Abstract
We report a case of Wohlfahrtiimonas chitiniclastica bacteremia and sepsis, in the setting of lower limb wounds with maggot infestation. This is the first documented infection by this organism in the Australasia/Pacific region, identified using matrix-assisted laser desorption ionization-time of flight mass spectrometry and 16S ribosomal ribonucleic acid sequencing. Clinicians should be aware of this emerging pathogen.
Keywords: Gram-negative bacillus, myiasis, Wohlfahrtiimonas chitiniclastica
INTRODUCTION
We present the case of a 54-year-old male diagnosed with Wohlfahrtiimonas chitiniclastica bacteremia secondary to maggot-infested wounds.
CASE REPORT
A 54-year-old wheelchair-bound male was brought to a rural Australian hospital following an unconscious collapse at home. His history was significant for chronic inflammatory demyelinating polyneuropathy with severe sensory and motor neuropathy, alcohol dependence, and hereditary hemochromatosis.
In the setting of polyneuropathy and a prolonged lie adjacent to a radiant heater, his collapse was complicated by a partial thickness burn affecting the dorsum of the right foot extending to the toes. The wound was discharging, malodorous, and infested with numerous maggots, which were removed on initial wound cleansing. He was febrile and hypotensive on presentation with a blood pressure of 90/60 mmHg.
Initial bloods revealed a C-reactive protein of 126 mg/L (normal <2.9 mg/L) and acute on chronic liver function derangement (alkaline phosphatase 417 U/L [normal range 20–120 U/L], gamma-glutamyltransferase 1890 U/L [normal range <45U/L], and alanine aminotransferase 82 U/L [normal range <45 U/L]). Full blood count, renal function, electrolytes, and coagulation studies were normal. Chest X-ray was unremarkable and lower limb X-rays revealed only old healed fractures.
Empirical piperacillin/tazobactam 4.5 g 8-h was commenced along with intravenous fluids. The wound was surgically debrided.
Superficial wound swabs identified Gram-negative bacilli (GNB) on Gram stain and cultured a mix of skin and enteric flora. Blood culture bottles were incubated in a BacT/Alert system (Becton Dickinson, New Jersey, US) and flagged positive after 9 h incubation. Nonmotile GNB were seen on Gram stain from the aerobic bottle and motile GNB were seen from the anaerobic bottle. Overnight incubation cultured two nonlactose fermenters from the aerobic bottle. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS (Biotyper 3.0 database, Bruker Daltonics, Bremen, Germany) identified W. chitiniclastica and Morganella morganii in the aerobic blood culture. The anaerobic blood culture bottle isolated only M. morganii. Growth of W. chitiniclastica on agar and Gram stain appearance is shown in Figure 1. Antimicrobial susceptibility testing was performed on Vitek 2 GN card (bioMérieux, Marcy l'Etoile, France) in accordance with the manufacturer's instructions. W. chitiniclastica was susceptible to all antibiotics tested, including amoxicillin, second- and third-generation cephalosporins, meropenem, gentamicin, ciprofloxacin, and trimethoprim–sulfamethoxazole.
Figure 1.
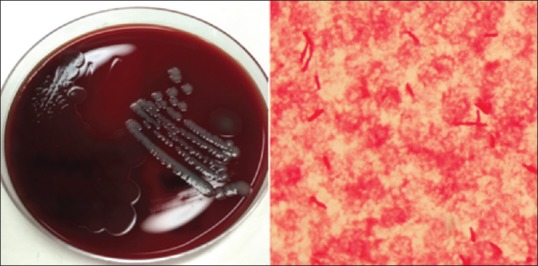
Wohlfahrtiimonas chitiniclastica growing on horse agar (left) and Gram stain of Wohlfahrtiimonas chitiniclastica (right)
Antibiotic treatment was broadened to meropenem to improve coverage of M. morganii. Subsequent blood cultures showed clearance of bacteremia and the patient recovered clinically. He was discharged home on day 7 with a further 2-week course of oral ciprofloxacin.
DISCUSSION
W. chitiniclastica is a strict aerobe, oxidase, and catalase-positive Gram-negative bacillus, first described in 2008.[1] We report on the fifth confirmed bacteremia and first documented W. chitiniclastica infection in Australia.
W. chitiniclastica was first identified in culture from the third-stage larvae of Wohlfahrtia magnifica flies which are known to cause myiasis, commonly in sheep and cattle. Their distribution is in Southern Europe, Asiatic Russia, the Middle East, North Africa, and China,[2] but they are not found in Australia. W. chitiniclastica has also been isolated from the Chrysomya megacephala and Lucilia sericata flies from the Calliphoridae, or blowfly family, as well as the Hermetia illucens fly, of the Stratiomyidae, or soldier fly family,[3] all of which are found in Australia.
W. chitiniclastica infection most commonly occurs via larval contamination of open wounds. It is an emerging human pathogen, with 12 documented human infections in the last decade.[3] Majority of infected patients have had poor personal hygiene, difficult social circumstances, alcohol dependence, and/or chronic wounds, and sites of isolation have ranged from the skin, bone, soft tissue, and blood.
W. chitiniclastica bloodstream infections are frequently polymicrobial. In the five reported cases of bacteremia including this case, three were polymicrobial, concurrently isolated with Escherichia coli in one case, M. morganii and Proteus mirabilis in the second case and Providencia rettgeri and Staphylococcus aureus in the third. Where antimicrobial susceptibility is reported in human infections, all the isolates have been highly susceptible to antimicrobial agents. No penicillin resistance has been detected in reported clinical isolates. An isolate carrying blaVEB-1 (Vietnamese extended-spectrum B-lactamase) gene cassette was isolated from the pancreas of a zebra in China.[4] blaVEB-1 confers high-level resistance to amoxicillin, ticarcillin, piperacillin, cefotaxime, ceftazidime, and aztreonam, which is reversed by clavulanic acid.
Maggots in the patient's wound were discarded during initial wound assessment and care, precluding entomologic identification and confirmation as the source of W. chitiniclastica. However, given that the Wohlfahrtia fly is not found in Australia, we can unfortunately only speculate regarding the vector species based on known domestic entomological data.
This is the first documented case of W. chitiniclastica infection in Australia, an emerging human pathogen associated with myiasis, a condition disproportionately associated with homelessness, chronic wounds, poor self-care, and social isolation. This pathogen may also be present in patients undergoing maggot debridement therapy where maggots have not been adequately sterilized and carry commensal organisms that may be pathogenic. Infections are frequently polymicrobial, with S. aureus and/or coliforms. It is readily identifiable on MALDI-TOF. Reported clinical isolates of W. chitiniclastica are broadly susceptible to antibiotics used for GN infections, including beta-lactams, aminoglycosides, carbapenems, and fluoroquinolone. We would encourage any clinician presented with a patient similarly infected to consider collection and storage of larvae for further analysis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the members of the Entomological Society of Victoria for their valuable information and expertise on the Wohlfahrtia magnifica, Chrysomya megacephala, Lucilia sericata, and Hermetia illucens flies.
REFERENCES
- 1.Tóth EM, Schumann P, Borsodi AK, Kéki Z, Kovács AL, Márialigeti K, et al. Wohlfahrtiimonas chitiniclastica gen. Nov. sp. Nov. a new Gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: Sarcophagidae) Int J Syst Evol Microbiol. 2008;58:976–81. doi: 10.1099/ijs.0.65324-0. [DOI] [PubMed] [Google Scholar]
- 2.Campisi L, Mahobia N, Clayton JJ. Wohlfahrtiimonas chitiniclastica bacteremia associated with Myiasis, United Kingdom. Emerg Infect Dis. 2015;21:1068–9. doi: 10.3201/eid2106.140007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schröttner P, Rudolph WW, Damme U, Lotz C, Jacobs E, Gunzer F, et al. Wohlfahrtiimonas chitiniclastica: Current insights into an emerging human pathogen. Epidemiol Infect. 2017;145:1292–303. doi: 10.1017/S0950268816003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou W, Li M, Zhu L, Hua F, Ji X, Sun Y, et al. Complete genome sequence of Wohlfahrtiimonas chitiniclastica strain BM-Y, isolated from the pancreas of a zebra in China. Genome Announc. 2016;4 doi: 10.1128/genomeA.00643-16. pii: e00643-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


