Abstract
Bone has well documented natural healing capacity that normally is sufficient to repair fractures and other common injuries. However, the properties of bone change throughout life, and aging is accompanied by increased incidence of bone diseases and compromised fracture healing capacity, which necessitate effective therapies capable of enhancing bone regeneration. The therapeutic potential of adult mesenchymal stem cells (MSCs) for bone repair has been long proposed and examined. Actions of MSCs may include direct differentiation to become bone cells, attraction and recruitment of other cells, or creation of a regenerative environment via production of trophic growth factors. With systemic aging, MSCs also undergo functional decline, which has been well investigated in a number of recent studies. In this review, we first describe the changes in MSCs during aging and discuss how these alterations can affect bone regeneration. We next review current research findings on bone tissue engineering, which is considered a promising and viable therapeutic solution for structural and functional restoration of bone. In particular, the importance of MSCs and bioscaffolds is highlighted. Finally, potential approaches for the prevention of MSC aging and the rejuvenation of aged MSC are discussed.
Keywords: MSC, aging, stem cell niche, bone healing, rejuvenation
1. Bone healing and aging
Annually, there are more than two million fragility-associated fractures with healthcare costs exceeding $20 billion in the United States [1]. There is a significant increase in the incidence of fractures as well as the associated morbidity with increasing age [2]. As an example, one-year mortality rates after hip fractures in the elderly have been reported as 33% [3]. FDA approved medical therapies for aging-related bone loss include estrogen and related agonists (e.g. raloxifene), bisphosphonates (e.g., alendronate, risendronate, ibandronate, zoledronic acid), recombinant parathyroid hormone (e.g. teriparatide, abaloparatide), and antibodies against receptor activator of NF-κβ (RANKL) (e.g. denosumab), as well as supplements such as vitamin D and calcium. Monoclonal antibodies targeting sclerostin (SOST) have recently shown promise in clinical trials [4]. Each of these therapies has its own limitations and side effects, including adverse cardiovascular events, risk of pathological fractures, increased incidence of bone tumors, and immune dysfunction, the majority of which are associated with the systemic administration of these agents [5].
To avoid these side effects, a variety of techniques have recently been developed that involve local delivery of combinations of cells, growth factors, and scaffolds to the site of fractures (Table 1). The Hernigou procedure involves application of bone marrow aspirate to the site of non-unions [6]. Recombinant bone morphogenetic protein-2 (BMP-2) is utilized in spine surgery to facilitate fusions [7], and recombinant human platelet-derived growth factor (rhPDGF) is used to help fill bony defects [8]. Finally, implantation of demineralized bone matrix, a frequent and long-practiced approach based on the well-known bone-inductive activity of bone tissue grafts, is used to facilitate fracture healing and spinal fusions, [9]. Thus, iliac crest bone autografts and fibula allograft are commonly utilized in clinical practice to facilitate spinal fusions, ankle arthrodesis, and the healing of long bone and pelvic fractures. Despite the availability of these approaches, the rate of delayed union and non-union for these procedures remains a significant 10–25% in elderly populations. [10].
Table 1.
FDA approved therapies for bone healing
| Systemic | Local |
|---|---|
| Bisphosphonates | INFUSE (rhBMP-2) |
| Recombinant parathyroid hormone | Regranex (rhPDGF-BB) |
| RANKL inhibitors | rhBMP-7* |
| SOST inhibitors (pending) | Healos (GDF-5) (pending) |
| Demineralized bone matrix | |
| Fibula allograft | |
| Iliac crest autograft |
FDA humanitarian device exemption in 2003, failed to pass FDA approval in 2009 (https://www.fda.gov/)
While fracture healing is significantly delayed in older versus younger patients, the underlying mechanisms are not fully understood. Some aging-associated changes have been proposed, such as altered interaction between macrophages and MSCs [11], reduced level of estrogen in postmenopausal females [12], and transition of bone marrow, which changes from red marrow to fatty yellow marrow during aging [13]. For example, a recent report demonstrated that the accumulation of adipocytes in bone marrow significantly impairs the stem cell participation in bone healing [14]. In this article, we will review the current understanding of MSC aging and how aging-associated changes in MSCs may lead to compromised bone healing in the elderly. In particular, we will address MSC niche and discuss how niche aging affects MSC population density and functionality. An in-depth review of how tissue engineering strategies are being used to promote bone healing is also provided.
2. Aging of bone marrow-derived MSCs
In 1970, Friedenstein et al. first identified and described colony-forming fibroblasts (CFU-Fs) able to adhere to plastic culture substrate from bone marrow [15]. The term, mesenchymal stem cells (MSCs), was suggested for this cell population by Caplan in 1991 [16]. The first detailed description of the tri-lineage differentiation capacity of MSCs, including osteogenesis, chondrogenesis and adipogenesis, was reported in 1999 [17], which soon became a key criterion to identifying MSCs. It should be noted that MSCs are rare in bone marrow, constituting less than 0.01% of the overall mononucleated cells [17]. Under normal conditions, they maintain a quiescent state. Upon stimulation by biological signals, e.g., as a result of tissue injuries, MSCs are activated and undergo symmetric or asymmetric division, and they are believed to be recruited to injured sites to replace or regenerate damaged tissues. Due to their robust reparative potential, MSCs have been applied in an expanding array of clinical indications, specifically for bone defects and cartilage loss [18]. The original rationale for the application of MSCs in tissue regeneration is the replenishment of damaged cells and their differentiation into tissue-specific cells for de novo tissue formation. Results from many animal studies have supported the clinical feasibility of MSC-based treatments, demonstrating the presence of MSCs in the newly-formed bone tissues. Whether the transplanted cells can directly engraft and become bone cells in the human body requires further investigation. In addition, MSC-produced agents, or the MSC secrectome, have recently been proposed as a more important biological mechanism than their differentiation capacity that contributes to the function of MSCs [19].
Currently, MSCs have been isolated from many different tissues, including from bone marrow, adipose, cartilage, muscle, bone, and umbilical cord and placenta. While cells derived from these different tissue sources all possess a tri-lineage (bone, cartilage, and adipose) differentiation capacity and display similar surface marker expression profiles [20, 21], differences in the biology of MSCs from different ontogenetic sources have also been extensively reported. For example, MSCs from human adipose tissue, and skin from both adults and newborns displayed significant differences in adipogenic and osteogenic potential [20]. Such origin-dependent characteristics of MSCs strongly implicate the critical role of the tissue microenvironment surrounding the MSCs, commonly referred to as the stem cell niche.
2.1. Contribution of MSCs to bone repair in vivo
The contribution of MSCs to bone repair has been long proposed. In particular, the crosstalk between MSCs and macrophages during the bone healing process has recently drawn investigative attention [22]. Studying the underlying mechanism has been facilitated by recent technical advancements in cell labeling and tracking, which allow the tracing of the lineage of a cell population in vivo to study their location and functions. For example, Mendez-Ferrer et al. exploited nestin as a marker of MSCs and tagged MSCs with a green fluorescent protein (GFP) transgene under regulation by the nestin promoter. Using this lineage-tracing method, they demonstrated that these GFP tagged MSCs directly contribute to bone remodeling by differentiating into osteoblasts [23]. Using a similar strategy, Park et al. showed Mx1-expressing bone marrow cells possessed all known MSC characteristics, and they could migrate to the injury site, supplying new osteoblasts during fracture healing [24]. Besides nestin and Mx1, leptin receptor (LepR) is another marker to identify MSCs. LepR positive cells were shown to be capable of giving rise to osteoblasts and adipocytes in bone marrow [25]. In a bone fracture model, 85% of the osteoblasts were shown to be derived from LepR+ MSCs after 8 weeks. It should be noted that MSCs in bone marrow is by nature heterogeneous. Recently, gremlin-1-expressing cells were isolated from bone marrow, which are capable of osteogenesis, but not adipogenesis [26, 27]. However, these cells still contribute to 28% of the osteoblasts found in the facture callus. Collectively, these studies indicate the critical roles of MSCs during the bone healing process.
2.2. MSC niches
The concept of stem cell niche was first described by Schofield et al. to refer to the specialized microenvironment around hematopoietic stem cells (HSCs) [28] and to propose a mechanism to maintain stem cells under a quiescent state [29]. Currently, it is generally accepted that the stem cell niche includes the supporting cells, extracellular matrix (ECM), vascular network, as well as biochemical and physical cues [30]. Under normal conditions, the niche maintains stem cells in a quiescent state to prevent them from being depleted. Upon stimulation by injury or other signals, changes in the niche will activate stem cells and promote stem cell proliferation, differentiation, and migration. These activated cells may either divide into daughter stem cells, which can maintain a stable stem cell pool, or divide into cells committed to differentiation, thereby decreasing the number of stem cells [31]. As mentioned above, MSC phenotypes and functions are distinct in accordance to their tissue origin, which suggests the presence of tissue-specific MSC niches. However, their specific nature and characteristics are incompletely understood.
In 2003, Gronthos et al. isolated a human MSC population from bone marrow using the antibodies targeting vascular cell adhesion molecule-1 (VCAM-1/CD106) and STRO-1 [32]. In that same year, Shi et al. performed the first study to identify the in vivo niche of MSCs, and the group suggested that MSCs may reside in the microvasculature of tissues [33]. In this study, expression of STRO-1 was confined to the vascular wall in both human bone marrow and dental pulp. Covas et al. performed a subsequent comparative study to investigate the difference between perivascular cells (CD146+) and MSCs as well as between non-perivascular stromal cells and MSCs. They concluded that the gene expression profiles and differentiation capacities were similar between MSCs and perivascular stromal cells [34]. Again, in the same year, a landmark study by Crisan et al. demonstrated the perivascular origin of MSCs [35]. Prospectively, these investigators identified perivascular cells, principally pericytes, in multiple human organs. Long-term in vitro cultures of these cells expressed MSC markers and possessed tri-lineage, i.e., osteogenic, chondrogenic, and adipogenic differentiation potentials [35]. Additional subsequent studies by other groups confirmed the isolation of MSC-like cells from the vascular wall [36–38].
Based on this evidence, we elaborated on the perivascular location theory of the MSCs in 2010 [39]. In fact, residing in a perivascular niche improves the migration capability of MSC in response to injury or the pathogenic signals. It should be noted that the perivascular niche theory is challenged by the fact that MSCs have also been isolated from avascular tissues. For example, MSC have been isolated from cartilage [40], which also displayed clonogenicity and multi-lineage differentiation ability [41]. In addition, the precise location of MSCs within the vascular wall remains unknown, although there is increasing evidence suggesting that MSCs may stay in the adventitia. The potential adventitia nature of MSCs was first described by Hoshino et al. in 2008 [42]. They isolated vascular adventitial fibroblasts (hVAFs) from human pulmonary arteries and showed that these hVAFs were positive for MSC markers, but negative for hematopoietic and endothelial cell markers. hVAFs also showed osteogenic and adipogenic differentiation upon appropriate stimulation. Furthermore, Corselli et al. used surface markers to further isolate cells from middle and outmost layers of blood vessels [43]. In their work, CD34+, CD31−, CD146−, and CD45− cells were sorted, which represented the cells in the outmost layer of blood vessels from adipose tissue. These cells expressed a surface marker prolife and differentiation potential identical to standard bone marrow-derived MSCs. In contrast, pericytes, defined based on the expression of CD34, CD31, and CD146, retained phenotypes and genotypes distinct from those of cells from adventitia. In addition, adventitia-derived cells treated with angiopoietin-2 upregulated the expression of pericyte markers, suggesting that MSCs may function as the precursors of pericytes. Based on the adventitial nature of MSCs and middle perivascular nature of pericytes, de Souza et al. proposed a model to describe the association between these two cell types [44]. In this model, MSCs serve as the progenitor cells of perivascular populations, including pericytes.
We have previously reviewed the potential interaction between MSCs and their niches, including supporting cells, ECM, soluble factors, etc. [39]. Next, we will briefly describe MSC aging, with special attention paid to how aging affects each niche component and the consequent influence on MSC characteristics.
2.3. Identification and characterization of MSC Aging
Aging is characterized by a global, gradual, and functional decline of the entire body. To date, there is no established method to indicate or monitor in vivo aging of cells. Unlike in vitro replicative senescence, which has been well characterized on the basis of chromosome telomere length, cell proliferative behavior, and differentiation potential [45], the debate of natural aging compromising MSC functionality in vivo is unsettled. To date, senescence-associated β-galactosidase (SA-β-gal) activity is the most widely used biomarker of aging cells, especially in combination with additional markers [46], although its role on the aging process is not known. In addition, p16 INK4a is a common marker of cell cycle arrest, which correlates with senescence both in vitro and in vivo [47, 48]. Based on these two markers, a protocol of quantitative analysis of cellular senescence has been described by Zhao et al. [49]. Recently, extracellular microvesicles (MVs) have been proposed as the potential biomarkers to identify MSC aging. Lei et al. reported that miR-146a-5p localized in MSC-MVs characterized the senescent state of late passage MSCs [50]. Expression of miR-335 was also reported to correlate with donor age of human MSCs (hMSCs), and overexpression of miR-335 resulted in a rapid senescent phenotype and abolished differentiation potential [51]. Furthermore, since the nuclear lamina undergoes aging-associated changes, prelamin A has emerged as a new marker to identify senescent MSCs [52]. McHugh et. al proposed that aging hallmarks should be divided into three categories: (1) primary, or the causes of age-associated damage; (2) antagonistic, or the responses to the damage; and (3) integrative, or the consequences of the responses and culprits of the aging phenotype [53]. In the future, more studies addressing these issues in MSCs are clearly needed.
MSC aging, as well as its effects on MSC properties such as telomere length, cell proliferation capacity, differentiation potential, epigenetics, and secretome (Figure 1), have been recently discussed in several review articles [54–57]. Depending on the criteria used to define young and old cells, the methods of cell isolation, cell culture, culture passage, sample size, and results are not consistent in practice. However, it is generally accepted that organismal aging reduces the density of MSCs in bone marrow and compromises their osteogenic potential based on in vitro standard osteogenesis assay and in vivo bone repair experiments. Interestingly, a study by Brusnahan et al. indicated that the loss of stem cells started with those that possessed the lowest biological quality [58]. In addition, there is increasing evidence suggesting that MSC aging benefits adipogenesis at the expense of osteogenesis, resulting in impaired bone formation capacity [59]. This is consistent with the fact that, in vivo, bone marrow contained increased adipose tissue during aging. Such a shift may be due to reduced expression of transcriptional coactivator with PDZ binding motif, thus enhancing expression of peroxisome proliferator-activated receptor gamma (PPAR-γ). PPAR-γ is associated with adipogenesis, and it inhibits expression of Runt-related transcription factor 2 (RUNX2) expression, which is associated with osteogenesis [60].
Figure 1.
Major changes during cell aging. Compared to young cells, old cells display reduced autophagy, proteostasis, and altered mitochondrial function. The proliferation capacity also declines with aging, due to the increase of P16 and P21 level. In addition, old cells produced SASP factors, which cause adverse effect on other cells.
To enable the function of MSCs to facilitate tissue repair, it is critical to direct MSCs to the site of injury, referred to as “homing”. When tissues are damaged, signaling factors are released into the body fluid and activate both residential and systemic MSCs, resulting in the elevation of the level of CD44 on the surface of MSCs. During this process, high expression of stromal derived factor-1 (SDF-1) at the injury site recruits MSCs and docks them to the right spot by binding with the cytokine receptor, CXCR4, on the surface of MSCs. Afterwards, integrin α4 and β1 combine together and form very late antigen-4 (VLA-4) in MSCs, further directing MSCs to endothelial cells by interacting with VCAM-1. Finally, lytic enzymes such as matrix metalloproteinases (MMPs) are produced to generate space to allow MSC migration [61, 62]. In a mouse study, expression of CXCR4 on the surface of MSCs was significantly reduced in old mice compared with young counterparts [63]. Interestingly, intravenous injection of old MSCs homed less to wound site with less neovascularization-promoting capacity than intravenous injections of young MSCs. MSCs isolated from mouse bone marrow were found to lose their homing ability rapidly after in vitro expansion [64], which may be associated with decreased expression of VCAM-1, an MSC surface marker that is known to regulate MSC interaction with endothelial cells [65]. Moreover, by measuring migration rates, Geibler et al. showed that young MSCs of each passage always demonstrated significantly higher migratory potential compared to old MSCs [66], suggesting an impaired response of aged MSCs to injury signals. Recently, we have collected young and aged MSCs from more than twenty donors and found similar decline of migration capacity (unpublished observation). Similar phenomenon is also observed in MSCs derived from adipose tissues, suggesting that this characteristic may be associated with aging-associated inferior microenvironment [67]. However, in a hMSC-based study, no difference in the migratory ability of MSC was reported with respect to the age of the donor, which could be due to the relatively “young” donors in the older group (38–58 years old) [68]. Similar to the findings from studies of natural aging, in vitro senescence was also found to severely impair the migratory capacity of human MSCs in response to proinflammatory signals [69].
As discussed above, MSC may function via the production and secretion of paracrine factors [70]. Autocrine and paracrine properties of senescent MSCs have been recently reviewed by Lunyak et al [71]. To date, how natural aging affects MSC secrectome has not received sufficient attention. In one study on adipose derived MSCs, adult MSCs (derived from >40 years old individuals) produced significantly higher interleukin-6 (IL-6) and IL-8 than those isolated from donors of <16 years of age [72]. The difference was further confirmed by a recent study [73]. Such enhanced pro-inflammatory secretome was likely to significantly diminished the immunomodulation capacity of MSCs [73]. In comparison, there have been a number of studies analyzing the secretome of early and late passage MSCs in vitro. Secretion of senescence-associated secretory phenotype (SASP) products from senescent MSCs has also been characterized. By generating senescent human bone marrow-derived MSCs with gamma irradiation, Sepúlveda et al. identified more than 27 SASP components, such as IL-17F, leptin, IL-8, Eotaxin, VCAM1, interferon β, IL4, and MCP1. In particular, IL-6 was robustly generated by these senescent cells, representing the most prominent SASP factors [69]. Severino et al. showed that conditioned media (CM) from passage 10 (P10) senescent MSCs could directly induce a senescent phenotype in P1 cells in association with insulin-like growth factor binding proteins (IGFBP) 4 and 7 [74]. Ozcan et al. used four different methods to generate in vitro senescence in human MSCs, including oxidative stress, doxorubicin treatment, X-ray irradiation, and replicative exhaustion, which were all confirmed by positive staining of SA-β-gal [75]. To date, how SASP from MSC affects bone healing has not yet been reported. However, IL-6, one of the most recognized SASP factors, has been shown to drive osteoclastogenesis [76] and negatively regulate osteoblast differentiation [77]. Knockdown of IL-6 significantly enhanced Runx2 and collagen type I gene expression in osteoblasts while decreasing the expression of osteoclast related genes such as tartrate resistant alkaline phosphatase (TRAP), MMP9, and CTSK [78]. Therefore, it can be extrapolated that SASP production would not be beneficial to bone regeneration.
Finally, it is noteworthy that most MSCs studied were isolated from surgical waste from patients with bone or osteochondral diseases, such as osteoarthritis. How important these disease states are as a variable that affects the experimental outcomes is unknown, and more rigorous and standardized methods to collect MSCs from “healthy” or “abnormal” tissues are clearly needed. Also, it is important to point out that aging or senescence does not always imply a negative physiological function. For example, acute or chronic injury is found to lead to the accumulation of senescent muscle stem cells, which could initiate in vivo reprogramming, leading to the repair of muscle injury [79].
2.4. Mechanisms Underlying MSC Aging
To date, the mechanism of in vivo natural MSC aging in humans is still not clear, as there is no feasible way to trace the in-body changes of MSCs. However, several factors have been proposed to be associated with this process, including diseases, over-division, and exposure to multiple stresses such as reactive oxygen species (ROS) [55]. Since MSCs reside in unique tissue niches within the body, their aging is expected to be associated with systemic and local factors. For example, natural aging is accompanied by increased levels of pro-inflammatory cytokines, called inflammaging [80], in particular IL-6 [81–83]. Although it is clear that IL-6 may disrupt bone formation, its effect on MSCs is apparently opposite. Previously, we, for the first time, showed that IL-6 gene expression is significantly higher in undifferentiated MSCs than those exposed to an osteogenic differentiation medium. Interestingly, treatment with IL-6 enhances MSC proliferation rates and protects MSCs from apoptosis, which may function through the activation of ERK1/2 pathway [84]. Moreover, IL-6 and the IL-6 receptor (IL-6R) complex activate STAT3 signaling pathway, which promotes osteogenic differentiation in BM-MSCs [85]. Therefore, whether elevated IL-6, which is a natural response to aging, is in fact acting to activate or rejuvenate the functionality of MSCs requires further investigation.
These potential risk factors then function on cell phenotype or functions through signaling pathways. For example, canonical Wnts and members of the forkhead family were shown to be significantly involved in the MSC aging process (see review in [55]). Recently, forkhead box P1 (FOXP1) has been shown to play a major role in transcriptional control of MSC senescence. The expression of FOXP1in bone marrow MSCs decreased with the aging progress, and the conditional depletion of FOXP1 significantly accelerated MSCs aging in vivo [86]. In fact, overexpression of FOXP1 increased the proliferation rate and resulted in higher osteogenic potential over adipogenesis of MSCs, which may function through the inhibition of p16.
2.5. Effect of niche aging on MSC
2.5.1. Effect of aged supporting cells
As shown in Figure 2, MSCs reside together with many other cell types, including osteoblasts, osteoclasts, fibroblasts, endothelial cells, adipocytes, and within bone marrow compartments [39]. The interactions between MSCs and niche supporting cells may be through direct physical contact, secreted soluble factors, or ECM proteins [30]. Due to well-recognized technical challenges, analysis and understanding of the cellular interactions within bone marrow in vivo are difficult. Therefore, most of the current studies have used in vitro culture system in an attempt to investigate the interactions of cells during the aging process, which may not mirror the situation in vivo.
Figure 2.
MSC Niche in bone marrow. In quiescent state, MSCs reside in a homeostatic microenvironment, which contains different cells, soluble factors and extracellular matrix.
Since aging represents a decline in systematic functions, all cells in bone marrow should undergo a similar “aging stress”, affecting neighboring cells. For example, conditioned media (CM) from passage 10 senescent MSCs could directly induce a senescent phenotype in P1 cells by secreting IGFBP 4 and 7 [74]. Additionally, aging decreased the expression level of fibroblast growth factor-2 (FGF-2) in most cells, which may cause the diminution of proliferative capacity of MSCs [87]. Furthermore, data from our laboratory showed that canonical and non-canonical Wnts, Wnt3a and Wnt5a, respectively, act in opposite ways for modulating the behavior of MSCs, i.e., Wnt3a maintains stemness, while Wnt5a induces osteogenesis [88]. With aging, increased Wnt/β-catenin signaling was seen in old mice, the function of which, however, requires further investigation [89].
Currently, the supporting function of MSCs to HSCs has been well demonstrated. Given their very close location in bone marrow, how HSCs affect MSCs, in particular during the aging process, requires further investigation. A study from June et al. suggests that HSCs do not rest passively in the bone marrow [90]. Instead, HSCs directly induce MSC osteogenesis through the secretion of BMP-2 and −6. In addition, aging causes the dysregulation of BMP expression by HSCs, which may contribute to the pathogenesis of osteoporosis.
Although the mechanism of generation of inflammaging is unclear, adipose tissue has been proposed as the major generator of systemic proinflammatory cytokines with advancing age [91–93]. After the discovery of leptin in 1994, adipose endocrine function and involvement in many physiologic and pathologic processes are now well recognized [94]. Aging significantly alters adipose tissues in both lean and obese individuals, including progenitor cell function decline, cellular senescence, adipose-derived hormone changes, and reduced miRNA processing [95]. In particular, through a senescence-associated secretory phenotype, senescent cells themselves generate many pro-inflammatory cytokines and chemokines [96, 97]. Accumulation of fat tissues and adipocytes has been observed in the bone marrow of older human [98] and mice subjects [14]. These cells impair the bone healing process through the excessive generation of dipeptidyl peptidase-4. It is noteworthy that the increased number of adipocytes may be due to the bias of aged MSC towards adipogenic differentiation [99], which increases the complexity in understanding the interaction between adipocytes and MSCs during aging.
MSCs also have direct physical contact with osteoblasts. In monolayer co-cultures, MSCs and osteoblasts were observed to actively establish cell-cell contact, which was sufficient to drive MSC osteogenesis [100]. Similar results were reported by Glueck et al. [101]. Based on next- generation sequencing and bioinformatics analysis, differentially expressed microRNAs and genes were identified between normal and senescent osteoblasts. In particular, miR-204–5p was identified as an upstream regulator, inhibiting the expression of Runx2 [102]. Aged osteoblasts showed compromised capacity of generating cell junction upon stimulation; in fact, connexin 43 was shown to be critical for MSC survival and migration [104, 105]. As discussed above, MSCs reside in a perivascular environment. By co-culturing MSCs with human umbilical vein endothelial cells (HUVEC), there was not only a significant increase of MSC proliferation, but also the promotion of osteogenic differentiation of MSCs [106]. In fact, we have previously shown that ECM generated from HUVECs directs differentiation of MSCs into vascular lineages [107]. Since endothelial cell dysfunction was shown to be associated with sedentary aging, such as the robust production of proinflammatory cytokines [108, 109], they may have the potential to change the functionality of MSCs as well.
2.5.2. Effect of aged ECM
The composition, mechanical properties, and topography of ECM have all been shown to affect the phenotype of MSCs to varying extents. We have previously reviewed the function of ECM on MSCs [39]. Recently, our results, drawn together with others, showed that the ECM derived from MSCs could promote MSC proliferation and maintain their potential [110, 111]. MSCs expanded on this ECM regenerated significantly higher bone in vivo than those maintained on tissue culture plastic. Interestingly, ECM generated by aged MSCs showed significantly decreased capacity in preserving normal MSC function, suggesting the critical role of ECM in MSC aging [112]. However, whether the amount of ECM generated by MSCs representing a major determining factor in bone marrow is not clear. In addition to stem cell-derived ECM, the aging of tissue matrix also affects stem cell function. Our recent study showed that aged muscle ECM displayed decreased collagen tortuosity and stiffness with aging, which increased the fibrogenic marker expression in muscle stem cells at the expense of myogenesis [113]. In 2006, a landmark work by Engler et al. showed that MSCs could sense the stiffness of culture substrate and differentiate into different lineages according to the stiffness of substrate, including neurogenesis on the soft surface and osteogenesis on the hard surface [114]. Afterwards, Winer et al. cultured MSC on a soft gel with 250Pa stiffness and found that the cells maintained a quiescent state without division. However, they were activated by being switched to a stiff substrate [115]. Similar results were reported in another independent study [116]. Taken together, these results suggest the effects of mechanical cues derived from the ECM on MSC aging. Unfortunately, how aging may change these aspects of the ECM within bone marrow is still unknown.
2.5.3. Effect of aged systemic factors
As mentioned, aging represents a systemic dysfunction. In addition, MSCs are physically located around the blood vessels. Therefore, the aging of MSCs must be associated with the circulating systemic factors. Zhang et al. showed that serum from old rats caused significantly higher senescent cell ratio in MSCs compared with young serum, which may functionally relate to enhanced β-catenin level [117]. By inhibiting canonical Wnt/β-catenin, the negative effect of old serum was mitigated. Similar inhibition by aged serum on MSC proliferation was also reported [118]. Interestingly, serum from aged rats treated with NT-020 did not show such inhibitory effects. Utilizing parabiotic pairing method to allow the transfer of blood borne cells, bone marrow stem cells from old animals significantly enhanced osteogenic differentiation upon stimulation, which also resulted in enhanced fracture repair [119]. Results from these studies also suggest that this effect is partially mediated through β-catenin. Recently, Rebo et al. has reported a new method, which allows heterochronic blood exchange between young and old mice without sharing other organs. They also showed that blood from old mice significantly inhibited the proliferation of stem cells in young mice [120]. Taken together, these results suggest that the old serum/plasma contains inhibitory factors that impede MSC function. Murphy et al. reviewed the potential mechanisms and proposed that CCL11, GDF11, mTOR, and insulin/IGF1 signaling pathways may significantly participate as the systematic factors on stem cell function [121].
3. Stem cell therapy in bone repair and regeneration
3.1. Current strategies for stem cell-based therapy
As discussed previously in this review article, bone has a unique and well-documented natural healing process that is normally sufficient to repair fractures and other common injuries [122, 123]. However, with aging, bone healing capacity is decreased, partially due to functional decline of MSCs. Therefore, an intervention is usually required to treat delayed union or nonunion. The current surgical methods of repairing bone defects are highly invasive and not always successful due to poor vascularization, soft tissue conditions, infection, and pre-existing bone malignancy [124]. Stem cell-based therapy is a viable alternative with promising therapeutic advantages in restoring both the structure and function of damaged bone [125]. In general, stem cells can be therapeutically used in one of three ways. First, freshly isolated stem cells can be transplanted directly into tissue and undergo in vivo differentiation to become a desired cell type. Second, stem cells can be manipulated in vitro before implantation. In many cases, stem cells are genetically engineered to express specific types of genes or pre-differentiated into a particular cell type prior to implantation to enhance lineage-specific differentiation [126]. Third, administration of specific cytokines can recruit circulating endogenous stem cells into injury sites and further facilitate cell proliferation, migration, adhesion, and differentiation [126, 127].
A review of the literature from the past fifteen years shows that various types of stem cells, including bone marrow-derived MSCs (BM-MSCs), adipose-derived stem cells (ADSCs), muscle- derived stem cells (MDSCs), umbilical cord blood-derived MSCs (UCB-MSCs), and dental pulp stem cells (DPSCs), have been used to enhance bone regeneration and repair (Table 2). These studies have utilized strategies to transplant different types of stem cells in animal models, with or without scaffolds; however, they all report similar stem cell implantation effects. Stem cell-based therapy has been shown to facilitate bone or blood vessel formations and/or decrease inflammation and lower immunogenicity. A good amount of literature has reported that the direct injection of autologous MSCs from different tissue sources were able to engraft and regenerate bone to repair critical-size bone defects after implantation in different types of animals [128–134]. Among these, some recent studies have reported that engrafted stem cells play a crucial role in improving the process of bone regeneration, primarily through the secretion of paracrine factors to create a pro-osteogenic microenvironment at the defect site [135–138]. For example, Gao et al., reported that MDSC implantation enhanced angiogenesis and bone regeneration in a critical size calvarial defect by promoting endothelial cell proliferation via secreting multiple growth factors. Moreover, the MDSCs were shown to suppress initial immune responses in the host animal by secretion of monocyte chemotactic protein 1 and attract macrophages [135]. Another group from Japan recently reported that secretomes in MSCs include various cytokines that are important in regulation of osteoclast differentiation and the recruitment and proliferation of osteogenic- and angiogenic- cells [136]. These studies suggest that investigating and developing drugs that can deliver the above-mentioned secreting factors from stem cells may lead to effective therapeutic modality for the treatment of bone fractures and defects.
Table 2.
Characteristics of studies involve stem cell therapy in bone repair and regeneration
| Cell Type | Source | Animal Model | Type of Defect | Study Design | Ref. |
|---|---|---|---|---|---|
| MSCs | Bone Marrow | Dog | Mandibular | MSCs + PRP injection | [142] |
| MSCs | Bone Marrow | Sheep | Metatarsal | MSC seeded PRP-based scaffold implantation | [144] |
| MDSCs | Skeletal Muscle | Mouse | Skull | BMP-2. VEGF. sFlt1 expressing MDSC transplantation | [147] |
| ASCs | Adipose Tissue | Dog | Parietal bones | MSCs seeded coral scaffold implantation | [141] |
| MSCs | Bone Marrow | Dog | Mandibular | MSCs seeded β-TCP scaffold implantation | [143] |
| ASCs | Fat Tissue | Dog | Ulna | BMP-2 expressing ASC transplantation | [149] |
| MSCs | Umbilical cord blood | Dog | Radial | MSC injection | [226] |
| MSCs | Bone Marrow | Dog | ONFH | MSC injection | [131] |
| DPSCs | Teeth | Pig | Mandibular | DPSC transplantation | [133] |
| MDSCs | Orbicular oris muscle | Rat | Cranial defect | hMDSC transplantation | [134] |
| MDSCs | Skeletal muscle | Mouse | Calvarial | BMP4 expressing MDSC transplantation | [148] |
| MSCs | Bone Marrow | Dog | Femoral head | MSC-seeded BCP scaffold implantation | [140] |
| MSCs | Bone Marrow | Rabbit | Femurs | Pre-osteogenically differentiated MSC transplantation | [146] |
| MSCs | Bone Marrow | Sheep | Tibial | hMSC transplantation | [132] |
| MPCs | Bone Marrow | Sheep | Tibial diaphyseal defect | MPC-seeded scaffold implantation | [128] |
| MSCs | Bone Marrow | Dog | Craniofacial | PRFG-MSCs injection | [139] |
| MSCs | Bone Marrow | Dog | Inferior orbital rim bone | Pre-osteogenically differentiated MSC-seeded β-TCP scaffold implantation | [145] |
| MSCs | Bone Marrow | Rabbit | Tibial | MSC-seeded bone scaffold implantation | [130] |
| ASCs | Adipose Tissue | Pig | Ulna | US2/US3 gene transfected ASC transplantation | [150] |
| MSCs | Bone Marrow | Sheep | Mandibular | MSC injection | [129] |
| MSCs | Adipose Tissue | Rabbit | Jaw bone | hMSC transplantation | [137] |
| MDSCs | Skeletal muscle | Mouse | Calvarial | BMP-4 expressing MDSC transplantation | [135] |
| ASCs | Adipose Tissue | Rabbit | OA-like damage | hASC injection | [138] |
| MSCs | Bone Marrow | Rabbits | Jaw bone loss | MSC injection | [136] |
MSCs, mesenchymal stem cells; MDSCs, muscle-derived stem cells; ASCs, adipose-derived stem cells; DPSCs, dental pulp stem cells; MPCs, mesenchymal progenitor cells; h, human; PRP, platelet rich plasma; β-TCP, beta-tricalcium phosphate; BCP, biphasic calcium phosphate ceramic.
In addition to direct stem cell injection, researchers have used tissue engineering approaches by delivering stem cells on biodegradable scaffolds [139–144]. In this case, several groups show that pre-differentiating or pre-manipulating stem cells in vitro prior to implantation could be beneficial to generate a particular cell phenotype [143, 145, 146]. Various genes, such as BMP-2 and BMP-4, have been introduced in stem cells to enhance osteogenic-specific differentiation. Additionally, US2/US3 genes, which can decrease the expression of MHC I protein in cells and reduce the activation of T-cells of the recipient animals, have been utilized for this goal as well [147–150]. Interestingly, in 2010, Lyons et al. reported that cell-free collagen-based scaffolds developed for bone repair showed excellent healing, relative to MSC-seeded constructs. Their results suggested that the matrix deposited by MSCs during the differentiation process may adversely affect healing by forming a barrier to healthy macrophage activity and thus preventing remodeling of the implanted tissue and formation of new bone by the host. This barrier prevents vascularization of the implanted tissue, resulting in the initiation of avascular necrosis at the center of the implanted scaffold [151]. From a bone healing and repair perspective, this study may provide a simpler solution, which is the development of a biomimetic scaffold with a composition similar to native bone tissue and without requiring any cell compartments. However, further studies will be needed to accurately access the effect of stem cells on scaffolds. Utilization and effects of different types of scaffolds and biomaterials for bone tissue engineering will be discussed in detail, in a later section of this review.
3.2. Effect of aging on stem cell therapy
3.2.1. Intrinsic and extrinsic factors affecting stem cell behavior and their niche
Although MSC-based therapy offers the possibility of a renewable source of replacement cells and tissues to treat various types of bone defects and injuries [152], aging and the aging-associated processes can significantly impact the beneficial effect of stem cell-based therapy. The active stem and progenitor cells in young adult tissues persist into old age; however, these populations are different from their younger counterparts. In old age, resident stem cells are affected by both intrinsic and extrinsic factors, which often compromise their functions [153]. For example, Baster et al. showed that the number of BM-MSCs with osteogenic potential decreases early during aging in humans, which may be responsible for the age-related reduction in bone formation and in the mechanical properties, and integrity of bone [154]. In addition to MSCs, neural stem cells (NSCs), satellite cells, and HSCs have also been reported to show significant age-related decrease in the proliferation and differentiation potentials both in vitro and in vivo [155–157]. The loss of a number of functional stem cells with age can lead to profound consequences on tissue viability. The mechanism of stem cell exhaustion/depletion is unclear; however, it may be caused by a combination of a number of both intrinsic and extrinsic factors, including a change in growth factors activity, accumulation of DNA damage, and decline in progenitor cell responsiveness.
Intrinsic factors
Independent lines of evidence suggest that forms of cell cycle regulators, cell DNA damage, and telomere shortening lead to the activation of the tumor-suppressor mechanism, such as senescence, and cause stem cell behavior change in old age [158, 159]. As with all dividing cells, tight regulation of cell cycle is important for regulating the rate of cell division and cell kinetics [158]. Some studies have shown that the inhibitor of cyclin-dependent kinase, p16Ink4a, which regulates the G1-S phase cell cycle transition, restricts stem cell self-renewal in aging systems, including NSCs [160] and HSCs [161]. Stem cell aging is also due to accumulation of heritable intrinsic events such as DNA damage. This damage is associated with exposure to ROS, ionizing radiation, chemical mutagens, and repeated DNA replication [158, 159]. Moreover, recent studies suggest that stem cell exhaustion, decreased proliferation, differentiation, and homing capabilities may be due to the shortening of telomere length in stem cells [162, 163]. Several studies have reported that there is a strong correlation between MSCs self-renewal capacity and telomere length in culture with donor age [162, 163].
Recently, the best strategy suggested to cure aging due to cell damage is a rapid and effective elimination of the damaged stem cells by apoptosis [164]. When the DNA repair mechanism falls apart due to the aging process, cells respond to innate changes and enter independent stress-response mechanisms including apoptosis and cellular senescence. Senescent cells (SCs) are permanently withdrawn from the cell cycle; however, unlike apoptotic cells, which are permanently eliminated, they are viable for prolonged periods of time, and accumulate with age. These persistent SCs are thought to accelerate aging and the onset of age-related diseases [164, 165]. Recently, a group of researchers have identified a molecular mechanism of SC viability and have reported that disruption of FOXO4-p53 interactions in SCs selectively induce cell-intrinsic apoptosis. Furthermore, targeted apoptosis of SCs restored fitness, hair density, and renal function in fast and naturally aged mice models [164]. Similarly, another group has reported that the clearance of SCs in a progeroid mouse model delays several age-associated disorders. They have identified a specific inhibitor of the anti-apoptotic proteins Bcl-2 and Bcl-xl, ABT263, as a potent drug and showed that ABT263 selectively kills senescent HSCs, and muscle stem cells (MuSCs) both in vitro and in vivo [166]. Taken these together, therapeutic targeting of SCs could counteract the loss of tissue homeostasis in response to aging.
Extrinsic factors
In addition to the intrinsic factors, the function and regenerative potential of MSCs are impacted by extrinsic factors in aging. Studies report that the age-associated impairment of stem cell function is induced to a significant extent by the molecular composition of the surrounding niche rather than by cell intrinsic changes alone [167]. Additionally, many studies show that morphological and functional changes within different tissues and organs due to aging result in deleterious changes in the stem cell niche and their microenvironments, which further inhibit their regenerative potential [55, 158, 168]. For example, in old age, the status of stem cells is altered with decreased niche cells, fragmented ECM, increased genomic, mitochondrial DNA damage, disruption in a hypoxic microenvironment, and increased ROS [169]. Additionally, stem cells require growth factors, cytokines, and mitogens for maintaining self-renewal and multipotency; however, changes in ECM components and structure will further cause subsequent modification on the availability of these factors [158, 159, 169].
A concrete example of the influence of the local and systemic environment on stem cell function during aging has been well demonstrated by Conboy et al. This group has shown that the rejuvenation of aged progenitor cells in old animals is possible by exposing the old to a young, systemic environment [156]. In their experiments, they have used strategies such as heterochronic parabiosis to expose cells in an aged mouse to the systemic environment of the young mouse. Their results clearly indicated that aged murine MuSCs and hepatocytes showed significant increase in their proliferation and regenerative capacities after heterochronic parabiosis with a young mouse [156]. More recently, using the same parabiosis technique, Villeda et al. has shown that when young stem cells were subjected to an aged systemic milieu, they exhibited functional decline in neurogenesis, which further negatively affects cognitive function in mice [170]. Lastly, a group of researchers have also shown that systemic inhibition of TGFβ2 signaling pathways, responsible for increased fibrosis and inhibit muscle regeneration in dystrophic skeletal muscles, rescue the satellite cell phenotypes and significantly reduce fibrogenesis of satellite cells in dystrophic mice [171]. Defective regeneration and accumulation of fibrotic tissue also characterize aging muscles [172]. Therefore, this study suggests that changes in stem cell milieu definitely have a vital effect on changing stem cell behavior and fate. These studies, taken together, clearly indicate that changes in the stem cell microenvironment that occur with aging affect stem cell behaviors.
The vast majority of stem cell-based therapies are currently being explored for cell replacement. However, the above studies strongly suggest that stem cell-based therapy might be less effective when the cells are derived from older individuals or when therapy is performed in older patients. Therefore, for any stem cell transplantation-based strategy, it may be crucial to consider the age of both the donor tissue and the recipient environment.
3.3. Stem cell rejuvenation for bone repair in aging
Similar to other stem cell types, bone marrow-derived MSCs are affected by aging, and MSCs from old patients show significantly limited fracture repair and osteoblast differentiation potential compared to MSCs from younger patients [154]. The cause of this difference in MSC bone repair and differentiation potential with age is still unknown; however, this phenomenon is irreversible and similar in mice [173]. Recently, Baht et al. has reported that exposure to youthful circulation by heterochronic parabiosis reverses the aged fracture repair phenotype and the diminished MSC-medicated osteoblastic differentiation capacity of old animals. They have also demonstrated that the engraftment of young bone marrow, particularly CD45+ hematopoietic cells, was able to rejuvenate bone repair and osteoblast differentiation. This rejuvenation was driven by a factor that was able to modulate Wnt/β-catenin signaling pathways through the downregulation of β-catenin expression in old fracture calluses early in fracture repair and subsequent revitalizion of fracture repair processes in mice [119]. It will be interesting to further investigate if rejuvenation affects the levels of any growth factors related to bone regeneration or inflammatory response during bone repair in these parabiosis experiments. Considering all these results together, this study strongly suggests that aged cells exposed to a youthful systemic milieu display more youthful characteristics, promising that it may be possible to mitigate certain aging features that hinder bone repair and regeneration in old patients. Furthermore, rejuvenating MSCs prior to the cell therapy for bone repair may be a possible option to consider.
4. Scaffolds for bone regeneration
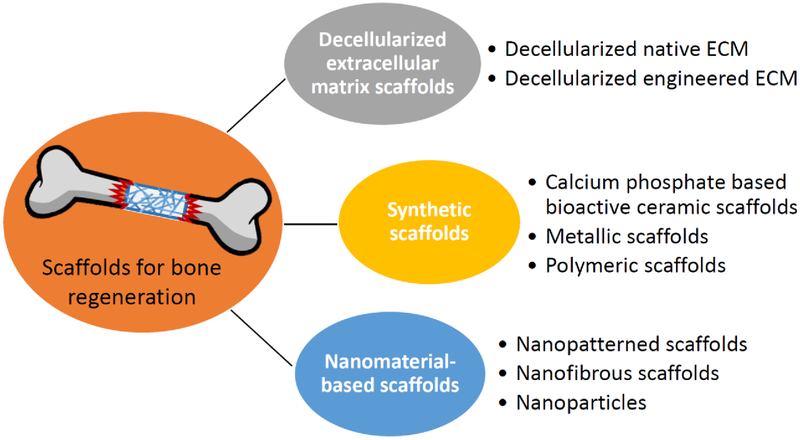
In addition to growth factors and stem cells, bioscaffolds are also needed. Often, bioscaffolds can function as the reservoir for multiple factors, the carrier for cells, the filler for the void space, and the template for bone regeneration. The requirements of an ideal scaffold for bone tissue engineering are as follows: (1) showing no local and systematic toxic effects to the host tissue; (2) supporting normal cellular activity; (3) allowing cell adhesion, proliferation, extracellular matrix deposition, and inducing new bone formation; (4) prompting the formation of blood vessels after several weeks implantation; and (5) appropriate mechanical properties, pore size, and in vivo biodegradation rate [174–177]. Many researchers have investigated the development of biomimetic scaffolds with osteogenic microenvironment to facilitate the ossification process and to improve clinical therapy (Figure 3).
Figure 3.
Application of engineered scaffolds to augment MSC-based bone repair.
4.1. Decellularized extracellular matrix scaffolds
Bone tissue ECM retains multiple bioactive molecules, such as pro-inflammatory cytokines and growth factors (e.g. BMPs and vascular endothelial growth factor (VEGF)) that will be beneficial for bone repair without adverse immune responses. In addition, decellularized ECM can be combined with growth factors or calcium phosphates (CaP) (e.g. β-tricalcium phosphate, β-TCP, and hydroxyapatite, HA) to enhance osteoinductivity [178, 179].
Decellularized native ECM (nECM), which is obtained from a mature organ (allograft or xenograft) after the removal of cells and cellular antigens, retains the structure and architecture of the original tissue and has the ability to facilitate bone repair [180]. Moreover, nECM is also considered a promising candidate structure for stem cell delivery system due to its functionality of growth factor regulation [181]. Another widely used bone ECM is demineralized bone matrix (DBM), which is obtained from allogeneic cortical bone after demineralization by acid [182]. DBM contains collagenous proteins and ECM-associated growth factors (BMPs and FGFs), which act to increase bone healing efficiency. Decellularized engineered ECM (eECM), which is a cell-free matrix generated by stem cells in vitro containing various components of the native ECM, has also been demonstrated to improve in vitro MSC expansion and osteogenic differentiation [183]. Additionally, combining bioactive eECM with synthetic scaffolds has been found to increase the osteoblastic hMSC differentiation, calcium deposition, and osteoid tissue formation, as compared to eECM-free scaffolds [184].
4.2. Synthetic scaffolds
Calcium phosphate based bioactive ceramic scaffolds
Calcium phosphate ceramics (CaP), such as HA and β-TCP, major components of bone, have good biocompatibility, high bioactivity, and osteoconductivity, and they are widely applied in bone tissue engineering. Zhang et al. found that composite porous scaffolds containing β-TCP as a matrix and HA nanofibers of different concentrations showed improved mechanical properties. The compressive strength of the β-TCP porous scaffold with the presence of 5% of HA nanofibers was 9.8 ± 0.3 MPa, which is comparable to that of human cancellous bone (2–10 MPa) [185]. A porous collagen-calcium phosphate scaffold fabricated via three-dimensional (3D) printing showed suitable mechanical properties and osteoconductivity for non-loading bone defect implantation and new bone formation [186].
Metallic scaffolds
Metallic materials, such as stainless steel and cobalt- and titanium-based alloys, are widely used in clinical orthopedics and demonstrate good biocompatibility and mechanical properties. However, the lack of bio-specific recognition epitopes on the metallic material surface makes these metallic scaffolds biologically less active. To improve cell-scaffold interaction and tissue repair/regeneration efficiency, growth factors and other bioactive factors have been coated onto the surface of the scaffold [187]. Moreover, development of magnesium and its alloy scaffolds has attracted great research interest, as magnesium-based scaffolds are bioresorbable and osteoconductive, and they do not elicit an inflammatory response [188]. Hybrid scaffolds, which combine two or more materials, endow the benefits of each type of material and therefore exhibit novel properties. For example, metal–ceramic–polymer hybrid scaffolds with porous structure have increased implant interface with preservation of the titanium implant lifetime, and they show improved tissue formation through load-sharing and stress distribution compared with fully dense titanium [189].
Polymeric scaffolds
Both natural and synthetic polymer-based (e.g. collagen and polycaprolactone (PCL)) scaffolds have been widely used in the bone tissue engineering field because the porosity, mechanical properties, and degradation behavior can be designed and controlled. Hutmacher [190] and Liu et al. [191] have provided extensively reviewed polymer materials for bone repair applications, including polymeric materials and the design, fabrication methods, and modification of scaffolds.
Hydrogels, formed by highly hydrated polymers, can provide an appropriate microenvironment for cell culture and are actively being explored as promising substrates for bone repair. Hydrogel scaffolds can be modified or bio-activated with growth factors (such BMP and FGF), gene constructs, and small inductive molecules to enhance the proliferative and osteogenic activities of the seeded stem cells [192]. Hydrogels can also be combined with calcium phosphate ceramics to improve the mechanical properties [193]. Additionally, 3D hydrogel scaffold itself also induces osteoblast differentiation and mineralization by virtue of the mechanical properties of the scaffold. For example, poly (ethylene glycol) dimethacrylate hydrogel with a gradient in compressive modulus (~10–300 kPa) and encapsulated osteoblasts resulted in gradient-dependent osteoblastic differentiation [194]. Shen et al. also found that methylcellulose-based scaffolds with different cross-linking densities and controlled Young’s modulus had a stimulatory effect on inducing osteogenic differentiation of seeded MSCs in the absence of inductive agents [195].
The development of 3D printed synthetic material-based scaffolds fabricated via computer aided design (CAD) using ceramic, metallic, polymeric, and composite materials has shown great potential for regenerative bone application due to controllable chemistry, shape, and porosity [196]. The precise, designed, 3D printed scaffold can mimic the structure and mechanical properties of trabecular bone, as well as support vessel formation [197].
4.3. Nanomaterial-based scaffolds
With a hierarchical structure ranging from nanoscale to macroscale by virtue of its composition of organic (e.g. collagen) and inorganic (e.g. nano-hydroxyapatite) materials, bone can be considered a “nanomaterial”. To overcome limitations in traditional therapies, nanomaterials are being explored in bone tissue engineering, as they provide a closer structural support approximation to native bone architecture and can also regulate cell fate [198]. Scaffolds composed of nanofibers, nanotubes, nanoparticles, and hydrogels with nanostructures/nanopatterns have recently attracted increasing research interest.
Nanopatterned scaffold
To mimic the native tissue, nanogrooved matrices were investigated. It has been reported that the body and nucleus of hMSCs cultured on substrate with sparser nanogrooved pattern were elongated and orientated along the direction of nanogrooves [199]. The nano-topographical density of the nanopattern scaffold also played an important role on the osteogenesis of hMSCs by regulating the formation of cytoskeleton, which is necessary for tension effects on cell morphology and stem cell differentiation mediated via the Rho-associated protein kinase (ROCK)-pathway. In addition, multi-scale hierarchical topography-based substrates could provide native ECM-like topographical signals to influence substrate adhesion and differentiation of hMSCs. For example, the poly (lactic-co-glycolic acid) (PLGA) patches with nanopatterned hierarchical topography showed enhancement of hMSC osteogenesis and in vivo bone regeneration [200]. The symmetry and order of the nanopits also present the capability of regulating the expression of bone-specific ECM proteins (osteopontin and osteocalcin) [201]. Interestingly, a nanopatterned substrate with surface immobilization of osteoinductive BMP-2 peptides exhibited additive effects of BMP-2 induction and nanotopographical stimulation on osteogenic differentiation of hMSCs [202].
Nanofibrous scaffold
Nanofibers fabricated via electrospinning technique have a wide range of diameter from tens of nanometers to microns. The diameter of the polymeric nanofibers can be affected by controlling the fabrication parameters, including polymer properties, solvent properties, solution flow rate, voltage, distance from the needle to the collector, and polymer concentration. Therefore, by tuning the parameters of electrospinning, the nanofibers produced are able to mimic the intricate fibrillar architecture of natural ECM components [203]. Synthetic polymers, such as poly-L-lactic acid (PLLA), polyglycolic acid (PGA), PLGA and PCL, and natural polymers, such as collagen, gelatin, alginate and chitosan, are used to design nanofibrous scaffolds. The synthetic polymers are more flexible in terms of synthesis, processing, and modification, while the natural polymers are more bioactive for stimulating cell adhesion [204, 205].
Our group has previously found that 3D PCL nanofibrous scaffolds supported and maintained osteogenic, chondrogenic, and adipogenic differentiation of hMSCs in vitro [205]. To endow the nanofibers with desired functionalities, hybrid nanofibers containing multiple components were explored. For example, chitosan/PCL nanofibers showed improved cell adhesion and osteogenic differentiation compared to pure PCL nanofibers [206]. PCL–CaCO3 hybrid nanofibers improved mechanical tensile properties and water affinity [207]. Furthermore, nanofibrous scaffolds with surface-functionalization or internal incorporation of growth factors, gene constructs, and small molecules were widely investigated as controlled delivery systems for the acceleration of bone regeneration [208]. The silk/polyethylene oxide nanofibrous substrate with BMP-2 encapsulation showed enhancement of osteogenesis and calcium deposition [209]. PLGA nanofibers with DNA encapsulated within polylactide–poly (ethylene glycol) incorporation were able to gene transfection of the cultures and subsequently encode protein β-galactosidase [210].
Nanoparticles
Nanomaterials were explored as factors/genes nanocarriers during bone regeneration. For example, gold nanoparticles promoted osteogenic differentiation and inhibited the adipogenic differentiation of MSCs by causing mechanical stress on the MSCs to activate p38 mitogen-activated protein kinase pathway (MAPK) signaling pathway through the interaction with cell membrane and cytosolic proteins [211]. Interestingly, Henstock et al. found that functionalized magnetic nanoparticles directly targeted cell-surface mechanosensors and transduced forces from an external magnetic field, thus providing mechanical stimuli to hMSCs. The functionalized magnetic nanoparticles binding to the mechanically gated TREK1 K(+) channel of hMSCs increased mineralization of the cells as a result of mechanotransduction. This study presented the potential of using nanoparticles to enhance bone formation via stimulation of mechanotransduction [212]. Besides metal nanoparticles, carbon nanoparticles, including carbon nanotube and graphene, have also been explored in acceleration osteogenesis. Due to the unique physicochemical properties of graphene and its derivatives, graphene-based nanomaterials not only acted as a nanoplatform for growth factor adsorption and delivery, but also provided mechanical support [213]. For example, we found that PLGA nanofibrous scaffold with graphene oxide incorporation accelerated hMSC osteogenesis as a result of preconcentration of protein and inductive agents by graphene oxide [214]. In addition, graphene-based nanomaterials have the capability of biomineralization under simulated body fluid environment [215], likely contributing to the enhancement of osteogenic differentiation of MSCs by graphene–biomineral materials [216].
4.4. Vascularization in engineered bone
Bone has a rich vascular supply. Thus, providing an artificial environment rich in functional vascular networks may achieve efficient osseointegration and accelerate bone repair after implantation. VEGF and FGF-2 are two key factors involved in angiogenesis [217]. A combination delivery and release of angiogenic and osteogenic factors (such as VEGF and BMP-2) by a PLGA scaffold combined with condensed plasmid DNA encoding for BMP-4 and VEGF [218], or a scaffold encapsulated with BMP and VEGF proteins [219], were shown to significantly promote bone formation after implantation. In addition, co-culturing MSCs and HUVECs on a 3D β-TCP scaffold also resulted in the formation of vessel-like structure and osteogenesis, which can be considered as a cell-based strategy for vascularization in the biomaterial scaffolds [220]. Interestingly, nanomaterials have shown the potential of promoting vascularization even without the use of growth factors. For instance, a self-assembling peptide amphiphile (PA) molecule, functionalized with bioactive groups mimicking heparin, was designed and synthesized for angiogenesis induction. Both in vitro and in vivo experiments demonstrated that the 3D nanofibers formed as a result of PA-mediated self-assembly have the capacity of angiogenesis and robust vascularization [221].
Future perspectives.
Because aging is accompanied by increased incidence of bone diseases and compromised fracture healing capacity, the successful development of strategies that maintain healthy or functional MSCs in vivo should be of great benefit. The strategy to maintain MSC functionality during the host aging process includes two major avenues: maintaining cell quantity within the bone marrow or rejuvenating aged cells. The second method, rejuvenating MSCs of declining functions, is as described above. Since aging causes inevitable stem cell exhaustion, the success of the first method may rely on the supplementation of functional MSCs. In fact, direct injection of MSCs into the bone marrow has been performed, and these cells were found to stay in the injection area and home to bone fracture site after injury occurred [222, 223]. Twenty-four hours after transplantation of uncultured MSC into sublethally irradiated mice, more than half of the injected cells were found in the bone marrow [64]. However, whether hMSCs also have such capacity and how long these transplanted cells could require further investigation. In addition, the compromised homing capacity of MSCs during aging may not be solely due to the aging of MSCs, and additional guiding signals may be required. For example, a synthetic high-affinity and specific peptidomimetic ligand (LLP2A), against integrin α4β1 on the MSC surface, was attached to a bisphosphonate (alendronate, Ale) with high affinity for bone. Interestingly, the introduction of LLP2A-Ale was able to increase the homing of transplanted MSCs and augment bone formation [224]. Finally, to completely overcome the aging-associated issues of MSCs, an unlimited supply of young and functional MSCs from other resources is required, such as induced pluripotent stem cell (iPSCs). Our previous study had generated MSC-like cells from iPSCs, which displayed a robust osteogenic potential similar to primary MSCs [225].
Acknowledgements
We thank Sreyas Ravi for editing the manuscript. This work was supported by the National Institutes of Health (1R21AG056819, 1UG3TR002136 and 5R01EB019430).
References
- [1].Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A, Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025, J Bone Miner Res 22(3) (2007) 465–75. [DOI] [PubMed] [Google Scholar]
- [2].Ensrud KE, Epidemiology of fracture risk with advancing age, J Gerontol A Biol Sci Med Sci 68(10) (2013) 1236–42. [DOI] [PubMed] [Google Scholar]
- [3].Nikitovic M, Wodchis WP, Krahn MD, Cadarette SM, Direct health-care costs attributed to hip fractures among seniors: A matched cohort study, Osteoporos Int 24(2) (2013) 659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A, romosozumab or alendronate for fracture prevention in women with osteoporosis, N Engl J Med 377(15) (2017) 1417–1427. [DOI] [PubMed] [Google Scholar]
- [5].Khosla S, Increasing options for the treatment of osteoporosis, N Engl J Med 361(8) (2009) 818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H, The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone, J Bone Joint Surg Br 87(7) (2005) 896–902. [DOI] [PubMed] [Google Scholar]
- [7].Schroeder GD, Hsu WK, Kepler CK, Kurd MF, Vaccaro AR, Patel AA, Savage JW, Use of recombinant human bone morphogenetic protein-2 in the treatment of degenerative spondylolisthesis, Spine (Phila Pa 1976) 41(5) (2016) 445–9. [DOI] [PubMed] [Google Scholar]
- [8].Dhote R, Charde P, Bhongade M, Rao J, Stem cells cultured on beta tricalciumphosphate (beta-TCP) in combination with recombinant human platelet-derived growth factor - BB (rh-PDGF-BB) for the treatment of human infrabony defects, J Stem Cells 10(4) (2015) 243–54. [PubMed] [Google Scholar]
- [9].Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO, Demineralized bone matrix in bone repair: History and use, Adv Drug Deliv Rev 64(12) (2012) 1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin SS, Yeranosian MG, The role of orthobiologics in fracture healing and arthrodesis, Foot Ankle Clin 21(4) (2016) 727–737. [DOI] [PubMed] [Google Scholar]
- [11].Gibon E, Lu L, Goodman SB, Aging, inflammation, stem cells, and bone healing, Stem Cell Res Ther 7 (2016) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rizzoli R, Reginster JY, Boonen S, Breart G, Diez-Perez A, Felsenberg D, Kaufman JM, Kanis JA, Cooper C, Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis, Calcif Tissue Int 89(2) (2011) 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ, Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: A stereological study, Exp Hematol 17(1) (1989) 34–7. [PubMed] [Google Scholar]
- [14].Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, Saraiva LR, Schulz TJ, Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration, Cell Stem Cell 20(6) (2017) 771–784 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Friedenstein AJ, Chailakhjan RK, Lalykina KS, The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells, Cell Tissue Kinet 3(4) (1970) 393–403. [DOI] [PubMed] [Google Scholar]
- [16].Caplan AI, Mesenchymal stem cells, J Orthop Res 9(5) (1991) 641–50. [DOI] [PubMed] [Google Scholar]
- [17].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Multilineage potential of adult human mesenchymal stem cells, Science 284(5411) (1999) 143–7. [DOI] [PubMed] [Google Scholar]
- [18].Squillaro T, Peluso G, Galderisi U, Clinical trials with mesenchymal stem cells: An update, Cell Transplant 25(5) (2016) 829–48. [DOI] [PubMed] [Google Scholar]
- [19].Caplan AI, MSCs: The sentinel and safe-guards of injury, J Cell Physiol 231(7) (2016) 1413–6. [DOI] [PubMed] [Google Scholar]
- [20].Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M, Aldahmash A, Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential, Stem Cell Rev 9(1) (2013) 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD, Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood, Exp Hematol 33(11) (2005) 1402–16. [DOI] [PubMed] [Google Scholar]
- [22].Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Lu L, Yao Z, Goodman SB, Mesenchymal stem cell-macrophage crosstalk and bone healing, Biomaterials (2018) DOI: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS, Mesenchymal and haematopoietic stem cells form a unique bone marrow niche, Nature 466(7308) (2010) 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT, Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration, Cell Stem Cell 10(3) (2012) 259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ, Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow, Cell Stem Cell 15(2) (2014) 154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, Gross S, Renz BW, Setlik W, Martinez AN, Chen X, Nizami S, Lee HG, Kang HP, Caldwell JM, Asfaha S, Westphalen CB, Graham T, Jin G, Nagar K, Wang H, Kheirbek MA, Kolhe A, Carpenter J, Glaire M, Nair A, Renders S, Manieri N, Muthupalani S, Fox JG, Reichert M, Giraud AS, Schwabe RF, Pradere JP, Walton K, Prakash A, Gumucio D, Rustgi AK, Stappenbeck TS, Friedman RA, Gershon MD, Sims P, Grikscheit T, Lee FY, Karsenty G, Mukherjee S, Wang TC, Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential, Cell 160(1–2) (2015) 269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT, Identification and specification of the mouse skeletal stem cell, Cell 160(1–2) (2015) 285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schofield R, The relationship between the spleen colony-forming cell and the haemopoietic stem cell, Blood Cells 4(1–2) (1978) 7–25. [PubMed] [Google Scholar]
- [29].Chakkalakal JV, Jones KM, Basson MA, Brack AS, The aged niche disrupts muscle stem cell quiescence, Nature 490(7420) (2012) 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jones DL, Wagers AJ, No place like home: Anatomy and function of the stem cell niche, Nat Rev Mol Cell Biol 9(1) (2008) 11–21. [DOI] [PubMed] [Google Scholar]
- [31].Augello A, Kurth TB, De Bari C, Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches, Eur Cell Mater 20 (2010) 121–33. [DOI] [PubMed] [Google Scholar]
- [32].Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ, Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow, J Cell Sci 116(Pt 9) (2003) 1827–35. [DOI] [PubMed] [Google Scholar]
- [33].Shi S, Gronthos S, Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp, J Bone Miner Res 18(4) (2003) 696–704. [DOI] [PubMed] [Google Scholar]
- [34].Covas DT, Panepucci RA, Fontes AM, Silva WA Jr., Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA, Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts, Exp Hematol 36(5) (2008) 642–54. [DOI] [PubMed] [Google Scholar]
- [35].Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B, A perivascular origin for mesenchymal stem cells in multiple human organs, Cell Stem Cell 3(3) (2008) 301–13. [DOI] [PubMed] [Google Scholar]
- [36].Covas DT, Siufi JL, Silva AR, Orellana MD, Isolation and culture of umbilical vein mesenchymal stem cells, Braz J Med Biol Res 36(9) (2003) 1179–83. [DOI] [PubMed] [Google Scholar]
- [37].Covas DT, Piccinato CE, Orellana MD, Siufi JL, Silva WA Jr., Proto-Siqueira R, Rizzatti EG, Neder L, Silva AR, Rocha V, Zago MA, Mesenchymal stem cells can be obtained from the human saphena vein, Exp Cell Res 309(2) (2005) 340–4. [DOI] [PubMed] [Google Scholar]
- [38].Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL, Multilineage potential of cells from the artery wall, Circulation 108(20) (2003) 2505–10. [DOI] [PubMed] [Google Scholar]
- [39].Kuhn NZ, Tuan RS, Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis, J Cell Physiol 222(2) (2010) 268–77. [DOI] [PubMed] [Google Scholar]
- [40].Jiang Y, Cai Y, Zhang W, Yin Z, Hu C, Tong T, Lu P, Zhang S, Neculai D, Tuan RS, Ouyang HW, Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration, Stem Cells Transl Med 5(6) (2016) 733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang Y, Tuan RS, Origin and function of cartilage stem/progenitor cells in osteoarthritis, Nat Rev Rheumatol 11(4) (2015) 206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A, Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells, Biochem Biophys Res Commun 368(2) (2008) 305–10. [DOI] [PubMed] [Google Scholar]
- [43].Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B, The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells, Stem Cells Dev 21(8) (2012) 1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Souza LE, Malta TM, Kashima Haddad S, Covas DT, Mesenchymal stem cells and pericytes: To what extent are they related?, Stem Cells Dev 25(24) (2016) 1843–1852. [DOI] [PubMed] [Google Scholar]
- [45].Oja S, Komulainen P, Penttila A, Nystedt J, Korhonen M, Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures, Stem Cell Res Ther 9(1) (2018) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J, Age‐related intrinsic changes in human bone‐marrow‐derived mesenchymal stem cells and their differentiation to osteoblasts, Aging Cell 7(3) (2008) 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Martin N, Beach D, Gil J, Ageing as developmental decay: Insights from p16INK4a, Trends Mol Med 20(12) (2014) 667–674. [DOI] [PubMed] [Google Scholar]
- [48].Zhang D.-y., Wang H.-j., Tan Y.-z., Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway, PloS one 6(6) (2011) e21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhao J, Fuhrmann-Stroissnigg H, Gurkar AU, Flores RR, Dorronsoro A, Stolz DB, St Croix CM, Niedernhofer LJ, Robbins PD, Quantitative analysis of cellular senescence in culture and in vivo, Curr Protoc Cytom 79 (2017) 9 51 1–9 51 25. [DOI] [PubMed] [Google Scholar]
- [50].Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, Ren W, Guo H, Zhang L, Wang H, Chen Z, Guo AY, Li Q, Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells, Theranostics 7(10) (2017) 2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tome M, Sepulveda JC, Delgado M, Andrades JA, Campisi J, Gonzalez MA, Bernad A, miR-335 correlates with senescence/aging in human mesenchymal stem cells and inhibits their therapeutic actions through inhibition of AP-1 activity, Stem Cells 32(8) (2014) 2229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bellotti C, Capanni C, Lattanzi G, Donati D, Lucarelli E, Duchi S, Detection of mesenchymal stem cells senescence by prelamin A accumulation at the nuclear level, Springerplus 5(1) (2016) 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].McHugh D, Gil J, Senescence and aging: Causes, consequences, and therapeutic avenues, J Cell Biol 217(1) (2018) 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Baker N, Boyette LB, Tuan RS, Characterization of bone marrow-derived mesenchymal stem cells in aging, Bone 70 (2015) 37–47. [DOI] [PubMed] [Google Scholar]
- [55].Boyette LB, Tuan RS, Adult stem cells and diseases of aging, J Clin Med 3(1) (2014) 88–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA, Age-related changes in bone marrow mesenchymal stromal cells: A potential impact on osteoporosis and osteoarthritis development, Cell Transplant 26(9) (2017) 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Charif N, Li YY, Targa L, Zhang L, Ye JS, Li YP, Stoltz JF, Han HZ, de Isla N, Aging of bone marrow mesenchymal stromal/stem cells: Implications on autologous regenerative medicine, Biomed Mater Eng 28(s1) (2017) S57–S63. [DOI] [PubMed] [Google Scholar]
- [58].Brusnahan SK, McGuire TR, Jackson JD, Lane JT, Garvin KL, O’Kane BJ, Berger AM, Tuljapurkar SR, Kessinger MA, Sharp JG, Human blood and marrow side population stem cell and Stro-1 positive bone marrow stromal cell numbers decline with age, with an increase in quality of surviving stem cells: Correlation with cytokines, Mech Ageing Dev 131(11–12) (2010) 718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Maredziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM, The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells, Stem Cells Inter 2016 (2016) 2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim J, Ko J, A novel PPAR gamma(2) modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation, Cell Death Differ 21(10) (2014) 1642–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].De Becker A, Riet IV, Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy?, World J Stem Cells 8(3) (2016) 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kolf CM, Cho E, Tuan RS, Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation, Arthritis Res Ther 9(1) (2007) 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shao H, Xu Q, Wu Q, Ma Q, Salgueiro L, Wang J, Eton D, Webster KA, Yu H, Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration, J Cell Mol Med 15(10) (2011) 2046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rombouts WJ, Ploemacher RE, Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture, Leukemia 17(1) (2003) 160–70. [DOI] [PubMed] [Google Scholar]
- [65].Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK, Min JK, Oh DB, Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells, Biochem Biophys Res Commun 404(1) (2011) 463–9. [DOI] [PubMed] [Google Scholar]
- [66].Geissler S, Textor M, Kuhnisch J, Konnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN, Functional comparison of chronological and in vitro aging: Differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells, Plos One 7(12) (2012) e52700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liu M, Lei H, Dong P, Fu X, Yang Z, Yang Y, Ma J, Liu X, Cao Y, Xiao R, Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties, Cell Transplant 26(9) (2017) 1505–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lund TC, Kobs A, Blazar BR, Tolar J, Mesenchymal stromal cells from donors varying widely in age are of equal cellular fitness after in vitro expansion under hypoxic conditions, Cytotherapy 12(8) (2010) 971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sepulveda JC, Tome M, Fernandez ME, Delgado M, Campisi J, Bernad A, Gonzalez MA, Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model, Stem Cells 32(7) (2014) 1865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hofer HR, Tuan RS, Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies, Stem Cell Res Ther 7(1) (2016) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lunyak VV, Amaro-Ortiz A, Gaur M, Mesenchymal stem cells secretory responses: Senescence messaging secretome and immunomodulation perspective, Front Genet 8 (2017) 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].O’Hagan-Wong K, Nadeau S, Carrier-Leclerc A, Apablaza F, Hamdy R, Shum-Tim D, Rodier F, Colmegna I, Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells’ homeostasis, Oncotarget 7(12) (2016) 13285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kizilay Mancini O, Lora M, Shum-Tim D, Nadeau S, Rodier F, Colmegna I, A proinflammatory secretome mediates the impaired immunopotency of human mesenchymal stromal cells in elderly patients with atherosclerosis, Stem Cells Transl Med 6(4) (2017) 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A, Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells, Cell Death Dis 4 (2013) e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ozcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, Galderisi U, Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses, Aging (Albany NY) 8(7) (2016) 1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Luo X, Fu Y, Loza AJ, Murali B, Leahy KM, Ruhland MK, Gang M, Su X, Zamani A, Shi Y, Lavine KJ, Ornitz DM, Weilbaecher KN, Long F, Novack DV, Faccio R, Longmore GD, Stewart SA, Stromal-initiated changes in the bone promote metastatic niche development, Cell Rep 14(1) (2016) 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, Koizumi K, Morimoto T, Yoshikawa H, Hashimoto J, IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro, J Bone Miner Metab 32(4) (2014) 378–92. [DOI] [PubMed] [Google Scholar]
- [78].Zhu S, He H, Gao C, Luo G, Xie Y, Wang H, Tian L, Xiang C, Xijie Y, He C, Ovariectomy induced bone loss in TNFalpha and IL6 knockout mice is regulated by different mechanisms, J Mol Endocrinol 60(3) (2018) 185–198. [DOI] [PubMed] [Google Scholar]
- [79].Chiche A, Le Roux I, von Joest M, Sakai H, Aguin SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P, Tajbakhsh S, Li H, Injury-induced senescence enables in vivo reprogramming in skeletal muscle, Cell Stem Cell 20(3) (2017) 407–414 e4. [DOI] [PubMed] [Google Scholar]
- [80].Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, Inflamm-aging. An evolutionary perspective on immunosenescence, Ann N Y Acad Sci 908 (2000) 244–54. [DOI] [PubMed] [Google Scholar]
- [81].Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S, The pro- and anti-inflammatory properties of the cytokine interleukin-6, Biochim Biophys Acta 1813(5) (2011) 878–88. [DOI] [PubMed] [Google Scholar]
- [82].Bektas A, Schurman SH, Sen R, Ferrucci L, Aging, inflammation and the environment, Exp Gerontol (2017) DOI: 10.1016/j.exger.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N, Inflammation and frailty in the elderly: A systematic review and meta-analysis, Ageing Res Rev 31 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [84].Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS, Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism, J Cell Biochem 108(3) (2009) 577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xie Z, Tang S, Ye G, Wang P, Li J, Liu W, Li M, Wang S, Wu X, Cen S, Zheng G, Ma M, Wu Y, Shen H , Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells, Stem Cell Res Ther 9(1) (2018) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li H, Liu P, Xu S, Li Y, Dekker JD, Li B, Fan Y, Zhang Z, Hong Y, Yang G, Tang T, Ren Y, Tucker HO, Yao Z, Guo X, FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging, J Clin Invest 127(4) (2017) 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hurley MM, Gronowicz G, Zhu L, Kuhn LT, Rodner C, Xiao L, Age-related changes in FGF-2, fibroblast growth factor receptors and beta-catenin expression in human mesenchyme-derived progenitor cells, J Cell Biochem 117(3) (2016) 721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Baksh D, Boland GM, Tuan RS, Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation, J Cell Biochem 101(5) (2007) 1109–24. [DOI] [PubMed] [Google Scholar]
- [89].White BD, Nguyen NK, Moon RT, Wnt signaling: It gets more humorous with age, Curr Biol 17(21) (2007) R923–5. [DOI] [PubMed] [Google Scholar]
- [90].Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS, Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche, Stem Cells 26(8) (2008) 2042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Starr ME, Evers BM, Saito H, Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6, J Gerontol A Biol Sci Med Sci 64(7) (2009) 723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN, Aging up-regulates expression of inflammatory mediators in mouse adipose tissue, J Immunol 179(7) (2007) 4829–39. [DOI] [PubMed] [Google Scholar]
- [93].Stout MB, Justice JN, Nicklas BJ, Kirkland JL, Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty, Physiology (Bethesda) 32(1) (2017) 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, Positional cloning of the mouse obese gene and its human homologue, Nature 372(6505) (1994) 425–32. [DOI] [PubMed] [Google Scholar]
- [95].Palmer AK, Kirkland JL, Aging and adipose tissue: Potential interventions for diabetes and regenerative medicine, Exp Gerontol 86 (2016) 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL, JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age, Proc Natl Acad Sci U S A 112(46) (2015) E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL, Targeting senescent cells enhances adipogenesis and metabolic function in old age, Elife 4 (2015) e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M, Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis, Biogerontology 2(3) (2001) 165–71. [DOI] [PubMed] [Google Scholar]
- [99].Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA, Age related changes in bone marrow mesenchymal stromal cells: A potential impact on osteoporosis and osteoarthritis development, Cell Transplant 6(9) (2017) 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Csaki C, Matis U, Mobasheri A, Shakibaei M, Co-culture of canine mesenchymal stem cells with primary bone-derived osteoblasts promotes osteogenic differentiation, Histochem Cell Biol 131(2) (2009) 251–66. [DOI] [PubMed] [Google Scholar]
- [101].Glueck M, Gardner O, Czekanska E, Alini M, Stoddart MJ, Salzmann GM, Schmal H, Induction of osteogenic differentiation in human mesenchymal stem cells by crosstalk with osteoblasts, Biores Open Access 4(1) (2015) 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yu C, Li L, Xie F, Guo S, Liu F, Dong N, Wang Y, LncRNA TUG1 sponges miR-204–5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification, Cardiovasc Res 114(1) (2018) 168–179. [DOI] [PubMed] [Google Scholar]
- [103].Genetos DC, Zhou Z, Li Z, Donahue HJ, Age-related changes in gap junctional intercellular communication in osteoblastic cells, J Orthop Res 30(12) (2012) 1979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang D, Shen W, Zhang F, Chen M, Chen H, Cao K, Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart, Cell Biol Int 34(4) (2010) 415–23. [DOI] [PubMed] [Google Scholar]
- [105].Zhang X, Sun Y, Wang Z, Huang Z, Li B, Fu J, Up-regulation of connexin-43 expression in bone marrow mesenchymal stem cells plays a crucial role in adhesion and migration of multiple myeloma cells, Leuk Lymphoma 56(1) (2015) 211–8. [DOI] [PubMed] [Google Scholar]
- [106].Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Amedee J, Granja PL, Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells, Stem Cell Res 7(3) (2011) 186–97. [DOI] [PubMed] [Google Scholar]
- [107].Lozito TP, Kuo CK, Taboas JM, Tuan RS, Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix, J Cell Biochem 107(4) (2009) 714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G, Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries, FASEB J 17(9) (2003) 1183–5. [DOI] [PubMed] [Google Scholar]
- [109].Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ, Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function, Am J Physiol Heart Circ Physiol 313(5) (2017) H890–H895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD, Reconstitution of marrow-derived extracellular matrix ex vivo: A robust culture system for expanding large-scale highly functional human mesenchymal stem cells, Stem Cells Dev 19(7) (2010) 1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lin H, Yang G, Tan J, Tuan RS, Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential, Biomaterials 33(18) (2012) 4480–9. [DOI] [PubMed] [Google Scholar]
- [112].Sun Y, Li W, Lu Z, Chen R, Ling J, Ran Q, Jilka RL, Chen XD, Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix, FA SEB J 25(5) (2011) 1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stearns-Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C, Barchowsky A, Rando TA, Tuan RS, Ambrosio F, Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion, Aging Cell 16(3) (2017) 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126(4) (2006) 677–89. [DOI] [PubMed] [Google Scholar]
- [115].Winer JP, Janmey PA, McCormick ME, Funaki M, Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli, Tissue Eng Part A 15(1) (2009) 147–54. [DOI] [PubMed] [Google Scholar]
- [116].Stolzing A, Colley H, Scutt A, Effect of age and diabetes on the response of mesenchymal progenitor cells to fibrin matrices, Int J Biomater 2011 (2011) 378034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhang DY, Wang HJ, Tan YZ, Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway, PLoS One 6(6) (2011) e21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bickford PC, Kaneko Y, Grimmig B, Pappas C, Small B, Sanberg CD, Sanberg PR, Tan J, Douglas Shytle R, Nutraceutical intervention reverses the negative effects of blood from aged rats on stem cells, Age (Dordr) 37(5) (2015) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, Wei Q, Alman BA, Exposure to a youthful circulaton rejuvenates bone repair through modulation of beta-catenin, Nat Commun 6 (2015) 7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM, A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood, Nat Commun 7 (2016) 13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Murphy T, Thuret S, The systemic milieu as a mediator of dietary influence on stem cell function during ageing, Ageing Res Rev 19 (2015) 53–64. [DOI] [PubMed] [Google Scholar]
- [122].Damien CJ, Parsons JR, Bone graft and bone graft substitutes: A review of current technology and applications, J Appl Biomater 2(3) (1991) 187–208. [DOI] [PubMed] [Google Scholar]
- [123].Calvert JW, Weiss LE, Sundine MJ, New frontiers in bone tissue engineering, Clin Plast Surg 30(4) (2003) 641–8, x. [DOI] [PubMed] [Google Scholar]
- [124].Amini AR, Laurencin CT, Nukavarapu SP, Bone tissue engineering: Recent advances and challenges, Crit Rev Biomed Eng 40(5) (2012) 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Liao Y, Zhang XL, Li L, Shen FM, Zhong MK, Stem cell therapy for bone repair: A systematic review and meta-analysis of preclinical studies with large animal models, Br J Clin Pharmacol 78(4) (2014) 718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zaidi N, Nixon AJ, Stem cell therapy in bone repair and regeneration, Ann NY Aca Sci 1117 (2007) 62–72. [DOI] [PubMed] [Google Scholar]
- [127].Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC, Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells, Blood 105(3) (2005) 1068–77. [DOI] [PubMed] [Google Scholar]
- [128].Field JR, McGee M, Stanley R, Ruthenbeck G, Papadimitrakis T, Zannettino A, Gronthos S, Itescu S, The efficacy of allogeneic mesenchymal precursor cells for the repair of an ovine tibial segmental defect, Vet Comp Orthop Traumatol 24(2) (2011) 113–21. [DOI] [PubMed] [Google Scholar]
- [129].Aykan A, Ozturk S, Sahin I, Gurses S, Ural AU, Oren NC, Isik S, Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis, J Craniofac Surg 24(2) (2013) e169–75. [DOI] [PubMed] [Google Scholar]
- [130].Ai J, Ebrahimi S, Khoshzaban A, Jafarzadeh Kashi TS, Mehrabani D, Tissue engineering using human mineralized bone xenograft and bone marrow mesenchymal stem cells allograft in healing of tibial fracture of experimental rabbit model, Iran Red Crescent Med J 14(2) (2012) 96–103. [PMC free article] [PubMed] [Google Scholar]
- [131].Yan Z, Hang D, Guo C, Chen Z, Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head, J Orthop Res 27(4) (2009) 442–6. [DOI] [PubMed] [Google Scholar]
- [132].Niemeyer P, Schonberger TS, Hahn J, Kasten P, Fellenberg J, Suedkamp N, Mehlhorn AT, Milz S, Pearce S, Xenogenic transplantation of human mesenchymal stem cells in a critical size defect of the sheep tibia for bone regeneration, Tissue Eng A 16(1) (2010) 33–43. [DOI] [PubMed] [Google Scholar]
- [133].Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL, Stem cells from deciduous tooth repair mandibular defect in swine, J Dent Res 88(3) (2009) 249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bueno DF, Kerkis I, Costa AM, Martins MT, Kobayashi GS, Zucconi E, Fanganiello RD, Salles FT, Almeida AB, do Amaral CE, Alonso N, Passos-Bueno MR, New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients, Tissue Eng A 15(2) (2009) 427–35. [DOI] [PubMed] [Google Scholar]
- [135].Gao X, Usas A, Proto JD, Lu A, Cummins JH, Proctor A, Chen CW, Huard J, Role of donor and host cells in muscle-derived stem cell-mediated bone repair: Differentiation vs. paracrine effects, FASEB J 28(8) (2014) 3792–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ogata K, Katagiri W, Hibi H, Secretomes from mesenchymal stem cells participate in the regulation of osteoclastogenesis in vitro, Clin Oral Investig 21(6) (2017) 1979–1988. [DOI] [PubMed] [Google Scholar]
- [137].Linero I, Chaparro O, Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration, PloS one 9(9) (2014) e107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kuroda K, Kabata T, Hayashi K, Maeda T, Kajino Y, Iwai S, Fujita K, Hasegawa K, Inoue D, Sugimoto N, Tsuchiya H, The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression, BMC Musculoskelet Disord 16 (2015) 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Liao HT, Chen CT, Chen CH, Chen JP, Tsai JC, Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction, J trauma 70(1) (2011) 228–37. [DOI] [PubMed] [Google Scholar]
- [140].Peng J, Wen C, Wang A, Wang Y, Xu W, Zhao B, Zhang L, Lu S, Qin L, Guo Q, Dong L, Tian J, Micro-CT-based bone ceramic scaffolding and its performance after seeding with mesenchymal stem cells for repair of load-bearing bone defect in canine femoral head, J Biomed Mater Res B Appl Biomater 96(2) (2011) 316–25. [DOI] [PubMed] [Google Scholar]
- [141].Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y, Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model, Biomaterials 28(36) (2007) 5477–86. [DOI] [PubMed] [Google Scholar]
- [142].Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T, Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration, Tissue Eng 10(5–6) (2004) 955–64. [DOI] [PubMed] [Google Scholar]
- [143].Yuan J, Cui L, Zhang WJ, Liu W, Cao Y, Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate, Biomaterials 28(6) (2007) 1005–13. [DOI] [PubMed] [Google Scholar]
- [144].Lucarelli E, Fini M, Beccheroni A, Giavaresi G, Di Bella C, Aldini NN, Guzzardella G, Martini L, Cenacchi A, Di Maggio N, Sangiorgi L, Fornasari PM, Mercuri M, Giardino R, Donati D, Stromal stem cells and platelet-rich plasma improve bone allograft integration, Clin Orthop Relat Res (435) (2005) 62–8. [DOI] [PubMed] [Google Scholar]
- [145].Zhou H, Xiao C, Wang Y, Bi X, Ge S, Fan X, In vivo efficacy of bone marrow stromal cells coated with beta-tricalcium phosphate for the reconstruction of orbital defects in canines, Invest Ophthalmol Vis Sci 52(3) (2011) 1735–41. [DOI] [PubMed] [Google Scholar]
- [146].Wang L, Fan H, Zhang ZY, Lou AJ, Pei GX, Jiang S, Mu TW, Qin JJ, Chen SY, Jin D, Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized beta-tricalcium phosphate scaffold and mesenchymal stem cells, Biomaterials 31(36) (2010) 9452–61. [DOI] [PubMed] [Google Scholar]
- [147].Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J, VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis, J Bone Miner Res 20(11) (2005) 2017–27. [DOI] [PubMed] [Google Scholar]
- [148].Usas A, Ho AM, Cooper GM, Olshanski A, Peng H, Huard J, Bone regeneration mediated by BMP4-expressing muscle-derived stem cells is affected by delivery system, Tissue Eng A 15(2) (2009) 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Li H, Dai K, Tang T, Zhang X, Yan M, Lou J, Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2, Biochem Biophys Res Commun 356(4) (2007) 836–42. [DOI] [PubMed] [Google Scholar]
- [150].Ren ML, Peng W, Yang ZL, Sun XJ, Zhang SC, Wang ZG, Zhang B, Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs, Cell Transplant 21(12) (2012) 2711–21. [DOI] [PubMed] [Google Scholar]
- [151].Lyons FG, Al-Munajjed AA, Kieran SM, Toner ME, Murphy CM, Duffy GP, O’Brien FJ, The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs, Biomaterials 31(35) (2010) 9232–43. [DOI] [PubMed] [Google Scholar]
- [152].Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF, Mesenchymal stem cells: A new trend for cell therapy, Acta Pharm Sin 34(6) (2013) 747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Goodell MA, Rando TA, Stem cells and healthy aging, Science 350(6265) (2015) 1199–204. [DOI] [PubMed] [Google Scholar]
- [154].D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA, Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow, J Bone Miner Res 14(7) (1999) 1115–22. [DOI] [PubMed] [Google Scholar]
- [155].Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S, Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination, J Neurosci 24(38) (2004) 8354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, Rejuvenation of aged progenitor cells by exposure to a young systemic environment, Nature 433(7027) (2005) 760–4. [DOI] [PubMed] [Google Scholar]
- [157].Warren LA, Rossi DJ, Stem cells and aging in the hematopoietic system, Mech Ageing Dev 130(1–2) (2009) 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Piccin D, Morshead CM, Potential and pitfalls of stem cell therapy in old age, Dis Model Mech 3(7–8) (2010) 421–5. [DOI] [PubMed] [Google Scholar]
- [159].Sharpless NE, DePinho RA, How stem cells age and why this makes us grow old, Nat Rev Mol Cell Biol 8(9) (2007) 703–13. [DOI] [PubMed] [Google Scholar]
- [160].Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ, Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing, Nature 443(7110) (2006) 448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT, Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a, Nature 443(7110) (2006) 421–6. [DOI] [PubMed] [Google Scholar]
- [162].Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I, Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion, Stem Cells 22(5) (2004) 675–82. [DOI] [PubMed] [Google Scholar]
- [163].Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM, Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice, Cell 143(7) (2010) 1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ, Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging, Cell 169(1) (2017) 132–147 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA, Dev Cell 31(6) (2014) 722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D, Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice, Nat Med 22(1) (2016) 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Asumda FZ, Age-associated changes in the ecological niche: Implications for mesenchymal stem cell aging, Stem Cell Res Ther 4(3) (2013) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Oh J, Lee YD, Wagers AJ, Stem cell aging: Mechanisms, regulators and therapeutic opportunities, Nat Med 20(8) (2014) 870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Wong TY, Solis MA, Chen YH, Huang LL, Molecular mechanism of extrinsic factors affecting anti-aging of stem cells, World J Stem Cells 7(2) (2015) 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T, The ageing systemic milieu negatively regulates neurogenesis and cognitive function, Nature 477(7362) (2011) 90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Biressi S, Miyabara EH, Gopinath SD, Carlig PM, Rando TA, A Wnt-TGFbeta2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice, Sci Transl Med 6(267) (2014) 267ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA, Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis, Science 317(5839) (2007) 807–10. [DOI] [PubMed] [Google Scholar]
- [173].Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ, Age-related changes in osteogenic stem cells in mice, J Bone Miner Res 11(5) (1996) 568–77. [DOI] [PubMed] [Google Scholar]
- [174].Murphy CM, Haugh MG, O’Brien FJ, The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering, Biomaterials 31(3) (2010) 461–466. [DOI] [PubMed] [Google Scholar]
- [175].Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB, Bone structure and formation: A new perspective, Mat Sci Eng R 58(3) (2007) 77–116. [Google Scholar]
- [176].Woodard JR, Hilldore AJ, Lan SK, Park C, Morgan AW, Eurell JAC, Clark SG, Wheeler MB, Jamison RD, Johnson AJW, The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity, Biomaterials 28(1) (2007) 45–54. [DOI] [PubMed] [Google Scholar]
- [177].Bose S, Roy M, Bandyopadhyay A, Recent advances in bone tissue engineering scaffolds, Trend Biotech 30(10) (2012) 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Barradas A, Yuan H, Blitterswijk CA, Habibovic P, Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms, Eur Cell Mater 21 (2011) 407–429. [DOI] [PubMed] [Google Scholar]
- [179].Papadimitropoulos A, Scotti C, Bourgine P, Scherberich A, Martin I, Engineered decellularized matrices to instruct bone regeneration processes, Bone 70 (2015) 66–72. [DOI] [PubMed] [Google Scholar]
- [180].Zimmermann G, Moghaddam A, Allograft bone matrix versus synthetic bone graft substitutes, Injury 42 (2011) S16–S21. [DOI] [PubMed] [Google Scholar]
- [181].Martino MM, Briquez PS, Maruyama K, Hubbell JA, Extracellular matrix-inspired growth factor delivery systems for bone regeneration, Adv Drug Deliv Rev 94 (2015) 41–52. [DOI] [PubMed] [Google Scholar]
- [182].Urist MR, Bone: formation by autoinduction, Science 150(3698) (1965) 893–899. [DOI] [PubMed] [Google Scholar]
- [183].Thibault RA, Baggett L. Scott, Mikos AG, Kasper FK, Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements, Tissue Eng A 16(2) (2009) 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Sadr N, Pippenger BE, Scherberich A, Wendt D, Mantero S, Martin I, Papadimitropoulos A, Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix, Biomaterials 33(20) (2012) 5085–5093. [DOI] [PubMed] [Google Scholar]
- [185].Ramay HR, Zhang M, Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering, Biomaterials 25(21) (2004) 5171–5180. [DOI] [PubMed] [Google Scholar]
- [186].Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA, 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration, Biomaterials 35(13) (2014) 4026–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Alvarez K, Nakajima H, Metallic scaffolds for bone regeneration, Materials 2(3) (2009) 790–832. [Google Scholar]
- [188].Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth C, Windhagen H, In vivo corrosion of four magnesium alloys and the associated bone response, Biomaterials 26(17) (2005) 3557–3563. [DOI] [PubMed] [Google Scholar]
- [189].Spoerke ED, Murray NG, Li H, Brinson LC, Dunand DC, Stupp SI, A bioactive titanium foam scaffold for bone repair, Acta Biomater 1(5) (2005) 523–533. [DOI] [PubMed] [Google Scholar]
- [190].Hutmacher DW, Scaffolds in tissue engineering bone and cartilage, Biomaterials 21(24) (2000) 2529–2543. [DOI] [PubMed] [Google Scholar]
- [191].Liu X, Ma PX, Polymeric scaffolds for bone tissue engineering, Ann Biomed Eng 32(3) (2004) 477–486. [DOI] [PubMed] [Google Scholar]
- [192].Park J-B, The use of hydrogels in bone-tissue engineering, Med Oral Patol Oral Cir Bucal 16(1) (2011) e115–8. [DOI] [PubMed] [Google Scholar]
- [193].Gkioni K, Leeuwenburgh SC, Douglas TE, Mikos AG, Jansen JA, Mineralization of hydrogels for bone regeneration, Tissue Eng B 16(6) (2010) 577–585. [DOI] [PubMed] [Google Scholar]
- [194].Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG, The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening, Biomaterials 31(19) (2010) 5051–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Shen H, Ma Y, Luo Y, Liu X, Zhang Z, Dai J, Directed osteogenic differentiation of mesenchymal stem cell in three-dimensional biodegradable methylcellulose-based scaffolds, Colloid Surface B 135 (2015) 332–338. [DOI] [PubMed] [Google Scholar]
- [196].Bose S, Vahabzadeh S, Bandyopadhyay A, Bone tissue engineering using 3D printing, Mater Today 16(12) (2013) 496–504. [Google Scholar]
- [197].Becker ST, Bolte H, Schünemann K, Seitz H, Bara J, Beck-Broichsitter B, Russo P, Wiltfang J, Warnke PH, Endocultivation: the influence of delayed vs. simultaneous application of BMP-2 onto individually formed hydroxyapatite matrices for heterotopic bone induction, Int J Oral Maxillofac Surg 41(9) (2012) 1153–1160. [DOI] [PubMed] [Google Scholar]
- [198].Christenson EM, Anseth KS, van den Beucken JJ, Chan CK, Ercan B, Jansen JA, Laurencin CT, Li WJ, Murugan R, Nair LS, Nanobiomaterial applications in orthopedics, J Orthop Res 25(1) (2007) 11–22. [DOI] [PubMed] [Google Scholar]
- [199].Kim J, Kim HN, Lim K-T, Kim Y, Seonwoo H, Park SH, Lim HJ, Kim D-H, Suh K-Y, Choung P-H, Designing nanotopographical density of extracellular matrix for controlled morphology and function of human mesenchymal stem cells, Sci Rep 3 (2013) 3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Kim J, Bae W-G, Choung H-W, Lim KT, Seonwoo H, Jeong HE, Suh K-Y, Jeon NL, Choung P-H, Chung JH, Multiscale patterned transplantable stem cell patches for bone tissue regeneration, Biomaterials 35(33) (2014) 9058–9067. [DOI] [PubMed] [Google Scholar]
- [201].Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO, The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder, Nat Mater 6(12) (2007) 997–1003. [DOI] [PubMed] [Google Scholar]
- [202].Kim M-J, Lee B, Yang K, Park J, Jeon S, Um SH, Kim D-I, Im SG, Cho S-W, BMP-2 peptide-functionalized nanopatterned substrates for enhanced osteogenic differentiation of human mesenchymal stem cells, Biomaterials 34(30) (2013) 7236–7246. [DOI] [PubMed] [Google Scholar]
- [203].Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D, Fiber-based tissue engineering: Progress, challenges, and opportunities, Biotech Adv 31(5) (2013) 669–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Khajavi R, Abbasipour M, Bahador A, Electrospun biodegradable nanofibers scaffolds for bone tissue engineering, J Appl Polym Sci 133(3) (2016) 42883. [Google Scholar]
- [205].Li W-J, Tuli R, Huang X, Laquerriere P, Tuan RS, Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold, Biomaterials 26(25) (2005) 5158–5166. [DOI] [PubMed] [Google Scholar]
- [206].Yang X, Chen X, Wang H, Acceleration of osteogenic differentiation of preosteoblastic cells by chitosan containing nanofibrous scaffolds, Biomacromolecules 10(10) (2009) 2772–2778. [DOI] [PubMed] [Google Scholar]
- [207].Fujihara K, Kotaki M, Ramakrishna S, Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers, Biomaterials 26(19) (2005) 4139–4147. [DOI] [PubMed] [Google Scholar]
- [208].Jang J-H, Castano O, Kim H-W, Electrospun materials as potential platforms for bone tissue engineering, Adv Drug Delivery Rev 61(12) (2009) 1065–1083. [DOI] [PubMed] [Google Scholar]
- [209].Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL, Electrospun silk-BMP-2 scaffolds for bone tissue engineering, Biomaterials 27(16) (2006) 3115–3124. [DOI] [PubMed] [Google Scholar]
- [210].Chu B, Liang D, Hadjiargyrou M, Hsiao BS, A new pathway for developing in vitro nanostructured non-viral gene carriers, J Phys Condens Mat 18(36) (2006) S2513. [Google Scholar]
- [211].Yi C, Liu D, Fong C-C, Zhang J, Yang M, Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway, ACS Nano 4(11) (2010) 6439–6448. [DOI] [PubMed] [Google Scholar]
- [212].Henstock JR, Rotherham M, Rashidi H, Shakesheff KM, El Haj AJ, Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: Applications for injectable cell therapy, Stem Cell Transl Med 3(11) (2014) 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Shen H, Zhang L, Liu M, Zhang Z, Biomedical applications of graphene, Theranostics 2(3) (2012) 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Luo Y, Shen H, Fang Y, Cao Y, Huang J, Zhang M, Dai J, Shi X, Zhang Z, Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly (lactic-co-glycolic acid) nanofibrous mats, ACS Appl Mater Interface 7(11) (2015) 6331–6339. [DOI] [PubMed] [Google Scholar]
- [215].Kim S, Ku SH, Lim SY, Kim JH, Park CB, Graphene–biomineral hybrid materials, Adv Mater 23(17) (2011) 2009–2014. [DOI] [PubMed] [Google Scholar]
- [216].Zhang T, Li N, Li K, Gao R, Gu W, Wu C, Su R, Liu L, Zhang Q, Liu J, Enhanced proliferation and osteogenic differentiation of human mesenchymal stem cells on biomineralized three-dimensional graphene foams, Carbon 105 (2016) 233–243. [Google Scholar]
- [217].Madeddu P, Therapeutic angiogenesis and vasculogenesis for tissue regeneration, Exp Physiol 90(3) (2005) 315–326. [DOI] [PubMed] [Google Scholar]
- [218].Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ, Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell‐driven bone regeneration, J Bone Miner Res 20(5) (2005) 848–857. [DOI] [PubMed] [Google Scholar]
- [219].Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ, Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration, Biomaterials 30(14) (2009) 2816–2825. [DOI] [PubMed] [Google Scholar]
- [220].Kang Y, Kim S, Fahrenholtz M, Khademhosseini A, Yang Y, Osteogenic and angiogenic potentials of monocultured and co-cultured human-bone-marrow-derived mesenchymal stem cells and human-umbilical-vein endothelial cells on three-dimensional porous beta-tricalcium phosphate scaffold, Acta biomater 9(1) (2013) 4906–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Mammadov R, Mammadov B, Toksoz S, Aydin B, Yagci R, Tekinay AB, Guler MO, Heparin mimetic peptide nanofibers promote angiogenesis, Biomacromolecules 12(10) (2011) 3508–3519. [DOI] [PubMed] [Google Scholar]
- [222].Shirley D, Marsh D, Jordan G, McQuaid S, Li G, Systemic recruitment of osteoblastic cells in fracture healing, J Orthop Res 23(5) (2005) 1013–21. [DOI] [PubMed] [Google Scholar]
- [223].Liu X, Liao X, Luo E, Chen W, Bao C, Xu HH, Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation, Tissue Eng A 20(3–4) (2014) 883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE, Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass, Nat Med 18(3) (2012) 456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Diederichs S, Tuan RS, Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor, Stem Cells Dev 23(14) (2014) 1594–610. [DOI] [PMC free article] [PubMed] [Google Scholar]