Graphical abstract
Keywords: Landrace pigs, Minipigs, Food effect, Fasting protocol, Gastric emptying
Abstract
A preclinical porcine model that reliably predicts human food effect of fenofibrate was developed. Fenofibrate was administered to pigs as model compound with a positive food effect. Two different types of fed conditions were explored: a FDA style breakfast and a standard pig pellet feed. In order to assess if complete stomach emptying had been achieved under the employed fasting protocol, the amount of gastric and intestinal content was evaluated post-mortem. In addition, the protocol was designed to evaluate gastric emptying in the pre- and postprandial state using paracetamol as a marker. The study confirmed that micronized fenofibrate displayed a positive food effect with a similar fold difference to humans in FDA style fed state. Post-mortem assessment of stomach and intestinal content confirmed significantly lower content in the fasted compared to the pig pellet fed state. In the case of paracetamol, a delayed gastric emptying in the fed state was not observed, which may suggest that the Magenstrasse phenomena reported in humans, may also occur in landrace pigs. The study demonstrated the utility of a food effect protocol in landrace pigs as a pre-clinical approach to predict human food effects and provided new insights into gastric emptying in pigs.
1. Introduction
Food dependent oral bioavailability of drugs can have a significant impact on patient treatment, hence there are clear clinical advantages to predicting food dependent bioavailability early in the drug development process. Recent observations indicate that over 40% of new medicines licensed since 2010 display variable food effects (O'Shea et al., 2018). In preclinical development, it is crucial to predict the impact of food on oral pharmacokinetics as early as possible in drug development, so as to allow optimisation of formulation design to migrate the risk of variable food effect bioavailability and avoid costly reformulation later in the product lifecycle (Fleisher et al., 1999, Koziolek et al., 2019). While there is a range of in vitro approaches to predict food effects, which have been reviewed recently (Pentafragka et al., 2018), current regulatory guidance mandate food effect studies in humans, which are time and resource intensive. In addition, with the increasing drive to ensure clinical studies in humans are confirmatory, rather than exploratory (Selen et al., 2014), there is a need for pre-clinical models to assess the interplay between formulation and food for a given drug candidate and its impact on drug levels in vivo.
Food intake can result in large variability of oral absorption by altering the physiological and physicochemical conditions in the gastrointestinal (GI) tract, including (a) slower gastric emptying influencing cmax and tmax, (b) increased gastric pH, altering the solubility of ionisable compounds, (c) increase of gastrointestinal fluid volume, which changes the volume available for solubilisation/dissolution, (d) increased secretion of bile, cholesterol, phospholipids etc. resulting in increased solubilisation for poorly soluble drugs. Therefore, the physiological differences in the postprandial state effects drug solubilisation and absorption depending on the drug and formulation characteristics (Abuhelwa et al., 2017, Custodio et al., 2008, Gu et al., 2007, Lentz, 2008, Varum et al., 2013).
Thus, the FDA provided guidelines on how to design clinical trials for new drug products, recommending dosing in both fasted and fed states in order to investigate food effects under standardised conditions. The FDA guidance defines that a food-effect is established if the 90% confidence intervals for the ratio of population geometric means, based on log-transformed data, for either AUC0→∞ or cmax fall outside the 80–125% bioequivalence limits (FDA, 2002). The standardised fed state conditions are represented by a high fat FDA breakfast, containing 800–1000 kcal, with approximately 150, 250, and 500–600 calories from protein, carbohydrate and fat, respectively (FDA, 2002). While this regulatory guidance establishes clearly the conditions for investigating the impact of food in a clinical setting, protocols for assessing food effects in preclinical models are less clearly defined, albeit it is expected that fed state conditions should generally match the standard FDA style fed state conditions.
The canine model is the most commonly preclinical model used for assessing food effects. Lentz et al. (2007) developed a protocol in a canine model for predicting food effects in humans. While in some cases dogs tended to overestimate food effects, in particular when 100 g of FDA breakfast were administered, the model was useful for the identification of both positive and negative food effects of nine drugs using a 50 g FDA breakfast (Lentz et al., 2007). Over the last decade, the pig has become an increasingly popular preclinical model in regulatory toxicology and pharmacokinetics due to the similarities in gastrointestinal anatomy and physiology to humans (Bode et al., 2010, Henze et al., 2018b, Puccinelli et al., 2011, Sjogren et al., 2014, Suenderhauf and Parrott, 2013, Walters et al., 2011). While there are various breeds of pigs used in pharmaceutical research, the domestic landrace pig and the Göttingen minipig are the most commonly employed. In general, both breeds are considered to be comparable to humans in terms of physiological pH and intestinal physiology, as recently reviewed (Henze et al. 2018b). Henze et al. (2018) recently reported that pigs compare more favourably than dogs at predicting the extent of absolute bioavailability in humans, albeit based on a limited set of 15 comparable studies (Henze et al., 2018b). However, the rate of drug absorption in pigs is tending to be slower than in humans, this most likely reflect a prolonged and more variable gastric emptying in pigs. The variability in estimates of gastric emptying in pigs has been reported to be influenced by the fasting period and methods employed (Suenderhauf et al., 2014). In many cases insufficient fasting protocols may have resulted in an incomplete stomach emptying of food prior to dosing. Therefore, there is a need to define a standardised protocol that ensures complete stomach emptying in the fasted state in food effect studies.
Paracetamol has previously been used as a marker for gastric emptying (Suenderhauf et al., 2014), as a BCS class I compound food is not expected to have any impact on the extent of paracetamol bioavailability, however food intake is anticipated to reduce the rate of absorption due to a delay gastric emptying. Suenderhauf et al. (2014) reported that gastric emptying rates in fasted Göttingen minipigs for paracetamol were longer and more variable than estimated in humans. Interestingly, post-mortem evaluation of the stomach contents revealed that even after a 12 h fasting period, a significant amount of food remained in the stomach and therefore dosing conditions were not reflective of the desired fasted conditions. Interestingly complete stomach emptying was observed in pigs fed a liquid meal the day before dosing or by pre-treating with metoclopramide, a pro-kinetic agent (Suenderhauf et al., 2014). Henze et al. (2018), attempted to extend these post-mortem observations to establishing a fasting/fed protocol in Göttingen minipigs by administering metoclopramide intramuscularly 2 h before oral dosing of paracetamol. However, the pre-treatment of metoclopramide did not appear to regulate gastric emptying under the fed and fasted conditions (Henze et al., 2018a).
The current study was designed with the objective of assessing the utility of landrace pigs to predict food effects, using the model drug fenofibrate, a BCS class II drug with a known positive food effect in humans. Two different fed state conditions were explored: firstly, a FDA style breakfast consisting of a high fat, high caloric meal and secondly, a standard pig pellet feed. An additional objective of the present study was to investigate the efficiency of the employed fasting protocol, as to date most of the studies observed as a cofounding factor that pigs may have not been dosed in a truly fasted state. Therefore, the gastrointestinal contents of pigs were evaluated post-mortem after the employed fasting regime and the pig standard feed. Additionally, pigs were co-administered paracetamol to evaluate if differences in gastric emptying between fasted and fed states resulted in changes in paracetamol absorption in vivo.
2. Materials and methods
2.1. Materials
Paracetamol, 2-acetamidophenol and fenofibric acid were purchased from Sigma-Aldrich (Ireland) Ltd. Fenofibrate was purchased from Kemprotec Ltd. (UK). Hard gelatine capsules (Size 0) were obtained from Capsugel (Coni-Snap®). Lipantil™ Micro 67 mg hard capsules and Paralief™ 500 mg tablets were commercially sourced from local pharmacies. All food components used in preparing FDA recommended breakfast were purchased commercially. All other chemicals and solvents were of analytical grade or HPLC grade, respectively, and were purchased from Sigma–Aldrich (Ireland) and used as received.
2.2. In vivo study
The study was carried out under the licence issued by the Health Products Regulatory Authority (HPRA), Ireland, as directed by the Cruelty to Animals Act, Ireland and EU Statutory Instruments (Licence number AE19130/P058). Local University ethical committee approval was obtained. Four male landrace pigs (16.8–18.7 kg, mean 17.96 kg) were sourced locally and housed individually at the University’s Biological Services Unit. Landrace pigs of this size were selected to get as close to the size of Göttingen minipigs and beagle dogs used in previously comparable studies (Lentz et al., 2007, Henze et al., 2018a). Pigs were fed approximately 175 g of standard weanling pig pellet feed twice daily. The final feed of 175 g was given 24 h prior to dosing. As part of the study design any remaining food was removed 16 h before dosing, however, no food remained at this point in any of the groups. The study was a non-randomised, one sequence, three-way crossover design, where the pigs were dosed in the fed state (350 g pig weanling food which was considered a normal or ‘standard’ feed for landrace pigs of this size – equivalent to 20% protein, 6.5% oil, 3.5% fibre or 987 kcal in total) in week one, fasted on week 2, fed a half portion of a standard high-caloric, high-fat FDA breakfast (444 kcal, 315 g, one slice of bacon, one slice buttered toast, one fried egg, 118 mL whole milk, 60 g hashed brown potatoes) in week 3. The mass of FDA breakfast fed equated to approximately 18–20 g/kg of body weight.
On day one, an indwelling intravenous catheter was inserted into the jugular vein via an ear vein under general anaesthesia, as previously described (Framstad and Aass, 2000, Pairis-Garcia et al., 2014, Swindle, 2016, Swindle, 2010, Swindle and Smith, 2013). Following an overnight fast on day three, oral formulations of 67 mg fenofibrate (Lipantil® Micro 67 mg hard gelatine capsules) and 500 mg of paracetamol tablets (Paralief™) were administered simultaneously with the aid of a dosing device, after which the pigs received 50 mL of water via a syringe. Under fed state conditions, pigs received the meal 30 min prior to oral dosing. After dosing, pigs were returned to their pens. Blood samples (4 mL) were collected at time zero (pre-dosing) and 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12 and 24 h post-dosing. Water was available ad libitum throughout the study period and the animals were fed with 175 g of pig weanling pellet feed 8 h post-dose. All blood samples were collected in heparinised tubes (Sarstedt, Germany) and immediately centrifuged at 3220 g for 5 min at 4 °C (Eppendorf 5810r swinging bucket rotor centrifuge, Eppendorf AG, Hamburg, Germany). Plasma was collected and stored at − 80 °C prior to analysis. A six-day washout period was used between each phases. All animals remained in good health throughout the study.
2.3. Bioanalysis of paracetamol
The plasma concentrations of paracetamol were determined by a reversed-phase HPLC. The Agilent 1200 series HPLC system comprised a binary pump, variable wavelength detector, autosampler and column oven. A Synergi, C18 reversed phase column (250 × 4.6 mm, 4 μm) (Phenomenex Inc., Macclesfield, UK) was used for the chromatographic separation. The mobile phase consisted of 0.1% (w/v) acetic acid: methanol (70:30, v/v) and was used at a flow rate of 1 mL/min. The detection wavelength was set at 254 nm. The retention time for paracetamol was approximately 6.5 min and 11 min for the internal standard (2-acetamidophenol). However, due to the presence of fenofibric acid, the main metabolite of fenofibrate, in the same sample a wash run was performed after every third sample. This was a 20-minute cleaning run with a gradient from 70% acetic acid and 30% methanol to 20% acetic acid and 80% methanol over five minutes. The system was held at this composition for five minutes before returning to the original gradient over five minutes and re-equilibrating the column at this gradient for five minutes.
Paracetamol was extracted from the plasma samples by liquid–liquid extraction. 100 µL of plasma sample was transferred to an Eppendorf microcentrifuge tube. 60 mg sodium chloride, 10 µL internal standard (250 µg/mL, 2-acetamidophenol) were added to the tube. Sodium chloride was added to precipitate and denature protein before extraction. The tubes were mixed using a benchtop vortex. 1000 µL of ethyl acetate was added to extract paracetamol. The tubes were shaken for 30 s using a vortex mixer followed by centrifugation at 11,500g for 9 min (Hermle z233M-2 fixed angle rotor centrifuge, HERMLE Labortechnik GmbH, Wehingen, Germany) at ambient temperature. The supernatant was transferred to a polypropylene vial and evaporated to dryness under a stream of nitrogen at 60 °C. A second extraction step was carried out by adding 1000 µL ethyl acetate to the precipitate again, followed by 30 s vortexing and centrifuging under the same conditions. The supernatant was transferred to the corresponding round-bottom polypropylene vial from the previous step and evaporated to dryness under a stream of nitrogen at 60 °C. The residue was dissolved in 100 µL mobile phase and 20 µL was injected into the column. The limit of quantification by this procedure was 500 ng/mL and the assay was linear between 500 ng/mL and 15,000 ng/mL. The extraction efficiency was greater than 89% across the concentration range.
2.4. Bioanalysis of fenofibrate
The pharmacokinetic evaluation of fenofibrate was based on the quantification of fenofibric acid, as fenofibrate is a prodrug and after absorption fenofibrate is rapidly hydrolysed to the major active metabolite fenofibric acid. Fenofibric acid was analysed from the plasma samples, using a validated HPLC-UV method, as previously described (Griffin et al., 2014, O'Shea et al., 2015). Briefly, 0.5 mL plasma was spiked with 20 μL of a suldinac 100 μg/mL solution in methanol as an internal standard. Proteins were precipitated through addition of 0.5 mL of 25% NaCl solution and 1 mL of 1% H3PO4 in methanol with thorough mixing. Samples were centrifuged at 11.500 g for 9 min (Hermle z233 M-2 fixed angle rotor centrifuge; HERMLE Labortechnik GmbH, Wehingen, Germany). The clear supernatants were injected onto a Synergi Fusion C18 reversed phase column (250 × 4.6 mm, 4 μm) (Phenomenex Inc., Macclesfield, UK) using the same system as described above for paracetamol. The mobile phase consisted of 80% methanol and 20% water (adjusted to pH 2.5 with phosphoric acid) at a flow rate of 1 mL/min, resulting in an elution of fenofibric acid at 6.5 min. UV detection was performed at 286 nm. The analysis showed linearity over the range of 50–2000 ng/mL with an LOQ of 50 ng/ml and extraction recoveries were ≥95%.
2.5. Gastric and intestinal fluid sample collection
For the post mortem assessment, 12 pigs were used – 4 pigs from the current in vivo study and 8 pigs from other unrelated in vivo studies. All pigs were euthanized humanely following completion of in vivo pharmacokinetic experiments. All animals were euthanized by intravenous injection of pentobarbital sodium followed by potassium chloride. The peritoneal cavity was exposed by midline incision and the stomach and small intestine were isolated. Occluding ligatures were applied to the proximal cardiac sphincter and distal to the pyloric sphincter as well as at the proximal and distal ends of the small intestine. Once both ends were secured, both the stomach and small intestine were removed from the peritoneal cavity. The luminal fluid was collected in sterile 50 mL sample tube, pH was measured using a calibrated Jenway 3510 pH meter and the samples were frozen until further analysis.
Thawed samples were homogenised using a T25 Ultra-Turrax® homogeniser (IKA®-Werke GmbH & Co. KG, Germany) probe for 5 min at 200 rpm. Samples were centrifuged at 3220 g for 10 min at ambient temperature (Eppendorf 5810r swinging bucket rotor centrifuge, Eppendorf AG, Hamburg, Germany) to separate solid content from liquid content. Liquid supernatant was macroscopically observed to be free from solids and was removed and placed in 50 mL sterile tubes. Solid content was measured using lab balances, while the volume of liquid content was measured in a graduated cylinder. The following feeding regimens were evaluated:
-
(i)
Fasted state: final feed was given 24 h prior to euthanasia. As part of the study design any remaining food was removed 16 h before euthanasia.
-
(ii)
Fed state: final feed (350 g pig pellet feed), was given 2 h prior to euthanasia.
2.6. Pharmacokinetic analysis
Exposure after oral administration was estimated by calculating the area under the plasma concentration curve (AUC) for 8 h and 24 h post dosing for fenofibric acid and paracetamol by using PKPlus (Gastroplus.9.5). The AUC 24h–∞ in the fasted state was calculated using the mean elimination constant from the i.v. dataset (Griffin et al., 2014). AUC and mean residence time (MRT) were determined from non-compartmental analysis. The peak plasma concentrations (cmax) and the time for their occurrence (tmax) were noted directly from the individual plasma concentration versus time profiles. The absolute bioavailability (Fa) of fenofibrate and paracetamol was calculated according to Eq. (1), using previously published intravenous data from our group (Griffin et al., 2014, Henze et al., 2018a). All pharmacokinetic parameters are reported as mean ± SD.
| (1) |
Mean absorption time (MAT) was calculated from intravenous and oral MRT, according Eq. (2):
| (2) |
Food effect was calculated using the fold difference (FD) in the AUC in fed vs the fasted state using Eq. (4):
| (3) |
Fold differences (FD) are presented, where possible, as mean FD ± standard error of the fold difference (SEFD) as calculated by Eq. (5):
| (4) |
where FD is the mean fold difference in food effect, AUCfed and AUCfasted are the represent the mean AUC in the fed and fasted states, respectively, and SEfed and SEfasted represent the standard errors corresponding to these values, respectively.
2.7. Deconvolution
The correlation between absorption and plasma concentration was previously described by Langenbucher et al. (1982). The oral administration can be treated as the response function R(t) and the weighting function W(t) is based on the data after an intravenous bolus administration. The correlation of the weighting and response function depends on the input and can be described according to Eq. (5):
| (5) |
The principal of numerical deconvolution was previously described by (Langenbucher, 1982):
| (6) |
I(Xi) combines the release and absorption process, T is the time interval, (Xk) and W(Xk) the average input rate and weight between the times Xk-1 and Xk. The method of numerical deconvolution was used to have a dynamic illustration of the absorption process. Mean time points for 25%, 50% and 75% absorption were calculated from the deconvolution profiles, to present the totally absorbed fraction of the compounds. The deconvolution was conducted in Excel, results are presented as means and their standard error mean (±S.E.M.).
2.8. Statistical analysis
After using the Bartlett’s test to check for equal variance, a one-way ANOVA was used to determine the statistical significance of calculated in vivo bioavailability and Cmax. Tukey’s post hoc test was used to identify pairwise statistical significance. A nonparametric statistical test was used, the Kruskal Wallis rank test, with Dunn’s multiple comparison rank sums to determine the significance in tmax. All statistical analyses were performed using GraphPad Prism version 5, p-values of <0.05 were considered significant.
3. Results
3.1. Effect of food on oral pharmacokinetics of fenofibrate in landrace pigs
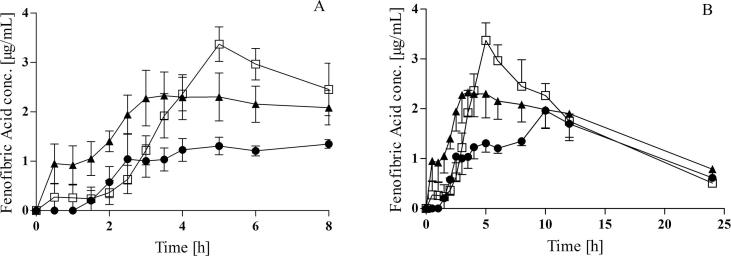
The mean plasma concentration profiles obtained after oral administration of Lipantil® Micro (67 mg micronized fenofibrate) to fasted and fed pigs are presented in Fig. 1. The mean plasma profiles display a similar ranking and trends as the individual plasma profiles for each pig. Two different feeding regimes were compared, a FDA style- high-fat, high caloric breakfast and a standard pig pellet feed, which were administered 30 min prior to the oral dosing of fenofibrate. The key pharmacokinetic parameters for fenofibric acid are summarised in Table 1.
Fig. 1.
(A) Plasma concentration profiles after oral administration of 67 mg fenofibrate to landrace pigs 0–8h, (B) Plasma concentration profiles after oral administration of 67 mg fenofibrate to landrace pigs over 24 h, 8 h post dosing animals were allowed access to food; fasted state (●), FDA breakfast (□), pig pellet feed (▲); (mean ± SEM, n = 4).
Table 1.
Pharmacokinetic parameters of fenofibrate (fenofibric acid) after oral administration of 67 mg per animal (mean ± SD, n = 4).
| Pharmacokinetic parameters | |||
|---|---|---|---|
| cmax (μg/mL) | 2.24 ± 0.68 | 3.24 ± 0.59 | 2.91 ± 0.60 |
| tmax (h)a | 7.5 (2.5–12.0) | 5.0 (4.0–8.0) | 6.5 (2.5 ± 12.0) |
| AUC0→8h (μg⋅h/mL) | 10.64 ± 2.90 | 19.14 ± 3.83c | 18.85 ± 5.62 |
| AUC0→24h (μg⋅h/mL) | 28.19 ± 7.01 | 36.79 ± 2.52 | 38.88 ± 12.63 |
| AUC0-∞ (μg⋅h/mL) | 31.36 ± 7.45 | 41.98 ± 3.89 | 51.63 ± 21.06 |
| Fa0-8h (%)b | 21.61 ± 5.59 | 38.89 ± 7.55c | 38.29 ± 11.42 |
| Fa0-∞ (%)b | 62.11 ± 14.75 | 83.13 ± 7.71 | 102.26 ± 41.71 |
| Food effect 0-8hd | – | 1.82 ± 0.18 | 1.94 ± 0.94 |
| Food effect 0-∞d | – | 1.39 ± 0.30 | 1.78 ± 0.96 |
| MAT (h)b | 13.42 ± 8.08 | 7.03 ± 3.25 | 10.73 ± 6.03 |
| MRT p.o. (h) | 19.22 ± 7.49 | 12.84 ± 1.45 | 16.54 ± 5.36 |
F0-∞ = (AUC(oral)0-∞/AUC(iv)0-∞) * (Dose(iv)/Dose(oral).
F0-8h = (AUC(oral)0-8h/AUC(iv)0-∞) * (Dose(iv)/Dose(oral).
Median (range).
Absolute bioavailability and MAT was calculated with i.v. data previously published from (Griffin et al., 2014).
Significant difference to the fasted equivalent parameter (p < 0.05).
Calculated according to Eq. (4), reported as mean values of the individual fed/fasted ratios.
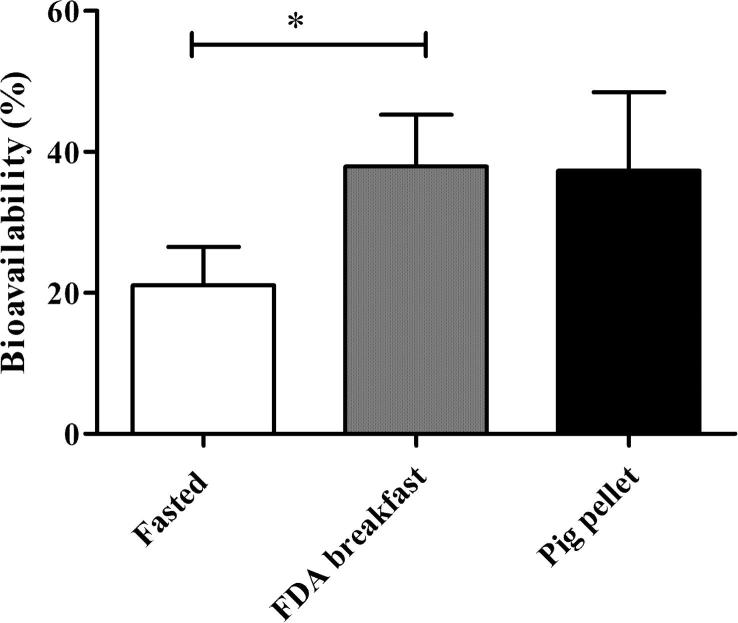
During the first 8 h post dose (Fig. 1A), a marked difference in the absorption profile of fenofibrate in fasted pigs versus pigs fed the FDA style breakfast was observed. A clear positive food effect was observed for fenofibrate following oral dosing after the FDA breakfast. The AUC 0-8h increased from 10.6 ± 2.9 μg⋅h/mL in the fasted state to 19.1 ± 3.8 μg⋅h/mL in the fed state, reflecting a statistically significant increase in bioavailability of 1.82 ± 0.18, (p < 0.05) (Fig. 2).
Fig. 2.
Bioavailability after 8 h of 67 mg fenofibrate orally administered to fasted pigs, pigs fed a FDA breakfast and pigs fed a standard pig pellet feed. Absolute bioavailability was calculated with i.v. data previously published from (Griffin et al., 2014); (mean ± SD, n = 4).
Pigs were allowed access to food 8 h post dosing, the food intake correlated with a distinct second peak in plasma concentration profiles at 10 h of the fasted study group. In pigs the secretion of bile acids has a similar pattern to humans (Henze et al. 2018b.). Therefore, the ingestion of food 8 h post dosing, results in an increase of bile salts in the intestine which leads to increased solubilisation capacity in the intestine. This observation of a second peak is similar to previous findings for Lipantil micro in fasted landrace pigs, most likely reflecting a post prandial induced secondary absorption phase of drug that remained in the gastrointestinal tract even 8–10 h post dosing (O'Shea et al., 2015). As a result of this secondary absorption peak in plasma concentration, a longer mean absorption time (MAT) (13.4 ± 8.1 h) and the time to reach peak concentrations (tmax) (5.5 ± 4.4 h) were obtained in the fasted study leg. Interestingly, the mean tmax found in fasted landrace pigs was comparable to recently published human data of 8.6 ± 4.0 h for a micronized fenofibrate formulation (Hens et al., 2015). A distinct secondary peak was not evident in the fed groups, suggesting that no secondary absorption phase of fenofibrate occurred in these pigs.
Additionally, over the full 0-∞ h period, given the secondary absorption phase in the fasted state, there was no significant difference in AUC0-∞ between both fed states and the fasted state. The individual mean fold fed/fasted difference for the FDA style breakfast was determined to be 1.39 ± 0.30, indicative of an overall positive food effect, however no longer statistically significant over the 0-∞ h. Interestingly this fold difference is comparable to the food effect of fenofibrate reported in humans, of approximately 35–50% increase in bioavailability in the fed state (Guivarc'h et al., 2004).
In pigs administered the pig pellet feed, a trend towards a faster absorption was evident from the average plasma concentration profile (Fig. 1A), however plasma concentrations were more variable within this study leg. As a result of this greater variability, differences in AUC between fasted and pig pellet fed groups were not statistically significant.
3.2. Post mortem assessment of gastrointestinal contents in fasted and fed state
Previous studies identified that Göttingen minipigs displayed an incomplete stomach emptying, even after an overnight fasting period (Suenderhauf et al., 2014). In order to assess if truly fasted state conditions were achieved in landrace pigs after an overnight fast, post mortem gastrointestinal content samples were collected. The fasted gastrointestinal content was compared to a fed state, where pigs were fed 350 g of a standard pig pellet feed in the morning 2 h pre euthanasia.
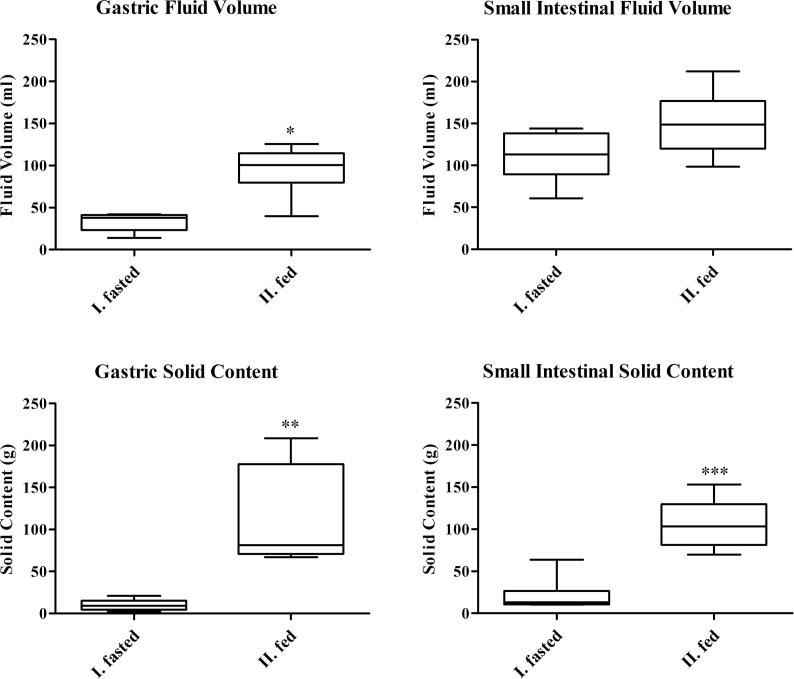
Fig. 3 illustrates the content samples collected from the stomach and the small intestine under fasted and fed conditions. On average the amount of solid content remaining in the stomach of the fasted group was 10.1 ± 6.4 g and a fluid volume of 33.4 ± 10.7 mL. The low overall amount of solid content in the stomach confirms that the fasting protocol in landrace pigs was sufficient to generate fasted state conditions, resulting in near-complete emptying of solid content from the stomach. In the case of the fed state group, a significant higher solid content of 112.9 ± 60.1 g and liquid volume of 95.1 ± 29.4 mL was observed in the stomach.
Fig. 3.
Post mortem assessment of fluid volume and solid content of landrace pigs under fasted and fed conditions. I. fasted state: overnight fasting period; II. fed state: pigs received standard pig pellet feed (350 g) 2 h before euthanasia. Plots show the median (solid line), Whiskers: min. to max. value, n = 6.
In the small intestine, average fluid volume in the fasted state was 111.4 ± 28.8 mL compared to a volume of 150.1 ± 39.3 mL in the fed state. Small intestine fluid volume was not significantly different in pre and post prandial conditions. On the other hand, the intestinal solid content was significantly different between the fasted and the fed state in the small intestine, with a solid content of 20.9 ± 18.5 g in the fasted animals and 106.1 ± 29.5 g in the fed animals.
3.3. Absorption of paracetamol in pre- and post-prandial state
Previously, paracetamol has been used to provide an estimate of gastric emptying (Christiansen et al., 2015, Henze et al., 2018a, Suenderhauf et al., 2014). Thus, in order to further compare gastric emptying in the current protocol for landrace pigs, paracetamol was co-administered with fenofibrate. Generally, the administration of a drug with food should lead to a slower gastric emptying, resulting in a longer tmax (Christiansen et al., 2015). Therefore, any differences in tmax and absorption rate of paracetamol between fasted and fed conditions should provide further insights into food influences on gastric emptying (Christiansen et al., 2015).
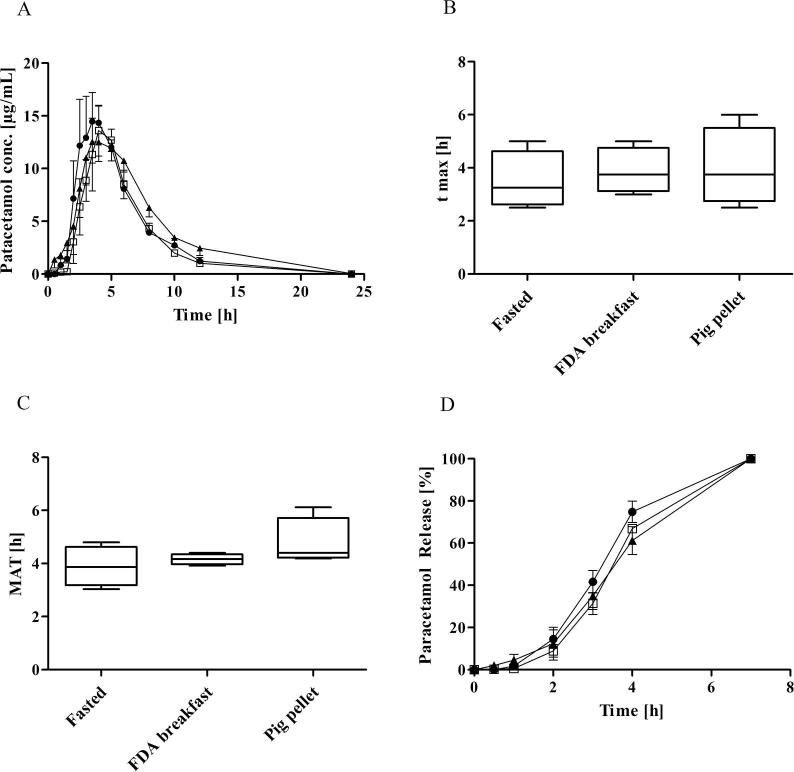
Table 2 summarises the pharmacokinetic parameters after oral administration of paracetamol (Paralief® 500 mg tablets). Fig. 4A represents the plasma concentration–time profile of paracetamol in landrace pigs in the fasted state, after a FDA breakfast and standard pig pellet feed, respectively. No significant difference was observed between the fasted state and the two feeding regimes. In the fasted state tmax ranged from 2.5 h to 5.0 h, with a mean value of 3.5 ± 1,1h. These estimates of paracetamol absorption rates in landrace pigs are comparable to values obtained in fasted minipigs, where Henze et al. reported a mean of 4.2 ± 1.6 h (Henze et al., 2018a). The values were also similar to the mean tmax of 3.5 h reported for a paracetamol capsule in male minipigs, albeit slower than the mean tmax of 1.4 h reported for a paracetamol tablet in the same study (Suenderhauf et al., 2014). Comparing tmax in fasted and fed states, no significant differences were found (Fig. 4B) and critically, a delayed absorption of paracetamol in the fed state was not evident. The reported mean data showed the same ranking as the individual profiles.
Table 2.
Pharmacokinetic parameters of paracetamol, oral administration 500 mg per animal (mean ± SD, n = 4). No statistical difference was observed between the study legs.
| Pharmacokinetic parameters | |||
|---|---|---|---|
| cmax (μg/mL) | 16.41 ± 5.48 | 11.88 ± 4.64 | 14.76 ± 2.46 |
| tmax (h)a | 3.25 (2.5–5.0) | 3.75 (3.0–5.0) | 3.75 (2.5–6.0) |
| AUC0→8h (μg⋅h/mL) | 70.02 ± 20.70 | 61.32 ± 13.07 | 74.71 ± 11.04 |
| AUC0→24h (μg⋅h/mL) | 81.40 ± 24.41 | 70.47 ± 14.97 | 95.64 ± 17.16 |
| MAT (h)b | 3.89 ± 0.77 | 4.16 ± 0.63 | 4.77 ± 0.96 |
| MRT p.o. (h) | 5.70 ± 0.74 | 5.97 ± 0.20 | 6.58 ± 0.90 |
Median (range).
MAT was calculated with i.v. data previously published from (Henze et al., 2018a).
Fig. 4.
(A) Plasma concentration profiles after oral administration of 500 mg paracetamol to landrace pigs, fasted state (●), FDA breakfast (□), pig pellet feed (▲), (mean ± SEM, n = 4), (B) tmax (median ± range), (C) Mean absorption time (median ± range), (D) mean deconvolution profiles: fasted state (●), FDA breakfast (□), pig pellet feed (▲), (mean ± SEM, n = 4).
This lack of difference between the fasted and the fed study legs after oral administration of paracetamol was also reflected in the comparable MAT (Fig. 4C) and the release profile of paracetamol, calculated by numerical deconvolution (Fig. 4D). The MAT of approximately 4 h of paracetamol was observed in fasted conditions, which was similar to a previous study in minipigs (Henze et al., 2018a). While a short MAT indicates a fast drug absorption, a longer MAT indicates a delayed or prolonged absorption. A key limitation of a previous study in minipigs was the high variability of MAT of paracetamol in both fed (% CV = 100.6) and fasted state (% CV = 30.2), which limited the power to detect differences in absorption between fasting and fed state conditions (Henze et al., 2018a). In this study, absorption of paracetamol in landrace pigs was more consistent as evidenced by lower variability in MAT with % CV = 19.8 for the fasted state and % CV = 15.1 in the fed state. Despite the lower overall variability within both treatment groups, no significant differences were observed between fasted and fed state.
4. Discussion
4.1. Utility of landrace pigs to predict food effects in humans
Bioavailability of fenofibrate increased significantly after the co-administration with the FDA style breakfast, relative to the fasted state during the 8 h post-dosing period (Fig. 2, Table 1). This study confirms that a standardised preclinical food-effect protocol can be employed in landrace pigs to predict the food-effect of fenofibrate in humans. This is to the best of our knowledge the first report in the literature, where a food effect was demonstrated in a pig model for a drug product with a known positive food effect in humans. Previously, Christiansen et al. (2015) did not detect a significant food effect with the FDA breakfast in minipigs for atazanavir (positive food effect) and pravastatin (negative food effect) (Christiansen et al., 2015). A key difference of the current study was to employ a higher caloric FDA breakfast (mean 19 g food/kg bodyweight), which was approximately twice the amount of food compared to previous studies in minipigs (10 g/kg). By comparison to the established canine food protocol, dogs receive 5 g/kg to mimic human food effects studies (Lentz et al., 2007). Therefore, the results obtained in the present study suggests that the caloric content in the fed state may need to be higher in pigs in order to mimic human food effects.
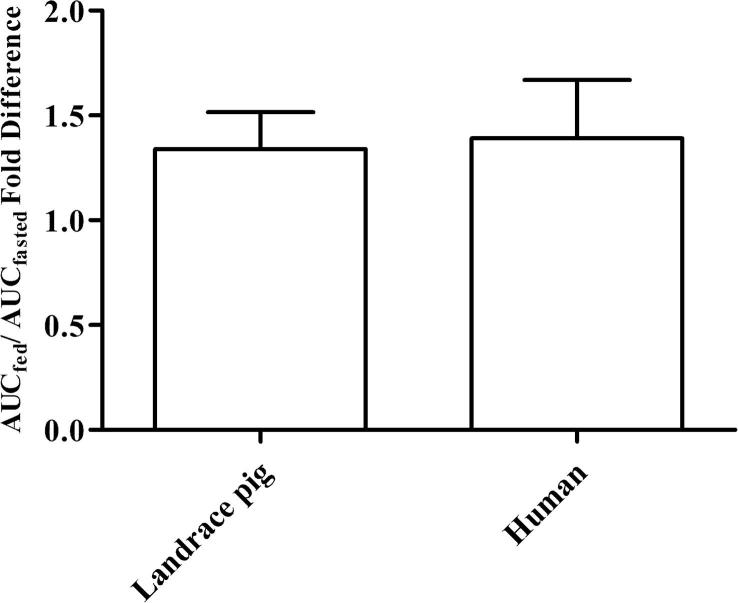
In humans, a ∼ 35% bioavailability increase under fed conditions for a micronized fenofibrate formulation has been reported in product labelling (FDA, 2017). Under fasted conditions fenofibrate is poorly absorbed because of the poor water solubility, the absorption in the fed state increased due to higher concentrations of bile salts and lipid digestion products (O'Shea et al., 2018). While a direct estimate of the individual AUC fold difference from cross over studies in humans has not been reported, Guivarc'h et al. (2004) reported the overall mean AUC0-∞ fold difference in fed versus fasted as 1.39 ± 0.74 for a micronized formulation in 7 human volunteers (Guivarc'h et al., 2004). This compares favourably to the mean AUC0-∞ fold difference under fed versus fasted conditions in this pig study, which is calculated as 1.34 ± 0.34, as illustrated in Fig. 5.
Fig. 5.
Food effect illustrated as fold difference in AUCfed/AUCfasted (0 h – ∞), data is presented as mean ± SD. In both studies a micronized fenofibrate formulation was used, the fed state was representative by a FDA style breakfast of 1000 kcal and 444 kcal in humans and pigs, respectively (Guivarc'h et al., 2004).
Additionally, a second feeding regime using standard pellet pig feed was included in the study to assess the suitability of the standard pig feed to predict food effect differences. Henze et al. (2018), previously suggested that the standard pig feed may have advantages in terms of less training and habituation to ingestion of the FDA style breakfast and the porcine digestive system may simply be better adjusted to normal pig food (Henze et al., 2018a). While in the current study the mean AUC of fenofibrate in the pig pellet group was higher with a trend of a positive food effect, overall the data was very variable resulting in no statistical significant difference between the fasted and pig pellet fed state. While pigs may be more familiar with a standard pig pellet feed as a food source, clearly consistency of the standard pig pellet food (e.g. rheological properties) will differ substantially from the FDA style breakfast. It may also be interesting to speculate whether the trend towards a faster rate of absorption of fenofibrate in pigs who received the standard pig pellet food reflected a faster emptying of pig feed from the stomach, leading to a triggering of bile secretion and subsequent enhanced solubilisation and absorption of fenofibrate. However overall, it would therefore appear that using the standard pig pellet feed as a food source in a protocol for food effect studies is of limited merit in matching FDA style post prandial conditions.
4.2. Assessing fasted state conditions in landrace pigs
In pigs gastric emptying has been reported to be more variable which can lead to prolonged gastric lag times, relative to human estimates. Even after an overnight fast, obtaining a truly fasted condition in the stomach may not be achieved (Suenderhauf et al., 2014). One reason can be that pigs have the ability to retain food for a long time due to torus pyloricus, a protuberance at the pylorus of pigs (Henze et al., 2018a). Using paracetamol as a marker of gastric emptying in the current study the pharmacokinetics results demonstrate no significant difference between the fasted and the fed state. The results are therefore similar to previous observations in minipigs, however in the previous study we could not discount that the lack of food effect in paracetamol could be masked by incomplete stomach emptying in the fasted state. The current study in landrace pigs therefore addresses a potential limitation of the previous work by confirming, via post mortem gastrointestinal content sampling, that the solid content in the stomach after an overnight fast was negligible (Fig. 3). Therefore, overall this study protocol confirms that the overnight fasting was sufficient to induce a fasted state. Paracetamol may not be suitable marker of food induced changes in gastric emptying, which will be discussed further in a subsequent section.
Suenderhauf et al. (2014) reported a total content of 76 ± 37 g was retained after an overnight fast in the stomach of Göttingen minipigs. In the present study in landrace pigs, the overall amount on contents remaining in the stomach after the fasting protocol was lower. In particular, by separating into liquid (33.4 ± 10.7 mL) and solid (10.1 ± 6.4 g) components, this study confirms near-complete stomach emptying of solid content was achieved. In the fed state a significant increase in solid (112.9 ± 60.1 g) and liquid (95.1 ± 29.4 mL) content was observed in the stomach. Collectively these studies have advanced the understanding towards establishing a standardised feeding and fasting protocol in a pig model for food effect studies, that allows for physiologically relevant comparisons to humans.
4.3. Paracetamol as a marker for gastric emptying
The present study evaluated the absorption of paracetamol in the fasted and fed state, as paracetamol has been previously used as a marker for gastric emptying rate. Pharmacokinetics of paracetamol in pigs were not significantly different between pre – and postprandial conditions.
It has previously been hypothesized that a lack of significant changes between the fasted and the fed pigs after the administration of paracetamol may reflect that a truly fasted state could not be achieved in pigs due to incomplete stomach emptying of food even after an overnight fast (Suenderhauf et al., 2014). However, based on the post-mortem assessment of the gastric content in landrace pigs, near-complete stomach emptying of solid content was confirmed and significant differences in solid content between the fasted and fed state were achieved, as discussed above (Section 4.2). While clear differences in rate and extent of absorption of fenofibrate were confirmed in pigs, the lack of differences in absorption of paracetamol between pre and post prandial conditions in pigs merits further discussion. As a BCS class I compound, paracetamol is neither permeability or solubility limited. Paracetamol, which was co-administered with 50 mL water under fasted and fed condition, is therefore expected to dissolve rapidly in gastric fluids following ingestion. One plausible explanation for the lack of a delayed absorption of paracetamol and hence no shift of tmax under fed conditions may reflect a phenomenon similar to the “Magenstrasse” (or stomach road) for gastric emptying of liquids in humans (Pal et al., 2007). The Magenstrasse in humans is considered a bypass route of liquids to travel directly from the fundus and passes through the antrum, where the liquid gastric contents are directed directly to the intestine, independent of normal gastric contraction patterns. Even under fed state conditions liquid gastric content can be emptied rapidly into the intestine (Weitschies and Wilson, 2011). Kelly et al. (2003) conducted a study in humans with radiolabelled paracetamol to assess gastric emptying in fasted and fed conditions (Kelly et al., 2003). Unexpectedly the absorption of paracetamol was similar and not statistically significant different between the pre- and post-prandial state, or even faster in the fed state compared to the fasted state for two subjects. The authors hypothesized that it reflected the Magenstrasse phenomenon, suggesting that a fast dissolution of paracetamol occurred on top of the stomach and paracetamol moved along the Magenstrasse with the liquid content of the stomach directly into the intestine (Weitschies and Wilson, 2011). Similarly, Grimm et al. (2017), recently used an MRI study to demonstrate that gastric emptying of water under fed state clinical trial was as fast as under fasted conditions, which supports the hypothesis that the Magenstrasse represents a shortcut through the fed stomach in humans and may explain why a delayed absorption is not evident in the fed state (Grimm et al., 2017). While the use of radiolabelled paracetamol or imaging tools would be required to confirm conclusively if the Magenstrasse effect is evident in pigs, the lack of a difference in paracetamol transit times between fasted and fed state observed in this study may be indicative of a similar emptying of liquids in the fasted and fed state pigs.
In contrast, in the case of the low solubility BCS Class II drug fenofibrate, the drug will not be soluble in gastric fluid and clearance from the stomach is likely to be dictated by food induced gastric contraction patterns. In summary, while the results support the utility of co-administrating paracetamol to monitor gastric emptying rate under fasted conditions, it is not suitable as a marker of gastric emptying under fed state conditions.
In addition to the limitations outlined above, further limitations of the in vivo pig model should be considered. While this study has demonstrated a positive food effect for micronized fenofibrate, further studies using a broader range of drugs are warranted before the full merits of the porcine model can be confirmed. Also, while factors in the study were adjusted to closely mimic human dosing conditions, there are inherent differences between pigs and humans that can lead to inaccurate scaling predictions. For example, while our data confirmed a positive food effect at a dose of 4 mg/kg in pigs, it is unclear if a similar effect would also be evident for the entire dose range recommended in humans (i.e. 1–4 mg/kg).
5. Conclusion
The primary objective of this study confirmed that a food effect protocol in pigs correctly predicted enhanced bioavailability of fenofibrate in humans. Post-mortem assessment of the gastrointestinal content, proved that the overnight fasting protocol was sufficient to achieve a near-complete stomach emptying and established fasting conditions in landrace pigs. No significant difference in paracetamol absorption between fasted and fed condition indicated that paracetamol absorption is not delayed under post prandial conditions. This may indicate that paracetamol was emptied into the intestine with the co-administered liquid similar to the well described Magenstrasse phenomenon in humans. Further investigations using more lipophilic model compounds or monitoring tools like a SmartPill may therefore be useful to evaluate gastric emptying rate under fasted and fed state conditions.
Declaration of Competing Interest
None.
Acknowledgements
The authors are part of the PEARRL European Training network, which has received funding from the Horizon 2020 Marie Sklodowska-Curie Innovative Training Networks programme under grant agreement No. 674909. The authors also like to thank Novo Nordisk for the advice on the surgical cannulation of the pigs in this study.
References
- Abuhelwa A.Y., Williams D.B., Upton R.N., Foster D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017;112:234–248. doi: 10.1016/j.ejpb.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Bode G., Clausing P., Gervais F., Loegsted J., Luft J., Nogues V., Sims J., Project R. The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Meth. 2010;62:196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Christiansen M.L., Mullertz A., Garmer M., Kristensen J., Jacobsen J., Abrahamsson B., Holm R. Evaluation of the use of gottingen minipigs to predict food effects on the oral absorption of drugs in humans. J. Pharm. Sci. 2015;104:135–143. doi: 10.1002/jps.24270. [DOI] [PubMed] [Google Scholar]
- Custodio J.M., Wu C.-Y., Benet L.Z. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv. Drug Deliv. Rev. 2008;60:717–733. doi: 10.1016/j.addr.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2002. Food-effect bioavailability and fed bioequivalence studies: guidance for industry. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126833.pdf, Food-effect bioavailability and fed bioequivalence studies: guidance for industry.
- FDA, 2017. TRICOR tablets [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021203s006lbl.pdf, TRICOR tablets [package insert].
- Fleisher D., Li C., Zhou Y., Pao L.H., Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration – Clinical implications. Clin. Pharmacokinet. 1999;36:233–254. doi: 10.2165/00003088-199936030-00004. [DOI] [PubMed] [Google Scholar]
- Framstad T.S.O., Aass R.A. The Norwegian School of Veterinary Science; 2000. Bleeding and Intravenous Techniques in Pigs. [Google Scholar]
- Griffin B.T., Kuentz M., Vertzoni M., Kostewicz E.S., Fei Y., Faisal W., Stillhart C., O'Driscoll C.M., Reppas C., Dressman J.B. Comparison of in vitro tests at various levels of complexity for the prediction of in vivo performance of lipid-based formulations: case studies with fenofibrate. Eur. J. Pharm. Biopharm. 2014;86:427–437. doi: 10.1016/j.ejpb.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Grimm M., Scholz E., Koziolek M., Kuhn J.P., Weitschies W. Gastric water emptying under fed state clinical trial conditions is as fast as under fasted conditions. Mol. Pharm. 2017;14:4262–4271. doi: 10.1021/acs.molpharmaceut.7b00623. [DOI] [PubMed] [Google Scholar]
- Gu C.-H., Li H., Levons J., Lentz K., Gandhi R.B., Raghavan K., Smith R.L. Predicting effect of food on extent of drug absorption based on physicochemical properties. Pharm. Res. 2007;24:1118–1130. doi: 10.1007/s11095-007-9236-1. [DOI] [PubMed] [Google Scholar]
- Guivarc'h P.H., Vachon M.G., Fordyce D. A new fenofibrate formulation: results of six single-dose, clinical studies of bioavailability under fed and fasting conditions. Clin. Ther. 2004;26:1456–1469. doi: 10.1016/j.clinthera.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Hens B., Brouwers J., Corsetti M., Augustijns P. Gastrointestinal behavior of nano- and microsized fenofibrate: in vivo evaluation in man and in vitro simulation by assessment of the permeation potential. Eur. J. Pharm. Sci. 2015;77:40–47. doi: 10.1016/j.ejps.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Henze L.J., Griffin B.T., Christiansen M., Bundgaard C., Langguth P., Holm R. Exploring gastric emptying rate in mini-pigs: effect of food type and pre-dosing of metoclopramide. Eur. J. Pharm. Sci. 2018 doi: 10.1016/j.ejps.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Henze L.J., Koehl N.J., O'Shea J.P., Kostewicz E.S., Holm R., Griffin B.T. The pig as a preclinical model for predicting oral bioavailability and in vivo performance of pharmaceutical oral dosage forms: a PEARRL review. J. Pharm. Pharmacol. 2018 doi: 10.1111/jphp.12912. [DOI] [PubMed] [Google Scholar]
- Kelly K., O'Mahony B., Lindsay B., Jones T., Grattan T.J., Rostami-Hodjegan A., Stevens H.N., Wilson C.G. Comparison of the rates of disintegration, gastric emptying, and drug absorption following administration of a new and a conventional paracetamol formulation, using gamma scintigraphy. Pharm. Res. 2003;20:1668–1673. doi: 10.1023/a:1026155822121. [DOI] [PubMed] [Google Scholar]
- Koziolek M., Alcaro S., Augustijns P., Basit A.W., Grimm M., Hens B., Hoad C.L., Jedamzik P., Madla C.M., Maliepaard M., Marciani L., Maruca A., Parrott N., Pavek P., Porter C.J.H., Reppas C., van Riet-Nales D., Rubbens J., Statelova M., Trevaskis N.L., Valentova K., Vertzoni M., Cepo D.V., Corsetti M. The mechanisms of pharmacokinetic food-drug interactions – A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019;134:31–59. doi: 10.1016/j.ejps.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Langenbucher F. Numerical convolution- deconvolution as atool for correlating in vitro with in vivo drug availability. Pharm. Ind. 1982;44:1166–1172. [Google Scholar]
- Lentz K.A. Current methods for predicting human food effect. AAPS J. 2008;10:282–288. doi: 10.1208/s12248-008-9025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz K.A., Quitko M., Morgan D.G., Grace J.E., Jr., Gleason C., Marathe P.H. Development and validation of a preclinical food effect model. J. Pharm. Sci. 2007;96:459–472. doi: 10.1002/jps.20767. [DOI] [PubMed] [Google Scholar]
- O'Shea J.P., Faisal W., Ruane-O'Hora T., Devine K.J., Kostewicz E.S., O'Driscoll C.M., Griffin B.T. Lipidic dispersion to reduce food dependent oral bioavailability of fenofibrate: in vitro, in vivo and in silico assessments. Eur. J. Pharm. Biopharm. 2015;96:207–216. doi: 10.1016/j.ejpb.2015.07.014. [DOI] [PubMed] [Google Scholar]
- O'Shea J.P., Holm R., O'Driscoll C.M., Griffin B.T. Food for thought: formulating away the food effect – a PEARRL review. J. Pharm. Pharmacol. 2018 doi: 10.1111/jphp.12957. [DOI] [PubMed] [Google Scholar]
- Pairis-Garcia M.D., Johnson A.K., Bates J.L., Stock M., Barth L.A., Brommel A.S., Stalder K.J., Karriker L.A. Development and refinement of a technique for short-term intravascular auricular vein catheter placement in mature sows. Lab. Anim. 2014;48:78–81. doi: 10.1177/0023677213514044. [DOI] [PubMed] [Google Scholar]
- Pal A., Brasseur J.G., Abrahamsson B. A stomach road or “Magenstrasse” for gastric emptying. J. Biomech. 2007;40:1202–1210. doi: 10.1016/j.jbiomech.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Pentafragka C., Symillides M., McAllister M., Dressman J., Vertzoni M., Reppas C. The impact of food intake on the luminal environment and performance of oral drug products with a view to in vitro and in silico simulations: a PEARRL review. J. Pharm. Pharmacol. 2018 doi: 10.1111/jphp.12999. [DOI] [PubMed] [Google Scholar]
- Puccinelli E., Gervasi P.G., Longo V. Xenobiotic metabolizing cytochrome P450 in pig, a promising animal model. Curr. Drug Metab. 2011;12:507–525. doi: 10.2174/138920011795713698. [DOI] [PubMed] [Google Scholar]
- Selen A., Dickinson P.A., Mullertz A., Crison J.R., Mistry H.B., Cruanes M.T., Martinez M.N., Lennernas H., Wigal T.L., Swinney D.C., Polli J.E., Serajuddin A.T., Cook J.A., Dressman J.B. The biopharmaceutics risk assessment roadmap for optimizing clinical drug product performance. J. Pharm. Sci. 2014;103:3377–3397. doi: 10.1002/jps.24162. [DOI] [PubMed] [Google Scholar]
- Sjogren E., Abrahamsson B., Augustijns P., Becker D., Bolger M.B., Brewster M., Brouwers J., Flanagan T., Harwood M., Heinen C., Holm R., Juretschke H.P., Kubbinga M., Lindahl A., Lukacova V., Munster U., Neuhoff S., Nguyen M.A., van Peer A., Reppas C., Hodjegan A.R., Tannergren C., Weitschies W., Wilson C., Zane P., Lennernas H., Langguth P. In vivo methods for drug absorption – Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur. J. Pharm. Sci. 2014;57:99–151. doi: 10.1016/j.ejps.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Suenderhauf C., Parrott N. A physiologically based pharmacokinetic model of the minipig: data compilation and model implementation. Pharm. Res. 2013;30:1–15. doi: 10.1007/s11095-012-0911-5. [DOI] [PubMed] [Google Scholar]
- Suenderhauf C., Tuffin G., Lorentsen H., Grimm H.P., Flament C., Parrott N. Pharmacokinetics of paracetamol in Gottingen minipigs: in vivo studies and modeling to elucidate physiological determinants of absorption. Pharm. Res. 2014;31:2696–2707. doi: 10.1007/s11095-014-1367-6. [DOI] [PubMed] [Google Scholar]
- Swindle, M.M., 2010. Blood collection in swine. Sample collection series. Sinclair Bio-Resources (Last Accessed February 2018).
- Swindle M.M. third ed. Crc Press-Taylor & Francis Group; Boca Raton: 2016. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. [Google Scholar]
- Swindle M.M., Smith A.C. Best practices for performing experimental surgery in swine. J. Invest. Surg. 2013;26:63–71. doi: 10.3109/08941939.2012.693149. [DOI] [PubMed] [Google Scholar]
- Varum F.J.O., Hatton G.B., Basit A.W. Food, physiology and drug delivery. Int. J. Pharm. 2013;457:446–460. doi: 10.1016/j.ijpharm.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Walters E.M., Agca Y., Ganjam V., Evans T. Animal models got you puzzled?: think pig. Ann. N. Y. Acad. Sci. 2011;1245:63–64. doi: 10.1111/j.1749-6632.2011.06345.x. [DOI] [PubMed] [Google Scholar]
- Weitschies W., Wilson C.G. In vivo imaging of drug delivery systems in the gastrointestinal tract. Int. J. Pharm. 2011;417:216–226. doi: 10.1016/j.ijpharm.2011.07.031. [DOI] [PubMed] [Google Scholar]