Abstract
Water pollution due to organic compounds is of great concern and efforts are being made to develop efficient adsorbents for remediation of toxic pollutants. The development of new functionalized materials with increased performance is growing to meet the regulatory standards in response to public concerns for environment. In this study, an attempt has been made to investigate the influence of synthesis parameters like the reaction temperature, the surfactant-to-silica ratio and reaction time on the structural and textural properties of novel ordered mesoporous silica hybrids. In order to understand the effect of different synthesis parameters, all the prepared materials were systematically characterized by various analytical, spectroscopic and imaging techniques such as XRD, BET, TG etc. It was deduced from these studies that the synthesis temperature influence greatly the structural order whereas both the P104/Na2SiO3 molar ratio and reaction time found to influence textural properties significantly. However, under optimized experimental condition, we could achieve the functionalized silica hybrids that offers successful incorporation of -Amino, -Glucidoxy, -Methacrylate, -Vinyl and -Phenyl moieties indicated by FTIR peaks at 793 cm−1, 2870 cm−1, 796 cm−1, 1630 cm−1 and 954 cm−1. XRD studies reveal orthorhombic and tetragonal symmetry for the hybrids and these materials were found to be thermally stable due to incorporation of organic moiety in silica matrix. Functionalized silica hybrids then applied as adsorbents demonstrated efficient and comparable removal of 4-aminophenol and p-nitrophenol in 20 min facilitated through organic moiety. Detailed modeling of the sorption using equilibrium and kinetic isotherms has been carried out to get an insight into the transport process. The adsorption isotherms of phenol derivatives are well-fitted with the Langmuir, Freundlich and Temkin Isotherms and the adsorption kinetics follows the pseudo second order model. The modeling confirms that the uptake is a chemisorption process.
Keywords: Silica hybrids, One-pot synthesis, Phenol derivatives, Adsorption, Equilibrium dynamics
1. Introduction
The development of Pakistan is very closely related to its effort for environmental protection. In this regard, Pakistan Environment Protection Ordinance (PEPO) in 1983 defined the legal framework. Pakistan being a member of the third world countries is still lacking pollution abatement standards, environmental management infrastructure, political will and public awareness. Despite this, a group of scientists and researchers are in continuous effort to eliminate the wastefulness and shift to principles of sustainable development to protect the environment and entire ecology from harmful effects of a wide variety of toxic inorganic and organic chemicals discharged as industrial wastes, causing serious water, air, and soil pollution.
In response to such challenges, efficient and cost‐effective treatment technologies are widely investigated. Adsorption is a well-known separation method and recognized as one of efficient and economic methods for water decontamination applications. A major advantage of adsorption lies in the fact that the persistent compounds are removed, rather than being broken down to potentially dangerous metabolites that may be produced by oxidation and reductive processes (Valderrama et al., 2007, Naureen et al., 2014, Noor et al., 2015).
A wide range of adsorbents are tested to be highly efficient due to simplicity, low cost, effectiveness and availability. In addition, the adsorbents can be regenerated by suitable desorption processes (Pan et al., 2009). Selection of novel adsorbents with multiple and diverse application range is a challenge. In the same spirit, promising organic-inorganic hybrids have been used for the removal of toxic species from wastewater (Wang et al., 2012, Wang et al., 2011, Gao et al., 2010, Zaitseva et al., 2013, Simsek et al., 2012, Suchithra et al., 2012, Repo et al., 2011, Ge et al., 2010, Pang et al., 2011). The characteristic feature of these compounds is the combined advantage of functional variation of organic materials and thermally stable inorganic substrate, resulting in strong binding affinities. Further, the Functionalized hybrids present the best properties of each of its components in a synergic way and have high performances of physical, chemical and mechanical properties (Mercier and Pinnavaia, 1998).
Phenols are important aromatic compounds having antioxidant properties. It can inhibit the oxidative degradation of organic materials. Phenols are naturally constituent in a number of biological aerobic organisms such as human blood plasma, mammalian urine, pine needles and oil from tobacco leaves. Phenol derivatives such as α-tocopherol are a component of vitamin E, thymol and carvacrol are components of lignin, from which phenol is liberated by hydrolysis.
Commercially, phenol is used in the production of phenolic resins phenol–formaldehyde resin called Bakelite phenolphthalein used as an indicator. Dilute solutions of Phenols are useful antiseptics, since Phenols are more acidic than aliphatic alcohols. However, its toxic fumes cause kidney damage. It is likely that Phenol solution contains dioxins. Consequently, phenol has only limited use in pharmaceuticals today because of its toxicity. Phenols penetrate deep into the tissue, leading to gangrene through damage to blood vessels (Nair et al., 2008). Ingestion of phenols in concentration from 10 to 240 mg/l for a long time causes mouth irritation, diarrhea, and excretion of dark urine and vision problems (Navarro et al., 2008).
Most of the phenol absorbed by the body is excreted in the urine as phenol and/or its metabolites. Only smaller quantities are excreted with feces or exhaled. The refractory Phenols form stable free radicals. Such a property is undesirable (Nguyen et al., 2003d) that makes it an important pollutant in wastewater. Phenols are released by industries that produce chlorophenols for use as fungicides and insecticides in agriculture sector.
A number of phenolic compounds like chlorinated, nitrated, methylated or alkylated are prevalent in the environment due to chemical processing industries and use of numerous pesticides. For instance, N-acetylated Aminophenol is a component of Paracetamol (Nagaraja et al., 2003). 3-aminophenol, 2-aminophenol and 2.4-diaminophenol are used as biomarkers for analysis of drugs (Vecchia and Tavani, 1995), precursor for indole synthesis, preparation of hair dyes (Chen et al., 2004), respectively. Nitrophenols result due to environmental reaction of phenol with nitrite ions.
Toxicity of Phenolic compounds is due to hydrophobic nature and property to generate reactive radicals. Phenolic compounds in potable water emit an unpleasant odor and flavor in concentration as low as 5 μg/l and are poisonous to aquatic life, plants and humans. Furthermore, the position of substitution in phenol molecule also affects the toxic action. p-Nitrophenol is classified as a priority pollutant and potential environmental toxicant (Leung et al., 1997) due to its rapid breakdown in water. The Maximum Contaminant Level of 1 μg/L in drinking water (US EPA, 1986) is defined for phenols.
The significant environmental risks urge for the rapid removal and detoxification of phenols. Different physical, chemical and biological treatment processes are frequently employed (Zylstra et al., 2000, Takahashi et al., 1994). For instance, adsorption, ultrasonic irradiation and microwave assisted oxidation are preferred for removal of p-Nitrophenol. Further, different materials for adsorption are investigated by a number of researchers. Roostaei and Tezel (2004) applied activated carbons, activated alumina, filtrasorb-400, silica gel and zeolites for adsorption of phenol from water. Cardenas et al. (2005) attempted porous clay heterostructure as adsorbents for removal of phenol and dichlorophenols.
The development of present research draws its essence from the increasing concentration of diverse toxic pollutants in different compartments of the environment. Further, there is a continuous effort to synthesize materials with multidimensional properties. The present research focuses on preparation of specialized materials based on silica (abundantly available natural precursor) with specific property to adsorb pollutants and can also be accepted as an economical and efficient substitute to conventional adsorbents (Surhio et al., 2014). The preference of silica over other materials for preparation of Hybrid materials is based on the fact that silicates reveal many advantages.
-
•
Silica is transparent and does not scatter light. Silica show low optical loss in comparison to zirconia or titanium in its rutile phase.
-
•
Silica has very high thermal resistance (Lygin, 1994).
-
•
Organic-inorganic hybrids are preferred as attachment on silica surface is easier due to high number of cross-linking bonds (Arakaki et al., 2000).
-
•
Immobilization of organic functional groups in the inorganic framework (Buszewski et al., 1998, Arakaki et al., 2000) of silica renders more stability.
The functionalized silica hybrids are synthesized in the present work to explore the opportunities for optimal route of and factors (choice of precursor and surfactant) affecting the structure-activity properties and adsorption efficiency. The present study reports the removal of 4-amino phenol and p-Nitrophenol using the functionalized Mesoporous silica based hybrids as adsorbents.
2. Material and methods
This research is an attempt to synthesize functionalized hybrids with P104 as non-ionic structure directing agent and sodium silicate as silica precursor with five different organosilanes.
The direct co-condensation synthesis procedure follows addition of surfactant (4.0 g of P104) and 8 g KCl in 100 mL of water and 15 mL of Acetic acid at room temperature. A known amount of precursor was added and pre-hydrolyzed for 2 h. Organosilane (1.08 g) with a known organic moiety (3-Aminopropyltrimethoxysilane, APTMS) was added to the mixture under stirring (20 h) at 60 °C and static conditions of heating (at 100 °C for 24 h) for functionalization. The material was collected by filtration, dried in air and extracted with ethanol. Excessive Pluronic was washed with ethanol to remove template, filtered and dried under vacuum at 100 °C for 3 h (Da’na and Sayari, 2012).
The same procedure was repeated for organosilanes with organic moieties of 3-Glucidoxypropyltrimethoxysilane (GPTMS); 3-Methacryloxypropyltrimethoxysilane (MPTMS); Vinyltrimethoxysilane (VTMS); and Phenyltrimethoxysilane (PTMS). Products obtained are coded as AM, GM, MM, VM and PM.
2.1. Characterization
Each of the functionalized hybrid Mesoporous silica materials was comprehensively characterized to determine the surface and bulk characteristics by a wide range of techniques like Attenuated total reflectance (ATR) Infrared spectroscopy (Thermo Nicolet NEXUS 670 FTIR), SEM coupled with EDX (Hitachi SU-70 Analytical UHR FEG-SEM), XRD (PANalytical Empyrean) and TG/DTA (Perkin Elmer).
2.2. Adsorption protocol
A known mass (3 mg) of each silica based hybrid is added to a known concentration of phenol (4-aminphenol and p-nitrophenol) solution. The contact of hybrid (adsorbent) and phenol (adsorbate) is made for a known time (20 min) on the shaker (Lab-companion SK-300). An aliquot is drawn after every 2 min, filtered and analyzed on UV-Vis spectrophotometer (UV-1601, Shimadzu) at the wavelength (λmax) of 275 nm and 317 nm for 4-aminphenol and p-nitrophenol, respectively. The concentration uptake was determined from the calibration curve constructed for standard phenol solution of five known dilutions.
The adsorbed concentration on the adsorbent or uptake on each hybrid is calculated by Eq. (1):
| (1) |
where Ci, Ct and Ce (mg/L) are the liquid-phase concentrations of adsorbate initially, at time t and at equilibrium, respectively. V is the volume (L) of the solution and W is the weight (g/L) of sorbent.
Removal of Metals (% R) by the synthesized silica based hybrids is determined from the relation given in Eq. (2):
| (2) |
3. Results and discussion
The successful synthesis of functionalized hybrids is attributed to diffusion of organic moiety into silica framework. The surface and bulk characteristics are revealed by a wide range of techniques.
3.1. ATR-FTIR
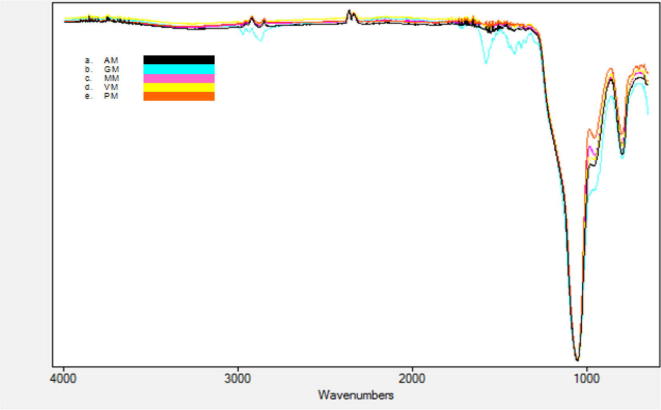
ATR-FTIR analysis of functionalized hybrids demonstrated significant peaks at 795 cm−1, 955 cm−1, 1348 cm−1 and 1839 cm−1 suggesting successful incorporation of the organic moiety (see Fig. 1a–e). These peaks also indicate the linkage of organic moiety to Silicon atom containing –OH, –NH2, –OC O, and –CH CH2 apical functional groups. This is also supported by the literature citing a number of such assignments for Mesoporous hybrid silica materials (Liu et al., 2002, Deng et al., 1995). Therefore, it is reasonable to consider that the reaction products obtained by the hydrolytic condensation are a mixture of species with different structures (Rikowski and Marsmann, 1997).
Figure 1.
ATR of functionalized silica hybrids (a) AM (b) GM (c) MM (d) VM (e) PM.
The linkage of surface functional (amino) group in functionalized silica hybrids (AM) is clearly indicated by a characteristic IR peak for vibration of Si-CH2R (R = NH2) at 793 cm−1 as shown in Fig. 1a. On the other hand, stretching of NH2 as broad band (3250–3450 cm−1) and N–H deformation peaks are exhibited at 1640–1560 cm−1. The organic moiety of methyl group is manifested as C–H stretching at 2940–2800 cm−1 and around 1450 cm−1 (Lee et al., 2001, Piers and Rochester, 1995). The introduction of organic moiety also results in a relative decrease in the silanol bands, with an associated increase in new bands characteristic of immobilized amino propyl groups. This is evident by a broader NH2 symmetric stretching at 3264 cm−1. Similar bands are identified in amino modified silica (OSU-6-W-APTMS-1) synthesized by grafting method.
Methyacryl (MM) functionalized silica demonstrates characteristic absorption peak at 796 cm-1 assigned to –Si–OCH3 (see Fig. 1c). This is also supported by (Mori and Pinnavaia, 2001, Yiu et al., 2001) for propyl methacrylate groups in two modified silica (OSU-6-W-TMSPMA-1 and OSU-6-W-TMSPMA-2). This also demonstrates that efficiency of one pot synthesis is comparable to the grafting method (Piers and Rochester, 1995). The disappearance or non-existence of –CH3 bending mode vibration (at 1331 cm−1) indicates that there is no unreacted –OCH3 (Lin et al., 2002). It further suggests the comparable rather better efficacy of direct co-condensation method (Qureshi et al., 2015).
A sharp absorption band characteristic of non-hydrogen bonded silanols (Zhao et al., 1997, Jentys et al., 1996) at 3746 cm−1 disappears in Glucidoxy (GM) functionalized hybrids. The new bands at 3696, 2935, 2971, 2870, and 1372 cm−1 appear at the expense of the band at 3746 cm−1. Further, epoxy group identified at 2971 and 2870 cm−1 may be assigned to νas(OC–H) and νs(OC–H) vibrations, respectively, in GPTMS (see Fig. 1b). Asymmetrical ring stretching at 794 cm−1 is also observed. Similar stretching is also marked by other authors (Lwoswsi, 1984, Grasselli and Ritchey, 1975). The high percent transmittance may allow adding more functional groups onto the surface and formation of a highly ordered structure (Kiyani et al., 2014).
On the other hand, functionalized silica hybrids also indicate the presence of strong Si–O–Si stretching vibration band at 1103 cm−1 due to pure silica precursor (Deng et al., 2009). The vinyl group (VM) is depicted by the peak at 1630 cm−1 due to C C stretching vibration, confirming that vinyl group (–CH CH2) exists and connects to silicon atom in organosilane.
Functionalization of silica with organic moiety of phenyl (PM) distinguished by aromatic ring vibrations at 795 cm−1 and 954 cm−1 confirms that phenyl ring is bonded to a silicon atom. Mono substituted phenyl rings in plane deformation is reported (Darga, 2007) at 1007 cm−1. The absence of this peak also indicates that presently synthesized hybrid is not mono-substituted.
3.2. SEM/EDX
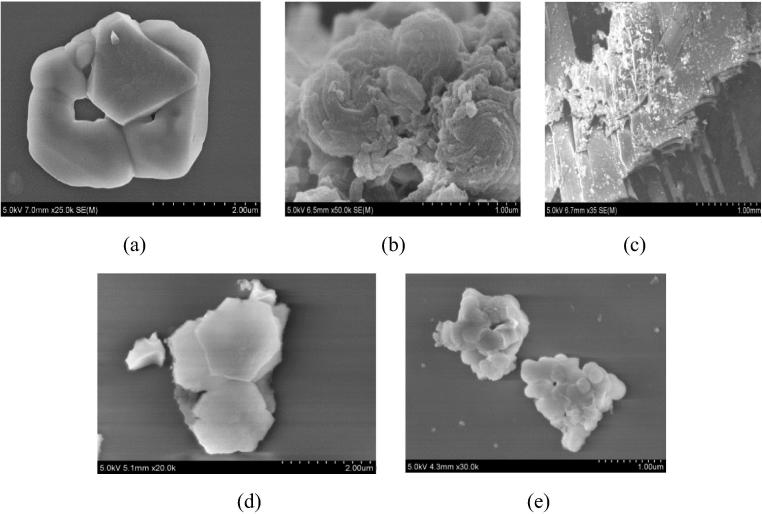
The morphological features including shape and size of functionalized hybrids were assessed under Scanning Electron Microscope (SEM) that revealed interesting features (see Fig. 2a–e). It is understood that morphology is dependent largely on the synthesis method, choice of ingredients and reaction conditions. Amino group (AM) induces a doughnut shape and rigorously blended morphology with silica material. Stacking of methacrylate hybrid (MM) is seen to turn into ladder. However, the spongy features are also visible reflecting the unreacted monodispersed particles. The proportion of unreacted particles may be attributed to relatively less blending imparting less linkage of two constituents. Bean shape whirling around each other is demonstrated by Glucidoxy silica hybrid (GM) distorting the hexagonal shape of silica. Furthermore, perforated layered structure and clearly distinguishing feature of vinyl sphere is the hallmark of phenyl and vinyl hybrids, respectively (Khaskheli et al., 2015).
Figure 2.
SEM of functionalized silica hybrids (a) AM (b) GM (c) MM (d) VM (e) PM.
Functionalized silica hybrids containing functional groups of amine, glucidoxy, methacrylate, vinyl and phenyl revealed interesting compositions as atom percentage of Si, O, and C as common elements (see Table 3.1). In addition, the presence of nitrogen in amine-hybrid is a clear indication of successful impregnation of organic moiety with silica framework. To a close approximation, the elements Si and O stand no significant variation with change in organic moiety.
Table 3.1.
EDX data of synthesized functionalized mesoporous silica hybrids.
| Sample codes | Si (%) | O (%) | C (%) | N (%) |
|---|---|---|---|---|
| AM | 27.55 | 65.77 | 0.78 | 5.90 |
| GM | 20.39 | 73.11 | 6.51 | – |
| MM | 21.24 | 64.72 | 14.04 | – |
| VM | 23.70 | 70.07 | 6.23 | – |
| PM | 19.30 | 75.53 | 5.17 | – |
This is based on the fact that silica source is used in each hybrid. The only variation is demonstrated by Carbon ranging from 0.78% to 14% in AM and MM, respectively. The lower C content is likely due to the presence of nitrogen in the earlier.
3.3. XRD
Functionalized silica hybrids exhibited symmetries that are orthorhombic and tetragonal (see Table 3.2). Both are of higher symmetry than hexagonal revealing extended network linkages in functionalized hybrids. So it might be concluded that addition of organosilanes to the basic framework of Mesoporous silica helps in stabilizing the hybrids. This stability is manifested and supported by the results of thermo gravimetric analysis.
Table 3.2.
XRD data of synthesized functionalized mesoporous silica hybrids.
| Sample codes | Planes (Å) | Angles (°) | hkl indices | d-spacing | 2θ (°) | Crystal system |
|---|---|---|---|---|---|---|
| AM | a – 22.2 | α – 90 | 020 | 7.56 | 0.758 | Orthorhombic |
| b – 15.0 | β – 90 | 111 | 9.20 | 0.623 | ||
| c – 13.6 | γ – 90 | 200 | 11.10 | 0.516 | ||
| GM | a – 9.52 | α – 90 | 100 | 9.54 | 0.601 | Tetragonal |
| b – 9.52 | β – 90 | 110 | 6.73 | 0.851 | ||
| c – 9.40 | γ – 90 | 200 | 4.75 | 1.206 | ||
| MM | a – 11.8 | α – 90 | 011 | 5.10 | 1.123 | Tetragonal |
| b – 11.8 | β – 90 | 020 | 5.93 | 0.966 | ||
| c – 5.65 | γ – 90 | 220 | 4.19 | 1.366 | ||
| VM | a – 13.7 | α – 90 | 020 | 11.93 | 0.601 | Orthorhombic |
| b – 23.8 | β – 90 | 120 | 9.01 | 0.636 | ||
| c – 3.99 | γ – 90 | 210 | 6.61 | 0.867 | ||
| PM | a – 13.2 | α – 90 | 111 | 7.39 | 0.775 | Orthorhombic |
| b – 18.5 | β – 90 | 021 | 6.82 | 0.840 | ||
| c – 10.0 | γ – 90 | 200 | 6.60 | 0.868 |
The literature (Wang et al., 2004) strongly supports the obsession of hkl indices of 100, 110 and 200 characteristics of Mesoporous silica (hexagonal geometry) even in the functionalized hybrids. The present study results are in accordance with the literature for hybrid (GM). The disagreement to this is also exhibited by functionalized hybrids (AM, MM, VM and PM). In addition to this agreement and disagreement of hkl indices, it is generally observed that symmetry is necessarily changed in each functionalized hybrids than Mesoporous silica. This change is directly attributed to the incorporation of organic moieties in hybrids. Further, the higher stabilized symmetries of hybrids are expected to exhibit better adsorption.
3.4. TG/DTA
Thermal analysis of synthesized Mesoporous silica on the basis of weight loss and enthalpy changes revealed important information. The recorded spectra of TG, DTG and DTA are presented (Table 3.3).
Table 3.3.
TG/DTA data of synthesized functionalized mesoporous silica hybrids.
| Sample codes | Wt. of sample g/mol | Weight loss (%) (1st step) | Weight loss (%) (2nd step) | Weight loss (%) Residue | Peak (min) | Area (mJ) | ΔH kJ/mol |
|---|---|---|---|---|---|---|---|
| AM | 1 | 5.509 | 18.014 | 0.040 | 76.217 | 44965.83 | 6.62 |
| 90–305 °C | 305–1000 °C | ||||||
| GM | 1 | 5.982 | 38.504 | 0.154 | 75.885 | 58372.12 | 11.04 |
| 130–250 °C | 250–1015 °C | ||||||
| MM | 1 | 5.627 | 24.362 | 0.085 | 39.2 | 45148.24 | 7.25 |
| 155–305 °C | 305–980 °C | ||||||
| VM | 1 | 4.244 | 24.241 | 0.148 | 74.783 | 89367.90 | 17.2 |
| 205–305 °C | 305–980 °C | ||||||
| PM | 1 | 5.833 | 17.670 | 0.184 | 75.317 | 57394.98 | 8.92 |
| 180–385 °C | 385–995 °C |
Functionalized silica hybrids revealed characteristic features on TG/DTA curves. The apparent 2-step decomposition is due to larger moisture loss initially (Step 1) followed by actual weight loss of hybrid itself. The result is encouraging and supportive to successful synthesis and complete conversion of ingredients into a unified product. Further, single entity compound also indicates the purity of compound. It may be deduced that the functional group is successfully impregnated as a component of Si framework (Batool et al., 2015).
It is also noted that relationship of weight loss with enthalpy cannot be inferred for functionalized silica. This may be due to the fact that organic moieties having different apical functional groups are inculcated to varying proportions into or onto the surface of material.
Results of Thermal studies can conveniently be compared with analysis done by range of other characterization techniques. For example, higher moisture loss in functionalized hybrids is satisfied by broad –OH peak assigned in ATR-FTIR studies. This is reflected by initial weight loss attributed to evolution of –OH group in moisture content (Ashraf et al., 2013a, Ashraf et al., 2013b, Ashraf et al., 2013c, Ashraf et al., 2013d).
Thermally stable oligomer-templated silica with wormlike porous structures similar to those obtained using TEOS were synthesized using sodium silicate as a silica source (Kim et al., 2000, Boissiere et al., 2000).
3.5. Adsorption application for phenols decontamination
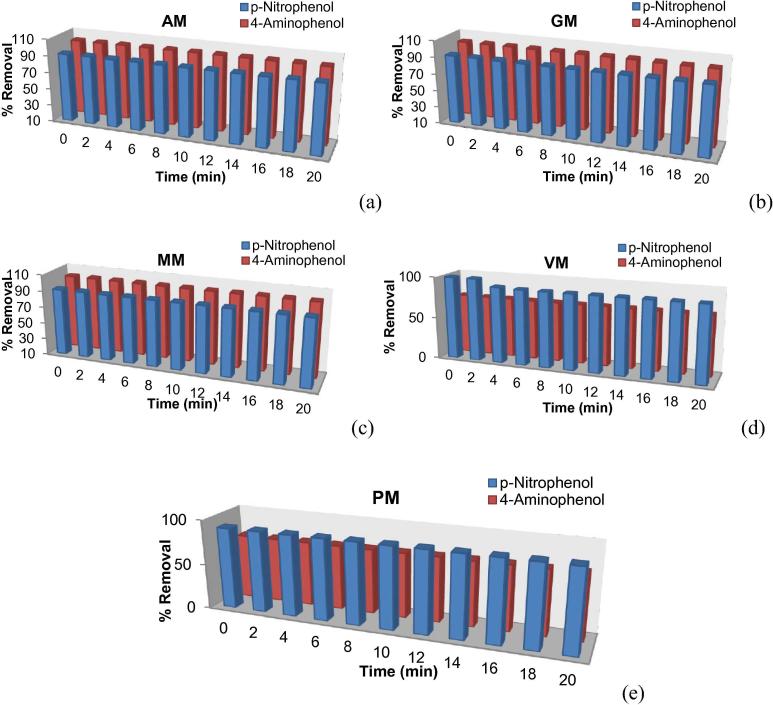
The application of synthesized Mesoporous silica and functionalized hybrids is extended for the decontamination of Phenolic compounds using protocol of batch adsorption process. The results are demonstrated graphically to exhibit different relations as a function of time.
The first observation is the dormant or insignificant role of varying contact time on percentage adsorption. It is encouraging to report that appreciable quantities of induced phenols on each synthesized material are adsorbed. This is evident by the removal percentage of more than 90% acquired on Mesoporous silica and functionalized hybrids (Ashraf et al., 2013a, Ashraf et al., 2013b, Ashraf et al., 2013c, Ashraf et al., 2013d).
It is understood from the literature that addition of surfactant (SDS) reduces the adsorption for phenols (Shawabkeh and Abu-Nameh, 2007). However, the enhanced adsorption in the present study may be explained on the basis that non-ionic surfactant (P104) facilitates adsorption in comparison to ionic (SDS).
On the bases of results of adsorption trends (see Fig. 3), Functionalized hybrids can conveniently be categorized as potential adsorbents for (a) 4-aminophenol (b) p-nitrophenol. It is clearly distinguished that AM, GM MM and VM, PM belong to class (a) and (b), respectively. The postulate again stands true that lower adsorption of 4-aminophenol owns to unsaturation of vinyl and phenyl (Ashraf et al., 2013a, Ashraf et al., 2013b, Ashraf et al., 2013c, Ashraf et al., 2013d).
Figure 3.
Removal (% age) of phenols on functionalized silica hybrids (a) AM (b) GM (c) MM (d) VM (e) PM.
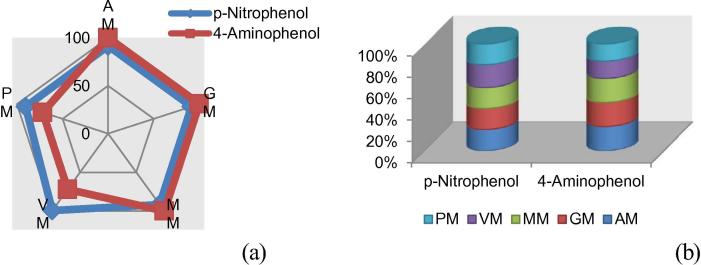
Fig. 4 (a and b) clearly draws the comparative analysis of Mesoporous silica and functionalized hybrids as adsorbents for the removal of phenols with nitro and amino groups. The Mesoporous silica develop comparable adsorption efficiency to functionalized hybrids (AM; GM and MM). This reflects that organic moiety of –amino, -glucidoxy and -methacrylate is not contributing significantly in providing binding sites for phenol in the silica framework (Ashraf et al., 2012).
Figure 4.
Adsorption trends (% R) as a function of (a) silica based hybrids (b) phenols.
On the extreme, the polarization of functionalized hybrids having organic moiety of –phenyl (PM) and –vinyl (VM) is clearly visible. This polarization from normalization of Mesoporous silica extends more disparity for the removal of p-nitrophenols in comparison to 4-aminophenols. Thus, it can be concluded that p-nitrophenols develop comparable and better retention than Mesoporous silica on adsorbent class (a) and (b), respectively.
3.6. Equilibrium and kinetic study
Application of Isotherms and Kinetic models is applied to explore the mode of adsorption for metal ions, phenols and PAHs onto functionalized silica based hybrid surfaces.
3.7. Batch sorption equilibrium dynamics
Equilibrium isotherms are applied to get an insight into sorption mechanism to propose surface properties and affinity of adsorbents. The treatment of present study data to Langmuir, Freundlich and Temkin Isotherms demonstrated R2 ≈ 1 for each of the synthesized silica hybrids, applied as adsorbent for the removal of both phenol derivatives; 4-aminophenol and p-nitrophenol (see Table 3.4).
Table 3.4.
Langmuir, Freundlich and Temkin Isotherms analysis against sorption variables for phenols.
| Sorbents | SBA-15 | MSU-H | VO | PO | AM | GM | MM | VM | PM |
|---|---|---|---|---|---|---|---|---|---|
| Langmuir parameters | |||||||||
| 4-Aminophenol | |||||||||
| qm (mg/g) | 9.980 | 9.980 | 5.117 | 5.128 | 9.990 | 9.920 | 9.950 | 5.141 | 5.117 |
| KL (L/mg) | 2004 | 1002 | 1.233 | −1.240 | −1001 | −1440 | 201 | 1.249 | −1.233 |
| R2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| p-Nitrophenol | |||||||||
| qm (mg/g) | 8.382 | 8.403 | 8.123 | 8.130 | 8.361 | 8.354 | 8.347 | 8.960 | 8.156 |
| KL (L/mg) | −14.03 | −14.87 | −10.25 | −10.33 | −13.59 | −13.44 | −13.46 | −124 | −10.66 |
| R2 | 0.999 | 1 | 1 | 1 | 1 | 1 | 1 | 0.999 | 1 |
| Freundlich parameters | |||||||||
| 4-Aminophenol | |||||||||
| n | −166 | −111 | −2.512 | −2.525 | −2500 | −5.076 | −500 | −2.538 | −2.512 |
| KF (mg/g) | −115 | −766 | 66.93 | 68.86 | −115 | 19.69 | −459 | 70.920 | 66.93 |
| R2 | 0.921 | 0.956 | 1 | 1 | 0.939 | 0.738 | 0.919 | 1 | 1 |
| p-Nitrophenol | |||||||||
| n | −10.85 | −10.68 | −9.174 | −9.174 | −10.68 | −5.076 | −10.63 | −27.02 | −9.345 |
| KF (mg/g) | −48.89 | −48.89 | −48.89 | −48.89 | −48.89 | 19.69 | −48.89 | −50.00 | −48.89 |
| R2 | 1 | 1 | 1 | 1 | 0.999 | 0.738 | 1 | 0.996 | 1 |
| Temkin parameters | |||||||||
| 4-Aminophenol | |||||||||
| B (KJ/mol) | −0.0064 | −0.009 | −2.847 | −2.839 | −0.0036 | −0.019 | −0.019 | −2.829 | −2.846 |
| KT (L/g) | 0 | 0 | 0.0284 | 0.0282 | 0 | 9.1E−227 | 5.4E−219 | 0.0280 | 0.0284 |
| R2 | 0.921 | 0.956 | 1 | 1 | 0.939 | 0.779 | 0.919 | 1 | 1 |
| p-Nitrophenol | |||||||||
| B (KJ/mol) | −0.843 | −0.855 | −0.987 | −0.984 | −0.856 | −0.86 | −0.862 | −0.351 | −0.97 |
| KT (L/g) | 2.28E−05 | 2.65E−05 | 0.0001 | 0.0001 | 2.68E−05 | 2.82E−05 | 2.89E−05 | 6.85E−12 | 9.34E−05 |
| R2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.997 | 1 |
This confirms the adsorption of phenols on the homogenous surface layer and also indicates multilayer adsorption for all the silica hybrids resulting in an intricate adsorbate-adsorbent interaction. The homogeneity of the adsorbent structure is attributed to the silica commonly present in all the hybrids synthesized. However, heterogeneity is attributed to the induction of organic moieties within the silica network. Fitness of Temkin Isotherm indicates uniform distribution of the pollutant into the pores of adsorbent. Overall results indicate that all adsorbents followed the three isotherms were almost best fitted, except that Freundlich isotherm data are not good fitted for adsorption of phenols on GM that may be attributed to the blockage due to bulkiness of organic moiety which leads to less adsorption (Ashraf et al., 2011).
3.8. Kinetic studies and adsorption capacity (qe)
Validation of zero order, pseudo-first order and pseudo-second order equations for Phenols adsorption is explored from linear plots. Kinetic studies suggest that for designing of a good adsorbent the variable parameters, the fitness of pseudo second order indicates dependence of adsorption on more than one factor (Ashraf et al., 2010).
In the present study the incorporation of different organic moieties into the silica network for functionalization provides more binding sites. Optimizations of available binding sites for adsorption are few parameters evaluated for the development of good adsorbents. For Phenols, the adsorption capacity (qe) values signify that synthesized silica hybrids adsorbents are classified for adsorption efficiency as good (23–30 mg/g).
The general sequence of adsorbent efficiency of silica hybrids for the Phenols removal follows:
AM > MM > GM > PM > VM
The study of adsorption capacity suggests the possible and potential application of each functionalized silica hybrid as successful adsorbent for remediation of phenols pollution (Ashraf et al., 2015).
4. Conclusions
-
➢
The synthesis of hybrids with diverse organic moieties is significant to provide opportunities to understand the role of each component in the hybrid. It also broadens the scope for application as potential adsorbents for the removal of organic pollutants.
-
➢
The emergence of new intense peaks in XRD spectra indicates the interaction of organic moiety with silica and crystal symmetry ranges from tetragonal to orthorhombic.
-
➢
EDX of functionalized hybrids containing amine, glucidoxy, methacrylate, vinyl and phenyl share common elements of Si, O, and C. In addition, presence of nitrogen in amine-hybrid is a clear indication of successful impregnation of amine moiety with silica framework.
-
➢
The Thermal degradation studies conclude that complexation of Mesoporous silica with organic functional groups gives strength and thermal stability to the hybrids.
-
➢
Functionalized hybrids can conveniently be categorized as potential adsorbents for (a) 4-aminophenol (b) p-nitrophenol. It is clearly distinguished that AM, GM MM and VM, PM belong to class (a) and (b), respectively. The postulate stands true that lower adsorption of 4-aminophenol is due to unsaturation of vinyl and phenyl.
-
➢
Adsorption isotherms of phenol derivatives are well-fitted with the Langmuir, Freundlich and Temkin Isotherms and the adsorption kinetics follows the pseudo second order model. The modeling confirms that the uptake is a chemisorption process.
Acknowledgments
The authors thank NISP Lab and FabLab at University of Maryland, College Park USA, Chemistry Labs in University of Malaya, Malaysia and Fatima Jinnah Women University, Rawalpindi, Pakistan, for providing the necessary techniques for characterization of synthesized materials. Authors also gratefully acknowledge HEC (Higher Education Commission, Pakistan) for providing IRSIP fellowship to Saima Nasreen for pursuing her doctoral research at UMD, USA.
Footnotes
Peer review under responsibility of King Saud University.
References
- Arakaki L.N.H., Nunes L.M., Simoni J.A., Airoldi C. Ethyleneimine anchored on thiol-modified silica gel surface – Adsorption of divalent cations and calorimetric data. J. Colloid Interface Sci. 2000;228(1):46–51. doi: 10.1006/jcis.2000.6842. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Khan A., Sarfraz M., Ahmad M. Effectiveness of silica based Sol–gel microencapsulation method for odorants and flavours leading to sustainable environment. Front. Chem. 2015;3:42. doi: 10.3389/fchem.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M.A., Yusoff I., Yusof I., Alias Y. Removal of Cd (II) onto Raphanus sativus peels biomass: equilibrium, kinetics, and thermodynamics. Desalin. Water Treat. 2013;51(2013):4402–4412. doi: 10.1080/19443994.2012.752333. [DOI] [Google Scholar]
- Ashraf M.A., Yusoff I., Yusof I., Alias Y. Removal of acid yellow17 dye from aqueous solution using eco-friendly biosorbent. Desalin. Water Treat. 2013;51(2013):4530–4540. doi: 10.1080/19443994.2012.747187. [DOI] [Google Scholar]
- Ashraf M.A., Qureshi A.K., Gharibreza M., Rehman M.A., Ahmad I., Yusoff I. Intercationic effect on biosorbent efficacy. Desalin. Water Treat. 2013;52(7–9):1504–1513. doi: 10.1080/19443994.2013.788456. [DOI] [Google Scholar]
- Ashraf M.A., Ullah S., Ahmad I., Qureshi A.K., Balkhair K.S., Rehman M.A. Green biocides, a promising technology: current and future applications. J. Sci. Food Agric. 2013;94(3):388–403. doi: 10.1002/jsfa.6371. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Maah M.J., Yusoff I. Removal of lead from synthetic solutions by protonated teleosts biomass. E-J. Chem. 2012;9(1):345–353. [Google Scholar]
- Ashraf M.A., Maah M.J., Yusoff I. Heavy metals accumulation in plants growing in ex tin mining catchment. Int. J. Environ. Sci. Technol. 2011;8(2):401–416. [Google Scholar]
- Ashraf M.A., Maah M.J., Yusoff I. Study of Mango Biomass (Mangifera Indica L) as cationic bio-sorbent. Int. J. Environ. Sci. Technol. 2010;7(3):581–590. [Google Scholar]
- Batool S., Khalid A., Chowdury A.J.K., Sarfraz M., Balkhair K.S., Ashraf M.A. Impacts of azo dye on ammonium oxidation process and ammonia oxidizing soil bacteria. RSC Adv. 2015;5:34812–34820. doi: 10.1039/C5RA03768A. [DOI] [Google Scholar]
- Boissiere C., Larbot A., Prouzet E. Synthesis of mesoporous MSU-X materials using inexpensive silica sources. Chem. Mater. 2000;12:1937–1940. [Google Scholar]
- Buszewski B., Jezierska M., Welniak M., Berek D. Survey and Trends in the preparation of chemically bonded silica phases for liquid-chromatographic analysis. J. High. Resolut. Chromatogr. 1998;21(5):267–281. [Google Scholar]
- Cardenas S.A., Velazquez T.G., Revilla G.O., Cortez M., Perera B.G. Adsorption of phenol and dichlorophenol from aqueous solutions by porous clay heterostructure. J. Mex. Chem. Soc. 2005;49(3):287–291. [Google Scholar]
- Chen J., Jiang J., Zhang F., Yu H., Zhang J. Cytotoxic environmentally relevant chlorophenols on L929 cells and their mechanisms. Cell Biol. Toxicol. 2004;20:183. doi: 10.1023/b:cbto.0000029468.89746.64. [DOI] [PubMed] [Google Scholar]
- Da’na E., Sayari A.H. Adsorption of heavy metals on amine-functionalized SBA-15 prepared by co-condensation: applications to real water samples. Desalination. 2012;285:62–67. [Google Scholar]
- Darga A. Faculty of Chemistry and Pharmacy; Dissertation, LMU München: 2007. Sorption Isotherms of Volatile Molecules on Micro- and Mesoporous Nanosized Siliceous Materials Based on Acoustic Wave Devices: Determination of Corresponding Isosteric Heats of Adsorption. [Google Scholar]
- Deng Q., Mauritz K.A., Moore R.B. (Perfluorosulfonate Ionomer)-(Inorganic Oxide) nanocomposites organic modification of surfaces of silicon oxide nanoparticles grown in situ. ACS Symp. Ser. 1995;585:66–84. [Google Scholar]
- Deng T.S., Zhang Q.F., Zhang J.Y., Shen X., Zhu K.T., Wu J.L. One-step synthesis of highly monodisperse hybrid silica spheres in aqueous solution. J. Colloid Interface Sci. 2009;329:292–299. doi: 10.1016/j.jcis.2008.09.063. [DOI] [PubMed] [Google Scholar]
- Gao B., Gao Y., Li Y. Preparation and chelation adsorption property of composite chelating material poly(amidoxime)/SiO2 towards heavy metal ions. Chem. Eng. J. 2010;158:542–549. [Google Scholar]
- Ge P., Li F., Zhang B. Synthesis of modified mesoporous materials and comparative studies of removal of heavy metal from aqueous solutions. Pol. J. Environ. Stud. 2010;19:301–308. [Google Scholar]
- Grasselli J.G., Ritchey W.M., editors. Atlas of Spectral Data and Physical Constants for Organic Compounds. 2nd ed. CRC Press Inc.; Cleveland, OH: 1975. Vol. 1. [Google Scholar]
- Jentys A., Pham N.H., Vinek H. Nature of hydroxy groups in MCM-41. J. Chem. Soc., Faraday Trans. 1996;92(17):3287–3291. [Google Scholar]
- Khaskheli A.A., Talpur F.N., Ashraf M.A., Cebeci A., Jawaid S., Afridi H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B: Enzym. 2015;113:56–61. doi: 10.1016/j.molcatb.2015.01.002. [DOI] [Google Scholar]
- Kiyani S., Ahmad M., Zafar M.A., Sultana S., Khan M.P.Z., Ashraf M.A., Hussain J., Yaseen G. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies – Abbottabad, Northern Pakistan. J. Ethnopharmacol. 2014;156:47–60. doi: 10.1016/j.jep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Kim S.S., Pauly T.R., Pinnavaia T.J. Non-ionic surfactant assembly of ordered, very large pore molecular sieve silicas from water soluble silicates. Chem. Commun. 2000;1:1661–1662. [Google Scholar]
- Lee B., Kim Y., Lee H., Yi J. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: the introduction of surface hydrophilicity onto the surface of adsorbents. Microporous Mesoporous Mater. 2001;50(1):77–90. [Google Scholar]
- Leung K.T., Tresse O., Errampalli D., Lee H., Trevors J.T. Mineralization of p-nitrophenol by pentachloro-phenol-degrading Sphingomonas spp. FEMS Microbiol. Lett. 1997;155:107–114. [Google Scholar]
- Lin V.S.Y., Radu D.R., Han M.K., Deng W., Kuroki S., Shanks B.H., Pruski M. Oxidative polymerization of 1,4-diethynylbenzene into highly conjugated poly(phenylene butadiynylene) within the channels of surface-functionalized mesoporous silica and alumina materials. J. Am. Chem. Soc. 2002;124(31):9040–9041. doi: 10.1021/ja025925o. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Yang C.C., Chen W.C., Dai B.T., Tsai M.S. The structural transformation and properties of spin-on poly(silsesquioxane) films by thermal curing. J. Non-Cryst. Solids. 2002;311(3):233–240. [Google Scholar]
- Lwoswsi W. ed. Pergamon Press; Oxford, U.K.: 1984. Comprehensive Heterocyclic Chemistry. 7, 99. [Google Scholar]
- Lygin V.I. The structure of the silica surface and its modification by thermal-treatment. Kinet. Catal. 1994;35(4):480–486. [Google Scholar]
- Mercier L., Pinnavaia T.J. Heavy metal ion adsorbents formed by the grafting of a thiol functionality to mesoporous silica molecular sieves: factors affecting Hg(II) uptake. Environ. Sci. Technol. 1998;32:2749–2754. [Google Scholar]
- Mori Y., Pinnavaia T.J. Optimizing organic functionality in mesostructured silica: direct assembly of mercaptopropyl groups in wormhole framework structures. Chem. Mater. 2001;13(6):2173–2178. [Google Scholar]
- Naureen R., Tariq M., Yusoff I., Choudhury A.J.K., Ashraf M.A. Synthesis, spectroscopic and chromatographic studies of sunflower oil biodiesel using optimized base catalyzed methanolysis. Saudi J. Biol. Sci. 2014;22:322–339. doi: 10.1016/j.sjbs.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor M.J., Ahmad M., Ashraf M.A., Zafar M., Sultana S. A review of the pollen analysis of South Asian honey to identify the bee floras of the region. Palynol. 2015;2015:1–12. doi: 10.1080/01916122.2014.988383. [DOI] [Google Scholar]
- Nagaraja P., Yathirajan H., Raju C., Vasantha R., Nagendra P., Kumar M. 3-Aminophenol as a novel coupling agent for the spectrophotometric determination of sulfonamide derivatives. Farmaco. 2003;58:1295. doi: 10.1016/S0014-827X(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Nair C.I., Jayachandran K., Shashidhar Biodegradation of phenol. Afr. J. Biotechnol. 2008;7(25):4951–4958. [Google Scholar]
- Navarro A.E., Portales R.F., Sun-Kou M.R., Llanos B.P. Effect of pH on phenol biosorption by marine seaweeds. J. Hazard. Mater. 2008;156(1–3):405–411. doi: 10.1016/j.jhazmat.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Nguyen M.T., Kryachko E.S., Vanquickenborne L.G. In: General and Theoretical Aspects of Phenols. Rappoport Z., editor. John Wiley and Sons Ltd; England: 2003. The chemistry of phenols. [Google Scholar]
- Pan B.J., Pan B.C., Zhang M.W., Lv L., Zhang Q.X., Zheng S.R. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009;151:19–29. [Google Scholar]
- Pang Y., Zeng G., Tang L., Zhang Y., Liu Y., Lei X., Li Z., Zhang J., Xie G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination. 2011;281:278–284. [Google Scholar]
- Piers A.S., Rochester C.H. Infrared study of the adsorption of 1-aminopropyltrialkoxysilanes on silica at the solid/liquid interface. J. Colloid Interface Sci. 1995;174(1):97–103. [Google Scholar]
- Qureshi T., Memon N., Memon S.Q., Ashraf M.A. Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin. Water Treat. 2015;2015:1–9. doi: 10.1080/19443994.2015.1006825. [DOI] [Google Scholar]
- Repo E., Warchoł J.K., Bhatnagar A., Sillanpää M. Heavy metals adsorption by novel EDTA-modified chitosan-silica hybrid materials. J. Colloid Interface Sci. 2011;358:261–267. doi: 10.1016/j.jcis.2011.02.059. [DOI] [PubMed] [Google Scholar]
- Rikowski E., Marsmann H.C. Cage-rearrangement of silsesquioxanes. Polyhedron. 1997;16:3357–3361. [Google Scholar]
- Roostaei N., Tezel F.H. Removal of phenol from aqueous solutions by adsorption. J. Environ. Manage. 2004;70:157–164. doi: 10.1016/j.jenvman.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Shawabkeh R.A., Abu-Nameh E.S.M. Absorption of phenol and methylene blue by activated carbon from pecan shells. Colloid J. 2007;69(3):355–359. [Google Scholar]
- Simsek E.B., Duranoglu D., Beker U. Heavy metal adsorption by magnetic hybrid-sorbent: an experimental and theoretical approach. Sep. Sci. Technol. 2012;47:1334–1340. [Google Scholar]
- Suchithra P.S., Vazhayal L., Mohamed A.P., Ananthakumar S. Mesoporous organic-inorganic hybrid aerogels through ultrasonic assisted sol-gel intercalation of silica-PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012;200–202:589–600. [Google Scholar]
- Surhio M.A., Talpur F.N., Nizamani S.M., Amin F., Bong C.W., Lee C.W., Ashraf M.A., Shahid M.R. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. 2014;4:55960–55966. [Google Scholar]
- Takahashi N., Nakai T., Satoh Y., Katoh Y. Variation of biodegradability of nitrogenous organic compounds by ozonation. Water Res. 1994;28:1563–1570. [Google Scholar]
- US Environmental Protection Agency; Washington, DC: 1986. US Environmental Protection Agency: Quality Criteria for Water. [Google Scholar]
- Valderrama C., Cortina J.L., Farran A., Gamisans X., Lao C. Kinetics of sorption of polyaromatic hydrocarbons onto granular activated carbon and Macronet hyper-cross-linked polymers (MN200) J. Colloid Interface Sci. 2007;310(1):35–46. doi: 10.1016/j.jcis.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Vecchia L.C., Tavani A. Epidemiologic evidence on hair dyes and the risk of cancer in humans. Eur. J. Cancer Prev. 1995;4:31. doi: 10.1097/00008469-199502000-00003. [DOI] [PubMed] [Google Scholar]
- Wang L., Wu X.L., Xu W.H., Huang X.J., Liu J.H., Xu A.W. Stable organic-inorganic hybrid of polyaniline/α-zirconium phosphate for efficient removal of organic pollutants in water environment. Appl. Mater. Interfaces. 2012;4:2686–2692. doi: 10.1021/am300335e. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang J., Wang A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination. 2011;266:33–39. [Google Scholar]
- Wang M., Zou H.K., Shao L., Chen J.F. Controlling factors and mechanism of preparing needlelike CaCO3 under high-gravity environment. Powder Technol. 2004;142:166–174. [Google Scholar]
- Yiu H.H.P., Botting C.H., Botting N.P., Wright P.A. Size selective protein adsorption on thiol-functionalized SBA-15 mesoporous molecular sieve. Phys. Chem. Chem. Phys. 2001;3(15):2983–2985. [Google Scholar]
- Zaitseva N., Zaitsev V., Walcarius A. Chromium(VI) removal via reduction-sorption on bi-functional silica adsorbents. J. Hazard. Mater. 2013;250–251:454–461. doi: 10.1016/j.jhazmat.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Zhao X.S., Lu G.Q., Whittaker A.K., Millar G.J., Zhu H.Y. Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, pyridine-TPD, and TGA. J. Phys. Chem. B. 1997;101(33):6525–6531. [Google Scholar]
- Zylstra G.J., Bang S.W., Newman L.M., Perry L.L. Lewis Publishers; Boca Raton, Florida: 2000. Biodegradation of Nitroaromatic Compounds and Explosives, Microbial Degradation of Mononitrophenols and Mononitrobenzoates. pp. 145–160. [Google Scholar]