Abstract
PURPOSE
Helicobacter pylori (HP) and Epstein Barr virus (EBV) infections induce chronic gastritis (CG) and are accepted carcinogenics of gastric cancer (GC). Our objective for this study was to determine the prevalence of these agents and clinicopathological features of GC and CG associated with the infection.
PATIENTS AND METHODS
A single-center cohort of 375 Peruvian patients with GC and 165 control subjects with CG were analyzed. Evaluation of HP and EBV genes was performed through quantitative polymerase chain reaction.
RESULTS
Prevalence of HP was 62.9% in the whole population and 60.8% in the GC subset. The cagA gene was detected in 79.9%; vacAs1 and vacAm1 alleles in 41.6% and 60.7%, respectively; and concurrent expression of vacAs1 and vacAm1 in 30.4% of infected patients in the whole series. The prevalence of EBV was 14.1% in the whole population and was higher in GC (P < .001). Coinfection of HP and EBV was found in 7.8% and was also higher in GC in univariate (P < .001) and multivariate (P = .011) analyses. Infection rates of HP and EBV were not associated with a geographic location in the whole series. Few clinicopathological features have been associated with infectious status.
CONCLUSION
Prevalence of HP infection and virulent strains are high in the Peruvian population. Infection by EBV was more frequent in patients with GC.
INTRODUCTION
There are large regional differences in gastric cancer (GC) rates, and the highest incidence rates are in East Asia and South America. More than two-thirds of cases occurred in middle- and low-income countries, according to GLOBOCAN 2018.1 In addition, areas with the highest GC rates have been described within countries.2,3
In the Andean country of Peru, GC is the third most common cancer and causes the highest absolute number of cancer deaths.4 Persistent infection with Helicobacter pylori (HP) induces chronic inflammation and gastric carcinogenesis. HP infection in gastric mucosa has been associated with low socioeconomic status, and its prevalence in adults living in developing Latin American countries is 70% to 80%.5-7
The genomes of HP are heterogeneous and encode different virulence factors that play an important role in the clinical outcome of the infection. Cytotoxin-associated gene A (cagA) is a protein encoded by the cagA gene. It translocates to epithelial cells and activates signaling pathways that induce cellular changes and the production of proinflammatory cytokines. The vacuolating cytotoxin A (vacA) of HP is encoded by the vacA gene, causes direct damage to the gastric epithelium, and stimulates an acute inflammatory process. The vacA gene has the s1 or s2 allele types in the signal region (s) whereas the m1 and m2 allele types are in the middle region (m). The combination of s1/m1 allele types results in high levels of cytotoxin. The proinflammatory potential of cagA-positive and vacA allele variants of HP may explain HP’s association with severe atrophic gastritis, peptic ulcer, and gastric adenocarcinoma.8
Gastric infection by Epstein-Barr virus (EBV) produces a severe mucosal inflammatory response, has demonstrated carcinogenic activity, and leads to a specific subtype of GC.9,10 Recent studies suggest that HP and EBV coinfection could have a synergistic carcinogenic effect and would be more frequently found in GC than chronic gastritis (CG).11-13
CONTEXT
Key Objective
The purpose of this study was to analyze the prevalence of HP and EBV infections in Peruvian patients with chronic gastritis and gastric cancer, and to evaluate clinicopathological features associated with the presence of the infectious agents.
Knowledge Generated
The infection rate of H. pylori is higher than 60% in our population, and most strains were cagA positive. Epstein-Barr virus infection was more frequent in gastric cancer than chronic gastritis, and its coinfection with H. pylori was also more frequently found in gastric cancer.
Relevance
Treating H. pylori infection in South American countries should be enforced in public health care to reduce gastric cancer rates. To our knowledge, the current study is one of the largest series of molecular epidemiology of infectious carcinogenic agents in gastric samples from one South American country. We expect our results can influence public policies in South American countries.
The prevalence of the genotypes of HP that express the most virulent factors and EBV change with geographic area, and there is limited information about rates of these infections in the Peruvian population. Information about prevalence differences of these infectious agents is even lower among the three Peruvian recognized regions: coast, highland and, rain forest. The coast (11.7% of the national territory) is the most urbanized area, has the highest population (56.3% of the total population), and is where the largest city in Peru, the crowded and capital city of Lima, is located. The Peruvian rain forest (Peruvian Amazon) represents 60.3% of the Peruvian territory but only 14.0% of the total population15.
The purpose of this study was to analyze the prevalence of HP and EBV infections in Peruvian patients with CG and GC, and to evaluate clinicopathological features associated with the presence of the infectious agents.
PATIENTS AND METHODS
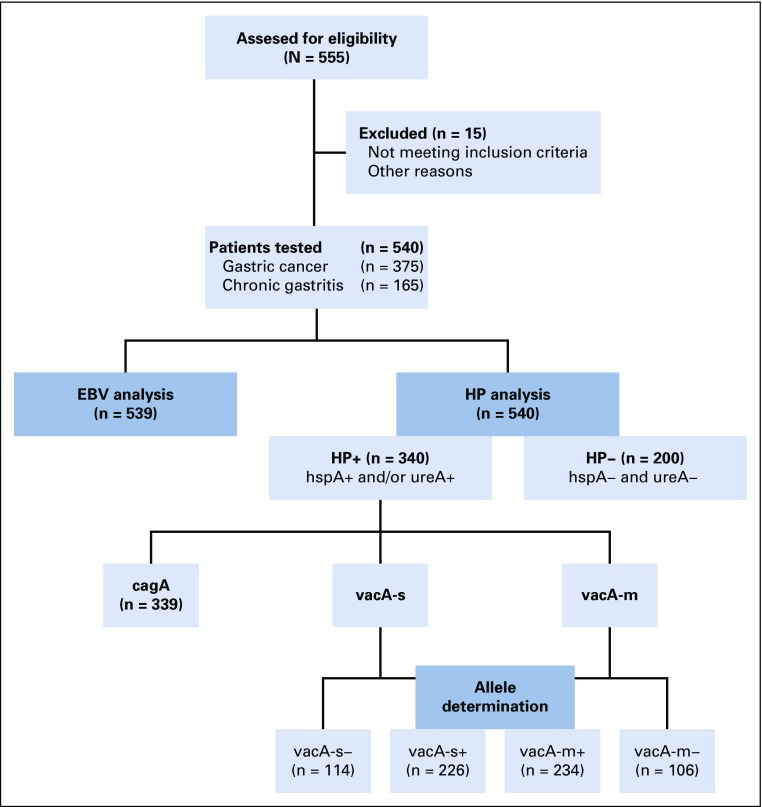
We developed a cross-sectional study with 540 patients who came to the Instituto Nacional de Enfermedades Neoplasicas (INEN), Lima, Peru, for screening or GC treatment from 2015 to 2018. We included 375 patients with GC who underwent diagnostic endoscopy (n = 67) or gastrectomy (n = 308) and 165 patients with CG who underwent screening endoscopies (nonmalignancies). Inclusion criteria were histology of adenocarcinoma and not being previously exposed to chemotherapy, in the cancer group. CONSORT methodology was followed in the study development (Fig 1).
FIG 1.
Flowchart showing screened patients with gastric cancer and chronic gastritis, and subgroups with allele determination. EBV, Epstein-Barr virus; HP, Helicobacter pylori.
The Human Subjects Committee of INEN approved the conduct of this study (Protocol No. 050-2015-CIE/INEN). Before their inclusion, patients received information regarding the purpose and the conduct of the study. Patients gave written consent for the storage of their samples at the INEN biobank and for their samples’ use in research.
We collected clinicopathological data from the INEN’s patient files and from an epidemiologic questionnaire, including the patient’s home address. Peru is divided into 25 provinces distributed among the three previously mentioned regions. In addition, Lima city is divided into districts and they were settled into North (seven districts), South,7 Center,15 and East7 sectors. Callao (the main harbor of the country) shares limits with Lima city and was considered for the Lima sectors analysis.15,16
Endoscopy and Histology
Biopsy specimens were obtained from tumoral, proximal, and distant nontumoral tissues in patients with GC and from only one area from those with CG. Samples were immediately saved and frozen at −70°C until processing.
Detection of HP Infection
Diagnosis of HP was accepted when at least the results of one of the areas were positive. The detection of HP in gastric tissue samples was carried out through the extraction of genomic DNA with the GeneJETGenomic DNA kit (Thermo Fisher Scientific, Waltham, MA), the quantification by fluorometry was conducted using the Quantus fluorometer (Promega, Madison, WI), and, subsequently, the standard detection of the colonizing genes (hspA and UreA) by quantitative polymerase chain reaction (qPCR) in the LightCycler 96 Instrument Thermal Cycler (Roche, Mannheim, Germany). The hspA gene sequence was detected with the following primers: reverse: 5′-GCT ATC TGA AAA TTT GAT TTC TTT TGC-3′ and forward: 5′-TGC GCT ATA GTT GTG TCG C-3′; and that of UreA: reverse: 5′-TTG TCT GCT TGT CTA TCA ACC-3′ and forward: 5′-GAG AAT GAG ATG AAA CTC ACC C-3′. The mixture for the qPCR was composed of PCR-grade water, Sybr GreenFastStartEssential DNA Green Master (Roche), the respective primers, and the sample of genomic DNA.17,18
Evaluation of HP Virulence Genes
Virulence of cagA, vacAs, and vacAm genes was tested in HP-positive patients detected by constitutive genes (hspA and ureA). The DNA of HP was detected with qPCR using oligonucleotides directed to the hspA and UreA genes. The signal and middle regions of vacA were genotyped by qPCR using the oligonucleotides previously described by Camorlinga-Ponce et al.18a
The cagA gene sequence was detected with the following primers: reverse: 5′-TCTAATCCTGTTTGCTCCCCA-3′ and forward: 5′-CTCATTGCGAAGGCGACCT-3′. The vacAs gene was detected with the following primers: reverse: 5′-GCGTCAAAATAATTCCAAGG-3′ and forward: 5′-CAATCTGTCCAATCAAGCGAG-3. And to detect the vacAm gene, the following primers were used: reverse: 5′-CTGCTTGAATGCGCCAAAC-3′ and forward: 5′-ATGGAAATACAACAAACACAC-3′.17,18
The amplification program included one cycle at 95°C for 10 minutes; 45 cycles at 95°C for 15 seconds, 57°C to 63°C (depending on the gene) for 15 seconds, and 72°C for 20 seconds; and a melting curve 95°C for 10 seconds, 65°C for 60 seconds, and 97°C for 1 second.19,20
A melting curve for vacA alleles was constructed for each primer pair to verify the presence of one gene-specific peak and the absence of primer dimmer. Genotype s1/m1 was identified in positive patients for gene expression by comparing them with the gene-specific peak obtained from strain ATCC700392D-5 HP vacA s1+m1+.
Evaluation of the EBV Gene
EBV qPCR was performed on DNA using the Primerdesign EBV (human herpesvirus 4) kit (genesig Advanced, Southampton, UK) with a region of BNRF1 as the target. ;The target sequence within the BNRF1 gene was a good genetic marker for EBV in other clinical real-time–PCR-based studies.21 The amplification program included one cycle at 95°C for 120 seconds, 50 cycles at 95°C for 10 seconds, and 60°C for 10 seconds.
Statistical Analysis
We used Pearson χ2 or Fisher’s exact tests to compare frequencies between groups. Kruskal-Wallis test was used for comparing number of copies HspA and UreA among tumoral, and proximal and distal nontumoral areas. Multivariate analysis was performed using a multiple logistic regression model for factors associated with GC compared with the CG subset and results were evaluated with odds ratios. P < .05 was considered statistically significant. We used the R, version 3.4.1 (https://www.r-project.org/), for the statistical analyses.
RESULTS
Patients and Histologic Diagnosis
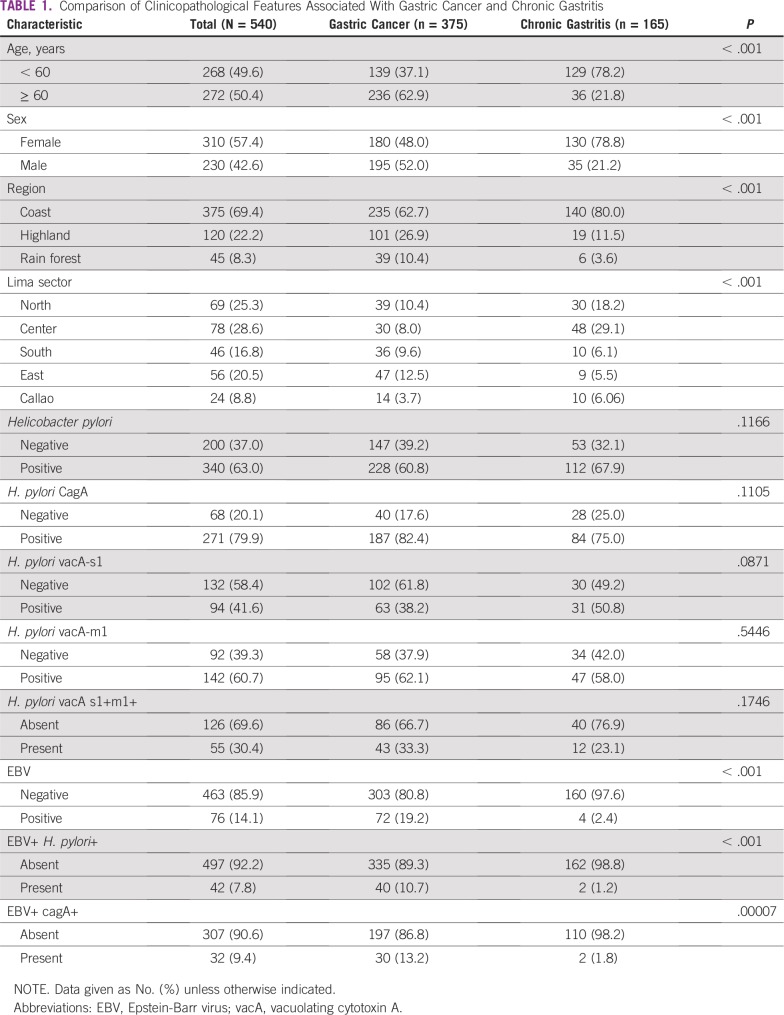
In the whole series, the median age was 60 years and 57.4% of the patients were women. Most came from the coast region (69.4%), and most people from Lima city came from central districts (28.6%).
In the group of patients with GC, most patients were of poorly and undifferentiated grade (57.8%), and had intestinal histology (47.6%), lymphovascular invasion (69.1%), and perineural invasion (46.1%). The most common clinical stages were III and IV (65.7%). Most patients had antrum involvement (62.6%) and evaluated samples came from biopsy (17.9%), or subtotal (51.5%) or total gastrectomy (30.7%). In the CG group, most patients were women (78.8%) and were at least 60 years old (33.2%). Metaplasia was found in 26.7% (Table 1).
TABLE 1.
Comparison of Clinicopathological Features Associated With Gastric Cancer and Chronic Gastritis
Prevalence of HP
The prevalence of HP was 62.9% in the whole population and 60.8% in GC subset. In the GC subset, the infection was detected in tumoral, proximal, or distal areas in 46.0%, 53.2%, and 49.5% of patients, respectively. The highest median number of ureA copies/μL was found in nontumoral regions (95.5 in tumoral, 149.6 in proximal, and 142.8 copies/μL in distal areas; P < .001). The highest median number of hspA copies/μL was found in nontumoral regions (220.9 in tumoral, 608.5 in proximal, and 632.2 copies/μL in distal areas; P = .003).
Infection prevalence in the coast (n = 239 of 375; 63.7%), highland (n = 70 of 120; 58.3%), and rain forest (n = 31 of 45; 68.8%) areas were similar in the whole series (P = .39). There were no associations between the presence of HP and age (P = .45), sex (P = .12), cancer stage (P = .059), or other evaluated clinicopathological features (P > .05) in the GC group.
Prevalence of HP With cagA Genotype
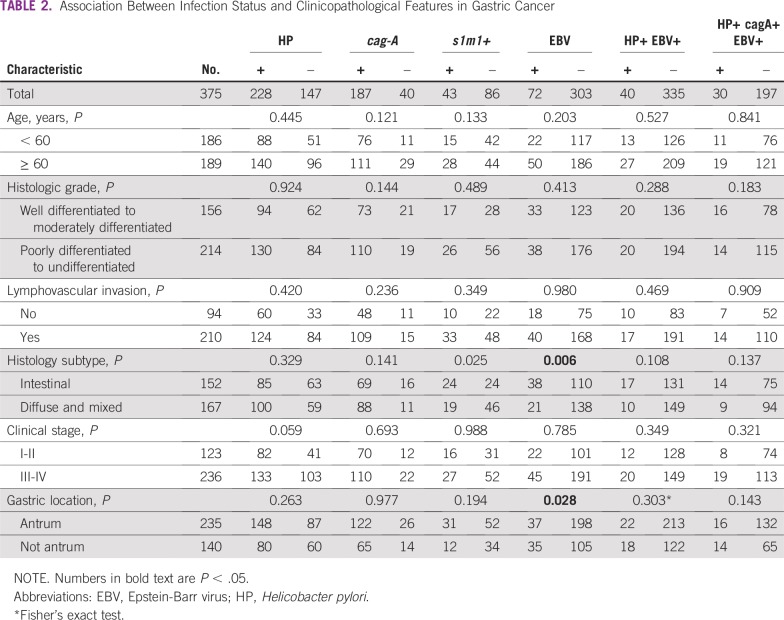
The cagA gene was evaluable in 339 samples and detected in 79.9% of HP-infected patients in the whole series (Fig 1). The protein, cagA, tended to be more frequent in GC than CG (82.4% v 75%; P = .12; Table 1). In the GC subset, presence of cagA was not associated with age (P = .12), sex (P = .49), cancer stage (P = .69), or other evaluated clinicopathological feature (P > .05; Table 2).
TABLE 2.
Association Between Infection Status and Clinicopathological Features in Gastric Cancer
Prevalence of HP With vacA Alleles
VacAs was detected in 226 and vacAm in 234 patients (Fig 1). VacAs1 and vacAm1 alleles were detected in 41.6% and 60.7%, respectively, in the whole series, and in 38.2% and 62.1%, respectively, in the GC subset. There was a correlation between presence of vacAs1 and both vacAm1 (P < .001) and cagA (P < .001). Concurrent expression of vacAs1+m1+ was found in 30% in the whole series and 33.3% in the GC subset (Table 1). In the GC subset, s1+m1+ was associated with intestinal histology (P = .03; Table 2).
Prevalence of EBV Infection
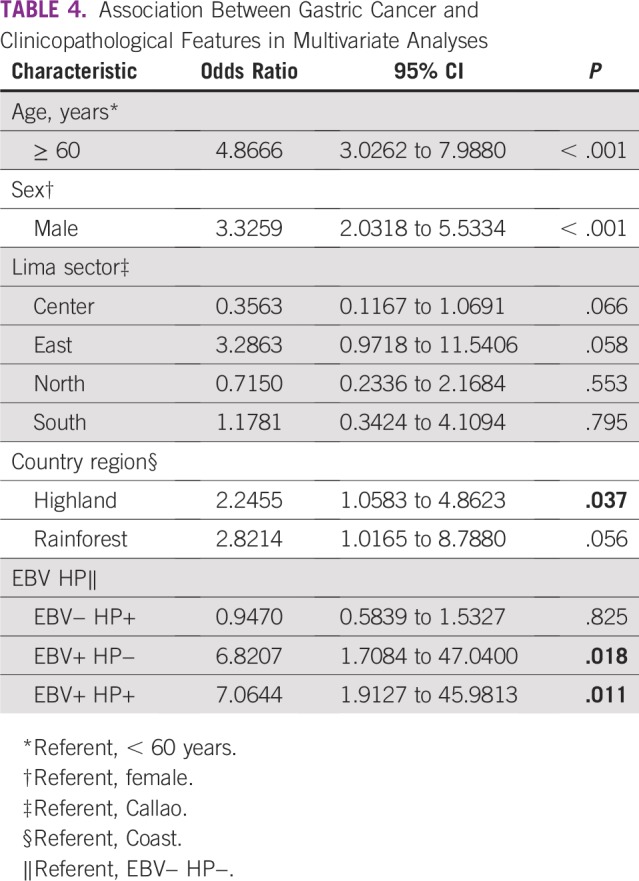
The prevalence of EBV was 14.1% in the whole population and was higher in the GC than in the CG subset (19.2% v 2.4%; P < .001; Table 1). Infection with EBV was associated with intestinal histology (P = .006) and nonantrum location (P = .028) in the GC subset (Table 2). Coinfection of HP and EBV was found in 42 patients (7.8%) in the whole series and was higher in patients with GC than in the subset with CG (10.7% v 1.2%; P < .001). Coinfection of HP cagA+ and EBV was found in 9.4% of patients and was higher in the GC group than in the CG subset (13.2% v 1.8%; P < .001). There was no association between presence of any infection and metaplasia in the CG subset (P > .05; Table 3). According to multivariate analysis, EBV+ HP+ was present seven times more frequently in patients with GC than was EBV− HP− (P = .011; Table 4).
TABLE 3.
Association Between Infection Status and Clinicopathological Features in Chronic Gastritis
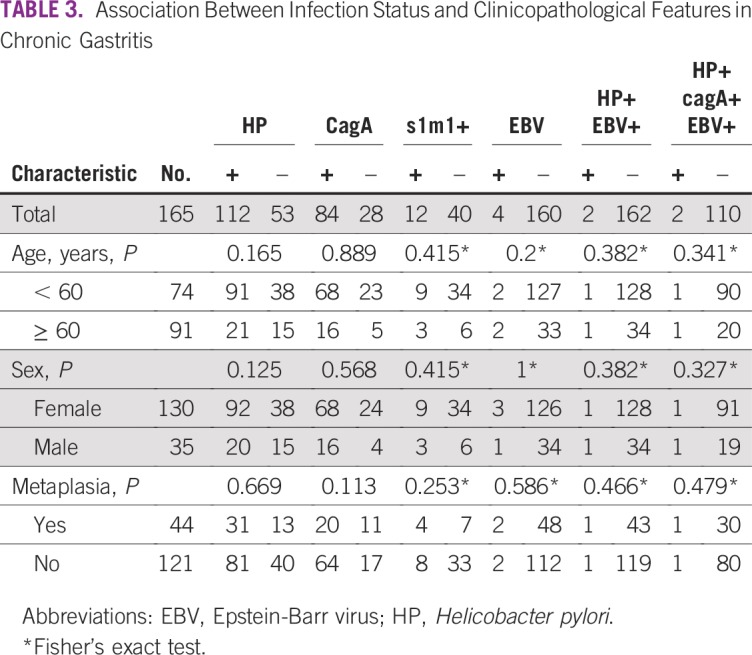
TABLE 4.
Association Between Gastric Cancer and Clinicopathological Features in Multivariate Analyses
DISCUSSION
The rates of HP infection in patients with GC (60.8%) in this study is similar to the reported prevalence in developing countries and similar to CG samples (67.9%). This prevalence is much lower than the prevalence reported three decades ago. This phenomenon of reduction has also been described in developed countries22-24 and could be related to low rates of HP contamination in drinking water because of an improvement in water potability, as recently described by Boehnke et al,25 as well as the increased use of antibiotics.24,26,27
Studies have described HP infection rates that differed among patients living in the coast, highland, and rain forest areas; this has been suggested to be as a result of higher HP infection rates in people living in high-altitude areas compared with those at sea level.22,24,28,29 Influence of racial factors in this phenomenon is probably not important, because a previous study did not find differences in HP infection rates among the Peruvian population and the Japanese colony resident in Peru (The Gastrointestinal Physiology Working Group of the Cayetano Heredia and the Johns Hopkins University, 1992).23 We found no difference among those patients from any of these areas in our whole series.
When we analyzed only patients from Lima city, we found that the districts with higher HP infection rates are different to those located in the areas previously described as GC rates.4 These differences, in addition to the absence of similarity between Peruvian regions with higher rates of HP infection and those with higher rates of GC diagnosis in our series and previous studies,22-24,28,29 could be related to the fact that patients from highland and rain forest regions constantly visit coastal cities, because the latter have better services, such as the Health Care Institute where diagnostics are conducted, and by a similar HP contamination rates of drinking water we have found in different districts (unpublished data). However, we cannot exclude that the differences could be related to the small sample size in our study.
We found that 79.9% of the HP strains in our whole series were cagA+. A previous study reported that more than 90% of HP strains in Lima were cagA+.22 The frequency we report is lower than that reported in a northeastern region of Brazil (96.7%)30,31 but higher than that reported in Mexico (47.6% to 73.9%).12,32-34 Differences in regional distribution of strains are clear: The frequency of cagA-positive HP is 90% to 95% in Asian countries, whereas only it is 50% to 60% in Western countries5,34a,35; however, we did not find differences in cagA+ infection rates among Peruvian regions nor among Lima sectors. Although cagA+ strains have been associated with strong carcinogenic activity,34b we did not find higher rates in GC samples compared with CG samples, which indicates that presence of aggressive strains of HP is not enough to develop GC. Our results can be explained by the fact that cancer is a long-term result of HP infection and it starts to disappear as hypochlorhydria sets in.35-37Prevalence of HP vacA s1+m1+ strains was 33.3%.This rate is similar to that reported in southern Mexico (38.5%)38 but lower than frequencies found in other regions of Mexico, Colombia, and Brazil.31,32,34 This strain has been described as more aggressive; however, we did not find higher rates in GC samples compared with CG samples.
On the other hand, 14% of gastric tissues had EBV infection in our whole series and the prevalence was 19.2% in the GC subset. These rates are higher than the previously reported rate of 3.9% (diagnosed by in situ hybridization) in a Lima population and close to the average rate of 10% EBV infection in patients with GC in South American countries.13,39-41 We selected qPCR instead of in situ hybridization because the former can detect fewer gene copies.42 A coinfection of EBV and HP was found in 7.8% of patients in the whole series and was also more frequent in the GC group than in the CG subset (P < .001). Furthermore, multivariate analysis showed the concurrence was seven times more frequently associated with GC than in patients negative for both EBV and HP infection. Rates of coinfection in our study were lower than those reported in two Mexican series; however, both series found that coinfection of HP and EBV was more frequent in patients with GC than in those with CG.11-13
The association between coinfection of HP and EBV with gastric malignancy could be explained by the preclinical finding of a synergy of both pathogens to induce carcinogenesis. Double infections of HP and EBV can synergistically act to enhance expression of IL-17 and maintain an inflammatory state that more severely damages gastric mucosa. HP also induces monochloramine generation, which could reactivate EBV.11,12,43-46 To our knowledge, the current study is one of the largest series of molecular epidemiology of infectious carcinogenic agents in gastric samples from one South American country. To produce confident values, the presence of HP infection was evaluated in tumoral and nontumoral regions; correlation of detection was high, but more copies of constitutive genes were found in distal nontumoral regions, probably as a result of environmental challenges generated by malignant cells over bacteria growth, like pH alteration. We expect our results can influence public policies in South American countries.
A weakness of our study is that we included every patient with CG or GC who came to the Institute for diagnosis in a convenience sample instead of paired selection. However, the multivariate analysis allowed us to better quantify the association with cancer compared with nonmalignant pathology for every feature.
In conclusion, our results indicate that HP infection and its virulent strains are frequent and widely spread among Peruvian regions. EBV and dual infection by HP and EBV are more frequent in patients with GC than in those with CG.
ACKNOWLEDGMENT
We thank Carolina Belmar, PhD, and Sandro Casavilca, MD, for providing scientific advice on development of the experiments.
Footnotes
Supported by the Programa Nacional de Innovación para la Competitividad y Productividad (Innóvate Perú; Contract No. 430-PNICP-PIAP-2014) and Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica (Contract No. 197-2015-FONDECYT).
The study was approved by the ethics committee of the Institutional of Instituto Nacional de Enfermedades Neoplasicas (Approval No. 025-2016-DI-DICON/INEN). Informed consent or substitute for it was obtained from all patients prior to the procedure.
AUTHOR CONTRIBUTIONS
Conception and design: Carlos A. Castaneda, Miluska Castillo, Emmanuel Dias-Neto
Administrative support: Miluska Castillo, Luis A. Bernabe
Provision of study material or patients: Fernando Barreda, Daniel Valdivia, Iván Chavez, Eloy Ruiz
Collection and assembly of data: Miluska Castillo, Nancy Suarez, Jais Nieves, Luis A. Bernabe, Maria P. Landa-Baella, Yaqueline Bazan, Carlos A. Rengifo
Data analysis and interpretation: Carlos A. Castaneda, Miluska Castillo, Luis A. Bernabe, Nancy Suarez, Emmanuel Dias-Neto, Maria P. Landa-Baella, Paola Montenegro
Manuscript writing: All authors
Final approval of manuscript: All authors
Agree to be accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Jais Nieves
Employment: Sanofi/Aventis
Paola Montenegro
Employment: Merck Serono (I)
Honoraria: Merck (I)
Consulting or Advisory Role: Tecnofarma
Speakers' Bureau: Bristol-Myers Squibb
Expert Testimony: Tecnofarma
Travel, Accommodations, Expenses: Tecnofarma
No other potential conflicts of interest were reported.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 8:394-424, 2018. [DOI] [PubMed]
- 2. Waye JD, Aabakken L, Armengol-Miro J, et al: Screening for GI cancer and payment mechanisms. Gastrointest Endosc 55:453-454, 2002. [DOI] [PubMed]
- 3. León-Barúa R: Factores geográficos y socioeconómicos en la orientación de la patología gastroduodenal asociada a la infección por Helicobacter pylori [in Spanish]. Acta Gastroenterol Latinoam 30:491-496, 2000. [PubMed] [Google Scholar]
- 4.Payet E, Pérez P, Poquioma E, et al. Registro de cáncer de Lima metropolitana. Incidencia y mortalidad 2010–2012. Lima, Peru: Instituto Nacional de Enfermedades Neoplásicas; 2016. http://www.inen.sld.pe/portal/documentos/pdf/banners_2014/2016/Registro%20de%20C%C3%A1ncer%20Lima%20Metropolitana%202010%20-%202012_02092016.pdf [Google Scholar]
- 5. Yamaoka Y, Kodama T, Gutierrez O, et al: Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: Studies in four different countries. J Clin Microbiol 37:2274-2279, 1999. [DOI] [PMC free article] [PubMed]
- 6. Huang JQ, Zheng GF, Sumanac K, et al: Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology125:1636-1644, 2003. [DOI] [PubMed]
- 7. Eslick GD, Lim LL-Y, Byles JE, et al: Association of Helicobacter pylori infection with gastric carcinoma: A meta-analysis. Am J Gastroenterol 94:2373-2379, 1999. [DOI] [PubMed]
- 8.Yamaoka Y, Kodama T, Gutierrez O, et al. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: Studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukayama M, Hayashi Y, Iwasaki Y, et al. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994;71:73–81. [PubMed] [Google Scholar]
- 10. Murphy G, Pfeiffer R, Camargo MC, et al: Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 137:824-833, 2009. [DOI] [PMC free article] [PubMed]
- 11. Cárdenas-Mondragón MG, Torres J, Flores-Luna L, et al: Case-control study of Epstein-Barr virus and Helicobacter pylori serology in Latin American patients with gastric disease. Br J Cancer 112:1866-1873, 2015. [DOI] [PMC free article] [PubMed]
- 12. Del Moral-Hernandez O, Castanon-Sanchez CA, Reyes-Navarrete S, et al: Multiple infections by EBV, HCMV and Helicobacter pylori are highly frequent in patients with chronic gastritis and gastric cancer from Southwest Mexico: An observational study. Medicine 98:e14124, 2019. [DOI] [PMC free article] [PubMed]
- 13. de Souza CRT, de Oliveira KS, Ferraz JJS, et al: Occurrence of Helicobacter pylori and Epstein-Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. BMC Gastroenterol 14:1-9, 2014. [DOI] [PMC free article] [PubMed]
- 14. Reference deleted.
- 15. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1251/Libro.pdf Instituto Nacional de Estadística e Informática Estado de la Población Peruana: Día mundial de la publación. 11 de julio.
- 16. Torres-Roman JS, Ruiz EF, Martinez-Herrera JF, et al: Prostate cancer mortality rates in Peru and its geographic regions. BJU Int 123:595-601, 2019. [DOI] [PubMed]
- 17. Figura N, Vindigni C, Covacci A, et al: cagA Positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: Relevance to histological damage. Gut 42:772-778, 1998. [DOI] [PMC free article] [PubMed]
- 18. Argent RH, Zhang Y, Atherton JC: Simple method for determination of the number of Helicobacter pylori CagA variable-region EPIYA tyrosine phosphorylation motifs by PCR. J Clin Microbiol 43:791-795, 2005. [DOI] [PMC free article] [PubMed]
- 18a. Camorlinga-Ponce M, Perez-Perez G, Gonzalez-Valencia G, et al: Helicobacter pylori Genotyping from American Indigenous Groups Shows Novel Amerindian vacA and cagA Alleles and Asian, African and European Admixture. PlosOne 6:e27212, 2011. [DOI] [PMC free article] [PubMed]
- 19. Martínez-Carrillo DN, Garza-González E, Betancourt-Linares R, et al: Association of IL1B-511C/-31T haplotype and Helicobacter pylori vacA genotypes with gastric ulcer and chronic gastritis. BMC Gastroenterol 10:1-8, 2010. [DOI] [PMC free article] [PubMed]
- 20. Park C-Y, Kwak M, Gutierrez O, et al: Comparison of genotyping Helicobacter pylori directly from biopsy specimens and genotyping from bacterial cultures. J Clin Microbiol 41:3336-3338, 2003. [DOI] [PMC free article] [PubMed]
- 21.Niesters HG, van Esser J, Fries E, et al. Development of a real-time quantitative assay for detection of Epstein-Barr virus. J Clin Microbiol. 2000;38:712–715. doi: 10.1128/jcm.38.2.712-715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mendoza D, Herrera P, Gilman RH, et al: Variation in the prevalence of gastric cancer in Peru. Int J Cancer 123:414-420, 2008. [DOI] [PubMed]
- 23.Ramírez-Ramos A, Watanabe-Yamamoto J, Takano-Morón J, et al. Decrease in prevalence of peptic ulcer and gastric adenocarcinoma at the Policlínico Peruano Japones, Lima, Peru, between the years 1985 and 2002. Analysis of 31,446 patients. Acta Gastroenterol Latinoam. 2006;36:139–146. [PubMed] [Google Scholar]
- 24. Ramírez Ramos A, Chinga Alayo E, Mendoza Requena D, et al: Variacion de la prevalencia del H. pylori en le Perú período (1985-2002), en una población de nivel socioeconómico medio y alto [in Spanish]. Rev Gastroenterol Peru 23:92-98, 2003. [PubMed] [Google Scholar]
- 25. Boehnke KF, Brewster RK, Sanchez BN, et al: An assessment of drinking water contamination with Helicobacter pylori in Lima, Peru. Helicobacter 23:e12462, 2018. [DOI] [PubMed]
- 26.Ramírez Ramos A, Leey Casella J, Mendoza Requena D, et al. Helicobacter pylori: Epidemiología-diagnóstico-tratamiento-consensos mundiales-experiencia en el Perú[in Spanish]Diagnóstico (Perú) 4223–37.2003 [Google Scholar]
- 27. Pareja Cruz A, Navarrete Mejía PJ, Parodi García JF: Seroprevalencia de infección por Helicobacter pylori en población adulta de Lima, Perú 2017 [in Spanish]. Horiz Med (Peru) 17:55-58, 2017.
- 28.Ramírez Ramos A, Mendoza Requena D, Leey Casella J, et al. Estudio del Helicobacter pylori en el Perú [in Spanish] Rev Peru Med Exp Salud Publica. 2002;19:209–214. [Google Scholar]
- 29.Ramirez-Ramos A, Leon-Barua R, Gilman R, et al. Helicobacter pylori and gastritis in Peruvian patients: Relationship to socioeconomic level, age, and sex. Am J Gastroenterol. 1990;85:819–823. [PubMed] [Google Scholar]
- 30. Cavalcante MQ, Silva CIS, Braga-Neto MB, et al: Helicobacter pylori vacA and cagA genotypes in patients from northeastern Brazil with upper gastrointestinal diseases. Mem Inst Oswaldo Cruz 107:561-563, 2012. [DOI] [PubMed]
- 31. Cittelly DM, Huertas MG, Martínez JD, et al: Helicobacter pylori genotypes in non atrophic gastritis are different of the found in peptic ulcer, premalignant lesions and gastric cancer in Colombia [in Spanish]. Rev Med Chil 130:143-151, 2002. [PubMed] [Google Scholar]
- 32. Martínez-Carrillo D, Atrisco-Morales J, Hernández-Pando R, et al: Helicobacter pylori vacA and cagA genotype diversity and interferon gamma expression in patients with chronic gastritis and patients with gastric cancer [in Spanish]. Rev Gastroenterol Mex 79:220-228, 2014. [DOI] [PubMed]
- 33. Meine GC, Rota C, Dietz J, et al: Relationship between cagA-positive Helicobacter pylori infection and risk of gastric cancer: A case control study in Porto Alegre, RS, Brazil. Arq Gastroenterol 48:41-45, 2011. [DOI] [PubMed]
- 34. Thomazini CM, Pinheiro NA, Pardini MI, et al: Helicobacter pylori and gastric cancer: Distribution of cagA and vacA genotypes in patients with gastric carcinoma [in Portuguese]. J Bras Patol Med Lab 42:25-30, 2006.
- 34a. Nomura M, Lee J, Stemmermann GN, et al: Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis 186:1138–1144, 2002. [DOI] [PubMed]
- 34b. Hatakeyama M, Higashi H: Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci 96: 835-843, 2005. [DOI] [PMC free article] [PubMed]
- 35. Nagiyev T, Yula E, Abayli B, et al: Prevalence and genotypes of Helicobacter pylori in gastric biopsy specimens from patients with gastroduodenal pathologies in the Cukurova Region of Turkey. J Clin Microbiol 47:4150-4153, 2009. [DOI] [PMC free article] [PubMed]
- 36. Atherton JC, Cao P, Peek RM, Jr, et al: Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 270:17771, 1995. [DOI] [PubMed]
- 37. El-Omar EM, Oien K, El-Nujumi A, et al: Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113:15-24, 1997. [DOI] [PubMed]
- 38. Román-Román A, Martínez-Carrillo DN, Atrisco-Morales J, et al: Helicobacter pylori vacA s1m1 genotype but not cagA or babA2 increase the risk of ulcer and gastric cancer in patients from Southern Mexico. Gut Pathog 9:1-2, 2017. [DOI] [PMC free article] [PubMed]
- 39.Yoshiwara E, Koriyama C, Akiba S, et al. Epstein-Barr virus-associated gastric carcinoma in Lima, Peru. J Exp Clin Cancer Res. 2005;24:49–54. [PubMed] [Google Scholar]
- 40. Corvalan A, Koriyama C, Akiba S, et al: Epstein-Barr virus in gastric carcinoma is associated with location in the cardia and with a diffuse histology: A study in one area of Chile. Int J Cancer 94:527-530, 2001. [DOI] [PubMed]
- 41.Herrera-Goepfert R, Reyes E, Hernández-Avila M, et al. Epstein-Barr virus-associated gastric carcinoma in Mexico: Analysis of 135 consecutive gastrectomies in two hospitals. Mod Pathol. 1999;12:873–878. [PubMed] [Google Scholar]
- 42. Chen X-Z, Chen H, Castro FA, et al: Epstein–Barr virus infection and gastric cancer: A systematic review. Medicine 94:e792, 2015. [DOI] [PMC free article] [PubMed]
- 43. Amedei A, Munari F, Della Bella C, et al: Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 9:303-309, 2014. [DOI] [PubMed]
- 44. Singh S, Jha HC: Status of Epstein-Barr virus coinfection with Helicobacter pylori in gastric cancer. J Oncol 2017:1-17, 2017. [DOI] [PMC free article] [PubMed]
- 45. Minoura-Etoh J, Gotoh K, Sato R, et al: Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein–Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J Med Microbiol 55:905-911, 2006. [DOI] [PubMed]
- 46. Rahal EA, Hajjar H, Rajeh M, et al: Epstein-Barr virus and human herpes virus 6 type a DNA enhance IL-17 production in mice. Viral Immunol 28:297-302, 2015. [DOI] [PubMed]