Abstract
Background
Smoking is a strong risk factor for the development of Graves’ ophthalmopathy (GO). Immediate early genes (IEGs) are overexpressed in patients with active GO compared to healthy controls. The aim of this study was to study the effects of tobacco smoking and simvastatin on preadipocytes and orbital fibroblasts (OFs) in the adipogenic process.
Methods
Cigarette smoke extract (CSE) was generated by a validated pump system. Mouse 3T3-L1 preadipocytes or OFs were exposed to 10% CSE with or without simvastatin. Gene expression was studied in preadipocytes and OFs exposed to CSE with or without simvastatin and compared to unexposed cells or cells treated with a differentiation cocktail.
Results
In 3T3-L1 preadipocytes, Cyr61, Ptgs2, Egr1 and Zfp36 expression levels were two-fold higher in cells exposed to CSE than in unexposed cells. Simvastatin downregulated the expression of these genes (1.6-fold, 5.5-fold, 3.3-fold, 1.4-fold, respectively). CSE alone could not stimulate preadipocytes to differentiate. Scd1, Ppar-γ and adipogenesis were downregulated in simvastatin-treated preadipocytes compared to nontreated preadipocytes 18-, 35- and 1.7-fold, respectively. In OFs, similar effects of CSE were seen on the expression of CYR61 (1.4-fold) and PTGS2 (3-fold). Simvastatin downregulated adipogenesis, PPAR-γ (2-fold) and SCD (27-fold) expression in OFs.
Conclusion
CSE upregulated early adipogenic genes in both mouse 3T3-L1 preadipocytes and human OFs but did not by itself induce adipogenesis. Simvastatin inhibited the expression of both early and late adipogenic genes and adipogenesis in preadipocytes and human OFs. The effect of simvastatin should be investigated in a clinical trial of patients with GO.
Keywords: Graves’ opthalmopathy (GO), cigarette smoke extract (CSE), CYR61, simvastatin, orbital fibroblasts
Introduction
Autoimmune thyroid disease is a prerequisite for the development of Graves’ ophthalmopathy (GO), and both thyroid and orbital tissues are infiltrated by immunocompetent cells. The thyroid-stimulating hormone receptor (TSHR) acts as an autoantigen and is expressed by both preadipocytes and adipocytes in orbital tissue (1, 2) as well as in the thyroid. Other autoantigens, such as the insulin-like growth factor 1 (IGF-1) receptor have been suggested to be involved in the pathogenesis of GO (3, 4).
Almost all patients with Graves’ disease (GD) (98%) already have morphological changes in the retrobulbar space at diagnosis when investigated with CT/MRI, but only 1/3 of the patients will develop clinical ophthalmopathy (5).
In the individual patient, it is difficult to predict who will develop clinical ophthalmopathy.
The triggering of the autoimmune response depends on the interplay between genetic (6, 7) and environmental factors (8).
One strong risk factor for development of GO is smoking, which is also shared with GD. Smoking increases the risk for GD approximately twofold and GO approximately threefold (9).
Inflammation and adipogenesis are both important processes in the pathogenesis of GO. Inflammation causes an increased synthesis of hyaluronic acid from OFs, which partly explains the orbital edema in the eye muscles and retrobulbar tissue. In parallel, de novo adipogenesis and an accumulation of lipids in adipocytes increase the volume of the intraorbital tissue. During the initial proliferation phase of adipogenesis, when induced by adipogenic factors, growth-arrested preadipocytes express immediate early genes (IEGs). IEGs are mitogen-responsive genes that rapidly and transiently, within 30–60 min, trigger the subsequent transcription cascade leading to the development of mature adipocytes (10, 11).
In gene expression studies with microarray and real-time PCR, we have shown that IEGs such as prostaglandin-endoperoxide synthase 2 (COX-2/PTGS2) and cysteine-rich angiogenic inducer 61 (CYR61) were overexpressed in intraorbital adipose/connective tissue in GO patients with optic nerve dysfunction compared to healthy controls (12) and in smokers with active severe GO compared to non-smokers (13). Both genes are important in adipogenesis and inflammation. CYR61 has also been found to be expressed in skin fibroblasts exposed to tobacco smoke (14). In addition, we have demonstrated that several single nucleotide gene polymorphisms (SNPs) in CYR61 are associated with an increased risk of both GD and GO (15). One of these SNPs, rs12756618, was strongly associated with an increased risk of developing GO in smokers (OR 4.75).
In vitro simvastatin has been shown to downregulate CYR61 in osteoblasts (16) and synovial fibroblasts (17). In newly diagnosed GD patients, the use of statins significantly reduced the risk of development of GO (18).
Based on the results of our expression studies from patients with GO, the aim of this study was to establish an in vitro model to study the effect of tobacco smoking on preadipocytes and OFs from patients with GO and the effect of simvastatin on the adipogenic process.
Materials and methods
Adipocyte differentiation of 3T3-L1 preadipocytes
The mouse cell line 3T3-L1 preadipocytes were kindly provided by Olga Göransson, Lund University. The cells were cultured as previously described (19). Briefly, preadipocytes were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (Gibco, Thermo Scientific) with 10% fetal bovine serum (FBS) (Gibco, Thermo Scientific) and passaged by trypsinization less than 12 times. In order to find the concentration of simvastatin that would give an effect on the mRNA expression of genes of interest without affecting the viability of the cells, 2 days post-confluent 3T3-L1 preadipocytes were incubated with 1 µM, 5 µM or 10 µM simvastatin (17) for either 2 h, 6 h, 10 h or 24 h. The effect of simvastatin on mRNA expression of CYR61 was seen after 10 µM simvastatin treatment for 24 h and the viability was measured to >80%. 3T3-L1 cells were then exposed to CSE in concentrations of 3, 5 and 10% for 30 min, 1 h, 2 h and 24 h, respectively. The effect of CSE and/or 10 µM simvastatin was investigated by mRNA expression of CYR61 and cell viability was measured. The effect of CSE on CYR61 expression was already seen at 30 min and cell viability was >90% at all time points. Cell viability for 10% CSE and 10 µM simvastatin combined was >80%. Based on these results, simvastatin 10 µM and CSE 10% was used for further experiments. Two days post-confluent cells were pretreated with 10 µM simvastatin followed, in some experiments, by incubation with cigarette smoke extract (CSE) for 30–120 min. Cigarette smoke was extracted using a validated peristaltic pump system (20, 21). Standard differentiation cocktail containing 1 µM rosiglitazone (Sigma), 1 µM dexamethasone (Sigma) and 1.7 µM insulin (Sigma) was then added and replaced by growth medium after 3 days. In some experiments, 1.7 µM insulin was replaced by 1.7 µM IGF-1. For mRNA expression analysis, cells were taken at different time points during the differentiation. On differentiation day 6, the lipid content in the differentiated adipocytes was measured by Oil Red O (Sigma) staining. For visualization and counting, the cells were stained with Giemsa (Sigma) and visualized under a light microscope at 10× magnification. The number of differentiated cells were counted in five fields of vision in three separate wells, and average number of cells per field of vision were calculated.
Adipocyte differentiation of orbital fibroblasts (OFs)
Intraorbital adipose/connective tissue was obtained from patients with GO operated with decompression surgery and from healthy controls during blepharoplasty after cutting ‘septum orbitale’ as described previously (12). All subjects provided written informed consent and permission was granted by the Lund University Ethics Committee. All patients had been treated with corticosteroids (Table 1). The tissue was cut into pieces of 1–3 mm, trypsinized and cultured in DMEM with 10% FBS and 1% penicillin-streptomycin (Gibco, Thermo Scientific). The medium was changed every third day, and orbital fibroblast (OFs) were grown to confluence and passaged with trypsin (less than ten times). Cell viability and mRNA expression were measured, as described for the 3T3-L1 cells. Pretreatment with 10 µM simvastatin for 24 h had an effect on the expression of SCD, PPAR-γ and adipogenesis without effecting the cell viability which was measured to >70%. Cell viability after exposure to both 10 µM simvastatin and 10% CSE for 24 h was >70%. 10 µM simvastatin and 10% CSE were therefore chosen for further experiments. Confluent OFs were pretreated with 10 µM simvastatin for 24 h and in some experiments, exposed to 10% CSE for 30–120 min. Media was then changed to serum-free Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM:F-12 Gibco, Thermo Scientific) supplemented with 1 µM dexamethasone (Sigma), 1.7 µM insulin (Sigma), 1 µM rosiglitazone (Sigma) and 1 µM 3-isobutyl-1-methylxanthine (IBMX) (Sigma). In some experiments, 1.7 µM insulin was replaced by 1.7 µM IGF-1. After 3 days, the medium was changed to DMEM:F-12 with 3% FBS, 0.1 µM insulin and 1 µM dexamethasone (22). In some experiments, 0.1 µM insulin was replaced by 0.1 µM IGF-1. The OFs were grown until fully differentiated. For mRNA expression analysis, cells were taken at different time points during the differentiation. Visualization and Oil Red O staining of the differentiated adipocytes was done at differentiation day 12.
Table 1.
Clinical data on patients with Graves’ ophthalmopathy operated with orbital decompression and included in the study.
| Sex | M | F | F | F |
|---|---|---|---|---|
| Age at operation | 67 | 38 | 82 | 81 |
| Smoking at diagnosis of ophthalmopathy | No | Yes | No | No |
| Treatment of thyrotoxicosis before op GO | ||||
| Thyrostatics | Yes | Yes | Yes | Yes |
| Radioactive iodine | No | No | No | No |
| Thyroidectomy | No | Yes | No | No |
| Treatment of ophthalmopathy | ||||
| Corticosteroids | Yes | Yes | Yes | Yes |
| Retrobulbar irradiation | No | No | No | No |
| Duration of ophthalmopathy before operation (months) | 8 | 4 | 13 | 60 |
Cytotoxicity assay
Cell viability was investigated using a Cell Proliferation Kit I (MTT assay, Sigma). 3T3-L1 preadipocytes were treated with either CSE (3, 5 and 10%) for 30 min, 1 h, 2 h and 24 h or simvastatin (1 µM, 5 µM and 10 µM), for 2 h, 6 h, 10 h and 24 h, or both combined for 24 h. OFs were treated with both CSE 10% and simvastatin 10 µM for 24 h followed by the cell viability test using the MTT assay.
Absorbance of the solution was measured at 490 nm.
Real-time RT-PCR
RNA was isolated using RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions, and the purity and concentration were determined spectrophotometrically using a NanoDrop ND-1000. In total, 0.2 µg of total RNA was reverse transcribed using a QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. RT-PCR was performed using the QuantStudio 7 Flex sequence detection system (Applied Biosystems) according to the manufacturer’s instructions as described previously (13, 23) using TaqMan mRNA Expression Master Mix (Applied Biosystems). The following assays (Applied Biosystems) were used for mRNA expression in the 3T3-L1 cell line (assay ID in brackets): early growth response 1 (Egr1)/Mm00656724_m1/; cysteine-rich angiogenic inducer 61 (Cyr61)/Mm00487499_g1/; prostaglandin-endoperoxide synthase 2 (Ptgs2)/Mm00478374_m1/; zinc finger protein (Zfp36)/Mm00457144_m1/; stearoyl-CoA desaturase (Scd1)/Mm00446190_m1/; peroxisome proliferator-activated receptor-gamma (Ppar-γ)/Mm00440940_m1/; interleukin-6 (Il-6)/Mm00446190_m1/; and interleukin-1beta (Il-1b)/Mm00434228_m1/. The following assays were used in human OF’s: EGR1/Hs00152928_m1/; CYR61/Hs00155479_m1/; PTGS2/Hs00153133_m1/; ZFP36/Hs00185658_m1/; SCD/Hs01682761_m1/; PPAR-γ/Hs01115513_m1/; IL-6/Hs00174131_m1/, and IL-1B/Hs01555410_m1/. All samples were run in duplicate, and data were analyzed with the standard curve method using β-actin and cyclophilin A as endogenous controls for the 3T3-L1 cell line and OFs, respectively. The results were confirmed in at least three individual experiments.
Statistical analysis
The mean gene expression value from three separate experiments was compared between cells exposed to CSE versus unexposed cells, CSE-exposed versus DC-treated cells, and those with and without simvastatin treatment, using the Mann–Whitney rank-sum test. The time points where gene expression reached the maximum level are presented in the figures. Statistical significance was defined as P < 0.05. The data were analyzed using IBM SPSS Statistics 22 software.
Results
Expression of mRNA in 3T3-L1 preadipocytes after treatment with CSE or simvastatin
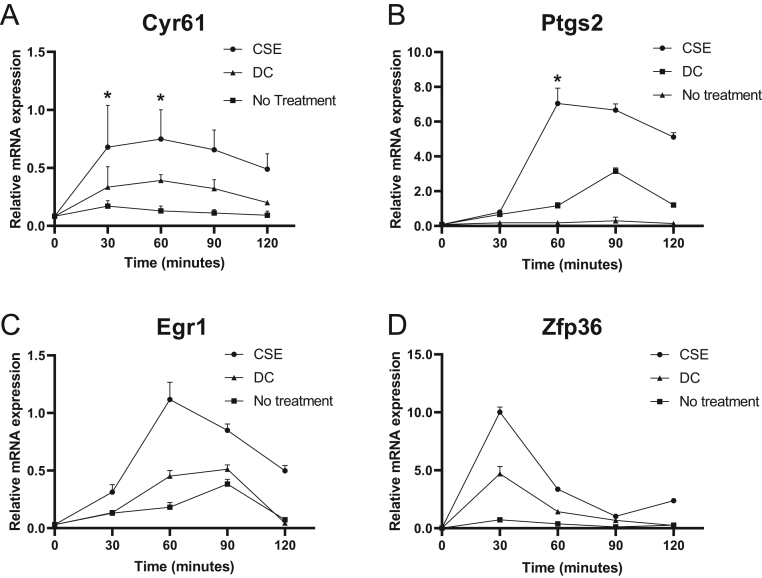
After adding CSE, a maximal increase in mRNA expression was already found after 30–60 min for Cyr61, Ptgs2, Egr1 and Zfp36 and was followed by a slow decrease over 2 h. The increase in mRNA expression after CSE exposure for Cyr61 was 1.7-fold (P = 0.04) (Fig. 1A) and for Ptgs2 was two-fold (P = 0.02) (Fig. 1B) compared to preadipocytes treated with DC and 3.7-fold (P = 0.02) and 11-fold (P = 0.02), respectively, compared to unexposed preadipocytes (Fig. 1A and B).
Figure 1.
Effects of cigarette smoke extract on early adipogenic genes in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were stimulated with differentiation cocktail (DC) or cigarette smoke extract (CSE) for 30–120 min. Gene expression was quantified with RT-PCR of Cyr61 (A), Ptgs2 (B), Egr1 (C) and Zfp36 (D). β-actin was used as endogenous control. Values are the mean ± s.d. of three independent experiments. *P < 0.05.
The mRNA expression of Egr1 was elevated two-fold (P = 0.06) in CSE-exposed preadipocytes compared to DC-treated preadipocytes and seven-fold (P = 0.06) compared to unexposed preadipocytes (Fig. 1C). Zfp36 mRNA expression in CSE-exposed preadipocytes increased two-fold (P = 0.08) compared to preadipocytes treated with DC and 12-fold (P = 0.07) compared to unexposed preadipocytes (Fig. 1D).
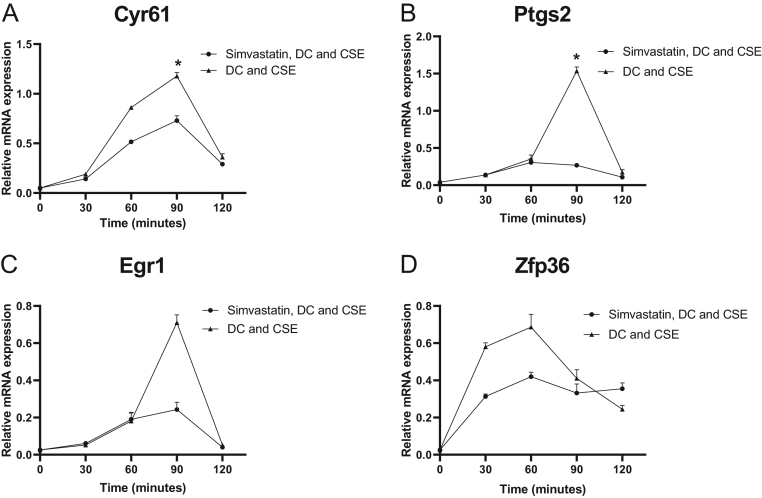
Simvastatin downregulated IEG expression in preadipocytes exposed to CSE. After CSE exposure, expression of Cyr61 decreased 1.6-fold (P = 0.03) in simvastatin-treated preadipocytes at 90 min compared to nontreated preadipocytes (Fig. 2A).
Figure 2.
Effects of simvastatin on early adipogenic genes in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes stimulated with differentiation cocktail (DC) and cigarette smoke extract (CSE) for 30–120 min in the presence or absence of simvastatin. Gene expression was quantified with RT-PCR. Cyr61 (A), Ptgs2 (B), Egr1 (C) and Zfp36 (D) with β-actin as endogenous control. Values are the mean ± s.d. of three independent experiments. *P < 0.05.
The expression of Ptgs2 was downregulated 5.5-fold (P = 0.03) at 90 min in simvastatin-treated preadipocytes compared to nontreated preadipocytes (Fig. 2B).
Egr1 was decreased 3.3-fold (P = 0.07) at 90 min in preadipocytes treated with simvastatin compared to nontreated preadipocytes, and Zfp36 was downregulated by 1.4-fold (P = 0.09) at 60 min (Fig. 2C and D).
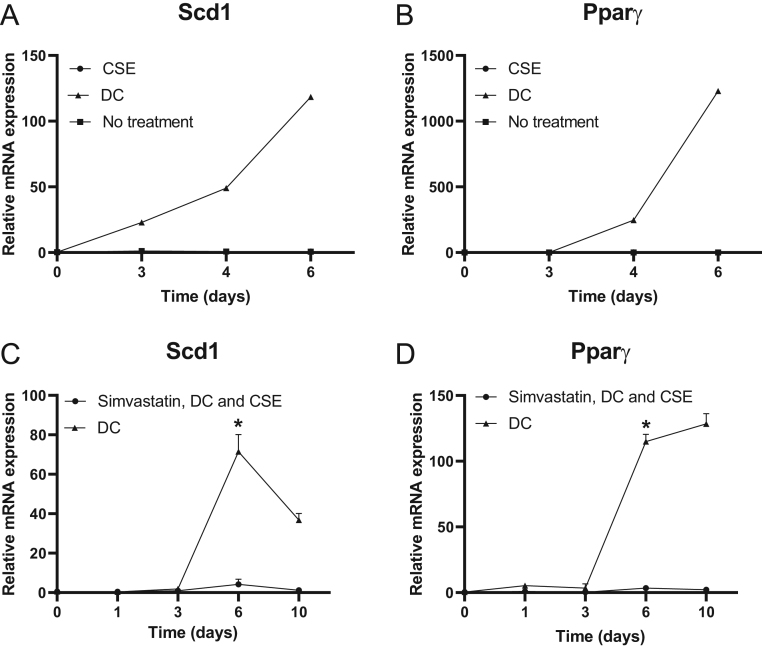
3T3-L1 preadipocytes were also grown for longer periods of time. The late adipogenic genes Scd1 and Ppar-γ showed an increase in expression after 3-4 days when treated with DC. However, their expression was not increased by CSE alone (Fig. 3), and no differentiated adipocytes could be visualized by light microscopy after CSE exposure (Fig. 4B).
Figure 3.
Effects of simvastatin on the expression of late adipogenic genes in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were stimulated with differentiation cocktail (DC) with or without cigarette smoke extract (CSE). Gene expression was quantified with RT-PCR in the absence of simvastatin, Scd1 (A) and Ppar-γ (B) after 3–6 days or in the presence of simvastatin after 3–10 days, Scd1 (C) and Ppar-γ (D) with β-actin as endogenous control. Values are the mean ± s.d. of three independent experiments. *P < 0.05.
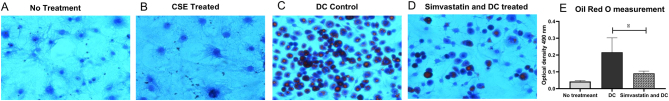
Figure 4.
Effects of simvastatin on the morphology in differentiated 3T3-L1 preadipocytes. Mature 3T3-L1 adipocytes stained with Oil Red O after differentiation for 6 days; no treatment (A); treatment with cigarette smoke extract (CSE) (B); treatment with differentiation cocktail (DC) (C); or treatment with simvastatin and DC (D). Cells were visualized by light microscope at ×10 magnification. Oil Red O was quantified from simvastatin-treated and untreated preadipocytes after extraction at 490 nm (E). Values are the mean ± s.d. of three independent experiments. *P < 0.05.
To study the effect of simvastatin on the expression of late adipogenic genes, Scd1 and Ppar-γ, cells were grown in the presence of DC and CSE for up to 10 days and analyzed after 1, 3, 6 and 10 days. The expression of Scd1 showed a maximal increase after 6 days and then a slow decrease, in contrast with Ppar-γ, which demonstrated a continuous increase from day 3 up to day 10 (Fig. 3C and D). The presence of simvastatin abrogated this effect. The expression of Scd1 was downregulated 18-fold (P = 0.02) and that of Ppar-γ was decreased 35-fold (P = 0.02) in simvastatin-treated differentiating preadipocytes on day 6 compared to nontreated cells.
After 6 days of treatment with DC, the preadipocytes differentiated into larger cells filled with lipid droplets. In the presence of simvastatin, the cells developed fewer lipid droplets but showed otherwise preserved morphology (Fig. 4D).
Simvastatin did not have any cytotoxic effect, which was tested with MTT. Oil Red O-visualized lipid droplets were used as a marker of mature adipocytes, and optical density was measured at 490 nm after extraction of cells. The measurement of Oil Red O was decreased 1.7-fold (P = 0.02) in simvastatin-treated adipocytes compared to nontreated adipocytes (Fig. 4E). In addition, when counting mature adipocytes visualized under a light microscope, the number of mature adipocytes was five times lower in simvastatin-treated adipocytes than in nontreated cells (Fig. 4).
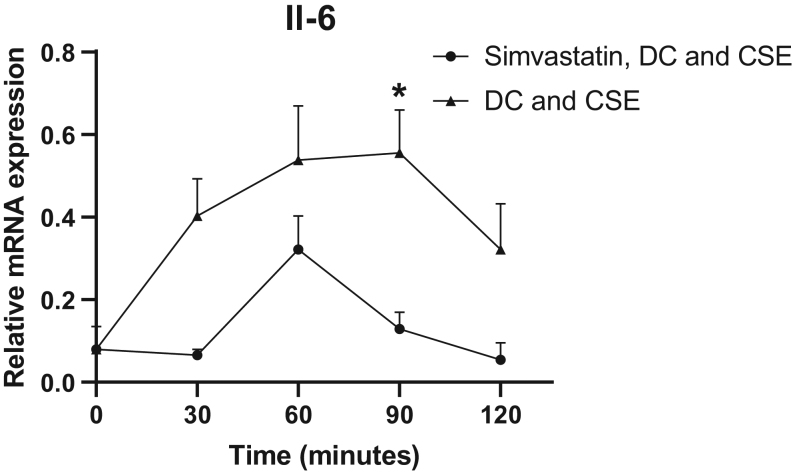
Furthermore, treatment with simvastatin also significantly reduced Il-6 expression four-fold (P = 0.03) at 90 min compared to nontreated preadipocytes (Fig. 5).
Figure 5.
Effects of simvastatin on Il-6 mRNA expression in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were stimulated with differentiation cocktail (DC) and cigarette smoke extract (CSE) for 30–120 min in the presence or absence of simvastatin. Gene expression of Il-6 was quantified with RT-PCR with β-actin as endogenous control. Values are the mean ± s.d. of three independent experiments. *P < 0.05.
Expression of mRNA in OFs from patients with GO and healthy controls after treatment with CSE or simvastatin
Orbital fibroblasts from patients with GO (OFs) were exposed to CSE (10%) or treated with DC for 30–120 min. The expression of IEGs reached maximum expression at 30–90 min (Fig. 6).
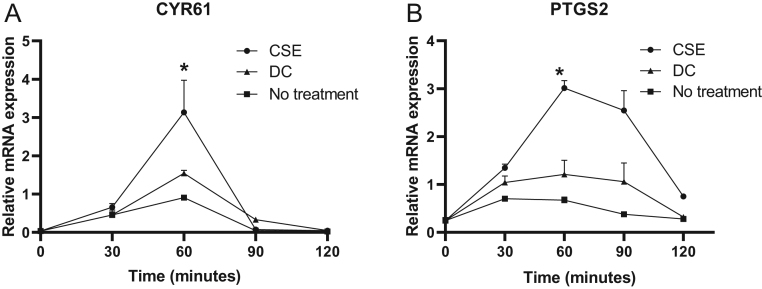
Figure 6.
Effects of cigarette smoke extract on early adipogenic genes in human primary orbital fibroblasts. Orbital fibroblasts from patients with GO were stimulated with cigarette smoke extract (CSE) or differentiation cocktail (DC) for 30–120 min. Gene expression was quantified with RT-PCR of CYR61 (A) and PTGS2 (B) with cyclophilin A as endogenous control. Values are the mean ± s.d. of three independent experiments. *P < 0.05.
CYR61 reached maximum expression at 60 minutes, and expression was increased 2.6-fold (P = 0.02) in cells exposed to CSE compared to unexposed OFs. The response in gene expression to CSE exposure compared to DC treatment was stronger and showed a 1.4-fold increase of CYR61 (P = 0.04) (Fig. 6A). The expression decreased rapidly after 90 min. At 60 min, PTGS2 was increased three-fold (P = 0.02) in cells exposed to CSE compared to unexposed OFs. In addition, here, the effect of CSE on gene expression was stronger than that of DC as shown by a two-fold increase (P = 0.03) (Fig. 6B).
In addition, OFs exposed to CSE showed increased expression of both IL-6 and IL-1β at 2 h compared to unexposed OFs (Supplementary Fig. 1, see section on supplementary data given at the end of this article). Expression of IL-6 was increased by 2.6-fold (P = 0.04) in OFs exposed to CSE compared to unexposed OFs at 2 h and 1.5-fold (P = 0.05) compared to OFs treated with DC. IL-1β expression was increased by eight-fold (P = 0.04) in OFs exposed to CSE compared to unexposed OFs at 2 h and two-fold (P = 0.04) in OFs treated with DC compared to CSE-exposed OFs.
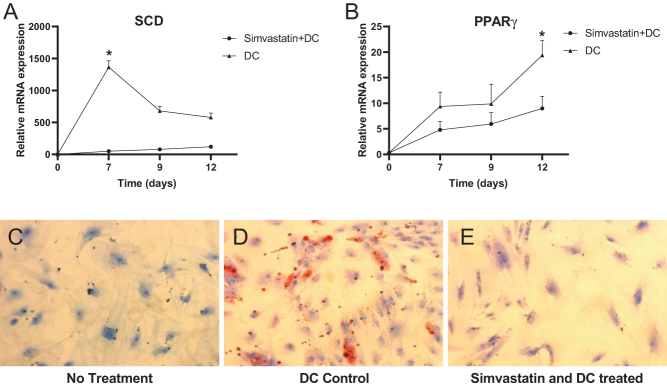
OFs were treated with simvastatin to investigate the effect on late adipogenic genes PPAR-γ and SCD in OFs. OFs were treated with DC and grown for 12 days. Cells were analyzed at day 3, 7, 9 and 12, and the expression of both SCD and PPAR-γ showed an increase after 6 days. Thereafter, SCD slowly decreased, which was in contrast to PPAR-γ, which showed a prolonged increase up to day 12. When exposed to simvastatin, the expression of both PPAR-γ and SCD was downregulated (Fig. 7).
Figure 7.
Effects of simvastatin on adipogenesis in human primary orbital fibroblasts. Orbital fibroblasts were treated with simvastatin and differentiation cocktail (DC) for 1–12 days. Gene expression was quantified with RT-PCR of late adipogenic genes SCD (A) and PPAR-γ (B) with cyclophilin A as endogenous control. Mature adipocytes at differentiation day 12 were stained with Oil Red O and visualized by light microscope at ×20. No treatment (C) treatment with DC (D) treatment with simvastatin and DC (E). Values are the mean ± s.d. of three independent experiments. *P < 0.05.
SCD was downregulated 27-fold (P = 0.02) at differentiation day 7, and PPAR-γ was downregulated two-fold (P = 0.04) at differentiation day 12 when compared to OFs not treated with simvastatin. The morphology of OFs was changed in differentiated adipocytes and showed signs of mature adipocytes filled with lipid droplets visualized with Oil Red O under a light microscope. The number of mature adipocytes was decreased when treated with simvastatin, but those that were present were observed with the preserved morphology of mature cells (Fig. 7).
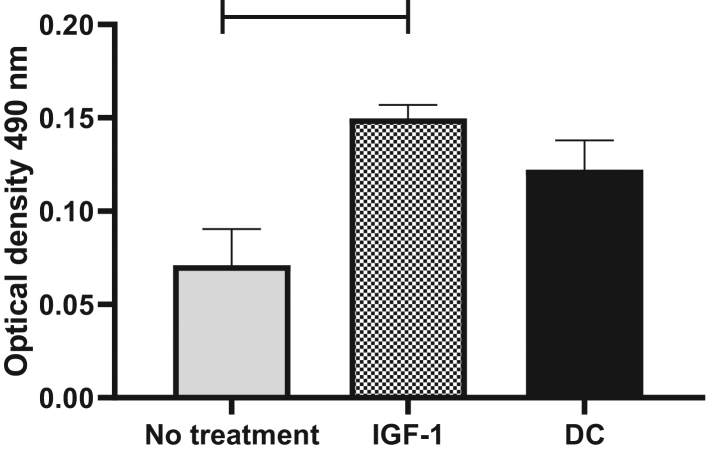
To study whether the effects of insulin could be mimicked with IGF-1, OFs were treated with DC containing either insulin or IGF-1. IGF-1 stimulated OFs to differentiate into mature adipocytes, and the response to IGF-1 was stronger than the response to insulin as measured by Oil Red O content after 12 days. OFs showed a 1.7-fold increase in Oil Red O content in response to insulin and a two-fold increase in response to IGF-1 (P = 0.02) (Fig. 8).
Figure 8.
Human primary orbital fibroblasts stimulated to differentiate with IGF-1. Differentiation in orbital fibroblasts was stimulated by IGF-1. Matured adipocytes were stained with Oil Red O at differentiation day 12. Oil Red O was quantified from IGF-1-treated, insulin-treated and -untreated orbital fibroblasts after extraction at 490 nm. Values are the mean ± s.d. of three independent experiments. *P < 0.05
Some of the experiments were repeated in OFs from healthy subjects. OFs from healthy subjects that underwent blepharoplasty surgery were exposed to CSE and both CYR61 and PTGS2 increased in expression at 60 min 1.5-fold (P = 0.01) and 90 min 2-fold (P = 0.02) respectively, compared to DC-treated OFs (Supplementary Fig. 2). The expression of both SCD and PPAR-γ was decreased 1.3-fold (P = 0.01 and P = 0.01, respectively) at day 8 after treatment with simvastatin compared to nontreated cells (Supplementary Figs 3 and 4).
Discussion
Tobacco smoking is a strong risk factor for the development of GO. In this study, we have established an in vitro model for the study of cigarette smoke-induced effects on mRNA expression and differentiation of preadipocytes (3T3-L1 cells) to mature adipocytes and in differentiation of human OFs from patients with GO to adipocytes. Cigarette smoke induced early mRNA expression of the transcription factors EGR1 and ZFP36 in parallel with expression of early adipogenic and inflammatory genes CYR61 and COX2 and cytokines IL6 and IL-1β. In late adipogenesis, upregulation of SCD and PPAR-γ was demonstrated. Simvastatin decreased the expression of all these genes after stimulation with cigarette smoke alone or in combination with the differentiation cocktail. An almost total suppressive effect was demonstrated on the expression of COX2, PPAR-γ and SCD1. Simvastatin also had a clear effect on the morphology in the differentiation process with a decreased number of mature adipocytes, without affecting the viability of the cells. However, cigarette smoke exposure alone had no effect on the maturation process and has to be seen as an enhancer in the adipogenic process. Taken together, simvastatin, in our model, had a very strong effect on mRNA expression of both early and late adipogenic genes, which resulted in a decreased number of mature adipocytes.
In a study by Kireeva et al. (24), dermal fibroblasts exposed to cigarette smoke showed upregulation of the IEGs EGR-1 and CYR61. In the present study, both of these genes were rapidly expressed after stimulation of preadipocytes with CSE alone, and the magnitude of the expression was similar to the expression in response to the differentiation cocktail. CYR61 is a multifunctional gene with roles in adipogenesis, inflammation, cell proliferation, extracellular matrix production and fibrosis (25, 26, 27). Thus, there are several mechanisms by which CYR61 could contribute to the pathogenesis of GO. Our group has previously shown that upregulation of CYR61 in smokers with active GO activates pathways associated with adipogenesis and inflammation (13).
The pro-inflammatory cytokines IL-1β and IL-6 are CYR61-responsive genes and are overexpressed in vivo in smokers with GO compared to non-smokers (13). This was also demonstrated in the present study in vitro.
Similar data on the effect of statins has been presented in other cell types such as osteoblastic and synovial cells in patients with rheumatoid arthritis (17, 28). These cells responded with downregulation of CYR61 in the presence of simvastatin, which is in line with our results. Kok et al. suggested that the suppressive effect was mediated by upregulation of the transcription factor FOXO3, which binds to the promoter of CYR61. If there is an imbalance in the transcription factors that regulate CYR61, with upregulation of the transcription factor EGR1 and downregulation by FOXO3, this may promote inflammation and adipogenesis, resulting in expansion in the retrobulbar tissue in GO. Smoking is a factor that may promote an imbalance, and treatment with simvastatin combined with the recommendation to stop smoking may restore the suggested imbalance.
In a study on patients with rheumatoid arthritis, atorvastatin decreased the activity by lowering CRP and SR (29). In a previous study on newly diagnosed GD patients, the use of statins for more than 60 days in the previous year substantially reduced the risk of GO (HR, 0.60) (18). It has also been hypothesized that cholesterol itself may have a role in the pathogenesis of GO, and in a recent study, GO patients with a higher clinical activity score had higher levels of cholesterol (30).
To start the differentiation process, we have used insulin as a mitogen, which is also known to have an affinity to the receptor for IGF-1 and has been suggested as an autoantigen in GO (4). It has also been shown that hyaluronic acid synthesis from TSH receptor-activated OFs from GD patients can be blocked by an antibody to the IGF-1 receptor. In our study, we found the same effects on expression of IEGs and late adipogenic genes when replacing insulin with IGF-1 in the differentiation cocktail. Recently, in a clinical study, a blocking antibody directed against the IGF-1 receptor was found to decrease the clinical activity score (CAS) and proptosis in patients with active moderate-to-severe GO (31). The effects of IGF-1 have also been studied in breast cancer, where CYR61 was upregulated in cells that showed an increased proliferation rate (32).
We did not study protein expression which is a limitation of our study. However, we have previously shown overexpression of the same group of early and late adipogenic genes in intraorbital adipose/connective tissue from operated patients with severe active GO compared to healthy controls and in smokers with GO compared to non-smokers by microarray and real-time PCR. Moreover, the desired endpoint, decreased adipogenesis, is clearly demonstrated in this study. Thus, we believe that the difference in expression presented in this study is clinically significant.
To conclude, we have found a distinct effect of cigarette smoke on both 3T3-L1 preadipocytes and human OFs with upregulation of both early and late adipogenic genes. Cigarette smoke enhanced adipogenesis but did not by itself induce the differentiation of mature adipocytes. Simvastatin inhibited the expression of both early and late adipocyte genes and decreased differentiation into mature adipocytes. Using IGF-1 as a mitogen mimicked the effects of insulin and stimulated preadipocytes to differentiate into mature cells. Simvastatin should be investigated in a clinical trial of patients with GO.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grants from the Faculty of Medicine at Lund University, the Skåne Research Foundation and the Research Funds of Skåne University Hospital.
Author contribution statement
T Planck and M Lantz: contributed equally.
Acknowledgments
The authors gratefully acknowledge the assistance of Helena Hansson with handling the tissue samples.
References
- 1.Lantz M, Vondrichova T, Capretz A, Nilsson E, Frenander C, Bondeson AG, Ridderstråle M, Aberg M, Asman P, Groop L, et al Thyrostimulin (a TSH-like hormone) expression in orbital and thyroid tissue. Thyroid 2007. 113–118. ( 10.1089/thy.2006.0155) [DOI] [PubMed] [Google Scholar]
- 2.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. Journal of Clinical Endocrinology and Metabolism 1998. 998–1002. ( 10.1210/jcem.83.3.4676) [DOI] [PubMed] [Google Scholar]
- 3.Smith TJ. The putative role of fibroblasts in the pathogenesis of Graves’ disease: evidence for the involvement of the insulin-like growth factor-1 receptor in fibroblast activation. Autoimmunity 2003. 409–415. ( 10.1080/08916930310001603000) [DOI] [PubMed] [Google Scholar]
- 4.Khoo TK, Bahn RS. Pathogenesis of Graves’ ophthalmopathy: the role of autoantibodies. Thyroid 2007. 1013–1018. ( 10.1089/thy.2007.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocrine Reviews 1993. 747–793. ( 10.1210/edrv-14-6-747) [DOI] [PubMed] [Google Scholar]
- 6.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocrine Reviews 2003. 694–717. ( 10.1210/er.2002-0030) [DOI] [PubMed] [Google Scholar]
- 7.Davies TF. Really significant genes for autoimmune thyroid disease do not exist – so how can we predict disease? Thyroid 2007. 1027–1029. ( 10.1089/thy.2007.1526) [DOI] [PubMed] [Google Scholar]
- 8.Weetman AP. Autoimmune thyroid disease: propagation and progression. European Journal of Endocrinology 2003. 1–9. ( 10.1530/eje.0.1480001) [DOI] [PubMed] [Google Scholar]
- 9.Wiersinga WM. Smoking and thyroid. Clinical Endocrinology 2013. 145–151. ( 10.1111/cen.12222) [DOI] [PubMed] [Google Scholar]
- 10.Inuzuka H, Nanbu-Wakao R, Masuho Y, Muramatsu M, Tojo H, Wakao H. Differential regulation of immediate early gene expression in preadipocyte cells through multiple signaling pathways. Biochemical and Biophysical Research Communications 1999. 664–668. ( 10.1006/bbrc.1999.1734) [DOI] [PubMed] [Google Scholar]
- 11.Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. PNAS 2003. 850–855. ( 10.1073/pnas.0337434100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantz M, Vondrichova T, Parikh H, Frenander C, Ridderstrale M, Asman P, Aberg M, Groop L, Hallengren B. Overexpression of immediate early genes in active Graves’ ophthalmopathy. Journal of Clinical Endocrinology and Metabolism 2005. 4784–4791. ( 10.1210/jc.2004-2275) [DOI] [PubMed] [Google Scholar]
- 13.Planck T, Shahida B, Parikh H, Strom K, Asman P, Brorson H, Hallengren B, Lantz M. Smoking induces overexpression of immediate early genes in active Graves’ ophthalmopathy. Thyroid 2014. 1524–1532. ( 10.1089/thy.2014.0153) [DOI] [PubMed] [Google Scholar]
- 14.Kim JN, Kim HJ, Jeong SH, Kye YC, Son SW. Cigarette smoke-induced early growth response-1 regulates the expression of the cysteine-rich 61 in human skin dermal fibroblasts. Experimental Dermatology 2011. 992–997. ( 10.1111/j.1600-0625.2011.01380.x) [DOI] [PubMed] [Google Scholar]
- 15.Planck T, Shahida B, Sjogren M, Groop L, Hallengren B, Lantz M. Association of BTG, CYR61, ZFP36, and SCD gene polymorphisms with Graves’ disease and ophthalmopathy. Thyroid 2014. 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin LD, Lin SK, Chao YL, Kok SH, Hong CY, Hou KL, Lai EH, Yang H, Lee MS, Wang JS. Simvastatin suppresses osteoblastic expression of Cyr61 and progression of apical periodontitis through enhancement of the transcription factor Forkhead/winged helix box protein O3a. Journal of Endodontics 2013. 619–625. ( 10.1016/j.joen.2012.12.014) [DOI] [PubMed] [Google Scholar]
- 17.Kok SH, Lin LD, Hou KL, Hong CY, Chang CC, Hsiao M, Wang JH, Lai EH, Lin SK. Simvastatin inhibits cysteine-rich protein 61 expression in rheumatoid arthritis synovial fibroblasts through the regulation of sirtuin-1/FoxO3a signaling. Arthritis and Rheumatism 2013. 639–649. ( 10.1002/art.37807) [DOI] [PubMed] [Google Scholar]
- 18.Stein JD, Childers D, Gupta S, Talwar N, Nan B, Lee BJ, Smith TJ, Douglas R. Risk factors for developing thyroid-associated ophthalmopathy among individuals with Graves disease. JAMA Ophthalmology 2015. 290–296. ( 10.1001/jamaophthalmol.2014.5103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vondrichova T, de Capretz A, Parikh H, Frenander C, Asman P, Aberg M, Groop L, Hallengren B, Lantz M. COX-2 and SCD, markers of inflammation and adipogenesis, are related to disease activity in Graves’ ophthalmopathy. Thyroid 2007. 511–517. ( 10.1089/thy.2007.0028) [DOI] [PubMed] [Google Scholar]
- 20.Cawood TJ, Moriarty P, O’Farrelly C, O’Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. Journal of Clinical Endocrinology and Metabolism 2007. 59–64. ( 10.1210/jc.2006-1824) [DOI] [PubMed] [Google Scholar]
- 21.Bernhard D, Huck CW, Jakschitz T, Pfister G, Henderson B, Bonn GK, Wick G. Development and evaluation of an in vitro model for the analysis of cigarette smoke effects on cultured cells and tissues. Journal of Pharmacological and Toxicological Methods 2004. 45–51. ( 10.1016/j.vascn.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 22.Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. Journal of Clinical Endocrinology and Metabolism 2002. 385–392. ( 10.1210/jcem.87.1.8164) [DOI] [PubMed] [Google Scholar]
- 23.Planck T, Parikh H, Brorson H, Martensson T, Asman P, Groop L, Hallengren B, Lantz M. Gene expression in Graves’ ophthalmopathy and arm lymphedema: similarities and differences. Thyroid 2011. 663–674. ( 10.1089/thy.2010.0217) [DOI] [PubMed] [Google Scholar]
- 24.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Molecular and Cellular Biology 1996. 1326–1334. ( 10.1128/mcb.16.4.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. Journal of Cell Communication and Signaling 2007. 159–164. ( 10.1007/s12079-008-0022-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas CS, Creighton CJ, Pi X, Maine I, Koch AE, Haines GK, Ling S, Chinnaiyan AM, Holoshitz J. Identification of genes modulated in rheumatoid arthritis using complementary DNA microarray analysis of lymphoblastoid B cell lines from disease-discordant monozygotic twins. Arthritis and Rheumatism 2006. 2047–2060. ( 10.1002/art.21953) [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Qi Q, Wang Y, Shi Y, Yang W, Cen Y, Zhu E, Li X, Chen D, Wang B. Cysteine-rich protein 61 regulates adipocyte differentiation from mesenchymal stem cells through mammalian target of rapamycin complex 1 and canonical Wnt signaling. FASEB Journal 2018. 3096–3107. ( 10.1096/fj.201700830RR) [DOI] [PubMed] [Google Scholar]
- 28.Kok SH, Hou KL, Hong CY, Wang JS, Liang PC, Chang CC, Hsiao M, Yang H, Lai EH, Lin SK. Simvastatin inhibits cytokine-stimulated Cyr61 expression in osteoblastic cells: a therapeutic benefit for arthritis. Arthritis and Rheumatism 2011. 1010–1020. ( 10.1002/art.27433) [DOI] [PubMed] [Google Scholar]
- 29.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004. 2015–2021. ( 10.1016/S0140-6736(04)16449-0) [DOI] [PubMed] [Google Scholar]
- 30.Sabini E, Mazzi B, Profilo MA, Mautone T, Casini G, Rocchi R, Ionni I, Manconi F, Leo M, Nardi M, et al High serum cholesterol is a novel risk factor for Graves’ orbitopathy: results of a cross-sectional study. Thyroid 2018. 386–394. ( 10.1089/thy.2017.0430) [DOI] [PubMed] [Google Scholar]
- 31.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, et al Teprotumumab for thyroid-associated ophthalmopathy. New England Journal of Medicine 2017. 1748–1761. ( 10.1056/NEJMoa1614949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkissyan S, Sarkissyan M, Wu Y, Cardenas J, Koeffler HP, Vadgama JV. IGF-1 regulates Cyr61 induced breast cancer cell proliferation and invasion. PLoS ONE 2014. e103534 ( 10.1371/journal.pone.0103534) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a