Abstract
Background
Non-adherence to r-hGH treatments occurs in a variable percentage of subjects. One problem found when evaluating adherence is the great variability in methods of detection and definitions utilized in studies. This study assessed the level of adherence in subjects receiving r-hGH with the easypod™ electronic device.
Methods
National, multicenter, prospective and observational study involving 238 subjects (144 with GH deficiency (GHD), and 86 with small for gestational age (SGA), 8 with Turner Syndrome), who received r-hGH with easypod™ for at least 3 months before inclusion. The follow-up period was 4 years.
Results
Overall adherence was 94.5%; 97.5% after 6 months, 95.3% after 1 year, 93.7% after 2, 94.4% after 3 and 95.5% after 4 years of treatment. No differences in adherence were observed between prepubertal and pubertal groups and GHD and SGA groups. Change in height after 1 and 2 years, change in height SDS after 1 and 2 years, HV after 1 year, HV SDS after at 1 and 4 years, change in BMI after 1 year and change in BMI SDS at 1 and 2 years showed significant correlation with adherence. No significant differences in adherence according to IGF-I levels were found in follow-up visits or between groups.
Conclusions
The easypod™ electronic device, apart from being a precise and objective measure of adherence to r-hGH treatment, allows high compliance rates to be achieved over long periods of time. Adherence significantly impacts growth outcomes associated with r-hGH treatment.
Keywords: adherence, r-hGH, easypod, electronic device, long-term, outcomes, pediatrics, e-health
Introduction
Since the 60s, GH has been the mainstay of treatment for children with GH deficiency (GHD) (1). With the discovery of recombinant human growth hormone (r-hGH), the treatment has been extended to other conditions, such as Turner syndrome (TS), chronic renal insufficiency (CRI) and children born small for gestational age (SGA) (2, 3). Pathologies requiring the administration of r-hGH in pediatric patients show a great variability in severity (especially among secretion pathologies) and in sensitivity to the hormone (4). Treatment with r-hGH has been shown to be effective in children and adolescents with GHD, increasing short-term growth and adult height (5, 6). GH therapy requires daily subcutaneous injections for long periods of time (7). Some studies have suggested that non-adherence to treatment occurs in a variable percentage of subjects (8, 9, 10). The main cause for minor efficacy in r-hGH therapy is the lack of adherence to treatment or non-persistence with the prescription (11). Since dosing has to be adjusted depending on the response to treatment, it is necessary, initially, to ensure adherence to treatment in order to evaluate its response. Non-adherence to the prescribed treatment is directly associated with worse clinical outcomes, increased healthcare costs and may affect the benefit–risk balance of treatments, especially in chronic diseases (12). One of the problems found at the time of evaluating adherence to r-hGH treatments is the great variability in methods of detection and definitions of adherence utilized in studies (8, 9, 10). To provide a precise and objective measure of adherence, electronic devices can be used to monitor and administer treatment. The r-hGH electronic auto-injector device, easypod™ (Merck Serono International S.A.), has been specifically designed to ease the use of these daily injectors and to provide reliability and convenience to users by recording the dosing history and using preprogrammed doses; in other words, to provide an e-health tool to patients (13). Despite this term has evolved in the last decade, e-health is defined by the World Health Organization (WHO) as ‘the use of information and communication technologies for health’ (14). Since easypod™ records the number of doses administered, adherence can be accurately assessed. The multinational easypod™ connect observational study (ECOS) was specifically designed to quantify adherence in subjects receiving r-hGH with easypod™ and to characterize the impact of adherence on clinical outcomes and identify adherence patterns (15). This study aimed to evaluate adherence as a factor involved in the response to treatments with r-hGH. The present manuscript shows results obtained from the Spanish ECOS study (ClinicalTrials.gov identifier: NCT01376921) and the main objective of the study was to assess the level of adherence in subjects receiving r-hGH with the electronic device, easypod™.
Subjects and methods
Patients
This national, multicenter, observational study involved subjects aged between 2 and 18 years old or over 18 years with no fusion of growth plates, who received r-hGH (Saizen®, Merck Serono S.A.) with the easypod™ electronic device for at least 3 months before inclusion into the study. The period of follow-up was up to 5 years; however, due the low number of patients at that time, results are only shown for up to 4 years. Exclusion criteria to participate in the study were previous treatment with r-hGH other than Saizen®; hypersensitivity to the active ingredient or excipients; subjects with fused growth plates; contraindications to r-hGH or participating in another clinical study. Subjects were identified from the investigator’s review of medical charts. Informed consent signed by parents or legal guardians was obtained from all subjects. All procedures were in accordance with the Declaration of Helsinki and approved by the Ethics Committee of each participating center.
Endpoints and variables
The primary endpoint was the adherence to treatment over a follow-up period of 4 years. Adherence data were collected by the means of easypod™ and uploaded at every follow-up visit or at any time point adjacent to the visit if collected remotely, depending on the clinical practice. Adherence was calculated as the percentage of injections received (days) with respect to planned injections. The compliant population was defined as those with ≥85% adherence to prescribed treatment (no more than 1 missed dose a week on average) (16). Secondary endpoints included evaluation of the impact of adherence on growth outcomes over the follow-up period; identification of demographic and auxological characteristics of subjects associated with adherence and assessment of the impact of insulin-like growth factor I (IGF-I) levels on adherence over the follow-up period. Demographic, auxological and diagnostic data were obtained from medical charts. All data were collected both retrospectively and prospectively. In contrast with the global ECOS study, demographics, historical information on growth hormone treatment, auxological parameters and adherence were transcribed by investigators onto paper case report forms and not reported electronically. Growth outcomes included height, height standard deviation score (SDS), change in height, change in height SDS, height velocity (HV), HV SDS, change in HV, change in HV SDS, weight, weight SDS, change in weight, change in weight SDS, BMI, BMI SDS, change in BMI and change in BMI SDS. The SDS variables were calculated by adjusting data with age and gender, according to child growth standards (17). Bone age was measured using the Greulich and Pyle method (18). The IGF-I concentration was classified as high, normal or low according to standard laboratory ranges (established on the heterogeneity of techniques used in the present multicenter study). The proportion of subjects with dose changes was calculated as a percentage of the number of visits with dose changes over the total number of visits.
Determination of sample size and statistical analysis
The ECOS Spain study was planned to be implemented in 50 centers and expected to recruit 300 subjects. Treatment adherence data of 300 subjects would be able to compute the 95% confidence interval (95% CI) around the observed mean percent of adherence with a precision of ±1.7% assuming a standard deviation (s.d.) of 16%. Statistical analysis was performed on the full analysis set (FAS) subpopulation. The FAS subpopulation included subjects with GHD, SGA and TS, according to the new definition established by investigators on June 3, 2016. The FAS subpopulation was also stratified by prepubertal (Tanner stage 1) and pubertal subjects (Tanner stage 2–4). Due to low numbers, subjects with TS were excluded from analyses. Categorical variables were expressed as absolute and relative (%) frequencies; whereas continuous variables as the mean, median, s.d. and 95% CI. Parametric or non-parametric analyses were carried out depending on results of the normality test of each variable. The impact of adherence on growth outcomes at the different follow-up visits was determined by non-parametric Spearman’s correlation analysis. The correlation between compliance and growth outcomes, and the impact of adherence on serum IGF-I levels was measured by using a t-test or the Wilcoxon–Mann–Whitney test. The identification of demographic and auxological characteristics associated with adherence was performed with a multiple linear regression model. The following predictor variables were considered: age (at inclusion and when starting r-hGH treatment with easypod™), gender, indication (GHD or SGA), person who performed the majority of injections, treatment duration, and height SDS before start of GH treatment. Those variables with a statistical significance (P > 0.1) were included in the model. All statistical procedures were performed using SAS version 9.4. Statistical significance was established when P ≤ 0.05.
Results
Characteristics of subjects and treatment
A total of 272 subjects consented to participate in the study. Among them, 238 (87.5%) were included in the FAS subpopulation; 212 (89.1%) in the prepubertal and 26 (10.9%) in the pubertal group. The main reason for discontinuation (80.6% of cases) was due to the closure of the study by the sponsor at which point subjects were followed up for at least one year. The FAS subpopulation included 235 (209 prepubertal and 26 pubertal) subjects at 6 months and 1 year after starting the treatment, 176 (160 and 16, respectively) subjects at 2 years, 84 (80 and 4, respectively) subjects at 3 years and 25 (24 and 1, respectively) subjects at 4 years. In the FAS subpopulation, there were no data from three patients regarding if prepubertal or pubertal. In total, 144 subjects presented with GHD, 86 with SGA and 8 with TS. Baseline sociodemographic and clinical characteristics of patients are shown in Table 1. Subjects had a mean age at inclusion of 9.0 ± 3.3 years; approximately half of them were male (51.7%), mainly Caucasian (94.1%). At the time of starting treatment with easypod™, mean height SDS and velocity SDS were −2.6 ± 0.8 and −2.0 ± 1.7, respectively. No differences in demographic and auxological variables in terms of SDS values were observed between prepubertal (n = 212) and pubertal (n = 26) groups. At baseline, 73.1, 23.1 and 3.8% of pubertal subjects had Tanner Stage 2, 3 or 4, respectively. Mean bone age for the prepubertal GHD population (n = 119) was 6.0 ± 3.1 years, whereas their mean chronological age at inclusion was 9.2 ± 3.1 years. The mean duration of treatment was 26.6 ± 11.6 months (27.2 ± 11.7 months in the prepubertal group, 22.0 ± 8.9 months in the pubertal group, 27.4 ± 11.9 months in the GHD population, and 25.5 ± 10.7 months in the SGA population). Changes in prescribed dose were documented in 61.7 ± 20.2% of visits over the treatment period; for adjusting it to the recommending dose (mg/kg/day). The main reasons for missed injections were forgetting to inject (78.0%), and holidays, long weekends or not sleeping at home (51.8%). No significant differences were found regarding missed injections and the person who performed the majority of injections.
Table 1.
Characteristics of subjects and treatment with easypod™.
| Total (n = 238) | Prepubertal (n = 212) | Pubertal (n = 26) | GHD (n = 144) | SGA (n = 86) | |
|---|---|---|---|---|---|
| Age | |||||
| At inclusion, mean years ± s.d. | 9.0 ± 3.3 | 8.5 ± 3.1 | 13.4 ± 3.1 | 9.9 ± 3.4 | 7.7 ± 2.7 |
| At start of treatment with easypod™, mean years ± s.d. | 7.9 ± 3.2 | 7.3 ± 2.9 | 12.5 ± 1.8 | 8.7 ± 3.3 | 6.6 ± 2.5 |
| Gender, n (%) | |||||
| Male | 123 (51.7) | 112 (52.8) | 11 (42.3) | 78 (54.2) | 45 (52.3) |
| Female | 115 (48.3) | 100 (47.2) | 15 (57.7) | 66 (45.8) | 41 (47.7) |
| Ethnicity, n (%) | |||||
| Caucasian | 224 (94.1) | 199 (93.9) | 25 (96.2) | 135 (93.8) | 81 (94.2) |
| African | 1 (0.4) | 1 (0.5) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Asian | 3 (1.3) | 3 (1.4) | 0 (0.0) | 2 (1.4) | 1 (1.2) |
| Other | 10 (4.2) | 9 (4.8) | 1 (3.8) | 6 (4.2) | 4 (4.7) |
| Auxological data before starting treatment | |||||
| Height SDS, mean (s.d.) | −2.6 (0.8) | −2.6 (0.7) | −2.2 (1.1) | −2.5 (0.8) | −2.6 (0.6) |
| Weight SDS, mean (s.d.) | −1.3 (0.9) | −1.3 (0.9) | 0.9 | −1.1 (1.0) | −1.5 (0.8) |
| HV SDS, mean (s.d.) | −2.0 (1.7) | −2.0 (1.6) | −2.3 (2.3) | −2.4 (1.7) | −1.5 (1.5) |
| BMI SDS | 0.2 (1.4) | 0.2 (1.4) | 0.3 (1.3) | 0.3 (1.5) | −0.2 (1.1) |
| Bone age, years (s.d.) | 6.0 (3.3) | 5.4 (2.9) | 11.0 (1.3) | 6.7 (3.4) | 4.8 (2.7) |
| Treatment with r-hGH | |||||
| Prescribed dose, mean mg/kg/day (s.d.) | 0.032 (0.005) | 0.032 (0.005) | 0.033 (0.006) | 0.030 (0.004) | 0.035 (0.004) |
| Percentage of reported changes in the prescribed doses over the treatment period, mean % ± s.d. | 61.7 ± 20.2 | 62.0 ± 20.5 | 58.8 ± 18.1 | 59.1 ± 22.4 | 66.1 ± 15.6 |
| Duration of treatment period, mean months (s.d.) | 26.6 (11.6) | 27.2 (11.7) | 22.0 (8.9) | 27.4 (11.9) | 25.5 (10.7) |
| Person who performed majority of injections, n (%) | |||||
| Parents/legal guardian | 161 (67.6) | 154 (72.6) | 7 (26.9) | 85 (59.0) | 70 (81.4) |
| Under parent supervision | 54 (22.7) | 42 (19.8) | 12 (46.2) | 40 (27.8) | 12 (14.0) |
| Self-injections | 17 (7.1) | 11 (5.2) | 6 (23.1) | 14 (9.7) | 3 (3.5) |
| Not available | 6 (2.5) | 5 (2.4) | 1 (3.8) | 5 (3.5) | 1 (1.2) |
| Missed some injection over the treatment period, n (%) | 195 (81.9) | 173 (81.6) | 22 (84.6) | 115 (79.9) | 73 (84.9) |
| Reasons for missing injections, n (%) | |||||
| Forgot injection | 152 (78.0) | 137 (79.2) | 15 (68.2) | 90 (78.3) | 55 (75.3) |
| Holidays/long weekend/not sleeping at home | 101 (51.8) | 90 (52.0) | 11 (50.0) | 57 (49.6) | 42 (57.5) |
| Medical reasons | 6 (3.1) | 5 (2.9) | 1 (4.6) | 4 (3.5) | 2 (2.7) |
| Tired of injections | 7 (3.6) | 5 (2.9) | 2 (9.1) | 4 (3.5) | 3 (4.1) |
| Technical problems with easypod™ | 14 (7.2) | 11 (6.4) | 3 (13.6) | 6 (5.2) | 8 (11.0) |
| Forgot drug/Easypod™ | 15 (7.7) | 12 (6.9) | 3 (13.6) | 10 (8.7) | 5 (6.9) |
| Ran out of needle/cartridge | 7 (3.6) | 7 (4.1) | 0 (0.0) | 3 (2.6) | 4 (5.5) |
| Others | 20 (10.3) | 18 (10.4) | 2 (9.1) | 11 (9.6) | 7 (9.6) |
BMI, body mass index; GHD, growth hormone deficiency; HV, height velocity; s.d., standard deviation; SDS, standard deviation score; SGA, small for gestational age.
Adherence rates
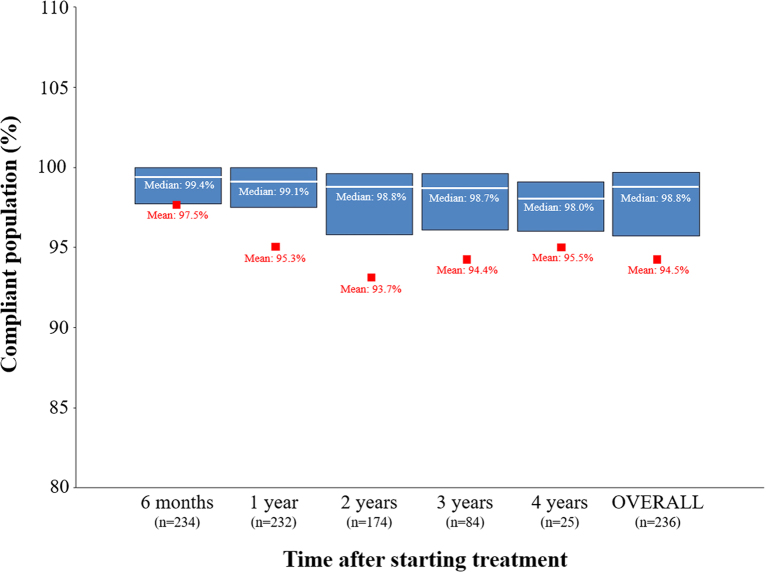
Mean overall adherence to the treatment was 94.5% (95% CI 92.7–96.3%; Table 2). Adherence was higher than 90% in all follow-up visits, that is 97.5% (95% CI 96.5–98.5%, n = 234) after 6 months, 95.3% (95% CI 93.3–97.2%, n = 232) after 1 year, 93.7% (95% CI 91.1–96.2%, n = 174) after 2 years, 94.4% (95% CI 91.5–97.3%, n = 84) after 3 years and 95.5% (95% CI 92.7–98.3%, n = 25) after 4 years of starting treatment with easypod™. Adherence for prepubertal and pubertal groups was 94.4% (95% CI 92.4–96.4%) and 95.5% (95% CI 92.8–98.1%), respectively. In the case of the GHD and SGA populations, adherence was 95.2% (95% CI 93.3–97.0%) and 93.0% (95% CI 89.1–96.9%). No differences in adherence were observed between the prepubertal and pubertal groups or the GHD and SGA populations. Adherence to treatment among the compliant population (≥85% adherence) was 97.5% after 6 months, 93.2% after 1 year, 92.1% after 2 years, 91.7% after 3 years and 92.0% after 4 years of starting treatment with easypod™ (Fig. 1).
Table 2.
Adherence to the treatment through different follow-up visits.
| Overall | 6 months | 1 year | 2 years | 3 years | 4 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n available | Mean (95% CI) | n available | Mean (95% CI) | n available | Mean (95% CI) | n available | Mean (95% CI) | n available | Mean (95% CI) | n available | Mean (95% CI) | |
| Total | 236 | 94.5% (92.7–96.3) | 234 | 97.5% (96.5–98.5%) | 232 | 95.3% (93.3–97.2%) | 174 | 93.7% (91.1–96.2%) | 84 | 94.4% (91.5–97.3%) | 25 | 95.5% (92.7–98.3%) |
| Prepubertal | 210 | 94.4% (92.4–96.4) | 208 | 97.7% (96.6–98.7%) | 207 | 95.1% (93.0–97.3%) | 158 | 93.6% (90.8–96.4%) | 80 | 94.3% (91.3–97.3%) | 24 | 95.6% (92.7–98.5%) |
| Pubertal | 26 | 95.5% (92.8–98.1) | 26 | 96.2% (93.4–99.1%) | 25 | 96.6% (94.1–99.2%) | 16 | 94.7% (90.4–98.9%) | 4 | 97.1% (94.4–99.8%) | 1 | 93.6% |
| GHD population | 142 | 95.2% (93.3–97.0) | 141 | 97.0% (95.5–98.6) | 142 | 93.9% (90.8–97.0) | 106 | 93.8% (90.4–97.1) | 55 | 95.2% (92.6–97.8) | 17 | 95.9% (92.5–99.2) |
| SGA population | 86 | 93.0% (89.1–96.9) | 85 | 98.1% (97.5–98.7) | 83 | 97.4% (96.1–98.7) | 63 | 93.1% (88.7–97.5) | 26 | 92.3% (84.5–100.1) | 7 | 94.4% (87.0–101.9) |
95% CI, 95% confidence interval; GHD, growth hormone deficiency; SGA, small for gestational age.
Figure 1.
Percentage of compliant population through 4 years of treatment. Blue boxes represent the limits between the 25 and 75% confidence interval. Median and mean values are shown as white lines and red squares, respectively.
IGF-I levels and adherence
The IGF-I values were below the laboratory range at baseline in 29.0% of subjects. Among these subjects, 76.6% showed IGF-I values within/above laboratory range after 6 months, 91.7% after 1 year, 93.0% at 2 years, 100% after 3 years and 75.0% after 4 years of starting treatment (Table 3). No significant differences in adherence rate according to IGF-I levels were found in any of the follow-up visits or between prepubertal and pubertal groups or GHD and SGA populations.
Table 3.
Baseline IGF-I and percentage of adherence regarding IGF-I levels after starting treatment with easypod™ (IGF-I levels were not associated with percentage of adherence).
| IGF-I laboratory range | Number of patients regarding IGF-I (%) | |||||
|---|---|---|---|---|---|---|
| Total | Prepubertal | Pubertal | GHD | SGA | ||
| Baseline IGF-I | Above | 3 (1.3) | 2 (0.9) | 1 (3.9) | 1 (0.7) | 1 (1.2) |
| Within | 144 (60.5) | 127 (59.9) | 17 (65.4) | 75 (52.1) | 62 (72.1) | |
| Below | 69 (29.0) | 64 (30.2) | 5 (19.2) | 55 (38.2) | 14 (16.3) | |
| Adherence, mean % (s.d.) | ||||||
| Total | Prepubertal | Pubertal | GHD | SGA | ||
| After 6 months | Above | 98.2 ± 2.3 | 98.6 ± 2.2 | 96.6 ± 2.1 | 97.7 ± 2.4 | 100.0 |
| Within | 97.0 ± 10.0 | 97.4 ± 10.1 | 93.6 ± 9.3 | 96.2 ± 13.0 | 98.0 ± 2.9 | |
| Below | 98.3 ± 2.6 | 98.0 ± 2.7 | 100.0 | 98.0 ± 2.7 | 100.0 | |
| After 1 year | Above | 97.6 ± 4.4 | 97.6 ± 4.8 | 97.7 ± 2.3 | 97.5 ± 5.2 | 97.7 ± 3.2 |
| Within | 96.5 ± 11.9 | 96.3 ± 12.5 | 97.9 ± 2.6 | 95.8 ± 14.6 | 97.3 ± 6.4 | |
| Below | 92.2 ± 15.4 | 92.2 ± 15.4 | N.A. | 92.2 ± 15.4 | N.A. | |
| After 2 years | Above | 91.0 ± 24.1 | 90.9 ± 25.3 | 91.4 ± 15.7 | 91.4 ± 24.4 | 89.2 ± 26.5 |
| Within | 95.4 ± 11.4 | 95.3 ± 11.9 | 96.4 ± 3.4 | 95.0 ± 13.6 | 95.9 ± 6.8 | |
| Below | 87.1 ± 12.1 | 87.1 ± 12.1 | N.A. | 87.1 ± 12.1 | N.A. | |
| After 3 years | Above | 98.2 ± 1.9 | 98.2 ± 1.9 | N.A. | 98.2 ± 2.4 | 98.3 ± 1.6 |
| Within | 93.6 ± 15.7 | 93.4 ± 16.1 | 96.8 ± 2.0 | 95.7 ± 9.3 | 87.9 ± 25.7 | |
| Below | N.A. | N.A. | N.A. | N.A. | N.A. | |
| After 4 years | Above | 96.0 ± 3.5 | 96.0 ± 3.5 | N.A. | 96.0 ± 3.5 | 96.2 ± 5.1 |
| Within | 98.2 ± 1.6 | 98.2 ± 1.6 | N.A. | 99.2 ± 0.7 | 97.9 ± 1.8 | |
| Below | 96.4 | 96.4 | N.A. | 96.4 | N.A. | |
GHD, growth hormone deficiency; IGF-I, insulin-like growth factor I; N.A., not available (n = 0); s.d., standard deviation; SGA, small for gestational age.
Impact of adherence on growth outcomes
Overall change in height after 1 and 2 years (Spearman’s correlation = 0.170 and 0.217; P = 0.010 and 0.04, respectively), change in height SDS after 1 and 2 years (correlation = 0.161 and 0.160; P = 0.015 and 0.035, respectively), HV after 1 year (correlation = 0.206; P = 0.002), HV SDS after at 1 and 4 years (correlation = 0.168 and −0.473; P = 0.011 and 0.041, respectively), change in BMI after 1 year (correlation = −0.193; P = 0.003) and change in BMI SDS at 1 and 2 years (correlation = −0.238 and −0.171; P = 0.002 and 0.051) showed significant correlation with adherence (Table 4). Regarding the GHD population, change in height after 2 years (Spearman’s correlation = 0.203; P = 0.037), height SDS after 1 year (correlation = 0.169; P = 0.046), HV after 1 year (correlation = 0.224; P = 0.008), change in BMI after 2 years (correlation = −0.193; P = 0.047) and change in BMI SDS after 2 years (correlation = −0.214; P = 0.047) showed a significant correlation with adherence. In the case of the SGA population, only change in BMI after 1 year (correlation = −0.246; P = 0.025) and change in BMI SDS after 1 year (correlation = −0.2350; P = 0.016) showed a significant correlation with adherence. The remaining growth outcomes showed no differences between the compliant and non-compliant populations. In the compliant GHD population, the following outcomes were higher than in non-compliant GHD population: height SDS after 2 years (−1.5 ± 0.8 vs −2.0 ± 0.6, P = 0.047), HV after 2 years (7.6 ± 1.6 cm/year vs 6.0 ± 1.5 cm/year, P = 0.013), weight after 3 years (35.8 ± 13.1 kg vs 24.5 ± 10.8 kg, P = 0.025) and change in weight after 3 years (13.0 ± 5.7 kg vs 8.4 ± 2.1 kg, P = 0.048). In the SGA population, change in weight after 2 years was significantly higher in the compliant subpopulation (7.1 ± 3.0 kg) than the non-compliant subpopulation (4.5 ± 0.3 kg, P = 0.017).
Table 4.
Significant impact of adherence on growth outcomes at different follow-up visits, groups and populations.
| Spearman’s correlation (P value) | |||||
|---|---|---|---|---|---|
| Total | Prepubertal | Pubertal | GHD | SGA | |
| Change in height after 1 year | 0.170 (0.010)a | 0.162 (0.021)a | 0.250 (0.229) | 0.147 (0.084) | 0.176 (0.111) |
| 2 years | 0.217 (0.004)a | 0.216 (0.007)a | 0.281 (0.292) | 0.203 (0.037)a | 0.242 (0.056) |
| Change in height SDS after 1 year | 0.161 (0.015)a | 0.157 (0.025)a | 0.171 (0.414) | 0.144 (0.089) | 0.173 (0.117) |
| 2 years | 0.160 (0.035)a | 0.119 (0.137) | 0.611 (0.012)a | 0.146 (0.136) | 0.227 (0.073) |
| Height SDS after 1 year | 0.092 (0.164) | 0.092 (0.189) | 0.144 (0.493) | 0.169 (0.046)a | −0.075 (0.499) |
| HV after 1 year | 0.206 (0.002)a | 0.186 (0.008)a | 0.349 (0.084) | 0.224 (0.008)a | 0.114 (0.304) |
| HV SDS after 1 year | 0.168 (0.011)a | 0.193 (0.006)a | −0.065 (0.759) | 0.154 (0.070) | 0.152 (0.171) |
| 4 years | −0.473 (0.041)a | −0.473 (0.041)a | N.A. | −0.283 (0.348) | −0.943 (0.005)a |
| Change in BMI after 1 year | −0.193 (0.003)a | −0.162 (0.020)a | −0.364 (0.074) | −0.150 (0.077) | −0.246 (0.025)a |
| 2 years | −0.126 (0.099) | −0.069 (0.389) | −0.609 (0.012)a | −0.193 (0.047)a | −0.064 (0.619) |
| Change in BMI SDS after 1 year | −0.238 (0.002)a | −0.214 (0.011)a | −0.327 (0.111) | −0.181 (0.055) | −0.350 (0.016)a |
| 2 years | −0.171 (0.051)a | −0.137 (0.146) | −0.636 (0.008)a | −0.214 (0.047)a | −0.214 (0.185) |
aSignificant correlation.
BMI, body mass index; GHD, growth hormone deficiency; HV, height velocity; N.A., not available (n = 0); SDS, standard deviation score; SGA, small for gestational age.
Subject’s characteristics associated with adherence
In the univariate analysis, age at inclusion and at start of treatment, duration of treatment and height SDS before starting treatment showed a P value of 0.10 and were therefore included in the multivariate analysis. This multiple linear regression model did not show significant differences on treatment adherence (data not shown). In the GHD population, female subjects (96.4%) showed a significant higher adherence than male subjects (94.2%, P = 0.037). Results of the multiple linear regression model in the GHD and SGA populations did not show significant differences on treatment adherence when adjusted for other factors such as age at inclusion and at start of treatment, duration of treatment and height SDS in the FAS subpopulation or the prepubertal and pubertal groups.
Discussion
Non-adherence is an important problem in chronic pathologies for national healthcare systems as it interferes with the effectiveness of treatments, leading to poor clinical outcomes and increased healthcare costs (10, 19, 20). A parent or guardian is required to perform the injections for pediatrics, and children and adolescents are usually reticent to receive them (19). Treatment devices are frequently used to address this issue, especially by favoring self-injection (such as the electronic ones). The prevalence of non-adherence has been reported to range from 5 to 82% (9), with overall values between 36 and 49% (8). A Spanish study involving 158 children (121 with GHD and 37 with SGA) receiving r-hGH showed that 33.5% presented a moderate-to-poor adherence (defined by authors as <92% adherence) (10). Adherence, measured according to issued r-hGH prescriptions, was indeed significantly correlated with HV and IGF-I levels. Despite these results, only electronic devices can provide a precise and objective measure of adherence (13, 15). To our knowledge, there are scarce studies specifically designed to measure adherence by using the electronic device, easypod™ (21, 22, 23, 24, 25, 26). Recently, the ECOS study (global, i.e. using overall data of 1190 children from 24 national ECOS studies) has evidenced a median overall rate of adherence of 93.7% (26). An international, multicenter, observational study that evaluated 824 children who received r-hGH as part of their normal care for 3 months, reported a rate of adherence (>92% of injections prescribed) of 87.5% using easypod™ (21). Interestingly, children also completed a questionnaire-based survey to measure adherence and the adherence rate was shown to be 90.2%. A retrospective multicenter study from Spain with 504 r-hGH-naïve children with growth disorders receiving r-hGH with easypod™ (in 81.5% of cases) showed that only 7.4% of children were not completely adherent (24). Adherence rates from our study (overall 94.5%) were as high as described in literature using easypod™, and maintained over 4 years of follow-up; however, rates were higher than previously reported in questionnaire-based retrospective studies (9, 16, 21). Furthermore, adherence rates were higher than those in studies using auto-injector devices in diseases such as diabetes and multiple sclerosis (ranging between 60 and 88%) (27, 28, 29). Studies using needle-free auto-injectors with r-hGH in children (such as Zoma-Jet) have reported adherence rates from 58% (30) to 97% (31). In our study, no significant differences in adherence to treatment were found between the prepubertal and pubertal groups or the GHD (95.2%) and SGA (93.0%) populations. This result is in concordance with ECOS study (global), which showed median adherences of 93.4 and 95.0%, respectively (26). The use of easypod™ might induce Hawthorne effect on patients. It would also be interesting to evaluate the Hawthorne effect in patients receiving r-hGH with easypod™. The Hawthorne effect refers to the change in the behavior of some individuals participating in clinical trials, feeling that they are being monitored and resulting in better adherence rates and results (32). According to some authors (33), levels of IGF-I are not good indicators for adherence because influenced by factors (such as weight, etiology of GHD or nutrition). This statement is supported by no correlation between levels of IGF-I and adherence found in our study. Furthermore, IGF-I levels only would demonstrate adherence of the treatment just one the previous week to the analysis (34).
On the other hand, in our study, change in height and height SDS, HV and HV SDS showed significant, although weak, correlations with adherence. These results are in concordance with literature and highlight the importance of adherence on clinical outcomes of the treatment (16, 21). In ECOS study (global), adherence showed a significant positive correlation with change in height, change in height SDS, HV and HV SDS; all of them after 1 year (26). It is interesting to note that change, in our study, height SDS after 3 years of treatment was higher in patients with >85% adherence. Given the weak correlations between adherence rate and growth parameters found in our study, conclusions (clinical implications) should be carefully considered.
Finally, some patient factors, such as age and gender, have been shown to influence the response to GH therapies (35). In our study, we failed to identify demographic and auxological characteristics in subjects associated with adherence and growth outcomes over 4-year follow-up. Nevertheless, our predictive variables, including age at inclusion, age when starting r-hGH treatment with easypod™, gender, indication, person who performed injections, treatment duration and height SDS before start of GH treatment, were consistent with those selected in prediction models for long-term growth responses (36). Besides this, in the GHD population, female subjects showed a higher adherence rate than males.
One limitation of the present study was its observational nature as it was not specifically designed (primary objective) to determine the impact of adherence to the treatment on clinical outcomes. The number of patients with low adherence rates (non-compliant population) was very low, which made it impossible to implement such an analysis. Another limitation was the lack of a control group for comparison purposes. A control group would improve the methodological design of the study and would inform whether or not easypod™ increases the adherence or increases the possibility to monitor the adherence. Nevertheless, in our opinion, comparing our results with data from other published studies was also a pertinent option. Moreover, our results are in concordance with literature, such as ECOS study (global) (26). Further studies, specifically designed to evaluate clinical implications of adherence to r-hGH that involve long periods of time (for example, beyond the fourth year of treatment to evaluate long-term adherence) are thus required. The goal of the present study was to evaluate prospectively the adherence to r-hGH treatment, accurately with easypod™, and identify the associations with growth outcomes and biomarkers, such as IGF-I, for an extended period of time.
Conclusions
Subjects receiving r-hGH with the electronic device easypod™ showed and maintained high adherence to the treatment through 4 years of follow-up, in both prepubertal and pubertal subjects, and in both GHD and SGA populations. Adherence significantly impacts growth outcomes of r-hGH treatment. Knowing accurately the adherence to treatment allows clinicians to establish the best approach and adequate dose-adjustment for patients with GHD. Electronic devices, such as easypod™, are the only tools to determine with precision the adherence to treatments with r-hGH. Moreover, they provide this information without the patient being physically present during the consultation. This emerging field of e-health allows activities to be performed that are aimed to improve adherence to treatments (such as supporting nursing programs or motivational messaging), through the use of digital health tools, the Internet and related technologies.
Declaration of interest
Merck, S.L.U. personnel work in the Medical Department that sponsored the study. The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This study was funded by Merck, S.L., Madrid, Spain.
Acknowledgements
Authors would like to thank all patients and relatives who kindly have participated in the study. Additional participants in the ECOS study were as follows: Dr Alfonso Maria Lechuga and Dr Francisco Suarez, Hospital Puerta del Mar, Cádiz, Spain; Dra Mª Ángeles Santos and Dr Francisco José Macías, Hospital Jerez de la Frontera, Cádiz, Spain; Dr José Antonio Bermúdez, Centro Nuevas Tecnologías, Sevilla, Spain; Dra Cristina Hernández, Hospital Virgen de la Macarena, Sevilla, Spain; Dra María Belén Jimenez, Hospital Juan Ramón Jiménez, Huelva, Spain; Dra Manuela Díaz, Hospital Infanta Elena, Madrid, Spain; Dra Marta Carmona and Dr Eugenio Fernández, Instituto Hispalense de Pediatría, Sevilla, Spain; Dra Mª Jose Martinez-Aedo, Hospital Regional Universitario de Málaga – Carlos Haya, Málaga, Spain; Dr Manuel Carranza, Hospital Nostra Senyora de Meritxell, Escaldes-Engordany, Andorra; Dr Esteban Mayayo and Dr José Ignacio Labarta, Hospital Miguel Servet, Zaragoza, Spain; Dra Gloria Bueno and Dr Jesús M Garagorri, Hospital Clínico Zaragoza, Zaragoza, Spain; Dra María Caimari and Dr Diego Sotto, Hospital Son Espases, Palma, Illes Balears, Spain; Dr Bartolomé Bonet, Hospital Can Misses, Eivissa, Illes Balears, Spain; Dra María Alija and Dra Pilar Sevilla, Hospital General de Guadalajara, Guadalajara, Spain; Dr Pablo Prieto, Hospital de Salamanca, Salamanca, Spain; Dr Carlos Hernando and Dra Inés Mulero, Hospital Rio Ortega, Valladolid, Spain; Dra Raquel Corripio and Dr Jacobo Perez, Hospital Parc Tauli, Barcelona, Spain; Dr Jordi Bosch, Hospital Arnau de Vilanova de Lleida, Lleida, Spain; Dr Abel López, Hospital Josep Trueta, Girona, Spain; Dr Albert Feliu, Hospital de Reus, Tarragona, Spain; Dr Alex Suarez and Dra Esperanza Moreno, Hospital Santa Caterina de Salt, Girona, Spain; Dr Diego Yeste, Hospital Vall d´Hebron, Barcelona, Spain; Dra Nuria Cabrinety, Hospital Sagrado Corazón, Madrid, Spain; Dr Javier Arroyo, Private Consultation; Dra Manuela Núñez, Hospital Materno Infantil de Badajoz, Badajoz, Spain; Dra Lydia Castro, Hospital Clínico de Santiago, Santiago de Compostela, Spain; Dr Jaime Sanchez, Hospital 12 de Octubre, Madrid, Spain; Dr José Luis Ruibal, Hospital Infanta Cristina, Madrid, Spain; Dra Carolina Bezanilla, Fundación Alcorcón, Madrid, Spain; Dra Dolores Rodriguez and Dra Amparo Rodríguez, Hospital Gregorio Marañón, Madrid, Spain; Dr Jesús Argente and Dr Gabriel Ángel Martos, Hospital Infantil Niño Jesús, Madrid, Spain; Dra Purificación Ros, Hospital Puerta de Hierro de Majadahonda, Madrid, Spain; Dra Amparo González, Hospital Severo Ochoa, Madrid, Spain; Dra Pilar Gutiérrez, Hospital de Getafe, Madrid, Spain; Dr Joaquín Ramírez, Hospital Príncipe de Asturias, Madrid, Spain; Dra Amparo Rodriguez, Hospital Montepríncipe Madrid, Madrid, Spain; Dr Fernando de la Vega, Hospital Sanitas La Zarzuela, Madrid, Spain; Dra Maria Chueca, Hospital Virgen del Camino, Pamplona, Spain; Dra Amaya Rodríguez, Hospital de Cruces, Baracaldo, Spain; Dra Elena Artola, Hospital Donostia; Donostia-San Sebastián, Spain; Dra Concepción Fernández, Hospital Basurto, Bilbao, Spain; Dr Ignacio Diez, Hospital Txagorritxu, Áraba, Spain.
References
- 1.Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone-IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clinical Endocrinology 2010. 721–728. ( 10.1111/j.1365-2265.2009.03775.x) [DOI] [PubMed] [Google Scholar]
- 2.Richmond E, Rogol AD. Current indications for growth hormone therapy for children and adolescents. Endocrine Development 2010. 92–108. ( 10.1159/000316130) [DOI] [PubMed] [Google Scholar]
- 3.Loche S, Carta L, Ibba A, Guzzetti C. Growth hormone treatment in non-growth hormone-deficient children. Annals of Pediatric Endocrinology and Metabolism 2014. 1–7. ( 10.6065/apem.2014.19.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH. & Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Hormone Research in Paediatrics 2016. 361–397. ( 10.1159/000452150) [DOI] [PubMed] [Google Scholar]
- 5.Lanes R. Long-term outcome of growth hormone therapy in children and adolescents. Treatments in Endocrinology 2004. 53–66. ( 10.2165/00024677-200403010-00006) [DOI] [PubMed] [Google Scholar]
- 6.Baxter L, Bryant J, Cave CB, Milne R. Recombinant growth hormone for children and adolescents with Turner syndrome. Cochrane Database of Systematic Reviews 2007. CD003887 ( 10.1002/14651858.CD003887.pub2) [DOI] [PubMed] [Google Scholar]
- 7.Rose SR, Cook DM, Fine MJ. Growth hormone therapy guidelines: clinical and managed care perspectives. American Journal of Pharmacy Benefits 2014. e134–e146. [Google Scholar]
- 8.Haverkamp F, Johansson L, Dumas H, Langham S, Tauber M, Veimo D, Chiarelli F. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clinical Therapeutics 2008. 307–316. ( 10.1016/j.clinthera.2008.02.017) [DOI] [PubMed] [Google Scholar]
- 9.Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Hormone Research in Paediatrics 2013. 189–196. ( 10.1159/000350251) [DOI] [PubMed] [Google Scholar]
- 10.De Pedro S, Murillo M, Salinas I, Granada ML, Martinez M, Puig-Domingo M, Andreu A, Bel J. Variability in adherence to rhGH treatment: socioeconomic causes and effect on children’s growth. Growth Hormone and IGF Research 2016. 32–35. ( 10.1016/j.ghir.2015.12.002) [DOI] [PubMed] [Google Scholar]
- 11.Saz-Parkinson Z, Granados M, Bouza C, Poveda Andrés JL, Amate JM. Self-administration of recombinant human growth hormone with an electronic device: clinical, economic and management benefits of objective adherence monitoring. Journal of Health Economics and Outcomes Research 2013. 296–307. [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes V, Neilson J, O’Flynn N, Calvert N, Kuntze S, Smithson H, Benson J, Blair J, Bowser A, Clyne W, et al Clinical Guidelines and Evidence Review for Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence. London, UK: National Collaborating Centre for Primary Care and Royal College of General Practitioners, 2009. (available at: https://www.nice.org.uk/guidance/cg76/resources/medicines-adherence-involving-patients-in-decisions-about-prescribed-medicines-and-supporting-adherence-pdf-975631782085) [PubMed] [Google Scholar]
- 13.Tauber M, Payen C, Cartault A, Jouret B, Edouard T, Roger D. User trial of Easypod, an electronic autoinjector for growth hormone. Annales d’Endocrinologie 2008. 511–516. ( 10.1016/j.ando.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization.eHealth. Geneva, Switzerland: World Health Organization, 2017. (available at: http://www.who.int/ehealth/en/) [Google Scholar]
- 15.Davies P, Kim HS, Borkenstein M, Du M, Kirk J, Kostalova L, Lebl J, Loche S, Luczay A, Nicolino M, et al Quantifying adherence to growth hormone treatment: the easypod™ connect observational study (ECOS). International Journal of Pediatric Endocrinology 2013. 46 ( 10.1186/1687-9856-2013-S1-P46) [DOI] [Google Scholar]
- 16.Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, Hofman PL. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS ONE 2011. e16223 ( 10.1371/journal.pone.0016223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization, 2006. (available at: http://www.who.int/childgrowth/standards/technical_report/en/index.html) [Google Scholar]
- 18.Greulich WW, Pyle SI. Radiograph Atlas of Skeletal Development of the Hand and Wrist, 2nd ed Stanford, CA, USA: Stanford University Press, 1959. [Google Scholar]
- 19.Matsui DM. Drug compliance in pediatrics: clinical and research issues. Pediatric Clinics of North America 1997. 1–14. ( 10.1016/S0031-3955(05)70459-4) [DOI] [PubMed] [Google Scholar]
- 20.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Medical Care 2002. 794–811. ( 10.1097/01.MLR.0000024612.61915.2D) [DOI] [PubMed] [Google Scholar]
- 21.Bozzola M, Colle M, Halldin-Stenlid M, Larroque S, Zignani M. & easypod™ survey study group. Treatment adherence with the easypod™ growth hormone electronic auto-injector and patient acceptance: survey results from 824 children and their parents. BMC Endocrine Disorders 2011. 4 ( 10.1186/1472-6823-11-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann K, Ittner J, Müller-Rossberg E, Schönau E, Stephan R, Ullrich KP, Hoppe B, Ramseger R, Brämswig J. Growth hormone treatment adherence in prepubertal and pubertal children with different growth disorders. Hormone Research in Paediatrics 2013. 1–5. ( 10.1159/000351800) [DOI] [PubMed] [Google Scholar]
- 23.Loche S, Salerno M, Garofalo P, Cardinale GM, Licenziati MR, Citro G, Caruso Nicoletti M, Cappa M, Longobardi S, Maghnie M, et al Adherence in children with growth hormone deficiency treated with r-hGH and the easypod™ device. Journal of Endocrinological Investigation 2016. 1419–1424. ( 10.1007/s40618-016-0510-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez A, Rodríguez-Arnao MD, Labarta JI, Martínez-Aedo MJ, Alija M, Díez-López I, Cañete R, Otero Villar J. Results after the first year of treatment with recombinant human growth hormone therapy in a group of Spanish children with short stature. Revista Española Endocrinología Pediátrica 2015. 41–52. [Google Scholar]
- 25.Arrabal Vela MA, García Gijón CP, Pascual Martin M, Benet Giménez I, Áreas Del Águila V, Muñoz Rodríguez JR, Palomo Atance E. Adherence to somatotropin treatment administered with an electronic device. Endocrinología, Diabetes y Nutrición 2018. 314–318. ( 10.1016/j.endinu.2018.02.003) [DOI] [PubMed] [Google Scholar]
- 26.Koledova E, Stoyanov G, Ovbude L, Davies PSW. Adherence and long-term growth outcomes: results from the easypod™ connect observational study (ECOS) in paediatric patients with growth disorders. Endocrine Connections 2018. 914–923. ( 10.1530/EC-18-0172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004. 1218–1224. ( 10.2337/diacare.27.5.1218) [DOI] [PubMed] [Google Scholar]
- 28.Costello K, Kennedy P, Scanzillo J. Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape Journal of Medicine 2008. 225. [PMC free article] [PubMed] [Google Scholar]
- 29.Lugaresi A, Florio C, Brescia-Morra V, Cottone S, Bellantonio P, Clerico M, Centonze D, Uccelli A, di Ioia M, De Luca G, et al Patient adherence to and tolerability of self-administered interferon β-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC Neurology 2012. 7 ( 10.1186/1471-2377-12-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spoudeas HA, Bajaj P, Sommerford N. Maintaining persistence and adherence with subcutaneous growth-hormone therapy in children: comparing jet-delivery and needle-based devices. Patient Preference and Adherence 2014. 1255–1263. ( 10.2147/PPA.S70019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weill J, Niez P. Adherence to the treatment with Zomajet, a needle-free device transjecting growth hormone: results of French observational survey. Poster presented at the European Society for Paediatric Endocrinology Annual Conference, Milan, Italy; September 19–22, 2013. [Google Scholar]
- 32.Davis SA, Feldman SR. Using Hawthorne effects to improve adherence in clinical practice: lessons from clinical trials. JAMA Dermatology 2013. 490–491. ( 10.1001/jamadermatol.2013.2843) [DOI] [PubMed] [Google Scholar]
- 33.Auer MK, Stieg MR, Hoffmann J, Stalla GK. Is insulin-like growth factor-I a good marker for treatment adherence in growth hormone deficiency in adulthood? Clinical Endocrinology 2016. 862–869. ( 10.1111/cen.13030) [DOI] [PubMed] [Google Scholar]
- 34.Léger J, Mohamed D, Dos Santos S, Ben Azoun M, Zénaty D, Simon D, Paulsen A, Martinerie L, Chevenne D, Alberti C, et al Impact of the underlying etiology of growth hormone deficiency on serum IGF-I SDS levels during GH treatment in children. European Journal of Endocrinology 2017. 267–276. ( 10.1530/EJE-17-0215) [DOI] [PubMed] [Google Scholar]
- 35.Ross J, Lee PA, Gut R, Germak J. Factors influencing the one- and two-year growth response in children treated with growth hormone: analysis from an observational study. International Journal of Pediatric Endocrinology 2010. 494656 ( 10.1155/2010/494656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Hormone and IGF Research 2009. 1–11. ( 10.1016/j.ghir.2008.08.001) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a