Abstract
The aim of this study is to evaluate the effect of sheep follicular fluid (SFF) supplementation of the in vitro maturation (IVM) media of sheep oocytes on the resumption of meiosis, glutathione (GSH) level, and expression of apoptosis (Bax, Bcl-2) as well as heat shock protein beta-1 (HSPB1) genes. Sheep ovaries were collected from the central slaughterhouse of Riyadh city, KSA. Oocytes were aspirated from 3 to 8 mm follicles. Sheep oocytes were cultured in maturation medium with different concentrations of sheep follicular fluid: 0% (control), 10%, 20% and 40% for 24 h. The results indicated that the maturation rate of oocytes was significantly (p ≤ .05) decreased in 40% SFF (36.87%) versus the control (61.3%), 10% SFF (63.95%) and 20% SFF (64.08%). The supplementation of the IVM medium with 10% SFF induced an intra-oocyte GSH concentration that was significantly higher than in sheep oocytes cultured with 20% and 40% SFF and similar to the GSH content in oocytes cultured without SFF. Real-time polymerase chain reaction analysis of gene expression revealed no significant differences in the Bax and HSPB1 genes between the control and 10% SFF, whereas they were significantly higher in 40% FF (p ≤ .05) compared to the control. The expression of Bax:Bcl-2 was significantly higher in 20% and 40% SFF compared to the control group. In conclusion, the addition of SFF to the IVM culture of sheep oocytes is recommended to support nuclear maturation and increase oocyte competence.
Keywords: In vitro maturation, Sheep follicular fluid, Glutathione, Apoptosis genes
1. Introduction
Oocytes must progress during folliculogenesis from the diplotene to the MII stage to become mature at the nuclear level. However, completing nuclear maturation does not ensure embryonic development. Oocyte maturation also includes cytoplasmic transformations to support fertilization and subsequent embryo development (Atef and Marc-Andre, 2002, Trounson et al., 2001, Yang et al., 1998).
The in vitro maturation (IVM) of oocytes has become a crucial step in the in vitro production (IVP) of sheep embryos. Although oocytes matured in vitro and in vivo have similar rates of nuclear maturation, fertilization and cleavage, they clearly differ in their developmental potential (Blondin and Sirard, 1995, Dieleman et al., 2002, Dunning et al., 2007, Kyasari et al., 2012, Rizos et al., 2002). Thus, the blastocyst rate after in vitro oocyte maturation, fertilization and embryo culture is 30–40%, whereas 70% of in vivo mature oocytes develop into blastocysts (Dieleman et al., 2002, Humblot et al., 2005, Rizos et al., 2002, Romar et al., 2011, Smiljakovic and Tomek, 2006, van de Leemput et al., 1999). Thus, several substances have been added to test different culture conditions for IVM. One possible way to improve IVM efficiency is to supplement IVM medium with follicular fluid.
The components of follicular fluid are mainly derived from the serum and synthesized products of follicular cells and oocytes (Avery et al., 2003, Li et al., 2000). Follicular fluid contains an abundance of steroid hormones, FSH, LH, growth factors, cytokines, glucose, cholesterol, triglycerides, albumin, globulin, electrolytes, enzymes and nutrients (Angelucci et al., 2006, Edwards, 1974, Kor and Moradi, 2013, Tabatabaei and Mamoei, 2011, Tabatabaei et al., 2011). These factors may affect oocyte quality, fertilization and embryonic development. Follicular fluid is a natural medium for oocyte maturation during folliculogenesis and may be suitable as a supplement to allow the in vitro culture medium to mimic the in vivo microenvironment and promote development (Coleman et al., 2007, Driancourt and Thuel, 1998, Knight et al., 1996).
Glutathione (GSH) is the major non-protein sulfhydryl compound found in mammalian cells that protects the cells from oxidative stress (Droge, 2002, Pastore et al., 2003). Many factors, when added to IVM medium, promote cysteine uptake in oocytes and enhance glutathione synthesis in the ooplasm (de Matos and Furnus, 2000, Gasparrini et al., 2000). Thus, the cytoplasmic glutathione concentration after IVM is a good marker of cytoplasmic maturation (Livingston et al., 2009, Lojkic et al., 2012).
On the other hand, gene expression can be altered by the culture conditions not only in the oocyte but also in the blastocyst stages (Lonergan et al., 2003, Nemcova et al., 2006, Rizos et al., 2002). Knowledge regarding gene expression during oocyte maturation is crucial for the optimization of in vitro embryo production (Aswal et al., 2008). Some conditions for in vitro maturation can induce the expression of apoptotic genes (e.g., Bcl-2: anti-apoptotic and Bax: pro-apoptotic) in oocytes. The sensitivity of cells to apoptotic stimuli may depend on the balance of pro- and anti-apoptotic genes (Ebrahimi et al., 2010, Kim and Tilly, 2004, Yang and Rajamahendran, 2002). Bax gene expression was increased when serum was added to the culture medium (Rizos et al., 2002, Sanna, 2009) and in degenerated embryos compared to good quality embryos (Yang et al., 1998).
Heat shock protein (HSPB1) is induced by heat shock, freezing and slight changes in pH (Chernik et al., 2004). This protein plays an important role not only in various stress conditions but also in certain biological events, including gene activation, cell cycle arrest, differentiation and apoptosis (Palasz et al., 2008). These genes (Bax, Bcl-2 and HSPB1) could be considered good markers of oocyte maturation.
Some studies have shown that the use of supplementary follicular fluid plays a key role in the ability of oocytes to undergo nuclear and cytoplasmic maturation, fertilization and embryonic development in cattle (Ali et al., 2004, Kim et al., 1996, Larocca et al., 1993), humans (Chi et al., 1998), sheep (Guler et al., 2000, Shabankareh and Sarsaifi, 2008, Sun et al., 1994, Thompson, 2000), pigs (Huang et al., 2002, Ito et al., 2008), buffalo (Gupta et al., 2005, Nandi et al., 2004), horses (Bogh et al., 2002), and goats (Cognie et al., 2003, Masudul et al., 2012). However, the effect of follicular fluid on the glutathione level and gene expression of apoptotic genes has not been tested in sheep oocytes after IVM. The objectives of the current study were to investigate the effects of sheep follicular fluid on in vitro maturation, glutathione concentration and expression of apoptosis (Bax, Bcl-2) and heat shock protein (HSPB1) genes in sheep oocytes.
2. Materials and methods
2.1. Chemicals
All chemicals and media used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
2.2. Preparation of follicular fluid
The follicular fluid was obtained by aspiration from normal follicles located on sheep ovaries collected from fresh slaughtered animals in abattoir. The fluid was pooled in 15-ml centrifuge tubes and centrifuged twice at 4000 rpm for 20 min to remove cellular debris. The supernatant was collected, filtered on a 0.45-µm filter, and then heat inactivated at 56 °C for 30 min and stored as aliquots in 1.5-ml Eppendorf tubes at −20 °C until use (Sun et al., 1994).
2.3. Experimental design
After oocyte collection from the ovaries, the cumulus oocyte complexes (COCs) were divided into four groups depending on the concentration of sheep follicular fluid (SFF) added to the in vitro maturation medium: 0% SFF (control); 10% SFF; 20% SFF; and 40% SFF. After maturation, all oocytes were subjected to 199 Hank's medium supplemented with 100 IU/ml hyaluronidase enzyme to remove the surrounding cumulus cells by repeat pipetting, and then, the denuded oocytes in each group were divided into 3 lines: first line, evaluation of nuclear maturation using aceto-orcine stain; second line, measurement of the intracellular glutathione concentration; and last line, detection of the expression of selected genes (Bax, Bcl-2, and HSPB1).
2.4. In vitro maturation
Sheep ovaries were collected from the central slaughterhouse in Riyadh, Saudi Arabia, and transported to the laboratory in normal saline at 30–35 °C in a thermos flask within 1–3 h after collection. The extra-ovarian tissues were removed, and the ovaries were washed three times with warm fresh normal saline (37◦C). Oocytes were obtained by the aspiration of follicles (3–6 mm in diameter) using a 10-ml sterile syringe with a 19 G needle containing 0.5 ml of handling medium {TCM-199 (Hank's salts), supplemented with 10% FCS, 0.5 mM sodium pyruvate, 140 µg/ml heparin and 50 µg/ml gentamycin}. The aspirated follicular fluid containing the oocytes was placed in 15-ml tubes in a water bath (37 °C) for approximately 10 min to allow settling in the bottom of the tube. The COCs with more than three layers of intact cumulus cells and uniform cytoplasm were collected under a stereomicroscope by mouth pipette and washed three times in maturation medium {TCM-199 (Earle's salts) with 10% FCS, 0.5 mM sodium pyruvate, 0.02 AU/ml FSH, 0.023 AU/ml LH, 1 µg/ml E2, 100 µM cystamine, 50 µg/ml gentamycin and 0% SFF for group 1, 10% for group 2, 20% for group 3, and 40% for group 4}. Groups of ten COCs were placed in 50-µl maturation drops, which were covered with mineral oil and incubated in a humidified atmosphere and 5% CO2 for 24 h at 38.5 °C.
2.5. Evaluation of oocyte meiotic maturation
Samples of denuded oocytes from each group were mounted on glass slides, covered and fixed on slides with 3 volumes of acetic acid to one volume of ethanol (v/v) for 24 h and stained with 1% aceto-orcein stain. The oocytes were examined using a phase contrast microscope (400x Olympus, CKX41 Japan) and assessed for oocyte nuclear status, including Germinal vesicle, Germinal vesicle Breakdown, Metaphase I and Metaphase II (Table 2).
Table 2.
Effect of the supplementation in vitro maturation medium with SFF on maturation rates of sheep oocytes.
| Nuclear status (Mean ± SEM) |
Deg. oocytes (%) | Total oocytes | Concentration of SFF | |||
|---|---|---|---|---|---|---|
| M II (%) | M I (%) | GVBD (%) | GV (%) | |||
| 276 (61.39 ± 3.50)a |
64 (14.23 ± 2.82)a |
44 (9.7 ± 1.4) a |
16 (3.55 ± 0.76)a,b |
49 (11.15 ± 2.1) |
449 | 0% SFF (control) |
| 301 (63.95 ± 1.84)a |
41 (6.99 ± 1.92)a |
51 (11.09 ± 1.64) a,b |
8 (1.58 ± 0.85)a |
69 (14.6 ± 1.1) |
470 | 10% SFF |
| 273 (64.08 ± 6.47) a |
78 (18.76 ± 2.31)b |
41 (9.92 ± 1.51)a,b |
8 (1.73 ± 0.78)a |
46 (10.92 ± 1.1) |
426 | 20% SFF |
| 170 (36.87 ± 2.14) b |
130 (28.32 ± 1.35)c |
73 (15.89 ± 1.4)b |
27 (5.86 ± 0.47)b |
59 (13.04 ± 1.6) |
459 | 40% SFF |
Data are presented as the Mean ± S.E.M. GV: germinal vesicle; GVBD: germinal vesicle break down; MI: metaphase-I; MII: metaphase-II; SFF sheep follicular fluid. Five replicates in each treatment. (a,b,c) letters with different superscript in the same column differ significantly (p ≤ .05).
2.6. Glutathione assay
Samples of 20 denuded oocytes from each groups (A, B, C and D) were washed three times in PBS, then transferred into a 1.5-ml microfuge tube containing 5 µl of PBS and stored at −80 °C until use. The experiments were performed in four replicates in each group. The frozen samples were thawed at room temperature and centrifuged. The quantity of glutathione (GSH) in the oocytes was assayed according to the Glutathione kit instructions (Cat. No. CS0260). The amount of GSH in each sample was determined based on the standards and calculated for 20 oocytes.
2.7. Sample collection, RNA isolation and reverse transcription
Samples of 20 denuded oocytes from each group (0% SFF (control); 10% SFF; 20% SFF; and 40% SFF) were washed in 0.1% PBS-PVA, then transferred into an Eppendorf tube containing 5 µL of 0.1% PBS-PVA and stored at −80 °C until RNA extraction. The experiments were performed in three replicates for each group. RNA was isolated from the oocytes, and complementary DNA (cDNA) was synthesized using a Cells-to-cDNA™II Kit (Cat. No. AM1722) as described by the manufacturer’s instructions. Briefly, pools of oocytes from each group were lysed by adding 100 µL of ice-cold Cell Lysis II Buffer to each sample in 1.5-ml Eppendorf tubes. After vortexing and centrifugation, the samples were incubated at 75 °C for 10 min. For genomic DNA removal, RNA samples were treated with 2 µL of DNase I per 100 µL of Cell Lysis II Buffer for 30 min at 37 °C. To inactivate DNase I, the samples were heated at 75 °C for 5 min. Reverse-transcription reactions were performed with 3 μg of total RNA, dNTP Mix, Oligo(dT)18 Primer, 10X RT Buffer, and RNase Inhibitor in a total reaction volume of 20 µL for 60 minutes at 42 °C, followed by 10 minutes at 95 °C to inactivate the reverse transcriptase. The integrity of the RNA was visualized on a 1% agarose gel using a gel documentation system (Universal Hood II, BioRad). Then, the RNA concentration was determined by spectrophotometry (NanoDrop, Wilmington, DE, USA) using 1-µL samples.
2.8. Real-time polymerase chain reaction (RT-PCR)
Real-time PCR was performed in 50 µL of reaction buffer containing 25 µL of SYBR® Green Master Mix (Applied Biosystems, 4367659), 2 µL of each of the forward and reverse primer pairs for each gene (10 pM), 2.5 µL of cDNA, and 18.5 µl of nuclease-free water. The primer sequences, annealing temperature, approximate sizes of the amplified fragments of all transcripts and GenBank® accession number are listed in Table 1. Histone H2AFZ (Histone 2A, member Z) mRNA was used as an internal standard. The program used for the amplification of the genes consisted of denaturing at 95 °C for 10 min followed by 45 cycles of PCR (denaturation at 95 °C for 15 s, annealing at 60 °C for one minute, and extension at 72 °C for one minute). All reactions were conducted using the LightCycler® 480 real-time PCR machine (Cat. No. 04 640 268 001). The expression of each gene was calculated by (Schmittgen and Livak, 2008).
Table 1.
The sequence of primers for quantitative Real-time PCR.
| Functions | Genes | Primer sequences (5′–3′) | Size (bp) | Ref. |
|---|---|---|---|---|
| Endogenous control | H2AFZ | (F) AGGACGACTAGCCATGGACGTGTG(R) CCACCACCAGCAATTGTAGCCTTG |
212 | Sanna (2009) |
| Apoptosis genes | BAX | (F) CTACTTTGCCAGCAAACTGG(R) TCCCAAAGTAGGAGAGGA |
158 | Sanna (2009) |
| Bcl-2 | (F): GCCGAGATGTCCAGTCAGC(R) :GACGCTCTCCACACACATGAC |
150 | Ebrahimi et al. (2010) | |
| Stress gene | HSPB1 | (F) TCCCTGGACGTCAACCACTTCG(R) AGGTTTGGCGGGTGAGGATGTC |
391 | Sanna (2009) |
2.9. Statistical analysis
Statistical analyses of data from at least three replicate trials for each treatment comparison were performed using the SPSS version 20 software package (SPSS Inc., Chicago, IL, USA). Data were first evaluated using the Kolmogrov-Smirnov normalization test. The means of GV, GVBD, MI, MII and degenerated oocytes after in vitro maturation, glutathione concentration and relative gene expression in all groups were compared by one-way analysis of variance (ANOVA) and post-LSD Dunnett’s test.
3. Results
The overall percentages of mature oocytes in the different groups are reported in Table 2. The results show that the different concentrations of follicular fluid in the first three groups does not affect the maturation rate significantly, whereas the maturation rate is significantly (p ≤ .05) decreased in 40% SFF (36.87%). Simultaneously, the proportions of GV, GVBD, and MI oocytes were found to be higher in 40% SFF than the other groups (Table 2).
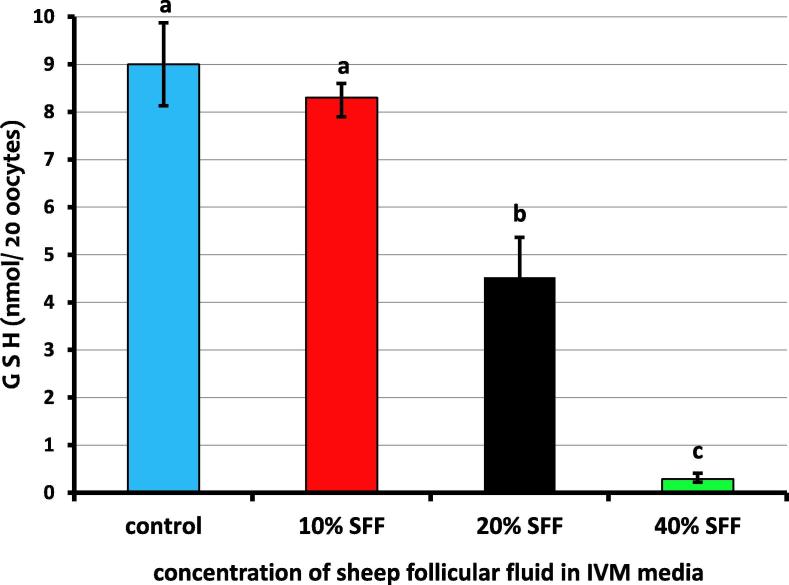
The glutathione levels measured in sheep oocytes after maturation using IVM media supplemented with different concentrations of follicular fluid are shown in Fig. 1. Our results indicate that the level of glutathione was significantly (p ≤ .05) lower in 20% SFF and 40% SFF compared to the control group. A decrease was also observed in the level of glutathione in 10% SFF compared to the control, but the difference was not significant. The maximum depletion of the glutathione level was observed in 40% SFF (Fig. 1).
Fig. 1.
Glutathione levels in sheep oocytes with different concentrations of sheep follicular fluid during IVM. Columns with different superscript (a, b, c) differ significantly (p ≤ .05).
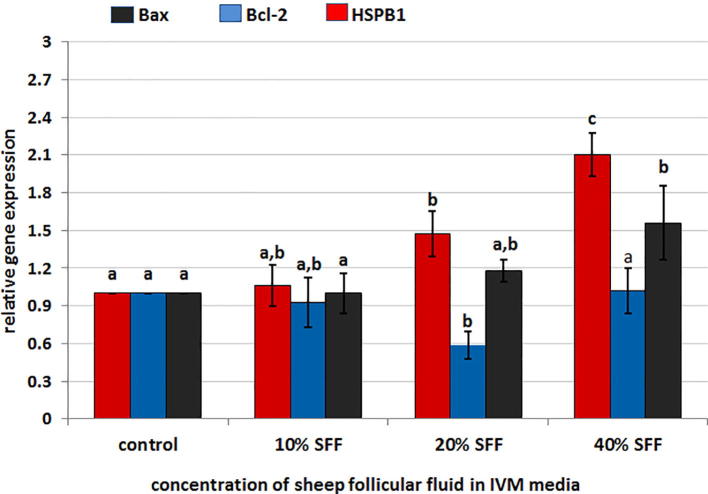
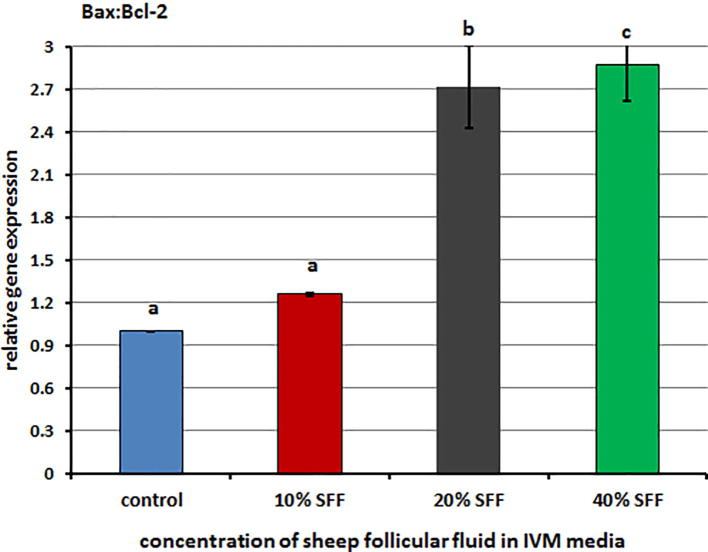
The expression levels of apoptosis marker genes and heat shock protein genes are summarized in Fig. 2, Fig. 3. The expression levels of the Bax and HSPB1 genes were significantly up-regulated in 40% SFF compared to the control group (Fig. 2), and the level of expression of the Bcl-2 gene was down-regulated in 20% SFF compared to the control group (Fig. 2). The Bax:Bcl-2 ratio was up-regulated in all groups and significantly up-regulated in 20% SFF and 40% SFF compared with the control group (Fig. 3).
Fig. 2.
Fold differences in relative gene expression for three different genes; Bax, Bcl-2 and HSPB1 genes in sheep oocytes matured in different concentrations of sheep follicular fluid during IVM. Columns with different superscript (a, b, c) for the same gene columns differ significantly (p ≤ .05).
Fig. 3.
Relative Quantitative RT-PCR representing the Bax/Bcl-2 ratio in sheep oocytes matured in different concentrations of sheep follicular fluid during IVM. Columns with different superscript (a, b, c) differ significantly (p ≤ .05).
4. Discussion
Follicular fluid contains many important hormones and growth factors that support the in vivo maturation of oocytes. The addition of follicular fluid to the in vitro maturation medium of sheep oocytes may increase oocyte competence. Here, for the first time, we report the use of real-time RT-PCR to characterize the changes in the relative gene expression of apoptosis genes (Bax, Bcl-2) and heat shock protein (HSPB1) and the estimation of the glutathione concentration in sheep oocytes cultured at different concentrations of follicular fluid in IVM media.
It was observed that the addition of SFF to IVM media at 10% and 20% concentrations could support the in vitro maturation of sheep oocytes. The maturation rates of oocytes in 10% SFF (63.95%) and 20% SFF (64.08%) were similar to the maturation rates of the oocytes in the control group (61.39%), with no significant difference among the groups. These results were in good agreement with observations in cattle (Coleman et al., 2007) and in horses (Caillaud et al., 2008). In another study, (Sun et al., 1994) observed that the addition of SFF to IVM at a concentration of 20% significantly increased the rate of matured oocytes. Concurrently, an inhibitory effect of FF on the maturation rate was observed in 40% SFF at a 40% concentration, which is consistent with the findings of Kim et al. (1993). They reported that adding 60% follicular fluid to the IVM medium inhibits bovine oocyte maturation, probably due to coagulating the cumulus cell mass with a fibrin-like substance in the follicular fluid, whereas 10% follicular fluid stimulated both the maturation and developmental capacity of bovine oocytes (Kim et al., 1993). Supporting these results, we found that the glutathione levels of oocytes in the first three groups, 0% SFF, 10% SFF and 20% SFF, were significantly higher than in the last group, 40% SFF. It is known that the intracellular GSH level increases during maturation and peaks when the oocytes reach metaphase II (de Matos and Furnus, 2000, Perreault et al., 1988, Yoshida et al., 1993).
The in vitro maturation conditions can alter the acquisition of oocyte developmental competence and could influence the levels of certain oocyte transcripts (Nemcova et al., 2006, Watson et al., 2000). Many studies have used the expression of apoptotic genes as a quality marker for oocyte viability (Tatone et al., 2006, Yang and Rajamahendran, 2002). We used the sheep histone H2AFZ gene as an endogenous control for quantifying the expression of other genes, as described in (Nemcova et al., 2006, Sanna, 2009). The expression levels of Bax and HSPB1 showed no significant differences between the control and 10% SFF, but were significantly higher (p ≤ .05) in 40% SFF compared to the control group. Lonergan et al. (2003) reported the Bax and Bcl-2 genes to be significantly altered by modification of the culture conditions. Furthermore, the ratio of Bax:Bcl-2 could potentially be used as a tool to compare various treatments (Rao et al., 2012). These results indicated the significantly heightened expression of Bax:Bcl-2 in 20% SFF and 40% SFF compared to the control group. As the expression of heat shock protein genes may be altered during oocyte culture, the expression of these genes can be used as an indicator of culture conditions (Warzych et al., 2007, Wrenzycki et al., 2005). It has been suggested that interaction in the expression levels of the Hsp-70 and Bax genes may occur under stress conditions, with Bax activation suppressed in cells with high Hsp-70 levels (Stankiewicz et al., 2005). Furthermore, oocytes undergoing apoptosis have poor developmental competence (Li et al., 2009). HSPB1 enhances the resistance of cells to the deleterious effects induced by heat shock, oxidative stress or conditions that trigger apoptosis (Paul et al., 2010). These data are similar to the findings of Avery et al. (2003), who reported that the maturation of cattle oocytes cultured in large quantities of bovine follicular fluid was inhibited compared with the maturation of cattle oocytes cultured in small amounts of follicular fluid. The sudden change of media in the in vitro environment probably caused sharp changes in oocyte metabolic pathways, negatively interfering with the maturation process (Cruz et al., 2014).
In conclusion, this study reports for the first time the use of real-time RT-PCR to characterize the changes in the relative gene expression of apoptosis genes (Bax, Bcl-2) and heat shock protein (HSPB1) and to estimate the glutathione concentration in sheep oocytes cultured at different concentrations of follicular fluid in IVM media. The data also suggest that the addition of a small amount of SFF to the IVM media of sheep oocytes may improve the progression of nuclear maturation and the developmental competence of sheep oocytes compared to the addition of large amount.
Acknowledgement
The project was supported by King Abdulaziz City for Science and Technology (KACST) (No: 33-282), Riyadh, Saudi Arabia. There was no conflict of interest in this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ali A., Coenen K., Bousquet D., Sirard M.A. Origin of bovine follicular fluid and its effect during in vitro maturation on the developmental competence of bovine oocytes. Theriogenology. 2004;62:1596–1606. doi: 10.1016/j.theriogenology.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Angelucci S., Ciavardelli D., Di Giuseppe F., Eleuterio E., Sulpizio M., Tiboni G.M., Giampietro F., Palumbo P., Di Ilio C. Proteome analysis of human follicular fluid. Biochimica et biophysica acta. 2006;1764:1775–1785. doi: 10.1016/j.bbapap.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Aswal A.P., Raghav S., De S., Thakur M., Goswami S.L., Datta T.K. Expression stability of two housekeeping genes (18S rRNA and G3PDH) during in vitro maturation of follicular oocytes in buffalo (Bubalus bubalis) Anim. Reprod. Sci. 2008;103:164–171. doi: 10.1016/j.anireprosci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Atef A., Marc-Andre S. Effect of the absence or presence of various supplements on further development of bovine oocytes during in vitro maturation. Biol. Reprod. 2002;66:901–905. doi: 10.1095/biolreprod66.4.901. [DOI] [PubMed] [Google Scholar]
- Avery B., Strobech L., Jacobsen T., Bogh I.B., Greve T. In vitro maturation of bovine cumulus-oocyte complexes in undiluted follicular fluid: effect on nuclear maturation, pronucleus formation and embryo development. Theriogenology. 2003;59:987–999. doi: 10.1016/s0093-691x(02)01139-1. [DOI] [PubMed] [Google Scholar]
- Blondin P., Sirard M.A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol. Reprod. Dev. 1995;41:54–62. doi: 10.1002/mrd.1080410109. [DOI] [PubMed] [Google Scholar]
- Bogh I.B., Bezard J., Duchamp G., Baltsen M., Gerard N., Daels P., Greve T. Pure preovulatory follicular fluid promotes in vitro maturation of in vivo aspirated equine oocytes. Theriogenology. 2002;57:1765–1779. doi: 10.1016/s0093-691x(02)00650-7. [DOI] [PubMed] [Google Scholar]
- Caillaud M., Dell'aquila M.E., De Santis T., Nicassio M., Lacalandra G.M., Goudet G., Gerard N. In vitro equine oocyte maturation in pure follicular fluid plus interleukin-1 and fertilization following ICSI. Anim. Reprod. Sci. 2008;106:431–439. doi: 10.1016/j.anireprosci.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Chernik I.S., Panasenko O., Li Y., Marston S.B., Gusev N.B. pH-induced changes of the structure of small heat shock proteins with molecular mass 24/27kDa (HspB1) Biochem. Biophys. Res. Commun. 2004;324:1199–11203. doi: 10.1016/j.bbrc.2004.09.176. [DOI] [PubMed] [Google Scholar]
- Chi H.J., Kim D.H., Koo J.J., Chang S.S. The suitability and efficiency of human follicular fluid as a protein supplement in human in vitro fertilization programs. Fert. Steril. 1998;70:871–877. doi: 10.1016/s0015-0282(98)00313-6. [DOI] [PubMed] [Google Scholar]
- Cognie Y., Baril G., Poulin N., Mermillod P. Current status of embryo technologies in sheep and goat. Theriogenology. 2003;59:171–188. doi: 10.1016/s0093-691x(02)01270-0. [DOI] [PubMed] [Google Scholar]
- Coleman N.V., Shagiakhmetova G.A., Lebedeva I.Y., Kuzmina T.I., Golubev A.K. In vitro maturation and early developmental capacity of bovine oocytes cultured in pure follicular fluid and supplementation with follicular wall. Theriogenology. 2007;67:1053–1059. doi: 10.1016/j.theriogenology.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Cruz M.H.C., Saraiva N.Z., Cruz J.F.d., Oliveira C.S., Collado M.D., Fernandes H., Castro F.C.d., Garcia J.M. Effect of follicular fluid supplementation during in vitro maturation on total cell number in bovine blastocysts produced in vitro. Revista Brasileira de Zootecnia. 2014;43:120–126. [Google Scholar]
- de Matos D.G., Furnus C.C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- Dieleman S.J., Hendriksen P.J., Viuff D., Thomsen P.D., Hyttel P., Knijn H.M., Wrenzycki C., Kruip T.A., Niemann H., Gadella B.M., Bevers M.M., Vos P.L. Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos. Theriogenology. 2002;57:5–20. doi: 10.1016/s0093-691x(01)00655-0. [DOI] [PubMed] [Google Scholar]
- Driancourt M.A., Thuel B. Control of oocyte growth and maturation by follicular cells and molecules present in follicular fluid. A review. Reprod. Nutr. Dev. 1998;38:345–362. doi: 10.1051/rnd:19980401. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dunning K.R., Lane M., Brown H.M., Yeo C., Robker R.L., Russell D.L. Altered composition of the cumulus-oocyte complex matrix during in vitro maturation of oocytes. Hum. Reprod. 2007;22:2842–2850. doi: 10.1093/humrep/dem277. [DOI] [PubMed] [Google Scholar]
- Ebrahimi B., Valojerdi M.R., Eftekhari-Yazdi P., Baharvand H. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J. Assist. Reprod. Genet. 2010;27:239–246. doi: 10.1007/s10815-010-9401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R.G. Follicular fluid. J. Reprod. Fertil. 1974;37:189–219. doi: 10.1530/jrf.0.0370189. [DOI] [PubMed] [Google Scholar]
- Gasparrini B., Neglia G., Palo R.D., Campanile G., Zicarelli L. Effect of cysteamine during in vitro maturation on buffalo embryo development. Theriogenology. 2000;54:1537–1542. doi: 10.1016/s0093-691x(00)00473-8. [DOI] [PubMed] [Google Scholar]
- Guler A., Poulin N., Mermillod P., Terqui M., Cognie Y. Effect of growth factors, EGF and IGF-I, and estradiol on in vitro maturation of sheep oocytes. Theriogenology. 2000;54:209–218. doi: 10.1016/s0093-691x(00)00342-3. [DOI] [PubMed] [Google Scholar]
- Gupta P.S.P., Ravindra J.P., Kumar V.G., Raghu H.M., Nandi S. Stimulation of in vitro ovine oocyte maturation with a novel peptide isolated from follicular fluid of the buffalo (Bubalus bubalis) Small Ruminant Res. 2005;59:33–40. [Google Scholar]
- Huang W.T., Lu S.G., Tang P.C. Biochemical compositions of follicular fluid and the effects of culture conditions on the in vitro development of pig oocytes. Asian Aust. J. Anim. Sci. 2002;15:1403–1411. [Google Scholar]
- Humblot P., Holm P., Lonergan P., Wrenzycki C., Lequarre A.S., Joly C.G., Herrmann D., Lopes A., Rizos D., Niemann H., Callesen H. Effect of stage of follicular growth during superovulation on developmental competence of bovine oocytes. Theriogenology. 2005;63:1149–1166. doi: 10.1016/j.theriogenology.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Ito M., Iwata H., Kitagawa M., Kon Y., Kuwayama T., Monji Y. Effect of follicular fluid collected from various diameter follicles on the progression of nuclear maturation and developmental competence of pig oocytes. Anim. Reprod. Sci. 2008;106:421–430. doi: 10.1016/j.anireprosci.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kim K., Mitsumizo N., Fujita K., Utsumi K. The effects of follicular fluid on in vitro maturation, oocyte fertilization and the development of bovine embryos. Theriogenology. 1996;45:787–799. doi: 10.1016/0093-691x(96)00008-8. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Tajima J., Yamaji T., Mitsumizo N., Fujita K., Cho H.J. Effects of supplemented follicular fluid to IVM serum free-medium and developmental of bovine oocytes. Jap. Soc. Anim. Reprod. Tech. 1993;15:41–48. [Google Scholar]
- Kim M.R., Tilly J.L. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochimica et biophysica acta. 2004;1644:205–210. doi: 10.1016/j.bbamcr.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Knight P.G., Muttukrishna S., Groome N.P. Development and application of a two-site enzyme immunoassay for the determination of 'total' activin-A concentrations in serum and follicular fluid. J. Endocrinol. 1996;148:267–279. doi: 10.1677/joe.0.1480267. [DOI] [PubMed] [Google Scholar]
- Kor N.M., Moradi K. A review of biochemical metabolites concentration and hormonal composition of ovarian follicular fluid in domestic animals. Annu. Rev. Res. Biol. 2013;3:246–255. [Google Scholar]
- Kyasari O.R., Valojerdi M.R., Farrokhi A., Ebrahimi B. Expression of maturation genes and their receptors during in vitro maturation of sheep COCs in the presence and absence of somatic cells of cumulus origin. Theriogenology. 2012;77:12–20. doi: 10.1016/j.theriogenology.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Larocca C., Kmaid S., Calvo J. Effect of follicular fluid and estrous cow serum on maturation, fertilization and development of the bovine oocyte in vitro. Theriogenology. 1993;3:239–253. [Google Scholar]
- Li H.J., Liu D.J., Cang M., Wang L.M., Jin M.Z., Ma Y.Z., Shorgan B. Early apoptosis is associated with improved developmental potential in bovine oocytes. Anim. Reprod. Sci. 2009;114:89–98. doi: 10.1016/j.anireprosci.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Li R., Norman R.J., Armstrong D.T., Gilchrist R.B. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol. Reprod. 2000;63:839–845. doi: 10.1095/biolreprod63.3.839. [DOI] [PubMed] [Google Scholar]
- Livingston T., Rich K., MacKenzie S., Godkin J.D. Glutathione content and antioxidant enzyme expression of in vivo matured sheep oocytes. Anim. Reprod. Sci. 2009;116:265–273. doi: 10.1016/j.anireprosci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Lojkic M., Getz I., Samardzija M., Matkovic M., Bacic G., Karadjole T., Macesic N., Folnozic I., Spoljaric B. Effect of cysteamine supplementation during in vitro culture of early stage bovine embryos on blastocyst rate and quality. Acta Vet. Brno. 2012;81:229–234. [Google Scholar]
- Lonergan P., Rizos D., Gutierrez-Adan A., Fair T., Boland M.P. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reproduction in domestic animals = Zuchthygiene. 2003;38:259–267. doi: 10.1046/j.1439-0531.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Masudul H., Khandoker M.A.M.Y., Kabiraj S.K., Asad L.Y., Fakruzzaman M., Tareq K.M.A. Effect of goat follicular fluid on in vitro production of embryos in black Bengal goats. Iran. J. Appl. Anim. Sci. 2012;2:287–294. [Google Scholar]
- Nandi S., Raghu H.M., Ravindranatha B.M., Gupta P.S., Sarma P.V. In vitro development of buffalo oocytes in media-containing fluids from different size class follicles. Reproduction in domestic animals = Zuchthygiene. 2004;39:33–38. doi: 10.1046/j.1439-0531.2003.00472.x. [DOI] [PubMed] [Google Scholar]
- Nemcova L., Machatkova M., Hanzalova K., Horakova J., Kanka J. Gene expression in bovine embryos derived from oocytes with different developmental competence collected at the defined follicular developmental stage. Theriogenology. 2006;65:1254–1264. doi: 10.1016/j.theriogenology.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Palasz A.T., Brena P.B., Fuente J.D.l., Gutierrez-Adan A. The effect of different zwitterionic buffers and PBS used for out-of-incubator procedures during standard in vitro embryo production on development, morphology and gene expression of bovine embryos. Theriogenology. 2008;70:1461–1470. doi: 10.1016/j.theriogenology.2008.06.092. [DOI] [PubMed] [Google Scholar]
- Pastore A., Federici G., Bertini E., Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clinica Chimica Acta; Int. J. Clin. Chem. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Paul C., Simon S., Gibert B., Virot S., Manero F., Arrigo A.P. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27) Exp. Cell Res. 2010;316:1535–1552. doi: 10.1016/j.yexcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Perreault S.D., Barbee R.R., Slott V.L. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev. Biol. 1988;125:181–186. doi: 10.1016/0012-1606(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Rao B.S., Mahesh Y.U., Charan K.V., Suman K., Sekhar N., Shivaji S. Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology. 2012;64:176–184. doi: 10.1016/j.cryobiol.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Rizos D., Ward F., Duffy P., Boland M.P., Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002;61:234–248. doi: 10.1002/mrd.1153. [DOI] [PubMed] [Google Scholar]
- Romar R., De Santis T., Papillier P., Perreau C., Thelie A., Dell'Aquila M.E., Mermillod P., Dalbies-Tran R. Expression of maternal transcripts during bovine oocyte in vitro maturation is affected by donor age. Reproduction in domestic animals = Zuchthygiene. 2011;46:e23–30. doi: 10.1111/j.1439-0531.2010.01617.x. [DOI] [PubMed] [Google Scholar]
- Sanna, D. 2009. Quality of in vitro ovine embryo production: lambing rate, body weight and gene expression. In: Produzione e Benessere Animale. Doctoral thesis. Università degli Studi di Sassari, Italy, p. 126.
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shabankareh K.H., Sarsaifi K. Developement potential of ovine oocytes cultured in different maturation media, effect of human monoposal serum. J. Bio. Tec. 2008;10:10–16. [Google Scholar]
- Smiljakovic T., Tomek W. Meiotic maturation and in vitro maturation of bovine oocytes. Biotechnol. Anim. Husbandry. 2006;22:29–34. [Google Scholar]
- Stankiewicz A.R., Lachapelle G., Foo C.P., Radicioni S.M., Mosser D.D. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Sun F.J., Holm P., Irvine B., Seamark R.F. Effect of sheep and human follicular fluid on the maturation of sheep oocytes in vitro. Theriogenology. 1994;41:981–988. doi: 10.1016/0093-691x(94)90513-i. [DOI] [PubMed] [Google Scholar]
- Tabatabaei S., Mamoei M. Biochemical composition of blood plasma and follicular fluid in relation to follicular size in buffalo. Comp. Clin. Pathol. 2011;20:441–454. [Google Scholar]
- Tabatabaei S., Mamoei M., Aghaei A. Dynamics of ovarian follicular fluid in cattle. Aghaei A. Comp. Clin. Pathol. 2011;20:591–595. [Google Scholar]
- Tatone C., Carbone M.C., Gallo R., Delle Monache S., Di Cola M., Alesse E., Amicarelli F. Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor BCL2. Biol. Reprod. 2006;74:395–402. doi: 10.1095/biolreprod.105.046169. [DOI] [PubMed] [Google Scholar]
- Thompson J.G. In vitro culture and embryo metabolism of cattle and sheep embryos – a decade of achievement. Anim. Reprod. Sci. 2000;60–61:263–275. doi: 10.1016/s0378-4320(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Trounson A., Anderiesz C., Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- van de Leemput E.E., Vos P.L., Zeinstra E.C., Bevers M.M., van der Weijden G.C., Dieleman S.J. Improved in vitro embryo development using in vivo matured oocytes from heifers superovulated with a controlled preovulatory LH surge. Theriogenology. 1999;52:335–349. doi: 10.1016/s0093-691x(99)00133-8. [DOI] [PubMed] [Google Scholar]
- Warzych E., Wrenzycki C., Peippo J., Lechniak D. Maturation medium supplements affect transcript level of apoptosis and cell survival related genes in bovine blastocysts produced in vitro. Mol. Reprod. Dev. 2007;74:280–289. doi: 10.1002/mrd.20610. [DOI] [PubMed] [Google Scholar]
- Watson A.J., De Sousa P., Caveney A., Barcroft L.C., Natale D., Urquhart J., Westhusin M.E. Impact of bovine oocyte maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol. Reprod. 2000;62:355–364. doi: 10.1095/biolreprod62.2.355. [DOI] [PubMed] [Google Scholar]
- Wrenzycki C., Herrmann D., Lucas-Hahn A., Korsawe K., Lemme E., Niemann H. Messenger RNA expression patterns in bovine embryos derived from in vitro procedures and their implications for development. Reprod. Fertil. Dev. 2005;17:23–35. doi: 10.1071/rd04109. [DOI] [PubMed] [Google Scholar]
- Yang M.Y., Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim. Reprod. Sci. 2002;70:159–169. doi: 10.1016/s0378-4320(01)00186-5. [DOI] [PubMed] [Google Scholar]
- Yang X., Kubota C., Suzuki H., Taneja M., Bols P.E., Presicce G.A. Control of oocyte maturation in cows – biological factors. Theriogenology. 1998;49:471–482. doi: 10.1016/s0093-691x(97)00419-6. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ishigaki K., Nagai T., Chikyu M., Pursel V.G. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol. Reprod. 1993;49:89–94. doi: 10.1095/biolreprod49.1.89. [DOI] [PubMed] [Google Scholar]