Summary
Mortality rate for burns patients in developing countries is approximately 34%. Data show that most patients in burn units will likely experience organ dysfunction. Sequential Organ Failure Assessment (SOFA) score assesses organ dysfunction and is frequently used in the ICU, but there are no previous studies regarding SOFA score in burn units in Indonesia specifically. This study was a retrospective study, conducted to assess the validity of the SOFA score in predicting mortality of critically ill burn patients in the Burn HDU and ICU of Cipto Mangunkusumo General Hospital between January 2012 to December 2017. This study included 169 subjects who met the inclusion and exclusion criteria. Medical records were used to identify the subjects’ characteristics, SOFA score within 24 hours, and outcome (deceased or survived) at day 30. SOFA score validity was assessed using Area Under Curve (AUC), Hosmer-Lemeshow goodness of fit and multivariate logistic regression. The mortality rate for burn patients was 32.5%. SOFA score had very good discrimination (AUC 96.4%, CI 95% 0.933 – 0.995) and good calibration (Hosmer-Lemeshow p = 0.561). SOFA variables which had a statistically significant effect on 30-day mortality in the Burn Unit were PaO2/FiO2 ratio < 400, PaO2/FiO2 ratio < 300, PaO2/FiO2 ratio < 200 with mechanical ventilation and platelet count < 150,000/mm3. SOFA score was a valid instrument for predicting 30 day mortality of critically ill burn patients in the Burn HDU and ICU of Cipto Mangunkusumo General Hospital, especially respiration and coagulation variables.
Keywords: burn, mortality, organ failure
Abstract
La mortalité après brûlure est d’environ 34% dans les pays en développement. La plupart des patients hospitalisés en CTB auront une défaillance d’organe. Le score SOFA est régulièrement utilisé en réanimation mais n’a pas été évalué spécifiquement chez les brûlés en Indonésie. Une étude rétrospective a été réalisée dans ce but au sein du CTB de l’hôpital général Cipto Mangunkusumo, entre janvier 2012 et décembre 2017. Elle a concerné 169 patients. A partir des dossiers, nous avons calculé les SOFA à h 24 et avons recherché les corrélations avec l’évolution. La validité du SOFA a été évaluée grâce à l’aire sous la courbe ROC, au test de corrélation de Hosmer- Lemeshow et à une régression logistique multivariée. La mortalité observée était de 32,5%. Le SOFA avait une bonne capacité discriminante (AUC ROC 96,4%- IC 95% 0,933-0,995) et une calibration correcte (Hosmer- Lemeshow p= 0,561). Les paramètres du score SOFA corrélés à la mortalité à J30 étaient P/F < 400, P/F < 300; P/F < 200 (sous ventilation mécanique) et une numération plaquettaire < 150 000/mm3. SOFA est un bon instrument pour prévoir la mortalité à 30 j des patients brûlés hospitalisés dans le CTB de l’hôpital général Cipto Mangunkusumo, en particulier ses paramètres ventilatoires et le taux de plaquettes.
Introduction
Burn injury is a destructive injury that mostly results in significant health and mental problems, disability and low quality of life.1 Based on previous studies, the mortality rate among adult patients with burn injury is still high in south-east Asian developing countries, such as in Indonesia where it is approximately 14.5-34%.1-3 Because of the high mortality rate among critically-ill burn patients, we need to identify risk factors using a simple and effective tool to predict mortality.
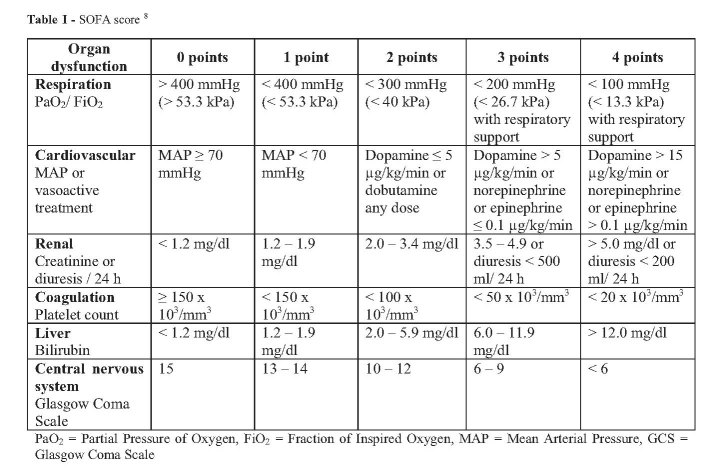
The ideal scoring system should have certain characteristics, such as easy-to-use routinely recorded variables, well validated, high discrimination value, be applicable to all populations and countries, and able to predict functional status and quality of life after discharge from the intensive care unit (ICU).4 Several scores for stratifying organ failure and predicting mortality have been developed over the past years. Sequential Organ Failure Assessment (SOFA) is one of the scores that is commonly used in the ICU. SOFA score can be used for all patients, not only for those with sepsis.5-7 The SOFA score (Table I) has been validated to assess organ failure or predict mortality in any critically-ill patient, such as post-surgery patients and those with sepsis, but it has rarely been used for burn injury patients.6,8–13 Just like in other trauma patients, organ failure from burn injury is a fatal complication during treatment. Lorente et al. stated in their study that early or delayed onset of organ failure in burn injury patients is similar to other trauma patients, and can increase mortality risk.14 Every critically-ill burn patient has a different response to the injury, which is related to the severity of organ failure. These differences can be assessed with the SOFA score.14
Table I. SOFA score 8.
Simplified tools to stratify the risk of mortality and to promote treatment are needed. Several studies have been conducted to prove the validity of SOFA scores in the general ICU but they have excluded burn patients.8,9,15 Our study retrospectively assessed the validity of the SOFA score and evaluated the ability of each variable to predict mortality in critically-ill burn patients.
Materials and methods
Study design and ethical issue
This retrospective study was conducted in the Cipto Mangunkusumo General Hospital using the medical records of adult patients who were admitted to the Burn High Dependency Unit (HDU) and ICU between January 2012 to December 2017. Data were collected under approval from the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (No. 0948/UN2.F1/ETIK/2018).
Subjects and study protocol
The minimum required sample size was calculated based on multivariate predictive categorical variables in the SOFA score, which required 162 subjects for this study. The inclusion criteria for this study were: adult, critically-ill burn patients aged 18 years or over. Exclusion criteria were: deceased patients or those who were discharged less than 24 hours from admission to the Burn Unit, medical records with inadequate or incomprehensible information regarding assessed variables, and unknown patient outcome (survived or deceased) during care in the Burn Unit. Patients who were referred to another hospital within 30 days of admission to the Burn Unit were excluded from the analysis.
Secondary data were obtained from the patient registry and medical records in the Burn Unit. Subject characteristics, SOFA score within 24 hours, and patient outcome (deceased or survived) at day 30 were recorded. If the patient was discharged within 30 days of admission, the outcome was also recorded. Unrecorded information regarding patient outcome was traced by contacting the patient’s relatives. Data were collected until the minimum sample size required was met.
Statistical analysis
Data were analysed using Statistical Package for Social Scientist (SPSS) and MedCalc Program. Data were processed and presented in tables, graphs and/or text. Variables with P value <0.25 and clinically important were screened for logistic regression analysis. External validation testing of the SOFA score was done by assessing discrimination and calibration. Discrimination testing was done by Receiver Operating Characteristic (ROC) curve analysis to obtain Area Under the Curve (AUC) value. Calibration testing was done using the Hosmer-Lemeshow test.
Results
There were 233 patient medical records for this study, but 38 of these did not meet the inclusion criteria. Of the remaining 195 medical records, 26 could not be analysed because of incomplete data (Fig. 1).
Fig. 1. Subject recruitment flow diagram.
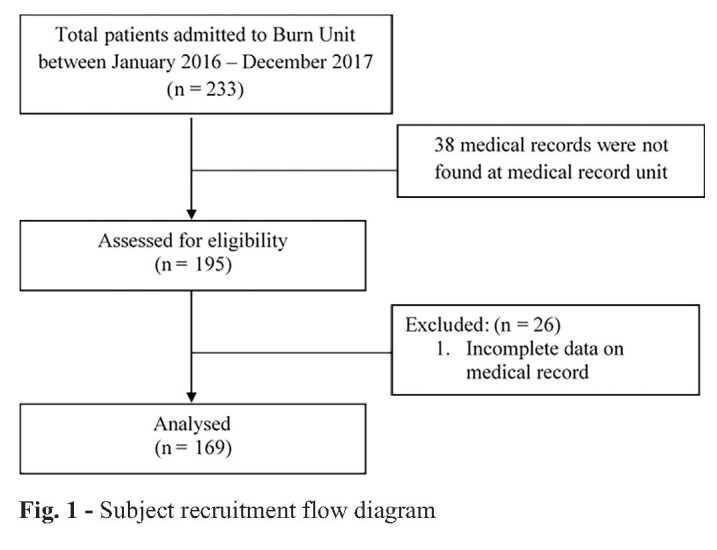
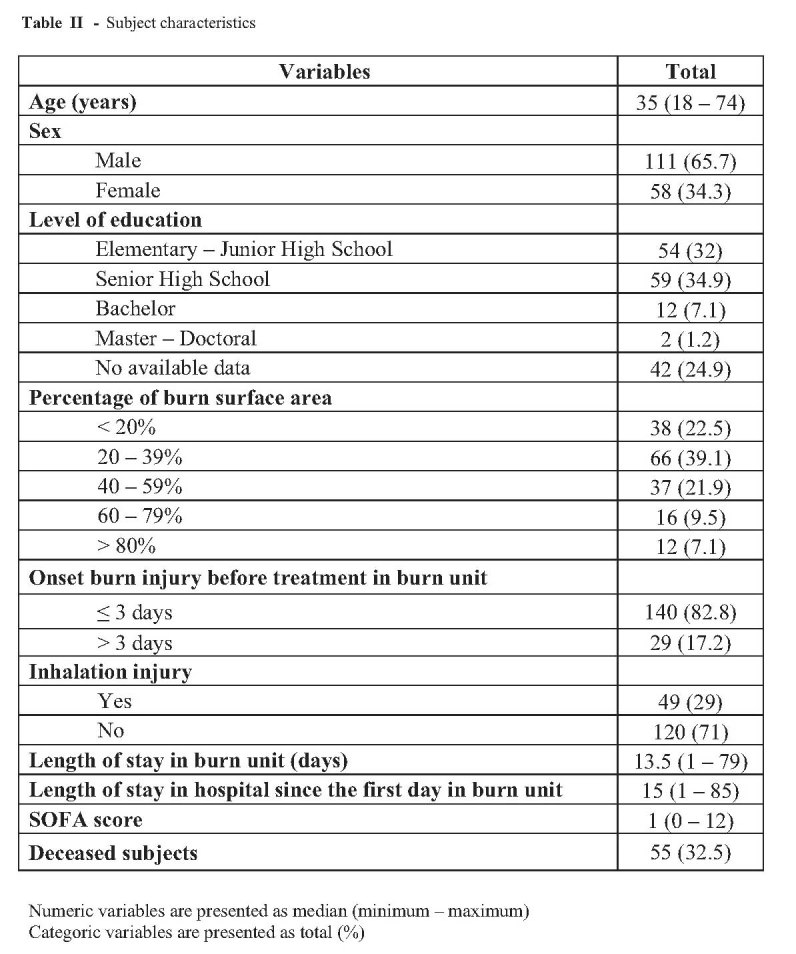
Table II shows subject characteristics in total and percentage. Our subjects were predominantly male (65.7%) with a mean age 35 years, and most had suffered 20-60% TBSA burns. Inhalation injury was diagnosed in 49 patients (29%) and the median length of stay in the Burn Unit was 13.5 days.
Table II. Subject characteristics.
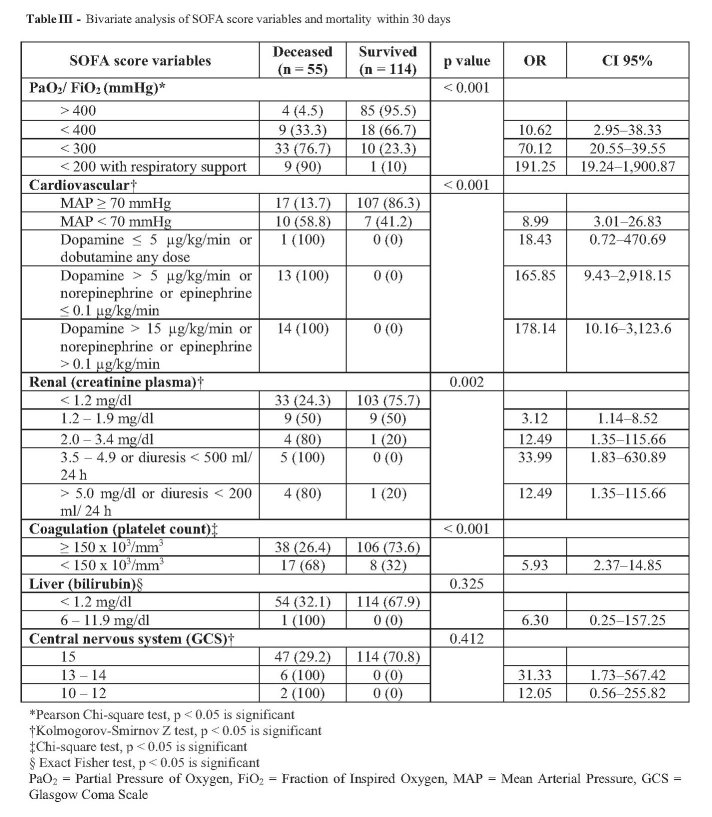
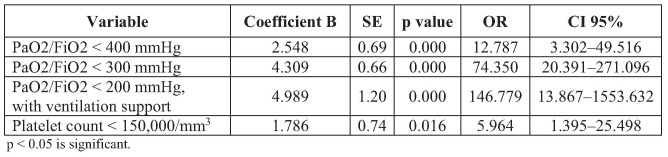
Bivariate analysis (Table III) shows that PaO2/FiO2 variable, platelet count, cardiovascular score and kidney variable in the SOFA score were significant. Logistic regression analysis (Table IV) shows that PaO2/FiO2 ratio with ventilation support; < 400 mmHg, < 300 mmHg, < 200 mmHg; and platelet count < 150,000/mm3 were the significant variables to predict mortality in 30 days, with the formula: y = -3.385 + (2.548 x PaO2/FiO2 < 400) + (4.309 x PaO2/FiO2 300) + (4.989 x PaO2/FiO2 < 200 with ventilation support) + (1.786 x platelet < 150,000/mm3). Hosmer-Lemeshow testing showed p = 0.138, which means that 4 variables in the SOFA score based on logistic regression analysis were suitable to predict mortality in 30 days. If those variables convert into a new SOFA score, PaO2/FiO2 < 400 and PaO2/FiO2 < 200 with ventilation support had a score of 2, PaO2/FiO2 < 300 had a score of 3, and platelet count <150,000/mm3 had a score of 1.
Table III. Bivariate analysis of SOFA score variables and mortality within 30 days.
Table IV. Logistic regression analysis in SOFA score variables and mortality within 30 days.
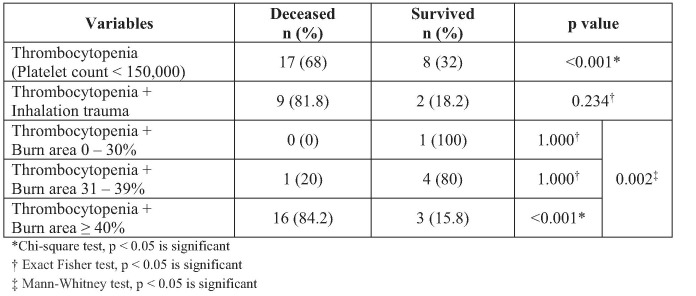
Table V indicates that the incidence of thrombocytopenia was significantly higher in deceased patients especially in thrombocytopenic patients with burn area ≥ 40% compared to patients with burn area < 40%. The extent of burn area and thrombocytopenia had a significant relationship (p = 0.002) on 30-days mortality. There was no significant difference between thrombocytopenic patients with inhalation trauma on 30-days mortality. However, all 9 deceased thrombocytopenic patients with inhalation trauma had burn area ≥ 40% while 2 thrombocytopenic survivors with inhalation trauma had burn areas of 30% and 38%.
Table V. Mortality rate of patients with and without thrombocytopenia.
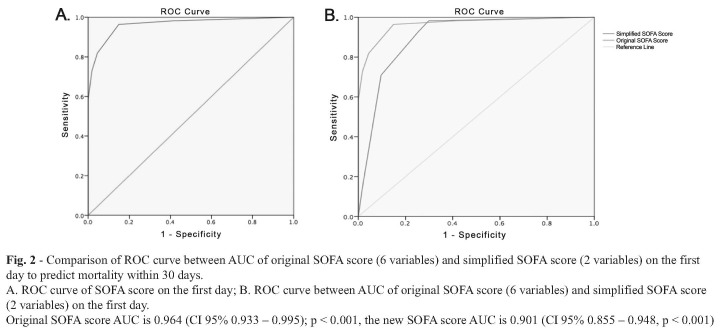
Fig. 2 shows SOFA score accuracy to predict mortality in 30 days among burn unit patients. AUC value is 0.964 (CI 95% 0.933 – 0.995) which means that the ability of the SOFA score at the first day to differentiate subjects who would die within 30 days is 96.4%. ROC curve comparison between the original SOFA score with 6 variables (AUC 0.964; CI 95% 0.933 – 0.995; p < 0.001) and the simplified SOFA score with 2 variables (respiratory PaO2/FiO2 ratios and platelet count) (AUC 0.901; CI 95% 0.855 – 0.948, p < 0.001) shows that both SOFA scores had a good performance and there were no significant differences in AUC (the difference is < 0.10) between both scores.
Fig. 2. Comparison of ROC curve between AUC of original SOFA score (6 variables) and simplified SOFA score (2 variables) on the first day to predict mortality within 30 days. A. ROC curve of SOFA score on the first day; B. ROC curve between AUC of original SOFA score (6 variables) and simplified SOFA score (2 variables) on the first day. Original SOFA score AUC is 0.964 (CI 95% 0.933 – 0.995); p < 0.001, the new SOFA score AUC is 0.901 (CI 95% 0.855 – 0.948, p < 0.001).
Goodness of fit testing using Hosmer-Lemeshow analysis shows p > 0.05 (Calibration Chi-square value = 2.98; p = 0.561) which means that SOFA score on the first day is fit enough to predict mortality within 30 days. A SOFA score cut-off value of 2.00 was taken from ROC analysis and determined based on clinical considerations, where cut-off value
Discussion
This study included 169 subjects selected by convenience sampling. Most of the patients were young adult males with a mean age of 35 years: the youngest was 18 years old and the oldest was 74 years old. Most of the subjects only reached high school education (34.9%), indicating that burns frequently occurred in patients with a low to middle education level due to their lack of understanding regarding burn prevention in daily life. This study showed that 38.5% of the subjects had a burn area ≥ 40%. The median SOFA score was 1, the lowest score was 0 and the highest was 12, out of the maximum SOFA score of 24. These results showed that the burn patients suffered at least one organ failure.
Our results showed the good calibration and discrimination of SOFA score on the first day of admission with a Hosmer-Lemeshow test result of p = 0.561 (p > 0.05) and AUC value of 0.96 (CI 95% 0.933 – 0.995), which means SOFA score on the first day is a fit for 30-day mortality prediction. The result was similar to a study by Karlie et al., which evaluated Belgian Outcome of Burn Injury (BOBI) and correlation with mortality in the Burn Unit, and gave an AUC value of 0.96.16 The SOFA score cut-off point is 2.00 in our study, based on clinical findings with good sensitivity and specificity. A SOFA score of 2 or more on the first day had good discrimination in patients who had a higher probability of 30-day mortality rate. This low cut-off point could have resulted from homogenous data; however, this may be beneficial as an early warning for the physician. From this result, the physician can anticipate close observation to prevent organ failure, which can increase the mortality risk of the patient.
In our centre, routine SOFA score evaluation has not been a standard procedure for burn patients due to cost, especially for patients without insurance cover. Logistic regression analysis was used to evaluate SOFA score variables that had a strong correlation with 30-day mortality outcome. This analysis showed two variables with a strong correlation; PaO2/FiO2 ratio and platelet count. PaO2/FiO2 ratio < 200 with ventilation support and platelet count < 150,000/mm3 made a large contribution to predicting 30-day mortality rate. The AUC comparison analysis showed that the new SOFA scores using 2 variables (PaO2/FiO2 ratio and platelet count) showed a comparable performance with the original SOFA score with 6 organ variables. Although there is currently no perfect scoring system, every scoring system has its own strengths and weaknesses. Implementation in a health centre depends on validity, feasibility, complexity and impact study of the score. Therefore, a simple scoring system is preferred over a complicated one.
In comparison to the BOBI score, which has good discrimination for burn patients, the SOFA score is simpler and more objective because the variables are measured from laboratory tests. SOFA score evaluates organ failure, which can help the physician’s judgement in managing the patient. Routine SOFA score evaluation helps the clinician to evaluate therapeutic management that may reduce mortality risk. The original SOFA score was more universal because it was renowned and many countries had implemented it. However, evaluating the 6 organ variables is frequently impractical, especially for burn units in developing countries. Based on this study result, we can use PaO2/FiO2 ratio and platelet count on the first day to predict 30-day mortality of burn patients in the Cipto Mangunkusumo General Hospital.
The cardiovascular system was measured from mean arterial pressure (MAP) and the usage of inotropic and vasoactive drugs. In this study, cardiovascular variables were remarkable in bivariate analysis, but unremarkable in multivariate analysis for 30-day mortality. This result might be due to the fact that most patients in the Cipto Mangunkusumo General Hospital had onset of burn less than 3 days before, and receiving early vasoactive or inotropic drugs along with adequate fluid resuscitation made no significant correlation between a cardiovascular variable with 30-day mortality in multivariate analysis. Acute kidney failure usually develops in the first 48 hours because of inadequate fluid resuscitation, resulting in low kidney perfusion and a 50%-70% mortality rate.17,18 This study estimates that most burn patients in the Cipto Mangunkusumo General Hospital received adequate fluid resuscitation using the Parkland formula within the first 24 hours. A clinician has to maintain kidney perfusion and minimize nephrotoxic agents to reduce the risk of kidney failure.19
Bivariate analysis in this study showed a statistically significant correlation (p < 0.05) between PaO2/FiO2 and 30-day mortality. This correlation was confirmed with multivariate analysis, which also showed a lower PaO2/FiO2 value, the greater mortality rate of the subject. These results were also observed in a retrospective study conducted in 2014 by Chen et al. His study involving 791 burn patients in 44 hospitals showed a greater mortality rate for patients with respiratory failure due to inhalation trauma (17.9%) compared to patients without inhalation trauma (0.7%). Silva et al. stated that inhalation injury is a risk factor for acute respiratory distress syndrome (ARDS) (OR = 9.75; p < 0.001).20-22 ARDS respiratory failure in burns is still one of the leading complications causing death among burn patients, with an incidence of about 20-56%. Most studies showed that the onset of ARDS was around 6 to 7 days post burn.6,10,12 In our study, the time point of ARDS was around the 3rd day or less post burn. A study by Lam et al. in a developing country showed that 66.7% of patients with ARDS progressed to severe ARDS, with inhalation injury, burn surface area over 40% and full thickness burn area over 20% TBSA determined as risk factors. The mortality rate for patients, especially burn patients with severe ARDS in a developing country, was extremely high (80%) (p < 0.01) since there are only a limited number of institutions that can perform prone-positioned ventilation or ECMO, so deaths are mostly due to multiple organ failure.22
Coagulation system was evaluated from platelet count. Thrombocytopenia is usually found in the first week of disease in critical burn patients, followed by increasing platelet values for 2–3 weeks afterwards. The bivariate and multivariate analysis of our study showed a good correlation between the platelet count of deceased subjects and subjects who survived (p < 0.001). Our study also showed that subjects with platelet count < 150,000/mm3 had a 5.93 times higher risk for 30-day mortality than subjects with a platelet value of more than 150,000/mm3. Our results showed that 65 (38.5%) subjects with burn area over 40% and the incidence of thrombocytopenia was significantly higher in non-survivors with burn area over 40% compared to non-survivors with burn area less than 40%. There was a significant relationship (p = 0.002) between the extent of burn area and thrombocytopenia on 30-days mortality. Marck et al. concluded from their study that thrombocytopenia was greater in patients with burn area over 30% compared to patients with burn area less than 30%. They also observed that patients who died had lower platelets than patients who survived.23
This result was also observed by Gou et al. who concluded that a decreased platelet count of 65% was a good predictive factor of 30-day mortality (AUC = 0.784, p = 0.05).24 Thrombocytopenia is associated with organ dysfunction and vascular leakage syndromes in burn patients. Most subjects (82.8%) were admitted to our Burn Unit within the 3rd day of injury, and most of them had already received fluid resuscitation before admission. Thrombocytopenia in burn injury can be caused by hemodilution from the administration of a large amount of fluids or as a complication of disseminated intravascular coagulation (DIC) due to inflammation or sepsis. After the injury, any damaged cells activate immune cells such as neutrophils, which promote platelet adhesion or aggregation and increase thrombocyte consumption.24,25
This was the first study to measure the validity of the SOFA score in burn patients, using a simplified score with 2 variables to predict 30-day mortality in critically ill burn patients in the Cipto Mangunkusumo General Hospital. The limitation of this study is that the SOFA score is not routinely assessed for predicting outcome in burn patients, therefore many of the variables are not routinely measured in burn patients. Although this study shows good validation of the SOFA score on the first day for predicting 30-days mortality, organ failure is a dynamic process that happens over time, therefore a routine evaluation with treatment adaptation to ongoing complications will be more beneficial in the therapeutic management of the patient.
Conclusion
The SOFA score showed very good prognostic accuracy for mortality in critically ill burn patients. Of the SOFA variables, respiratory and coagulation dysfunction on admission had a very good ability to predict 30-day in-hospital mortality in critically ill burn patients.
References
- 1.Wardhana A, Basuki A, Prameswara ADH, Rizkita DN. The epidemiology of burns in Indonesia’s national referral burn center from 2013 to 2015. Burns Open. 2017;1(2):67–73. [Google Scholar]
- 2.Querioz LF. Epidemiology and outcome analysis of burn patients admitted to an Intensive Care Unit in a University Hospital. Burns. 2016;42(3):655–662. doi: 10.1016/j.burns.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.De Macedo JLS, Santos JB. Predictive factors of mortality in burn patients. Rev Inst Med Trop Sao Paulo. 2007;49(6):365–370. doi: 10.1590/s0036-46652007000600006. [DOI] [PubMed] [Google Scholar]
- 4.Bouch DC, Thompson JP. Severity scoring systems in the critically ill. Contin Educ Anaesth Crit Care Pain. 2008;8(5):181–185. [Google Scholar]
- 5.Vincent J. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Snell JA, Loh N-HW, Mahambrey T, Shokrollahi K. Clinical review: the critical care management of the burn patient. Crit Care. 2013;17:241. doi: 10.1186/cc12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A. Sequential organ failure assessment scoring and prediction of patient’s outcome in Intensive Care Unit of a tertiary care hospital. J Anesthesiol Clin Pharmacol. 2016;32(3):364–368. doi: 10.4103/0970-9185.168165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taofik S, Senapathi TGA, Wiryana M. Comparison of validity APACHE II, SOFA and Customized Sequential Organ Failure Assessment (CSOFA) for predicting non-surgical patient. J Anestesiol Indones. 2015;7(2):102–113. [Google Scholar]
- 9.Sunaryo A, Redjeki IS, Bisri T. Perbandingan validasi APACHE II dan SOFA Score untuk memperkirakan mortalitas pasien yang dirawat di Ruang Perawatan Intensif. Majalah Kedokteran Terapi Intensif. 2012;2(1):11–20. [Google Scholar]
- 10.Cárdenas-Turanzas M. Cross-validation of a Sequential Organ Failure Assessment score–based model to predict mortality in patients with cancer admitted to the intensive care unit. J Crit Care. 2012;27(6):673–680. doi: 10.1016/j.jcrc.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 11.de Brito MR. Evaluation of Sequential Organ Failure Assessment (SOFA) performance in neurocritical care patients overtime: A retrospective cohort study. J Brain Disord. 2017;1(1):38–43. [Google Scholar]
- 12.Nair R, Bhandary NM, D’Souza AD. Initial Sequential Organ Failure Assessment score versus Simplified Acute Physiology score to analyze multiple organ dysfunction in infectious diseases in Intensive Care Unit. Indian J Crit Care Med. 2016;20(4):210–215. doi: 10.4103/0972-5229.180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabré L, Mancebo J. Multicenter study of the multiple organ dysfunction syndrome in intensive care units: the usefulness of Sequential Organ Failure Assessment scores in decision making. Intensive Care Med. 2005;31(7):927–933. doi: 10.1007/s00134-005-2640-2. [DOI] [PubMed] [Google Scholar]
- 14.Lorento J. Organ dysfunction as estimated by the sequential organ failure assessment score is related to outcome in critically ill burn patients. Shock. 2009;31(2):125–131. doi: 10.1097/SHK.0b013e31817fc3ef. [DOI] [PubMed] [Google Scholar]
- 15.Andrias A, Hanafie A, Wijaya DW. Comparison of APACHE II, SOFA, and CSOFA scoring system validity as mortality predictor in ICU patients in H. Adam Malik General Hospital. J Anestesi Perioper. 2017;5(1):17–23. [Google Scholar]
- 16.Karlie J, Wardhana A. External validation of Belgian Outcome of Burn Injury Score on burned patient in Burn Unit Cipto Mangunkusumo General Hospital. New Ropanasuri J Surg. 2017;2(1):90. [Google Scholar]
- 17.Emara SS, Alzaylai AA. Renal failure in burn patients: a review. Ann Burns Fire Disasters. 2013;26(1):12–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim AE, Sarhane KA, Fagan SP, Goverman P. Renal dysfunction in burns: a review. Ann Burns Fire Disasters. 2013;26(1):16–25. [PMC free article] [PubMed] [Google Scholar]
- 19.Carson J, Goverman J, Fagan S. Acute renal failure in association with thermal injury. Total Burn Care. 2018:318–327. [Google Scholar]
- 20.Nielson CB. Burns: Pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38(1):e469–e481. doi: 10.1097/BCR.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enkhbaatar P, Sousse L, Cox R, Herndon D. The pathophysiology of inhalation injury. Total Burn Care. 2018:174–183. [Google Scholar]
- 22.Lam NN, Hung TD, Hung DK. Acute respiratory distress syndrome among severe burn patients in a developing country: application result of the Berlin definition. Ann Burns Fire Disasters. 2018;31(1):9–12. [PMC free article] [PubMed] [Google Scholar]
- 23.Marck RE, Montagne HL, Tuinebreijer WE, Breederveld RS. Time course of thrombocytes in burn patients and its predictive value for outcome. Burns. 2013;39(4):714–722. doi: 10.1016/j.burns.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Guo F. Association of platelet counts decline and mortality in severely burnt patients. J Crit Care, 2012;27(5):529.e1–529.e7. doi: 10.1016/j.jcrc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Thachil J, Warkentin TE. How do we approach thrombocytopenia in critically ill patients? Br J Haematol. 2017;177(1):27–38. doi: 10.1111/bjh.14482. [DOI] [PubMed] [Google Scholar]