Abstract
Quaternary-ammonium-salt-type amphiphilic gemini compounds (Cm-2-Cn X, where m and n represent the alkyl chain lengths; m = 4, 6, 8, 10; n = 2, 4, 6, 8, 10; m ≥ n; and X indicates the counterion BF4, PF6, OTf, FSA, or NTf2) were synthesized by the quaternization of N,N,N′,N′-tetramethylethylenediamine and n-alkyl bromide and a subsequent ion-exchange reaction with five different counterions. For comparison, the corresponding monomeric compounds (Cn X, n = 2, 4, 6, 8, and 10) were also synthesized. The melting points of the compounds were evaluated using differential scanning calorimetry, and those with melting points lower than 100 °C were treated as ionic liquids during the subsequent measurements. The amphiphilic gemini compounds exhibited the lowest melting points (44–49 °C) when bulky NTf2– was the counterion and the degree of dissymmetry between the two alkyl chains was 0.4 < n/m < 0.75. However, their melting points were not similar to those of the monomeric compounds with NTf2– and n = 4–10 (<29 °C). The gemini ionic liquids exhibited significantly lower conductivities and higher viscosities than those of the corresponding monomeric ionic liquids. This is because of the decrease in the mobility of the cation molecules caused by the gemini structure, in which the two monomeric compounds are connected by a spacer. The gemini ionic liquids also showed higher densities than those of the corresponding monomeric ionic liquids, owing to the dimer of the gemini structure. Further, the gemini ionic liquids were adsorbed readily at the air/water interface and oriented themselves but did not show the critical micelle concentration for the concentration range over which they could be dissolved in water. The amphiphilic monomeric and gemini ionic liquids also tended to form ion pairs in aqueous solutions, as the length of their alkyl chain was relatively short.
Introduction
Ionic liquids are salts consisting only of cation and anion and exhibit melting points lower than 100 °C. Ionic liquids were first reported by Wilkes et al. in 1992.1 These ionic liquids are stable in both air and water and are in the liquid state at room temperature. Of late, they have been the subject of extensive research efforts.2−4 They are known to be environmentally friendly solvents because of their properties of nonvolatility and nonflammability and are attracting interest as novel solvents that differ from water and conventional organic solvents.2−7 There can exist several combinations of cation and anion in ionic liquids. Hence, the physicochemical properties of these liquids, such as their melting point, density, viscosity, polarity, and hydrophobicity, can be controlled readily. Therefore, they are being explored extensively for use as reaction solvents,2 electrolytes,2,5 catalysts,4−6 lubricants,8,9 and drug delivery systems.10 Ionic liquids with different structures and functions, such as external-stimulus-responsive ionic liquids,11 magnetic ionic liquids,12 and cellulose-dissolving ionic liquids,13 have been reported, and the development of other novel ionic liquids with better performance and greater functionality is expected in the future.
Surfactants get adsorbed at the air/water or water/oil interface so that the surface free energy is lowered and form aggregates of different morphologies, such as micelles and vesicles in aqueous solutions, because of their amphiphilic structure, which consists of both hydrophobic and hydrophilic groups. They are used widely in many applications and products, including detergents, cosmetics, food, and paint, based on their properties of interfacial adsorption and aggregate formation. Of late, gemini surfactants, in which two conventional monomeric surfactants are connected by a spacer, have attracted a lot of interest because they show excellent surface-active properties, such as lower critical micelle concentrations (CMCs) and a greater ability to lower the surface tension than those of the corresponding monomeric surfactants.14−18
It is known that amphiphilic ionic liquids containing an alkyl chain show surface activity and form aggregates in aqueous solutions in a manner similar to surfactants.19 For instance, it has been reported that amphiphilic monomeric ionic liquids of 1-alkyl-3-methylimidazolium and 1-alkylpyridinium salts show surface activities in aqueous solutions similar to those of conventional surfactants.20,21 However, these amphiphilic ionic liquids are mostly imidazolium-based20,21 and amine-salt-containing protic-type compounds.22 So far, only gemini surfactants with hydrophobic, hydrophilic, and spacer groups have been reported, and there have been few reports on gemini ionic liquids,23−25 including quaternary-ammonium salt-type amphiphilic gemini ionic liquids.25
In this study, quaternary-ammonium-salt-type amphiphilic gemini compounds with alkyl chains of identical and nonidentical lengths and bulky counterions (Cm-2-Cn X, where m and n represent the alkyl chain length; m = 4, 6, 8, 10; n = 2, 4, 6, 8, 10; m ≥ n, X indicates counterion, which is one of the following: BF4, PF6, OTf, FSA, or NTf2; see Figure 1a) were designed and synthesized along with the corresponding monomeric compounds (Cn X, n = 2, 4, 6, 8, 10; see Figure 1b). This was done with the aim of developing novel amphiphilic ionic liquids that show the properties of both ionic liquids and surfactants as well as those of gemini surfactants. We investigated their properties as ionic liquids as well as their surface-active properties in aqueous solutions. Further, we discuss the effects of the alkyl chain length, number of alkyl chains, degree of dissymmetry of the chains, and structure of the counterion of the ionic liquids on their properties.
Figure 1.
Chemical structures of (a) quaternary-ammonium-salt-type amphiphilic compounds gemini-type Cm-2-Cn X, (b) monomeric-type Cn X, and (c) counterion X.
Results and Discussion
Melting Point
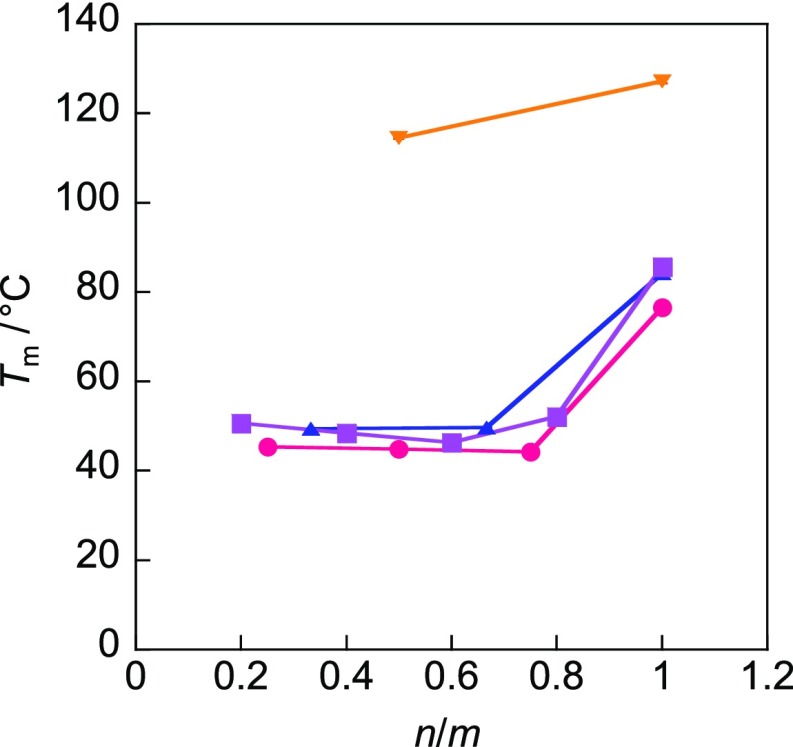
The melting points of the gemini and monomeric compounds could be arranged in the following order, based on the counterion present: PF6–, OTf– > BF4– > FSA–, NTf2–. Thus, the compounds containing FSA– and NTf2– had the lowest melting points (Table S1). In general, the radii of the above-mentioned anions can be arranged as follow: BF4– (3.44 Å) < PF6– (3.60 Å) < OTf– (3.79 Å) < NTf2– (4.39 Å).26 The radius of FSA– can be assumed to lie between those of OTf– and NTf2–, based on their structures. The melting points of the compounds containing counterions FSA– and NTf2–, which have a bulky structure, were very low. As stated previously, ionic liquids are salts consisting of cations and anions, which undergo electrostatic interactions.27,28 Ionic liquids show low melting points as compared to inorganic salts because they contain organic ions, which have bulky structures. On the other hand, the melting points of the monomeric compounds, Cn X with n = 2, were higher than 100 °C and could be arranged in the following order based on the counterion present: Br–, BF4–, PF6– > OTf– > FSA– > NTf2– (Figure S1). Cn NTf2 compounds with n = 8 and 10 were liquid at temperatures below 0 °C, and their melting points could not be obtained. An increase in the length of the alkyl chain from 2 to 4 resulted in a significant decrease in the melting point; this was true for all counterions. The attraction between the cation and the anion is greater for shorter alkyl chains, while the van der Waals interaction between the alkyl chains is stronger for longer alkyl chains, resulting in a high melting point.29 The melting point increased with an increase in the alkyl chain length from 6 to 8 because the van der Waals interactions between the alkyl chains became stronger than the electrostatic interaction between the cations and anions. A similar behavior has been reported in the literature.30,31Figure 2 shows the relationship between the melting point and degree of dissymmetry of the two alkyl chains, n/m (m ≥ n), for the amphiphilic gemini compounds Cm-2-Cn NTf2 (the values of the melting points of the amphiphilic gemini compounds containing the other counterions, namely, BF4–, PF6–, OTf–, and FSA–, are listed in Table S1). The error in the melting points of the amphiphilic gemini ionic liquids Cm-2-Cn NTf2 was ±0.6 °C. The melting points of Cm-2-Cn NTf2 were high for n/m = 1 (alkyl chains of the same length) but were low for 0.2 ≤ n/m < 1 (except for m = 4). This can be ascribed to the fact that the corresponding ionic liquids are packed tightly because of the strong van der Waals interactions between the alkyl chains owing to their high symmetry. It is known that a dissymmetric molecular structure or the presence of flexible alkyl chains leads to a decrease in the melting point because the lattice energy in this case is low, resulting in poor crystallinity.27 Thus, it can be concluded that the amphiphilic gemini compounds that exhibited low melting points did so because of the presence of dissymmetric alkyl chains. It is worth noting that the melting point of amphiphilic gemini compounds can be lowered to approximately 40 °C without causing an increase in the van der Waals force by varying the length (degree of dissymmetry) between the two alkyl chains.
Figure 2.
Relationship between melting point and degree of dissymmetry of two alkyl chains, n/m, for Cm-2-Cn NTf2: orange triangle, down, solid, m = 4; blue triangle, up, solid, m = 6; pink circle, solid, m = 8; and purple square, solid, m = 10.
For example, despite their asymmetric structure, amphiphilic gemini compounds C4-2-C2 BF4 (n/m = 0.5), C6-2-C2 BF4 (n/m = 0.33), and C6-2-C2 FSA (n/m = 0.33) showed higher melting points than those of C4-2-C4 BF4, C6-2-C6 BF4, and C6-2-C6 FSA, respectively, which contained the same alkyl chains. It can be surmised that the former gemini compounds exhibit high ionicity owing to their short chain (n = 2) and are crystalized readily, even though the van der Waals force between their chains is smaller. This is in spite of the fact that the difference between the lengths of the two alkyl chains is large. That is to say, there is a high degree of asymmetry between the structures of the alkyl chains. On the other hand, the melting-point behavior of Cm-2-Cn OTf was different from those of the compounds with the other counterions, whose melting point increased as the chains became more symmetric. This is because the van der Waals force increases with an increase in the alkyl chain length. Thus, it was found that the melting point of amphiphilic gemini compounds is significantly influenced by the degree of asymmetry between the two alkyl chains as well as the structure of the counterion present.
Characterization of Amphiphilic Gemini Ionic Liquids
The amphiphilic gemini compounds Cm-2-Cn X (with the exception of C4-2-C4 FSA, C6-2-C2 FSA, C4-2-C2 NTf2, and C4-2-C4 NTf2) and the monomeric ones Cn X with FSA– or NTf2– (with the exception of C2 FSA and C2 NTf2) were ionic liquids because their melting points were lower than 100 °C. The water contents of the amphiphilic gemini and monomeric compounds were determined to be less than 500 ppm (Table S2); this suggested that investigation of the properties of these ionic liquids would not be problematic. Figure 3 shows the plots of the zero-shear viscosity and conductivity as functions of n/m (C10-2-C4 FSA and C6-2-C4 NTf2) and n (Cn FSA and Cn NTf2). Here, the zero-shear viscosity was obtained from the relationship between the viscosity and shear rate (Figures S2 and S3). The viscosities of the amphiphilic ionic liquids did not change with increasing shear rate, indicating that they are Newtonian fluids (Figures S2 and S3). The zero-shear viscosities differed according to the structure of the ionic liquids. The viscosity of gemini ionic liquid C6-2-C4 NTf2 was 3.35 × 103 mPa s and 30–40 times higher than that of the corresponding monomeric ionic liquid. This can be attributed to the fact that the van der Waals force between the two alkyl chains enhances their degree of entanglement, resulting in high viscosity. The viscosities of the quaternary-ammonium-salt-type ionic liquids (both gemini and monomeric types) were higher than that of the imidazolium-based ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide (50 mPa s) as well as those of the protic ionic liquids such as ethylammonium nitrate (32 mPa s) and propylammonium nitrate (66.6 mPa s).32 The viscosity of ionic liquids depends on the interactions that occur between their molecules, such as collisions, hydrogen-bond formation, van der Waals interactions, and electrostatic interactions: the stronger these interactions are, the higher the viscosity will be.33,34 In general, it is known that ionic liquids show viscosities 100–1000 times higher than those of water and organic solvents because ionic liquids consist of a cation and an anion and the electrostatic interactions between them are strong. This, in turn, determines the viscosity.27,34,35 However, the amphiphilic gemini ionic liquids showed extremely high viscosities, suggesting that their gemini-type structure enhanced the van der Waals interactions between the two alkyl chains.
Figure 3.
Relationships between (a) zero-shear viscosity and (b) conductivity and degree of dissymmetry of two alkyl chains, n/m (gemini type), and alkyl chain length, n (monomeric type): black triangle, up, solid, C6-2-C4 FSA (50 °C); red triangle, up, solid, C6-2-C6 FSA (50 °C); black triangle, open, down, C8-2-C6 FSA (60 °C); orange titled square, solid, C10-2-C4 FSA (50 °C); sky blue square, solid, C6-2-C2 NTf2 (50 °C); purple square, solid, C6-2-C4 NTf2 (50 °C); green square, solid, C8-2-C6 NTf2 (60 °C); red circle, solid, Cn FSA; and blue circle solid, Cn NTf2 (25 °C).
The conductivity of the amphiphilic gemini ionic liquid C6-2-C4 NTf2 (2.84 mS m–1 at 50 °C) was 1/58 and 1/32 of those of the corresponding monomeric ionic liquids, namely, C4 NTf2 and C6 NTf2, respectively. That is to say, the gemini ionic liquid showed significantly lower conductivity than did the corresponding monomeric ones. The charge mobility of gemini ionic liquids is small because of their structure, in which two cation molecules are connected by a spacer. The conductivity of gemini ionic liquid C8-2-C6 FSA was 5.2 times that of C8-2-C6 NTf2 at 65 °C; thus, the conductivity of the ionic liquid containing FSA– was higher than that of the ionic liquid containing NTf2–, in keeping with the conductivity behavior of monomeric ionic liquids (Table S3). In the counterion NTf2–, the fluorine (−F) of FSA– is replaced with the trifluoromethyl group (−CF3). Further, FSA– has a more flexible structure as compared to that of NTf2–. Therefore, the ionic liquid with FSA– showed higher conductivity and lower viscosity.36 The conductivity of the gemini ionic liquids C8-2-Cn X reduced as their structure became more symmetric. This was also the case when the length of the alkyl chains of the monomeric ionic liquids was increased. This can be ascribed to the fact that the van der Waals force becomes stronger and the mobility reduces with these changes.
The quaternary-ammonium-salt-type amphiphilic monomeric ionic liquids Cn X exhibited lower conductivities compared to those of the imidazolium-type ionic liquid 1-buthyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide (4.06 × 102 mS m–1)37 and protic ionic liquids such as methylammonium formate (4.38 × 103 mS m–1),38 ethylammonium nitrate (2.69 × 103 mS m–1),39 and ethylammonium acetate (222 mS m–1).40 In general, ionic liquids with an aromatic part, where charge is delocalized, such as imidazolium-type and pyridinium-type ionic liquids, show high conductivities and low viscosities. However, quaternary-ammonium-salt-type gemini ionic liquids show low conductivities and high viscosities. This is because of the effects of the localized charge of the ammonium ion and the van der Waals force between the alkyl chains. The conductivity of the ionic liquids also increased with an increase in the temperature (Table S3), in keeping with the behavior of conventional ionic liquids.
Ionic liquids show high densities (more than 1.0 g cm–3) because their structure consists only of ions.29,41 The density of gemini ionic liquid C6-2-C4 NTf2 was 1.45 g cm–3 and higher than those of the corresponding monomeric ionic liquids C4 NTf2 (1.40 g cm–3) and C6 NTf2 (1.33 g cm–3) (Table S3), indicating that the gemini ionic liquid was more densely packed than the monomeric ones. The densities of the monomeric ionic liquids with counterion NTf2– were higher than those of the ionic liquids with FSA– and decreased with an increase in the length of the alkyl chain. This result was consistent with the trends seen in the case of conventional ionic liquids.
Solubilities in Various Organic Solvents
The solubilities of the amphiphilic gemini and monomeric ionic liquids in various organic solvents such as dimethyl sulfoxide (DMSO), methanol, acetone, ethyl acetate, chloroform, benzene, and hexane were measured by adding 0.01–10 mL of water or the organic solvents to 0.01–0.1 g of the ionic liquids and then allowing them to settle down for at least 24 h. The amphiphilic gemini ionic liquids showed low solubilities in water, and the solubilities could be arranged in the following order based on the counterion present: FSA > NTf2 (Table S4). The solubility decreased with an increase in the alkyl chain length as well as when the two alkyl chains became more symmetric. The gemini ionic liquids (Cm-2-Cn X, X = FSA and NTf2) showed low solubilities in water but were soluble in organic solvents such as DMSO and methanol at 25 °C (Table S5). The gemini and monomeric ionic liquids with FSA– and NTf2– also showed high solubilities in acetone and ethyl acetate and excellent solubilities in organic solvents with a wide range of polarities (DMSO, methanol, acetone, and ethyl acetate). The monomeric ionic liquids also showed solubilities similar to those of the gemini ionic liquids, with those having n ≥ 4 being soluble in chloroform and benzene. Further, the amphiphilic gemini and monomeric ionic liquids were insoluble in hexane, whose polarity is low.
Solution Properties of Amphiphilic Gemini Ionic Liquids
The solution properties of the amphiphilic gemini ionic liquids Cm-2-Cn X (m = 4, 6, 8, 10; n = 2, 4, 6, 8, 10; X = FSA and NTf2) (with the exception of C4-2-C4 FSA, C6-2-C2 FSA, C4-2-C2 NTf2, and C4-2-C4 NTf2) and monomeric ionic liquids Cn X (n = 4, 6, 8, 10; X = FSA and NTf2), including their Krafft temperature, conductivity, surface tension, and pyrene fluorescence values, were determined.
Krafft Temperature (TK)
Clear aqueous solutions of the amphiphilic gemini ionic liquids and the corresponding monomeric ionic liquids (0.020–0.20 wt %) were prepared by dissolving them in hot water and placing the formed solution in a refrigerator at ∼5 °C for at least 24 h. All of the amphiphilic ionic liquids with FSA– and NTf2–, except for C4-2-C2 FSA and C6-2-C4 FSA, precipitated in the solution. Next, the temperature of the cooled solution was raised gradually under constant stirring, and the conductance (κ) was measured over the temperature range of 0.5–5.0 °C. Further, TK was determined based on the relationship between the electrical conductivity and the temperature (Figure S4). Initially, the conductivity increased rapidly with an increase in the temperature because of the gradual dissolution of the amphiphilic ionic liquids. Subsequently, it increased gradually owing to the increase in the ionic mobility. TK was taken as the temperature at which the conductivity versus temperature plot exhibited a break point. Tables 1 and 2 show the values of TK for the amphiphilic gemini and monomeric ionic liquids with FSA− and NTf2−, respectively. The TK values of all of the amphiphilic monomeric ionic liquids were less than 5 °C, with the exception of C10 NTf2. The TK values of the amphiphilic gemini ionic liquids were higher than those of the corresponding monomeric ionic liquids. The TK values of the gemini ionic liquids Cm-2-Cn FSA containing alkyl chains of the same length (m = n) increased with an increase in the alkyl chain length. As the length of one of the alkyl chains, m, of the gemini ionic liquids Cm-2-Cn FSA was fixed at 6 or 8, TK was dependent on the length of the other alkyl chain, that is, on n. Thus, it was found that the amphiphilic gemini ionic liquids showed poor solubility in water as compared to the corresponding monomeric ionic liquids because of the dimer structure formed in the former case by the two hydrophobic alkyl chains as well as owing to the presence of a hydrophobic counterion.
Table 1. Melting Point (Tm), Krafft Temperature (TK) at 0.20 wt %, Concentration for Ion-Pair Formation (CI.P.), α, Surface Excess Concentration (Γ), Occupied Area per Molecule (A), and Adsorption Efficiency (pC20) Values of Amphiphilic Ionic Liquids Cm-2-Cn FSA and Cn FSA at 25 °C.
| ionic liquid | Tm/°C | TK/°C | CI.P./mmol dm–3 | α | Γ × 106/mol m–2 | A/nm2 | pC20 |
|---|---|---|---|---|---|---|---|
| C4-2-C2 FSA | 61.7 | <5 | 3.92 | 0.72 | |||
| C6-2-C4 FSA | 44.1 | <5 | 0.69 | 0.79 | |||
| C6-2-C6 FSA | 45.4 | 24.7b | 0.57 | 0.84 | |||
| C8-2-C2 FSA | 44.1 | 21.3 | 1.13 ± 0.02 | 1.47 ± 0.02 | 2.56 ± 0.02 | ||
| C8-2-C4 FSA | 65.5 | 18.2b | 1.27 ± 0.05 | 1.31± 0.05 | 2.86 ± 0.07 | ||
| C8-2-C6 FSA | 51.5 | 23.3c | 1.18 ± 0.03 | 1.40 ± 0.04 | 3.31 ± 0.06 | ||
| C8-2-C8 FSAa | 66.7 | 43.3c | 1.21 ± 0.01 | 1.38 ± 0.02 | 3.64 ± 0.02 | ||
| C8-2-C8 FSAa (in NaBr solution) | 2.93 ± 0.08 | 0.568 ± 0.016 | 4.10 ± 0.03 | ||||
| C10-2-C4 FSA | 48.5 | 20.2c | 1.13 ± 0.14 | 1.47 ± 0.19 | 3.29 ± 0.22 | ||
| C4 FSA | 17.9 | <5 | 26.3 | 0.61 | |||
| C6 FSA | 46.7 | <5 | 5.32 | 0.88 | |||
| C8 FSA | 7.18 | <5 | 2.15 | 0.75 | 1.81 ± 0.07 | 0.917 ± 0.035 | 2.44 ± 0.05 |
| C10 FSA | 27.4 | <5b | 2.54 ± 0.08 | 0.653 ± 0.020 | 3.09 ± 0.48 |
45 °C.
0.10 wt %.
0.050 wt %.
Table 2. Melting Point (Tm), Krafft Temperature (TK) at 0.20 wt %, Concentration for Ion-Pair Formation (CI.P.), α, Surface Excess Concentration (Γ), Occupied Area per Molecule (A), and Adsorption Efficiency (pC20) Values of Amphiphilic Ionic Liquids Cm-2-Cn NTf2 and Cn NTf2 at 25 °C.
| ionic liquid | Tm/°C | TK/°C | CI.P./mmol dm–3 | α | Γ × 106/mol m–2 | A/nm2 | pC20 |
|---|---|---|---|---|---|---|---|
| C6-2-C2 NTf2 | 49.4 | 37.1 | 0.555 | 0.77 | 1.22 ± 0.07 | 1.36 ± 0.08 | 2.92 ± 0.11 |
| C6-2-C4 NTf2 | 49.7 | 41.3 | 1.03 ± 0.01 | 1.61 ± 0.01 | 3.25 ± 0.02 | ||
| C6-2-C6 NTf2 | 84.2 | 62.1b | 1.03 ± 0.05 | 1.61 ± 0.08 | 3.65 ± 0.09 | ||
| C8-2-C2 NTf2 | 45.4 | 28.1 | 0.408 | 0.87 | 1.20 ± 0.05 | 1.39 ± 0.05 | 3.38 ± 0.08 |
| C8-2-C4 NTf2 | 44.9 | 75.5c | |||||
| C4 NTf2 | 8a | <5 | 6.93 | 0.83 | 1.46 ± 0.07 | 1.14 ±0.06 | 2.13 ± 0.07 |
| C6 NTf2 | 29.2 | <5 | 3.04 | 0.73 | 1.85 ± 0.06 | 0.898 ± 0.027 | 2.63 ± 0.05 |
| C8 NTf2 | <0 | <5b | 2.00 ± 0.06 | 0.829 ± 0.025 | 2.95 ± 0.09 | ||
| C10 NTf2 | <0 | 38.8c |
Conductivity, Surface Tension, and Pyrene Fluorescence
Figure 4 shows the plots of the conductivity, surface tension, and pyrene fluorescence intensity ratio, I1/I3, as functions of the concentration for the gemini ionic liquids C8-2-Cn FSA and monomeric ionic liquid C8 FSA in aqueous solution. Figures S5–S8 show the plots of the conductivity, surface tension, and pyrene fluorescence intensity ratio, I1/I3, of ionic liquids C4-2-C2 FSA, C6-2-Cn FSA (n = 4, 6), C10-2-C4 FSA (Figure S5), Cn FSA (n = 4, 6, 8, 10) (Figure S6), C6-2-Cn NTf2 (n = 2, 4, 6), C8-2-Cn NTf2 (n = 2, 4) (Figure S7), and Cn NTf2 (n = 4, 6, 8, 10) (Figure S8). The conductivity increased and surface tension decreased with an increase in the concentration of C8-2-Cn FSA (n = 2–8) and C8 FSA, and a break point was not observed for any of the compounds with the exception of C8 FSA. Therefore, the solubilities of the ionic liquids in water were poor because of the presence of the hydrophobic counterion. Further, micelle formation was not observed for the range of concentrations over which the ionic liquids did not dissolved in water. This result was in keeping with the pyrene fluorescence data, which showed that the I1/I3 value remained constant (did not decrease) even with an increase in the concentration. For C8 FSA, although a break point was observed in the conductivity plot, it was not observed in the plots for I1/I3 and the surface tension. Thus, the break point did not correspond to the CMC but was indicative of the formation of an ion pair because ion pairs do not readily form micelles owing to the shortness of their alkyl chains. This behavior was also observed in the cases of gemini ionic liquids C4-2-C2 FSA, C6-2-Cn FSA (n = 4, 6), and Cm-2-C2 NTf2 (m = 4, 6, 8) and the monomeric ionic liquids Cn FSA (n = 4, 6, 8) and Cn NTf2 (n = 4, 6) (Table 1 and Table 2). It has been reported that imidazolium-based ionic liquids (n ≥ 8) with Cl–, PF6–, or NTf2– as the counterion form micelles in aqueous solutions, while that with n = 6 does not form, instead forming a monolayer at the air/water interface.42 It has also been reported that amphiphilic compounds with a short alkyl chain (n = 8) exhibit two break points in their conductivity plots.43,44 The break points at the higher concentration corresponded to the CMC, as confirmed by the results of the surface tension and fluorescence measurements. Further, the break points occurring at concentrations lower than the CMC were attributable to the formation of an ion pair between the ammonium cation and the counterion. The amphiphilic ionic liquids with a short alkyl chain did not exhibit a second break point in their conductivity plot because of their poor solubility in water. However, they show a single break point, which corresponded to the formation of an ion pair.
Figure 4.
Variations in (a) conductivity, (b) surface tension, and (c) pyrene fluorescence intensity ratio, I1/I3, with ionic liquid concentration for C8-2-Cn FSA and C8 FSA: green circle, solid, n = 2; orange titled square, open, n = 4; blue square, solid, n = 6; red triangle, up, solid, n = 8; red triangle, up, open, n = 8 (in the presence of NaBr); and black circle, open, C8 FSA.
The adsorption and orientation of amphiphilic compounds at the air/water interface are related to the surface excess concentration, Γ, and the occupied area per molecule, A, which can be calculated from the slope for the linear relationship between the surface tension and the concentration and the Gibbs adsorption isotherm equations. Tables 1 and 2 show the degree of dissociation (α), Γ, A, and pC20 values as determined from the surface tension plots. The values of A of the amphiphilic gemini ionic liquids C8-2-Cn FSA were larger than that of the corresponding amphiphilic monomeric ionic liquid C8 FSA. The addition of NaBr (0.50 mmol dm–3) to the C8-2-C8 FSA solution increased its solubility and led to a decrease in the surface tension and the occupied area per molecule. However, a clear break point, which would correspond to the CMC, was not observed at the concentrations at which the ionic liquid dissolved in water. The occupied area per molecule of C8-2-C8 FSA in the presence of NaBr became smaller than that of C8 FSA in the absence of NaBr, indicating that the former would get adsorbed readily at the air/water interface because of the decrease in the electrostatic interaction between its hydrophilic groups after the addition of the salt. It has been reported that imidazolium-based ionic liquids with BF4– as the counterion form micelles in aqueous solutions, exhibit CMC values lower than those of ionic liquids containing Br–, and are readily adsorbed at the air/water interface.19 The values of the efficiency of adsorption, pC20, of the gemini ionic liquids (2.6–3.6) were larger than that of the corresponding monomeric one (2.4). This was particularly true as the lengths of the two alkyl chains became similar. The pC20 value increased further with the addition of the salt. The larger the pC20 value is, the more efficiently amphiphilic ionic liquids are adsorbed at the air/water interface.45 Thus, it was confirmed that these quaternary-ammonium-salt-type amphiphilic gemini ionic liquids would be adsorbed efficiently at the air/water interface, even though they did not form micelles in aqueous solutions. The A values of gemini ionic liquids C6-2-Cn NTf2 (n = 2, 4, 6) increased in the order of n = 2 (1.44 nm2) < n = 6 (1.50 nm2) < n = 4 (1.73 nm2), while their pC20 values increased in the order of n = 2 (2.9) < n = 4 (3.2) < n = 6 (3.7). Thus, it can be concluded that C6-2-Cn NTf2 will be adsorbed readily at the air/water interface as n is increased from 2 to 4, while for C6-2-Cn NTf2 with n = 6, the symmetric alkyl chains are adsorbed in a large number at the interface.
Conclusions
In this study, quaternary-ammonium-salt-type amphiphilic gemini compounds with two alkyl chains having identical and nonidentical lengths and their corresponding monomeric amphiphilic compounds were synthesized using five different counterions (BF4–, PF6–, OTf–, FSA–, and NTf2–). Their physicochemical properties, such as their melting point, conductivity, viscosity, density, and solubility, in various organic solvents, as well as their aqueous-solution properties, such as their conductivity, surface tension, and pyrene fluorescence, were investigated.
It was found that the melting points of the quaternary-ammonium-salt-type amphiphilic gemini compounds Cm-2-Cn X are significantly affected by the degree of dissymmetry between the two alkyl chains (n/m). Further, they can be decreased significantly through the ion exchange of the bromide ion with a bulky counterion (BF4–, PF6–, OTf–, FSA–, or NTf2–). The melting points of the amphiphilic gemini compounds were high when the two alkyl chains in the molecules had the same length (n/m = 1) and were low for 0.2 ≤ n/m <1. More notably, the melting point of the gemini ionic liquids could be decreased to approximately 40 °C by changing the n/m ratio as well as the counterion. The amphiphilic gemini ionic liquids showed lower conductivities, higher viscosities, higher densities, and higher solubilities in organic solvents as compared to the corresponding monomeric ionic liquids. Further, the amphiphilic gemini and monomeric ionic liquids with short alkyl chains showed a small break point in their conductivity–concentration curves, which corresponded to the formation of an ion pair. The addition of a salt to aqueous solutions of the amphiphilic gemini ionic liquids resulted in enhanced adsorption and improved orientation at the air/water interface as compared to the case for aqueous solutions of the gemini ionic liquids and the corresponding monomeric ionic liquids free of a salt.
In this study, we elucidated the effects of the alkyl chain length, number of alkyl chains, structural dissymmetry, and counterion structure on the physicochemical and aqueous-solution properties of quaternary-ammonium-salt-type amphiphilic ionic liquids. The development of amphiphilic ionic liquids that exhibit properties of both ionic liquids and surfactants is difficult, given the challenges faced in lowering their melting point and improving their solubility in water. In the future, it is expected that it will be possible to synthesize novel amphiphilic ionic liquids with high performance and functionality through molecular design for use in various industrial applications.
Experimental Section
Materials
N,N,N′,N′-Tetramethylethylenediamine, n-ethyl bromide, n-butyl bromide, n-hexyl bromide, n-octyl bromide, and n-decyl bromide were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Trimethylamine hydrochloride, silver tetrafluoroborate (AgBF4), potassium hexafluorophosphate (KPF6), potassium trifluoromethanesulfonate (KOTf), acetone, acetonitrile, chloroform, dichloromethane, dimethyl sulfoxide (DMSO), ethanol, ethyl acetate, hexane, methanol, and sodium hydroxide were purchased from FUJIFILM Wako Pure Chemical Co., Ltd. (Osaka, Japan). Potassium bis(trifluoromethanesulfonyl)amide (KNTf2) and thymolphthalein were purchased from Kanto Chemicals Co., Inc. (Tokyo, Japan), and potassium bis(fluorosulfonyl)amide (KFSA) was supplied by Nippon Shokubai Co., Ltd. (Osaka, Japan). The deuterated chloroform, deuterium oxide, and deuterated dimethyl sulfoxide used in the 1H NMR measurements were purchased from Cambridge Isotope Laboratories Inc. (Andover). All chemicals were used without further purification. Water from a Merck KGaA Direct-Q UV system (resistivity = 18.2 MΩ cm, Darmstadt, Germany) was used in all of the experiments.
Synthesis of N,N-Dimethyl-N-[2-(N′,N′-dimethylamino)ethyl]alkylammonium Bromide
First, n-butyl bromide, n-hexyl bromide, n-octyl bromide, or n-decyl bromide (1.0 equiv) was added in a dropwise manner to a heated solution of N,N,N′,N′-tetramethylethylenediamine (5.0 equiv) in methanol, and the solution was stirred. The mixture was then refluxed for 5 h. Next, the solvent was evaporated under reduced pressure, the residue was washed several times with hexane, and the hexane phase was removed by decantation. Then, the residue was dried under reduced pressure to obtain N,N-dimethyl-N-[2-(N′,N′-dimethylamino)ethyl]alkylammonium bromide (alkyl chain length = 4, 6, 8, and 10, respectively) as a yellow or white viscous solid (Supporting Information: yields and 1H NMR data).
Synthesis of N,N-Dimethyl-N-[(N′,N′-dimethyl-N′-(alkylammonio)ethyl)]alkylammonium Dibromide (Cm-2-Cn Br)
First, n-ethyl bromide, n-butyl bromide, n-hexyl bromide, n-octyl bromide, or n-decyl bromide (2.0 equiv) was added in a dropwise manner to a solution of N,N-dimethyl-N-[(N′,N′-dimethylamino)ethyl] alkylammonium bromide (1.0 equiv) in acetonitrile, then the solution was heated under stirring, and the mixture was refluxed for over 30 h. Next, the solvent was evaporated under reduced pressure. The residue was then washed several times, first with hexane and then with ethyl acetate, and recrystallized using a mixture of hexane and ethanol (5:1, vol/vol). The residual solid was dried under reduced pressure, yielding N,N-dimethyl-N-[(N′,N′-dimethyl-N′-(alkylammonio)ethyl)]alkylammonium dibromide Cm-2-Cn Br as a white solid (Supporting Information: yields, 1H NMR data, and elemental analysis).
Ion Exchange of Tetrafluoroborate, Hexafluorophosphate, Trifluoromethanesulfonate, Bis(fluorosulfonyl)amide, and Bis(trifluoromethanesulfonyl)amide Ions with Bromide Ion for Cm-2-Cn Br
AgBF4, KPF6, KOTf, KFSA, or KNTf2 (2.2 equiv) dissolved in water was added to N,N-dimethyl-N-[(N′,N′-dimethyl-N′-(alkylammonio)ethyl)]alkylammonium dibromide (1.0 equiv) in water, and the solution was heated under stirring for over 10 h. The purification procedure for each of the counterions is described below.
Cm-2-Cn BF4
After the precipitated gray solid had been removed by filtration, the solvent was evaporated under reduced pressure. For C4-2-C2 BF4, C4-2-C4 BF4, C6-2-C2 BF4, C6-2-C6 BF4, and C8-2-C8 BF4, the residue was washed repeatedly with hot ethyl acetate, yielding the corresponding compound. For C6-2-C4 BF4, C8-2-C4 BF4, and C8-2-C6 BF4, the residue was dissolved in water and extracted using ethyl acetate. This procedure was repeated several times, and the ethyl acetate phase was evaporated under reduced pressure to obtain the desired compound.
Cm-2-Cn OTf
After the solution had been evaporated under reduced pressure to remove the solvent, acetone was added to the residue. The inorganic salt was removed by filtration, and the filtrate was evaporated. This procedure was repeated twice. Next, the residue was washed with hot ethyl acetate (in the case of C6-2-C4 OTf, it was washed thrice with water) and recrystallized using a mixture of hexane and ethanol (7:1, vol/vol).
Cm-2-Cn X (X = PF6, FSA, NTf2)
After the white solid had been precipitated, the solution was filtered to obtain the solid. On the other hand, in the case where a viscous material was formed, the upper-phase solution was removed by decantation to obtain the viscous material. The residue was washed five times with water and then dried. Acetone was added to the dried residue, which was then filtered to remove the inorganic salt, while the solvent in the filtrate was removed by evaporation. This procedure was repeated twice. In the case of Cm-2-Cn PF6, the residue was further recrystallized using methanol and dried under reduced pressure, yielding N,N-dimethyl-N-[(N′,N′-dimethyl-N′-(alkylammonio)ethyl)]alkylammonium [tetrafluoroborate, hexafluorophosphate, trifluoromethanesulfonate, bis(fluorosulfonyl)amide, or bis(trifluoromethanesulfonyl)amide] as a white or brown solid as well as a yellow or orange-yellow viscous material (Supporting Information: yields, 1H NMR data, and elemental analysis).
Synthesis of Alkyltrimethylammonium Bromide (Cn Br)
First, n-ethyl bromide, n-butyl bromide, n-hexyl bromide, n-octyl bromide, or n-decyl bromide (1.0 equiv) was added in a dropwise manner to trimethylamine hydrochloride (1.5 equiv) dissolved in a methanol solution containing sodium hydroxide. The mixture was stirred at room temperature for 10 h and then refluxed for 5 h under alkaline conditions by adding sodium hydroxide with thymolphthalein as an indicator. The mixture solution was filtered to remove the inorganic salt (NaCl), and the solvent in the filtrate was removed. To the residue was added methanol (alkyl chain length, n = 2, 4, 8, 10) or acetone (n = 6), and the mixture was filtered to remove the inorganic salt. This procedure was performed twice. The solvent in the filtrate was removed by evaporation, and the blue residue was washed several times with hexane and then ethyl acetate, recrystallized using a mixture of hexane and ethanol (1:3, vol/vol, for n = 2 and 4; 3:1, vol/vol, for n = 6 and 8; and 3:1, vol/vol; containing a small amount of methanol for n = 10), and dried under reduced pressure to obtain alkyltrimethylammonium bromide (Cn Br) as a white solid (Supporting Information: yields, 1H NMR data, and elemental analysis).
Ion Exchange of Tetrafluoroborate, Hexafluorophosphate, Trifluoromethanesulfonate, Bis(fluorosulfonyl)amide, and Bis(trifluoromethanesulfonyl)amide Ion with Bromide Ion for Cn Br
Alkyltrimethylammonium bromide (1.0 equiv) dissolved in water was added to AgBF4, KPF6, KOTf, KFSA, or KNTf2 (1.1 equiv) dissolved in water, and the mixture solution was stirred under heating for 10 h. The purification procedure for each counterion is described below.
Cn BF4
After the precipitated gray solid had been removed by filtration, the solvent of the filtrate was evaporated under reduced pressure, resulting in a white solid in the cases of C2 BF4, C4 BF4, C8 BF4, and C10 BF4 and a brown viscous material in the case of C6 BF4. The residue was dissolved in methanol for C2 BF4, C4 BF4, and C6 BF4 and in chloroform for C8 BF4 and C10 BF4. The insoluble inorganic salt was removed by filtration. This procedure was repeated until the salt had been removed completely. The solvent in the filtrate was evaporated; in the cases of C2 BF4, C4 BF4, C8 BF4, and C10 BF4, the residue was washed twice with ethyl acetate. On the other hand, for C6 BF4, the residue was dissolved in water, extracted using chloroform, and the chloroform phase was evaporated under reduced pressure to obtain the target compound.
Cn OTf
After the solvent in the filtrate had been evaporated under reduced pressure, acetone was added to the residue. Next, the inorganic salt was removed by filtration, and the filtrate was evaporated. This procedure was repeated twice. Next, the residue was washed with ethyl acetate (in the case of C2 OTf, it was washed with hot ethyl acetate). For C4 OTf, C6 OTf, C8 OTf, and C10 OTf, dichloromethane was added to the residue. The mixture was filtered to remove the inorganic salt, and the filtrate was evaporated. This operation was repeated twice to obtain the target compound.
Cn X (X = PF6, FSA, NTf2)
Once the white solid had precipitated, the solution was filtered to obtain the solid. On the other hand, in the case where the solution separated into two phases, the upper phase was removed by decantation to obtain the liquid. The residue was washed five times with water and then dried. Acetone was added to the dried residue, which was then filtered to remove the inorganic salt, and the solvent in the filtrate was removed by evaporation. This procedure was repeated twice. In the case of Cn PF6, the residue was recrystallized using methanol and dried under reduced pressure, yielding alkyltrimethylammonium [tetrafluoroborate, hexafluorophosphate, trifluoromethanesulfonate, bis(fluorosulfonyl)amide, or bis(trifluoromethanesulfonyl)amide] as a white or brown solid as well as a clear liquid (Supporting Information: yields, 1H NMR data, and elemental analysis).
In the 1H NMR spectrum, the chemical shifts of the CH2 peaks of the ethylene spacer and alkyl chain and peak of CH3 next to the ammonium group were observed at a high magnetic field upon ion-exchange. Elemental analysis showed that the errors in the C, H, and N contents of the compounds were within ±0.3% of their theoretical values. Therefore, these ionic liquids and compounds are highly pure.
General Methods
The melting points of the amphiphilic gemini and monomeric compounds were measured using a Shimadzu DSC-50 system (Kyoto, Japan). For the measurements, 2 mg of the compound in question was placed in a hermetically sealed aluminum pan; the empty aluminum pan was used as the reference. The measurements were performed at a heating rate of 0.2 °C min–1 in a nitrogen atmosphere, and the obtained data was corrected and analyzed using software Shimadzu TA-60WS (Kyoto, Japan). The water contents of the amphiphilic gemini and monomeric ionic liquids were determined using a coulometric titration system (Hiranuma AQV-200, Karl Fischer, Tokyo, Japan). The viscosities were measured at 50 °C (gemini ionic liquids) and 25 °C (monomeric ionic liquids) using a Brookfield DV-2T system (Middleborough). The densities were measured using an Anton-Paar DMA 35 system (Graz, Austria). It should be noted that the densities of the gemini ionic liquids could not be measured at 25 °C because of their high melting point.
The electrical conductivities of the amphiphilic gemini and monomeric ionic liquids in neat and aqueous solutions were measured using a TOA CM-30R system (Tokyo, Japan); this was done to determine the conductivities of the neat ionic liquids and the Krafft temperatures and CMC values of the ionic liquids in aqueous solutions. The surface tensions of ionic liquids in neat and aqueous solutions were measured with a Teclis Tracker tensiometer (Lyon, France) using the pendant drop technique. The surface excess concentration (Γ/mol m–2) and occupied area per molecule (A) values of the amphiphilic ionic liquids at the air/water interface were calculated using the Gibbs adsorption isotherm equations: Γ = −(1/iRT)(dγ/dln C) and A = 1/(NΓ), where γ is the surface tension, C is the ionic liquid concentration, R is the gas constant (8.31 J K–1 mol–1), T is the absolute temperature, and N is Avogadro’s constant. The value of i, which is the number of ion species assumed to be completely dissociated in the aqueous solution, was taken to be 3 and 2, respectively, for the amphiphilic gemini and monomeric ionic liquids investigated in this study. The fluorescence of pyrene in the amphiphilic ionic liquid solutions was measured using a JASCO FP-6300 system (Tokyo, Japan). The concentration of pyrene in the ionic liquid solutions was 1 × 10–6 mol dm–3.
The aqueous solutions of the amphiphilic gemini and monomeric ionic liquids were prepared using water obtained from a Merck KGaA Direct-Q UV system (resistivity = 18.2 MΩ cm), and the measurements were performed at 25 °C, with the exception of those for C8-2-C8 FSA, which were performed at 45 °C.
Acknowledgments
This work was supported by JSPS KAKENHI Grant No. 17K05948. We thank Editage (www.editage.jp) for English language editing.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01660.
1H NMR and elemental analysis data for synthesized compounds along with details of experimental methods and melting point, electrical conductivity, surface tension, and fluorescence data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. All authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Wilkes J. S.; Zaworotko M. J. Air and Water Stable 1-Ethyl-3-Methylimidazolium Based Ionic Liquids. J. Chem. Soc., Chem. Commun. 1992, 965–967. 10.1039/c39920000965. [DOI] [Google Scholar]
- Welton T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2083. 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Wilkes J. S. A Short History of Ionic Liquids – From Molten Salts to Neoteric Solvents. Green Chem. 2002, 4, 73–80. 10.1039/b110838g. [DOI] [Google Scholar]
- Dupont J.; Souza R. F.; Suarez P. A. Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102, 3667–3692. 10.1021/cr010338r. [DOI] [PubMed] [Google Scholar]
- Seddon K. R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. . [DOI] [Google Scholar]
- Gordon C. M. New Developments in Catalysis Using Ionic Liquids. Appl. Catal., A 2001, 222, 101–117. 10.1016/S0926-860X(01)00834-1. [DOI] [Google Scholar]
- Araos M. U.; Warr G. G. Structure of Nonionic Surfactant Micelles in the Ionic Liquid Ethylammonium Nitrate. Langmuir 2008, 24, 9354–9360. 10.1021/la801603t. [DOI] [PubMed] [Google Scholar]
- Torimoto T.; Tsuda T.; Okazaki K.; Kuwabata S. New Frontiers in Materials Science Opened by Ionic Liquids. Adv. Mater. 2010, 22, 1196–1221. 10.1002/adma.200902184. [DOI] [PubMed] [Google Scholar]
- Minami I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. 10.3390/molecules14062286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M.; Tahara Y.; Tamura M.; Kamiya N.; Goto M. Ionic Liquid-Assisted Transdermal Delivery of Sparingly Soluble Drugs. Chem. Commun. 2010, 46, 1452–1454. 10.1039/b907462g. [DOI] [PubMed] [Google Scholar]
- Gong Y.; Hu Q.; Wang C.; Zang L.; Yu Li. Stimuli-Responsive Polyoxometalate/Ionic Liquid Supramolecular Spheres: Fabrication, Characterization, and Biological Applications. Langmuir 2016, 32, 421–427. 10.1021/acs.langmuir.5b03883. [DOI] [PubMed] [Google Scholar]
- Hayashi S.; Hamaguchi H. Discovery of a Magnetic Ionic Liquid [bmim]FeCl4. Chem. Lett. 2004, 33, 1590–1591. 10.1246/cl.2004.1590. [DOI] [Google Scholar]
- Fukaya Y.; Hayashi K.; Wada M.; Ohno H. Cellulose Dissolution with Polar Ionic Liquids Under Mild Conditions: Required Factors for Anions. Green Chem. 2008, 10, 44–46. 10.1039/B713289A. [DOI] [Google Scholar]
- Menger F. M.; Littau C. A. Gemini Surfactants: Synthesis and Properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. 10.1021/ja00004a077. [DOI] [Google Scholar]
- Alami E.; Beinert G.; Marie P.; Zana R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) Surfactants. 3. Behavior at the Air-Water Interface. Langmuir 1993, 9, 1465–1467. 10.1021/la00030a006. [DOI] [Google Scholar]
- Zana R. Gemini (Dimeric) Surfactants. Curr. Opin. Colloid Interface Sci. 1996, 1, 566–571. 10.1016/S1359-0294(96)80093-8. [DOI] [Google Scholar]
- Rosen M. J.; Song L. D. Dynamic Surface Tension of Aqueous Surfactant Solutions. 8. Effect of Spacer on Dynamic Properties of Gemini Surfactant Solutions. J. Colloid Interface Sci. 1996, 179, 261–268. 10.1006/jcis.1996.0212. [DOI] [Google Scholar]
- Zana R. Dimeric (Gemini) Surfactants: Effect of the Spacer Group on the Association Behavior in Aqueous Solution. J. Colloid Interface Sci. 2002, 248, 203–220. 10.1006/jcis.2001.8104. [DOI] [PubMed] [Google Scholar]
- Dong B.; Li N.; Zheng L.; Yu L.; Inoue T. Surface Adsorption and Micelle Formation of Surface Active Ionic Liquids in Aqueous Solution. Langmuir 2007, 23, 4178–4182. 10.1021/la0633029. [DOI] [PubMed] [Google Scholar]
- Behera K.; Pandey S. Ionic Liquid Induced Changes in the Properties of Aqueous Zwitterionic Surfactant Solution. Langmuir 2008, 24, 6462–6469. 10.1021/la800141p. [DOI] [PubMed] [Google Scholar]
- Jungnickel C.; Łuczak J.; Ranke J.; Fernàndez J. F.; Müller A.; Thöming J. Micelle Formation of Imidazolium Ionic Liquids in Aqueous Solution. Colloids Surf., A 2008, 316, 278–284. 10.1016/j.colsurfa.2007.09.020. [DOI] [Google Scholar]
- Santos D. Micelle Formation of Protic Ionic Liquids in Aqueous Solution. J. Chem. Eng. Data 2018, 63, 1480–1487. 10.1021/acs.jced.7b01053. [DOI] [Google Scholar]
- Anderson J. L.; Ding R.; Ellen A.; Armstrong D. W. Structure and Properties of High Stability Geminal Dicationic Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 593–604. 10.1021/ja046521u. [DOI] [PubMed] [Google Scholar]
- Pitawala J.; Matic A.; Martinelli A.; Jacobsson P.; Koch V.; Croce F. Thermal Properties and Ionic Conductivity of Imidazolium Bis(trifluoromethanesulfonyl)imide Dicationic Ionic Liquids. J. Phys. Chem. B 2009, 113, 10607–10610. 10.1021/jp904989s. [DOI] [PubMed] [Google Scholar]
- Verma P. L.; Bartolotti L. J.; Gejji S. P. Probing Molecular Interactions in Functionalized Asymmetric Quaternary Ammonium-Based Dicationic Ionic Liquids. J. Phys. Chem. A 2016, 120, 7732–7744. 10.1021/acs.jpca.6b07337. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Sun N.; He X.; Lu X.; Zhang X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 1475–1517. 10.1063/1.2204959. [DOI] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. Ionic Liquids Based Upon Metal Halide/Substituted Quaternary Ammonium Salt Mixtures. Inorg. Chem. 2004, 43, 3447–3452. 10.1021/ic049931s. [DOI] [PubMed] [Google Scholar]
- Faria L. F. O.; Matos J. R.; Ribeiro M. C. C. Thermal Analysis and Raman Spectra of Different Phases of the Ionic Liquid Butyltrimethylammonium Bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. B 2012, 116, 9238–9245. 10.1021/jp3051824. [DOI] [PubMed] [Google Scholar]
- Holbrey J. D.; Reichert W. M.; Nieuwenhuyzen M.; Johnston S.; Seddon K. R.; Rogers R. D. Crystal Polymorphism in 1-Butyl-3-Methylimidazolium Halides: Supporting Ionic Liquid Formation by Inhibition of Crystallization. Chem. Commun. 2003, 1636–1637. 10.1039/b304543a. [DOI] [Google Scholar]
- Trohalaki S.; Pachter R.; Drake G. W.; Hawkins T. Quantitative Structure-Property Relationships for Melting Points and Densities of Ionic Liquids. Energy Fuels 2005, 19, 279–284. 10.1021/ef049858q. [DOI] [Google Scholar]
- Tokuda H.; Hayamizu K.; Ishii K.; Susan M. A. B. H.; Watanabe M.; et al. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazorium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. 10.1021/jp044626d. [DOI] [PubMed] [Google Scholar]
- Greaves T. L.; Drummond C. J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2008, 108, 206–237. 10.1021/cr068040u. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Matsumoto H.; Tatsumi K. Low-Melting, Low-Viscous, Hydrophobic Ionic Liquids: Aliphatic Quaternary Ammonium Salts with Perfluoroalkyltrifluoroborates. Chem. - Eur. J. 2005, 11, 752–766. 10.1002/chem.200400817. [DOI] [PubMed] [Google Scholar]
- Belieres J.-P.; Angell C. A. Protic Ionic Liquids: Preparation, Characterization, and Proton Free Energy Level Representation. J. Phys. Chem. B 2007, 111, 4926–4937. 10.1021/jp067589u. [DOI] [PubMed] [Google Scholar]
- Bonhôte P.; Dias A.; Papageorgiou N.; Kalyanasundaram K.; Gra1tzel M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. 10.1021/ic951325x. [DOI] [PubMed] [Google Scholar]
- Tagiuri A.; Sumon K. Z.; Henni A.; Zanganeh K.; Shafeen A. Effect of Cation on the Solubility of Carbon Dioxide in Three Bis(fluorosulfonyl)imide Low Viscosity ([FSI]) Ionic Liquids. Fluid Phase Equilib. 2014, 375, 324–331. 10.1016/j.fluid.2014.05.010. [DOI] [Google Scholar]
- Widegren J. A.; Saurer E. M.; Marsh K. N.; Magee J. W. Electrolytic Conductivity of Four Imidazolium-Based Room-Temperature Ionic Liquids and the Effect of a Water Impurity. J. Chem. Thermodyn. 2005, 37, 569–575. 10.1016/j.jct.2005.04.009. [DOI] [Google Scholar]
- Greaves T. L.; Drummond C. J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2008, 108, 206–237. 10.1021/cr068040u. [DOI] [PubMed] [Google Scholar]
- Greaves T. L.; Drummond C. J. Protic Ionic Liquids: Evolving Structure–Property Relationships and Expanding Applications. Chem. Rev. 2015, 115, 11379–11448. 10.1021/acs.chemrev.5b00158. [DOI] [PubMed] [Google Scholar]
- Hou M.; Xu Y.; Han Y.; Chen B.; Zhang W.; Ye Q.; Sun J. Thermodynamic Properties of Aqueous Solutions of Two Ammonium-Based Protic Ionic Liquids at 298.15 K. J. Mol. Liq. 2013, 178, 149–155. 10.1016/j.molliq.2012.11.030. [DOI] [Google Scholar]
- Song D.; Chen J. Density and Viscosity Data for Mixtures of Ionic Liquids with a Common Anion. J. Chem. Eng. Data 2014, 59, 257–262. 10.1021/je400332j. [DOI] [Google Scholar]
- Blesic M.; Marques M. H.; Plechkova N. V.; Seddon K. R.; Rebelo L. P. N.; Lopes A. Self-Aggregation of Ionic Liquids: Micelle Formation in Aqueous Solution. Green Chem. 2007, 9, 481–490. 10.1039/b615406a. [DOI] [Google Scholar]
- Frindi M.; Michels B.; Levy H.; Zana R. Alkanediyl-alpha, Omega-bis(dimethylalkylammonium bromide) Surfactants. 4. Ultrasonic Absorption Studies of Amphiphile Exchange between Micelles and Bulk Phase in Aqueous Micellar Solutions. Langmuir 1994, 10, 1140–1145. 10.1021/la00016a028. [DOI] [Google Scholar]
- Łudzik K.; Kustrzepa K.; Piekarski H. Thermodynamics of Micelle Formation of Gemini Surfactants Hexylene-1,6-bis(dimethyloctylammonium bromide) and Dodecylene-1,12-bis(dimethyloctylammonium bromide) by Electric Conductance Measurements. J. Chem. Eng. Data 2014, 59, 4165–4172. 10.1021/je500804j. [DOI] [Google Scholar]
- Rosen M. J.Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley and Sons: New York, 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.