Abstract
Background
Alzheimer’s disease (AD) is the primary cause of dementia in the elderly. The imbalance between production and clearance of amyloid β (Aβ) is a very early, often initiating factor in AD. Dendrobium nobile Lindl. alkaloids (DNLA) extracted from a Chinese medicinal herb, which have been shown to have anti-aging effects, protected against neuronal impairment in vivo and in vitro. Moreover, we confirmed that DNLA can improve learning and memory function in elderly normal mice, indicating that DNLA has potential health benefits. However, the underlying mechanism is unclear. Therefore, we further explored the effect of DNLA on neurons, which is closely related to learning and memory, based on Aβ.
Methods
We exposed cultured hippocampal neurons to DNLA to investigate the effect of DNLA on Aβ in vitro. Cell viability was evaluated by MTT assays. Proteins were analyzed by Western blot analysis.
Results
The cell viability of hippocampal neurons was not changed significantly after treatment with DNLA. But DNLA reduced the protein expression of amyloid precursor protein (APP), disintegrin and metalloprotease 10 (ADAM10), β-site APP cleaving enzyme 1 (BACE1) and Aβ1–42 of hippocampal neurons in rats and increased the protein expression of ADAM17.
Conclusions
DNLA decreases Aβ by regulating α- and β-secretase in hippocampal neurons of SD rats.
Keywords: Dendrobium nobile Lindl. alkaloids, α-secretase, Hippocampal neurons, β-secretase, γ-secretase
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease of the central nervous system that causes dementia in a large percentage of the aged population and for which there are only symptomatic treatments. Clinically, AD is typically characterized by progressive loss of memory, declining cognitive function, decreasing physical function and ultimately death. The biology of AD is characterized by two major protein abnormalities in the brain of affected individuals: the extracellular accumulation of amyloid β (Aβ) plaques and intraneuronal deposits of neurofibrillary tangles (NFTs) (De-Paula et al., 2012). At present, the pathogenesis of AD has yet to be fully elucidated, and the main hypotheses proposed include deposition of Aβ protein, loss of choline neurons, abnormal activation of inflammatory reactions, disturbance of energy metabolism, genetic abnormalities and oxidative stress (Choudhry et al., 2012; Mouton-Liger et al., 2012). In addition, environmental factors, diet and diseases increase the risk of AD (Ashok et al., 2015; Banerjee et al., 2014; Cansu et al., 2017; Cheng et al., 2015; Faraco et al., 2016; Yousof Ali, Jung & Choi, 2015). The Aβ peptide cascade plays a critical role in the development of AD (Baranello et al., 2015).
Aβ is generated by amyloid precursor protein (APP) metabolism. APP in its mature form can be processed by at least two proteolytic pathways (Kowalska, 2004), the so-called “non-amyloidogenic” and the “amyloidogenic” pathways. In the first pathway, α-secretases cleaves APP with the γ-secretases, thus impeding the formation of the toxic Aβ peptide. In the second pathway, Aβ, which mainly consists of the Aβ1–40 and Aβ1–42 peptides, is produced in a two-step proteolytic process initiated by β-secretase and next mediated by γ-secretase. In brief, α-, β- and γ-secretases play key roles in the metabolic processing of APP. Strategies to reduce the formation of toxic Aβ are an obvious approach to prevent AD. Activation of α-secretase promotes the cleavage of APP, which can reduce the production of Aβ (Lichtenthaler, 2012). And the inhibition of β-secretase (Hilpert et al., 2013) and γ-secretase (Wolfe, 2012) activity can also reduce the production of Aβ. Therefore, targeting α-, β- and γ-secretases is a promising focus of AD research.
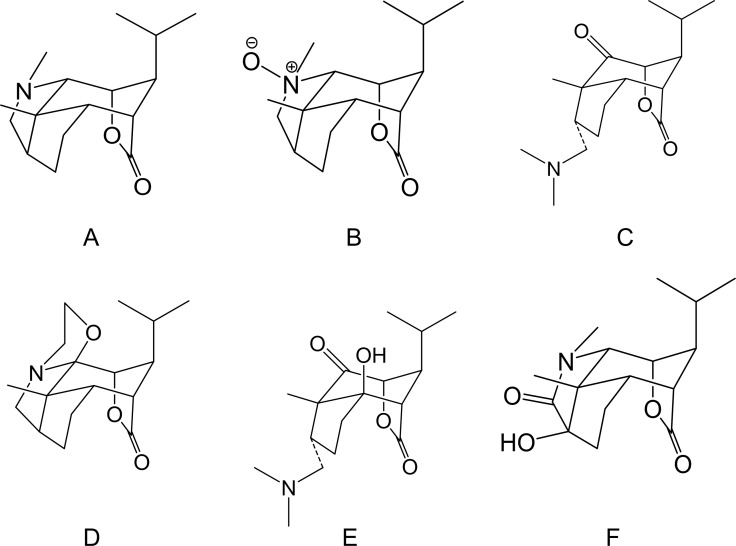
Dendrobium (D.) nobile Lindl. is a traditional Chinese herbal medicine and medicinal material of Guizhou Province. The chemical constituents of D. nobile Lindl. include alkaloids, sesquiterpene, bibenzyl, fluorenone, phenolic acid, phenylpropanoid, phenanthrene, polysaccharides, and lignans. An ancient medical book notes that this treatment can strengthen the body and extend life. Most compounds had good physiological activity, could significantly improve memory loss and cerebral ischemia, and showed anti-fatigue, antioxidative, blood sugar lowering, antitumor and anti-inflammatory effects. As a pharmacologically active ingredient of D. nobile Lindl., D. nobile Lindl. alkaloids (DNLA) was originally extracted from D. nobile Lindl and has significant protective effects in the nervous system. The chemical structures of DNLA are shown in the Fig. 1, and the chromatograms of the sample solutions are shown in Fig. 1B (Nie et al., 2016). Previous studies indicated that DNLA can improve the neuronal disruption caused by lipopolysaccharide (Li et al., 2011b), oxygen-glucose deprivation (Wang et al., 2010) and reperfusion and decrease neuronal apoptosis, hyperphosphorylation of tau protein, and Aβ deposition in the rat brain (Nie et al., 2016). Furthermore, we observed amelioration of the spatial learning performance in AD model rats induced by Aβ25–35, and this effect may be related to decreases in the generation of Aβ1–42 (Zhang et al., 2016) and alleviation of Aβ25–35-induced axonal injury by improving autophagic flux in neurons in vitro (Li et al., 2017; Zhang et al., 2017). Notably, DNLA improved learning and memory function in elderly normal mice (Linshan et al., 2016). This caught our attention and we really want to know why there is such an improvement.
Figure 1. Chemical structures of Dendrobium nobile Lindl alkaloids.
(A) Dendrobine, (B) Dendrobine-N-oxide, (C) Nobilonine, (D) Dendroxine, (E) 6-Hydroxy-nobilonine, and (F) 13-Hydroxy-14-oxodendrobine.
Therefore, based on the background of AD and our previous works, we designed the following experiment that aims to investigate the effect of DNLA on Aβ and related secretases in the normal hippocampal neurons of rats and to further explore the mechanism underlying the regulation of the APP metabolic pathway.
Materials & Methods
Materials
Dendrobium was purchased from Xintian Traditional Chinese Medicine Industry Development Co., Ltd., of Guizhou Province. DNLA was isolated from the extracts and analyzed by LC MS/MS. Alkaloids accounted for 79.8% of the DNLA and were comprised of 92.6% dendrobine (C16H25O2N), 3.3% dendrobine-N-oxide (C16H25O3N), 2.0% nobilonine (C17H27O3N), 0.9% dendroxine (C17H25O3N), 0.32% 6-hydroxy-nobilonine (C17H27O4N), and 0.07% 13-hydroxy-14-oxodendrobine (C16H23O4N) (Nie et al., 2016). Trypsin (SH30042.01) and DMEM/F12 medium (SH3002301B) were purchased from HyClone (America). FBS (S9030) was purchased from Solarbio (China). DES, Neurobasal A (A2477501), and B27 (17504044) were purchased from Gibco (America). The goat anti-mouse IgG H&L (Alexa Fluor® 488) was purchased from Life Technologies (America). Anti-beta III tubulin antibody (ab14545), goat anti-rabbit IgG H&L (ab150113), anti- disintegrin and metalloprotease (ADAM)10 (ab1997), anti-β-site APP cleaving enzyme 1(BACE1) (ab2007), anti-presenilin 1(PS1) (ab76083), and anti-Aβ1–42 (ab201060) were purchased from Abcam (America). Anti-APP (D260097) and anti-ADAM17 (D151531) were purchased from Sangon Biotech (China).
Animals
Sprague-Dawley (SD) rats (approximately 200∼250 g) were purchased from the Laboratory Animal Center, Chongqing, China [grade: specific pathogen-free (SPF), certificate no. SCXK 2012-0005] and housed at 22∼23 °C with a 12-hour light/dark cycle. Five SD rats (male: female = 1:4) were housed in each cage to propagate the newborn SD rats and had free access to food and water. All procedures were undertaken with the approval of the Animal Experimental Ethical Committee of Zunyi Medical University [No. (2019)2-995, 11 Mar 2019].
Pretreatment of DNLA
DNLA was soluble in dimethyl sulfoxide (DMSO) and was stored at −20 °C. When used, DNLA was diluted to different concentrations with Neurobasal A supplemented with 2% B27. The final concentration of DMSO was 0.01% (v/v) in our experimental system.
Culture of hippocampal primary neurons and identification
Hippocampal tissues from newborn SD rats born within 24∼48 h were separated on ice and then incubated in 0.125% trypsin at 37 °C with 5% CO2 for 15 min. Hippocampal tissues were triturated by passing repeatedly through a 1-mL pipette tip and filtered through a sieve with 200, 300, and 400 mesh. Then, the cells were collected by centrifugation at 179 g for 8 min. Cells were seeded on poly-L-lysine-coated (four mg/mL) 6-well, 24-well plates or 96-well plates and cultured in DMEM/F12 medium supplemented with 10% FBS, 10% DES, 100 U/mL penicillin and streptomycin at 37 °C with 5% CO2. After 4 h, DMEM/F12 medium was replaced with Neurobasal A supplemented with 2% B27 for the duration of the experiments. Half of the liquid was exchanged every two or three days. On the 3rd day, neurons were treated with cytarabine. On the 8th day, neurons in good condition were used for the following experiments. The animal procedures were approved by the Animal Experimentation Ethics Committee of Zunyi Medical University. The profile of neurons was visualized by immunofluorescence staining using mouse monoclonal anti-beta III tubulin antibody (1:1,000) and goat anti-mouse IgG H&L (1:1,000). DAPI (1:20) was used to mark the cell nucleus.
Assessment of cell viability by MTT assays
Neurons were seeded into 96-well plates and treated with DNLA for a desired time period at the indicated concentrations. Five replicates were made for each treatment. After treatment, cell viability was evaluated by MTT assays as previously described. The cell viability is expressed as a percentage of the OD of cells with the indicated treatments to that in cells treated with the DMSO control.
Western blot assay
Total protein was extracted from cultured neurons using a total protein extraction kit and quantified by a BCA protein assay kit. Equal amounts of protein (20 µg) per lane were separated by SDS-PAGE gels and then transferred to a PVDF (0.45 µm) membrane. The membranes were incubated with the following primary antibodies: anti-APP (1:500), anti-ADAM10 (1:1,000), anti-ADAM17 (1:1,000), anti-BACE1 (1:1,000), anti-PS1 (1:1,000), anti-Aβ1–42 (1:1,000), anti-β-actin (1:2,000), and anti-GAPDH (1:2,000) at 4 °C overnight, followed by incubation with secondary antibody at 4 °C for 1 h. The membranes were visualized using the chemiluminescence reagent ECL Plus (E003-100). The image was scanned, and band densities were quantified using Quantity One 1D analysis software v4.52 (Bio-Rad). GAPDH or β-actin was used to normalize protein loading.
Statistical analysis
All data are expressed as the mean ± SD and were analyzed statistically by SPSS 17.0 software. The normally distributed data were first analyzed statistically via one-way analysis of variance (ANOVA). P < 0.05 was considered to be statistically significant.
Results
The purity of hippocampal neurons in SD rats
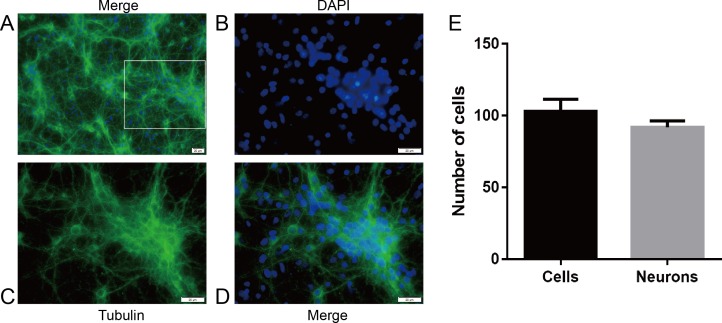
First, we examined the purity of neurons by immunofluorescence staining to ensure the accuracy and feasibility of the experiment. The neuronal purity was at least 92.5% (Fig. 2).
Figure 2. The purity of hippocampal neurons from SD rats.
Primary cultured hippocampal neurons were cultured for 8 days before DNLA treatment. (A) hippocampal neurons 1,000×. (B) DAPI 2,000×. (C)Tubulin 2,000×. (D) hippocampal neurons 2,000×. (E) Neuron purity was more than 92.5% (n = 3). White squares represent enlarged areas.
Effects of DNLA on the cell viability of hippocampal neurons in SD rats
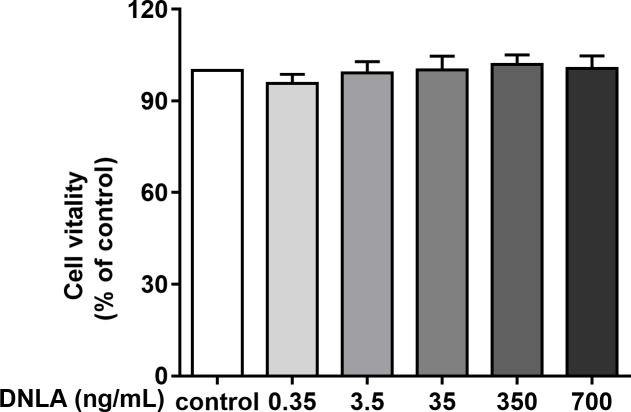
Second, to determine whether DNLA improved the cell viability of hippocampal neurons from SD rats, we treated primary hippocampal neurons with DNLA for 48 h and detected the viability of neurons in each group by MTT assays. The results showed that DNLA did not significantly change the cell vitality of hippocampal neurons (Fig. 3). We concluded that DNLA does not alter the overall state of the cells in premature aging hippocampal neurons. However, did the internal state of the neurons change? We further examined this question based on the “Aβ cascade hypothesis”.
Figure 3. Effects of DNLA on the cell viability of hippocampal neurons from SD rats.
DNLA did not significantly change the cell vitality of hippocampal neurons (n = 3).
DNLA decreased the accumulation of Aβ by decreasing APP
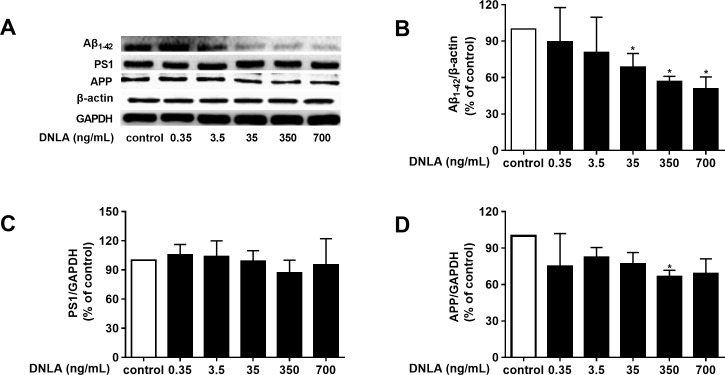
As mentioned above, dementia is attributed to synaptic dysfunction and neuronal loss in the hippocampus and its associated cortex, which are caused by the accumulation of Aβ oligomers. Thus, we detected the protein expression of Aβ1–42, and found that DNLA decreased the protein expression of Aβ1–42 (Fig. 4B). To determine whether the effects of DNLA on the protein expression of Aβ1–42 in hippocampal neurons of SD rats were related to APP, which is the source of Aβ and is cleaved to Aβ1–42 and the other Aβ fragment, we examined the protein expression of APP in hippocampal primary neurons by Western blot analysis. Furthermore, PS1 (γ-secretase) participates in both the “non-amyloidogenic” and the “amyloidogenic” pathways. Therefore, we also examined the protein expression of PS1 by Western blot analysis. As shown in Fig. 4, DNLA decreased the protein expression of APP in hippocampal primary neurons of rats but did not change the protein expression of PS1. We found that DNLA decreased the accumulation of Aβ by decreasing APP.
Figure 4. DNLA reduced the production of Aβ by decreasing APP.
The protein expression levels of Aβ1–42 (B), PS1 (C) and APP (D) as determined from densitometric scans of Western blots. DNLA significantly decreased the protein expression of Aβ1–42 (35, 350, 700 ng/mL) and APP (350 ng/mL). However, DNLA did not change the protein expression of PS1. (A) Representative strips of these proteins. Data are presented as the mean ± SD (n = 4). *P < 0.05 versus the sham group.
DNLA decreased the accumulation of Aβ by regulating α-secretase
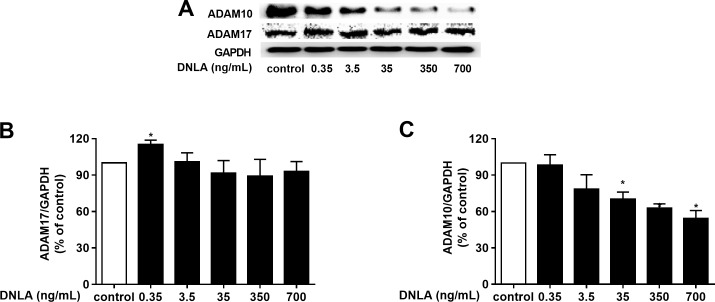
As a protective factor for the progression of AD, α-secretase plays a significant role in the “non-amyloidogenic” pathway. Moreover, ADAM10 and ADAM17 are considered to be the most important α-secretases involved in the physiological processing of APP in the brain. We further probed the effect of DNLA on α-secretases (ADAM10 and ADAM17). And we examined the protein expression of ADAM10 and ADAM17 by Western blot analyses. Our results indicated that DNLA increased the protein expression of ADAM17 but decreased the protein expression of ADAM10 (Fig. 5). Thus, DNLA decreased the accumulation of Aβ by regulating α-secretase.
Figure 5. DNLA reduced the accumulation of Aβ through non-amyloidogenic pathways.
The protein expression levels of ADAM17 (B) and ADAM10 (C) as determined from densitometric scans of Western blots analyses. DNLA significantly decreased the protein expression of ADAM10 (35, 350, 700 ng/mL). However, this treatment significantly increased the protein expression of ADAM17 (0.35 ng/mL). (A) Representative strips of these proteins. Data are presented as the mean ± SD (n = 4). *P < 0.05 versus the sham group.
DNLA decreased the accumulation of Aβ by inducing BACE1 (β-secretase)
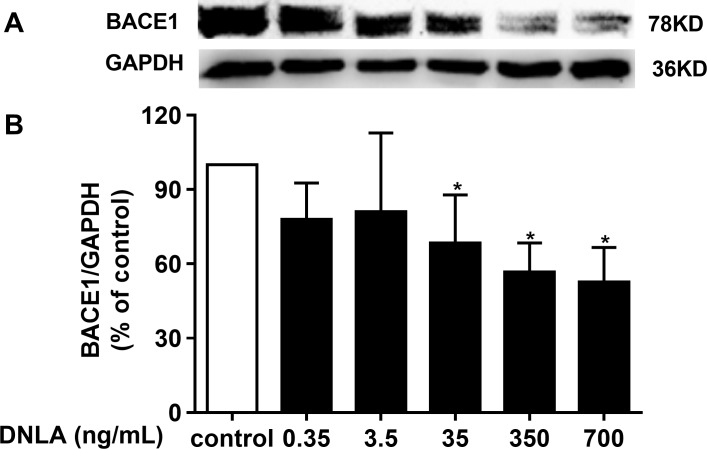
In addition to γ-secretase, β-secretase, the crucial factor in the “amyloidogenic” pathways, is related to APP metabolism, which is the source of Aβ. BACE1 was shown to have important physiological roles in Aβ production. Inhibiting the activity of BACE1 can reduce the accumulation of Aβ, which triggers the exacerbation of AD. We confirmed that DNLA could decrease the production of Aβ. Moreover, the reduction of Aβ involved APP and α-secretase. Thus, we examined whether this effect was related to BACE1. Accordingly, we detected the protein expression of BACE1 by Western blots analyses. As indicated in Fig. 6, DNLA could decrease the accumulation of Aβ by reducing the protein expression of BACE1 in hippocampal neurons from SD rats.
Figure 6. DNLA reduced the production of Aβ through amyloidogenic pathways.
Representative strip of the protein (A). The protein expression level of BACE1 (B) as determined from densitometric scans of Western blots. DNLA significantly decreased the protein expression of BACE1 (35, 350, 700 ng/mL). Data are presented as the mean ± SD (n = 4). *P < 0.05 versus the sham group.
Discussion
DNLA was extracted from D. nobile Lindl, which is a traditional Chinese medicinal material and is produced in Chishui, Guizhou. As a pharmacologically active ingredient of D. nobile Lindl, DNLA has a protective effect on the nervous system. Previous studies showed that APP, β-secretase and Aβ protein in the hippocampus of Tg2576 transgenic mice were reduced. Additionally, DNLA substantially improved the learning and memory of middle-aged APP/PS1 transgenic mice and wild-type mice, suggesting that DNLA has potential health benefits (Linshan et al., 2016; Nie et al., 2018). In this experiment, neurons in the normal growth state were treated with different concentrations of DNLA, and the effects of DNLA on cell viability, APP, Aβ and its related secretases (α-, β- and γ-secretases) in normal conditions were preliminarily explored.
The senile plaque formed by extracellular deposition of Aβ is one of the main pathological characteristics of AD. Moreover, with increasing age, Aβ deposition increases (Niedowicz et al., 2014). Aβ mainly includes Aβ1–40 and Aβ1–42. Aβ1–42 has stronger neurotoxicity and hydrophobicity than Aβ1–40, and it has a strong tendency to oligomerize than Aβ1–40 and is thus more easily polymerized in the brain. Aβ1–42 accumulates to a high level, forming “senile plaques”, which produce neurotoxic effects that injure the neurons and cause damage. Thus, inhibiting the production of Aβ1–42 can delay the pathogenesis of AD (Zhu et al., 2011). In this study, the protein expression of Aβ1–42 in hippocampal neurons of SD rats was detected. The results showed that DNLA can reduce the protein expression of Aβ1–42 in hippocampal neurons.
APP is the precursor protein of Aβ and is a member of a family of related proteins, including amyloid precursor-like proteins (APLP1 and APLP2) in mammals and amyloid precursor protein-like (APPL) in Drosophila, all with large extracellular structures. These proteins have a one-way transmembrane domain, but only APP produces amyloidosis fragments (O’Brien & Wong, 2011). In theory, reductions in APP, as the precursor protein of Aβ, could decrease the production of Aβ from the source. In this study, DNLA decreased the protein expression of APP.
Regardless, γ-secretase participates in the “non-amyloidogenic” and the “amyloidogenic” pathways and plays an indispensable role in the progression of APP metabolism to generate Aβ. Notably, DNLA reduced APP in a dose-dependent manner, but the effects were only significant at a dose of 350 ng/mL. One possible reason is that the data for these statistics are not sufficient to produce significant differences at other doses. Another possible reason is that the other dosages did not result in a significant change in this indicator. Simultaneously, some studies have suggested that the inhibition of γ-secretase activity could reduce the production of Aβ. Moreover, γ-secretase contains PS (including PS1 and PS2), nicastrin (NCT), PSEN enhancer 2 (Pen-2) and either anterior pharynx 1 (Aph-1) and is also the rate-limiting enzyme of APP production of Aβ (Li et al., 2011a). Many studies have shown that inhibition of γ-secretase activity helps reduce Aβ production (Wolfe, 2012). As the core catalytic subunit of γ-secretase, PS1 is the main active component (Garcia-Ayllon et al., 2013) of this enzyme and has an independent γ-secretase role (Woodruff et al., 2013). PS1 overexpression may be a risk factor for late-onset SAD (Li et al., 2011a). Conversely, inhibition of PS1 expression reduced Aβ production (Futai et al., 2016). In our study, the protein expression of PS1 showed no significant changes. These results indicated that DNLA did not affect PS1. Aβ1–42 produced by γ-secretase did not increase and was reduced. Therefore, we concluded that DNLA affects APP metabolism to Aβ in other ways. We further explored this phenomenon.
Next, to explore the effect of DNLA on the non-amyloidogenic pathway, we detected the α-secretases, mainly the ADAM10 and ADAM17 proteins. ADAM17 is an important α-secretase involved in the non-amyloidogenic pathway of APP. Enhancing the activity of ADAM17 increased the secretion of a soluble sAPP α fragment with neuroprotective activity and decreased Aβ production. Therefore, ADAM17 is considered a potential therapeutic target for AD (Qian, Shen & Wang, 2016). The results of this experiment showed that DNLA could increase the protein expression of ADAM17 and reduce the production of Aβ. Additionally, ADAM10 also degrades APP by the non-amyloidogenic pathway. On one hand, it cleaves APP to produce sAPP α and avoids the production of Aβ. On the other hand, studies have also found an age-dependent increase in ADAM10 levels (Schuck et al., 2016). However, experiments by Shackleton, Crawford & Bachmeier (2016) suggested that inhibition of ADAM10 promoted the clearance of Aβ1–40 and Aβ1–42 in the brains of mice with AD, thereby reducing the level of Aβ. This result may be because the other α-secretase process APP when ADAM10 is reduced or absent. Other studies have reported that other members of the α-secretase family, such as ADAM9 and ADAM17, can compensate for the reduction in ADAM10 activity (Asai et al., 2003; Hartmann et al., 2002). When ADAM10 was absent or reduced, sAPP α production was shown to be reduced or Aβ was significantly increased, which may be due to the lack of compensation for α-secretase (Kuhn et al., 2010; Postina et al., 2004; Suh et al., 2013). In addition, inhibition of BACE1 increased ADAM10 cleavage of APP, but decreasing ADAM10 activity increased the risk of AD by increasing β-secretase cleavage of APP (Colombo et al., 2013). In this experiment, ADAM10 protein expression in hippocampal neurons treated with DNLA was decreased. However, interestingly, ADAM17 protein expression was increased, and the Aβ protein level was decreased. These findings suggested that DNLA can reduce the expression of ADAM10 protein, which promotes the clearance of Aβ and reduces the Aβ level in hippocampal neurons of SD rats.
In the amyloidogenic pathway, APP finally produces Aβ by β- and γ-secretase cleavage. β-secretase, including BACE1, cathepsin S (Cat S), cathepsin L (Cat L) (Schechter & Ziv, 2011), cathepsin D (Cat D) and cathepsin B (Cat B) (Zhou et al., 2012), is the rate-limiting enzyme of APP production of Aβ. BACE2 was previously suggested to belong to the β-secretase family, but it was later proven to be a newly discovered η-secretase (Willem et al., 2015). Although Cat S, Cat L and Cat B may be further developed as targets for the treatment of AD (Schechter & Ziv, 2011), the current main target for β-secretase remains BACE1. Numerous studies have shown that inhibition of BACE1 can reduce the production of Aβ (Adwan, Subaiea & Zawia, 2014; Yan & Vassar, 2014; Yun et al., 2013; Zhu et al., 2012). The results of this experiment showed that BACE1 protein expression was decreased after treatment with DNLA, suggesting that DNLA can reduce the protein expression of BACE1 in neurons. This change likely caused the reduced protein expression of Aβ1–42, which is crucial for the development of AD.
Under the experimental conditions, there are some drawbacks in our study. We need further direct evidence showing that DNLA reduces Aβ by affecting α- and β-secretase. For further analysis of the effects of DNLA on α-, β- and γ-secretase, the products of APP, such as sAPP α, P3, sAPPβ and CTF- γ, which were lysed by the corresponding secretases, should be further tested to explain the effect of DNLA on related secretases and its functions. In addition, the α-, β-, and γ-secretase activity should be directly detected for further confirmation of our results. Moreover, Aβ was reduced by decreasing its source and promoting its clearance. In this experiment, Aβ1–42 protein expression in hippocampal neurons of SD rats was reduced, which was the result of various mechanisms. Therefore, the exact mechanism of Aβ1–42 reduction requires further elucidation.
In summary, DNLA decreases Aβ accumulation by regulating α- and β-secretases in hippocampal neurons from SD rats. We verified that DNLA has potential health benefits and provided a theoretical basis for the anti-aging effect of DNLA.
Conclusions
The results showed that DNLA can decrease Aβ1–42 in hippocampal neurons of rats by regulating α- and β-secretase.
Supplemental Information
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81473201), the Fund of Zunyi Medical University (No. F-900), the Fund of Zunyi Science and Technology Bureau & Zunyi Medical University (No. 2018-23), The Science and Technology Foundation of Guizhou Province of China, (No. JZ [2014]2016), and Shijingshan’s Tutor Studio of Pharmacology (No. GZS-2016-07). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Juan Huang and Nanqu Huang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Minghui Zhang performed the experiments, analyzed the data, prepared figures and/or tables.
Jing Nie contributed reagents/materials/analysis tools.
Yunyan Xu performed the experiments, contributed reagents/materials/analysis tools.
Qin Wu conceived and designed the experiments, contributed reagents/materials/analysis tools.
Jingshan Shi conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All animal procedures were approved by the Animal Experimental Ethical Committee of Zunyi Medical University [(2019)2-995].
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Adwan, Subaiea & Zawia (2014).Adwan L, Subaiea GM, Zawia NH. Tolfenamic acid downregulates BACE1 and protects against lead-induced upregulation of Alzheimer’s disease related biomarkers. Neuropharmacology. 2014;79:596–602. doi: 10.1016/j.neuropharm.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai et al. (2003).Asai M, Hattori C, Szabo B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochemical and Biophysical Research Communications. 2003;301:231–235. doi: 10.1016/S0006-291X(02)02999-6. [DOI] [PubMed] [Google Scholar]
- Ashok et al. (2015).Ashok A, Rai NK, Tripathi S, Bandyopadhyay S. Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicological Sciences. 2015;143:64–80. doi: 10.1093/toxsci/kfu208. [DOI] [PubMed] [Google Scholar]
- Banerjee et al. (2014).Banerjee P, Sahoo A, Anand S, Ganguly A, Righi G, Bovicelli P, Saso L, Chakrabarti S. Multiple mechanisms of iron-induced amyloid beta-peptide accumulation in SHSY5Y cells: protective action of negletein. Neuromolecular Medicine. 2014;16:787–798. doi: 10.1007/s12017-014-8328-4. [DOI] [PubMed] [Google Scholar]
- Baranello et al. (2015).Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, Greig NH, Pappolla MA, Sambamurti K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Current Alzheimer Research. 2015;12:32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansu et al. (2017).Cansu GB, Atilgan S, Balci MK, Sari R, Ozdem S, Altunbas HA. Which type 2 diabetes mellitus patients should be screened for subclinical Cushing’s syndrome? Hormones. 2017;16:22–32. doi: 10.14310/horm.2002.1716. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2015).Cheng XJ, Gao Y, Zhao YW, Cheng XD. Sodium chloride increases abeta levels by suppressing abeta clearance in cultured cells. PLOS ONE. 2015;10:e0130432. doi: 10.1371/journal.pone.0130432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry et al. (2012).Choudhry F, Howlett DR, Richardson JC, Francis PT, Williams RJ. Pro-oxidant diet enhances beta/gamma secretase-mediated APP processing in APP/PS1 transgenic mice. Neurobiology of Aging. 2012;33:960–968. doi: 10.1016/j.neurobiolaging.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Colombo et al. (2013).Colombo A, Wang H, Kuhn PH, Page R, Kremmer E, Dempsey PJ, Crawford HC, Lichtenthaler SF. Constitutive alpha- and beta-secretase cleavages of the amyloid precursor protein are partially coupled in neurons, but not in frequently used cell lines. Neurobiology of Disease. 2013;49:137–147. doi: 10.1016/j.nbd.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Paula et al. (2012).De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. Alzheimer’s disease. Sub-Cellular Biochemistry. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- Faraco et al. (2016).Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. Journal of Cerebral Blood Flow and Metabolism. 2016;36:241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai et al. (2016).Futai E, Osawa S, Cai T, Fujisawa T, Ishiura S, Tomita T. Suppressor mutations for presenilin 1 familial alzheimer disease mutants modulate gamma-secretase activities. Journal of Biological Chemistry. 2016;291:435–446. doi: 10.1074/jbc.M114.629287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ayllon et al. (2013).Garcia-Ayllon MS, Campanari ML, Brinkmalm G, Rabano A, Alom J, Saura CA, Andreasen N, Blennow K, Saez-Valero J. CSF Presenilin-1 complexes are increased in Alzheimer’s disease. Acta Neuropathologica Communications. 2013;1 doi: 10.1186/2051-5960-1-46. Article 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann et al. (2002).Hartmann D, De Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, Von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Human Molecular Genetics. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Hilpert et al. (2013).Hilpert H, Guba W, Woltering TJ, Wostl W, Pinard E, Mauser H, Mayweg AV, Rogers-Evans M, Humm R, Krummenacher D, Muser T, Schnider C, Jacobsen H, Ozmen L, Bergadano A, Banner DW, Hochstrasser R, Kuglstatter A, David-Pierson P, Fischer H, Polara A, Narquizian R. β-secretase (BACE1) inhibitors with high in vivo efficacy suitable for clinical evaluation in alzheimer’s disease. Journal of Medicinal Chemistry. 2013;56:3980–3995. doi: 10.1021/jm400225. [DOI] [PubMed] [Google Scholar]
- Kowalska (2004).Kowalska A. The beta-amyloid cascade hypothesis: a sequence of events leading to neurodegeneration in Alzheimer’s disease. Neurologia i Neurochirurgia Polska. 2004;38:405–411. [PubMed] [Google Scholar]
- Kuhn et al. (2010).Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO Journal. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2011a).Li T, Li YM, Ahn K, Price DL, Sisodia SS, Wong PC. Increased expression of PS1 is sufficient to elevate the level and activity of gamma-secretase in vivo. PLOS ONE. 2011a;6:e28179. doi: 10.1371/journal.pone.0028179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2011b).Li Y, Li F, Gong Q, Wu Q, Shi J. Inhibitory effects of Dendrobium alkaloids on memory impairment induced by lipopolysaccharide in rats. Planta Medica. 2011b;77:117–121. doi: 10.1055/s-0030-1250235. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li LS, Lu YL, Nie J, Xu YY, Zhang W, Yang WJ, Gong QH, Lu YF, Lu Y, Shi JS. Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Abeta25-35 in hippocampus neurons in vitro. CNS Neuroscience & Therapeutics. 2017;23:329–340. doi: 10.1111/cns.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler (2012).Lichtenthaler SF. Alpha-secretase cleavage of the amyloid precursor protein: proteolysis regulated by signaling pathways and protein trafficking. Current Alzheimer Research. 2012;9:165–177. doi: 10.2174/156720512799361655. [DOI] [PubMed] [Google Scholar]
- Linshan et al. (2016).Linshan J, Fei L, Jing N, Qin W, Jingshan S. Effect of Dendrobium nobile Lindl. alkaloids on the learning and memory function of APP/PS1 transgenic mice. Journal of Zunyi Medical University. 2016;3:246–249. doi: 10.14169/j.cnki.zunyixuebao.2016.0057. [DOI] [Google Scholar]
- Mouton-Liger et al. (2012).Mouton-Liger F, Paquet C, Dumurgier J, Bouras C, Pradier L, Gray F, Hugon J. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway. Biochimica et Biophysica Acta. 2012;1822:885–896. doi: 10.1016/j.bbadis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Nie et al. (2018).Nie J, Jiang LS, Zhang Y, Tian Y, Li LS, Lu YL, Yang WJ, Shi JS. Dendrobium nobile Lindl. alkaloids decreases the level of intracellular β-amyloid by improving impaired autolysosomal proteolysis in APP/PS1 mice. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.01479. Article 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie et al. (2016).Nie J, Tian Y, Zhang Y, Lu YL, Li LS, Shi JS. Dendrobium alkaloids prevent Abeta25-35-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ. 2016;4:e2739. doi: 10.7717/peerj.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedowicz et al. (2014).Niedowicz DM, Reeves VL, Platt TL, Kohler K, Beckett TL, Powell DK, Lee TL, Sexton TR, Song ES, Brewer LD, Latimer CS, Kraner SD, Larson KL, Ozcan S, Norris CM, Hersh LB, Porter NM, Wilcock DM, Murphy MP. Obesity and diabetes cause cognitive dysfunction in the absence of accelerated β-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathologica Communications. 2014;2 doi: 10.1186/2051-5960-2-64. Article 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien & Wong (2011).O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annual Review of Neuroscience. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina et al. (2004).Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, Leuven Fvan, Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. Journal of Clinical Investigation. 2004;113:1456–1464. doi: 10.1172/jci20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Shen & Wang (2016).Qian M, Shen X, Wang H. The distinct role of ADAM17 in app proteolysis and microglial activation related to alzheimer’s disease. Cellular and Molecular Neurobiology. 2016;36:471–482. doi: 10.1007/s10571-015-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter & Ziv (2011).Schechter I, Ziv E. Cathepsins S, B and L with aminopeptidases display beta-secretase activity associated with the pathogenesis of Alzheimer’s disease. Biological Chemistry. 2011;392:555–569. doi: 10.1515/bc.2011.054. [DOI] [PubMed] [Google Scholar]
- Schuck et al. (2016).Schuck F, Wolf D, Fellgiebel A, Endres K. Increase of alpha-secretase ADAM10 in platelets along cognitively healthy aging. Journal of Alzheimer’s Disease. 2016;50:817–826. doi: 10.3233/jad-150737. [DOI] [PubMed] [Google Scholar]
- Shackleton, Crawford & Bachmeier (2016).Shackleton B, Crawford F, Bachmeier C. Inhibition of ADAM10 promotes the clearance of Abeta across the BBB by reducing LRP1 ectodomain shedding. Fluids Barriers CNS. 2016;13 doi: 10.1186/s12987-016-0038-x. Article 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh et al. (2013).Suh J, Choi SH, Romano DM, Gannon MA, Lesinski AN, Kim DY, Tanzi RE. ADAM10 missense mutations potentiate beta-amyloid accumulation by impairing prodomain chaperone function. Neuron. 2013;80:385–401. doi: 10.1016/j.neuron.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2010).Wang Q, Gong Q, Wu Q, Shi J. Neuroprotective effects of Dendrobium alkaloids on rat cortical neurons injured by oxygen-glucose deprivation and reperfusion. Phytomedicine. 2010;17:108–115. doi: 10.1016/j.phymed.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Willem et al. (2015).Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LD, Moore S, Daria A, Hampel H, Muller V, Giudici C, Nuscher B, Wenninger-Weinzierl A, Kremmer E, Heneka MT, Thal DR, Giedraitis V, Lannfelt L, Muller U, Livesey FJ, Meissner F, Herms J, Konnerth A, Marie H, Haass C. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443–447. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe (2012).Wolfe MS. Gamma-Secretase inhibitors and modulators for Alzheimer’s disease. Journal of Neurochemistry. 2012;120(Suppl 1):89–98. doi: 10.1111/j.1471-4159.2011.07501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff et al. (2013).Woodruff G, Young JE, Martinez FJ, Buen F, Gore A, Kinaga J, Li Z, Yuan SH, Zhang K, Goldstein LS. The presenilin-1 DeltaE9 mutation results in reduced gamma-secretase activity, but not total loss of PS1 function, in isogenic human stem cells. Cell Reports. 2013;5:974–985. doi: 10.1016/j.celrep.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan & Vassar (2014).Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurology. 2014;13:319–329. doi: 10.1016/s1474-4422(13)70276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousof Ali, Jung & Choi (2015).Yousof Ali M, Jung HA, Choi JS. Anti-diabetic and anti-Alzheimer’s disease activities of Angelica decursiva. Archives of Pharmacal Research. 2015;38:2216–2227. doi: 10.1007/s12272-015-0629-0. [DOI] [PubMed] [Google Scholar]
- Yun et al. (2013).Yun SM, Cho SJ, Song JC, Song SY, Jo SA, Jo C, Yoon K, Tanzi RE, Choi EJ, Koh YH. SUMO1 modulates Abeta generation via BACE1 accumulation. Neurobiology of Aging. 2013;34:650–662. doi: 10.1016/j.neurobiolaging.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang M, Fei L, Wei Z, Qin W, Jingshan S. Effect of Dendrobium nobil Lindl. Alkaloids on the product of Aβ in hippocampus of rats induced by Aβ25–35. Journal of Zunyi Medical University. 2016;39:18–21. doi: 10.14169/j.cnki.zunyixuebao.2016.0005. [DOI] [Google Scholar]
- Zhang et al. (2017).Zhang W, Wu Q, Lu YL, Gong QH, Zhang F, Shi JS. Protective effects of Dendrobium nobile Lindl. alkaloids on amyloid beta (25-35)-induced neuronal injury. Neural Regeneration Research. 2017;12:1131–1136. doi: 10.4103/1673-5374.211193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2012).Zhou JW, Cheng XR, Cheng JP, Zhou WX, Zhang YX. The activity and mRNA expression of beta-secretase, cathepsin D, and cathepsin B in the brain of senescence-accelerated mouse. Journal of Alzheimer’s Disease. 2012;28:471–480. doi: 10.3233/jad-2011-111469. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2012).Zhu HC, Wang LM, Wang M, Song B, Tan S, Teng JF, Duan DX. MicroRNA-195 downregulates Alzheimer’s disease amyloid-beta production by targeting BACE1. Brain Research Bulletin. 2012;88:596–601. doi: 10.1016/j.brainresbull.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2011).Zhu F, Wu F, Ma Y, Liu G, Li Z, Sun Y, Pei Z. Decrease in the production of beta-amyloid by berberine inhibition of the expression of beta-secretase in HEK293 cells. BMC Neuroscience. 2011;12:125. doi: 10.1186/1471-2202-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.