Abstract
PURPOSE
Mammography is not always available or feasible. The purpose of this systematic review and meta-analysis is to assess the diagnostic performance of ultrasound as a primary tool for early detection of breast cancer.
MATERIALS AND METHODS
For this systematic review and meta-analysis, we comprehensively searched PubMed and SCOPUS to identify articles from January 2000 to December 2018 that included data on the performance of ultrasound for detection of breast cancer. Studies evaluating portable, handheld ultrasound as an independent detection modality for breast cancer were included. Quality assessment and bias analysis were performed with the Quality Assessment of Diagnostic Accuracy Studies-2 tool. Sensitivity analyses and meta-regression were used to explore heterogeneity. The study protocol has been registered with the international prospective register of systematic reviews (PROSPERO identifier: CRD42019127752).
RESULTS
Of the 526 identified studies, 26 were eligible for inclusion. Ultrasound had an overall pooled sensitivity and specificity of 80.1% (95% CI, 72.2% to 86.3%) and 88.4% (95% CI, 79.8% to 93.6%), respectively. When only low- and middle-income country data were considered, ultrasound maintained a diagnostic sensitivity of 89.2% and specificity of 99.1%. Meta-analysis of the included studies revealed heterogeneity. The high sensitivity of ultrasound for the detection of breast cancer was not statistically significantly different in subgroup analyses on the basis of mean age, risk, symptoms, study design, bias level, and study setting.
CONCLUSION
Given the increasing burden of breast cancer and infeasibility of mammography in certain settings, we believe these results support the potential use of ultrasound as an effective primary detection tool for breast cancer, which may be beneficial in low-resource settings where mammography is unavailable.
INTRODUCTION
Breast cancer is the leading cause of cancer-related deaths among females worldwide. In 2018, 2.1 million new breast cancer cases and 626,679 deaths were reported.1 Adequate access to detection of breast cancer with imaging is the first step in the diagnostic pathway to decrease mortality from this disease. Mammography, which has long been considered the gold standard for screening and early detection of breast cancer, is not always feasible, especially in limited-resource settings. This may be due to the high cost of purchasing and maintaining equipment as well as difficulty training and retaining skilled technologists and interpreting radiologists. Data from 2014 show that that per 1 million women between 50 and 69 years old, highly developed areas of the world have anywhere from 40 to 600 mammography units, whereas there is an average of 0 to 12 mammography units in most of sub-Saharan Africa and approximately 12 to 41 units in many developing areas in Asia.2 In the United States, where 70% of women undergo mammography, the most recent estimates of the overall sensitivity and specificity of diagnostic digital mammography are 87.8% and 90.5%, respectively.3,4 In low- and middle-income countries (LMICs), the reported sensitivity of mammography ranges from 63% to 95%; higher sensitivity is seen when examining palpable lumps, and lower sensitivity is seen in cases of dense breasts.4
Breast ultrasound, which is used in high-resource settings to supplement mammography in certain clinical scenarios, offers a potentially viable alternative for early breast cancer detection in some resource-limited areas because it is portable, lower cost than mammography, and versatile across a wider range of clinical applications. Breast ultrasound has been proven to be an exceptionally effective tool for imaging palpable abnormalities in the breast. It distinguishes cystic from solid masses and demonstrates those features of solid masses that would denote the mass as suspicious and warranting biopsy.5-7 Ultrasound is a particularly useful diagnostic modality in dense breast tissue, often detecting breast cancers obscured on mammography.8,9 Furthermore, if biopsy is required, ultrasound is the ideal imaging tool to guide subsequent procedures, further enhancing its utility in breast cancer diagnosis.5-7
CONTEXT
Key Objective
Is portable ultrasound a viable breast cancer detection modality?
Knowledge Generated
A comprehensive literature review and meta-analysis revealed portable ultrasound to have an overall high sensitivity of 80.1% and specificity of 88.4% for detection of breast cancer in a variety of patient populations. When the available data from low-resource countries were considered, ultrasound maintained a diagnostic sensitivity of 89.25% and specificity of 99.1%.
Relevance
Portable ultrasound could serve as a global primary detection modality and triage method for breast lesions, particularly in low-resource areas where mammography is currently unavailable or infeasible.
The deployment of ultrasound as a diagnostic modality could be most helpful in LMICs, because they carry a disproportionate burden of disease. In 2012, 52.9% of the 1.7 million breast cancer cases were classified as global, and 62.1% of the breast cancer–related deaths occurred in LMICs.10 Although breast cancer incidence is highest in high-income countries, mortality rates are lower in these locations as a result of advances in early detection, diagnosis, and treatment.11,12 In 2018, the age-standardized incidence rate of breast cancer (per 100,000 women) in Northern America was 84.8, with a mortality rate (per 100,000 women) of 12.6, whereas for Western Africa the estimates were 37.3 and 17.8, respectively.1 It is estimated that by 2020, 70% of all breast cancer cases worldwide will occur in LMICs, with a projected estimate to more than 1 million new cases per year in these areas.5,13 These disproportionately high mortality-to-incidence ratios in LMICs are due to scarcity of available detection, diagnosis, and treatment of breast cancer.14,15 It must also be acknowledged that data are severely lacking in LMICs, which can result even in an underestimation of the disease burden and barriers to care in these areas.16 This situation is further exacerbated by insufficient patient education about breast health and the importance of early detection.5,14 Identifying breast cancer at an early stage, before local, regional, or systemic spread, offers the potential for initiation of earlier, more effective treatment and is thus vital to improving outcomes in LMICs.6
Although the literature consistently reports increased breast cancer detection with use of supplementary screening ultrasound, few direct comparisons of mammography and ultrasound for average-risk patients have been reported.3 The purpose of this systematic review and meta-analysis is to assess the potential of ultrasound, indicated by sufficiently high diagnostic performance against histologic confirmation and benchmarked against the highly accepted performance of mammography, for breast cancer detection, which could be particularly applicable in LMICs.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
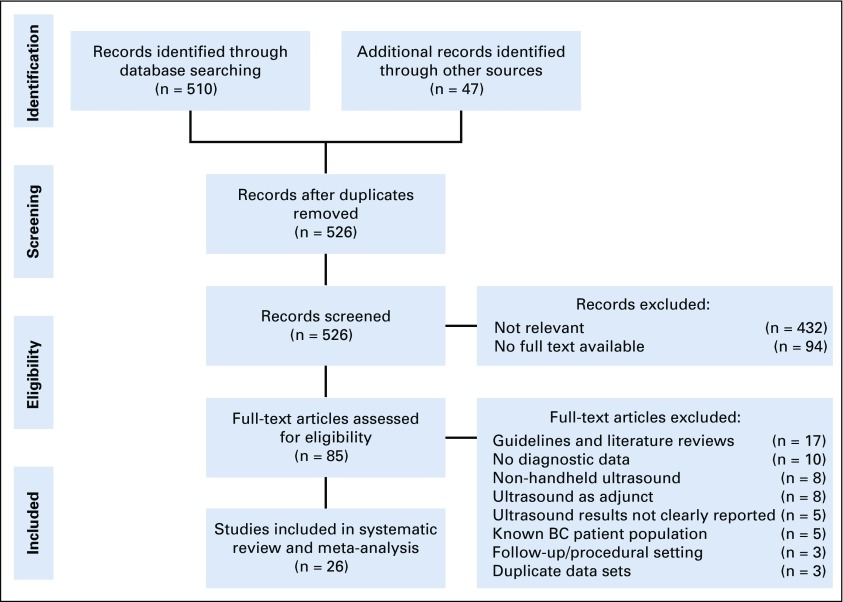
We conducted a systematic literature review and meta-analysis following Cochrane Guidelines for Screening and Diagnostic tests and the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines. We identified eligible studies in PubMed and Scopus (Amsterdam, the Netherlands) published between January 2000 and December 2018. The search was designed to identify all studies in which ultrasound was evaluated as a primary detection modality for breast cancer, both in a screening and diagnostic capacity. A comprehensive search strategy including free text and MeSH terms was developed in consultation with an experienced librarian specialist. Search terms included: “breast neoplasms,” “breast cancer,” “breast lesions,” “mammary ultrasound,” “breast ultrasound,” “breast diagnostic,” “mammography,” “low resource,” and “screening.” Titles and abstracts were screened to determine primary eligibility on the basis of the Preferred Reporting Items for Systematic reviews and Meta-Analysis algorithm (Fig 1). Reference lists of the retrieved publications were also screened for any additional relevant studies. If there were several publication reports for a specific study, the author was contacted to determine which study was most comprehensive for inclusion in this meta-analysis.
FIG 1.
Flowchart of study selection. BC, breast cancer.
The inclusion criteria were discussed between authors, and joint consensus was achieved. The studies eligible for inclusion in this systematic review and meta-analysis were peer-reviewed studies in human participants in which portable ultrasound was evaluated as a primary detection modality for breast cancer. The search was restricted to English language articles, and studies with portable ultrasound estimates were included. Prospective, retrospective, and cross-sectional studies published between January 2000 and December 2018 were included. The required reference standard was biopsy with histopathology results. Each manuscript was required to have extractable data to calculate true positives, false negatives, true negatives, and false positives so that sensitivity, specificity, positive predictive value, and negative predictive value (NPV) could be determined. Studies that compared mammography and ultrasound against verified histopathology were included, and data for both modalities were extracted. In comparative studies with other modalities, such as automated whole-breast ultrasound or magnetic resonance imaging, only handheld ultrasound estimates were extracted. Studies in populations with proven breast cancers were excluded, because they were deemed to bias diagnostic accuracy of the modality being evaluated. In addition, any study in which ultrasound was automated or examined only as a supplemental diagnostic modality, such as after mammography or magnetic resonance imaging screening, with only combined estimates recorded, were excluded. Studies in mammographically negative tissue with ultrasound diagnostic parameter values were included. When authors were unable to extract data for diagnostic parameter estimate calculations, primary authors of the considered article were contacted for clarification and raw data. In addition, duplicate articles were removed based on verified author, journal, title, and year of the study.
Data Extraction and Quality Assessment
One investigator (R.S.) extracted all study demographic data, including study type, study country, study setting, population, mean age, positive case definition, blinded image interpretation, and reference standard from each study. Two investigators (R.S. and D.S.) extracted data related to the number of ultrasound and mammography examinations, including quantification of true positives, true negatives, false positives, and false negatives. Two independent readers (R.S. and S.C.H.) performed the quality assessment and bias analysis with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.17 Three domains were used to identify applicability concerns and risk for bias: patient selection, performance of index test, and standard of reference. Discrepancies were resolved by consensus review or a third reader (D.S.). Each study was given a final designation of low or high bias on the basis of these categories.
Data Analyses
Meta-analysis for assessing the diagnostic performance of ultrasound alone and also comparatively between ultrasound and mammography was conducted using STATA (version 15; STATA, College Station, TX). Extracted data for all included studies were pooled to yield summary estimates of sensitivity and specificity of ultrasound for detection of breast cancer. Heterogeneity was investigated with calculated Higgins I2 values, with greater than 50% considered indicative of substantial heterogeneity among studies.18 Forest plots were drawn to visually represent overall diagnostic estimates and heterogeneity using the computing software R (version 3.5.2). Because significant variation was present among all studies, a Spearman correlation was performed between the sensitivity and false-positive rate (1 − specificity) to investigate the possibility of a threshold effect as the reason for the observed high heterogeneity. The result confirmed no positive correlation (Spearman rho, −0.1; P value = .4), so a bivariate random effects model was used to calculate pooled values for sensitivity, specificity, and diagnostic odds ratios (DORs) in contrast to using a summary receiver operating characteristic (ROC) model.19-21 An ROC curve was constructed, and an area under the curve was calculated using Reviewing Manager (RevMan), version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, 2014; Copenhagen, Denmark). Funnel plots of DORs were created, and the Deeks funnel plot asymmetry test was performed to evaluate for publication bias ,with P values < .1 considered statistically significant.22,23 To account for presence of possibly missing studies, the trim and fill method was used to obtain the adjusted DORs for both ultrasound and mammography.24
To assess for possible improvement in ultrasound technology over the years, regression analysis of the DORs was performed and trended across publication years of the studies. Sensitivity analyses to assess for sources of heterogeneity were also performed by stratifying studies on the basis of covariates including study design, mean age, high-risk features, symptoms, tissue density, and bias level (on the basis of QUADAS-2). To evaluate for differences between the sensitivity and specificity of ultrasound and mammography for cancer detection in certain subgroups, a two-tailed test of proportion was used. DORs were linearly regressed against a covariate, and relative DORs (rDORs) were estimated to provide a comparison of diagnostic performance between different subgroups. P values < .05 were considered statistically significant.
RESULTS
The literature search with PubMed and SCOPUS yielded 557 studies. After removal of duplicates, 526 studies remained and were screened. Four hundred forty-one studies were excluded based on irrelevance (432 studies) or unavailable full texts (nine studies). Eighty-five full-text articles were reviewed. Among these, 17 were guidelines or reviews of the literature, 10 lacked data for two by two diagnostic table calculations, eight focused on alternative ultrasound modalities, eight discussed ultrasound only as an adjunctive screening modality, five were about methods for reporting ultrasound findings, five were in patient populations with proven breast cancer, three were about the use of ultrasound in follow-up care or procedures, and three were derived from similar data sets. After excluding these ineligible studies, 26 studies were included in the final systematic review and meta-analysis (Fig 1).
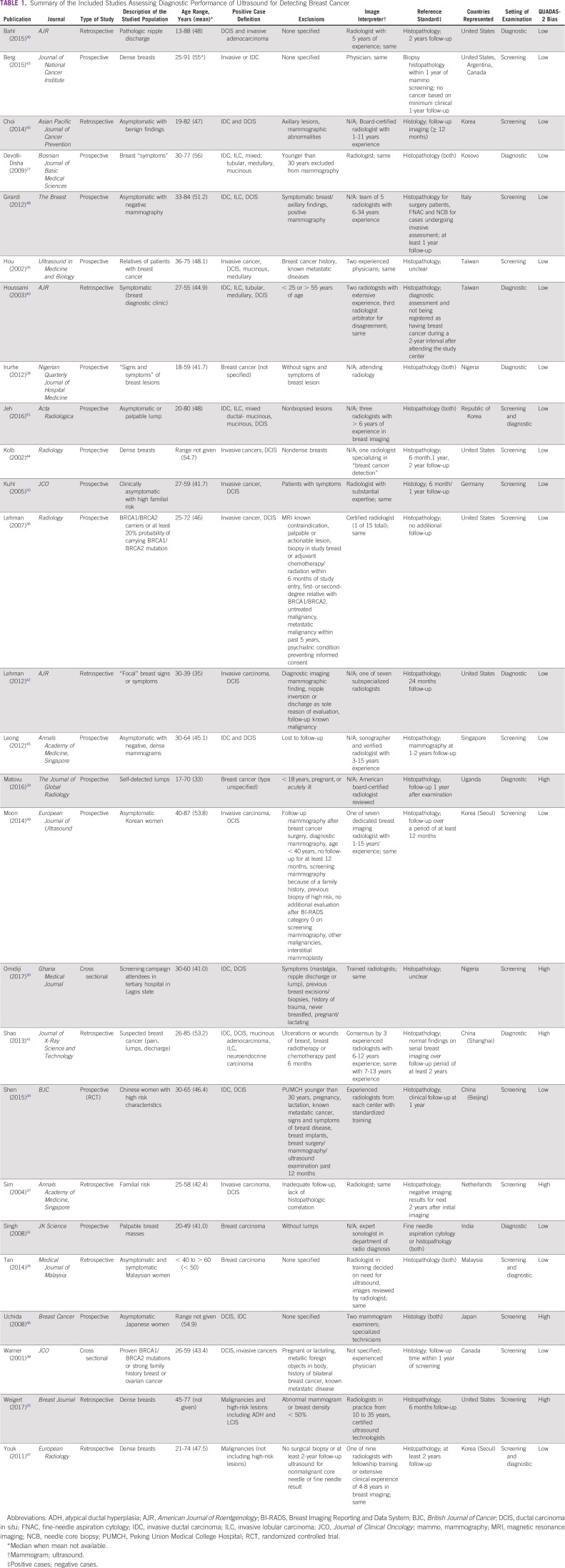
The 26 included studies included a total of 76,026 ultrasound examinations and 29,178 mammography examinations (Data Supplement). Weigert et al25 provided data from all 4 years of the study, but to avoid duplication of data, only the final year data were analyzed as patients carried over from the first year through the final year. Seventeen studies reported mean age younger than 50 years, and seven reported mean age older than 50 years. Of the included studies, two did not provide a mean age.25,26 The selected studies were geographically diverse, representing a total of 17 countries. Five of the 26 studies took place in LMICs (Kosovo, Nigeria, Uganda, and India), as determined by the 2018 World Bank classification.27-32 In our classification for this meta-analysis, high risk was defined as positive family history, BRCA mutation, elevated risk scores, or a combination of these, which six of the studies met.33-38 Symptomatic was defined as nipple discharge, palpable lump, unspecified, or a combination thereof in eight included studies.27-29,31,39-42 Eighteen studies were in asymptomatic women. Six of the 26 studies were given a dense tissue designation, because 50% or more of all included patients were reported to have dense breast tissue.25,43-47 In terms of setting, 14 took place in the screening setting, nine studies in the diagnostic setting, and three in a combination of both settings. Six studies took place in mammographically negative population subsets.25,44,45,47-49 In regard to study design, 15 were prospective and nine were retrospective. Two were cross-sectional in nature (Table 1).30,38
TABLE 1.
Summary of the Included Studies Assessing Diagnostic Performance of Ultrasound for Detecting Breast Cancer
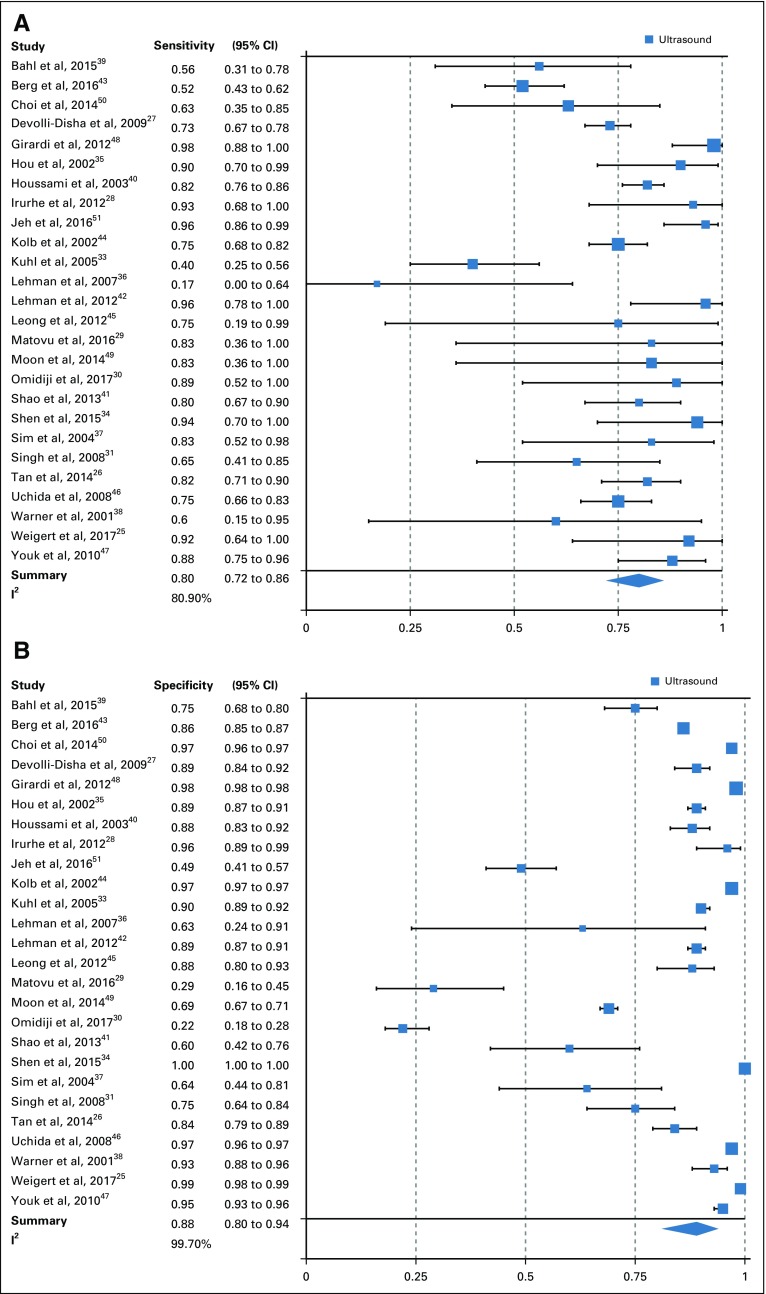
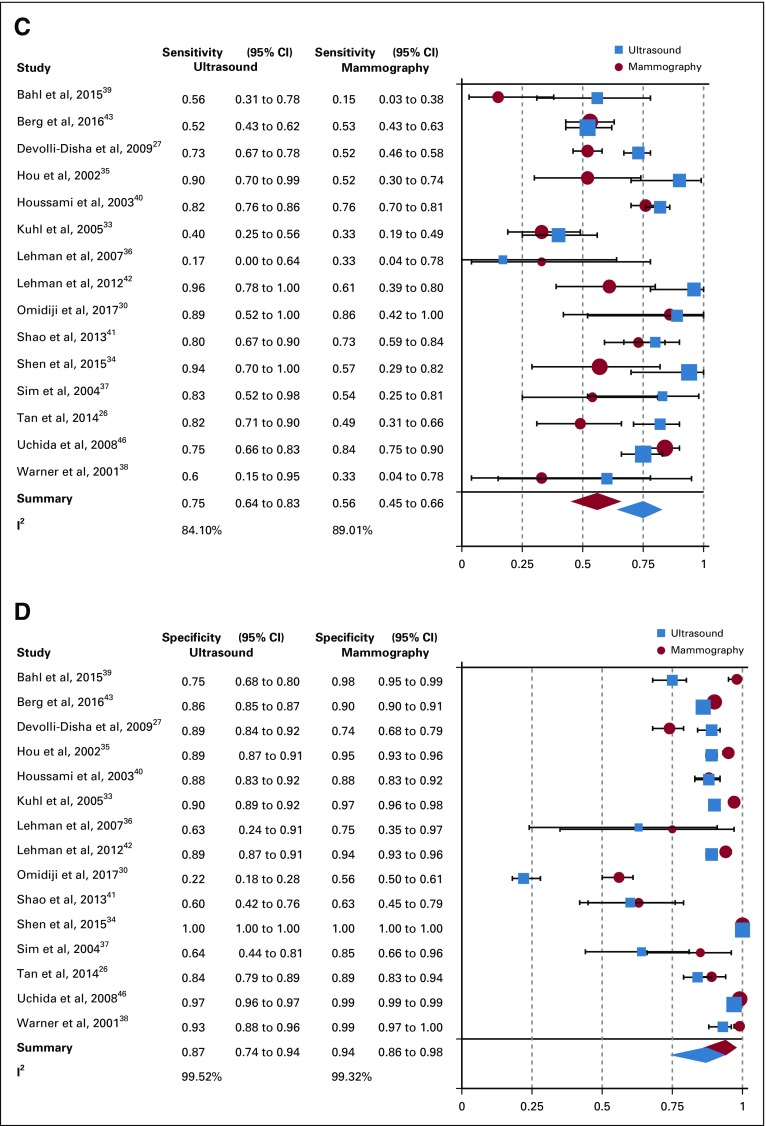
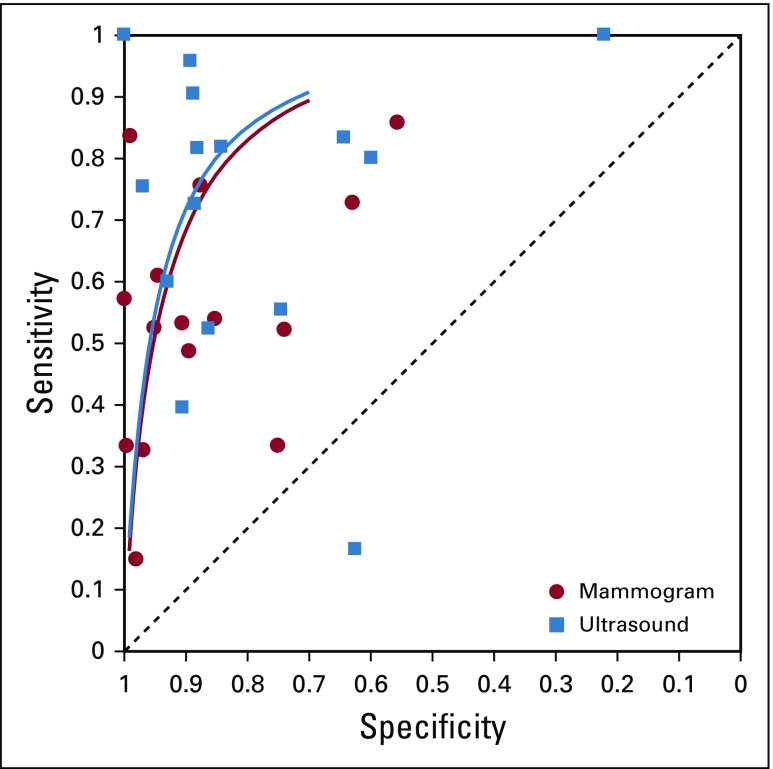
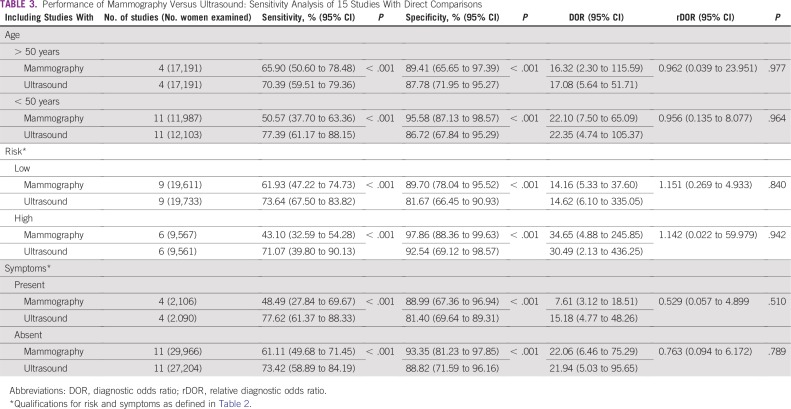
From the 26 studies reporting findings on the use of ultrasound as a detection modality for breast cancer, the pooled sensitivity, specificity, DOR, positive predictive value, and NPV (95% CI) were 80.1% (72.2% to 86.3%), 88.4% (79.5% to 93.6%), 30.7 (13.0 to 72.3), 0.86 (0.81 to 0.91), and 0.80 (0.75 to 0.85), respectively (Figs 2A and 2B; Data Supplement). Of the 15 studies that concurrently assessed the performance of ultrasound and mammography as compared with the gold standard of histopathology, sensitivity and specificity (95% CI) of ultrasound were 74.9% (63.9% to 84.5%) and 87.0% (73.7% to 94.1%), whereas for mammography estimates were 55.8% (44.9% to 66.3%) and 94.3% (86.3% to 97.7%), respectively (Figs 2C and 2D; Data Supplement). In the comparative studies, both the sensitivity and specificity of ultrasound was significantly higher (P < .001). Among these 15 studies, overall DOR (95% CI) of ultrasound was 20.0 (6.5 to 61.2), whereas for mammography it was 20.8 (7.9 to 55.0), with a comparative rDOR of 1.5 (0.2 to 5.0) that was not statistically significant (P value = .90). As illustrated by the ROC curves for the studies comparing ultrasound and mammography against histopathology in the same patients, the areas under the curves were statistically significantly higher for ultrasound (P value < .001), at 0.87 (0.84 to 0.90) and 0.77 (0.73 to 0.81; Fig 3).
FIG 2.
(A) Sensitivity of ultrasound overall. (B) Specificity of ultrasound overall. (C) Comparative sensitivity of ultrasound and mammography. (D) Comparative specificity of ultrasound and mammography. I2, Higgins I2 index.
FIG 3.
Summary receiver operating curves, fifteen studies comparing ultrasound and mammography.
Visual funnel plot assessment revealed no evidence of publication bias (Data Supplement). In addition, the slopes of the Deeks funnel plot asymmetry tests for ultrasound alone and ultrasound versus mammography did not support any presence of publication bias. Using the trim and fill method, adjusted DORs (95% CI) were 30.0 (16.5 to 54.5) and 19.2 (7.9 to 46.6) for overall ultrasound and mammography, respectively. Comparing these values with the original calculated DORs (ultrasound: 20.0 [6.5 to 61.2] and mammography: 20.8 [7.9 to 55.0]), we observed no statistically significant difference, and thus we concluded our results were not biased by publication bias. On the basis of the QUADAS-2 assessment, there were no concerns regarding applicability to clinical practice in any of the included studies (Data Supplement). Regarding internal validity, 20 of the studies (76.9%) had a low risk of bias, and six (23.1%) had a high risk of bias. The most common reason for risk of high bias was inconsistent blinding of the radiologists to final pathology results. The second most common risk factor for bias was observed in the flow and timing assessment; specifically, not all patients received the same reference standard.
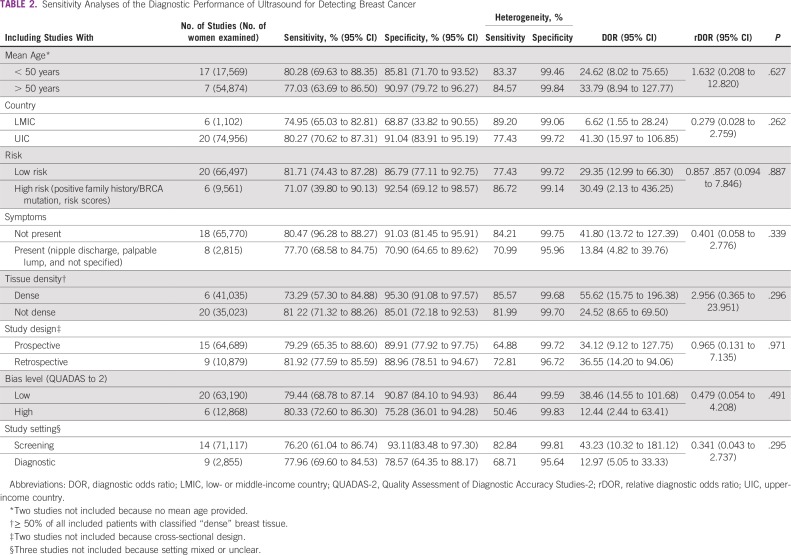
The set of studies was heterogeneous for overall pooled ultrasound sensitivity (I2 = 80.9%) and specificity estimates (I2 = 99.7%). Potential sources of heterogeneity were investigated using meta-regression, with covariates of mean age younger than or older than 50 years, country of study (LMIC v high-income country), risk category (high v low), presence of symptoms, breast tissue density (high v low), study design (retrospective v prospective), bias level (QUADAS-2), and study setting (screening v diagnostic). The performance of ultrasound for detection of breast cancer did not significantly statistically differ (P value cutoff = .05) by these covariates (Table 2). In the five studies from LMICs, ultrasound maintained a high pooled diagnostic sensitivity of 89.2% and specificity of 99.1%. For comparison, the pooled estimates from studies performed in high-income country populations were 80.3% and 91.0% for sensitivity and specificity, respectively. None of the LMIC studies included patients in the previously defined high-risk category, but four of the five studies were in symptomatic patients with palpable lumps. Among comparative studies of portable ultrasound and mammography, there was no statistically significant difference in terms of rDOR when these data were stratified by age, risk, and presence of symptoms (Table 3). However, subanalyses by age, risk, and symptom categories yielded a significantly higher sensitivity and specificity for ultrasound as compared with mammography (P values < .001; Table 3).
TABLE 2.
Sensitivity Analyses of the Diagnostic Performance of Ultrasound for Detecting Breast Cancer
TABLE 3.
Performance of Mammography Versus Ultrasound: Sensitivity Analysis of 15 Studies With Direct Comparisons
DISCUSSION
Although mammography is widely accepted as the gold standard for early breast cancer detection, it is not widely available globally. In contrast, ultrasound is accessible, versatile, and cost effective. To our knowledge, our systematic review and meta-analysis of 26 studies with a total of 76,058 patients is the most comprehensive analysis of the sensitivity of ultrasound as a primary modality for breast cancer detection. Our meta-analysis showed that ultrasound had an overall pooled sensitivity and specificity (95% CI) of 80.1% (72.2% to 86.3%) and 88.4% (79.8% to 93.6%), respectively, for the detection of breast cancer. In addition, this high sensitivity and specificity did not differ based on subgroup analyses. Our findings add to a growing body of literature describing ultrasound’s detection capacity for breast cancer. It is known that ultrasound is effective for the detection of small, invasive, node-negative cancers in dense breast tissue, where the sensitivity of mammography drops from 85% to 47.8% to 64.4%.52,53 In addition, numerous studies report high sensitivity and NPV up to 100% when ultrasound is used for cancer detection at the site of focal breast symptoms.42,54 However, there is limited information on the value of ultrasound as an early detection tool in primarily asymptomatic women. Our study evaluated the diagnostic potential of breast ultrasound, in a variety of patient populations, as a primary detection modality to provide supportive data for implementation in settings where mammography is unavailable.
As a detection modality, ultrasound has particular potential to affect early detection rates in areas that lack access to mammography. When LMIC-only data were considered, ultrasound maintained a diagnostic pooled sensitivity of 89.2% and pooled specificity of 99.1%. However, it is important to note that this high sensitivity and specificity may partly be due to the small number of studies in these countries and lack of diagnostic data of other modalities in these settings. The 15 included studies comparing mammography and ultrasound against the gold standard of histopathology further indicated that ultrasound has statistically significantly increased sensitivity and specificity in the studied populations. Because of the current approved uses of ultrasound, the comparative studies were predominantly in populations where mammography is known to perform on par with or more poorly than ultrasound, namely in women with dense breasts, with symptoms, and those at high risk for breast cancer. In areas where access to breast care is limited by resource constraints and mammography is generally not available, these population subsets are much more common. For example, Asian women tend to have dense breast tissue and a younger peak age of breast cancer incidence, making ultrasound a potentially more accurate breast cancer detection modality in this population.34 In addition, women presenting with late-stage disease and symptoms are known to represent the largest proportion of the women presenting for breast cancer concerns in LMICs or low-resource regions of high-income countries.55,56
Because patients in LMICs often present with locally advanced disease and have a younger average age at diagnosis, there is impetus for using ultrasound as the first imaging modality in these areas.11 The findings from this study on the diagnostic utility of ultrasound may therefore be most viable in these settings. Guidelines specific for detection of breast cancer in the LMIC setting further support the use of ultrasound. The Breast Health Global Initiative advocates for the introduction of diagnostic ultrasound at the resource-limited level, given that it is more widely available than mammography in these countries and extremely useful in women with palpable lesions.57 For example, in Uganda, where organized breast health programs are widely absent, only four mammography units exist for a population of 6 to 7 million women.58 Ultrasound could enable greater access to breast cancer detection in Uganda, where the alternative is a complete lack of breast imaging in the absence of mammography.
The studies included in this meta-analysis were highly heterogeneous. We hypothesized that the heterogeneity could be caused by variability in study definitions from geographically diverse locations and populations. Thus, we performed meta-regression to further investigate the source of heterogeneity within the studies. In addition, we completed subset analyses with variables that capture these differences, including participant mean age, country, risk category, symptom presence, tissue density, study bias level (QUADAS-2), and study setting. This evaluation yielded no significant difference between categories as an explanation for the heterogeneity. We further investigated the heterogeneity by confirming no threshold effect with a Spearman correlation coefficient. As a result of these additional analyses, we find that the heterogeneity is related to diversity of studies and likely has limited impact on our results and conclusions.
Strengths of this study include use of broad, comprehensive search terms and multiple databases to capture all potentially relevant studies. Uniquely, this study evaluates the performance of ultrasound alone and compares mammography and ultrasound estimates for cancer detection, whereas most of the literature reviews ultrasound as a supplemental detection technique.53 This work adds to the growing body of evidence identifying regions and populations where breast ultrasound would be an effective, available early detection tool. This could facilitate a path to earlier detection of breast cancer for the 50% of women globally who currently have no access to breast imaging. However, as evidenced in this study, direct data from LMICs is sparse, so estimates from high-income countries are more heavily weighted in the pooled analyses. In addition, some included studies are subject to verification bias, because only patients with suspicious imaging findings received the gold standard of histopathology, which could lead to overestimation of sensitivity. Although there are other methods to determine the ground truth, such as a 2-year cancer-free interval for benign-appearing lesions, not all of the manuscripts reviewed provided this level of detail. To limit potential bias, papers with study populations that included only patients with previously diagnosed breast cancer were excluded, because these studies in higher-risk women would potentially yield a higher accuracy of breast ultrasound.
It is evident that additional research is needed to investigate the full potential of breast ultrasound for early breast cancer detection where mammography is infeasible. It is possible that the use of portable ultrasound could assist in development of a breast cancer care program that could ultimately include mammography or partner with programs that include mammography. More recent studies focus on comparing portable ultrasound to automated whole-breast ultrasound instead of comparing estimates to mammography.50,51 Ideally, future research would include direct comparisons of mammography and ultrasound in population-based settings and focus on asymptomatic women for early detection, yet this would require large populations and would be costly. In addition, false positives are persistently a concern with all breast imaging modalities, and more research is needed to define the best practices in resource-limited settings to avoid unnecessary use of resources, which ultimately does not change patient outcomes. Technical capacity and training programs for handheld, portable breast ultrasound are additional important considerations when using an ultrasound-based detection program. There is evidence this can be achieved on a small scale and could be scalable.59
In conclusion, on the basis of the existing literature, our review demonstrates ultrasound has the potential to yield high sensitivity and specificity for breast cancer detection. Ultrasound is widely available, easy to maintain, economical, durable, and easily portable. Given the increasing global burden of breast cancer and lack of access to timely detection with imaging, ultrasound may be an effective primary detection tool and triage method for breast lesions, particularly in low-resource settings where mammography is not available.
Footnotes
Presented at the Society of Breast Imaging, Los Angeles, CA, April 6-9 2017; the Symposium on Global Cancer Research, New York, NY, March 15-17, 2018; and the Breast Health Global Initiative Summit, Seattle, WA, October 15-17 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Rupali Sood, Delaram Shakoor, Kara-Lee Pool, Erica Pollack, Daniel J. Mollura, Lisa A. Mullen, Susan C. Harvey
Administrative support: Daniel J. Mollura
Collection and assembly of data: Rupali Sood, Delaram Shakoor, Kara-Lee Pool, Erica Pollack
Data analysis and interpretation: Rupali Sood, Anne F. Rositch, Delaram Shakoor, Emily Ambinder, Kara-Lee Pool, Erica Pollack, Daniel J. Mollura
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Anne F. Rositch
Consulting or Advisory Role: UE Life Sciences
Lisa A. Mullen
Research Funding: IBM Research (Inst), Cepheid (Inst)
Susan C. Harvey
Employment: Hologic
Stock and Other Ownership Interests: Hologic
Honoraria: Hologic
Speakers' Bureau: Hologic
Patents, Royalties, Other Intellectual Property: I have several patents in collaboration with biomedical engineering at Johns Hopkins as well with the Johns Hopkins Applied Physics.
Expert Testimony: Hologic
Travel, Accommodations, Expenses: Hologic
Other Relationship: Hologic
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization: Medical equipment: Mammography units per million females aged between 50-69 years old. World Health Organization, 2014. https://www.who.int/diagnostic_imaging/collaboration/mammunitpermill_14.jpg?ua=1.
- 3.Sprague BL, Arao RF, Miglioretti DL, et al. National performance benchmarks for modern diagnostic digital mammography: Update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283:59–69. doi: 10.1148/radiol.2017161519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Costa Vieira RA, Biller G, Uemura G, et al. Breast cancer screening in developing countries. Clinics (São Paulo) 2017;72:244–253. doi: 10.6061/clinics/2017(04)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetty MK. Screening and diagnosis of breast cancer in low-resource countries: What is state of the art? Semin Ultrasound CT MR. 2011;32:300–305. doi: 10.1053/j.sult.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: Early detection resource allocation. Cancer. 2008;113(8) suppl:2244–2256. doi: 10.1002/cncr.23842. [DOI] [PubMed] [Google Scholar]
- 7.Athanasiou A, Tardivon A, Ollivier L, et al. How to optimize breast ultrasound. Eur J Radiol. 2009;69:6–13. doi: 10.1016/j.ejrad.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Bae MS, Moon WK, Chang JM, et al. Breast cancer detected with screening US: Reasons for nondetection at mammography. Radiology. 2014;270:369–377. doi: 10.1148/radiol.13130724. [DOI] [PubMed] [Google Scholar]
- 9.Nothacker M, Duda V, Hahn M, et al. Early detection of breast cancer: Benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009;9:335. doi: 10.1186/1471-2407-9-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends--An update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 11.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(8) suppl:2221–2243. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 12.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 13.Anderson BO. Breast cancer--Thinking globally. Science. 2014;343:1403. doi: 10.1126/science.1253344. [DOI] [PubMed] [Google Scholar]
- 14.Yip CH, Buccimazza I, Hartman M, et al. Improving outcomes in breast cancer for low and middle income countries. World J Surg. 2015;39:686–692. doi: 10.1007/s00268-014-2859-6. [DOI] [PubMed] [Google Scholar]
- 15. Ginsburg O, Rositch AF, Conteh L, et al: Breast cancer disparities among women in low- and middle-income countries. Current Breast Cancer Reports 10:179-186, 2018.
- 16. Sloan FA, Gelband H (eds): The cancer burden in low- and middle-income countries and how it is measured, in Cancer Control Opportunities in Low- and Middle-Income Countries. Washington, DC, National Academies Press, 2007. [PubMed] [Google Scholar]
- 17.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 19.Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: Didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–2884. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 22.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Mavridis D, Salanti G. How to assess publication bias: Funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. 2014;17:30. doi: 10.1136/eb-2013-101699. [DOI] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Weigert JM. The Connecticut Experiment; the third installment: 4 years of screening women with dense breasts with bilateral ultrasound. Breast J. 2017;23:34–39. doi: 10.1111/tbj.12678. [DOI] [PubMed] [Google Scholar]
- 26.Tan KP, Mohamad Azlan Z, Rumaisa MP, et al. The comparative accuracy of ultrasound and mammography in the detection of breast cancer. Med J Malaysia. 2014;69:79–85. [PubMed] [Google Scholar]
- 27.Devolli-Disha E, Manxhuka-Kërliu S, Ymeri H, et al. Comparative accuracy of mammography and ultrasound in women with breast symptoms according to age and breast density. Bosn J Basic Med Sci. 2009;9:131–136. doi: 10.17305/bjbms.2009.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irurhe NK, Adekola OO, Awosanya GO, et al. The accuracy of ultrasonography in the diagnosis of breast pathology in symptomatic women. Nig Q J Hosp Med. 2012;22:236–239. [PubMed] [Google Scholar]
- 29.Matovu A, Scheel JR, Shadrak PA, et al. Pilot study of a resource-appropriate strategy for downstaging breast cancer in rural Uganda. The Journal of Global Radiology. 2016;2:1–6. [Google Scholar]
- 30.Omidiji OA, Campbell PC, Irurhe NK, et al. Breast cancer screening in a resource poor country: Ultrasound versus mammography. Ghana Med J. 2017;51:6–12. doi: 10.4314/gmj.v51i1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh K, Azad A, Gupta D. The accuracy of ultrasound in diagnosis of palpable breast lumps. JK Science. 2008;10:186–188. [Google Scholar]
- 32. World Bank: World Bank List of Economies. 2018. [Google Scholar]
- 33.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23:8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 34.Shen S, Zhou Y, Xu Y, et al. A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br J Cancer. 2015;112:998–1004. doi: 10.1038/bjc.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou MF, Chuang HY, Ou-Yang F, et al. Comparison of breast mammography, sonography and physical examination for screening women at high risk of breast cancer in Taiwan. Ultrasound Med Biol. 2002;28:415–420. doi: 10.1016/s0301-5629(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 36.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: Prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–388. doi: 10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 37.Sim LS, Hendriks JH, Fook-Chong SM. Breast ultrasound in women with familial risk of breast cancer. Ann Acad Med Singapore. 2004;33:600–606. [PubMed] [Google Scholar]
- 38.Warner E, Plewes DB, Shumak RS, et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol. 2001;19:3524–3531. doi: 10.1200/JCO.2001.19.15.3524. [DOI] [PubMed] [Google Scholar]
- 39.Bahl M, Baker JA, Greenup RA, et al. Diagnostic value of ultrasound in female patients with nipple discharge. AJR Am J Roentgenol. 2015;205:203–208. doi: 10.2214/AJR.14.13354. [DOI] [PubMed] [Google Scholar]
- 40.Houssami N, Irwig L, Simpson JM, et al. Sydney Breast Imaging Accuracy study: Comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol. 2003;180:935–940. doi: 10.2214/ajr.180.4.1800935. [DOI] [PubMed] [Google Scholar]
- 41.Shao H, Li B, Zhang X, et al. Comparison of the diagnostic efficiency for breast cancer in Chinese women using mammography, ultrasound, MRI, and different combinations of these imaging modalities. J XRay Sci Technol. 2013;21:283–292. doi: 10.3233/XST-130376. [DOI] [PubMed] [Google Scholar]
- 42.Lehman CD, Lee CI, Loving VA, et al. Accuracy and value of breast ultrasound for primary imaging evaluation of symptomatic women 30-39 years of age. AJR Am J Roentgenol. 2012;199:1169–1177. doi: 10.2214/AJR.12.8842. [DOI] [PubMed] [Google Scholar]
- 43. doi: 10.1093/jnci/djv367. Berg WA, Bandos AI, Mendelson EB, et al: Ultrasound as the primary screening test for breast cancer: analysis from ACRIN 6666. J Natl Cancer Inst . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 45.Leong LC, Gogna A, Pant R, et al. Supplementary breast ultrasound screening in Asian women with negative but dense mammograms-a pilot study. Ann Acad Med Singapore. 2012;41:432–439. [PubMed] [Google Scholar]
- 46.Uchida K, Yamashita A, Kawase K, et al. Screening ultrasonography revealed 15% of mammographically occult breast cancers. Breast Cancer. 2008;15:165–168. doi: 10.1007/s12282-007-0024-x. [DOI] [PubMed] [Google Scholar]
- 47.Youk JH, Kim EK, Kim MJ, et al. Performance of hand-held whole-breast ultrasound based on BI-RADS in women with mammographically negative dense breast. Eur Radiol. 2011;21:667–675. doi: 10.1007/s00330-010-1955-8. [DOI] [PubMed] [Google Scholar]
- 48.Girardi V, Tonegutti M, Ciatto S, et al. Breast ultrasound in 22,131 asymptomatic women with negative mammography. Breast. 2013;22:806–809. doi: 10.1016/j.breast.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Moon HJ, Jung I, Park SJ, et al. Comparison of cancer yields and diagnostic performance of screening mammography vs. supplemental screening ultrasound in 4394 women with average risk for breast cancer. Ultraschall Med. 2015;36:255–263. doi: 10.1055/s-0034-1366288. [DOI] [PubMed] [Google Scholar]
- 50.Choi WJ, Cha JH, Kim HH, et al. Comparison of automated breast volume scanning and hand- held ultrasound in the detection of breast cancer: An analysis of 5,566 patient evaluations. Asian Pac J Cancer Prev. 2014;15:9101–9105. doi: 10.7314/apjcp.2014.15.21.9101. [DOI] [PubMed] [Google Scholar]
- 51.Jeh SK, Kim SH, Choi JJ, et al. Comparison of automated breast ultrasonography to handheld ultrasonography in detecting and diagnosing breast lesions. Acta Radiol. 2016;57:162–169. doi: 10.1177/0284185115574872. [DOI] [PubMed] [Google Scholar]
- 52. doi: 10.3390/diagnostics8010020. Thigpen D, Kappler A, Brem R: The role of ultrasound in screening dense breasts-a review of the literature and practical solutions for implementation. Diagnostics (Basel) 10.3390/diagnostics8010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rebolj M, Assi V, Brentnall A, et al. Addition of ultrasound to mammography in the case of dense breast tissue: Systematic review and meta-analysis. Br J Cancer. 2018;118:1559–1570. doi: 10.1038/s41416-018-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: Outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472–1477. doi: 10.2214/AJR.10.4396. [DOI] [PubMed] [Google Scholar]
- 55.Birnbaum JK, Duggan C, Anderson BO, et al. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: A modelling study. Lancet Glob Health. 2018;6:e885–e893. doi: 10.1016/S2214-109X(18)30257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silber JH, Rosenbaum PR, Ross RN, et al. Disparities in breast cancer survival by socioeconomic status despite medicare and medicaid insurance. Milbank Q. 2018;96:706–754. doi: 10.1111/1468-0009.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shyyan R, Masood S, Badwe RA, et al. Breast cancer in limited-resource countries: Diagnosis and pathology. Breast J. 2006;12(suppl 1):S27–S37. doi: 10.1111/j.1075-122X.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 58.Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10:89–92. [PMC free article] [PubMed] [Google Scholar]
- 59.Dickerson LK, Rositch AF, Lucas S, et al. Pilot educational intervention and feasibility assessment of breast ultrasound in rural South Africa. J Glob Oncol. 2017;3:502–508. doi: 10.1200/JGO.2016.008086. [DOI] [PMC free article] [PubMed] [Google Scholar]