Abstract
Context and objective
Males with Klinefelter syndrome (KS) are typically hypogonadal with a high incidence of metabolic disease, increased body fat and mortality. Testosterone treatment of hypogonadal patients decrease fat mass, increase lean body mass and improve insulin sensitivity, but whether this extends to patients with KS is presently unknown.
Research design and methods
In a randomized, double-blind, placebo-controlled, BMI-matched cross-over study, 13 males with KS (age: 34.8 years; BMI: 26.7 kg/m2) received testosterone (Andriol®) 160 mg per day (testosterone) or placebo treatment for 6 months. Thirteen age- and BMI-matched healthy controls were recruited. DEXA scan, abdominal computed tomography (CT) scan and a hyperinsulinemic–euglycemic clamp, muscle strength and maximal oxygen uptake measurement were performed.
Results
Total lean body mass and body fat mass were comparable between testosterone-naïve KS and controls using DEXA, whereas visceral fat mass, total abdominal and intra-abdominal fat by CT was increased (P < 0.05). Testosterone decreased total body fat (P = 0.01) and abdominal fat by CT (P = 0.04). Glucose disposal was similar between testosterone-naïve KS and controls (P = 0.3) and unchanged during testosterone (P = 0.8). Free fatty acid suppression during the clamp was impaired in KS and maximal oxygen uptake was markedly lower in KS, but both were unaffected by treatment. Testosterone increased hemoglobin and IGF-I.
Conclusion
Testosterone treatment in adult males with KS for 6 months leads to favorable changes in body composition with reductions in fat mass, including abdominal fat mass, but does not change measures of glucose homeostasis.
Keywords: Klinefelter syndrome, testosterone, body composition, rare diseases/syndromes, insulin sensitivity
Introduction
Klinefelter syndrome (KS, 47,XXY) is the most common sex chromosomal disorder with a prevalence of 1 in 660 men (1, 2). Classically, the KS phenotype is characterized by eunuch body proportions, increased height and hypergonadotropic hypogonadism (3). The incidence of the metabolic syndrome and insulin resistance is considerably higher in patients with KS (4, 5), and epidemiological studies show an increased mortality due to diabetes as well as diseases of the cardiovascular, respiratory and digestive systems (6).
The association between KS and diabetes was first reported in 1969, with 39% of patients with KS having a diabetic oral glucose tolerance test (7). Others have described decreased insulin sensitivity, with increased fasting insulin levels (8). At the same time cross-sectional studies have shown an inverse relationship between plasma testosterone and insulin resistance in normal males (9). We previously found that the increased occurrence of insulin resistance and diabetic glucose tolerance testing among males with KS, translates into an increased frequency of type 2 diabetes among patients with KS, which are often hypogonadal (10). Among normal males, hypogonadism is also more prevalent among patients with type 2 diabetes than in age-matched controls (9), which could indicate a bi-directional relationship between testosterone and diabetes. This effect is less pronounced (11) or even absent (4, 12) when one takes into account BMI or waist-to-hip ratio. This lack of an association between testosterone and diabetes, when adding BMI or the amount of body fat into the equation, begs the question whether the association between testosterone and type 2 diabetes is through adiposity rather than testosterone itself, not forgetting the distinct genetic background present in KS with an additional X chromosome. It has been shown that treatment with testosterone in hypogonadal patients with type 2 diabetes improves insulin sensitivity in obese patients (13), but not in lean patients (14). Which point toward the fact that changes in insulin sensitivity during therapy largely depend on the amount of ‘modifiable fat’, especially visceral fat.
Studies on male hypogonadal patients of different etiologies have shown an increase in muscle mass and a decreased fat mass following 6-month testosterone treatment (15) and that withdrawal of testosterone supplementation for just 14 days lead to lower insulin sensitivity (16). However, the genetic make-up of males with KS is unique and one study found that increased insulin resistance in KS was related to gene dosage of the CSF2RA gene located on both the X and Y chromosome (17). Also higher leptin levels in KS compared with controls were not lowered after 3 months of testosterone treatment (18), and finally, it has been suggested that overproduction of CCL2, a small chemokine expressed at sites of inflammation and associated with insulin resistance, could explain insulin resistance in KS (19). Recently, we found profound changes in the methylation and RNA expression pattern throughout the genome among males with KS and enrichment analyses showed that the observed methylation changes was related to development of diabetes (20).
The present study is the first randomized placebo-controlled study to investigate the effects of testosterone treatment on insulin sensitivity, body composition, muscle strength and maximal oxygen uptake in patients with KS using gold standard methods. We hypothesized that testosterone treatment would improve insulin sensitivity and body composition.
Materials and methods
Subjects
Twenty males with KS were included in the study. Men with KS were recruited from the outpatient clinic at the Department of Endocrinology and Internal Medicine at Aarhus University Hospital. Inclusion criteria were age above 18 years with verified KS karyotype. Exclusion criteria were BMI above 35 kg/m2 or below 20 kg/m2, diabetes, history of malignant disease, prostate-specific antigen above 4 ng/mL, symptomatic heart disease, familial history of thromboembolic conditions, clinical hepatic disease (alanine aminotransferase more than four times above upper limit value), known allergic condition toward the medicine used in the project, heavy smoking (more than 30 cigarettes a day), alcoholism (more than 21 units of alcohol per week), severe mental illness or any other condition with a known influence on the investigated parameters.
Most males with KS had received testosterone treatment before entering the study, while a few were testosterone naïve at inclusion. During the study one was excluded due to a high serum testosterone. Four withdrew their informed consent, and two withdrew their consent to participate due to side effects of the study medication, which was perceived to be normal effects of testosterone by the study investigators. Thus the final study group consisted of 13 patients (age: 34.8 (22–56 years); BMI: 26.7 ± 8.8 (kg/m2)) with cytogenetically verified KS.
The control group consisted of 13 age- (age: 34.8 (21–53) years) and BMI-matched (27.0 ± 6.9 (kg/m2)) healthy controls, which were recruited through advertisement on the Internet.
Before study start, participants (KS and controls) were screened and a complete physical examination was performed. All volunteers received oral and written information prior to giving written, informed consent. The study was conducted from 2005 to 2012. The protocol was approved by the Region Midt Ethical Scientific Committee (#20030077) and performed in accordance with the Helsinki Declaration II.
Study design
The study was a 12-month randomized, double-blinded, placebo-controlled, cross-over study without a washout period (Fig. 1). Randomization was performed in blocks of six in order to ensure equal numbers in each group, and by an independent body. Study days consisted of two coupled days; Day 1 or ‘metabolic day’ and Day 2, allocated for DEXA estimation of body composition, CT measuring the amount of intra-abdominal (visceral) fat mass, a VO2max test, muscle strength and 24-h ambulatory blood pressure measurement (AMBP) (Fig. 1). The KS group was randomized to either testosterone in the form of Andriol® (testosterone undecanoat) or placebo treatment and subsequently crossed over. The Andriol® dose was 160 mg/day divided in two doses of 80 mg and participants were instructed to ingest the medicine with a fatty meal. Compliance was measured by counting the remaining pills after every 6-month period. The control group did not receive any treatment and was examined once. The KS group was examined before and after each 6-month period. KS participants received their last tablet the evening before the examinations.
Figure 1.
Outline of the study design and the two separate study days.
Three days before the study, volunteers were instructed not to participate in heavy physical exercise or to drink alcoholic beverages. After an overnight fast (>10 h) subjects were admitted to the clinical research unit and confined to bed.
Hyperinsulinemic euglycemic clamp
At 08:00 h (t = −180 min) a catheter was placed into an antecubital vein for infusion of saline to maintain catheter patency. Another catheter was inserted into a vein on the contralateral hand and kept in a thermo-regulated heating box (60°C) for sampling of arterialized venous blood. After collection of baseline blood samples, plasma was collected for metabolites at t = 0, 150, 165 and 180 min. At t = 0 min, a 3-h infusion of human insulin (Actrapid; Novo Nordisk A/S) commenced (1.0 mU/kg total body mass/min). Plasma glucose was measured every 10 min and clamped at 5 mmol/L by a variable infusion of 20% glucose. The glucose infusion rate during the last hour of the clamp (M value) was used as an index of insulin sensitivity. Insulin and metabolite concentrations were determined every 60 min. Respiratory exchange rates (RQ) and total energy expenditure (EE) were calculated from indirect calorimetry (Deltatrac; Datex Instrumentarium, Helsinki, Finland) and urea excretion. Oxidation rates of glucose was calculated based on non-protein EE and RQ (21). Indirect calorimetry was performed from t = −30 to t = 0 min and from t = 150 to t = 180 min. At 180 min, all catheters were removed and plasma glucose stabilized and the participants had lunch and were discharged.
Analytical techniques
Plasma glucose was analyzed in duplicate using the glucose oxidase method (Beckman Coulter). Measurements were performed immediately during the study. Insulin was measured with an immunoassay (DAKO). HOMA2 calculator was downloaded from https://www.dtu.ox.ac.uk/homacalculator/. Plasma glucagon was measured by a RIA (22). Testosterone, DHEAS, androstenedione and 17-hydroxy-progesterone were measured by liquid-chromatography tandem mass spectrometry. The limit of detection for testosterone was 0.1 nmol/L, and the working ranges were 0.2–100 nmol/L. The working range was assessed from the precision profile, and defined as the concentration in which the coefficient of variation (CV) was <10%. Sex hormone-binding globulin, FSH and LH were analyzed on the Architect i2000 platform (Abbott) by chemiluminescent micro particle immunoassay method using the corresponding kits. The working ranges were 0.1–250 nmol/L, 0.05–150 IE/L and 0.07-250 IE/L, respectively. Serum IGF-1 was measured by noncompetitive TR-IFMA and serum IGFBP-3 was measured by an immunoradiometric assay (IRMA) (Diagnostic System Laboratories Inc.). C-reactive protein (CRP) was measured by a high-sensitive CRP TR-IFMA with a detection limit of 0.1 μg/L. Plasma lipids and triglycerides were measured using an automated commercially available system (Aeroset, Abbott Diagnostics, Abbott Park Laboratories). The CV was <5%. Serum FFA was determined by a colorimetric method using a commercial kit (Wako Chemicals).
Body composition, maximal aerobic capacity and 24-h ambulatory blood pressure measurement
Body weight was measured on trial day before confinement to bed (with the participants wearing underwear) to the nearest 0.1 kg, height was measured to the nearest 0.5 cm at inclusion and BMI was calculated. On the following day we measured total and regional fat mass (g) and lean body mass (g) by dual X-ray densitometry (DEXA) using a Hologic 2000/w osteodensitometer (Hologic, Waltham, MA, USA). The system software provided the mass of lean body, fat and bone mineral for the whole body and specific regions. Appendicular, trunk and visceral trunk fat mass and trunk and appendicular lean body mass were extracted. The CV for the DEXA scans was <2% as estimated by repeated measurements (23).
The amount of intra-abdominal (visceral) fat was evaluated by CT with a Somatom Plus-S scanner (Siemens). The subjects were studied in the supine position. The areas scanned comprised 6 and 12 mm cross-sectional slices at the umbilicus using 120 kV and 330 mAs. The same technician performed all the scans, which subsequently were analyzed by the same radiologist (AGJ). Then a 6-min submaximal exercise test with continuous monitoring of heart rate was performed on a bicycle ergometer (Monark Ergometric 829 E, Monark Exercise, Varberg, Sweden). We used a workload of 300–1200 kpm/min, depending on age and reported physical activity by the subject, and maximal aerobic capacity (VO2max) was calculated (24) based on extrapolation of heart rate, age of the participant and weight. The isometric strength of the right biceps and quadriceps muscles was assessed on Day 2 by means of a dynamometer (Good strength, Metitur Ltd, Jyväskylä, Finland), which electronically measures the isometric muscle functions in the upper and lower limbs. The strength was calculated as the mean of three voluntary maximum isometric contractions separated by 1 min intervals.
All the participants, KS and controls, underwent 24-h AMBP using an automatic portable apparatus (Spacelabs 90207, Redmond, Washington, USA) at the end of the examination program. The apparatus used an oscillometric method of BP measurement. An appropriate cuff size placed on the left arm was used and readings were obtained every 20 min for a period of 24 h on a normal weekday. Time of bed and time of rise in the morning was noted by the participant.
Microdialysis
After application of a local analgesic (Lidocaine), microdialysis catheters (CMA-60; CMA, Stockholm, Sweden) were inserted in subcutaneous adipose tissue in the periumbilical region. The microdialysis catheters have a molecular cutoff of 20 kDa and a membrane length of 30 mm and were perfused at a flow rate of 1 mL/min using CMA-107 perfusion pumps (CMA). Changes in interstitial glycerol concentration can be taken as an index of lipolysis (25). Glycerol, glucose and lactate in the microdialysis dialysate was measured in duplicate by an automated spectrophotometric kinetic enzymatic analyzer (CMA-600).
Statistics
All standard statistical calculations were performed using SPSS for Windows version 18.01 (SPSS Inc.). Data were checked for normality with Kolmogorov–Smirnov’s test and by plotting. Comparisons between groups were done with Student’s unpaired t-test or the Mann–Whitney U test, as appropriate. The development over time during the clamp in different analytes was analyzed with mixed models, as detailed below. We first compared placebo-treated KS with controls, and then we compared the effect of treatment (placebo or testosterone) within the KS group. The comparison of the treated KS and the controls was based on a test for interaction between the group and time factors, i.e. a test for parallel time curves. The effect of treatment within the KS group was based on a mixed model on the differences at each time point for each individual. Due to inhomogeneity between the standard deviations at different time points and also between the treated KS and the controls, we used mixed models with unstructured covariance matrices and Satterthwaite approximation when calculating P values with SAS/STAT 15.1 PROC MIXED (SAS Institute Inc. 2018. SAS/STAT® 15.1 User’s Guide. Cary, NC: SAS Institute Inc. at http://support.sas.com). We used a per-protocol analysis approach and thus not included the drop-outs in the final analysis. Data are shown as mean ± s.d. or median (range). P values <0.05 were regarded as statistically significant.
Results
Characteristics of study subjects and body composition during treatment
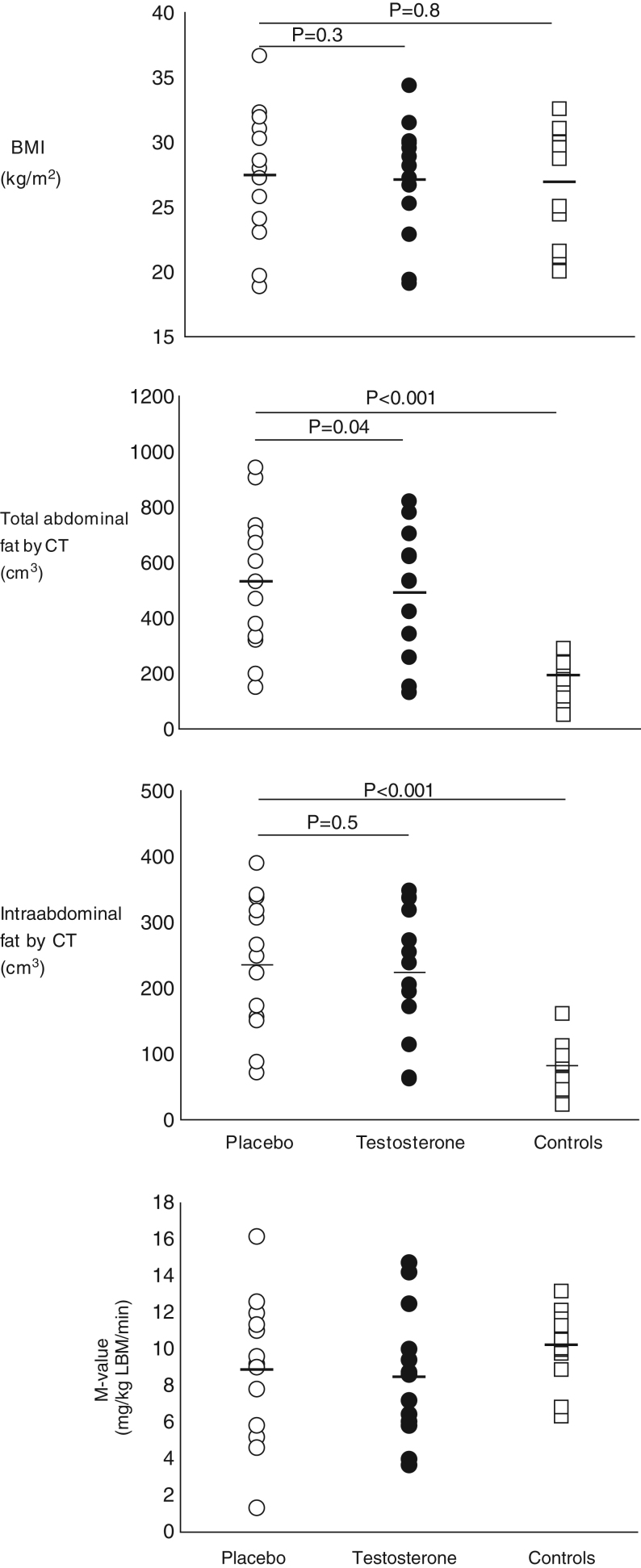
At baseline, males with KS and controls were similar on most body anthropometric composition measures (Table 1). Placebo-treated KS had greater total and intra-abdominal fat mass (P ≤ 0.001) and lower lumbar bone mineral density (P = 0.02) (Table 2) compared with controls at the end of the study. Testosterone treatment did not affect crude measures of body composition compared to baseline in males with KS, but weight, BMI, hip and waist circumference increased significantly during placebo treatment (Table 1). After 6 months, no difference was observed regarding the same measures comparing testosterone and placebo-treated men with KS (Table 1). However, abdominal fat and total body fat was higher in placebo versus testosterone-treated KS patients evaluated both by DEXA and CT (P ≤ 0.05 for all, Table 2). Total lean body mass increased during testosterone compared with placebo treatment, approaching statistical significance. Two participants dropped out of the study due to perceived side effects. These side effects were not considered to be serious. There were no other reported side effects.
Table 1.
Age of participants, anthropometric data at baseline in males with Klinefelter syndrome and controls at baseline.
| Klinefelter syndrome (mean ± s.d.) | Control (mean ± s.d.) | P value | ||||
|---|---|---|---|---|---|---|
| Age (years) | 34.8 ± 20.2 | 35 ± 20 | 1.00 | |||
| Height (cm) | 184 ± 15 | 182 ± 13 | 0.3 | |||
| Weight (kg) | 91 ± 15 | 89 ± 16 | 0.8 | |||
| BMI (kg/m2) | 27 ± 9 | 27 ± 7 | 0.9 | |||
| Baseline | Testosterone | Baseline vs testosterone | Placebo | Baseline vs placebo | Testosterone vs placebo | |
| Weight (kg) | 91 ± 15 | 91 ± 14 | 0.4 | 93 ± 16 | 0.03 | 0.2 |
| BMI (kg/m2) | 27 ± 5 | 27 ± 5 | 0.3 | 28 ± 5 | 0.03 | 0.2 |
| Hip (cm) | 95 ± 11 | 97 ± 7 | 0.2 | 99 ± 9 | 0.01 | 0.2 |
| Waist (cm) | 101 ± 17 | 104 ± 11 | 0.2 | 106 ± 14 | 0.01 | 0.4 |
In the lower part of the table anthropometric data at baseline and during treatment with either placebo or testosterone in Klinefelter syndrome is presented.
Table 2.
Body composition determined by CT and DEXA, muscle strength, androgens and other hormones, lipids, 24-h ambulatory blood pressure measurements (mmHg), and VO2max in males with Klinefelter syndrome during testosterone or placebo treatment and in controls.
| Placebo | Testosterone | P value | Controls | P value | ||
|---|---|---|---|---|---|---|
| placebo vs testosterone | placebo vs controls | |||||
| CT | Total abdominal fat cm3 | 533 ± 408 | 495 ± 366 | 0.04 | 194 ± 142 | <0.001 |
| Intra-abdominal fat cm3 | 233 ± 163 | 223 ± 166 | 0.5 | 82 ± 79 | <0.001 | |
| Ratio visceral vs total fat | 0.46 ± 0.12 | 0.46 ± 0.13 | 0.4 | 0.43 ± 0.17 | 0.4 | |
| DEXA | Total body fat (kg) | 26.8 ± 16.8 | 24.6 ± 15.3 | 0.01 | 21.3 ± 15.2 | 0.1 |
| Abdominal fat (kg) | 7.8 ± 5.8 | 7.2 ± 5.1 | 0.05 | 5.4 ± 4.6 | 0.06 | |
| Abdominal lean body mass (g) | 16.6 ± 4.9 | 17.0 ± 4.3 | 0.5 | 15.7 ± 3.5 | 0.3 | |
| Abdominal total mass (g) | 24.7 ± 10.6 | 24.3 ± 8.9 | 0.4 | 21.4 ± 8.1 | 0.1 | |
| Abdominal fat (%) | 29.9 ± 14.9 | 28.1 ± 16.8 | 0.03 | 24.5 ± 11.1 | 0.1 | |
| Visceral fat (g) | 3.5 ± 2.4 | 3.3 ± 2.3 | 0.2 | 2.3 ± 1.9 | 0.05 | |
| Total body lean mass (g) | 61.1 ± 12.1 | 62.1 ± 10.7 | 0.09 | 64.0 ± 13.0 | 0.4 | |
| Total lumbar BMD (g/cm2) | 0.98 ± 0.29 | 0.98 ± 0.27 | 0.5 | 1.11 ± 0.23 | 0.02 | |
| Total hip BMD (g/cm2) | 0.99 ± 0.16 | 0.99 ± 0.19 | 0.7 | 1.07 ± 0.22 | 0.1 | |
| Muscle strength | Arm – right biceps (Nm) | 300 ± 41 | 301 ± 50 | 0.9 | 322 ± 49 | 0.08 |
| Leg – right quadriceps (Nm) | 557 ± 121 | 556 ± 140 | 1.0 | 627 ± 115 | 0.2 | |
| Bio-chemistry | Testosterone (nmol/L) | 10.2 ± 5.5 | 8.5 ± 4.1 | 0.2 | 17.4 ± 5.0 | 0.002 |
| DHEAS (nmol/L) | 4.7 ± 2.3 | 4.0 ± 1.8 | 0.02 | 5.9 ± 3.2 | 0.3 | |
| 17-Hydroxy-progesterone (nmol/L) | 2.5 ± 1.3 | 1.8 ± 1.0 | 0.02 | 2.8 ± 1.2 | 0.5 | |
| Androstenedione (nmol/L) | 2.9 ± 1.0 | 2.8 ± 0.8 | 0.7 | 3.5 ± 1.5 | 0.3 | |
| LH (IU/L) | 19.6 ± 5.1 | 19.1 ± 5.2 | 0.7 | 4.0 ± 1.4 | <0.0001 | |
| FSH (IU/L) | 36.9 ± 9.1 | 35.7 ± 11.2 | 0.4 | 3.9 ± 1.6 | <0.0001 | |
| SHBG (nmol/L) | 31.6 ± 13.8 | 24.5 ± 10.6 | 0.001 | 30.3 ± 9.6 | 0.8 | |
| Hemoglobin (mmol/L) | 8.7 ± 0.6 | 9.0 ± 0.6 | 0.05 | 9.6 ± 0.6 | 0.001 | |
| IGF-I (µg/L) | 163 ± 51 | 178 ± 47 | 0.03 | 165 ± 54 | 0.9 | |
| IGFBP-3 (µg/L) | 4329 ± 794 | 4155 ± 710 | 0.2 | 3707 ± 397 | 0.02 | |
| CRP (mg/L) | 1.7 ± 1.7 | 2.1 ± 2.3 | 0.3 | 1.6 ± 1.6 | 1.0 | |
| Total cholesterol (mmol/L) | 4.7 ± 0.7 | 4.6 ± 0.8 | 0.6 | 4.9 ± 0.9 | 0.4 | |
| LDL cholesterol (mmol/L) | 2.7 ± 0.8 | 2.7 ± 1.0 | 1.0 | 2.8 ± 1.1 | 0.9 | |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.2 ± 0.6 | 0.4 | 1.3 ± 0.3 | 0.9 | |
| Triglycerides (mmol/L) | 1.3 ± 0.8 | 1.5 ± 0.6 | 0.4 | 1.3 ± 0.5 | 0.9 | |
| Alanine aminotransferase (U/L) | 36 ± 23 | 27 ± 11 | 0.1 | 42 ± 32 | 0.5 | |
| Blood Pressure | Day systolic AMBP | 125 ± 13 | 129 ± 9 | 0.2 | 129 ± 10 | 0.4 |
| Night systolic AMBP | 112 ± 12 | 113 ± 11 | 0.8 | 114 ± 10 | 0.7 | |
| 24-h systolic AMBP | 122 ± 9 | 124 ± 9 | 0.2 | 125 ± 8 | 0.4 | |
| Day diastolic AMBP | 77 ± 9 | 82 ± 13 | 0.8 | 80 ± 9 | 0.4 | |
| Night diastolic AMBP | 66 ± 10 | 65 ± 10 | 0.8 | 65 ± 10 | 0.9 | |
| 24-h diastolic AMBP | 74 ± 7 | 75 ± 7 | 0.4 | 76 ± 9 | 0.6 | |
| Exercise capacity | VO2max (mL O2/kg/min) | 33.1 ± 7.3 | 31.3 ± 7.4 | 0.1 | 45.2 ± 6.6 | 0.004 |
Insulin sensitivity and circulating metabolites
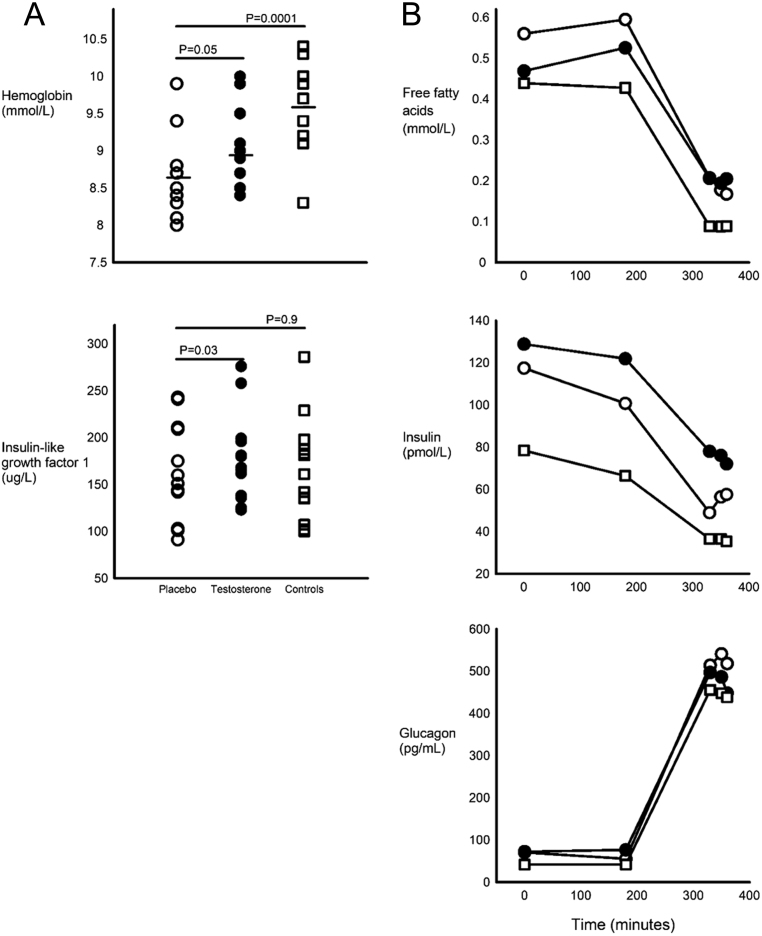
Insulin sensitivity was similar in placebo-treated KS and controls (Fig. 2), while HOMA-IR tended to be higher (P = 0.06). Glucagon, insulin and FFA showed a similar curvature in KS and controls during the clamp (non-significant interaction term group * time, P values not shown). Glucagon and insulin levels were similar in placebo-treated KS and controls during basal (P = 0.7 and P = 0.2, respectively) (Fig. 3) and during the entire clamp, including hyperinsulinemic circumstances (P = 0.2 and P = 0.2, respectively), whereas FFA levels were not suppressed to similar levels among males with KS (P = 0.0002) (Fig. 3). IGFBP3, but not IGF-1, was higher in placebo-treated KS as compared to controls (Table 2). CRP was also similar between placebo-treated KS and controls.
Figure 2.
(A) BMI, total and intra-abdominal fat in males with Klinefelter syndrome and controls. Under figure: Open circles indicate placebo-treated Klinefelter syndrome, closed circles indicate testosterone-treated Klinefelter syndrome and open squares indicate controls. P values are indicated in the figure. (B) Insulin sensitivity in males with Klinefelter syndrome and controls. Under figure: Round circles indicate placebo-treated KS, black circles indicate testosterone-treated KS and open squares indicate controls. There were no significant difference between groups.
Figure 3.
(A) Hemoglobin and IGF-I in males with Klinefelter syndrome during placebo or testosterone treatment and in controls. (B) FFA, insulin and glucagon at baseline and during clamp conditions. Under figure: Open circles indicate placebo-treated KS, closed circles indicate testosterone-treated KS and open squares indicate controls.
Testosterone treatment did not affect insulin sensitivity in KS (P = 0.6) (Fig. 2) or HOMA-IR (P = 0.8). Insulin and FFA levels were similar in placebo-treated and testosterone-treated KS during basal (P = 0.4 and P = 0.7) and hyperinsulinemic circumstances (P = 0.4 and P = 0.1) (Fig. 3). Glucagon was similar at baseline (P = 0.5), but slightly lower during testosterone treatment (P = 0.04). IGF-I (P < 0.03), but not IGFBP3 (P = 0.2), was higher among testosterone-treated KS compared with placebo (Fig. 3).
Sex hormones, gonadotrophins, hemoglobin and hematocrit
Testosterone and hemoglobin was lower in placebo-treated KS compared with controls, while levels of LH and FSH were higher, whereas levels of SHBG, 17-OH progesterone, DHEAS and androstenedione were comparable (Table 2).
Testosterone, LH and FSH levels were comparable between placebo and testosterone-treated KS patients, whereas SHBG levels were suppressed by testosterone treatment in KS (Table 2). Likewise, androstenedione levels were comparable, whereas 17-OH progesterone and DHEAS were higher in placebo versus testosterone-treated KS (Table 2). Hemoglobin was higher during testosterone treatment (Fig. 3), while levels of total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides and alanine aminotransferase were unchanged.
Resting EE, physical fitness and muscle strength
Resting EE was similar between placebo-treated KS and controls during basal and hyperinsulinemic circumstances (results not shown), and likewise, muscle strength was also similar, although numerically lower among placebo-treated KS (Table 2). VO2max was markedly lower in placebo-treated KS compared to controls (P = 0.004).
Resting EE and RQ during basal and hyperinsulinemic circumstances was unaffected by testosterone treatment (all P > 0.7). VO2max was only marginally affected by testosterone treatment (P = 0.12), while muscle strength in biceps and quadriceps muscle were comparable between groups (Table 2).
Microdialysis
Interstitial concentrations of glucose
Baseline levels of interstitial glucose (mmol/L) in abdominal adipose tissue were comparable between placebo-treated KS and controls (4.83 ± 0.98 vs 5.16 ± 1.22, respectively, interstitial concentrations of lactate, P = 0.55), and there were no effects over time (P = 0.3 and P = 0.68, basal and clamp periods, respectively). Baseline concentrations did not differ between placebo and testosterone-treated KS (4.83 ± 0.98 vs 5.09 ± 2.66, P = 0.8), and no change was seen with time (P = 0.15 and P = 0.49, basal and clamp periods, respectively).
Interstitial concentrations of glycerol
Baseline concentrations of interstitial glycerol (µmol/L) in abdominal adipose tissue was marginally higher among KS compared with controls (445 ± 297 vs 287 ± 108, respectively, P = 0.08). There were no effects over time (P = 0.8 and P = 0.7, basal and clamp periods, respectively). Likewise there was no difference between placebo and testosterone-treated KS (445 ± 297 vs 479 ± 251, P = 0.7), and no effect over time (P = 0.3 and P = 0.8 basal and clamp periods, respectively).
Interstitial concentrations of lactate
Baseline concentrations of interstitial lactate (mmol/L) in abdominal adipose tissue did not differ between placebo-treated KS and controls (2.38 ± 0.67 vs 2.1 ± 1.1, P = 0.4). There was no difference over time (P = 0.1 and P = 0.6, basal and clamp periods, respectively). There was no difference between placebo and testosterone-treated KS (2.38 ± 0.67 vs 2.86 ± 1.5, P = 0.3), and no effect over time for the comparison (P = 0.3 and P = 0.09, basal and clamp periods, respectively).
24-h ambulatory blood pressure
No differences between placebo- and testosterone-treated KS patients were detected with respect to any measures from 24-h AMBP. Likewise, no differences was seen between placebo-treated KS and controls (Table 2).
Discussion
This study, to our knowledge, is the first randomized trial of testosterone and placebo in KS studying insulin sensitivity, metabolism, body composition, muscle strength and maximal oxygen uptake. The principal results demonstrate distinct effects of testosterone on body composition, especially reductions in abdominal fat mass and total body fat during a 6-month course of testosterone treatment in KS. Testosterone treatment, on the other hand, did not change glucose homeostasis, circulating levels of free fatty acids or other measures of intermediate metabolism, but we speculate that longer term testosterone treatment would lead to greater changes in body composition and reductions in visceral fat, and in turn eventually increase insulin sensitivity.
The reductions in fat mass was accompanied by other testosterone-responsive changes, such as an increase in hemoglobin and IGF-I, reductions in SHBG and discrete changes in adrenal androgens such as 17-hydroxy progesterone and DHEAS, but not androstenedione, showing that active testosterone treatment influences adrenal steroidogenesis in a specific manner (26). Although hemoglobin increased significantly among males with KS during active treatment, the level was still somewhat lower than among controls. The testosterone-induced increase in hemoglobin seems to occur via increased erythropoietin and decreased ferritin and hepcidin (27). Due to the design of the study with withdrawal of study medication the day before examinations, we saw no changes in testosterone, LH and FSH.
Previously, short-term testosterone gel treatment (90 days) of hypogonadal men of different etiologies resulted in increments in lean body mass and reductions in fat mass measured by DEXA. The changes occurred in a dose-dependent fashion, but with increments in lean body mass only occurring with testosterone levels in the higher normo-physiological range (15). Woodhouse et al. studied experimental hypogonadism and 20-week graded testosterone enanthate treatment (28). Here, low physiological testosterone levels were associated with gains in subcutaneous, intermuscular and intra-abdominal adipose tissue, whereas testosterone levels in the normo- to supra-physiolgical range of 18.8–87.9 nmol/L increased lean body mass and reduced subcutaneous fat mass, but not total body fat or intra-abdominal fat mass (28). Our results are in line with the latter study, since only subcutaneous abdominal and total body fat, but not intra-abdominal fat mass was reduced during testosterone treatment. Although lean body mass, physical fitness and maximal muscle strength increased nominally, these changes were not statistically significantly affected by treatment in this study, perhaps because of a limited study sample. Notably, males with KS had much lower exercise capacity as evaluated by measurement of VO2max, as also previously documented in observational studies (26, 29). Of note, we saw nominal increases in weight and BMI from baseline through both treatment arms. Albeit only significant for the comparison with the placebo arm, these data underline the deleterious body compositional effects of a continuous hypogonadal milieu in these patients. In young boys with KS (4–12 years of age), treatment with oxandrolone, a non-aromatizable androgen not converted to estradiol, also led to lower body fat as measured by skin fold caliper (30).
In a previous study among 70 males with KS we described a high incidence of the metabolic syndrome and insulin resistance compared with an age-matched control group. About 50% of KS patients fulfilled the criteria for the metabolic syndrome, compared with 10% among controls (4, 31). Similar findings were later reported by Ishikawa et al. (5). In the current study, testosterone treatment had no effect on insulin-mediated glucose disposal (M value) or insulin sensitivity as expressed by HOMA-IR index, although a trend toward higher HOMA-IR was detected in placebo-treated KS compared to controls (P = 0.06). Of note, the males with KS studied here were not overtly obese, but overweight. In an uncontrolled setup, 18-month testosterone treatment in a group of males with KS with similar body composition was shown to improve HOMA-IR without changing BMI or waist circumference (32). In that study, however, HOMA-IR values were markedly higher than here (2.8 ± 1.7 vs 1.3 ± 1.0, respectively for placebo treated KS), and unfortunately, no data on regional body composition were provided. Thus, it is not clear whether reductions in intra-abdominal adipose tissue, which is normally believed to reflect insulin sensitivity, did in fact occur in that study following treatment. Intermediate metabolism, reflected by free fatty acid and circulating hormones was also unaffected by active treatment. Some studies have indicated that testosterone treatment of hypogonadal patients with type 2 diabetes may primarily help obese patients with improvements in insulin sensitivity (13), while this may not happen in lean patients (14), which could indicate that testosterone therapy works by affecting ‘modifiable fat’, which usually would be visceral fat. However, a meta-analysis including about 2900 men from cross-sectional studies and 850 with type 2 diabetes, showed that testosterone levels were significantly lower in patients with type 2 diabetes even after controlling for age and BMI. Here it was concluded that higher testosterone levels lead to a decreased risk of type 2 diabetes mellitus (33). Testosterone may also have direct effects on insulin sensitivity, because in patients with hypogonadotropic hypogonadism, removal of testosterone replacement therapy leads to reductions in insulin sensitivity within only 14 days (16). We previously showed short-term hypogonadism in healthy males did not affect insulin sensitivity when studied with the gold standard technique, hyperinsulinemic–euglycemic clamp technique (34, 35) and neither during 4 weeks induced hypogonadism (36) or during 20 weeks of treatment with a range of different doses (37), but 1-week treatment of healthy lean men with aromatase inhibitors resulted in slight elevation of testosterone, decreased estradiol levels and decreased insulin sensitivity (38), indicating a role for estradiol in determining insulin sensitivity in males. Previously, we have found normal levels of estradiol in males with KS, with higher levels in testosterone-treated KS (26, 31, 39). Interestingly, we observed only partial suppression of FFAs during the clamp when comparing placebo-treated KS with controls, an indirect sign of insulin resistance. To conclude, there is both epidemiological and clinical evidence that shows a four-fold increased risk of diabetes and metabolic syndrome in KS. Presently, there is no evidence to suggest that testosterone replacement therapy of KS patients improves insulin sensitivity, although it is likely that indirect effects on body composition and physical fitness in the longer term will lead to lowered insulin resistance and thus could protect from development of type 2 diabetes. The dissociation reported here between positive changes in body composition and no changes in glucose homeostasis may indicate that the high prevalence of type 2 diabetes in males with KS is a syndrome-specific trait that perhaps need much longer treatment and more precise normalization of testosterone levels to overcome. Longer term and larger studies will be needed to show this.
We saw a significant effect of testosterone treatment on circulating IGF-I, which is otherwise within normal levels in KS, both in childhood and adulthood (29, 40), without any change in IGFBP-3. Testosterone acts both via an effect on GH secretion and action, which subsequently enhances and augments the beneficial effects on fat oxidation and protein synthesis (41). IGFBP-3 was somewhat higher among males with KS, for which we have no ready explanation.
We used oral testosterone undecanoate which can show varying bioavailability, but nevertheless has been shown to have effects on body composition before (42), as also shown here. It may have been more efficacious to use either transdermal or injectable testosterone, which might have led to more pronounced effects on different androgen-responsive measures. Although we carefully matched KS and controls, we cannot be sure that the two groups are comparable on socioeconomic factors, habitual physical activity and diet, factors that could for instance affect measures of glucose homeostasis. There were several drop-outs during the study, but there was no common theme in their background for stopping participation in the study. Two males with KS dropped out due to reported side effects of the study medication. These were naïve to the effect of testosterone and thought it unpleasant to feel the normal effects of testosterone treatment. We did not find that there were any consistent differences between drop-outs and those that continued participation. The fact that some patients had never received testosterone, while others had and were using testosterone until inclusion in the study, can thus also be seen as a limitation of the study, and although all males with KS were only examined after 6 months of either active treatment or placebo, we cannot exclude that 6 months is an insufficient washout period for effects of testosterone on for example insulin sensitivity. Thus, we cannot exclude a type II error in the present study and inclusion of a larger study group would have been advantageous. Originally, it was our intent to include more males with KS, but we experienced a great deal of reluctance because of the 6 months of placebo treatment, which especially the males that were treated before entering the trial saw as problematic. We did not experience any serious adverse effects during the study.
In conclusion, testosterone for 6 months induces significant and positive effects on body composition in males with KS, including a reduced abdominal fat mass. We observed no changes in measures of glucose homeostasis, but prolonged treatment with testosterone in KS will likely lead to further positive changes also regarding glucose homeostasis. We observed no serious adverse effects of testosterone treatment.
Declaration of interest
C H is a Senior Editor for Endocrine Connections. C H was not involved in the review or editorial process for this paper, on which he is listed as an author. The other authors have nothing to disclose.
Funding
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that this study was supported by grants from the Danish Medical Research Council, an unconditional research grant from Ipsen Pharma, grants from the Novo Nordisk Foundation, Aase and Einar Danielsen foundation and the Danish Diabetes Association. None of the study funders had any role in study design, collection, analysis, interpretation, preparation of the manuscript or in the decision to submit the article for publication.
Author contribution statement
C H, A B, K K, A G J, N H B and C H G contributed substantial to conception and design of the study. C H, K A G, M E and C H G analyzed the data, and C H drafted the manuscript and C H G designed the tables. All authors were involved in the interpretation of the data, critically revised the article and approved the final version for publishing. All authors had full access to the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. CHG is the guarantor of the study and affirms that the manuscript is honest, accurate and transparent and no important aspects of the study have been omitted.
Acknowledgements
The technical assistance of Lone Svendsen, Lene Christensen, Susanne Sørensen, Hanne Petersen, Hanne Mertz, Joan Hansen (Medical Research Laboratories) and the staff at the Osteoporosis Clinic, Aarhus University Hospital is highly appreciated. The authors also wish to thank the doctors who referred their KS patients to us.
References
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. Journal of Clinical Endocrinology and Metabolism 2003. 622–626. ( 10.1210/jc.2002-021491) [DOI] [PubMed] [Google Scholar]
- 2.Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebaek A. Klinefelter syndrome – integrating genetics, neuropsychology and endocrinology. Endocrine Reviews 389–423. ( 10.1210/er.2017-00212) [DOI] [PubMed] [Google Scholar]
- 3.Smyth CM, Bremner WJ. Klinefelter syndrome. Archives of Internal Medicine 1998. 1309–1314. ( 10.1001/archinte.158.12.1309) [DOI] [PubMed] [Google Scholar]
- 4.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen JS, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 2006. 1591–1598. ( 10.2337/dc06-0145) [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa T, Yamaguchi K, Kondo Y, Takenaka A, Fujisawa M. Metabolic syndrome in men with Klinefelter’s syndrome. Urology 2008. 1109–1113. ( 10.1016/j.urology.2008.01.051) [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA. & United Kingdom Clinical Cytogenetics Group. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. Journal of Clinical Endocrinology and Metabolism 2005. 6516–6522. ( 10.1210/jc.2005-1077) [DOI] [PubMed] [Google Scholar]
- 7.Nielsen J, Johansen K, Yde H. Frequency of diabetes mellitus in patients with Klinefelter’s syndrome of different chromosome constitutions and the XYY syndrome. Plasma insulin and growth hormone level after a glucose load. Journal of Clinical Endocrinology and Metabolism 1969. 1062–1073. ( 10.1210/jcem-29-8-1062) [DOI] [PubMed] [Google Scholar]
- 8.Pei D, Sheu WH, Jeng CY, Liao WK, Fuh MM. Insulin resistance in patients with Klinefelter’s syndrome and idiopathic gonadotropin deficiency. Journal of the Formosan Medical Association 1998. 534–540. [PubMed] [Google Scholar]
- 9.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, MacIsaac RJ, Clarke S, Zajac JD, Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. Journal of Clinical Endocrinology and Metabolism 2008. 1834–1840. ( 10.1210/jc.2007-2177) [DOI] [PubMed] [Google Scholar]
- 10.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. Journal of Clinical Endocrinology and Metabolism 2006. 1254–1260. ( 10.1210/jc.2005-0697) [DOI] [PubMed] [Google Scholar]
- 11.Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity 2010. 247–256. ( 10.1097/MED.0b013e32833919cf) [DOI] [PubMed] [Google Scholar]
- 12.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 2007. 234–238. ( 10.2337/dc06-1579) [DOI] [PubMed] [Google Scholar]
- 13.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. Journal of Andrology 2009. 726–733. ( 10.2164/jandrol.108.007005) [DOI] [PubMed] [Google Scholar]
- 14.Gopal RA, Bothra N, Acharya SV, Ganesh HK, Bandgar TR, Menon PS, Shah NS. Treatment of hypogonadism with testosterone in patients with type 2 diabetes mellitus. Endocrine Practice 2010. 570–576. ( 10.4158/EP09355.OR) [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. & Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Journal of Clinical Endocrinology and Metabolism 2000. 2839–2853. ( 10.1210/jcem.85.8.6747) [DOI] [PubMed] [Google Scholar]
- 16.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. Journal of Clinical Endocrinology and Metabolism 2007. 4254–4259. ( 10.1210/jc.2007-0454) [DOI] [PubMed] [Google Scholar]
- 17.Zitzmann M, Bongers R, Werler S, Bogdanova N, Wistuba J, Kliesch S, Gromoll J, Tuttelmann F. Gene expression patterns in relation to the clinical phenotype in Klinefelter syndrome. Journal of Clinical Endocrinology and Metabolism 2015. E518–E523. ( 10.1210/jc.2014-2780) [DOI] [PubMed] [Google Scholar]
- 18.Ozata M, Ozisik G, Caglayan S, Yesilova Z, Bingol N, Saglam M, Turan M, Beyhan Z. Effects of gonadotropin and testosterone treatments on plasma leptin levels in male patients with idiopathic hypogonadotropic hypogonadism and Klinefelter’s syndrome. Hormone and Metabolic Research 1998. 266–271. ( 10.1055/s-2007-978881) [DOI] [PubMed] [Google Scholar]
- 19.Rotondi M, Coperchini F, Renzullo A, Accardo G, Esposito D, Groppelli G, Magri F, Cittadini A, Isidori AM, Chiovato L, et al. High circulating levels of CCL2 in patients with Klinefelter’s syndrome. Clinical Endocrinology 2014. 465–467. ( 10.1111/cen.12245) [DOI] [PubMed] [Google Scholar]
- 20.Skakkebaek A, Nielsen MM, Trolle C, Vang S, Hornshoj H, Hedegaard J, Wallentin M, Bojesen A, Hertz JM, Fedder J, et al. DNA hypermethylation and differential gene expression associated with Klinefelter syndrome. Scientific Reports 2018. 13740 ( 10.1038/s41598-018-31780-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 1983. 628–634. ( 10.1152/jappl.1983.55.2.628) [DOI] [PubMed] [Google Scholar]
- 22.Orskov H, Thomsen HG, Yde H. Wick chromatography for rapid and reliable immunoassay of insulin, glucagon and growth hormone. Nature 1968. 193–195. ( 10.1038/219193b0) [DOI] [PubMed] [Google Scholar]
- 23.Abrahamsen B, Gram J, Hansen TB, Beck-Nielsen H. Cross calibration of QDR-2000 and QDR-1000 dual-energy X-ray densitometers for bone mineral and soft-tissue measurements. Bone 1995. 385–390. ( 10.1016/8756-3282(94)00054-9) [DOI] [PubMed] [Google Scholar]
- 24.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiologica Scandinavica: Supplementum 1960. 1–92. [PubMed] [Google Scholar]
- 25.Hagstrom Toft E, Enoksson S, Moberg E, Bolinder J, Arner P. Absolute concentrations of glycerol and lactate in human skeletal muscle, adipose tissue, and blood. American Journal of Physiology 1997. E584–E592. ( 10.1152/ajpendo.1997.273.3.E584) [DOI] [PubMed] [Google Scholar]
- 26.Chang S, Skakkebaek A, Trolle C, Bojesen A, Hertz JM, Cohen A, Hougaard DM, Wallentin M, Pedersen AD, Ostergaard JR, et al. Anthropometry in Klinefelter syndrome – multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. Journal of Clinical Endocrinology and Metabolism 2015. E508–E517. ( 10.1210/jc.2014-2834) [DOI] [PubMed] [Google Scholar]
- 27.Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, Connor Westfall J, Bae H, Gordeuk V, Bhasin S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 2014. 725–735. ( 10.1093/gerona/glt154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, Bhasin S. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. Journal of Clinical Endocrinology and Metabolism 2004. 718–726. ( 10.1210/jc.2003-031492) [DOI] [PubMed] [Google Scholar]
- 29.Bojesen A, Birkebaek N, Kristensen K, Heickendorff L, Mosekilde L, Christiansen JS, Gravholt CH. Bone mineral density in Klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporosis International 2011. 1441–1450. ( 10.1007/s00198-010-1354-7) [DOI] [PubMed] [Google Scholar]
- 30.Davis SM, Cox-Martin MG, Bardsley MZ, Kowal K, Zeitler PS, Ross JL. Effects of oxandrolone on cardiometabolic health in boys with Klinefelter syndrome: a randomized controlled trial. Journal of Clinical Endocrinology and Metabolism 2017. 176–184. ( 10.1210/jc.2016-2904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Host C, Bojesen A, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH. Effect of sex hormone treatment on circulating adiponectin and subforms in Turner and Klinefelter syndrome. European Journal of Clinical Investigation 2010. 211–219. ( 10.1111/j.1365-2362.2009.02250.x) [DOI] [PubMed] [Google Scholar]
- 32.Selice R, Caretta N, Di Mambro A, Torino M, Palego P, Ferlin A, Foresta C. Prostate volume and growth during testosterone replacement therapy is related to visceral obesity in Klinefelter syndrome. European Journal of Endocrinology 2013. 743–749. ( 10.1530/EJE-13-0488) [DOI] [PubMed] [Google Scholar]
- 33.Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, Forti G, Mannucci E, Maggi M. Type 2 diabetes mellitus and testosterone: a meta-analysis study. International Journal of Andrology 2011. 528–540. ( 10.1111/j.1365-2605.2010.01117.x) [DOI] [PubMed] [Google Scholar]
- 34.Host C, Gormsen LC, Christensen B, Jessen N, Hougaard DM, Christiansen JS, Pedersen SB, Jensen MD, Nielsen S, Gravholt CH. Independent effects of testosterone on lipid oxidation and VLDL-TG production: a randomized, double-blind, placebo-controlled, crossover study. Diabetes 2013. 1409–1416. ( 10.2337/db12-0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Host C, Gormsen LC, Hougaard DM, Christiansen JS, Pedersen SB, Gravholt CH. Acute and short-term chronic testosterone fluctuation effects on glucose homeostasis, insulin sensitivity, and adiponectin: a randomized, double-blind, placebo-controlled, crossover study. Journal of Clinical Endocrinology and Metabolism 2014. E1088–E1096. ( 10.1210/jc.2013-2807) [DOI] [PubMed] [Google Scholar]
- 36.Rabiee A, Dwyer AA, Caronia LM, Hayes FJ, Yialamas MA, Andersen DK, Thomas B, Torriani M, Elahi D. Impact of acute biochemical castration on insulin sensitivity in healthy adult men. Endocrine Research 2010. 71–84. ( 10.3109/07435801003705601) [DOI] [PubMed] [Google Scholar]
- 37.Singh AB, Hsia S, Alaupovic P, Sinha-Hikim I, Woodhouse L, Buchanan TA, Shen R, Bross R, Berman N, Bhasin S. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. Journal of Clinical Endocrinology and Metabolism 2002. 136–143. ( 10.1210/jcem.87.1.8172) [DOI] [PubMed] [Google Scholar]
- 38.Gibb FW, Homer NZ, Faqehi AM, Upreti R, Livingstone DE, McInnes KJ, Andrew R, Walker BR. Aromatase inhibition reduces insulin sensitivity in healthy men. Journal of Clinical Endocrinology and Metabolism 2016. 2040–2046. ( 10.1210/jc.2015-4146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanbhogue VV, Hansen S, Jorgensen NR, Brixen K, Gravholt CH. Bone geometry, volumetric density, microarchitecture and estimated bone strength assessed by HR-pQCT in Klinefelter syndrome. Journal of Bone and Mineral Research 2014. 2474–2482. ( 10.1002/jbmr.2272) [DOI] [PubMed] [Google Scholar]
- 40.Aksglaede L, Skakkebaek NE, Juul A. Abnormal sex chromosome constitution and longitudinal growth: serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the Y chromosome (SRY)-positive 46,XX karyotypes. Journal of Clinical Endocrinology and Metabolism 2008. 169–176. ( 10.1210/jc.2007-1426) [DOI] [PubMed] [Google Scholar]
- 41.Birzniece V, Ho KKY. Sex steroids and the GH axis: implications for the management of hypopituitarism. Best Practice and Research: Clinical Endocrinology and Metabolism 2017. 59–69. ( 10.1016/j.beem.2017.03.003) [DOI] [PubMed] [Google Scholar]
- 42.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 2008. 39–52. ( 10.1001/jama.2007.51) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a