Abstract
Lung carcinoids (LCs) are rare and slow growing primary lung neuroendocrine tumors. We performed targeted exome sequencing, mRNA sequencing and DNA methylation array analysis on macro-dissected lung carcinoids. Recurrent mutations were enriched for genes involved in covalent histone modification/chromatin remodeling (34.5%; MEN1, ARID1A, KMT2C and KMT2A) as well as DNA repair (17.2%) pathways. Unsupervised clustering and principle component analysis on gene expression and DNA methylation profiles showed three robust molecular subtypes (LC1, LC2, LC3) with distinct clinical features. MEN1 gene mutations were found to be exclusively enriched in the LC2 subtype. LC1 and LC3 subtypes were predominately found at peripheral and endobronchial lung respectively. The LC3 subtype was diagnosed at a younger age than LC1 and LC2 subtypes. Immunohistochemical staining of two biomarkers, ASCL1 and S100, sufficiently stratified the three subtypes. This molecular classification of lung carcinoids into three subtypes may facilitate understanding of their molecular mechanisms and improve diagnosis and clinical management.
Introduction
Lung carcinoids are an indolent and rare type of primary lung neoplasms that are, in general, understudied. The 2015 World Health Organization(1) (WHO) classification of lung carcinoids (LCs) includes atypical carcinoids (AC) (~0.2% prevalence) and typical carcinoids (TC) (~2% prevalence). TCs are slow growing tumors that rarely spread beyond the lungs while ACs are faster growing tumors and have a greater chance of metastasizing to other tissues(2). The WHO classification relies mainly on morphology, proliferation rate (mitotic index) and necrosis assessment(3). This current method of classification has its drawbacks as studies have shown that the reproducibility of cancer classification and its prognostic efficacy have high inter-observer variability(3,4), especially for differentiating between TC and AC(5). Recent WHO classifications highlight use of the Ki67 cell proliferation marker to distinguish ACs from TCs(1). However, overlapping distribution of Ki67 between ACs and TCs does not enable reliable stratification between well-differentiated lung carcinoids,(6,7). It has also been reported that TCs and ACs are over-diagnosed as small cell lung carcinomas (SCLC) in small crush biopsy specimens(8), a situation where artifacts in specimens appear as bluish clusters in which cellular details are not recognizable. As SCLCs are highly malignant, incorrect diagnosis of TC and AC tumors as SCLC can subject patients to unnecessary stress and treatment(8). More accurate molecular diagnostic tools and stratification for lung carcinoids will help ensure more appropriate treatment and clinical management.
Previous genomic analysis of lung carcinoid tumors has identified recurrent mutations in MEN1, PSIP1, and ARID1A(9), while no significant mutations or focal copy alterations were observed in genes that are frequently mutated in non-small cell lung cancer (NSCLC), large cell neuroendocrine carcinoma (LCNEC) and SCLC (e.g. KRAS, TP53, EGFR and RB1)(10). The different mutation spectrum and low mutation burden(9) of lung carcinoids indicate they are distinct from NSCLC and high-grade lung neuroendocrine tumors (NETs). It is not known if there are distinct molecular subtypes of lung carcinoids or their cells of origin.
In this study, we performed genotyping on 29 LCs to detect mutations in a 354-cancer gene panel, mRNA sequencing (n=30) and DNA methylation 450K-array analysis (n=18) and identified three molecular subtypes with distinct clinical features. We also identified two key biomarkers (ASCL1 and S100) to stratify these subtypes. Integration of genetic and epigenetic signatures distinguishes each subtype of carcinoid, providing deeper insight into their distinctive molecular signatures of tumorigenesis as well as cells of origin.
Materials and Methods
Patient’s data
Retrospective and prospective reviews of 30 lung carcinoid neoplasms were performed using the pathology files and institutional database at Memorial Sloan Kettering Cancer Center (MSKCC, New York). All studies were conducted in accordance with appropriate ethical guidelines (following U.S Common Rule) and with IRB approval. Written informed consent was obtained from the patients. All patients were evaluated clinically with confirmed pathologic diagnoses, appropriate radiological and laboratory studies, and surgical or oncological management. Fresh-frozen tumor and paired normal tissues were obtained from MSKCC’s tissue bank under IRB protocol. Targeted cancer gene panel DNA sequencing, RNAseq and DNA methylation array were performed on fresh-frozen samples. Relevant clinical and pathologic information is presented in Supplementary File 1.
DNA sequencing and analysis
DNA extraction from microdissected tumor samples and normal adjacent tissues was performed using a commercially available DNA extraction kit (DNeasy Blood and Tissue Kit, cat. 69504, Qiagen), according to the manufacturer’s protocol. Targeted sequencing on 29 LCs was performed using MSK-IMPACT(11) hybrid capture cancer gene panel (n=354). Single nucleotide variants and short indels (<30bp) were identified and annotated using MSK-IMPACT pipeline(11). Briefly, raw reads were filtered based on quality, mapped to NCBI b37 genome using BWA–MEM version 0.7.5a (http://arxiv.org/abs/1303.3997), post-processed using GATK(12) and variant identification using MuTect(13). Variants were filtered based on its entry in NCBI-dbSNPs (http://www.ncbi.nlm.nih.gov/snp), 1000G project (http://www.1000genomes.org/) and COSMIC (http://cancer.sanger.ac.uk/cosmic). Filtered variants were manually reviewed on IGV. We created the mutational OncoPrint plot for our 29 LCs dataset using the online cBioPortal website (http://www.cbioportal.org/oncoprinter.jsp).
RNA sequencing and data analysis
Total RNA extraction from microdissected frozen tumor samples was performed using RNeasy Mini Kit (cat. 74106) followed by Illumina HiSeq sequencing (2 × 100 bp). We performed RNAseq data analysis as previously described(14),(15). Briefly, raw fastq files were examined for sequencing quality control using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). Sequencing reads were mapped to human transcripts corresponding to hg19 Genepattern(16) GTF annotations using RSEM(17) with default parameters. STAR(18) aligner was used to map reads in RSEM algorithm followed by calculating gene expression from mapped BAM files. Gene transcripts mapped data were normalized to TPM (Transcript Per Million). We used log2(TPM+1) values for all downstream analysis. For unsupervised clustering, we used Pearson distance metric and hclust (ward.D2) method (unless stated otherwise). PCA was done using prcomp in R. R (http://www.r-project.org/) was used for all analysis and visualization of data. The R package DeSeq2(19) was used to identify differentially expressed genes using Benjamini & Hochberg (BH) corrected p-value (< 0.05) and fold change of >=2. We used DAVID bioinformatics resources (20) version 6.8 and Gene Set Enrichment Analysis (GSEA) (21) (including PreRanked test) to find significant pathways, gene ontology terms and transcription factor motif analysis with default parameter.
Independent Dataset of Lung Carcinoids
To validate our novel molecular subtyping, we used gene expression and mutation data from independent lung carcinoids (n=65) dataset. The gene expression and mutational data was downloaded from supplement data files (https://www.nature.com/articles/ncomms4518#supplementary-information) reported in ref.9. This gene expression data was reported as transcript expression instead of gene expression. We used callapseRows(22) on transcript expression to convert to respective gene expression using MaxVariance option. Gene expression for LCs signature (top 100 variant genes from our 30 LCs dataset) was extracted from this RNAseq dataset. Unsupervised clustering and PCA analysis on this dataset were performed as previously described for our 30 LCs dataset.
DNA methylation and analysis
DNA were extracted from LC samples and interrogated for CpG DNA methylation using the Illumina 450K array platform (Illumina Inc. San Diego, CA). For CpG DNA methylation data analysis, we used ChAMP(23) package in R with default parameters. Briefly, IDAT raw data files were imported in R and filtered to exclude samples with detection p-value <0.01 and beadcount <3 in at least 5% of samples and normalized using FunctionNormalization (24). Additional filtering was performed to remove probes that have annotated SNPs, present on X and Y chromosome, and have CpG probes mapped to multiple locations. Beta values for 413,176 probes were used for all subsequent analysis. Subtype specific differentially methylated CpG probes (DMP) and CpG island were identified using COHCAP(25) (default parameter). For DMP, we focused on probes present at TSS1500/200 and first exon for subsequent analysis. We integrated gene expression and DNA methylation to investigate subtype specific gene expression using default parameter for COHCAP.integrate.avg.by.island with FDR p-value <0.01.
Immunohistochemical staining
Immunohistochemical staining was performed using commercially available antibodies at optimal dilutions as follows: ASCL1 (a-MASH1) [monoclonal, 1:300, BD] and S100 [monoclonal, 1:4000, BG]. Briefly, immunohistochemistry was performed by a standard protocol on Ventana Discovery XT automated stainer (Ventana Medical Systems Inc, Tucson, AZ) for the S100 antibody. Antigen retrieval was performed with Cell Conditioning 1 buffer (CC1; citrate buffer pH 6.0, Ventana). For the ASCL1 (a-MASH1) antibody, ER2 pre-treatment was used and the Leica Bondmax autostainer (Leica, Buffalo, IL). Immunoreactivity was performed on whole tissue sections of formalin-fixed and paraffin-embedded tissue section as well as on tissue microarray (TMA) constructed from 173 pulmonary carcinoid tumors formalin-fixed, paraffin-embedded tumor specimens were used for TMA construction. Briefly, four representative tumor areas were marked on H&E-stained slides, and cylindrical 0.6-mm tissue cores were arrayed from the corresponding paraffin blocks into a recipient block using an automated tissue arrayer ATA-27 (Beecher Instruments, Sun Prairie, WI, USA). From each tissue microarray, 4-μm-thick paraffin sections were prepared for immunohistochemistry. In all, 173 cases with adequate cores were available for immunohistochemical analysis(26). The results were semi-quantitatively scored based on the percentage of reactive tumor cells (≥ 25% tumor cells) and the intensity of staining.
Data Availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary data and figures. Data generated in this study were deposited to NCBI under GEO SuperSeries GSE118133 (GSE118131 for RNAseq and GSE118132 for 450 K methylation). We do not impose any restrictions on data availability.
Results
Patient cohort, clinical annotations and mutational profile of lung carcinoids
We analyzed 30 randomly selected and histologically confirmed, well-differentiated LCs (17 TCs and 13 ACs) comprising the discovery dataset. Most specimens were from pulmonary lobectomy with lymph node detection. Tumor locations, i.e. peripheral versus central (endobronchial), were assessed by combination of radiographic reveal and pathologic observations. Fifty-four percent (7/13) of ACs had either lymph node or distant metastasis while 6% (1/17) of TCs had local lymph node metastasis. The 5-year disease specific survival was 89% and 55% for TC and AC, respectively. Clinical information and features are presented in Supplementary File 1. In addition, a tissue microarray (TMA) containing 173 cases of lung carcinoids had been prepared previously(26) and used for study of clinical correlates.
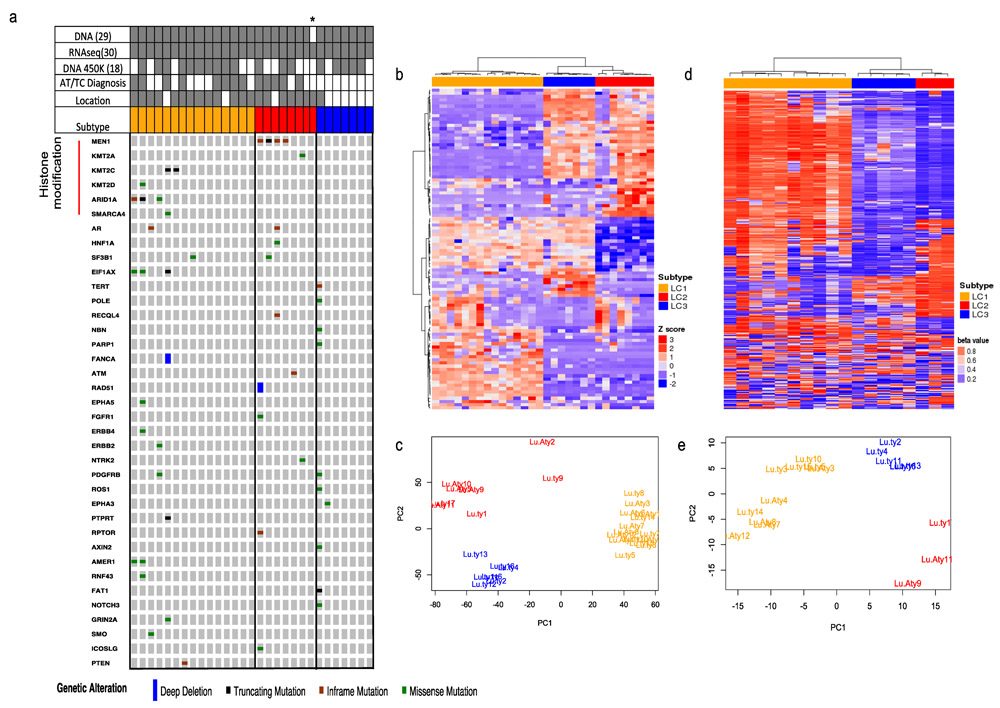
We performed targeted sequencing of a 354-cancer gene panel (MSK-IMPACT(11)) on 29 LCs from the discovery dataset. The mutated genes were enriched for those implicated in covalent histone modification/chromatin remodeling and found in 10 samples (MEN1 (13.8%), ARID1A (10%), KMT2A (3%), KMT2C (7%), KMT2D (3%) and SMARCA4 (3%)) reproducing the results from a previous study(9). We also found mutations in DNA repair pathways (17.2% of samples) (Figure 1a) (Supplementary File 2). Mutations were not detected in the 354-cancer gene panel for 13 LC samples. Mutations in MEN1, the most frequently mutated gene, were found in four samples (4 ACs) and four of these mutations had variant allele frequencies higher than 70% indicating loss of heterozygosity (LOH) (Supplementary Figure 1). One sample (Lu-Aty9) has two MEN1 mutations (an in-frame deletion and a missense substitution) a few bases apart on the same copy of MEN1 (Supplementary File 2). The ARID1A gene is mutated in three samples with LOH occurring in one of the three samples. Using variant allele frequencies and LOH status of MEN1 and ARID1A, we found median tumor purity to be 91% (Supplemental File 1), consistent with our pathology based estimates (Supplementary Table 1). In addition to MEN1, other genes encoding SET1/MLL complex proteins are also found mutated (KMT2A (3%), KMT2C (7%), KMT2D (3%)). One sample (Lu-ty4) has the highest number of mutations (nine) including mutations in POLE (DNA polymerase B domain: V1016M), ROS1, FAT1, NBN, PARP1, and TERT (in-frame deletion close to Telomerase RBD). We found homozygous deletions only in the FANCA and RAD51 genes in two different ACs. The most recurrent CNV are single copy deletions in FANCA (17%), FAT1 (10%), MEN1 (7%), ATM (17%), SDHD (17%), and CHEK1 (17%), many of which reside on chr11q. We did not observe changes in the transcription levels of these genes with hemizygous deletions in comparison to wild type samples. There are 18 samples (4 ACs and 14 TCs) with normal karyotype, 6 samples (4 ACs and 2 TCs) with nearly normal karyotype (aneuploid for only one or two different chromosomes), and 6 samples (5 ACs and 1 TCs) with aneuploidy in more than two different chromosomes in our dataset (Supplementary File 2). We did not find any known pathogenic germline mutations in the panel of cancer-associated genes in our samples. TP53 and RB1 genes were not mutated in this cohort, unlike high-grade lung NETs and SCLC.
Figure 1. Three novel molecular subtypes of lung carcinoids with mutational, gene expression and DNA CpG methylation with distinct clinical features.
a) Mutated genes in lung carcinoids on a 354-cancer gene panel. Samples summary from DNA (n=29), methylation (n=18), RNAseq (n=30), carcinoids samples (atypical (n=13, grey) and typical (n=17, white)) and specimen location (endobronchial in white (n=9) and peripheral in grey (n=21)). Samples are grouped according to their gene expression and DNA methylation pattern. Orange = subtype 1 (LC1), Red = subtype 2 (LC2), Blue = subtype 3 (LC3). Column represents sample and row represents gene name. Gene expression (n=30) and DNA CpG methylation (n=18) analysis revealed three lung carcinoid subtypes using unsupervised clustering and PCA analysis: b) Heatmap of unsupervised clustering of top 100 variably expressed gene across all samples. c) PCA of top 3000 variably expressed genes. d) Heatmap of unsupervised clustering of top 500 variable methylated CpG probes. e) PCA on top 3000 variable methylated CpG probes.
Transcriptome and methylome profiles reveal three distinct subtypes
We performed RNA sequencing on 30 LCs (13 ACs and 17 TCs) and DNA methylation analysis on 18 LCs (12 of the 30 samples did not have sufficient material for analysis) from the discovery dataset. Unsupervised clustering and principal component analysis (PCA) on the top 3000 variable genes showed three distinct clusters (Figure 1b and 1c). These clusters are robust when different numbers of top variable genes were used for clustering (Supplementary Figure 2). We named these subtypes LC1, LC2 and LC3. Heatmap of pearson correlation on top 3000 variable genes shows three blocks representing the three evident subtypes (Supplementary Figure 3). The top 100 variable genes (Supplementary Figure 4) across all LCs show enrichment for gene ontologies related to hormonal secretions, endogenous stimulus, wound healing and developmental processes (Supplementary Table 2). Gene expression analysis revealed greater similarity between LC2 and LC3 as compared to LC1 (Supplementary Figure 3 and 4).
We investigated the DNA methylation profiles of LCs (n=18), using the Illumina 450K microarray. Unsupervised clustering and principal component analysis of the top 3000 variable CpG sites revealed three distinct subtypes in complete agreement with the gene expression based subtypes (Figure 1d and 1e). Consistent with gene expression, we also observed greater similarity of DNA methylation levels for LC2 and LC3 subtypes when compared to LC1. The three grouping of subtypes was robust and reproducible using different numbers of top variable CpG sites (Supplementary Figure 5). Total genome-wide DNA methylation level is not different between the three subtypes (Supplementary Table 3).
Subtype–specific molecular characterization of lung carcinoids
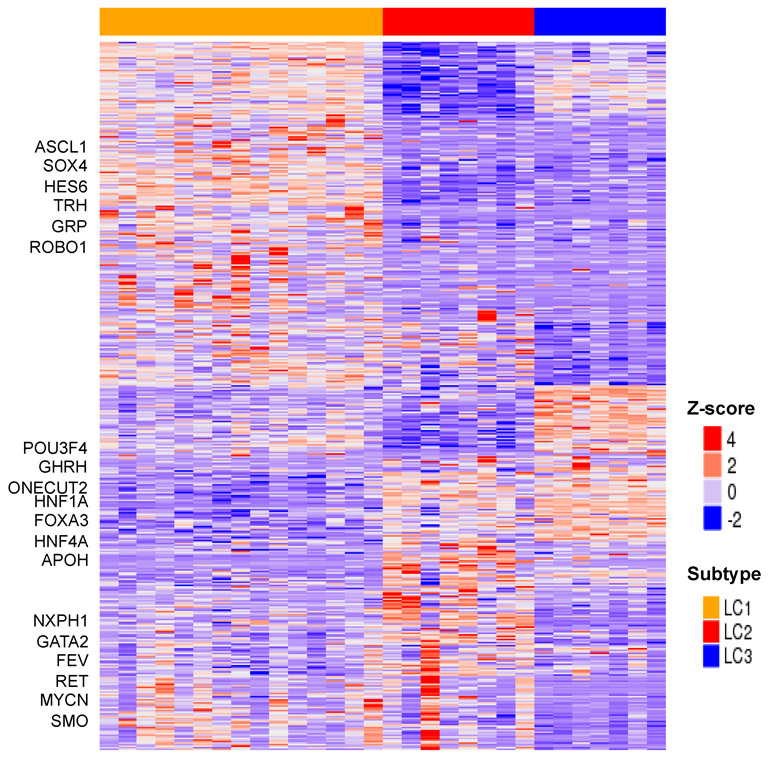
We investigated gene expression and CpG DNA methylation profiles to determine subtype-specific molecular alterations (see Materials and Methods section). Genes up-regulated in LC2 and LC3 as compared to LC1 are enriched for having the transcription factor motifs for HNF1 (FDR q-value < 0.001) and HNF4 (FDR q-value < 0.001)(Figure 2; Supplementary File 3). This is in agreement with the observed high gene expression and DNA hypomethylation of HNF1A, FOXA3 and HNF4A in LC2 and LC3 as compared to LC1 (Figure 3a and 3b, Supplementary Figure 6). In addition, many of the most highly expressed genes (APOH, GC, HAO1, G6PC, TM4SF4, PKLR, UGT2B17, CDH1, and SERPINA1/2/6) in LC2 and LC3 are targets of these hepatocyte nuclear factors (Figure 2). Cancer hallmark gene set enrichment analysis shows complement and coagulation, xenobiotic, retinol and bile acid metabolism to be significantly up-regulated in LC2 and LC3 as compared to LC1, a gene signature also found in subset of pancreatic neuroendocrine tumors(14) (Supplementary File 3). However, we also identified TFs that are differentially expressed between LC2 and LC3 (FEV and POU3F4 are more highly expressed in LC2 and LC3 respectively)(Figure 2; Supplementary File 3). MEN1 gene is required for regulation of several members of the HOX gene family(27). Indeed, the LC2 subtype, which included all of the MEN1 mutant samples, has low expression of HOXB2/¾/5/6 genes as compared to LC1 and LC3 (Supplementary Figure 7).
Figure 2.
Heatmap of differentially expressed genes between LC subtypes. Supervised analysis on LC subtypes reveals differential expression of transcription factor and neuropeptide (some are highlighted on the left side of the heatmap). Heatmap expression level is in z-score.
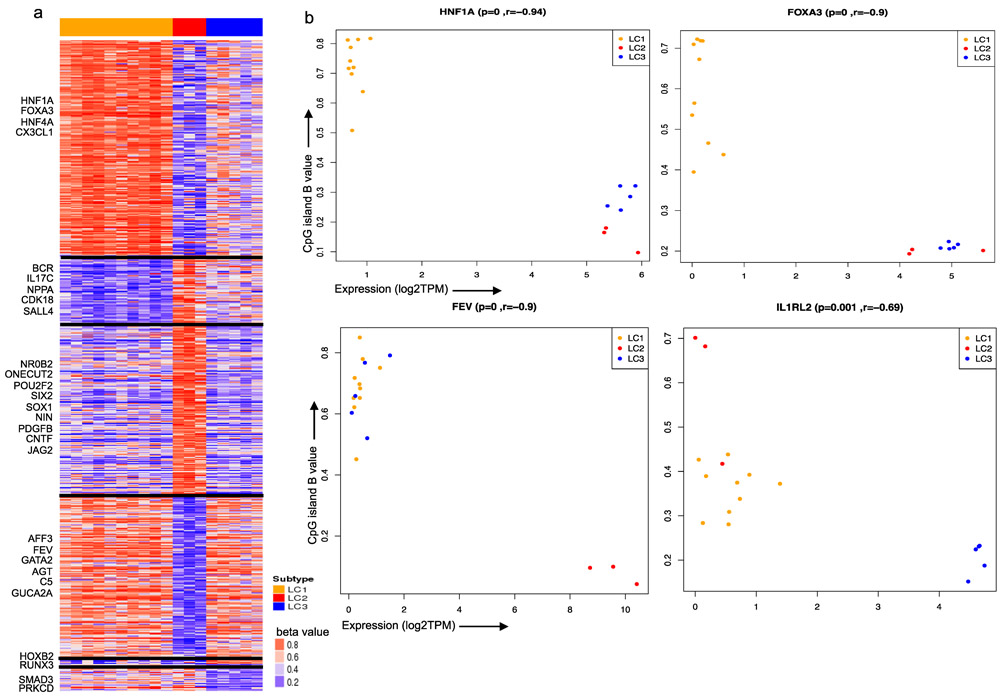
Figure 3. Subtype specific molecular characterization of gene expression and DNA methylation profiles.
a) Heatmap of differentially methylated CpG sites (probes from TSS1500, TSS200 and first exon) of genes among the three LC subtypes. Some genes with altered gene expression and CpG sites are highlighted on the left of heatmap. Dark black line represent subtype specific blocks b) Anti-correlation of gene expression and respective CpG island methylation (18 matched samples) for HNF1A, FOXA3, FEV and ILRL2 across three subtypes. Each plot represents gene expression on x-axis and average CpG island beta value on y-axis along with Pearson correlation (r) and p-value (p) on top of the plot.
We integrated subtype-specific CpG DNA methylation (see method section) with gene expression by focusing on CpG sites between 1500 bps and 200 bps upstream to the transcription start site (TSS) and in the first exon, which have been shown to inversely correlate with gene expression(28). Figure 3a shows subtype-specific differentially methylated CpG probes (DMP) and their inverse correlation with neighboring gene expression. We found 75 genes with expression to be significantly anti-correlated with respective CpG island methylation level (FDR P-value<0.01) (Supplementary File 4). HNF1A and FOXA3 are hypermethylated and low-expressed in LC1. FEV, GATA2 and PROCR are hypomethylated and highly expressed in LC2. SOX1 is hypermethylated and low-expressed in LC2. SIX2, ONECUT2 and IL1RL2 are hypomethylated and highly expressed in LC3 (Figure 3b). Many of these observations suggest further mechanistic studies but there are currently no appropriate lung carcinoid cell lines or animal models available.
Independent validation of lung carcinoid classification
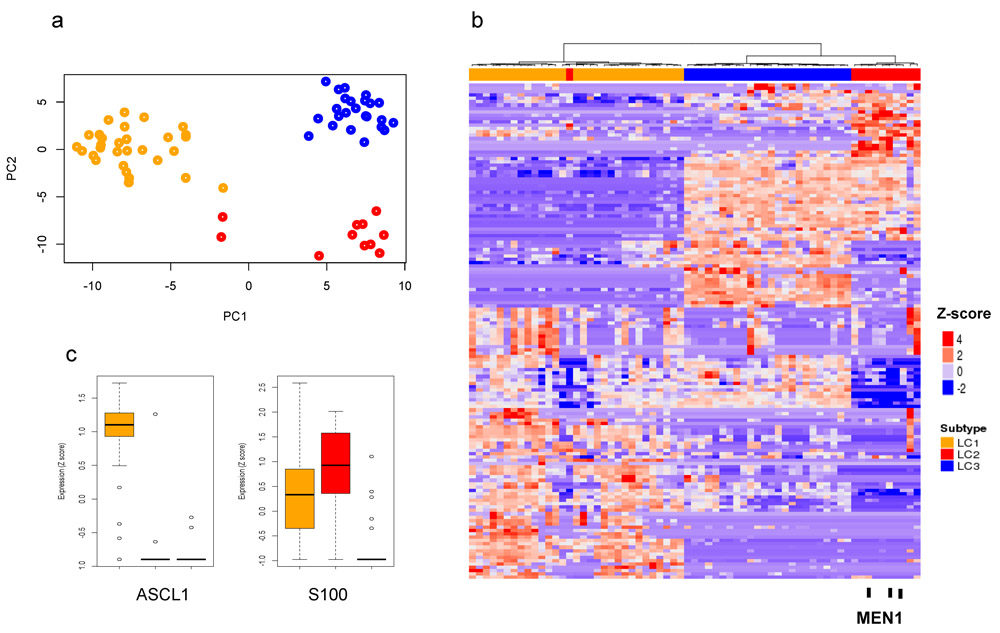
We validated our novel classification and gene expression biomarkers using published lung carcinoid data from Fernandez-Cuesta et al.(9) which include genome/exome and RNA sequencing of 65 samples (56 TCs, 6 ACs and 3 carcinoids). Using our gene signatures derived from the top 100 most variable genes (Supplementary File 5) across lung carcinoids, we found three distinct subtypes using unsupervised clustering and PCA that are consistent with the subtypes identified from our data (Figure 4a,b) (Supplementary Figure 8). Moreover, all MEN1 mutated lung carcinoids are found exclusively in LC2 (Figure 4b). In addition, we found HNF1A and FOXA3 are more highly expressed in LC2 and LC3 as compared to LC1 whereas FEV is highly expressed only in LC2 consistent with our data (Supplementary Figure 9).
Figure 4. Validation of novel classification of LC on an independent collection of LCs from Fernandez-Cuesta et al.
a) and b) PCA and Heatmap of hierarchical clustering of gene expression of LCs from Fernandez-Cuesta et al., using our top 100 variable gene set signature shows three distinct subtypes LC1 (orange), LC2 (red) and LC3 (blue). Black sticks represent samples with MEN1 mutations and they are all found in subtype LC2. c) Boxplot of ASCL1 and S100 gene expression from Fernandez-Cuesta et al. is consistent with LC subtypes. Centerline, median; bounds of box, the 1st and 3rd quartiles; and upper and lower whisker is defined to be 1.5 × IQR more than the third and first quartile.
ASCL1 and S100 are novel biomarkers for lung carcinoid subtypes
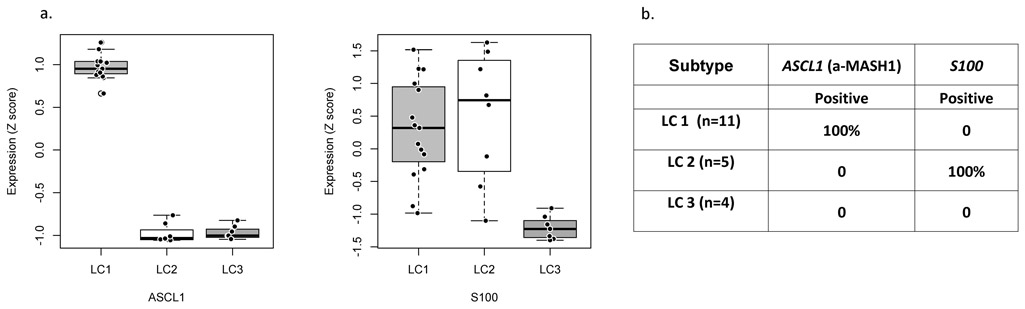
We selected genes with distinct subtype-specific expression to test for use as biomarkers. ASCL1 encodes a transcription factor that plays a role in neuronal differentiation and proliferation(29), neuroepithelial bodies formation(30) and is a lineage specific oncogene for high-grade neuroendocrine lung cancer(31). ASCL1 is significantly highly expressed in LC1 along with its transcriptional targets (Figure 4c and 5a; Supplementary Figure 10). S100, a family of proteins containing two EF-hand calcium-binding motifs, is implicated in tumor progression and poor prognosis(32). Its gene expression levels are significantly higher in subtype LC2 (Figure 5a). We performed IHC staining of ASCL1 and S100 to use as biomarkers. ASCL1 stained positively only for LC1 samples (n=11) and S100 stained positively only for LC2 samples (n=5)(Figure 5b). Both of these genes stained negatively for LC3 samples (n=4)(Supplementary Table 4).
Figure 5. Gene expression and immunohistochemistry for ASCL1 and S100 biomarker genes.
a) Boxplot of ASCL1 and S100 gene expression. Centerline, median; bounds of box, the 1st and 3rd quartiles; and upper and lower whisker is defined to be 1.5 × IQR more than the third and first quartile. b) IHC staining results for ASCL1 and S100 in LC samples: LC1 (n=11), LC2 (n=5) and LC3 (n=4). Supplementary table 4 has IHC results for all samples.
Additionally, we performed ASCL1 and S100 IHC staining on a panel of 173 lung carcinoids tissue microarray (TMA) (Supplementary File 6). ASCL1 positive and S100 negative samples (n=54) were designated LC1. ASCL1 negative and S100 positive samples (n=15) were designated LC2. ASCL1 negative and S100 negative samples (n=71) were designated LC3. Fifteen percent of the TMA samples stained positive for ASCL1 and S100, which are not represented in our discovery dataset.
Cell cycle and mitotic genes are highly expressed in atypical carcinoids of LC1
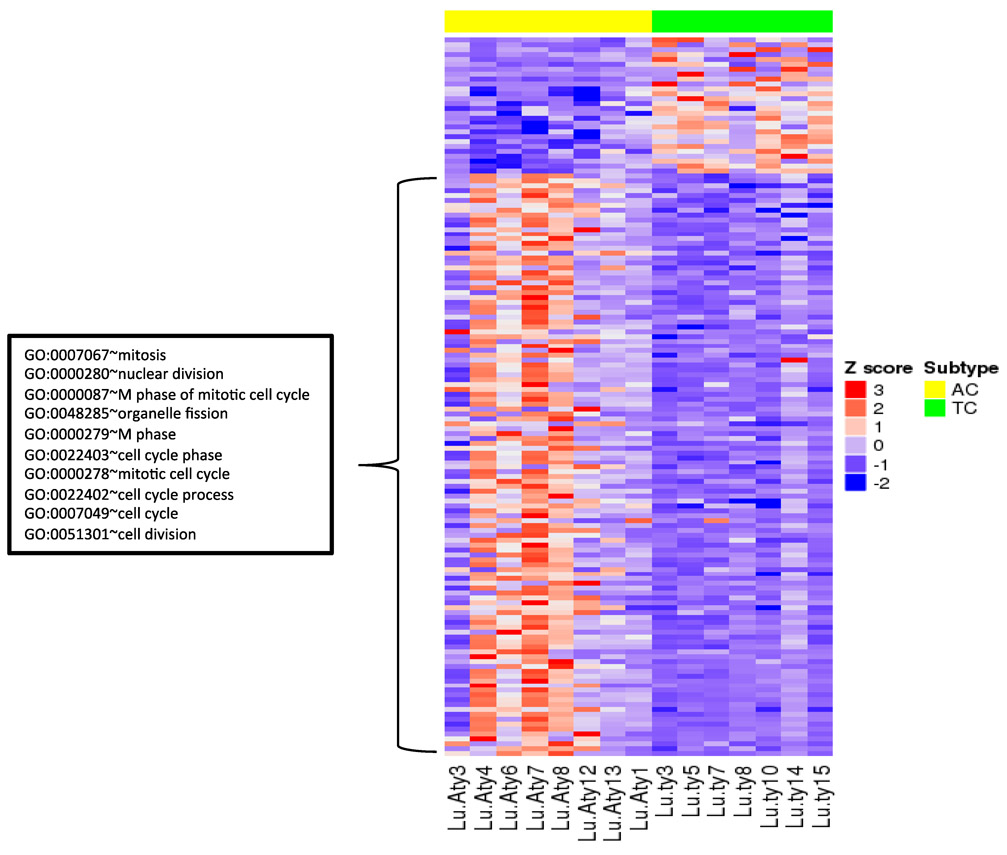
Pathologically, atypical carcinoids are more aggressive and have a higher mitotic index in comparison to TCs. To find the gene signature responsible for these features of ACs, we compared ACs (n=13) and TCs (n=17) from our 30 LC cohort. Surprisingly, we did not find cell cycle or mitosis-related genes to be differentially expressed. We then controlled for LC subtypes and compared ACs (n=8) and TCs (n=7) from subtype LC1 and found differentially expressed genes (Figure 6) were then enriched for mitotic and cell cycle related pathways with high expression in the ACs (Supplementary File 7). Of the eight AC tumors, the three with highest gene expression signature for mitotic/cell-cycle pathway have metastases or recurrences while only one of the five with lower gene expression signature have recurrence or metastases (Figure 6). We also observed high aneuploidy in the ACs with high gene expression signature of mitotic/cell-cycle pathway. We performed the same analysis for ACs and TCs from LC2 and did not find any significant gene signatures, which may be due to the small sample size of LC2.
Figure 6.
Heatmap of differentially expressed genes between ACs and TCs within LC1 subtype. Upregulated genes in ACs (of LC1) are significantly enriched for genes involved in cell-cycle/mitosis.
Lung carcinoid subtypes have distinct clinical phenotypes
The three subtypes of lung carcinoids have distinct clinical phenotypes. Subtype LC1 is enriched for peripheral lung (p-value <0.003 in discovery dataset; p-value < 0.002 in TMA) while subtype LC3 is found predominately at endobronchial lung (p-value < 0.054 in discovery dataset; p-value < 3.8e-5 in TMA) (Figure 1a box)(Supplementary File 1). Subtype LC3 has significantly younger age of diagnosis (median age of 33, 44.5, and 48 years in discovery, Fernandez-Cuesta et al, and TMA datasets respectively) than LC1 (median age of 67, 66, and 60 years respectively) and LC2 (median age of 62.5, 57, and 65 years respectively)(Supplementary Figure 11a and b). LC1 subtype was enriched for female patients (6.5 fold, p-value < 0.007 in discovery dataset; 3.9 fold, p-value < 1.4e-5 in TMA) but not for LC2 or LC3 (Supplementary File 1).
Discussion
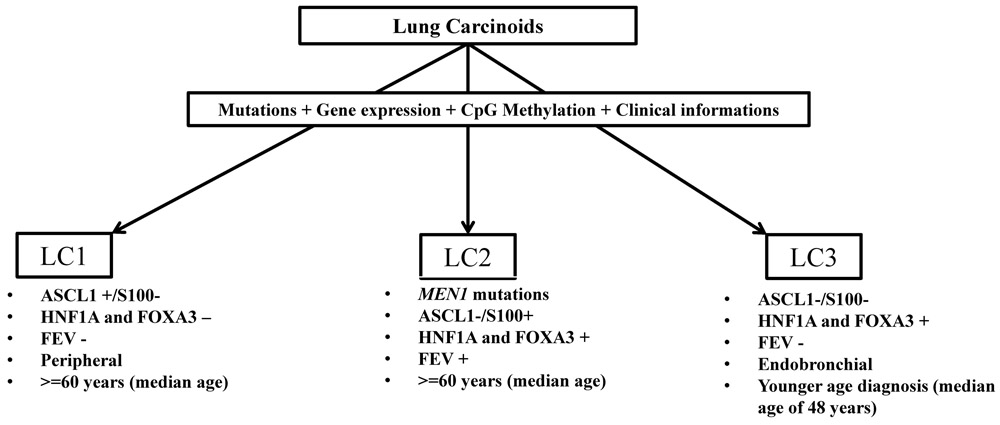
We identified three novel molecular subtypes of lung carcinoids with distinct clinical features using gene expression, DNA methylation and mutational profiles (Figure 7). Integrative analysis of gene expression and DNA methylation identified subtype specific transcriptional profiles of key differentiation transcription factors (ASCL1, HNF1A, FOXA3) and their downstream target genes. Mutational analysis revealed recurrent mutations in chromatin remodeling genes found in all subtypes of LCs with exception of MEN1 mutations occurring only in subtype LC2 tumors. Importantly, we found mutations in DNA repair genes in 17% of our LC samples. Subtype LC3 has younger age of diagnosis and is predominantly endobronchial, whereas subtypes LC1 are predominantly found in peripheral regions of the lung. These findings may argue that subtypes of lung carcinoid potentially originate from different neuroendocrine cell lineages although the lack of available cell-type-specific gene markers prevents a definitive validation beyond speculation at this time. Nevertheless, we believe that a more comprehensive single cell approach can uncover lung NE cell-type-specific gene signatures and reveal the cells of origin for the LC subtypes. The younger age of diagnosis for LC3 by 15–20 years as compared to LC1 and LC2 may be due to earlier diagnosis from the clinically symptomatic tumors located in the central lung as compared to asymptomatic tumors at the peripheral lung or suggest a possible distinct pathogenesis predisposing to LC3, including germline mutations. However, we were not able to detect any pathogenic germline mutations in the panel of cancer associated genes used in the MSK IMPACT testing for any of the lung carcinoids. Our classification and gene expression biomarkers were validated in 65 additional lung carcinoid samples from Fernandez-Cuesta et al (9). Using our subtype classification, we found gene signature for cell cycle and mitotic processes activated in ACs as compared to TCs of the LC1 subtype and those ACs with the high gene signature have worst outcome (Figure 6)(Supplementary File 7). This gene signature will need to be reproduced with a larger sample size but may potentially serve as a diagnostic and prognostic biomarker to differentiate malignant from more benign ACs from subtype LC1. This gene signature is specific to LC1 and would not have been found from comparing ACs to TCs from all lung carcinoids demonstrating the need to study distinct subtypes individually.
Figure 7.
Schematic representation of novel molecular subtypes of lung carcinoids with the principal genomic and clinical characteristics.
Our molecular classification introduces three subtypes of lung carcinoids with distinct clinical phenotypes. This can refine and complement the WHO classification of lung carcinoids into typical or atypical carcinoids and help diagnose ambiguous cases of lung carcinoids from the more malignant LCNEC and SCLC. In addition, the stratification of LCs into distinct molecular subtypes will help with future study of their tumorigenesis, prognosis and treatment options.
Supplementary Material
Acknowledgements
We would like to thank Starr Cancer Consortium Grant (LHT), Raymond and Beverly Sackler Foundation (LHT), Caring for Carcinoid Foundation (LHT), Mushett Family Foundation (LHT), MSKCC Support Grant/Core Grant (P30 CA008748), and Stand Up to Cancer (CSC). This material is based upon work supported in part by the National Science Foundation under Grant No. 1546101. This research was also supported by the Biomedical Informatics shared resource of Rutgers Cancer Institute of New Jersey (P30CA072720). Computational resources were provided by the Office of Advanced Research Computing (OARC) at Rutgers, The State University of New Jersey, under the National Institutes of Health Grant (S10OD012346).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243–60 [DOI] [PubMed] [Google Scholar]
- 2.Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604–20 [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol 1998;22:934–44 [DOI] [PubMed] [Google Scholar]
- 4.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol 2010;120:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swarts DR, van Suylen RJ, den Bakker MA, van Oosterhout MF, Thunnissen FB, Volante M, et al. Interobserver variability for the WHO classification of pulmonary carcinoids. Am J Surg Pathol 2014;38:1429–36 [DOI] [PubMed] [Google Scholar]
- 6.Pelosi G, Papotti M, Rindi G, Scarpa A. Unraveling tumor grading and genomic landscape in lung neuroendocrine tumors. Endocr Pathol 2014;25:151–64 [DOI] [PubMed] [Google Scholar]
- 7.Volante M, Gatti G, Papotti M. Classification of lung neuroendocrine tumors: lights and shadows. Endocrine 2015;50:315–9 [DOI] [PubMed] [Google Scholar]
- 8.Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005;29:179–87 [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Cuesta L, Peifer M, Lu X, Sun R, Ozretic L, Seidal D, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vollbrecht C, Werner R, Walter RF, Christoph DC, Heukamp LC, Peifer M, et al. Mutational analysis of pulmonary tumours with neuroendocrine features using targeted massive parallel sequencing: a comparison of a neglected tumour group. Br J Cancer 2015;113:1704–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015;17:251–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013;31:213–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CS, Laddha SV, Lewis PW, Koletsky MS, Robzyk K, Da Silva E, et al. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 2018;9:4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, et al. A survey of best practices for RNA-seq data analysis. Genome Biol 2016;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet 2006;38:500–1 [DOI] [PubMed] [Google Scholar]
- 17.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JA, Cai C, Langfelder P, Geschwind DH, Kurian SM, Salomon DR, et al. Strategies for aggregating gene expression data: the collapseRows R function. BMC Bioinformatics 2011;12:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, et al. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics 2014;30:428–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortin JP, Labbe A, Lemire M, Zanke BW, Hudson TJ, Fertig EJ, et al. Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol 2014;15:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warden CD, Lee H, Tompkins JD, Li X, Wang C, Riggs AD, et al. COHCAP: an integrative genomic pipeline for single-nucleotide resolution DNA methylation analysis. Nucleic Acids Res 2013;41:e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rekhtman N, Pietanza CM, Sabari J, Montecalvo J, Wang H, Habeeb O, et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod Pathol 2018;31:111–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005;123:207–18 [DOI] [PubMed] [Google Scholar]
- 28.Brenet F, Moh M, Funk P, Feierstein E, Viale AJ, Socci ND, et al. DNA methylation of the first exon is tightly linked to transcriptional silencing. PLoS One 2011;6:e14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro DS, Martynoga B, Parras C, Ramesh V, Pacary E, Johnston C, et al. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev 2011;25:930–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci U S A 2012;109:12592–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borromeo MD, Savage TK, Kollipara RK, He M, Augustyn A, Osborne JK, et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep 2016;16:1259–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Xu C, Jin Q, Liu Z. S100 protein family in human cancer. Am J Cancer Res 2014;4:89–115 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary data and figures. Data generated in this study were deposited to NCBI under GEO SuperSeries GSE118133 (GSE118131 for RNAseq and GSE118132 for 450 K methylation). We do not impose any restrictions on data availability.