v/v) as an eluent to afford the desired product 3a.
Control Experiment
An undivided cell was equipped with a graphite anode and a graphite cathode and connected to a DC power supply. Into the cell flushed with nitrogen were taken α-methyl styrene 1a (0.5 mmol), sodium 4-methylbenzenesulfinate 2a (1.0 mmol), TsOH (0.5 mmol), TEMPO (1.5 mmol), 4 Å MS (200 mg), and MeOH (8 mL). The mixture was electrolyzed under constant current (10 mA) at room temperature for 2.5 h. No desired product 3a was detected.
Acknowledgments
We gratefully acknowledge the financial supports from the National Natural Science Foundation of China (No. 21761132021) and Nanjing Forestry University start-up funding.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02442.
Copies of 1H NMR and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Magnus P. D. Recent developments in sulfone chemistry. Tetrahedron 1977, 33, 2019–2045. 10.1016/0040-4020(77)80311-6. [DOI] [Google Scholar]; b Meadows D. C.; Gervay-Hague J. Vinyl sulfones: Synthetic preparations and medicinal chemistry applications. Med. Res. Rev. 2006, 26, 793–814. 10.1002/med.20074. [DOI] [PubMed] [Google Scholar]; c Aziz J.; Messaoudi S.; Alami M.; Hamze A. Sulfinate derivatives: dual and versatile partners in organic synthesis. Org. Biomol. Chem. 2014, 12, 9743–9759. 10.1039/C4OB01727G. [DOI] [PubMed] [Google Scholar]; d Liu N. W.; Liang S.; Manolikakes G. Recent advances in the synthesis of sulfones. Synthesis 2016, 48, 1939–1973. 10.1055/s-0035-1560444. [DOI] [Google Scholar]
- a Abouimrane A.; Belharouak I.; Amine K. Sulfone-based electrolytes for high-voltage Li-ion batteries. Electrochem. Commun. 2009, 11, 1073–1076. 10.1016/j.elecom.2009.03.020. [DOI] [Google Scholar]; b Caldwell J. J.; Craig D. Sulfone-mediated total synthesis of (±)-lepadiformine. Angew. Chem., Int. Ed. 2007, 46, 2631–2634. 10.1002/anie.200604670. [DOI] [PubMed] [Google Scholar]; c Wen Z. H.; Chao C. H.; Wu M. H.; Sheu J. H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. 10.1016/j.ejmech.2010.09.067. [DOI] [PubMed] [Google Scholar]
- a Braghiroli D.; Avallone R.; DiBella M. D. Asymmetric synthesis of (R)-and (S)-2-pyrrolidinemethanesulfonic acid. Tetrahedron: Asymmetry 1997, 8, 2209–2213. 10.1016/S0957-4166(97)00196-1. [DOI] [Google Scholar]; b Nakamura H.; Wu H.; Kobayashi J.; Kobayashi M.; Ohizumi Y.; Hirata Y. Agelasidines. Novel Hypotaurocyamine Derivatives from the Okinawan Sea Sponge Agelas nakamurai Hoshino. J. Org. Chem. 1985, 50, 2494–2497. 10.1021/jo00214a017. [DOI] [Google Scholar]; c Oida S.; Tajima Y.; Konosu T.; Nakamura Y.; Somada A.; Tanaka T.; Habuki S.; Harasaki T.; Kamai Y.; Fukuoka T.; Ohya S.; Yasuda H. Synthesis and antifungal activities of R-102557 and related dioxane-triazole derivatives. Chem. Pharm. Bull. 2000, 48, 694–707. 10.1248/cpb.48.694. [DOI] [PubMed] [Google Scholar]; d Guerrini A.; Tesei A.; Ferroni C.; Paganelli G.; Zamagni A.; Carloni S.; Donato M. D.; Castoria G.; Leonetti C.; Porru M.; Cesare M. D.; Zaffaroni N.; Beretta G. L.; Rio A. D.; Varchi G. A new avenue toward androgen receptor pan-antagonists: C2 sterically hindered substitution of hydroxy-propanamides. J. Med. Chem. 2014, 57, 7263–7279. 10.1021/jm5005122. [DOI] [PubMed] [Google Scholar]
- a Zhao G.; Hu J.-B.; Qian Z.-S.; Yin W. X. Enantioselective reduction of β-keto sulfones using the NaBH4/Me3SiCl system catalyzed by polymer-supported chiral sulfonamide. Tetrahedron: Asymmetry 2002, 13, 2095–2098. 10.1016/S0957-4166(02)00546-3. [DOI] [Google Scholar]; b Zhang H.-L.; Hou X.-L.; Dai L.-X.; Luo Z.-B. Synthesis of a biferrocene diphosphine ligand with only planar chirality and its application in the Rh-catalyzed asymmetric hydrogenation of β-keto sulfones. Tetrahedron: Asymmetry 2007, 18, 224–228. 10.1016/j.tetasy.2007.01.009. [DOI] [Google Scholar]; c Brace N. O. Preparation, reactions and physical properties of segmented 2-(perfluoroalkyl)ethanesulfinic acids and their derivatives. The role of the perfluoroalkyl group in finding new and useful compounds and in searching out new chemistry. J. Fluorine Chem. 2000, 102, 21–41. 10.1016/S0022-1139(99)00239-0. [DOI] [Google Scholar]; d Berrisford D. J.; Lovell P. A.; Sulimanab N. R.; Whiting A. Latent reactive groups unveiled through equilibrium dynamics and exemplified in crosslinking during film formation from aqueous polymer colloids. Chem. Commun. 2005, 5904–5906. 10.1039/b512073j. [DOI] [PubMed] [Google Scholar]; e Murthy S. N.; et al. An approach toward the synthesis of β-hydroxy sulfones on water. Tetrahedron Lett. 2009, 50, 5009–5011. 10.1016/j.tetlet.2009.06.078. [DOI] [Google Scholar]; f Moure A. L.; Arrayas R. G.; Carretero J. C. Catalytic asymmetric conjugate boration of α, β-unsaturated sulfones. Chem. Commun. 2011, 47, 6701–6703. 10.1039/c1cc11949d. [DOI] [PubMed] [Google Scholar]
- a Wang X.; Yang M.; Xie W.; Fan X.; Wu J. Photoredox-catalyzed hydrosulfonylation reaction of electron-deficient alkenes with substituted Hantzsch esters and sulfur dioxide. Chem. Commun. 2019, 55, 6010–6013. 10.1039/C9CC03004B. [DOI] [PubMed] [Google Scholar]; b Taniguchi N. Aerobic nickel-catalyzed hydroxysulfonylation of alkenes using sodium sulfinates. J. Org. Chem. 2015, 80, 7797–7802. 10.1021/acs.joc.5b01176. [DOI] [PubMed] [Google Scholar]; c Lu Q.; Zhang J.; Wei F.; Qi Y.; Wang H.; Liu Z.; Lei A. Aerobic Oxysulfonylation of Alkenes Leading to Secondary and Tertiary β-Hydroxysulfones. Angew. Chem., Int. Ed. 2013, 52, 7156–7159. 10.1002/anie.201301634. [DOI] [PubMed] [Google Scholar]; d Pagire S. K.; Paria S.; Reiser O. Synthesis of β-hydroxysulfones from sulfonyl chlorides and alkenes utilizing visible light photocatalytic sequences. Org. Lett. 2016, 18, 2106–2109. 10.1021/acs.orglett.6b00734. [DOI] [PubMed] [Google Scholar]; e Xi C.; Lia C.; Chen C.; Wang R. Acid-promoted reaction of sulfonyl chlorides with alkenes: New approach to the regioselective synthesis of β-hydroxyl sulfone derivatives. Synlett 2004, 1595–1597. 10.1055/s-2004-829090. [DOI] [Google Scholar]; f Gong X.; Wang M.; Ye S.; Wu J. Synthesis of 3-(Methylsulfonyl)benzo[b]thiophenes from Methyl(2-alkynylphenyl)sulfanes and Sodium Metabisulfite via a Radical Relay Strategy. Org. Lett. 2019, 21, 1156–1160. 10.1021/acs.orglett.9b00100. [DOI] [PubMed] [Google Scholar]; g Zhang J.; Li Xi.; Xie W.; Ye S.; Wu J. Photoredox-Catalyzed Sulfonylation of O-Acyl Oximes via Iminyl Radicals with the Insertion of Sulfur Dioxide. Org. Lett. 2019, 21, 4950–4954. 10.1021/acs.orglett.9b01323. [DOI] [PubMed] [Google Scholar]
- a Sauer G. S.; Lin S. An electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal. 2018, 8, 5175–5187. 10.1021/acscatal.8b01069. [DOI] [Google Scholar]; b Mei H.; Yin Z.; Liu J.; Sun H.; Han J. Recent Advances on the Electrochemical Difunctionalization of Alkenes/Alkynes. Chin. J. Chem. 2019, 37, 292–301. 10.1002/cjoc.201800529. [DOI] [Google Scholar]; c Martins G. M.; Shirinfar B.; Hardwick T.; Ahmed N. A Green Approach: Vicinal Oxidative Electrochemical Alkene Difunctionalization. ChemElectroChem 2019, 6, 1300–1315. 10.1002/celc.201801466. [DOI] [Google Scholar]
- For selected recent reviews, see:; a Yoshida Ji.; Kataoka K.; Horcajada R.; Nagaki A. Modern strategies in electroorganic synthesis. Chem. Rev. 2008, 108, 2265–2299. 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]; b Yan M.; Kawamata Y.; Baran P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017, 117, 13230–13319. 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yoshida Ji.; Shimizu A.; Hayashi R. Electrogenerated cationic reactive intermediates: The pool method and further advances. Chem. Rev. 2018, 118, 4702–4730. 10.1021/acs.chemrev.7b00475. [DOI] [PubMed] [Google Scholar]; d Jiang Y.; Xu K.; Zeng C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018, 118, 4485–4540. 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]; e Feng R.; Smith J. A.; Moeller K. D. Anodic Cyclization Reactions and the Mechanistic Strategies That Enable Optimization. Acc. Chem. Res. 2017, 50, 2346–2352. 10.1021/acs.accounts.7b00287. [DOI] [PubMed] [Google Scholar]; f Tang S.; Liu Y.; Lei A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem 2018, 4, 27–45. 10.1016/j.chempr.2017.10.001. [DOI] [Google Scholar]
- a Xu H. C.; Moeller K. D. Intramolecular anodic olefin coupling reactions: The use of a nitrogen trapping group. J. Am. Chem. Soc. 2008, 130, 13542–13543. 10.1021/ja806259z. [DOI] [PubMed] [Google Scholar]; b Perkins R. J.; Xu H. C.; Campbell J.; Moeller K. D. Anodic coupling of carboxylic acids to electron-rich double bonds: A surprising non-Kolbe pathway to lactones. Beilstein J. Org. Chem. 2013, 9, 1630–1636. 10.3762/bjoc.9.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Qiu Y.; Tian C.; Massignan L.; Rogge T.; Ackermann L. Electrooxidative Ruthenium-Catalyzed C–H/O–H Annulation by Weak O-Coordination. Angew. Chem., Int. Ed. 2018, 57, 5818–5822. 10.1002/anie.201802748. [DOI] [PubMed] [Google Scholar]; b Tian C.; Massignan L.; Meyer T. H.; Ackermann L. Electrochemical C– H/N– H Activation by Water-Tolerant Cobalt Catalysis at Room Temperature. Angew. Chem., Int. Ed. 2018, 57, 2383–2387. 10.1002/anie.201712647. [DOI] [PubMed] [Google Scholar]
- a Huang P.; Wang P.; Wang S.; Tang S.; Lei A. Electrochemical oxidative [4+2] annulation of tertiary anilines and alkenes for the synthesis of tetrahydroquinolines. Green Chem. 2018, 20, 4870–4874. 10.1039/C8GC02463D. [DOI] [Google Scholar]; b Yuan Y.; Chen Y.; Tang S.; Huang Z.; Lei A. Electrochemical oxidative oxysulfenylation and aminosulfenylation of alkenes with hydrogen evolution. Sci. Adv. 2018, 4, eaat5312 10.1126/sciadv.aat5312. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lu F.; Yang Z.; Wang T.; Wang T.; Zhang Y.; Yuan Y.; Lei A. Electrochemical Oxidative Csp3-H/S-H Cross-Coupling with Hydrogen Evolution for Synthesis of Tetrasubstituted Olefins. Chin. J. Chem. 2019, 37, 547–551. 10.1002/cjoc.201900113. [DOI] [Google Scholar]
- a Siu J. C.; Parry J. B.; Lin S. Aminoxyl-Catalyzed Electrochemical Diazidation of Alkenes Mediated by a Metastable Charge-Transfer Complex. J. Am. Chem. Soc. 2019, 141, 2825–2831. 10.1021/jacs.8b13192. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fu N.; Shen Y.; Allen A. R.; Song L.; Ozaki A.; Lin S. Mn-Catalyzed Electrochemical Chloroalkylation of Alkenes. ACS Catal. 2019, 9, 746–754. 10.1021/acscatal.8b03209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Xiong P.; Xu H. H.; Xu H. C. Metal-and reagent-free intramolecular oxidative amination of tri-and tetrasubstituted alkenes. J. Am. Chem. Soc. 2017, 139, 2956–2959. 10.1021/jacs.7b01016. [DOI] [PubMed] [Google Scholar]; b Xiong P.; Long H.; Song J.; Wang Y.; Li J. F.; Xu H. C. Electrochemically Enabled Carbohydroxylation of Alkenes with H2O and Organotrifluoroborates. J. Am. Chem. Soc. 2018, 140, 16387–16391. 10.1021/jacs.8b08592. [DOI] [PubMed] [Google Scholar]
- a Wang Y.; Deng L.; Mei H. B.; Du B. N.; Han J. L.; Pan Y. Electrochemical oxidative radical oxysulfuration of styrene derivatives with thiols and nucleophilic oxygen sources. Green Chem. 2018, 20, 3444–3449. 10.1039/C8GC01337C. [DOI] [Google Scholar]; b Gao Y.; Mei H. B.; Han J. L.; Pan Y. Electrochemical Alkynyl/Alkenyl Migration for the Radical Difunctionalization of Alkenes. Chem.–Eur. J. 2018, 24, 17205–17209. 10.1002/chem.201804157. [DOI] [PubMed] [Google Scholar]; c Chen C.; Kang J. C.; Mao C.; Dong J. W.; Xie Y. Y.; Ding T. M.; Tu Y. Q.; Chen Z. M.; Zhang S. Y. Electrochemical halogenation/semi-pinacol rearrangement of allylic alcohols using inorganic halide salt: an eco-friendly route to the synthesis of β-halocarbonyls. Green Chem. 2019, 21, 4014–4019. 10.1039/C9GC01152H. [DOI] [Google Scholar]; d Zheng M. W.; Yuan X.; Cui Y. S.; Qiu J. K.; Li G.; Guo K. Electrochemical Sulfonylation/Heteroarylation of Alkenes via Distal Heteroaryl ipso-Migration. Org. Lett. 2018, 20, 7784–7789. 10.1021/acs.orglett.8b03191. [DOI] [PubMed] [Google Scholar]; e Zhang S.; Li L.; Wu P.; Gong P.; Liu R.; Xu K. Substrate-Dependent Electrochemical Dimethoxylation of Olefins. Adv. Synth. Catal. 2019, 361, 485–489. 10.1002/adsc.201801173. [DOI] [Google Scholar]; f Sun C. C.; Xu K.; Zeng C. C. Transition Metal- and Base-Free Electrochemical aza-Michael Addition of Aromatic aza-Heterocycles or Ts-Protected Amines to α,β-Unsaturated Alkenes Mediated by NaI. ACS Sustainable Chem. Eng. 2019, 7, 2255–2261. 10.1021/acssuschemeng.8b04934. [DOI] [Google Scholar]; g Luo M. J.; Liu B.; Li Y.; Hu M.; Li J. H. Electrochemical Three-Component 1, 2-Aminosulfonylation of Alkenes: Entry to 2-sulfonylethan-1-amines. Adv. Synth. Catal. 2019, 361, 1538–1542. 10.1002/adsc.201801492. [DOI] [Google Scholar]; h Wan C.; Song R. J.; Li J. H. Electrooxidative 1,2-Bromoesterification of Alkenes with Acids and N-Bromosuccinimide. Org. Lett. 2019, 21, 2800–2803. 10.1021/acs.orglett.9b00771. [DOI] [PubMed] [Google Scholar]
- Yuan Y.; Cao Y.; Lin Y.; Li Y.; Huang Z.; Lei A. Electrochemical oxidative alkoxysulfonylation of alkenes using sulfonyl hydrazines and alcohols with hydrogen evolution. ACS Catal. 2018, 8, 10871–10875. 10.1021/acscatal.8b03302. [DOI] [Google Scholar]
- Zhang Z.; Yang J.; Ma D.; Sun J. Electrochemical synthesis of β-hydroxy-, β-alkoxy-, and β-carbonyloxy sulfones by vicinal difunctionalization of olefins. Chin. Chem. Lett. 2019, 30, 1509–1511. 10.1016/j.cclet.2019.04.023. [DOI] [Google Scholar]
- a Dong D. Q.; Hao S. H.; Yang D. S.; Li L. X.; Wang Z. L. Sulfenylation of C-H Bonds for C-S Bond Formation under Metal-Free Conditions. Eur. J. Org. Chem. 2017, 2017, 6576–6592. 10.1002/ejoc.201700853. [DOI] [Google Scholar]; b Li W.; Yin G.; Huang L.; Xiao Y.; Fu Z.; Xin X.; Liu F.; Li Z.; He W. Regioselective and stereoselective sulfonylation of alkynylcarbonyl compounds in water. Green Chem. 2016, 18, 4879–4883. 10.1039/C6GC01196A. [DOI] [Google Scholar]; c Zhang J.; Liang Z.; Wang J.; Guo Z.; Liu C.; Xie M. Metal-Free Synthesis of Functionalized Tetrasubstituted Alkenes by Three-Component Reaction of Alkynes, Iodine, and Sodium Sulfinates. ACS Omega 2018, 3, 18002–18015. 10.1021/acsomega.8b02966. [DOI] [PMC free article] [PubMed] [Google Scholar]; d He X.; Yue X.; Zhang L.; Wu S.; Hu M.; Li J. H. Multiple-functionalizations of terminal alkynes with sodium sulfinates and tert-butyl nitrite: facile synthesis of 2H-azirines. Chem. Commun. 2019, 55, 3517–3520. 10.1039/C9CC00625G. [DOI] [PubMed] [Google Scholar]; e Ansari M. Y.; Kumar N.; Kumar A. Regioselective Intermolecular Sulfur–Oxygen Difunctionalization (Phenoxysulfonylation) of Alkynes: One-Pot Construction of (Z)-β-Phenoxy Vinylsulfones. Org. Lett. 2019, 21, 3931–3936. 10.1021/acs.orglett.9b01041. [DOI] [PubMed] [Google Scholar]; f Du B.; Qian P.; Wang Y.; Mei H.; Han J. L.; Pan Y. Cu-Catalyzed Deoxygenative C2-Sulfonylation Reaction of Quinoline N-Oxides with Sodium Sulfinate. Org. Lett. 2016, 18, 4144–4147. 10.1021/acs.orglett.6b02289. [DOI] [PubMed] [Google Scholar]
- a Ye S.; Qiu G.; Wu J. Inorganic sulfites as the sulfur dioxide surrogates in sulfonylation reactions. Chem. Commun. 2019, 55, 1013–1019. 10.1039/C8CC09250H. [DOI] [PubMed] [Google Scholar]; b Xie L. Y.; Peng S.; Tan J. X.; Sun R. X.; Yu X.; Dai N. N.; Tang Z. L.; Xu X.; He W. M. Waste-Minimized Protocol for the Synthesis of Sulfonylated N-Heteroaromatics in Water. ACS Sustainable Chem. Eng. 2018, 6, 16976–16981. 10.1021/acssuschemeng.8b04339. [DOI] [Google Scholar]; c Qian P.; Deng Y.; Mei H.; Han J.; Zhou J.; Pan Y. Visible-Light Photoredox Catalyzed Oxidative/Reductive Cyclization Reaction of N-Cyanamide Alkenes for the Synthesis of Sulfonated Quinazolinones. Org. Lett. 2017, 19, 4798–4801. 10.1021/acs.orglett.7b02163. [DOI] [PubMed] [Google Scholar]; d Ye S.; Zheng D.; Wu J.; Qiu G. Photoredox-catalyzed sulfonylation of alkyl iodides, sulfur dioxide, and electron-deficient alkenes. Chem. Commun. 2019, 55, 2214–2217. 10.1039/C9CC00347A. [DOI] [PubMed] [Google Scholar]; e Yang F. L.; Tian S. K. Sulfonyl hydrazides as sulfonyl sources in organic synthesis. Tetrahedron Lett. 2017, 58, 487–504. 10.1016/j.tetlet.2016.12.058. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.