Abstract
Silver nanoparticles (AgNPs) are gaining considerable importance due to their attractive physicochemical properties for many applications. In the present study, (Ag NPs) were synthesized by the reduction of aqueous solutions of silver nitrate (AgNO3) with powder and solvent extracts of Padina pavonia (brown algae). The obtained nanoparticles exhibited high stability, rapid formation of the biogenic process (2 min -3 h), small size (49.58–86.37 nm) (the diameter of formed nanoparticles was measured by TEM and DLS) and variable shapes (spherical, triangular, rectangle, polyhedral and hexagonal). Preliminary characterization of nanoparticles was monitored by using UV–visible spectroscopy (UV–vis), Transmission Electron Microscopy (TEM), Dynamic Light Scattering (DLS) and finally by Fourier Transform Infrared spectroscopy (FTIR). The ratios of converted Ag NPs were recorded as 88.5; 86.2 and 90.5% in case of P. pavonia powder. extract and chloroform extract, respectively.
Keywords: Biogenic, AgNPs, Padina pavonia
1. Introduction
The novel properties of chemical nanoparticles are highly sought after in a number of new and existing industries. The application of chemical methods in synthesis of nanoparticle have shown limited success and substituted by the use of a biological approach to overcome many of the obstacles (Dhas et al., 2012). So, the bioreduction method as a novel nanoparticle synthesizing technology has attracted increasing attention because it overcame the disadvantages of conventional chemical methods such as formation, monodispersity of the particles and thermodynamic stability (Li et al., 2009). Furthermore, the bioreduction green chemistry processes led to environmental friendly synthesis and safe process as compared to other methods (Mohanpuria et al., 2008, Ibraheem et al., 2016). Biological sources such as marine algae can catalyze specific reactions as a part of modern and realistic biosynthetic strategies (Govindaraju et al., 2008). These can be alternatives to other non-biological methods which used ultraviolet, irradiation, lithography, laser ablation, ultrasonic fields and photochemical reduction techniques. The use of marine natural products in the synthesis of metallic nanoparticles has the wide range of applications in the field of nanotechnology. Biogenic synthesis of NPs prolongs superiority over the chemical processes, whereas the biogenic synthesis doesn't need to use energy, temperature, high pressure or toxic chemicals (Parashar et al., 2009). The use of biological source like the brown alga Padina pavonia for a synthesis of AgNPs submits numerous benefits for pharmaceutical and biomedical applications as they do not use toxicity. Marine macroalgae have various chemicals such as flavonoids, alkaloids, steroids, phenols, polysaccharides, saponins, hydroxyl, carboxyl, and amino functional groups, which can serve as effective metal-reducing and capping agent's to provide a robust coating on the metal nanoparticles in a single step (Abdel-Raouf et al., 2017). The present study aid to the synthesis of AgNPs by using of the marine macro alga Padina pavonia.
2. Materials and methods
2.1. Collection and preparation of algal material
The brown alga Padina pavonia (commonly distributed at Umluj seashore) was collected from marine water seashore, Umluj coast of Saudi Arabia during our previous studies in this region (Ibraheem et al., 2014). Collected sample was immediately brought to the laboratory in new plastic bags containing pond water to prevent evaporation. The algal material was washed thoroughly with tap water and distilled water to remove extraneous materials and shade-dried for 5 days and oven dried at 60 °C until constant weight was obtained, then was grind into a fine powder using the electric mixer and stored at 4 °C for future use.
2.2. Biosynthesis of silver nanoparticles by algal powder
Formation of Ag0 was carried out according to Meena et al. (2007) by mixing 1 g of P. pavonia dry matter in a 250 ml Erlenmeyer flask with 100 ml of 10–3 M AgNO3 aqueous solution. The bioreduction of AgNO3 ions occurred within 3hr in light at room temperature with the continuous stirring condition. The change in color was envisaged as the evidence of bioreduction.
2.3. Biosynthesis of silver nanoparticles by ethanol and chloroform extracts
The extraction was achieved using two different solvents separately (Ethanol and Chloroform) where; the dry algal biomass was crushed and then fed to a Soxhlet extractor. The extraction was executed on a water bath for 20 h using 200 g of algal powder in a 1 L solvent. After them, all crude extracts were collected and filtered through Whatman No. 1 filter paper and the solvents of the filtrate were evaporated by rotary evaporator under vacuum (50 °C). The resulting dried sample was crushed into powder. Two hundreds mg of the algal extract was added to 100 ml of 10−3 M aqueous AgNO3 solution and kept at room temperature for 2 min with stirring. After the reaction, the residual solution was centrifuged at 5000 rpm for 10 min (Meena et al., 2007).
2.4. Characterization of silver nanoparticles
Characterization of the purified particles was carried out according to the method described previously (Kalimuthu et al., 2008, Kalishwaralal et al., 2008) as following:
2.4.1. UV–visible spectroscopy analysis
The color change in the reaction mixture of the silver nitrate solution with Padina pavonia (powder or extract) was recorded through visual observation and the reduction of Ag+ ions was recorded by UV–vis spectrum. The analysis was done using UV–vis spectrophotometer (Shimadzu UV-1601PC) in the range of 300–700 nm. NPs solution was centrifuged at 10,000 rpm for 15 min for three times then washed with deionized water for further purification to get the pure form. The sample was completely dried at 60 °C.
2.4.2. TEM analysis
The structural characterization of the silver nanoparticles was carried by Transmission Electron Microcopy using JEOL JEM 1011 high-resolution transmission electron microscope (Japan). The sample was prepared by dispensing a drop of silver nanoparticles solution directly onto a carbon coated copper grids and allowed to dry completely at room temperature and the size and morphology of the AgNPs were characterized.
2.4.3. Dynamic Light Scattering measurements (DLS)
Size average formed algal AgNPs was evaluated by using of Dynamic Light Scattering (DLS) studies (Malvern-Zetasizer, Nano-Z590 instrument, United Kingdom).
2.4.4. FTIR measurement
Identification of the possible biomolecules responsible for the reduction of the Ag ions and capping of the reduced AgNPs synthesized by algal powder or extract was recorded by Fourier Transforms Infrared Spectroscopy (FTIR). Silver nanoparticles powder samples were prepared by centrifuging the synthesized Ag nanoparticles solution at 14,000 rpm for 10 min. The pellet which contains AgNPs was re-dispersed with sterile deionized water three times to get rid of the unattached biological impurities and remove the free proteins/enzymes (to get rid of any impurities) that are not capping ligands for the silver nanoparticles. The samples were dried in an oven overnight at 60 °C and grinded with KBr pellets and analyzed on a NICOLET 6700, Thermo, USA, in the diffuse reflectance mode operating at a resolution of 4 cm−1.
3. Result and discussion
3.1. Synthesis and characterization of AgNPs synthesized by P. pavonia
The formation of AgNPs was visually approved by color change (Plate 1) from colorless (A) to yellowish brown (B), or brownish black color (C and D). The formation AgNPs was very fast (2 min and 3 h) for algal extracts and powder, respectively and keep stable in its color for a long time at room temperature. These results are in harmony with many researchers (Gopinath et al., 2013) who advertise that the appearance of brownish black color in solution suggested the formation of silver nanoparticles. But in the current work, the algal nanoparticles recorded high stability and formed within a short time compared with that formed in the other works. In this respect, Abdel-Raouf et al., reported that the colure of Galaxaura elongate extracts changed to intense brown after 3hrs of incubation with AgNO3 (Abdel-Raouf et al., 2017).
Plate 1.

Tubes containing the aqueous solution of 10−3 M of AgNO3 with colorless at the beginning of reaction (A), after adding 1 g of P. pavonia powder with color change to yellowish brown color within 3 h (B), after adding 200 mg of P. pavonia ethanolic extract with color change to brownish black color within 2 min (C) and after adding 200 mg of P. pavonia chloroform extract with color change to dark brown color within 2 min (D).
3.2. Characterization of synthesized AgNPs
3.2.1. UV–visible spectroscopy analysis of AgNPs synthesized by P. pavonia
The UV–visible spectroscopic analysis is an honorable technicality to achieve the formation and stability of metal nanoparticles in aqueous solution. UV–visible spectra of the AgNPs synthesized by algal powder indicated that AgNPs surface Plasmon band occurs in the range of 400–500 nm in an aqueous medium (Fig. 1a). It is observed that the silver surface Plasmon resonance band of NPs in alga powder occurs at 401 nm after 3hrs of reaction and steadily increases in intensity as a function of reaction time. Also, UV–visible absorption spectra in Fig. 1b&c indicated a strong plasma resonance bands that are located at 408 and 400 nm for AgNPs formed by both P. pavonia ethanol and chloroform extracts, respectively. AgNPs have free electrons which give rise to an SPR absorption band due to the combined vibration of electrons of metal nanoparticles in resonance with the light wave (Dubey et al., 2010). Sharma et al. reported that the frequency and width of the surface Plasmon absorption be based on the size and shape of the metal nanoparticles as well as on the dielectric constant of the metal itself and the surrounding medium (Sharma et al., 2009). It is interesting; the formed P. pavonia nanoparticles were highly stabilized with no complexity of the formed AgNPs (see Fig. 1). The resulting resonances were severe and indicating no evidence for accumulation of the AgNPs in the three studied cases (powder and ethanol or chloroform extracts). It is clear from the previous results that, the alga under investigating contains some reducing agents which releasing into solutions and responsible for the formation of AgNPs.
Fig. 1.
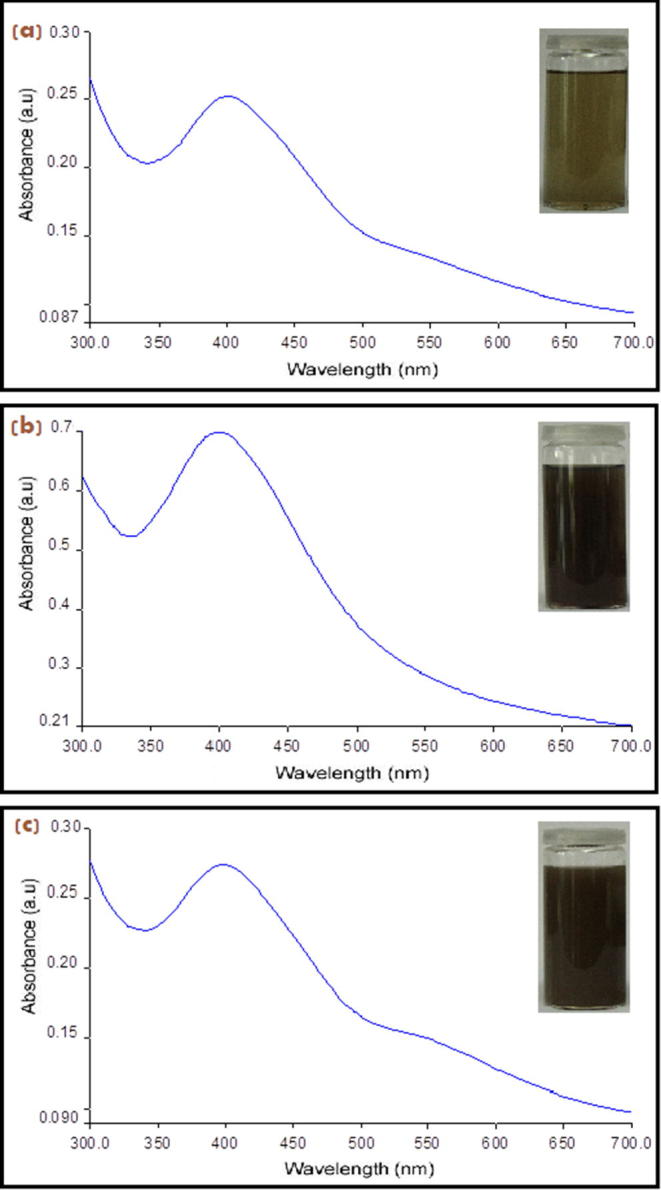
UV–visible rang spectra of AgNPs synthesized from (a) P. pavonia powder, (b) ethanol extract and (c) chloroform extract of P. pavonia.
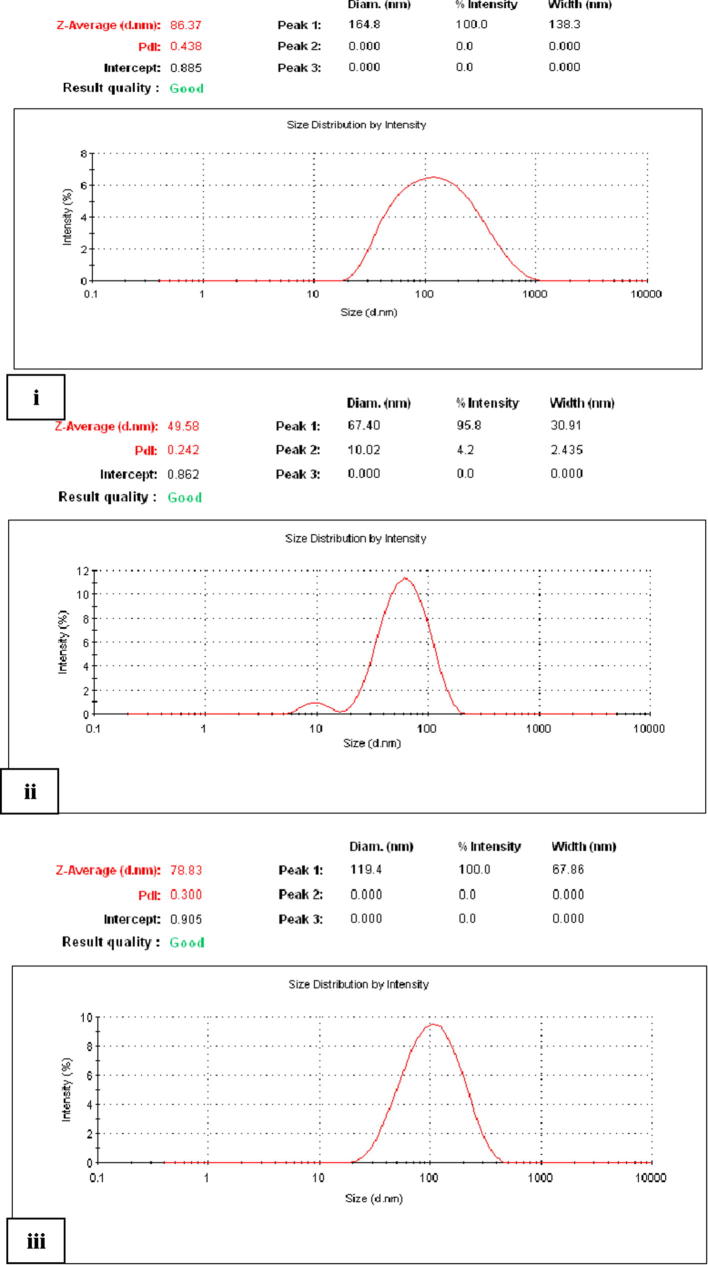
3.2.2. TEM and DLS analyses of the synthesized AgNPs by P. pavonia
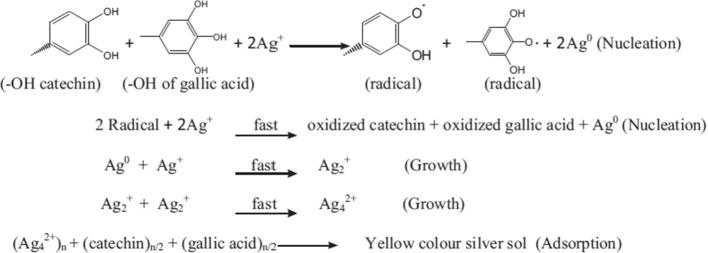
TEM and Zetasizer measurements were applied to characterize the nanoparticles size and morphology. TEM micrograph of representative silver nanoparticles synthesized by powder, ethanol and chloroform extracts of P. pavonia are shown in Plate 2 A, B, and C. The images were recorded at different magnifications. These results showed that ethanol extract of P. pavonia strongly affected the size and shape of the silver nanoparticles. Plate 2A revealed that silver nanoparticles produced by P. pavonia powder were more uniform mostly spherical and polygonal in shape with size ranging from 0.5 to 50 nm. These results confirmed by the characteristic peak observed in Zetasizer (Fig. 2i) which indicated the average size of silver nanoparticles of P. pavonia powder was 86.3 nm. Plate 2B appeared that the silver nanoparticles formed from the ethanol extract of P. pavonia were not uniform in size and shape. The nanoparticles were polydispersed with many different shapes such as pyramidal, spherical, polygonal, rod and hexagonal with highly smooth edges. The previous image contains SNPs in smaller size ranging from 1 to 49.5 nm according to TEM and Zetasizer estimation, respectively (Fig. 2ii). It is known that polygonal and pyramidal nanoparticles more active that the spherical shape because they have more active sites than the spherical shape. Also, Plate 2C of TEM observed that the smaller size of chloroform AgNPs at 0.8 nm in regular spherical shape with average size 78.8 nm which measured by Zetasizer (Fig. 2iii). These results indicated that the powder, ethanol and chloroform extracts of P. pavonia strongly affected the size and shape of the silver nanoparticles we hypothesize that the bioactive constituents of the studied alga play a pivotal reducing and controlling role in the formation of AgNPs in the solution. Ethanol and chloroform extracts of P. pavonia contain an amide group, carboxylation of the amino acids aspartate, glutamate and fatty acids. In addition to we conclude that the presence of powder and extracts of the studied alga in solution prevented the formation of aggregates as a result of algal bioactive constituents induced stabilization under the experiment. These results were confirmed and supported by the authors through chemical analyses for the same algal extracts by HPLC and GC–MS (Alenazi, 2013) who showed the presence of high percentages of Neophytadiene (41.82 and 57.8%) as a terpenoids and trans-phytol (21.6 and 16.9%) as diterpenes in ethanol and chloroform extracts, respectively. These results in agreement with Plaza et al., who reported that macro marine algae especially, brown algae contain high values of natural functional compounds as terpenes, alkaloids amino acids and fatty acids which act as stabilizers and prevent the formation of nanoparticles aggregations (Plaza et al., 2010). Padina pavonia as recorded by Alenazi contains high values of bioflavonoids as Epigallocatechin (EGC) 78.2, Epicatechin (EC) 10.69, Catechin (C) 7.9, Gallic acid 2.96 and Epigallocatechin gallate (EGCG) 2.62 ppm (Alenazi, 2013). These bioflavonoids are known as excellent stabilizing and reducing agent to reduce Ag+ to corresponding Ag0 nanoparticles. These reactions proceed to complete within 2 min and 3 h for algal extracts and powder, respectively. So, it was the novelty of our study that the identification of the compounds which were responsible for the synthesis of silver nanoparticles and act as reducing, stabilizing and capping agent. On the basis of above results, a possible mechanism was proposed to the reduction of Ag+ ions by the catechin group and gallic acid of P. pavonia extracts. In Scheme 1, first reaction nucleation, represent the reduction of Ag+ ions into metallic Ag0 by —OH of catechin group and gallic acid which were main constituents of extract (one step one electron oxidation – reduction mechanism to Ag0 and radical, nucleation, the rate determining step) (Tamuly et al., 2013). After the slow electron transfer step, reaction growth to adsorption may follow. The complexation of the formation of Ag0 atoms with Ag+ ions yield Ag2+ ions and Ag2+ dimerize to give yellow solution Ag4+. The adsorption step was further supported by the presence of a faint layer of organic molecules onto the surface of the Ag nanoparticles.
Plate 2.
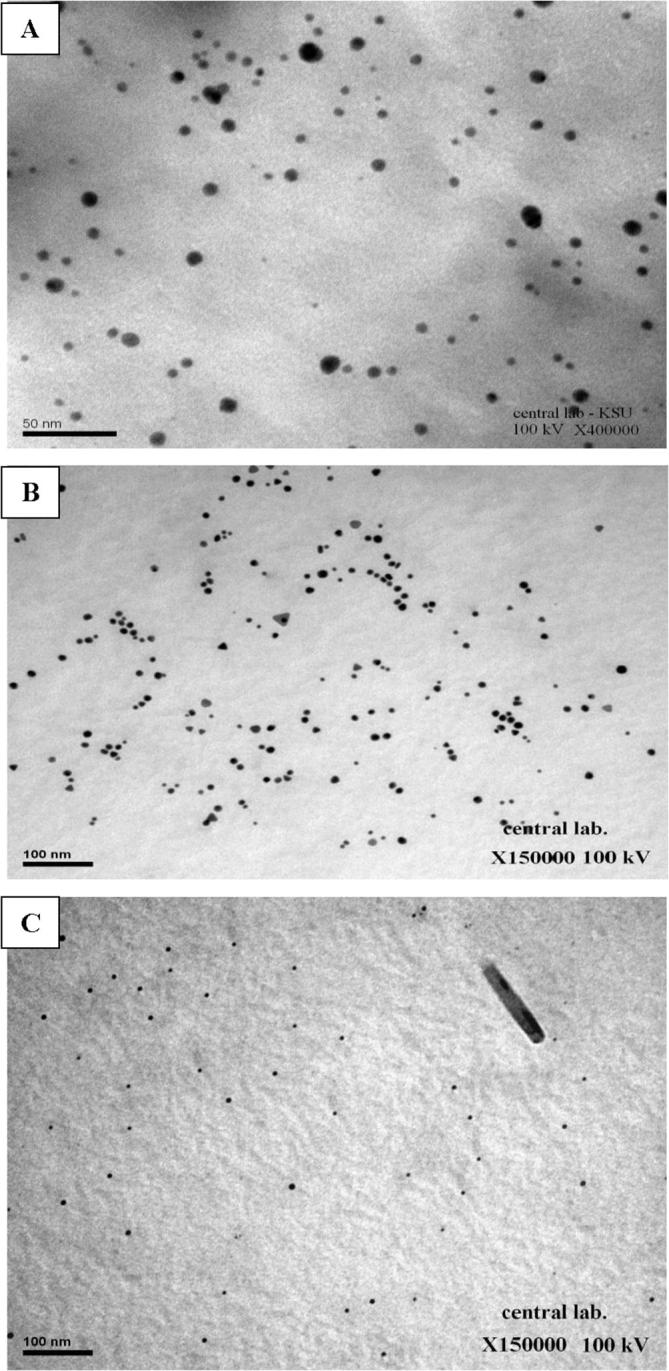
TEM micrograhs of AgNPs synthesized by (A) P. pavonia powder, (B) ethanol extract of P. pavonia and (C) chloroform extract of P. pavonia.
Fig. 2.
Zetasizer illustration of AgNPs produced by (i) P. pavonia powder, (ii) ethanol extract and (iii) chloroform extract of P. pavonia.
Scheme 1.
Mechanism to the reduction of Ag+ ions by the —OH groups of Catechin group (Epigallocatechin, Epicatechin, Catechin and Epigallocatechin gallate) and Gallic acid of P. pavonia extracts.
3.2.3. FT-IR analysis of AgNPs synthesized by P. pavonia
3.2.3.1. FT-IR analysis of AgNPs synthesized by P. pavonia powder
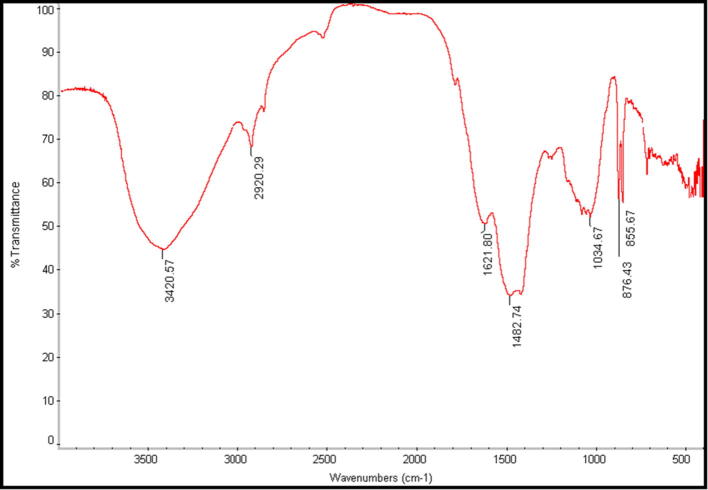
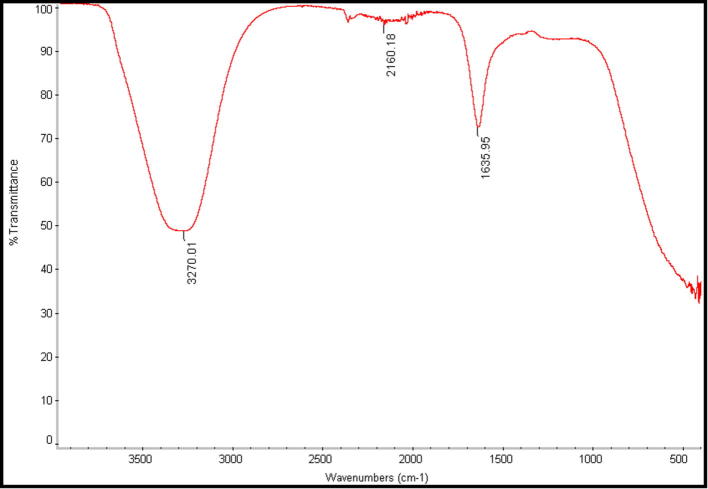
The control spectra (Fig. 3) showed a number of peaks thus reflecting a complex nature of the algal powder. On the other hand, the band intensities in different regions of the spectrum were analyzed and are shown in Fig. 3, Fig. 4. There was a shift in the following peaks: 3420–3274 and 1621–1635 cm−1. The peak located at around 3420 cm−1 was attributed to N—H formation of strong hydrogen bonding between two molecules. It is worthily mentioned that the U-shape peak around 3200–3500 cm−1 contains both N—H amine and O—H hydroxyl groups. So, this peak more observed on silver nanoparticles which synthesized from algal powder comparing to its control. Accordingly, the peak shift from 3420 to 3274 cm−1 implicated that these groups may be involved in the process of nanoparticles synthesis (Dubey et al., 2010). The untreated algal powder sample shows strong absorption bands about at 2920 cm−1 suggesting aliphatic C—H stretching (Kaswar et al., 2009). A clear shift in this peak (from 2920 to 2159 cm−1) indicated the possible involvement of amino or amide groups of algal powder in nanoparticles synthesis. The peak located at 1621 cm−1 could be assigned to the stretch vibration of C C [(in-ring) aromatic]. A broad peak shift from 1621 to 1635 cm−1 indicated the presence of amide I band in the AgNPs synthesized by algal powder.
Fig. 3.
FT-IR spectrum of P. pavonia powder.
Fig. 4.
FT-IR spectrum of AgNPs synthesized by P. pavonia powder.
The vibration shift around 1482 cm−1 of untreated P. pavonia powder (Fig. 3) was suggestive of the involvement of aliphatic – CH and aromatic – OH groups such as hydroxy flavones and hydroxy xanthones. Also, the control spectra (Fig. 3) showed that the peak located at around 1034 cm−1 was attributed to the primary amine C—N stretching vibrations of aliphatic amines. The total disappearance of these two bands (1482 and 1034 cm−1) in Fig. 4 after the bio-reduction may be due to the fact that the polyols are mainly responsible for the reduction of Ag ions. As stated earlier, cell walls of macro marine algae are mainly composed of pectin, cellulose, and hemicellulose, especially P. pavonia contains large quantities of fucoidan (it is a reducing and stabilizing agent) (polysaccharides) which used as reducing and stabilizing agent (Narayanan and Sakthivel, 2011). The functional groups associated with these polymers as well as the proteinaceous and polysaccharides matters may be involved in reducing the silver salt to Ag0. Biological components are known to interact with metal salts via these functional groups and mediate their reduction to nanoparticles (Bar et al., 2009).
3.2.3.2. FT-IR analysis of AgNPs synthesized by P. pavonia ethanol extract
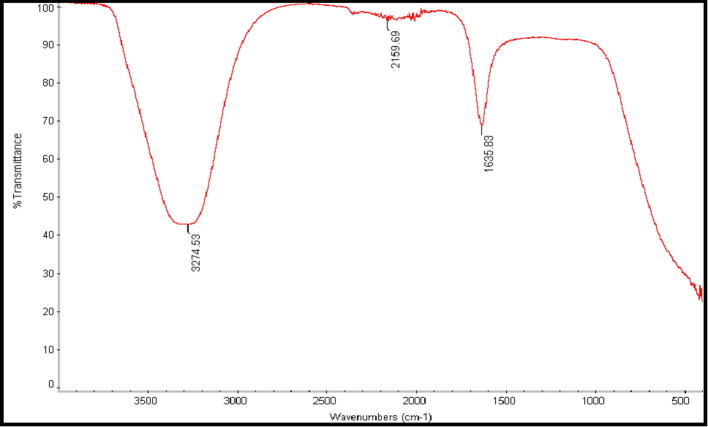
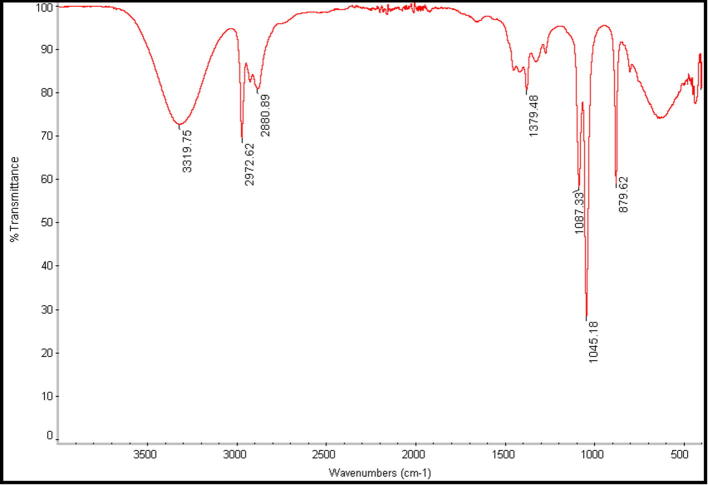
Fourier Transforms Infrared Spectroscopy analysis was performed in order to detect the functional groups on P. pavonia ethanol extract and predict their role in the synthesis of AgNPs. Data in Fig. 5, Fig. 6 revealed the band intensities in different regions of the spectrum. It was showing that there was a shift in the following peaks: 3319–3426, 2972–2919, and 2880–2850 and 1379–1384 cm−1. Also, it was observed that less intense peaks with slight shifts around 3319–3426 cm−1 due to a presence of O—H stretching. Further, supports by Mata et al. who described that hydroxyl groups (OH) are very abundant in polysaccharides which are majorly responsible for reduction process (Mata et al., 2009).
Fig. 5.
FT-IR spectrum of P. pavonia ethanol extract.
Fig. 6.
FT-IR spectrum of AgNPs synthesized by P. pavonia ethanol extract.
The IR bands at 2972 and 2880 cm−1 are characteristic of the C—H stretching symmetric and antisymmetric aliphatic and aromatic modes, respectively (Fig. 5). Also, these bands appeared as very strong bands in AgNPs of P. pavonia ethanol extract with a low shift to 2919 and 2850 cm−1, respectively (see Fig. 6). In addition, there are significant peaks at 2850, 1384 and 1467 cm−1 were indicative of the role of aromatic groups in reduction of silver ions. It is notable that a new band at about 1736 cm−1 corresponding to carbonyl stretch vibrations in ketones, aldehydes, and carboxylic acids indicating that the reduction of the silver ions is coupled to the oxidation of the hydroxyl groups in ethanol algal extract. These results were in agreement with Sanghi and Verma (2009).
Fig. 6 showed that the most useful IR band for the direct measurement of the secondary structure of the protein is a wide band found between 1736 and 1236 cm−1. The maximum absorbances of the amide I band of proteins were found in SNPs of ethanol P. pavonia extract samples near at 1736 and 1626 cm−1. Also, the amides II and III appeared at 1540 and 1236 cm−1 respectively. The amide II band is a combination of N—H in-plane bending and C—N stretching and is centered on a small peak at 1540 cm−1. This indicated that the secondary structure of the proteins is affected as a consequence of reaction with the Ag ions or binding with the algal silver NPs (Binupriya et al., 2010).
3.2.3.3. FT-IR analysis of AgNPs synthesized by P. pavonia chloroform extract
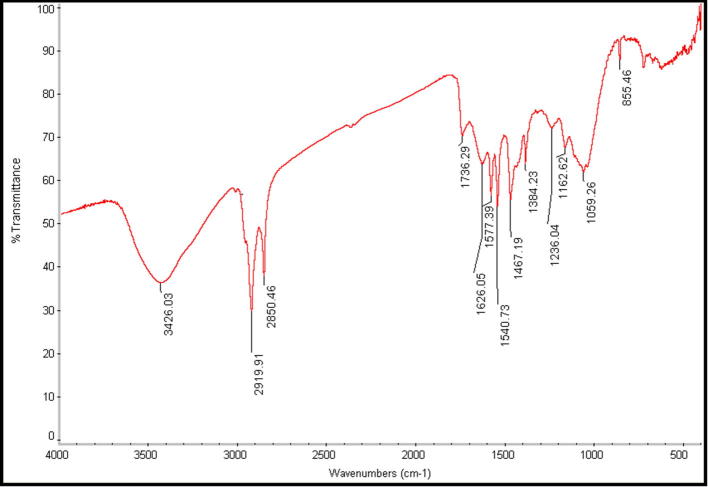
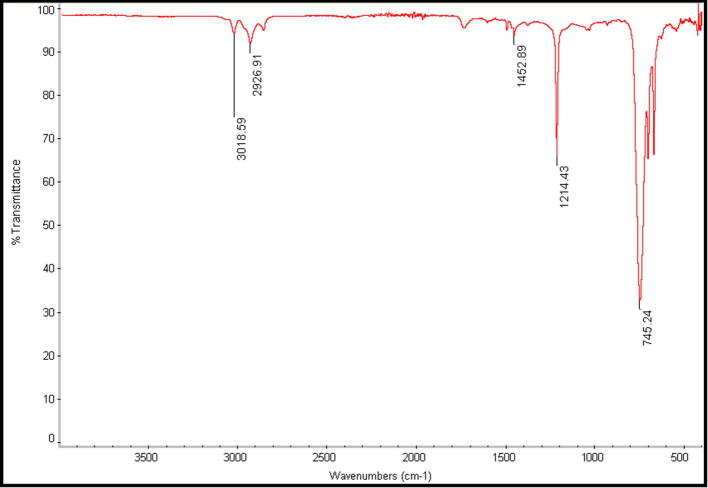
The FTIR of untreated and treated chloroform P. pavonia extract samples containing AgNPs are depicted in Fig. 7, Fig. 8. The untreated chloroform extract sample shows absorption bands at 3018, 2926, 1452 and 1214 cm−1. For untreated sample (Fig. 7) the weaker absorption band at 3018 cm−1 corresponding to H group. Also, weak aldehydic CH stretching was recorded at 2926 cm−1. Furthermore, a thinner strong band of Amide III shown at 1214 cm−1 while weak symmetric stretching vibrations of —COO— absorbed at 1452 cm−1. Fig. 8 showed that formation of strong U-shape peak occurred at 3270 cm−1 which included amide bands and O—H Stretching comparing with its control (Fig. 7). Additionally, slight amide bands of proteins recorded at 2160 cm−1 and moderate amide I bands absorbed at 1635 cm−1. Comparison between spectra of untreated to the treated samples, AgNps (Fig. 7, Fig. 8) indicated that only a major shift between untreated P. pavonia extract at 3018 to treated sample at 3270 cm−1 but a minor shift in amine groups of proteins. These results revealed that the studied alga should be the origin of the bioactive groups which may be responsible for the reduction of metal ion to metal nanoparticles. Also, the used solvent may play an important role in the extraction of these compounds (Abdel-Raouf et al., 2017, Al-Saif et al., 2014).
Fig. 7.
FT-IR spectrum of P. pavonia chloroform extract.
Fig. 8.
FT-IR spectrum of AgNPs synthesized by P. pavonia chloroform extract.
4. Conclusions
Synthesis of AgNPs using biological resources like marine algae is a challenging alternative to chemical synthesis since this novel biogenic method were eco-friendly methods. The obtained data clearly indicate the algal extracts can be used as an effective capping as well as the reducing agent for the synthesis of AgNPs. Silver nanoparticles synthesized by P. pavonia are quite stable and no visible changes in a long time. This can be supportive for medical uses. All used analysis showed that there is a major distribution of particle size with many different shapes such as pyramidal, spherical, polygonal, rod and hexagonal with highly smooth edges. Their size ranged from 49.58 to 86.37 nm.
Acknowledgment
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Neveen Abdel-Raouf, Email: neveenabdelraouf@science.bsu.edu.eg.
Nouf Mohammad Al-Enazi, Email: no_sa2007@hotmail.com.
Ibraheem Borie Mohammad Ibraheem, Email: ibraheemborie@science.bsu.edu.eg.
Reem Mohammed Alharbi, Email: r_0660@hotmail.com.
Manal Mohammed Alkhulaifi, Email: manalk@ksu.edu.sa.
References
- Abdel-Raouf N., Al-Enazi N.M., Ibraheem B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arabian J. Chem. 2017;10:S3029–S3039. [Google Scholar]
- Alenazi, N.M., 2013. Biogenic synthesis of nanoparticles and their synergistic effect with some benthic marine macroalgal extracts isolated from Umluj (KSA) seashore against some pathogenic bacteria. Ph.D. Thesis, Collage of Scie. KSU, KSA. pp. 83–90.
- Al-Saif S.S., Abdel-Raouf N., El-Wazanani H.A., Aref I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 2014;21:57–64. doi: 10.1016/j.sjbs.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar H., Bhui D., Sahoo G., Sarkar P., Pyne S., Misra A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A: Physicochem. Eng. Aspects. 2009;348:212–216. [Google Scholar]
- Binupriya A.R., Sathishkumar M., Vijayaraghavan K., Yun S.I. Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J. Hazard. Mater. 2010;177:539–545. doi: 10.1016/j.jhazmat.2009.12.066. [DOI] [PubMed] [Google Scholar]
- Dhas T., Kumar V., Abraham L., Karthick V., Govindaraju K. Sargassum myriocystum mediated biosynthesis of gold nanoparticles. Spectrochim. Acta Part A. Mol. Biomol. Spectrosc. 2012;99:97–101. doi: 10.1016/j.saa.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Dubey S.P., Lahtinen M., Sillanpaa M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010;45:1065–1071. [Google Scholar]
- Gopinath V., Priyadarshini S., Priyadharssini N., Pandian K., Velusamy P. Biogenic synthesis of antibacterial silver chloride nanoparticles using leaf extracts of Cissus quadrangularis Linn. Mater. Lett. 2013;91:224–227. [Google Scholar]
- Govindaraju K., Kiruthiga V., Ganesh K.V., Singaravelu G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii, Grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2008;9:5497–5501. doi: 10.1166/jnn.2009.1199. [DOI] [PubMed] [Google Scholar]
- Ibraheem I.B.M., Abd-Elaziz B.E.E., Saad W.F., Fathy W.A. Green biosynthesis of silver nanoparticles using marine Red Algae Acanthophora specifera and its antimicrobial activity. J. Nanomed. Nanotechnol. 2016;7:409. doi: 10.4172/2157-7439.1000409. [DOI] [Google Scholar]
- Ibraheem I.B.M., Alharbi R.M., Abdel-Raouf N., Al-Enazi N.M. Contributions to the study of the marine algae inhabiting Umluj Seashore, Red Sea. Beni-Suef Univ. J. Basic Appl. Sci. 2014;3(4):278–285. [Google Scholar]
- Kalimuthu K., Babu R. Suresh, Venkataraman D., Bilal M., Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces. 2008;65(1):150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kalishwaralal K., Deepak V., Ramkumarpandian S., Nellaiah H., Sangiliyandi G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008;62(29):4411–4413. [Google Scholar]
- Kaswar S.M.A., Mostafa G., Huq E., Nahar N., Ozeki Y. Chemical constituents and hemolytic activity of Macrotyloma uniflorum L. Int. J. Biol. Chem. 2009;3:42–48. [Google Scholar]
- Li H., Carter J.D., Labean T.H. Nanofabrication by DNA self-assembly. Mater. Today. 2009;12(5):24–32. [Google Scholar]
- Mata Y., Torres E., Blazques M., Ballester A., Gonzalez F., Munoz J. Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2009;166:612–618. doi: 10.1016/j.jhazmat.2008.11.064. [DOI] [PubMed] [Google Scholar]
- Meena R., Siddhanta A.K., Prasad K., Ramavat B.K., Eswaran K., Thiruppathi S., Ganesan M., Mantri V.A., Subba Rao P.V. Preparation, characterization and benchmarking of agarose from Gracilaria dura of Indian waters. Carbohydr. Polym. 2007;69:179–188. [Google Scholar]
- Mohanpuria P., Rana K.N., Yadav S.K. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 2008;10:507–517. [Google Scholar]
- Narayanan K., Sakthivel N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 2011;10:1–21. doi: 10.1016/j.cis.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Parashar U.K., Saxena S.P., Srivastava A. Bioinspired synthesis of silver nanoparticles. Digest J. Nanomater. Biostruct. 2009;4(1):159–166. [Google Scholar]
- Plaza M., Santoyo S., Jaime L., Garcia G., Herrero M., Senorans F., Ibanez E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010;51:450–455. doi: 10.1016/j.jpba.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Sanghi R., Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009;100:501–504. doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Tamuly C., Hazarika M., Borah S., Das M., Boruah M. In situ biosynthesis of Ag, Au and bimetallic nanoparticles using Piper pedicellatum C.DC: green chemistry approach. Colloids Surf. B: Biointerfaces. 2013;102:627–634. doi: 10.1016/j.colsurfb.2012.09.007. [DOI] [PubMed] [Google Scholar]