Abstract
Aim:
To evaluate the Inadequate oxygen delivery (IDO2) index dose as a predictor of cardiac arrest (CA) in neonates following congenital heart surgery.
Methods:
Retrospective cohort study in 3 US pediatric cardiac intensive units (1/2011-8/2016). Calculated IDO2 index values were blinded to bedside clinicians and generated from data collected up to 30 days postoperatively, or until death or ECMO initiation. Control event data was collected from patients who did not experience CA or require ECMO. IDO2 dose was computed over a 120-minute window up to 30 minutes prior to the CA and control events. A multivariate logistic regression prediction model including the IDO2 dose and presence or absence of a single ventricle (SV) was used. Model performance metrics were the odds ratio for each regression coefficient and receiver operating characteristic area under the curve (ROC AUC).
Results:
Of 897 patients monitored during the study period, 601 met inclusion criteria: 29 patients had CA (33 events) and 572 patients were used for control events. Seventeen (59%) CA and 125 (26%) control events occurred in SV patients. Median age/weight at surgery and level of monitoring were similar in both groups. Median postoperative event time was 0.73 days [0.05-22.39] in CA patients and 0.82 days [0.08 25.11] in control patients. Odds ratio of the IDO2 dose coefficient was 1.008 (95% CI: 1.006 - 1.012, p=0.0445), and 2.952 (95% CI: 2.952 - 3.258, p=0.0079) in SV. The ROC AUC using both coefficients was 0.74 (95% CI: 0.73 - 0.75). These associations of IDO2 dose with CA risk remained robust, even when censored periods prior to arrest were 10 and 20 minutes.
Conclusion:
In neonates post-CPB surgery, higher IDO2 index dose over a 120-minute monitoring period is associated with increased risk of cardiac arrest, even when censoring data 10, 20 or 30 minutes prior to the CA event.
INTRODUCTION
Postoperative care of neonates undergoing repair of congenital heart disease (CHD) demands integration and constant interpretation by multiple interdisciplinary bedside healthcare providers of high volume physiologic, laboratory, and diagnostic data. In busy intensive care units, caregivers are challenged by high acuity and low provider-to-patient ratio, information and interpretation overload, subtle vital sign trends, and distractions. These factors may contribute to late recognition of inadequate oxygen delivery to critical tissues, progressive shock and escalating risk for cardiac arrest (CA).
CA occurs more frequently in hospitalized children with acquired and congenital cardiac disease compared to children without cardiac disease [1,2]. From the Pediatric Cardiac Critical Care Consortium (PC4) database [3], neonates (< 28 days of age) have double the prevalence of CA compared to infants (28 days to 1 year of age), and 70% of postoperative CA events occur between admission from the operating room and postoperative day 7. Although not currently captured in the PC4 database, there may be circumstances in which there is evolving inadequate oxygen delivery leading to clinical deterioration and CA that is not appreciated by conventional monitoring and caregivers at the bedside. The capture of time-series physiologic data at the bedside for real-time analysis and predictive modelling may be one way to integrate diverse physiologic signals and provide an early warning related to the trajectory of a patient in response to their clinical state or to specific treatments. One such algorithm that has recently been FDA 510(k) cleared and has the potential to be displayed at bedside by the T3 Visualization Platform (Etiometry, Inc., Boston, MA) is the probability of Inadequate oxygen delivery (IDO2) index.
The IDO2 algorithm utilizes ten physiologic values in the full dataset captured from the bedside monitor and laboratory values streaming into the T3 platform from the local laboratory system to compute the IDO2 index in real time. The index is calculated at 5 second intervals, and provides a probability between 0 and 100 of the measured mixed systemic venous saturation being lower than 40% [Appendix]. IDO2 has been previously validated by positive correlation with other indices of oxygen perfusion (serum lactate) and longer length of stay [Appendix,4]. We hypothesized the cumulative IDO2 (“IDO2 dose”) during a 120-minute monitoring interval would identify the potential risk for CA, and evaluated whether dose of IDO2 between 150 minutes and 30 minutes prior to cardiac arrest were associated with progression to full CA in full-term neonates who had undergone cardiac surgery with cardiopulmonary bypass (CPB).
METHODS
Design, setting and patients
This is a retrospective cohort study of post-CPB full-term neonates (0-28 days of age) with CHD admitted to three tertiary care pediatric cardiac intensive care units (CICUs) in the United States. The study protocol was approved by the institutional review board at all three institutions. Patients were enrolled prior to FDA 510(k) clearance of the IDO2 algorithm and thus IDO2 was retrospectively calculated using de-identified data. Clinicians therefore did not have real-time or bedside access to displayed IDO2 values during the study period.
Two of the institutions used the T3 platform (Etiometry Inc, Boston, MA) for data capture and one institution used the BedMaster platform (Excel Medical electronics Inc, Jupiter, Florida). Review and analysis of de-identified data including demographics, physiologic trends, diagnostic information, procedures, and outcomes were limited to the first 30 days following the operation, death or ECMO cannulation. Our Appendix contain the data elements included in the IDO2 calculation, and further details about the collection and preprocessing of data including: IDO2 calculation, approach to artifacts, level of monitoring, and missing data. Criteria for inclusion as a CA case were: 1. Event location in the CICU, 2. Event requiring chest compressions and/or defibrillation, 3. The CA is documented in the medical records and T3/BedMaster data are available for the calculation of IDO2. Exclusion criteria were: 1. Prematurity, 2. Birth weight < 2 kg, 3. Initiation of ECMO in the operating room or prior to the event, 4. Incomplete data, 5. Event occurred after the rollout of display of IDO2 values on bedside monitors in the unit.
A cardiac arrest (CA) was defined as an acute event requiring chest compressions and/or defibrillation as a result of loss of spontaneous circulation or acute bradycardia. CA event data were retrieved from the electronic medical record and reconciled with the T3 high definition bedside monitor-captured data. Event onset time stamp was obtained from the high definition data. Neonates from the same cohort of post-CPB patients who did not experience CA in the operating room or the first 30 postoperative days served as controls. A control event was defined as a random time window of 150 minutes (120 minutes analysis window and 30 minutes censored time interval) during a control patient’s postoperative time. From the possible control patients, we generated 1000 different cohorts of control events. Control event time windows were chosen to ensure monitoring was similar to the monitoring of the CA patients at the time of the event and that the distribution of control event times in the cohort was statistically similar to the distribution of CA event times in the exposed cohort. Figure 1 depicts the distribution of event times in a sample control cohort as compared to the exposed cohort. Distribution quartiles were matched between the exposed cohort and the control cohorts. Each control cohort included events from on average 489 of the 572 possible control patients to maintain a 15 to 1 ratio of control events to CA events. All 572 control patients were included in at least 1 of the 1000 cohorts generated. Each control patient selected for a cohort contributed one control event to the cohort.
Figure 1.
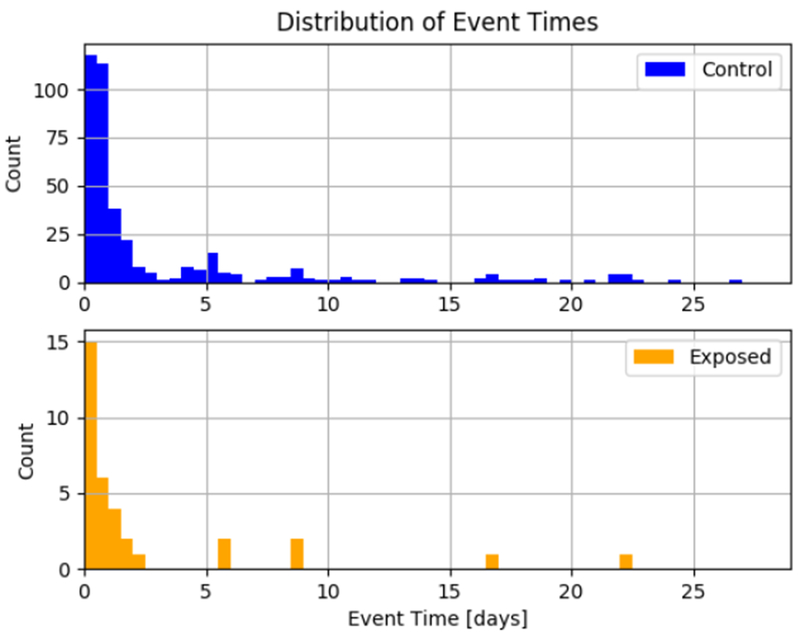
An example distribution of event times in the exposed group and control group for one of the control group cohorts generated
As the IDO2 reflects a physiologic state that is dynamic, rather than calculating a rate of change or defined level of IDO2, we prospectively calculated the exposure or “dose” of IDO2 which we defined as the area under the IDO2 curve computed over a 120-minute window that preceded the CA and “control” event by a censored pre-event window of 30 minutes (Figure 2). Secondary/sensitivity analyses assessed alternative definitions of “dose of IDO2” with 20 minute and 10-minute censored pre-event windows substituted for 30 minutes.
Figure 2.
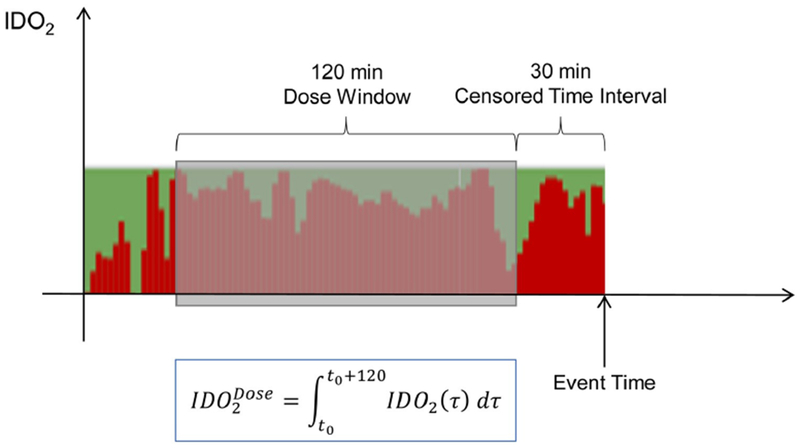
An example of the IDO2 index prior to a cardiac arrest. The value of the index at a given time slice is represented by the height of the red bars. The cardiac arrest occurs at Event Time. IDO2 Dose is computed by integrating the IDO2 index over the 120-minute window highlighted with the shaded box There is a 30-minute censored time interval prior to the cardiac arrest that is not included in the dose calculation
Statistical Analysis
The probability of IDO2 depends on the monitoring data utilized by the algorithm. Hence, in order to allow comparison between CA events and control events it was important that similar level of monitoring be available for each patient during the CA or control event time window. Due to the minimum data set requirement of IDO2, every event for which IDO2 was calculated had, at a minimum, heart rate, SpO2, and blood pressure measurements, either from an invasive or non-invasive source.
To analyze the relationship between 120 minutes IDO2 dose window and risk of CA, we performed a multivariate logistic regression analysis. In the analysis, the response variable was a binary variable indicating whether or not a CA event occurred following the prediction window (1=CA occurred or 0=CA did not occur), and the regression variables were IDO2 dose () as calculated in Figure 2 and a binary label (SV) indicating whether or not the patient that the event corresponded to was a single ventricle patient (1= Single Ventricle, 0 = Non-Single Ventricle). The single ventricle label was included to adjust for the increase in CA risk associated with this type of physiology [5]. The regression model was as follows:
| Equation 1. |
Where, pCA is the probability of CA occurring following the prediction window, and β0 is a constant offset, and βIDO2, βSV are the regression coefficients for each of the regression variables. The logit(pCA) is defined as the natural log of the odds of a CA event:
| Equation 2. |
To examine how these odds are affected by each model term, we compute the odds ratio (OR), which is calculated from the regression model in equation 1 by taking the exponential of both sides and computing the ratio of the odds for one unit change of the model variable, while holding the other variable constant. Specifically, taking the exponential results in,
| Equation 3. |
And then the OR for each component is calculated as,
| Equation 4. |
And,
| Equation 5. |
Results are reported as the OR for each of the regression coefficients and as the c-statistic for the receiver operator curve. The OR represents the change in odds of CA for each unit change in IDO2 dose. The c-statistic represents the ability of the logistic regression model to distinguish between patients who suffered a CA event and control patients. [6].
After fitting the regression model to the data, to assess how well the model discriminated between CA events and control events, we computed the Receiver Operating Characteristic (ROC) curve and associated area under the curve (AUC). Confidence intervals for the AUC value were computed from the AUC distribution for all cohorts
All analyses were performed using the Python software programming language. The regression analysis was performed using the Generalized Linear Model regression tools that are a part of the StatsModels statistical analysis package, and the ROC AUC analysis was performed using the scikit-learn Machine Learning package.
RESULTS
Review of medical records between 01/10/2011 – 8/28/2016 identified 897 patients who met inclusion criteria. Following exclusions, 601 eligible patients remained (Figure 3A). Table 1 provides a comparison between CA event and control patients.
Figure 3.
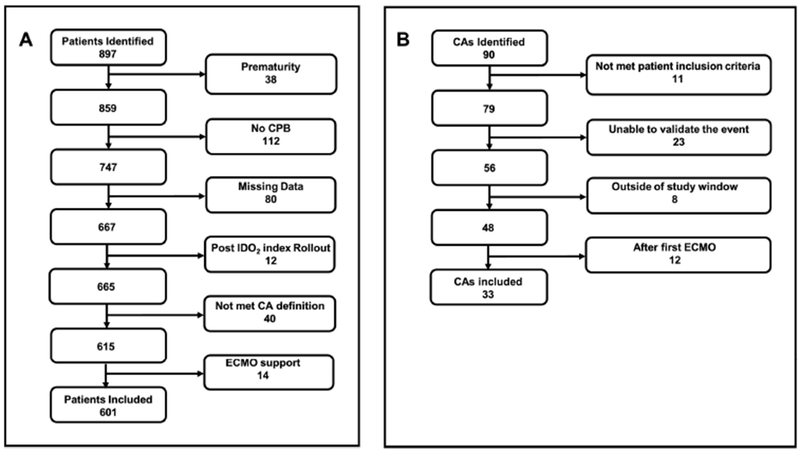
Flow charts outlining study patient selection (A) and cardiac arrest event selection (B). CA, cardiac arrest; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; IDO2,inadequate oxygen delivery
Table 1.
Characteristics of study patients. For control patients, average values across he cohorts are depicted
| Variable | Exposed | Control |
|---|---|---|
| No. (Percentage) Median [min – max] | Average no. (Percentage) Average median [min – max] | |
| Age at day of operation, days | 5.8[2.79 21.15] | 5.85[1.58 28.35] |
| Age at arrest, days | 7.83[2.84 26.18] | 8.18[2.05 28.93] |
| Weight at day of operation, kg | 3.0[2.2 4.2 | 3.2[2.0 4.66] |
| Event time relative to operation, days | 0.73 [0.05 22.39] | 0.82[0.08 25.11] |
| Diagnosis: | 17(59%) | 125 (26%) |
| Single ventricle | 12 (41%) | 364 (74%) |
| Two ventricle | 0 (0%) | 13 (3%) |
| STAT 1 | 1 (3%) | 33 (7%) |
| STAT 2 | 3 (10%) | 122 (25%) |
| STAT 3 | 9 (31%) | 213 (43%) |
| STAT 4 | 16 (55%) | 108 (22%) |
| STAT 5 | ||
Of the initial cohort, 90 CA events in 61 of all patients who met inclusion criteria were identified (6.8%). Following exclusions, 33 CA events in 29 of all eligible patients remained (4.8%) (Figure 3B) and were compared to on average 489 matched control events from 572 control patients. Seventeen (59%) of CA events and 125 (26%) of control events were in single ventricle patients. Median age and weight at the day of the operation was similar in both groups. Median postoperative event time was 0.73 days [0.05-22.39] in CA patients and average median event time of all 1000 control cohorts was 0.82 days [0.08 25.11], p = 0.4469.
Comparison of the level of monitoring in CA event versus control patients during the 150-minute time window in CA and control patients is provided in Table 2. Invasive continuous arterial and central venous pressure data were available in 18 CA events and 319 control events. For this subset of events, the calculated AUC for CA event prediction using IDO2 dose and single ventricle status was 0.81 (95% CI: 0.79 - 0.83). In 7 CA events and 83 control events invasive central venous pressure data were not available. For this subset of events, the calculated AUC for event prediction using IDO2 dose and single ventricle label was 0.84 (95% CI: 0.78 - 0.89). For CA patients and control patients who had only central venous pressure data (0 and 2 respectively) we could not calculate the AUC because of insufficient data. In 8 CA events and 85 control events both invasive arterial or invasive central venous pressure data were not available and for this subset of events, the calculated AUC for event prediction using IDO2 dose and single ventricle label was 0.54 (95% CI: 0.49 - 0.57). Lastly, in 15 CA events and 216 control events, hemoglobin data were not available, and for this subset of events, the calculated AUC of event prediction using IDO2 dose and single ventricle label was 0.63 (95% CI: 0.61 - 0.66).
Table 2.
Comparison of the monitoring level during cardiac arrest events and matched control events.
| Case | Total Events | Exposed Events | Control Events | AUC |
|---|---|---|---|---|
| Arterial line/central line | 337 | 18 | 319 | 0.81 (0.79-0.83) |
| Arterial line/no central Line | 90 | 7 | 83 | 0.84 (0.78-0.89) |
| No arterial line/central line | 2 | 0 | 2 | Insufficient Data |
| No arterial line/no central Line | 93 | 8 | 85 | 0.54 (0.49-0.57) |
| No hemoglobin | 231 | 15 | 216 | 0.63 (0.61-0.66) |
For all CA events from the logistic regression analysis, the OR of the coefficient associated with IDO2 dose over the 120-minute prediction window was 1.008 (95% CI: 1.006 - 1.012, p=0.045), indicating that the odds of CA increase by 0.8% for every unit increase of IDO2 dose. The OR of the coefficient associated with single ventricle physiology was 2.952 (95% CI: 2.675 - 3.258, p=0.008) indicating 2.952 greater odds of CA compared to a patient with two-ventricle physiology. The calculated AUC for event prediction using IDO2 dose and single ventricle label was 0.74 (95% CI: 0.73 - 0.75) (Figure 4). Our secondary sensitivity analyses, which assessed alternative definitions of “dose of IDO2 with 20 minute and 10-minute censored windows prior to CA event substituted for 30 minutes (Table 3) revealed no significant difference in odds of CA between these 3 windows.
Figure 4.
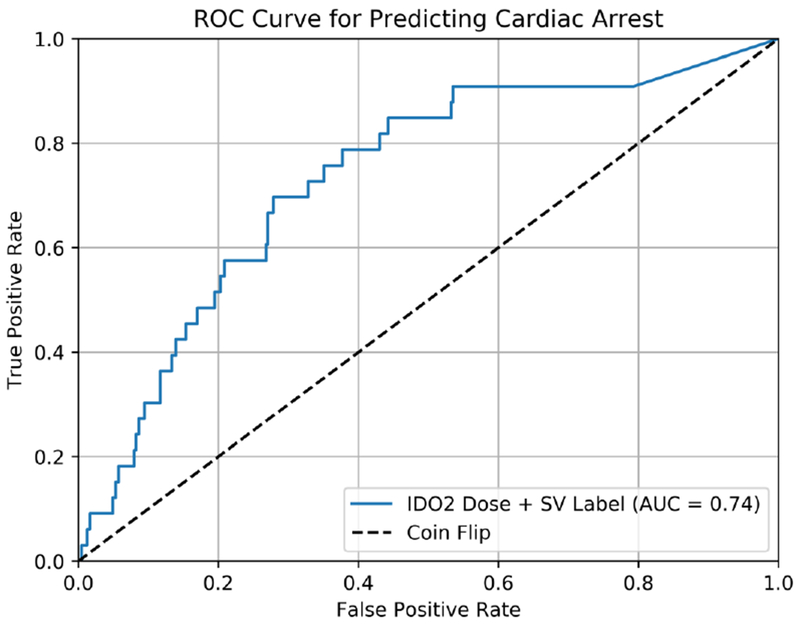
Receiver Operating Characteristic Curve (ROC) that assesses how well IDO2 Dose predicts cardiac arrest (blue line).The Area Under the Curve (AUC) of 0.74 is included in the legend. The ROC was generated using a logistic regression model that included IDO2 Dose, and a single ventricle label for risk adjustment. The ROC for an unbiased coin is also shown for comparison (black dashed line).
Table 3.
Secondary sensitivity analyses, which assessed alternative definitions of “dose of IDO2” with 20 minute and 10 minute censored windows prior to cardiac event substituted for 30 minutes. AUC, area under the receiver operating characteristic curve; O.R., odds ratio; SV, single ventricle. Average values reported and 95% confidence interval in parenthesis
| Censored Time Interval | AUC ROC | IDO2 O.R. | p-value | SV Label O.R. | p-value |
|---|---|---|---|---|---|
| 10 min | 0.74 (0.73-0.75) | 1.010 (1.007-1.013) | 0.0231 | 2.825 (2.538-3.125) | 0.0113 |
| 20 min | 0.74 (0.73-0.75) | 1.009 (1.006-1.012) | 0.0344 | 2.900 (2.618-3.203) | 0.0091 |
| 30 min | 0.74 (0.73-0.75) | 1.008 (1.006-1.012) | 0.0445 | 2.952 (2.675-3.258) | 0.0079 |
DISCUSSION
In this retrospective study utilizing streaming analytics platform that calculated IDO2 in neonates following cardiac operations, we demonstrated that a higher IDO2 dose over a 120-minute monitoring period is associated with a higher risk for cardiac arrest, even when the pre-event censored prediction window is 10, 20, or 30 minutes prior to the event. We also showed that higher level of monitoring and availability of hemoglobin level increased the performance of the algorithm (Table 2).
The IDO2 dose was calculated retrospectively for the purposes of our study, and was not displayed for the bedside healthcare providers. We refer to the IDO2 dose as the area under the IDO2 curve, computed as the integration of IDO2 values over a 120-minute prediction window that ends 30 minutes prior to the event (CA) or the start of retrograde data collection for control events (Figure 2). By using the area under the curve method, we account for both the magnitude and duration of IDO2 exposure, allowing for better quantification of exposure compared to maximum or mean IDO2 values. For example, a transient increase in IDO2 reflecting a transient increase in the probability that a patient’s oxygen delivery is inadequate may not be clinically significant. In contrast, persistent elevation of IDO2 representing higher probability of low oxygen delivery for a prolonged period of time may reflect a clinically significant change in the patient’s clinical state that may lead to end organ injury and culminate in CA. Bedside clinicians alerted by this quantitative risk assessment of exposure to low oxygen delivery over time (i.e. early warning alert) could be prompted to investigate possible etiologies underlying that change and adjust care accordingly. Furthermore, our analysis is based on a 120-minute dose window terminating 30 minutes before event time. Based on our primary analysis and secondary/sensitivity analysis we propose that our method may provide some lead time for the clinicians before a CA event occurs. Hence, providing them the opportunity to evaluate and possibly intervene.
As this study was conducted prior to implementation of IDO2 real-time display at bedside, we could not measure the effect of display of this index on patient management, prevention of CA, and patient outcomes. However, because IDO2 was not available to clinicians during the study period, we could test our hypothesis without bias introduced by clinician awareness of the IDO2 information. Indeed, our retrospective analysis suggests that the IDO2 signal could potentially provide warning for adverse changes in patient trajectory.
The importance of early recognition of low oxygen delivery states that may lead to CA is emphasized by the impact of CA on outcomes in this patient population. Children with cardiac disease suffer CA at rates of 2.6-6% with corresponding survival ranging from 32%-50.6% [1,2,7–10]. Development of progressive shock following open-heart surgical repair or palliation of critical CHD may lead to CA if not recognized in time. A recent analysis of the epidemiology and outcomes of CA in pediatric CICUs by Alten and colleagues [3] revealed that CAs were more prevalent in surgical CICU encounters. Their data revealed that the frequency of CA was higher in infants and especially in neonates. Cases involving greater surgical complexity and/or single ventricle palliation of any type had a disproportionally greater prevalence of CA. These data reinforce the value of our focus on this patient population.
Timely recognition of low oxygen delivery trajectories may allow prevention of CA events. Unfortunately, standard ICU monitoring systems are designed to alarm when age dependent set vital sign thresholds are crossed, but the sensitivity and specificity of such alarms to predict or prevent CA has not been established, and over 90% of such alarms may not be not relevant to patient safety [11].
Clinical support algorithms for identification of adverse clinical trajectories that increase the risk of CA are not available in the pediatric CICU in spite of major technological advances in high resolution data collection, storage, and graphic representation [12,13]. Kennedy et al [14] described CA prediction models using time series analysis as input. Their models were superior to traditional models built with multivariate data and a regression algorithm [15–20]. Machine learning models are likely to generate even more robust predictions, but the clinical application of time series analysis and machine learning models will require the ability to feed processed data back to the bedside in a timely fashion, and such functionality is to our knowledge not currently available. In contrast, the IDO2 dose approach can deliver risk prediction information to the bedside in near-real time, with a pre-clinical deterioration window of sufficient duration to allow potential intervention.
The use of multi-institutional datasets also constitutes a strength of our approach [21], since testing the external validity of predictive models requires data aggregation from multiple institutions. The T3 platform demonstrated the feasibility of combining high resolution physiologic datasets from separate institutions, an important consideration given the relative low frequency of CA events.
The current study shows an association of higher IDO2 dose from 150 to 30 minutes prior to a CA, and this may be useful in our patient population. We propose that this signal could warn the CICU providers of ongoing low oxygen delivery states that increase the risk of CA events. Future studies will be needed to further validate this approach and test the ability of the IDO2 to effectively influence care and prevent CA events.
There are several limitations to our study. First, the retrospective nature of our data limits our ability to track real time management decisions, changes in support, and other confounding factors. While we can hypothesize that these CA events were preventable, a future interventional clinical trial would be needed to test such hypothesis. A second limitation is the small number of CAs in our patient cohort. This is related to the known incidence of CA in neonates undergoing CPB operations. This limitation is offset by the homogeneous nature of the study group but also justifies the need for larger prospective validation studies.
In conclusion, we showed that in neonates post-CPB surgery, higher IDO2 index dose over a 120-minute monitoring period is associated with increased risk of cardiac arrest. We propose that a model-based index derived from physiologic data streams and represented by a calculated IDO2 index provides potentially actionable early warning information to clinicians at the bedside. Our findings support the need for prospective work to test the effect of display of this early warning signal on timely patient assessment, decision making, patient management, prevention of cardiac arrests, and important patient outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R43HL117340. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Avihu Gazit and Jose Pineda thank the Saint Louis Children’s Hospital Foundation for funding the initial installation of BedMaster and the T3 platform at their institution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Peter Laussen and Melvin Almodovar are the lead developers of the T3 platform which is owned by Boston Children’s Hospital, Boston, Massachusetts, and licensed to Etiometry Inc, Boston, Massachusetts; both Peter Laussen and Melvin Almodovar have received royalties from Boston Children’s Hospital following deployment of the T3 platform. Peter Laussen serves as the Medical Officer and advisor to Etiometry Inc, for which he has received options in the company, and is the co-developer of the Inadequate Oxygen Index displayed on the T3 platform.
Michael McManus is an employee of Etiometry, Inc, the company that created the IDO2 Index. He was heavily involved in the index’s development and also owns shares in the company.
Dimitar Baronov is one of the founders and the Chief Technology Officer of Etiometry Inc. the company that created the IDO2 Index. He was heavily involved in the index’s development and also owns shares in the company.
Craig Futterman, Joshua Salvin, Dimitar Baronov, Melvin Almodovar, Vinay Nadkarni, Peter Laussen, and Avihu Gazit are Co-Investigators (sub-contract) on the NIH SBIR Grant: Risk Assessment Using Noninvasive Measurements in Postoperative Pediatric Patients. NIH - National Heart, Lung, and Blood Institute. Small Business Innovation Research Program. 2R44HL117340-03A1/04/05. 2,000,000 USD.
Contributor Information
Craig Futterman, Division of Cardiac Critical Care Medicine, George Washington University, Children’s National Medical Center, 111 Michigan Avenue, NW Washington DC, 200010 United States.
Joshua W. Salvin, Department of Cardiology, Division of Cardiovascular Critical Cares Medicine, Harvard Medical School, Boston Children’s Hospital, 300 Longwood Avenue, Boston MA 02115 United States
Michael McManus, Etiometry, Inc 280 Summer St fl 4, Boston, MA 02210, United States.
Adam W. Lowry, Department of Cardiovascular Services, Division of Cardiac Critical Care, University of Central Florida, Nemours Cardiac Center, Nemours Children’s Hospital, 13535 Nemours Parkway, Orlando, FL 32827, United States
Dimitar Baronov, Etiometry, Inc 280 Summer St fl 4, Boston, MA 02210, United States.
Melvin C. Almodovar, Department of Pediatrics, Divisions of Cardiology and Critical Care Medicine, University of Miami Miller School of Medicine, Holtz Children’s Hospital/Jackson Health System, 1611 NW 12th Avenue, North Wing, Suite 109 Miami, FL 33136 United States
Jose A. Pineda, Department of Pediatrics and Neurology, Washington University School of Medicine, Saint Louis Children’s Hospital, 1 Children’s Place, St. Louis, MO 63110, United States
Vinay M. Nadkarni, Department of Anesthesiology, Critical Care, and Pediatrics, The Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, 3401 Civic Center Boulevard, Philadelphia, PA, 19104 United States
Peter C. Laussen, Department of Critical Care Medicine, The Hospital for Sick Children, 555 University Ave, Toronto, Ontario, M5G 1X8, Canada
Avihu Z. Gazit, Department of Pediatrics, Divisions of Critical Care Medicine and Cardiology, Washington University School of Medicine, Saint Louis Children’s Hospital, 1 Children’s Place St. Louis MO 63110, United States
REFERENCES
- 1.Lowry AW, Knudson JD, Cabrera AG, et al. : Cardiopulmonary resuscitation in hospitalized children with cardiovascular disease: Estimated prevalence and outcomes from the kids’ inpatient database. Pediatr Crit Care Med 2013; 14:248–255. [DOI] [PubMed] [Google Scholar]
- 2.Berg RA, Nadkarni VM, Clark AE, et al. : Incidence and outcomes of cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med 2016; 44:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alten JA, Klugman D, Raymond TT, et al. : Epidemiology and Outcomes of Cardiac Arrest in Pediatric Cardiac ICUs. Pediatr Crit Care Med 2017; 18:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baronov B, McManus M, Butler E, Douglas C, and Almodovar MC: Next Generation Patient Monitor Powered by In-Silico Physiology. Conf Proc IEEE Eng Med Biol Soc. 2015; 2015: 4447–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham EM, Forbus GA, Bradley SM, Shirali GS, Atz AM, Charleston SC. Incidence and outcome of cardiopulmonary resuscitation in patients with shunted single ventricle: Advantage of right ventricle to pulmonary artery shunt. J Thorac Cardiovasc Surg 2006; 131: e7–8 [DOI] [PubMed] [Google Scholar]
- 6.Bewick V, Cheek K, Ball J: Statistics Review 14: Logistic Regression. Critical Care 2005; 9(1) 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parra DA, Totapally BR, Zahn E, et al. : Outcome of cardiopulmonary resuscitation in a pediatric cardiac intensive care unit. Crit Care Med 2000; 28:3296–3300 [DOI] [PubMed] [Google Scholar]
- 8.Rhodes JF, Blaufox AD, Seiden HS, et al. : Cardiac arrest in infants after congenital heart surgery. Circulation 1999; 100 (19 Suppl): II194–II199 [DOI] [PubMed] [Google Scholar]
- 9.Ortmann L, Prodhan P, Gossett J, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators: Outcomes after in-hospital cardiac arrest in children with cardiac disease: A report from Get With the Guidelines–Resuscitation. Circulation 2011; 124:2329–2337 [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Jacobs JP, Pasquali SK, et al. : Epidemiology and outcomes after in-hospital cardiac arrest after pediatric cardiac surgery. Ann Thorac Surg 2014; 98:2138–2143; discussion 2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonafide CP, Lin R’Zander M, Graham CS, Paine CW, Rock W, Rich A, Roberts KE, Fortino M, Nadkarni VM, Localio AR, Keren R. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med 2015. June;10(6):345–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShea M, Holl R, Badawi O, et al. : The eICU research institute-a collaboration between industry, health care providers, and academia. IEEE Eng Med Biol Mag 2010; 29:18–25. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Scott DJ., Villaroel M, et al. : Open access MIMIC-II database for intensive care research. Conf Proc IEEE Eng Med Biol Mag 2011; 2011:8315–8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy CE, Aoki N, Mariscalco M, Turley JP. Using Time Series Analysis to Predict Cardiac Arrest in a PICU. Pediatr Crit Care Med 2015; 16:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egdell P, Finlay L, Pedley DK: The PAWS score: Validation of an early warning scoring system for the initial assessment of children in the emergency department. Emerg Med J 2008; 25:745–749 [DOI] [PubMed] [Google Scholar]
- 16.Hodgetts TJ, Kenward G, Vlachonikolis IG, et al. : The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation 2002; 54:125–131 [DOI] [PubMed] [Google Scholar]
- 17.Subbe CP, Kruger M, Rutherford P, et al. : Validation of a modified Early Warning Score in medical admissions. QJM 2001; 94:521–526 [DOI] [PubMed] [Google Scholar]
- 18.Pollack MM, Patel KM, Ruttimann UE: PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 19.Pollack MM, Ruttimann UE, Getson PR: Pediatric risk of mortality (PRISM) score. Crit Care Med 1988; 16:1110–1116 [DOI] [PubMed] [Google Scholar]
- 20.Leteurtre S, Martinot A, Duhamel A, et al. : Validation of the paediatric logistic organ dysfunction (PELOD) score: Prospective, observational multicentre study. Lancet 2003; 362:192–197 [DOI] [PubMed] [Google Scholar]
- 21.Eitan D, Goodwin A, Laussen P, Guerguerian AM: Insights from multi-dimensional physiological signals to predict and prevent cardiac arrests. Pediatr Crit Care Med 2016; 17:81–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


