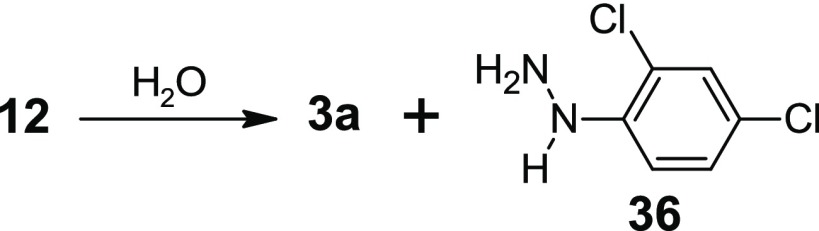Abstract
Microbial resistance to antibiotics is an urgent and worldwide concern. Several pyrazole-derived hydrazones were synthesized by using benign reaction conditions. Several of these molecules are potent growth inhibitors of drug-resistant strains of Staphylococcus aureus and Acinetobacter baumannii with minimum inhibitory concentration values as low as 0.39 μg/mL. Furthermore, these molecules are nontoxic to human cells at high concentrations. Some of these molecules were tested for their ability to disrupt the bacterial membrane by using the SYTO-9/propidium iodide (BacLight) assay.
Introduction
Antibiotic resistance is escalating to dangerously high levels all over the world. Without an imperative and coordinated action, we are heading for a post-antibiotic era, in which common infections and minor injuries could kill a person. Scarcity of effective antibiotics to treat antibiotic-resistance infections is putting modern medicine such as chemotherapy, surgeries, and organ transplantation at risk. To prevent and control the spread of antibiotic resistance, investment in research and development of new antibiotics, vaccines, and diagnostic tools is urgent.1
Staphylococcus aureus, a Gram-positive bacterium, is a leading cause of infection in US healthcare facilities. Methicillin-resistant S. aureus (MRSA) is resistant to several antibiotics. In the community, MRSA often causes skin infections as well as more serious infections such as pneumonia (lung infection). Untreated MRSA can cause sepsis and even death. In healthcare settings, such as hospitals and nursing homes, this drug-resistant bacterium can cause bloodstream infections, pneumonia, and surgical site infections. Approximately, 2% of the general population and 5% of patients in the US carry MRSA in their nares or on their skin. More than 119 000 bloodstream staphylococcal infections occurred, and nearly 20 000 people died with this infection in 2017.2Acinetobacter baumannii, a Gram-negative bacterium, can cause a variety of diseases including pneumonia or blood or wound infections. This bacterium may colonize in patients without causing infection or symptoms in healthy people. Acinetobacter may cause severe problems to people with weakened immune systems, chronic lung disease, or diabetes. A. baumannii is often resistant to commonly prescribed antibiotics.3 Since 2003, the beginning of the Iraq war, several US service members have been infected by A. baumannii making an enormous problem in veterans. So, this bacteria is also called Iraquibacter.4,5 In 2017, the World Health Organization (WHO) has released a list of 12 drug-resistant bacteria that pose the greatest threat to human health and for which new antibiotics are desperately needed. Carbapenem-resistant A. baumannii (CRAB) is on top of the list.6,7 CRAB infection is associated with high morbidity and mortality as well as it has the potential to cause outbreaks and spread resistance.8 It is very challenging to find novel molecules that can inhibit the growth of nonfermenting bacteria such as A. baumannii and P. aeruginosa, which are often multidrug resistant.9A. baumannii has a highly impermeable outer membrane that prevents the uptake of most molecules including several antibiotics.10
Results and Discussion
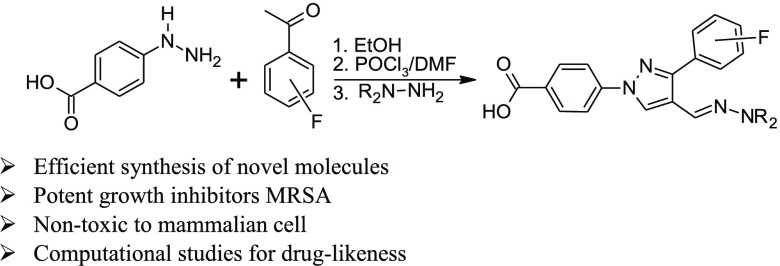
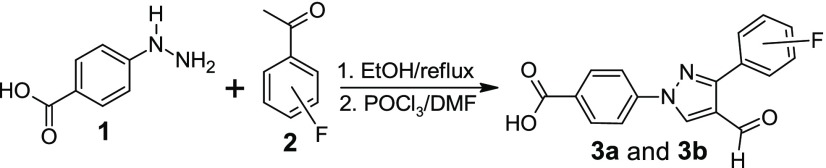
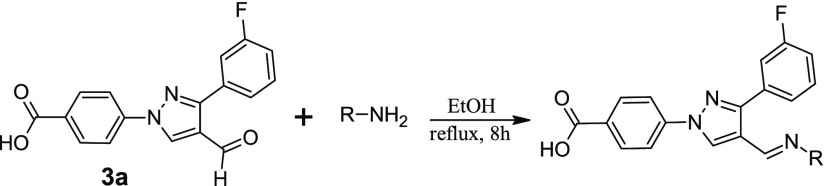
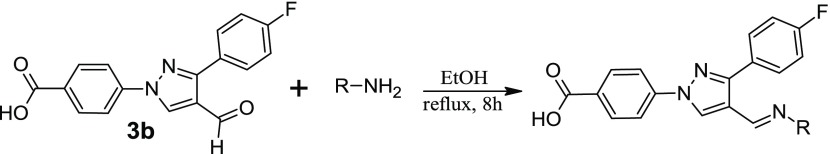
In our pursuit to find novel small-molecule heterocycles,11−15 we have reported the synthesis and antimicrobial activities of several pyrazole-derived hydrazones.16−19 Based on our reported molecules, we designed and synthesized fluorine-substituted aldehydes to synthesize hydrazone derivatives for antimicrobial studies. Fluorine substitution has been extensively studied in drug discovery to enhance the biological activity and increase the chemical and metabolic stability of the resultant molecules.20 The starting materials, fluorophenyl derivatives (1), were synthesized in the multigram scale by using our established procedure.17,18 Reactions of 4-hydrazinobenzoic acid with fluorophenyl acetophenones (2) afford the hydrazone derivatives. The evaporation of the solvent followed by the reaction with POCl3/DMF affords the aldehyde derivatives (3a and 3b) in a one-pot reaction (Scheme 1). Reactions of the aldehyde derivatives with different hydrazines afforded the target molecules. All of the synthesized molecules were tested against a total of 14 Gram-positive and Gram-negative bacterial strains.
Scheme 1. Synthesis of Fluorophenyl-Derived Pyrazole Aldehyde.
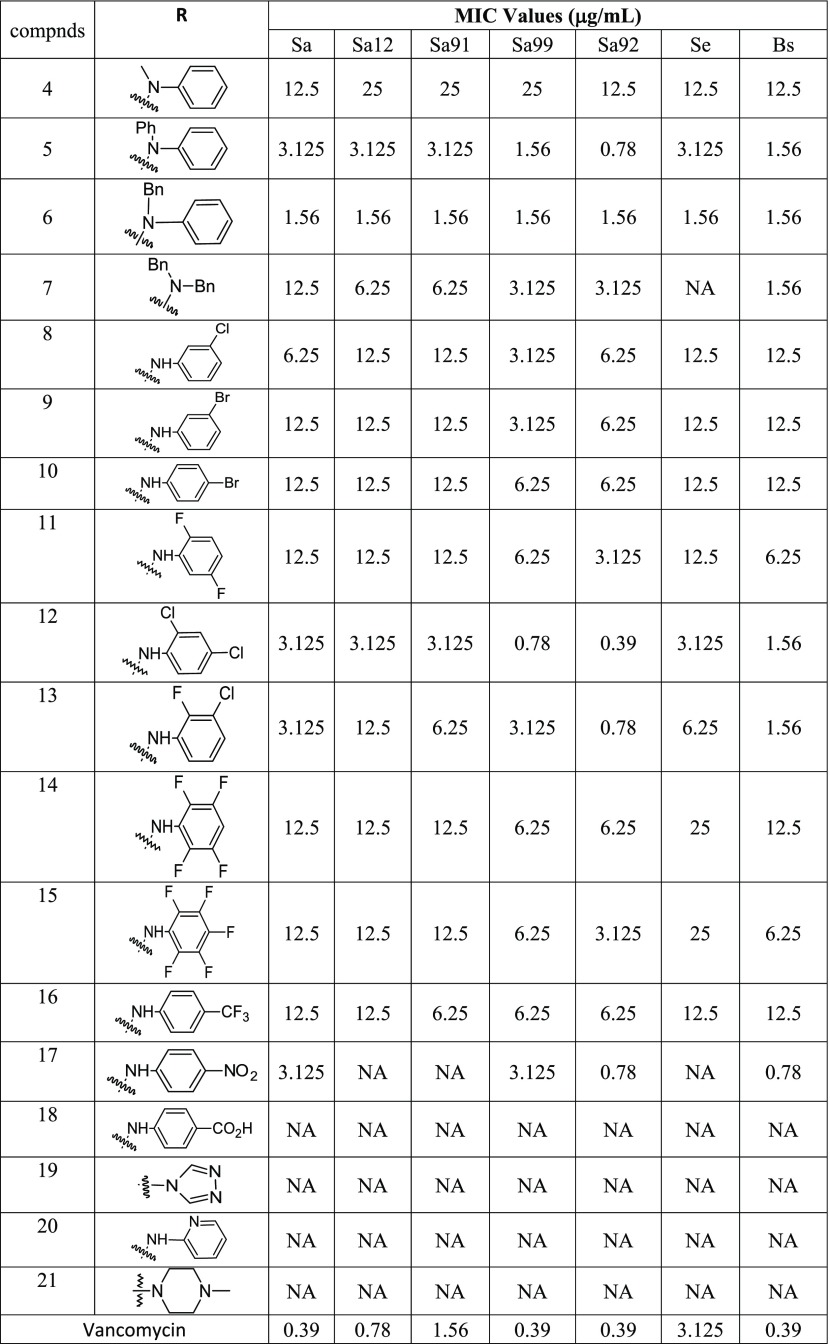
The first compound, N-methyl-N-phenyl derivative (4), showed moderate growth inhibition of the tested bacteria. N,N-Bisphenyl derivative (5) showed very good activity against a methicillin-sensitive S. aureus strain with an MIC value of 3.125 μg/mL concentration. This molecule also showed excellent activity against methicillin-resistant S. aureus strains: S. aureus ATCC 33591 (Sa91), S. aureus ATCC 33592 (Sa92), S. aureus ATCC 700699 (Sa99) with MIC value as low as 0.78 μg/mL. Potent growth inhibition of these three MRSA strains is very significant as they have SCC mec II or III genomic islands conferring multidrug resistance.21 This compound (5) inhibited the growth of S. epidermis 700296 (Se) with an MIC value of 3.125 μg/mL. S. epidermidis is an opportunistic pathogen, which is a part of normal human microbiota.22 Last but not the least, this novel molecule (5) inhibited the growth of B. subtilis with an MIC value of 1.56 μg/mL, making it a good candidate for further testing against B. anthracis, a potential biological warfare agent.23N-Benzyl-N-phenyl derivative (6) was found to be very potent across all of the tested Gram-positive strains with an MIC value as low as 1.56 μg/mL. N,N-Dibenzyl derivative (7) showed less potency against the staphylococcal species but inhibited the growth of B. subtilis at 1.56 μg/mL concentration. Chloro substitution on the phenyl hydrazone moiety decreased the activity of the molecule (8) with an MIC value as low as 3.125 μg/mL against MRSA. Bromo substitution (9 and 10) also showed similar results with decreased MIC values. Bisfluoro-substituted derivative (11) showed activity with an MIC value of 3.125 μg/mL against the multi-drug-resistant strain, S. aureus ATCC 33592 (Sa92). Bischloro substitution (12) increased the activity of the resultant compound several fold. This compound (12) is the most potent compound in the series with an MIC value of 0.39 μg/mL against the multiresistant staph strain (Sa92). 3-Chloro-2-fluoro derivative (13) also showed very good activity against these antibiotic-resistant strains with an MIC value as low as 0.78 μg/mL. Polyfluorinated derivatives (14 and 15) showed moderate activity against the tested strains as did the 4-trifluoromethy phenyl derivative (16). 4-Nitro substitution (17) showed strong activity against some of the tested strains with an MIC value of 0.78 μg/mL. The addition of carboxylic acid (18) and heterocycles (19, 20, and 21) completely ceased the activity of the resultant hydrazone derivatives (Table 1).
Table 1. Synthesis and Antimicrobial Activity of 3-Fluorophenyl-Substituted Hydrazone Derivatives, Gram-Positive Bacteria: Antibiotic Susceptible Strain S. aureus ATCC 25923 (Sa), and Antibiotic-Resistant Strains: S. aureus BAA-2312 (Sa12), S. aureus ATCC 33591 (Sa91), S. aureus ATCC 700699 (Sa99), S. aureus ATCC 33592 (Sa92), S. epidermidis 700296 (Se), B. subtilis ATCC 6623 (Bs), and NA = No Activity.
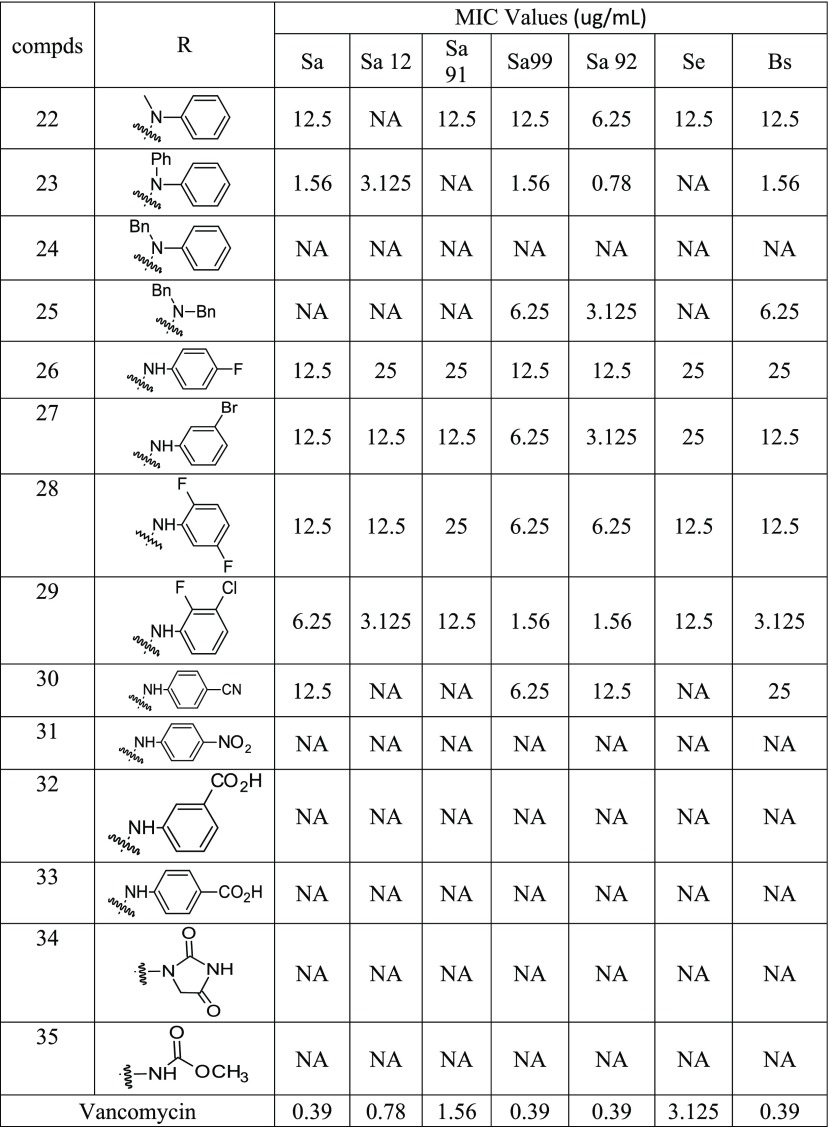
Likewise, the 4-fluorophenyl-derived aldehyde (3b) was treated with different hydrazine derivatives to synthesize the corresponding hydrazones (Table 2). Due to solubility issues, we could not characterize and test several compounds of this series. Decreased solubility in dimethyl sulfoxide solvent may be due to the symmetrical nature of the 4-flurophenyl substituent. Nevertheless, the following compounds were synthesized in good yields, and these compounds were tested for their growth inhibition properties against 14 bacterial strains. N-Methyl-N-phenyl derivative (22) showed moderate activity with an MIC value as low as 6.25 μg/mL against one of the MRSA strains. N,N-Diphenyl derivative (23) showed very potent activity with an MIC value as low as 0.78 μg/mL. This compound (23) also inhibited the growth of B. subtilis with an MIC value of 1.56 μg/mL concentration. Surprisingly, no activity was observed against S. epidermidis. N-Benzyl-N-phenyl derivative (24) failed to show any significant activity against the tested strains of bacteria. N,N-Dibenzyl derivative (25) showed moderate activity against some of the strains with an MIC value as low as 3.125 μg/mL. Fluoro- and bromo-substituted derivatives (26, 27, and 28) showed moderate activity against the tested strains. 3-Chloro-2-fluorophenyl-substituted derivative (29) showed good activity against the two strains of MRSA with an MIC value as low as 1.56 μg/mL. Cyano-substituted derivative (30) showed moderate activity against the tested Gram-positive strains of bacteria, and no activity was observed for the nitro derivative (31). Similar to the previous class of compounds, carboxylic acid and other substitution (32–35) did not show any significant potency against the tested strains. We have reported several pyrazole derivatives as anti-MRSA agents,16,17,19,24 but compound 13 is the most potent compound found to date with an MIC of 0.39 μg/mL.
Table 2. Synthesis and Antimicrobial Activity of 4-Fluorophenyl-Substituted Hydrazone Derivatives, Gram-Positive Bacteria: Antibiotic Susceptible Strain S. aureus ATCC 25923 (Sa), and Antibiotic-Resistant Strains: S. aureus BAA-2312 (Sa12), S. aureus ATCC 33591 (Sa91), S. aureus ATCC 700699 (Sa99), S. aureus ATCC 33592 (Sa92), S. epidermidis 700296 (Se), B. subtilis ATCC 6623 (Bs), and NA = No Activity.
All of the above compounds were tested against the seven Gram-negative bacterial strains. Some of these compounds showed moderate activity against A. baumannii strains, but none of the compounds showed any activity against the other four strains of Gram-negative bacteria: Escherichia coli ATCC 25922 (Ec), E. aerogenes ATCC 13048 (Ea), Pseudomonas aeruginosa 27833 (Pa), and Klebsiella pneumoniae ATCC 700603 (Kp). Only three derivatives (8, 9, and 10) of 3-fluorphenyl-derived aldehyde showed moderate activity against the three strains of A. baumannii with the lowest MIC value being 3.125 μg/mL. 4-Fluorophenyl-derived hydrazones (3) also showed moderate activity against the strains of A. baumannii (Table 3).
Table 3. MIC Values of Compounds against Gram-Negative Bacterial Strains: A. baumannii ATCC 19606 (Type Strain, AB06), A. baumannii ATCC BAA-1605 (Ab05), A. baumannii ATCC 747 (Ab47), E. coli ATCC 25922 (Ec), E. aerogenes ATCC 13048 (Ea), P. aeruginosa 27833 (Pa), K. pneumoniae ATCC 700603 (Kp), and NA = No Activity.
| MIC (μg/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| compounds | Ab06 | Ab47 | Ab05 | Ec | Ea | Pa | Kp |
| 8 | 6.25 | 12.5 | 6.25 | NA | NA | NA | NA |
| 9 | 6.25 | 12.5 | 12.5 | NA | NA | NA | NA |
| 10 | 3.125 | 6.25 | 6.25 | NA | NA | NA | NA |
| 26 | 12.5 | >25 | 25 | NA | NA | NA | NA |
| 27 | 6.25 | 25 | 12.5 | NA | NA | NA | NA |
| 30 | 12.5 | 12.5 | >25 | NA | NA | NA | NA |
| Colistin | 3.125 | 1.56 | 3.125 | ||||
Although hydrazones possess greater intrinsic hydrolytic stability than the corresponding imines but still these molecules can be converted into the starting materials by reacting with water under the physiological condition (Scheme 2).25 The antimicrobial activity of the compounds, hydrazones, may due to the hydrolyzed products, aldehydes, and hydrazines. To test this possibility, the starting materials of one of the most potent molecules (i.e., 12) were tested against S. aureus ATCC 700699 (Sa99). The aldehyde derivative (3a) did not show any activity, but the hydrazine (36) showed weak growth inhibition with an MIC value of 25 μg/mL. The reaction mixture (3a and 36) also showed the similar activity of hydrazine. Thus, we can conclude that the antimicrobial activity of the compounds is due to their hydrazone functional groups not due to their possible hydrolyzed products.
Scheme 2. Possible Hydrolysis of a Hydrazone to the Corresponding Aldehyde (3a) and Hydrazine (36).
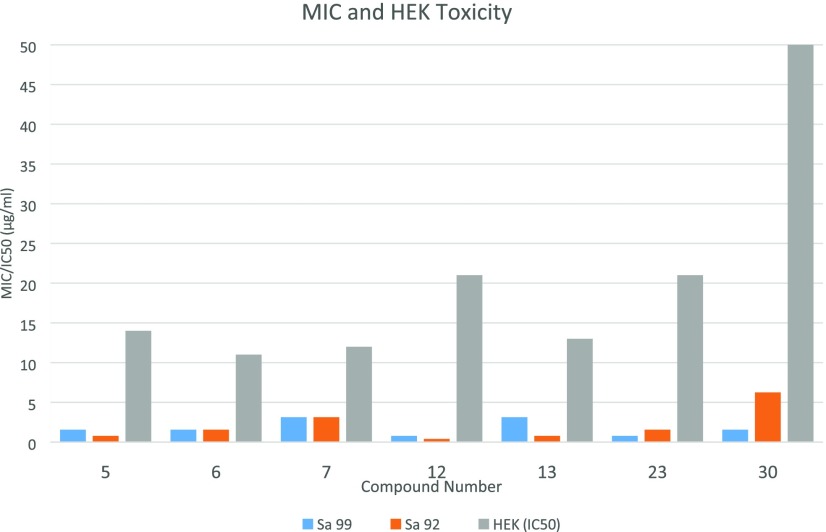
Compounds showing potent antimicrobial activity were tested against a human embryonic kidney (HEK293) cell line for their in vitro toxicity. Potent compounds did not show any noticeable cytotoxicity at the MIC concentration. IC50 values for the compounds are more than 10 μg/mL. High IC50 values and low MIC values for compounds 5, 12, and 23 indicate suitability for further drug development (Figure 1). Furthermore, these new compounds were submitted to the National Cancer Institute (NCI) for cytotoxicity studies against NCI-60 cancer cell lines. None of the compounds showed any substantial growth inhibition activity against these cancer cell lines at 10 μM concentration. Hence, these fluorophenyl-derived pyrazole derivatives (4–35) are nontoxic to human cell lines.
Figure 1.
Cytotoxicity studies of some potent compounds.
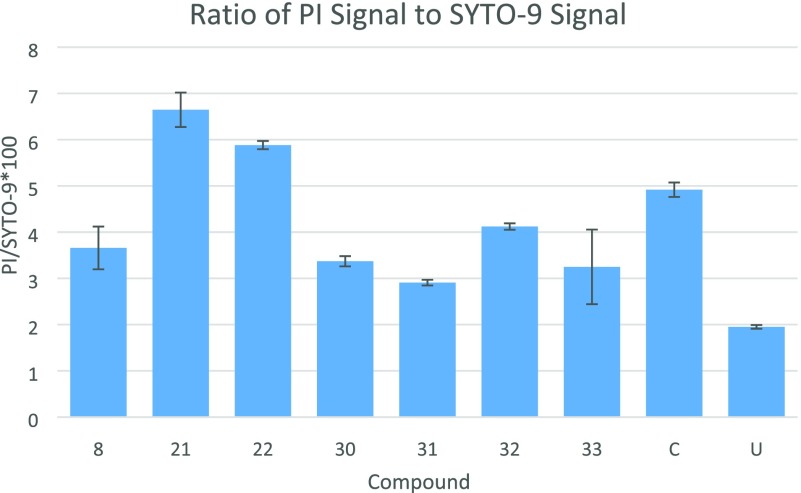
The bacterial membrane is one of the key targets of antibacterial agents. Antibiotics targeting bacterial membranes could be the key to solve bacterial resistance to antibiotics.26 Seven potent compounds (8, 21, 22, 30, 31, 32, and 33) were tested for their membrane permeability for the A. baumannii ATCC 19606 strain by using the SYTO/9-PI assay (Figure 2). Compounds 21 and 22 have shown better membrane disruption ability than the positive control, Colistin (C).
Figure 2.
Membrane disruption: ratios of PI to SYTO-9 signals with Colistin 6.25 μg/mL (C) and untreated controls (U). Bars indicate the standard deviation of the duplicate samples.
Calculated Physicochemical Properties
Physicochemical properties of the six most potent compounds (5, 6, 12, 13, 23, and 29) were calculated by using the SwissAMDE, a freely available online software (http://www.swissadme.ch/index.php). The n-octanol/water partition coefficient (ilogP) is an important parameter for the drug design and development. The ilogP values are mostly <4.0, which are within the acceptable range (Table 4). Topological total surface area of these molecules is less than 140 Å, which indicates the potential for very good passive transport through the cell membranes.27
Table 4. Calculated Physicochemical Properties.
| compounds | ilogP | TPSA |
|---|---|---|
| 5 | 3.91 | 70.72 |
| 6 | 3.77 | 70.72 |
| 13 | 3.56 | 79.51 |
| 14 | 3.48 | 79.51 |
| 23 | 4.05 | 70.72 |
| 29 | 3.45 | 79.51 |
Correlations of antimicrobial activity and the energy of lowest unoccupied molecular orbital (LUMO) orbitals have been reported previously.26,28 We calculated the energy of highest occupied molecular orbital (HOMO), LUMO orbital and HOMO–LUMO energy gap by using the density functional theory calculation (see the Supporting Information). Unfortunately, for our molecules, we did not find any good correlation.
Conclusions
In summary, we have efficiently synthesized several potential antibiotics by using benign reaction conditions and readily available starting materials. Several of these molecules have been found to be potent growth inhibitors of MRSA and B. subtilis. These molecules are significantly less toxic to human cell lines, which make them potential candidates for further antibiotic development. Design, synthesis, and antimicrobial studies of novel molecules based on the lead structures of this manuscript are in progress and will be reported soon.
Experimental Section
General Consideration
All of the reactions were carried out under the air atmosphere in round-bottom flasks. Solvents for reactions, recrystallizations and deuterated solvents for 1H and 13C NMR spectroscopy were purchased from Fisher Scientific and Oakwood chemical.
Synthesis of Hydrazone Derivatives16,17,24
A mixture of 4-hydrazinobenzoic acid (1, 1.597 g, 10.5 mmol) and 1-(3-fluorophenyl)ethanone (2, 1.381 g, 10 mmol) in ethanol was refluxed for 8 h. The solvent was removed under reduced pressure, and the hydrazone derivative was further dried in vacuo. The dried product was subjected to the next reaction without further isolation or purification. The fluorophenyl-derived product in anhydrous N,N-dimethyl formamide (30 mL) was stirred for 15 min to dissolve the solid material completely. The clear solution was chilled in an ice bath, and phosphorous oxychloride (POCl3, 4.67 mL, 50 mmol) was added dropwise. The reaction mixture was brought to an ambient temperature after 30 min and then heated for 8 h at 80 °C. After the completion of the reaction, the hot reaction mixture was decanted onto the ice in a beaker and the mixture was stirred for 12 h to precipitate the product. The solid product was filtered and washed with water repeatedly until the filtrate was clear. The aldehyde product (3a and 3b) was further dried in vacuo to get the pure pyrazole-derived aldehyde in a very good overall yield.
MIC Studies17,24
All of the synthesized compounds were tested for antimicrobial properties, and the minimum inhibitory concentration (MIC) was determined by broth microdilution plate-based technique as per Clinical and Laboratory Standards Institute (CLSI) procedures. In short, bacterial strains were streaked onto Tryptic Soy Agar (TSA) or TSA with 5% sheep blood plates. The streaked plates were incubated at 35 °C overnight. Isolated colonies were suspended in 0.9% NaCl sterile saline to match a 0.5 MacFarland standard. The suspension was further diluted 1:100 with the Mueller Hinton broth to an approximate 1 × 106 CFU/mL concentration. Compounds were dissolved in dimethyl sulfoxide (DMSO) to a 2 mg/mL concentration and further diluted serially 1:2 in microplates such that after the addition of bacteria solution in broth, the final concentrations began at 50 μg/mL. The inhibition of bacterial growth or viability was determined using resazurin as a colorimetric assay.29 Resazurin solution was added with the bacterial suspension to 8 μg/mL final concentration, and the microplates were incubated at 35 °C for 20–24 h to read them visually. Negative controls (bacteria without inhibitors), technical control (bacteria plus serially diluted DMSO), and positive controls (bacteria plus a serially diluted antibiotic) were included on every plate. The MIC value was the lowest concentration where there was no color change of resazurin from purple (resorufin) to red (resazurin) (evidence of the bacterial growth).
Cytotoxicity Studies
In vitro toxicity studies of the potent compounds were accomplished according to our reported modified procedure.17,24 HEK293 cells were counted using a Countess automated cell counter, and 4000 cells per well were seeded in black 96-well plates. Compounds were serially diluted starting at 50 μg/mL, added in triplicate, and incubated for 24 h. Resazurin solution (5%) for a final volume of 240 μL in each well was added after 24 h. After the addition of 5% resazurin, plates were incubated for 4 and 6 h before taking the readings for cell viability. Fluorescence was measured at 544 nm (excitation) and 590 nm (emission) using a BMG Labtech Fluostar Optima plate reader.
SYTO-9/PI Studies
BacLight assay (Invitrogen) was used to assess the membrane permeability of the bacterium A. baumannii ATCC 19606 following the treatment with our pyrazole-derived compounds using the reported procedure26,30 with a slight modification described below. SYTO-9 is a green fluorescent protein, which freely permeates into all bacterial cells and increases in fluorescent intensity when bound to DNA. This provides a baseline measurement of the total bacteria found in a single well, which can then be compared to the fluorescent intensity of propidium iodide (PI). Propidium iodide is much less permeable to the cell membrane but also increases in fluorescent intensity once it enters the cell and binds to DNA. PI is expected to only show a strong signal when significant membrane damage is present, so our compound treatments can be compared to negative controls and we can conclude if membrane damage has or has not occurred. In earlier experiments, our compounds have demonstrated a bleaching effect on the PI signal (results not shown), therefore we must remove our compounds from the cell suspension by centrifugation prior to reading our plates.
Ab 19606 was grown in cation adjusted Mueller Hinton broth at 37 °C with shaking to reach the log phase growth and an OD 600 nm of 0.2. The cultures were centrifuged at 10 000g for 10 min, the cell pellet was washed once with sterile water, resuspended so that the final optical density of the cell suspension was 0.2 OD, and aliquoted into separate wells of a 24-well-plate (780 μL/well), then test compounds were added (20 μL/well) at the MIC concentration as well as double the MIC and two 1:2 dilution factors below MIC. Suspensions were incubated at 37 °C with shaking for 1 h and then moved to individual microcentrifuge tubes and were centrifuged at 10 000g for 10 min, washed once with sterile water, and resuspended in 800 μL of water. A 1:1 mixture of SYTO-9 (1 μg/mL) and propidium iodide (1 mg/mL) was added to the suspension (3 μL/mL) and mixed well. The mixture (100 μL) was added to each well of a black opaque 96-well plate, and the plates were incubated in the dark for 15 min at room temperature. Green fluorescence (SYTO-9) was read at 530 nm, and red fluorescence (PI) was read at 645 nm (excitation wavelength, 485 nm). The ratio of green to red fluorescence was expressed as a percentage of the control.
Experimental Data
4-[3-(3-Fluorophenyl)-4-formyl-pyrazol-1-yl]benzoic Acid (3a)
Yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.98 (s, 1H), 9.47 (s, 1H), 8.10 (s, 4H), 7.82 (d, J = 8.4 Hz, 2H), 7.58–7.51 (m, 1H), 7.32 (t, J = 9.42 Hz, 1H); 13C NMR 75 MHz (DMSO-d6); δ 184.9, 166.9, 162.5 (1J = 241.6 Hz), 151.7 (4J = 2.51 Hz), 141.8, 136.8, 133.6 (3J = 8.53 Hz), 131.3, 131.0 (3J = 8.3 Hz), 130.1, 125.2 (4J = 2.6 Hz), 123.09, 119.36, 116.63 (2J = 20.8 Hz), 115.77 (2J = 23 Hz). HRMS (ESI-FTMS, m/z): calcd for C17H11FN2O3 [M + H]+ 311.0826, found 311.0825. Yield (2.8 g, 90%).
4-{3-(3-Fluorophenyl)-4-[(E)-(2-methyl-2-phenylhydrazinylidene)methyl]-1H-pyrazol-1-yl}benzoic Acid (4)
Light yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 8.99 (s, 1H), 8.10 (s, 4H), 7.62–7.54 (m, 4H), 7.36–7.22 (m, 4H), 6.87–6.83 (m. 1H), 2.49 (s, 3H); 13C NMR (75 MHz, DMSO-d6); δ 167.3, 162.7 (1J = 242.0 Hz), 150.0, 147.7, 142.4, 135.4 (3J = 8.2 Hz), 131.3, 131.1 (3J = 8.4 Hz), 129.4, 129.2, 127.3, 124.8, 124.5, 120.4 (3J = 14.1 Hz), 118.4, 115.7, 115.5, 115.2, 114.9, 32.8. HRMS (ESI-FTMS, m/z): calcd for C24H19FN4O2 [M + H]+ 415.1564, found. 415.1563. Yield (377 mg, 91%).
4-[4-[(E)-(Diphenylhydrazono)methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (5)
Yellow Solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.09 (s, 1H), 8.13–8.05 (m, 4H), 7.46–7.41 (m, 7H), 7.27–7.15 (m, 8H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.5 (1J = 242.3 Hz), 150.3, 143.2, 142.4, 134.9 (3J = 8.3 Hz), 131.3, 130.9 (3J = 8.4 Hz), 130.6, 130.4, 129.6, 129.0, 128.0 (3J = 7.4 Hz), 124.9, 124.7, 122.5, 119.2, 118.5, 115.7 (2J = 20.9 Hz), 115.3 (2J = 22.4 Hz). HRMS (ESI-FTMS, m/z): calcd for C29H21FN4O2 [M + H]+ 477.1721, found 477.1725. Yield (419 mg, 88%).
4-[4-[(E)-[Benzyl(phenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (6)
White solid, 1H NMR, 300 MHz (DMSO-d6); δ 9.02 (s, 1H), 8.14–8.05 (m, 4H), 7.47–7.18 (m, 13H), 7.06 (d, J = 7.5 Hz, 1H), 6.90 (t, J = 7.1 Hz, 1H), 5.29 (s, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 242.6 Hz), 149.9 (4J = 2.3 Hz), 147.7, 142.5, 136.4, 134.9 (3J = 8.3 Hz), 131.3, 131.0 (3J = 8.4 Hz), 129.5, 129.3, 128.9, 127.5, 127.1, 126.5, 125.5, 124.3, 120.6, 120.0, 118.6, 115.6 (2J = 20.7 Hz), 114.9, 114.6, 49.0. HRMS (ESI-FTMS, m/z): calcd for C30H24FN4O2 [M + H]+ 492.1950, found 492.1954. Yield (437 mg, 89%).
4-[4-[(2,2-Dibenzylhydrazino)methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (7)
White solid, 1H NMR, 300 MHz (DMSO-d6); δ 13.08 (br s, 1H), 8.78 (s, 1H), 8.11–8.03 (m, 4H), 7.61–7.15 (m, 13H), 7.09 (s, 1H), 6.97 (d, J = 7.4 Hz, 1H), 4.32 (s, 4H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.5 (1J = 242.4 Hz), 149.4, 142.5, 138.2, 134.9 (3J = 8.1 Hz), 131.3, 130.9 (3J = 8.2 Hz), 129.0, 128.8, 127.8, 127.5, 125.9, 124.0, 123.8, 120.4, 118.5, 115.4 (2J = 20.9 Hz), 114.6 (2J = 22.2 Hz), 58.2. HRMS (ESI-FTMS, m/z): calcd for C31H26FN4O2 [M + H]+ 506.2107, found 506.2107. Yield (465 mg, 92%).
4-[4-[(E)-[(3-Chlorophenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (8)
Yellow Solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.49 (s, 1H), 9.08 (s, 1H), 8.15–8.07 (m, 4H), 7.98 (s, 1H), 7.64–7.53 (m, 3H), 7.31 (t, J = 7.6 Hz, 1H), 7.18 (t, J = 7.9 Hz, 1H) 7.08 (s, 1H), 6.87 (d, J = 8.04, 1H), 6.72 (d, J = 7.7 Hz, 1H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.5 (1J = 242.0 Hz), 150.1, 147.0, 142.4, 135.0, 134.4, 131.3, 130.9, 130.4, 129.0, 127.7, 124.9, 119.2, 118.4, 118.2, 115.8, 115.6, 115.3, 111.1 (2J = 37.7 Hz). HRMS (ESI-FTMS, m/z): calcd for C23H17ClFN4O2 [M + H]+ 437.0991, found 437.0990. Yield (340 mg, 78%).
4-[4-[(E)-[(3-Bromophenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (9)
Green solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.46 (s, 1H), 9.07 (s, 1H), 8.10 (m, 5H), 7.61 (t, J = 4.8 Hz, 3H), 7.31 (s, 1H), 7.21 (s, 1H), 7.11 (d, J = 7.6 Hz, 1H), 6.92–6.85 (m, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 242 Hz), 150.2 (4J = 2.2 Hz), 147.2, 142.4, 135.1 (3J = 8.3 Hz), 131.3, 131.0 (3J = 8.4 Hz), 130.5, 129.0, 127.9, 125.0 (4J = 2.4 Hz), 123.0, 121.2, 119.2, 118.6, 115.9, 115.6, 115.4, 114.2, 111.3, HRMS (ESI-FTMS, m/z): calcd for C23H16BrFN4O2 [M + H]+ 481.0494, found 481.0485. Yield (384 mg, 80%).
4-[4-[(E)-[(4-Bromophenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (10)
1H NMR, 300 MHz (DMSO-d6): δ 10.44 (s, 1H), 9.05 (s, 1H), 8.15–8.06 (m, 4H), 7.97 (s, 1H), 7.63–7.53 (m, 3H), 7.35–7.32 (m, 3H), 6.98–6.96 (m, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 241.9 Hz), 150.1 (4J = 2.4 Hz), 145.0, 142.4, 135.0 (3J = 8.3 Hz), 132.0, 131.3, 131.1 (3J = 8.4 Hz), 129.9, 129.0, 127.6, 124.9 (4J = 2.5 Hz), 119.4, 118.5, 115.8 (2J = 21.1 Hz), 115.4 (2J = 22.5 Hz), 114.1, 109.7. HRMS (ESI-FTMS, m/z): calcd for C23H16BrFN4O2 [M + H]+ 481.0494, found 481.0485. Yield (393 mg, 82%).
4-[4-[(E)-[(2,5-Difluorophenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (11)
Brownish yellow solid, 1H NMR, 300 MHz (DMSO-d6); δ 13.11 (br s, 1H), 9.04 (s, 1H), 8.13–8.05 (m, 4H), 7.46–7.18 (m, 13H), 7.04 (d, J = 7.38 Hz, 1H), 6.90 (t, J = 7.02 Hz, 1H), 5.29 (s, 2H); 13C NMR 75 MHz (DMSO-d6); δ 167.1, 162.6 (1J = 241.9 Hz), 159.7 (1J = 234.8 Hz), 150.3 (4J = 2.4 Hz), 145.5 (1J = 234.8 Hz), 142.4, 135.3 (t, 3J = 11.9 Hz), 134.9 (3J = 8.2 Hz), 133.1, 131.3, 131.1 (3J = 8.4 Hz), 129.0, 127.9, 124.9 (4J = 2.5 Hz), 119.2, 118.6, 116.3 (2J = 20.5 Hz), 115.8 (2J = 20.7 Hz), 115.4 (2J = 22.4 Hz), 103.9 (2J = 24.4 Hz), 100.7 (2J = 29.3 Hz). HRMS (ESI-FTMS, m/z): calcd for C23H15F3N4O2 [M + H]+ 437.122, found 437.1221. Yield (332 mg, 76%).
4-[4-[(E)-[(2,4-Dichlorophenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (12)
Light Yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.06 (s, 1H), 9.09 (s, 1H), 8.63 (s, 1H), 8.15–8.07 (m, 4H), 7.60 (m, 4H), 7.43 (s, 1H), 7.27 (t, J = 8.9 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 242.0 Hz), 150.4 (4J = 2.2 Hz), 142.4, 141.0, 134.9 (3J = 8.3 Hz), 133.7, 131.1 (3J = 8.4 Hz), 129.1, 128.9, 128.2, 127.6, 124.9 (4J = 2.6 Hz), 122.3, 119.3, 118.6, 116.7, 115.8 (2J = 20.9 Hz), 115.5, 115.3 (3J = 8.3 Hz). HRMS (ESI-FTMS, m/z): calcd for C23H15Cl2FN4O2 [M + H]+ 469.0628, found 469.0630. Yield (360 mg, 77%).
4-[4-[(E)-[(3-Chloro-2-fluorophenyl)hydrazono]methyl]-3-(3-fluorophenyl)pyrazol-1-yl]benzoic Acid (13)
Yellow Solid, 1H NMR, 300 MHz (DMSO-d6); δ 10.42 (s, 1H), 9.09 (s, 1H), 8.31 (s, 1H), 8.15–8.06 (m, 4H), 7.61–7.56 (m, 3H), 7.44 (t, J = 8.01 Hz, 1H), 7.35 (t, J = 2.82 Hz, 1H), 7.07 (t, J = 8.13 Hz, 1H), 6.86 (t, J = 7.95 Hz, 1H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 241.8 Hz), 150.3 (4J = 2.5 Hz), 144.7 (1J = 239.8 Hz), 142.4, 135.3 (3J = 9.3 Hz), 134.9 (3J = 8.2 Hz), 133.2, 131.3, 131.1 (3J = 8.4 Hz), 129.1, 127.8, 125.8 (4J = 4.0 Hz), 124.9 (4J = 2.6 Hz), 119.9 (2J = 14.2 Hz), 119.2, 118.8, 118.6, 115.8 (2J = 20.5 Hz), 115.4 (2J = 22.4 Hz), 112.9. HRMS (ESI-FTMS, m/z): calcd for C23H15ClF2N4O2 [M + H]+ 453.0924, found 453.0928.
4-[3-(3-Fluorophenyl)-4-[(E)-[(2,3,5,6-tetrafluorophenyl)hydrazono]methyl]pyrazol-1-yl]benzoic Acid (14)
Light yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 13.11 (br s, 1H), 10.37 (s,1H), 8.88 (s, 1H), 8.32 (s, 1H), 8.14–8.01 (m, 4H), 7.64–7.48 (m, 3H), 7.33–7.14 (m, 2H); 13C NMR (75 MHz, DMSO-d6); δ =167.0, 162.6 (1JC-F = 241.8 Hz), 150.4 (4JC-F = 2.4 Hz), 146.6 (dt, 1J = 241.8 Hz, 3J = 10.6 Hz), 142.3, 136.5 (dd, 1J = 233.1 Hz, 2J = 20.1 Hz), 135.3, 134.7 (3JC-F = 8.3 Hz), 131.3, 131.0 (3JC-F = 8.4 Hz), 129.1, 127.8, 125.7 (m), 124.9 (4JC-F = 2.5 Hz), 118.8, 118.5, 115.8 (2J = 21.0 Hz), 115. 5 (2JC-F = 22.5 Hz), 95.5 (t, 2J = 24.1 Hz). HRMS (ESI-FTMS, m/z): calcd for C23H13F5N4O2 [M + H]+ 473.1031, found 473.1032. Yield (405 mg, 86%).
4-[3-(3-Fluorophenyl)-4-[(E)-[(2,3,4,5,6-pentafluorophenyl)hydrazono]methyl]pyrazol-1-yl]benzoic Acid (15)
Brown yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.13 (s, 1H), 8.76 (s, 1H), 8.21 (s, 1H), 8.04 (s, 4H), 7.58–7.47 (m, 3H), 7.26 (t, J = 7.62, 1H); 13C NMR (75 MHz, DMSO-d6); δ 167.0, 162.5 (1J = 241.9 Hz), 150.2 (4J = 2.3 Hz), 142.2, 138.1 (m), 137.9 (m), 135.3, 134.6 (3J = 8.3 Hz), 131.3 (m), 130.9 (3J = 8.4 Hz), 129.0, 127.5, 124.8 (4J = 2.4 Hz), 121.6 (3J = 10.2 Hz), 118.9, 118.5 (3J = 9.8 Hz), 115.7 (2J = 21.0 Hz), 115.3 (2J = 22.6 Hz). HRMS (ESI-FTMS, m/z): calcd for C23H12F6N4O2 [M + H]+ 491.0937, found 491.0937. Yield (432 mg, 88%).
4-[3-(3-Fluorophenyl)-4-[(E)-[[4-(trifluoromethyl)phenyl]hydrazono]methyl]pyrazol-1-yl]benzoic Acid (16)
1H NMR, 300 MHz (DMSO-d6): δ 10.74 (s, 1H), 9.09 (s, 1H), 8.15–8.07 (m, 4H), 8.04 (s, 1H), 7.65–7.54 (m, 3H), 7.51 (d, J = 8.5 Hz, 2H), 7.33 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 8.4 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 241.9 Hz), 150.3 (4J = 2.3 Hz), 148.6, 142.4, 135.0 (3J = 8.3 Hz), 131.6, 131.3, 131.1 (3J = 8.4 Hz), 129.1, 127.9, 125.5 (q, 1J = 268.4 Hz), 125.0 (4J = 2.6 Hz), 119.1, 118.6, 118.4 (q, 2J = 31.6 Hz) 115.8 (2J = 20.8 Hz), 115.5 (2J = 22.4 Hz), 111.8. HRMS (ESI-FTMS, m/z): calcd for C24H16F4N4O2 [M + H]+ 469.1282, found 469.1282. Yield (369 mg, 79%).
4-[3-(3-Fluorophenyl)-4-[(E)-(4-nitrophenyl)methyl]pyrazol-1-yl]benzoic Acid (17)
Orange solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.12 (s, 1H), 8.09 (s, 7H), 7.62–7.53 (m, 3H), 7.35–7.30 (m, 1H), 7.10–7.07 (m, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.2, 162.6 (1J = 242.3 Hz), 150.9, 150.6, 142.1, 138.5, 134.8 (3J = 8.3 Hz), 134.6, 131.3, 131.2, 131.1, 129.8, 128.4, 126.5, 125.0, 118.6, 115.9 (2J = 20.6 Hz), 115.5 (2J = 22.2 Hz), 111.4. HRMS (ESI-FTMS, m/z): calcd for C23H16FN5O4 [M + H]+ 446.1259, found 446.1259. Yield (400 mg, 90%).
4-[(2E)-2-[[1-(4-Carboxyphenyl)-3-(3-fluorophenyl)pyrazol-4-yl]methylene]hydrazino]benzoic Acid (18)
Yellowish solid, 1H NMR, 300 MHz (DMSO-d6); δ 10.75 (s, 1H), 9.08 (s, 1H), 8.11–8.05 (m, 5H), 7.79 (d, J = 8.2 Hz, 2H), 7.62 (d, J = 7.8 Hz, 3H), 7.32 (s, 1H), 7.05 (d, J = 8.1 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.7, 167.1, 162.6 (1J = 241.9 Hz), 150.3, 149.2, 142.4, 135.0 (3J = 8.3 Hz), 131.4 (2J = 15.5 Hz), 131.1 (3J = 8.5 Hz), 129.1, 127.9, 125.0 (4J = 2.5 Hz), 120.5, 119.2, 118.6, 115.9, 115.7, 115.4, 111.4. HRMS (ESI-FTMS, m/z): calcd for C24H17FN4O4 [M + H]+ 445.1306, found 445.1310. Yield (386 mg, 87%).
4-[3-(3-Fluorophenyl)-4-[(E)-1,2,4-triazol-4-yliminomethyl]pyrazol-1-yl]benzoic Acid (19)
White solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.33 (s, 1H), 9.12 (s, 2H), 8.97 (s, 1H), 8.19–8.08 (m, 4H), 7.72–7.69 (m, 2H), 7.57 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.1 Hz, 1H);(done) 13C NMR (75 MHz, DMSO-d6); δ 167.0, 162.7 (1J = 242.3 Hz), 152.3, 151.5, 142.0, 139.4, 133.8 (3J = 8.5 Hz), 131.4, 130.6, 129.9, 125.3, 119.3, 116.5, 116.2, 115.7 (2J = 22.3 Hz). HRMS (ESI-FTMS, m/z): calcd for C19H13FN6O2 [M + H]+ 377.1157, found 377.1160. Yield (270 mg, 72%).
4-[3-(3-Fluorophenyl)-4-{(E)-[2-(pyridin-2-yl)hydrazinylidene]methyl}-1H-pyrazol-1-yl]benzoic Acid (20)
Light yellow solid, 1H NMR (300 MHz, DMSO-d6): 13.08 (be s, 1H), 11.18 (s, 1H), 9.02 (s, 1H), 8.45 (d, J = 4.4 Hz, 2H), 8.31 (s, 1H), 8.17 (d, J = 8.2 Hz, 2H), 8.07 (d, J = 8.2 Hz, 2H), 7.73–7.68 (m, 2H), 7.60–7.56 (m, 1H), 7.31 (t, J = 7.7 Hz, 1H), 6.82 (s, 1H); 13C APT NMR (75 MHz, DMSO-d6): 167.0, 162.7 (d, 1J = 241.6 Hz), 160.2, 158.7, 150.6, 142.4, 134.7, 134.6, 131.3, 131.2 (d, 3J = 8.0 Hz), 129.1, 127.9, 125.0, 119.0, 118.8, 116.0, 115.7, 115.4, 113.3. HRMS (ESI-FTMS, m/z): calcd for C22H16FN5O2 [M + H]+ 402.1360, found 402.1365. Yield (300 mg, 75%).
4-[3-(3-Fluorophenyl)-4-[(E)-(4-methylpiperazin-1-yl)iminomethyl]pyrazol-1-yl]benzoic Acid (21)
White solid, 1H NMR, 300 MHz (DMSO-d6); δ 8.81 (s, 1H), 8.07 (s, 4H), 7.63–7.61 (m, 3H), 7.57–7.50 (m, 1H), 7.28 (t, J = 2.2 Hz, 1H), 3.08 (s, 4H), 2.5 (s, 4H), 2.2 (s, 3H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 241.8 Hz), 149.9, 142.4, 135.1 (3J = 8.1 Hz), 131.3, 131.1 (3J = 8.2 Hz), 129.2, 127.6, 127.2, 124.8, 120.2, 118.5, 115.6 (2J = 21.0 Hz), 115.2 (2J = 22.7 Hz), 115.1, 54.3, 50.8, 45.9. HRMS (ESI-FTMS, m/z): calcd for C22H22FN5O2 [M + H]+ 408.1830, found 408.1835. Yield (321 mg, 79%).
4-[3-(4-Fluorophenyl)-4-formyl-pyrazol-1-yl]benzoic Acid (3b)
Yellowish Solid, 1H NMR, 300 MHz (DMSO-d6); δ 12.37 (br s, 1H), 9.84 (s, 1H), 7.82 (d, J = 8.6 Hz, 2H), 7.65–7.58 (m, 2H), 7.46–7.39 (m, 1H), 7.31–7.28 (m, 2H), 7.15 (t, J = 8.3 Hz, 1H); 13C NMR (75 MHz, DMSO-d6); δ 185.0, 166.9, 163.2 (1J = 245.0 Hz), 152.2, 141.9, 136.5, 131.4, 131.3, 130.0, 127.9 (4J = 3.0 Hz), 122.9, 119.3, 115.9 (2J = 21.4 Hz). HRMS (ESI-FTMS, m/z): calcd for C17H11FN2O3 [M + H]+ 311.0826, found 311.0825. Yield (2.88 g, 93%).
4-[3-(4-Fluorophenyl)-4-[(E)-[methyl(phenyl)hydrazono]methyl]pyrazol-1-yl]benzoic Acid (22)
Yellowish solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.0 (s, 1H), 8.14–8.06 (m, 4H), 7.84 (t, J = 7.23 Hz, 2H), 7.6 (s, 1H), 7.37–7.23 (m, 6H), 6.86 (t, J = 7.05 Hz, 1H), 2.5 (s, 3H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 244.0 Hz), 150.5, 147.7, 142.5, 131.3, 130.8 (3J = 8.2 Hz), 129.6, 129.5, 129.2, 128.9, 127.0, 124.6, 120.3, 118.4, 116.0 (2J = 21.3 Hz), 114.9, 32.8. HRMS (ESI-FTMS, m/z): calcd for C24H19FN4O2 [M + H]+ 415.1565, found 415.1561. Yield (376 mg, 91%).
4-[4-[(E)-(Diphenylhydrazono)methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (23)
Light Yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.07 (s, 1H), 8.08 (s, 4H), 7.63–7.58 (m, 2H), 7.44 (t, J = 7.86 Hz, 4H), 7.27–7.12 (m, 9H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.6 (1J = 244.2 Hz), 150.7, 143.2, 142.5, 131.3, 130.8 (3J = 8.2 Hz), 130.4, 129.2 (4J = 3.0 Hz), 128.9, 128.1, 127.8, 124.9, 122.5, 119.0, 118.4, 115.8 (2J = 21.3 Hz). HRMS (ESI-FTMS, m/z): calcd for HRMS (ESI-FTMS, m/z): calcd for C29H21FN4O2 [M + H]+ 477.1721, found 477.1722. Yield (428 mg, 90%).
4-[4-[(2-Benzyl-2-phenyl-hydrazino)methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (24)
Light yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.01 (s, 1H), 8.13–8.05 (m, 4H), 7.46–7.27 (m, 10H), 7.19 (d, J = 7.1 Hz, 2H), 7.12 (t, J = 8.8 Hz, 2H), 6.9 (t, J = 7.1 Hz, 1H), 5.28 (s, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.4 (1J = 244.0 Hz), 150.4, 147.7, 142.5, 136.4, 131.3, 130.2 (3J = 8.2 Hz), 129.5, 129.4, 129.1 (4J = 3.0 Hz), 128.8, 127.6, 126.8, 126.6, 125.7, 120.6, 119.7, 119.3, 118.5, 115.9 (2J = 21.3 Hz), 114.6, 56.4. HRMS (ESI-FTMS, m/z): calcd for HRMS (ESI-FTMS, m/z): calcd for C30H23FN4O2 [M + H]+ 491.1877, found 491.1875. Yield (441 mg, 90%).
4-[4-[(E)-(Dibenzylhydrazono)methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (25)
Light yellow Solid, 1H NMR, 300 MHz (DMSO-d6 + CDCl3): δ 8.77 (s, 1H), 8.06–8.05 (m, 4H), 7.35–7.29 (m, 12H), 7.09–7.04 (m, 3H), 4.55 (s, 4H); 13C NMR (75 MHz, DMSO-d6 + CDCl3); δ 167.1, 162.7 (1J = 270 Hz), 149.9, 142.6, 138.3, 131.4, 131.3, 130.1 (3J = 8.2 Hz), 129.0, 128.6, 127.8, 127.5, 125.7, 124.2, 120.1, 118.4, 115.8 (2J = 21.4 Hz), 58.2. HRMS (ESI-FTMS, m/z): calcd for HRMS (ESI-FTMS, m/z): calcd for C31H25FN4O2 [M + H]+ 505.2034, found 505.2044. Yield (463 mg, 92%).
4-[3-(4-Fluorophenyl)-4-[(E)-[(4-fluorophenyl)hydrazono]methyl]pyrazol-1-yl]benzoic Acid (26)
Yellowish solid, 1H NMR, 300 MHz (DMSO-d6): δ 9.0 (s, 1H), 8.10 (s, 4H), 7.91 (s, 1H), 7.80 (t, J = 7.9 Hz, 2H), 7.36 (t, J = 8.4 Hz, 2H), 7.07–7.00 (m, 3H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.7 (1J = 244 Hz), 156.1 (1J = 232 Hz), 150.5, 142.5, 142.4, 131.3, 130.9 (3J = 8.3 Hz), 129.3 (4J = 2.9 Hz), 129.1, 128.8, 127.1, 119.4, 118.4, 116.7 (2J = 22.7 Hz), 115.9 (2J = 21.3 Hz), 113.1 (3J = 7.2 Hz). HRMS (ESI-FTMS, m/z): calcd for HRMS (ESI-FTMS, m/z): calcd for C23H16F2N4O2 [M + H]+ 419.1314, found 419.1311. Yield (326 mg, 78%).
4-[4-[(E)-[(3-Bromophenyl)hydrazono]methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (27)
Light Yellow Solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.43 (s, 1H), 9.04 (s, 1H), 8.13–8.06 (m, 4H), 7.93 (s, 1H), 7.83–7.78 (m, 2H), 7.36 (t, J = 8.8 Hz, 2H), 7.17–7.09 (m, 2H), 6.86 (t, J = 7.4 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.7 (1J = 244.2 Hz), 150.7, 147.2, 142.5, 131.4, 131.1, (3J = 8.2 Hz), 130.6, 129.31, 129.3 (3J = 12.4 Hz), 128.9, 127.8, 123.0, 121.2, 119.0, 118.5, 115.9 (2J = 21.4 Hz), 114.2, 111.3. HRMS (ESI-FTMS, m/z): calcd for C23H16BrFN4O2 [M + H]+ 479.0513, found 479.0510. Yield (377 mg, 79%).
4-[4-[(E)-[(2,5-Difluorophenyl)hydrazono]methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (28)
Light yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.33 (s, 1H), 8.85 (s, 1H), 8.28 (s, 1H), 8.12–8.04 (m, 5H), 7.83–7.78 (m, 2H), 7.34 (t, J = 3H), 7.50–7.10 (m, 1H); 13C NMR (75 MHz, DMSO-d6); δ 167.0, 162.8 (1J = 244.2 Hz), 150.9, 146.6 (m), 142.4, 138.4 (3J = 16.3 Hz), 135.7, 131.3, 131.0 (3J = 8.2 Hz), 129.0, 128.9 (4J = 3.0 Hz), 127.5, 125.7, 118.7, 118.3, 115.9 (2J = 21.3 Hz), 95.8, 95.5, 95.2. HRMS (ESI-FTMS, m/z): calcd for C23H15F3N4O2 [M + H]+ 437.1219, found 437.1220. Yield (331 mg, 76%).
4-[4-[(E)-[(3-Chloro-2-fluorophenyl)hydrazono]methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (29)
Light Yellow Solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.39 (s, 1H), 9.05 (s, 1H), 8.25 (s, 1H), 8.09 (s, 4H), 7.78 (t, J = 7.8 Hz, 2H), 7.44–7.33 (m, 3H), 7.07 (t, J = 8.1 Hz, 1H), 6.85 (t, J = 6.9 Hz, 1H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.7 (1J = 244.2 Hz), 150.8, 144.6 (1J = 239.8 Hz), 142.4, 135.3 (3J = 9.1 Hz), 133.3, 131.4, 130.9 (3J = 8.3 Hz), 129.0 (4J = 2.9 Hz), 129.0, 127.6, 125.8 (4J = 3.7 Hz), 119.8 (3J = 14.1 Hz), 119.0, 118.8, 118.5, 116.0 (2J = 21.4 Hz), 112.9. HRMS (ESI-FTMS, m/z): calcd for C23H15ClF2N4O2 [M + H]+ 453.0924, 455.0897, found 453.0926, 455.0895.Yield (334 mg, 74%).
4-[4-[(E)-[(4-Cyanophenyl)hydrazono]methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (30)
Greenish yellow solid, 1H NMR, 300 MHz (DMSO-d6): δ 10.88 (s, 1H), 9.07 (s, 1H), 8.13–8.06 (m, 4H), 8.01 (s, 1H), 7.83–7.78 (m, 2H), 7.59 (d, J = 8.7 Hz, 2H), 7.36 (t, J = 8.8 Hz, 2H), 7.08 (d, J = 8.6 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.8 (1J = 244.3 Hz), 150.9, 148.9, 142.4, 134.0, 132.8, 131.3, 131.0 (3J = 8.2 Hz), 129.1, 127.9, 120.6, 118.7, 118.5, 116.0 (2J = 21.4 Hz), 112.3, 99.4. HRMS (ESI-FTMS, m/z): calcd for C24H16FN5O2 [M + H]+ 426.1367, found 426.1364. Yield (352 mg, 83%).
4-[3-(4-Fluorophenyl)-4-{(E)-[2-(4-nitrophenyl)hydrazinylidene]methyl}-1H-pyrazol-1-yl]benzoic Acid (31)
Orange solid, 1H NMR, 300 MHz (DMSO-d6): δ 11.23 (br s, 1H), 9.10 (s, 1H), 8.11–8.05 (m, 7H), 7.82–7.78 (m, 2H), 7.37 (J = 8.7 Hz, 2H), 7.07 (d, J = 8.5 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 167.1, 162.8 (1J = 244.4 Hz), 151.0, 150.9, 142.3, 138.5, 134.7, 131.3, 131.0 (3J = 8.3 Hz), 129.1, 129.0 (4J = 3.0 Hz), 128.2, 126.5, 118.5, 118.4, 116.0 (2J = 21.4 Hz), 111.4. HRMS (ESI-FTMS, m/z): calcd for C23H16FN5O4 [M + H]+ 446.1259, found 446.1265. Yield (396 mg, 89%).
3-[(2E)-2-[[1-(4-Carboxyphenyl)-3-(4-fluorophenyl)pyrazol-4-yl]methylene]hydrazino]benzoic Acid (32)
Yellow solid, 1H NMR, 300 MHz (DMSO-d6); δ 10.44 (s, 1H), 9.01 (s, 1H), 8.14–8.06 (m, 4H), 7.96 (s, 1H), 7.89–7.84 (m, 2H), 7.57 (s, 1H), 7.41–7.30 (m, 4H), 7.23 (m, 1H); 13C NMR (75 MHz, DMSO-d6) δ 168.1, 167.1, 162.7 (1J = 244.2 Hz), 150.6, 145.9, 142.5, 132.1, 131.3, 131.0 (3J = 8.3 Hz), 130.3, 129.6, 129.2 (4J = 3.1 Hz), 129.0, 127.8, 119.8, 119.1, 118.5, 116.4, 115.9 (2J = 21.3 Hz), 112.7. HRMS (ESI-FTMS, m/z): calcd for C24H17FN4O4 [M + H]+ 445.1307, found 445.1308. Yield (364 mg, 82%).
4-[(2E)-2-[[1-(4-Carboxyphenyl)-3-(4-fluorophenyl)pyrazol-4-yl]methylene]hydrazino]benzoic Acid (33)
Yellow solid, 1H NMR, 300 MHz (DMSO-d6); δ 12.71 (br s, 2H), 10.74 (s, 1H), 9.07 (s, 1H), 8.14–8.07 (m, 4H), 8.01 (s, 1H), 7.84–7.78 (m, 4H), 7.40–7.34 (m, 2H), 7.04 (d, J = 8.6 Hz, 2H); 13C NMR (75 MHz, DMSO-d6), δ 167.7, 167.1, 162.8 (1J = 244.3 Hz), 150.8, 149.2, 142.5, 131.7, 131.6, 131.3, 131.0 (3J = 8.3 Hz), 129.1 (4J = 2.9 Hz), 128.9, 127.6, 120.4, 119.0, 118.5, 116.0 (2J = 21.4 Hz), 111.4. HRMS (ESI-FTMS, m/z): calcd for C24H17FN4O4 [M + H]+ 445.1307, found 445.1308. Yield (381 mg, 86%).
4-[4-[(E)-[(2,4-Dioxoimidazolidin-1-yl)hydrazono]methyl]-3-(4-fluorophenyl)pyrazol-1-yl]benzoic Acid (34)
White solid, 1H NMR, 300 MHz (DMSO-d6): δ 11.26 (s, 1H), 9.02 (s, 1H), 8.15–8.05 (m, 4H), 7.89–7.85 (m, 2H), 7.73 (s, 1H), 7.33 (t, J = 8.8 Hz, 2H); 13C NMR (75 MHz, DMSO-d6); δ 169.5, 167.0, 162.8 (1J = 244.5 Hz), 153.7, 151.4, 142.3, 135.8, 131.3, 131.0 (3J = 8.3 Hz), 129.2, 128.6, 128.3, 118.8, 117.8, 116.1 (2J = 21.3 Hz), 49.0. HRMS (ESI-FTMS, m/z): calcd for C20H14FN5O4 [M + H]+ 408.1103, found 408.1105. Yield (317 mg, 78%).
3-[(2E)-2-[[1-(4-Carboxyphenyl)-3-(4-fluorophenyl)pyrazol-4-yl]methylene]hydrazino]benzoic Acid (35)
White solid, 1H NMR, 300 MHz (DMSO-d6); δ 11.02 (br s, 1H), 9.01 (s, 1H), 8.14–8.04 (m, 5H), 7.81–7.76 (m, 2H), 7.34 (t, J = 8.79 Hz, 2H), 3.67 (s, 3H); 13C NMR (75 MHz, DMSO-d6); δ 167.0, 162.8 (1J = 244.3 Hz), 154.2, 151.4, 142.4, 131.3, 131.0 (3J = 8.2 Hz), 129.1, 128.7, 128.1, 118.8, 117.9, 116.0 (2J = 21.4 Hz), 52.3. HRMS (ESI-FTMS, m/z): calcd for C19H15FN4O4 [M + H]+ 383.1150, found 383.1157. Yield (267 mg, 70%).
Acknowledgments
This publication was made possible by the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health. ABI mini-grant 200136 also helped to complete this manuscript.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01967.
1H and 13C NMR spectra of new compounds; computational data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- WHO. Antibiotic Resistance. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed May 31, 2019).
- CDC. Methicillin-resistant Staphylococcus aureus (MRSA). https://www.cdc.gov/mrsa/community/index.html (accessed May 31, 2019).
- CDC Acinetobacter in Healthcare Settings. Acinetobacter in Healthcare Settings (accessed Sept 22, 2016).
- Huang X. Z.; Chahine M. A.; Frye J. G.; Cash D. M.; Lesho E. P.; Craft D. W.; Lindler L. E.; Nikolich M. P. Molecular analysis of imipenem-resistant Acinetobacter baumannii isolated from US service members wounded in Iraq, 2003–2008. Epidemiol. Infect. 2012, 140, 2302–2307. 10.1017/S0950268811002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P.; Deye G.; Srinivasan A.; Murray C.; Moran K.; Hulten E.; Fishbain J.; Craft D.; Riddell S.; Lindler L.; Mancuso J.; Milstrey E.; Bautista C. T.; Patel J.; Ewell A.; Hamilton T.; Gaddy C.; Tenney M.; Christopher G.; Petersen K.; Endy T.; Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 2007, 44, 1577–1584. 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed Feb 28, 2017).
- Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- WHO . Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities; WHO: Geneva, 2017; p 76. [PubMed] [Google Scholar]
- Su S. C.; Vaneechoutte M.; Dijkshoorn L.; Wei Y. F.; Chen Y. L.; Chang T. C. Identification of non-fermenting Gram-negative bacteria of clinical importance by an oligonucleotide array. J. Med. Microbiol. 2009, 58, 596–605. 10.1099/jmm.0.004606-0. [DOI] [PubMed] [Google Scholar]
- Tommasi R.; Brown D. G.; Walkup G. K.; Manchester J. I.; Miller A. A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discovery 2015, 14, 529–542. 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- Okolo C.; Ali M. A.; Newman M.; Chambers S. A.; Whitt J.; Alsharif Z. A.; Day V. W.; Alam M. A. Hexafluoroisopropanol-Mediated Domino Reaction for the Synthesis of Thiazolo-androstenones: Potent Anticancer Agents. ACS Omega 2018, 3, 17991–18001. 10.1021/acsomega.8b02840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. A.; Okolo C.; Alsharif Z. A.; Whitt J.; Chambers S. A.; Varma R. S.; Alam M. A. Benign Synthesis of Thiazolo-androstenone Derivatives as Potent Anticancer Agents. Org. Lett. 2018, 20, 5927–5932. 10.1021/acs.orglett.8b02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharif Z. A.; Alam M. A. Modular synthesis of thiazoline and thiazole derivatives by using a cascade protocol. RSC Adv. 2017, 7, 32647–32651. 10.1039/C7RA05993K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharif Z.; Ali M. A.; Alkhattabi H.; Jones D.; Delancey E.; Ravikumar P. C.; Alam M. A. Hexafluoroisopropanol mediated benign synthesis of 2H-pyrido[1,2-a]pyrimidin-2-ones by using a domino protocol. New J. Chem. 2017, 41, 14862–14870. 10.1039/C7NJ03376A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. A.; Alsharif Z.; Alkhattabi H.; Jones D.; Delancey E.; Gottsponer A.; Yang T. Hexafluoroisopropyl alcohol mediated synthesis of 2,3-dihydro-4H-pyrido[1,2-a]pyrimidin-4-ones. Sci. Rep. 2016, 6, 36316 10.1038/srep36316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brider J.; Rowe T.; Gibler D. J.; Gottsponer A.; Delancey E.; Branscum M. D.; Ontko A.; Gilmore D.; Alam M. A. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Med. Chem. Res. 2016, 25, 2691–2697. 10.1007/s00044-016-1678-8. [DOI] [Google Scholar]
- Allison D.; Delancey E.; Ramey H.; Williams C.; Alsharif Z. A.; Al-khattabi H.; Ontko A.; Gilmore D.; Alam M. A. Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg. Med. Chem. Lett. 2017, 27, 387–392. 10.1016/j.bmcl.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeyah A. A.; Whitt J.; Duke C.; Gilmore D. F.; Meeker D. G.; Smeltzer M. S.; Alam M. A. Synthesis and antimicrobial studies of hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Bioorg. Med. Chem. Lett. 2018, 28, 2914–2919. 10.1016/j.bmcl.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt J.; Duke C.; Sumlin A.; Chambers S. A.; Alnufaie R.; Gilmore D.; Fite T.; Basnakian A. G.; Alam M. A. Synthesis of Hydrazone Derivatives of 4-[4-Formyl-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid as Potent Growth Inhibitors of Antibiotic-resistant Staphylococcus aureus and Acinetobacter baumannii. Molecules 2019, 24, 2051. 10.3390/molecules24112051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P.; Westwell A. D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. 10.1080/14756360701425014. [DOI] [PubMed] [Google Scholar]
- Saber H.; Jasni A. S.; Jamaluddin T. Z. M. T.; Ibrahim R. A Review of Staphylococcal Cassette Chromosome mec (SCCmec) Types in Coagulase-Negative Staphylococci (CoNS) Species. Malays. J. Med. Sci. 2017, 24, 7–18. 10.21315/mjms2017.24.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey P. D.; Olson M. E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. C. Bacillus anthracis. J. Clin. Pathol. 2003, 56, 182–187. 10.1136/jcp.56.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeyah A. A.; Whitt J.; Duke C.; Gilmore D. F.; Meeker D. G.; Smeltzer M. S.; Alam M. A. Synthesis and antimicrobial studies of hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Bioorg. Med. Chem. Lett. 2018, 28, 2914–2919. 10.1016/j.bmcl.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia J.; Raines R. T. Hydrolytic stability of hydrazones and oximes. Angew. Chem., Int. Ed. 2008, 47, 7523–7526. 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L.; Battini N.; Bheemanaboina R. R. Y.; Zhang S. L.; Zhou C. H. Design and synthesis of aminothiazolyl norfloxacin analogues as potential antimicrobial agents and their biological evaluation. Eur. J. Med. Chem. 2019, 167, 105–123. 10.1016/j.ejmech.2019.01.072. [DOI] [PubMed] [Google Scholar]
- Palm K.; Stenberg P.; Luthman K.; Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. 1997, 14, 568–571. 10.1023/A:1012188625088. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Saini V.; Maurya I. K.; Sindhu J.; Kumari M.; Kataria R.; Kumar V. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: Potential standalone or adjuvant antimicrobial agents. PLoS One 2018, 13, e0196016 10.1371/journal.pone.0196016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker S. D.; Nahar L.; Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel P.; Schmidt-Emrich S.; Maniura-Weber K.; Ren Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. 10.1186/s12866-015-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.