Abstract
Objective
Sleep disturbance may be involved in symptom progression across multiple domains of psychopathology and could represent a target for treatment development in youth. Our objective was to identify sleep patterns that longitudinally change in conjunction with psychiatric symptom severity in at-risk youth.
Method
The study included 484 Pittsburgh Bipolar Offspring Study youth with at least two sleep assessments occurring between 10 and 18 years-old: 267 offspring of parents with bipolar I or II disorder and 217 community comparison offspring. Assessments occurred approximately every 2 years (mean number of assessments=2.8±0.8, mean follow-up duration= 3.8±1.6 years). Offspring had a range of psychiatric diagnoses at baseline. Multivariate lasso regression was implemented to select offspring-reported sleep patterns associated with changes in five psychiatric symptom measures from baseline through last follow-up (mania, depression, mood lability, anxiety, inattention/externalizing). Analyses accounted for parent psychiatric diagnoses and offspring demographics, psychiatric diagnoses, and medications.
Results
Follow-up duration, baseline socioeconomic status, parental history of bipolar disorder, offspring attention deficit hyperactivity disorder and disruptive behavior disorder, and five sleep patterns were identified as predictors of change in all five psychiatric symptom measures. Decreasing sleep duration, later sleep timing preference, longer sleep latency, increasing nighttime awakenings, and greater sleepiness over follow-up were associated with increasing severity the five psychiatric symptom outcomes over follow-up. These ten predictors explained 16% of the variance in longitudinal psychiatric symptom change, 33% of which was accounted for by sleep predictors.
Conclusion
A constellation of sleep features were associated with psychiatric symptom changes in youth, and may represent viable targets for future interventions.
Keywords: Sleep, Circadian, Youth, Psychiatric Symptoms, Longitudinal
Introduction
Incidence rates of major psychiatric disorders begin to rise from childhood into adolescence1, and these rates are even higher among youth who have a parent with psychiatric illness, particularly mood disorders.2 Studies examining psychopathology dimensionally have demonstrated that psychiatric symptom domains have differing, but inter-related, trajectories during this developmental period (e.g.,3,4). To identify more potent intervention targets for mental illness in youth, it is imperative to characterize modifiable processes that may underlie symptom progression across multiple domains of psychopathology, particularly among those at highest risk.
Sleep disturbances have been proposed as promising transdiagnostic intervention targets for psychiatric illness.5 In particular, studies have highlighted the relevance of insomnia and poor sleep continuity (e.g., difficulty falling and staying asleep)5,6 as well as irregular sleep wake patterns7,8 to the onset and course of most forms of psychopathology. Likewise, psychopathology can exacerbate sleep problems, suggesting a bi-directional cycle.9 There is encouraging evidence that treatment of insomnia with behavioral intervention promotes psychiatric symptom improvement among adults with mental illness.10 Sleep disturbances may also represent a modifiable target for treatment development for youth with psychiatric illnesses.
Sleep patterns undergo dramatic change from childhood into adolescence.11,12 Sleep timing behavior (bedtime, risetime) and preferences (eveningness) grow later due to a delay in endogenous circadian phase.11 In tandem, sleep duration decreases, sleep patterns become more variable, and daytime sleepiness increases due to a mismatch between sleep timing and psychosocial demands (e.g., school schedules, homework, socializing).12 Because of the concurrent changes in sleep and mental health during childhood and adolescence, it is particularly critical to understand how changes in sleep and psychopathology may intersect during this developmental period. Poor sleep duration, continuity, variability, timing, and sleepiness can each be targeted with behavioral intervention,13 thus a key step is to identify which sleep constructs may be the most pertinent to the broad spectrum of changes in psychopathology occurring among youth. Yet, most existing studies in youth are largely cross-sectional, emphasize categorical diagnoses, and focus on a narrow set of sleep-wake constructs, often derived from other symptom measures rather than validated sleep scales (for review see12,14,15). To more comprehensively evaluate which sleep patterns may relate to the longitudinal progression of psychiatric symptom severity in youth, analytic approaches that permit testing large numbers of sleep variables in combination with sociodemographic and clinical features are needed.
The Pittsburgh Bipolar Offspring Study (BIOS) includes detailed longitudinal assessments of psychiatric diagnoses, dimensional psychopathology, and sleep patterns in a cohort of offspring of parents with bipolar disorder (BP) and community comparison parents who are psychiatrically healthy or diagnosed with non-BP psychiatric disorders. Within the BIOS cohort, sleep patterns at baseline and the assessment proximal to conversion have been linked to future bipolar spectrum disorder onset (BPSD),16,17 including sleep continuity disturbances, extreme early chronotype, and a poor sleep phenotype. However, nearly 75% of high-risk offspring develop at least one axis I disorder; and, for 67% this includes non-BP diagnoses such as major depressive disorder (MDD), anxiety disorders, attention deficit hyperactivity disorder (ADHD), and disruptive behavior disorders (DBDs).18 Accordingly, a spectrum of dimensional psychopathology is expressed in the BIOS cohort; offspring of parents with BP display relatively higher baseline levels of anxiety/depression, inattention/disinhibition, externalizing, subsyndromal manic, and mood lability symptoms than offspring of community controls.19 The relevance of sleep patterns to longitudinal changes in the broad range of psychopathology experienced by BIOS youth has not been examined. Changing sleep patterns may parallel longitudinal changes across multiple domains of psychiatric symptom severity among these youth, who carry a range of familial risk for psychiatric disorder. In the BIOS cohort, our study objective was to identify a set of sleep patterns associated with longitudinal changes in dimensional psychiatric symptom severity (improvement or worsening) from baseline though follow-up using a lasso regression approach.
Method
Sample
Previous reports provide a detailed description of the methods of BIOS.20 All study procedures were approved by the University of Pittsburgh Institutional Review Board. Parents with DSM-IV bipolar disorder (BP; types I and II) living within 200 miles of Pittsburgh were recruited through advertisements, research studies, and outpatient clinics. Comparison parents were recruited from the community using random-digit dialing and matched to the parents with BP by age, sex, and neighborhood. Exclusion criteria for all parents included a lifetime diagnosis of schizophrenia, intellectual disability, or a mood disorder secondary to a medical condition, substance use disorder, or medication. Comparison parents could not be diagnosed with BP or have a co-parent or first-degree relative with BP, but otherwise were recruited regardless of non-BP psychopathology. Youth offspring of parents with BP and community comparison parents could be healthy or have one or more of a range of psychiatric diagnoses, including BPSDs, MDD, anxiety disorders, ADHD, DBDs, and substance use disorders. (SUDs) Table 1 describes offspring and parent demographic and clinical features (Table S1 presents this information by offspring group).
Table 1.
Baseline Clinical and Demographic Features of Pittsburgh Bipolar Offspring Study Youth (n=484) aged 10–18 Years-Old Included in Main Analysis
| Total Sample (n=484) | |
|---|---|
| Mean (SD) or n(%) | |
| Demographic Information | |
| Age at Baseline (Years) | 12.07(1.57) |
| Follow-up Duration (Years) | 3.83(1.67) |
| Sex (Female) | 239(49.4%) |
| Race (White/Caucasian) | 392(81.0%) |
| Lives with Biological Parent | 263(54.3%) |
| Socioeconomic Statusa | 36.79(14.50) |
| Available Sleep Assessments | 2.75(0.78) |
| Pubertal Status (Mid-Post)b | 123(25.4%) |
| Parent Lifetime Diagnoses | |
| Bipolar Spectrum Disorder | 267(55.2%) |
| Depressive Disorder | 83(17.1%) |
| Anxiety Disorder | 281(58.1%) |
| ADHD | 94(19.4%) |
| Disruptive Behavior Disorder | 112(23.1%) |
| Substance Use Disorder | 218(45.0%) |
| Offspring Baseline Lifetime Diagnoses | |
| Bipolar Spectrum Disorder | 47(9.7%) |
| Depressive Disorder | 48(9.9%) |
| Anxiety Disorder | 129(26.7%) |
| ADHD | 145(30.0%) |
| Disruptive Behavior Disorder | 75(15.5%) |
| Substance Use Disorder | 2(0.4%) |
| Psychotic Disorder | 4(0.8%) |
| Offspring Baseline Psychotropic Medication | |
| Antipsychotic | 18(3.7%) |
| Antidepressant | 24(5.0%) |
| Mood Stabilizer | 10(2.1%) |
| Stimulant/Non-Simulant ADHD medication | 56(11.6%) |
| Sedative/Hypnotic | 8(1.7%) |
Note. SD= Standard Deviation; SES = socioeconomic status; ADHD=attention deficit hyperactivity disorder
SES was assessed using the Hollingshead Four-Factor Index54
Pubertal status was measured using the self-report Petersen Pubertal Development Scale55 and categorized as pre/early versus mid/late/post-pubertal.
Prior to the initiation of study procedures, informed consent from the parents and assent from the children were obtained.20 Study assessments were performed approximately every 2 years. Our sleep measure (School Sleep Habits Survey [SSHS;21]) was added to BIOS in the 2nd year of recruitment, and subsequently completed in youth until age 18 years-old. Because offspring received assistance completing the SSHS prior to age 10, only data collected between 10 and 18 years-old were included for this analysis.17 Of the 782 in the sample (n=462 offspring of parents with BP; n=320 offspring of community comparison parents), 682 offspring between ages 10–18 years-old had completed an offspring- and parent-reported sleep assessment. Of those participants, 484 offspring (of 282 families) who had at least two sleep assessments between 10 and 18 were included in the analyses (267 offspring of 163 of parents with BP and 217 offspring of 119 community comparison parents). For this sample of 484 youth with two or more sleep assessments, the mean number of assessments was 2.8±0.8 and mean follow-up duration was 3.8±1.6 years. Table S2 (available online) compares demographic and clinical features between offspring with sleep assessments that were included (n=484) versus excluded (n=198).
Measures
Parent Psychopathology
Parents and participating biological co-parents (31%) were assessed by direct interview using the Structured Clinical Interview for DSM-IV (SCID) and the ADHD, oppositional defiant disorder, conduct disorder, and separation anxiety disorder sections of the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL).22 The psychiatric history of non-participating biological co-parents was obtained from the participant parent using the Family History Research Diagnostic Criteria23 plus the aforementioned K-SADS-PL modules. Assessments were performed by trained interviewers and reviewed by a child psychiatrist; both were blind to parental diagnoses.
Offspring Psychopathology
At baseline and subsequent assessments, parents (about their children) and offspring were interviewed using the K-SADS-PL22 for non-mood disorders, along with the Mania Rating Scale (KMRS) and Depression Rating Scale (KDRS) from the K-SADS-Present Version.24 Operationalized criteria were used to diagnose BP not otherwise specified (Supplemental Methods, available online).25 All K-SADS summary ratings were based on clinical consensus, which integrated parent and offspring responses. Parents and offspring also completed several dimensional symptom rating scales. The present analyses included child reports of mood lability, using the Child Affective Lability Scale, (CALS;26) and anxiety symptoms, using the Screen for Child Anxiety Related Emotional Disorders (SCARED;27) as well as parent-reported symptoms of Inattention/Externalizing in their offspring, using the Disruptive Behavior Disorders Rating Scale. (DBDRS;28) For all scales, total scores were calculated excluding items pertaining to sleep patterns. Symptom rating scales were selected based on a factor analysis of all dimensional psychopathology scales in BIOS.19
Offspring Medications
Offspring psychotropic medications were reported by parents at each assessment. Psychotropic medication use was categorized into a dichotomous (yes/no) variable for each class: antidepressant, antipsychotic, mood stabilizer (lithium and anticonvulsants), ADHD medication (stimulant/non-stimulant), and sedatives/hypnotic (anxiolytic and sleep medications).
Offspring Sleep-Wake Patterns
Offspring and their parents completed an abridged School Sleep Habits Survey (SSHS;21) assessing offspring habitual sleep patterns over the prior two weeks; it was abridged to reduce participant burden. School-day and weekend bedtime, risetime, sleep latency, and sleep duration were assessed, along with nighttime awakenings, napping, daytime sleepiness, and a morningness-eveningness scale indexing sleep timing preference. The SSHS has been used in large-scale community surveys of youth worldwide.29,30 SSHS-assessed sleep patterns are strongly correlated gold-standard prospective self-report (sleep diaries) and objective (actigraphy) sleep measures,31,32 however youth are typically more accurate reporters than their parents when compared against these measures.31
Analytic Approach
Analyses included 484 BIOS offspring aged 10–18 years-old with at least two sleep assessments. The BIOS cohort was considered as a whole to maximize the range of psychiatric symptom severities expressed in this sample. Sleep predictor selection was comparable among the two offspring groups when considered separately. As in our previous reports,16,17 the Baseline assessment was defined as the first available follow-up after age 10 at which sleep (offspring-reported and parent-reported) and psychiatric symptom measures were completed. Follow-up was defined as the last available follow-up assessment prior to age 18 at which sleep and psychiatric symptom measures were completed; this approach was used maximize sample size. To mitigate the impact of missing data, data were imputed using multivariate imputation by chained equations using the MICE package in R (Supplemental Methods, available online).33,34 Demographic and clinical features did not differ between youth with complete (N=178) versus partially imputed data (N=306; Table S3, available online).
Outcome Variables
Our goal was to identify sleep measures that pertain to a broad spectrum of psychopathology (i.e., transdiagnostically). As such, our five psychiatric symptom outcome measures (KMRS, KDRS, CALS, SCARED, DBDRS) were considered as a multivariate outcome rather than as separate univariate outcomes. As in a prior report from our group,35 all five psychiatric symptoms were considered as outcomes simultaneously and predictors had to be associated with all five outcomes. Correlations among the psychiatric symptom measures at baseline further supports the use of a multivariate outcome (r values 0.29–0.56). Residualized change scores were used to quantify change over follow-up, accounting for baseline symptom severity. Given considerable heterogeneity in baseline symptom severities in this at-risk sample, a residualized change approach was selected over absolute difference metrics, though we recognize there are caveats to both approaches.36,37 For each psychiatric symptom outcome, a residualized change score was defined as the residual from a linear regression of the outcome measurement (Follow-Up) on a prior measurement. (Baseline).37 Positive change scores reflect higher scores at follow-up and worsening over time, while negative change scores indicate improvement over time. Psychiatric symptom trajectories from ages 10 to 18 years-old can be found in Figure S1 (available online).
Sleep Variables
For SSHS-derived sleep variables, weighted weekly averages (e.g., [(5*school day value) + (2*weekend value)]/7) and weekend-school day differences were calculated for bedtime, risetime, sleep latency, sleep duration, and sleep midpoint. (Risetime - Sleep Duration/2) Morningness-eveningness (sleep timing preference), frequency of nighttime awakenings, regular napping (yes/no), and problems with daytime sleepiness were also derived. Residualized change scores were computed for all continuous sleep variables. For sleep variables, positive scores indicate increasing values over time, while negative scores indicate decreasing values.
Main Analysis
Predictors of change in the five psychiatric symptom outcome measures were selected by a single multivariate penalized least squares (lasso) regression implemented in the R GLMNET package.35,38 Lasso is a modified form of least squares regression that penalizes complex models with a regularization parameter (λ); the regularization parameter shrinks coefficients toward zero and eliminates unimportant terms entirely.38–41 This approach has two key advantages over traditional least squares regression: (1) it allows one to consider many predictors without the issue of multiple comparisons and (2) mitigates issues regarding predictor inter-correlation in the feature selection process.35,38 Ten-fold cross-validation identifies the optimal penalty term (λ) that minimizes mean cross-validated error (Lambda.min). Lambda.1se corresponds to the λ that is 1 standard error from the lambda.min and provides the most regularized (parsimonious) model;41 lambda.1se was used for predictor selection. Exponentiated non-zero (exp[B]) coefficients are reported for non-zero predictors. Similar to a rate ratio (odds ratio), exponentiated coefficients reflect how much the dependent variable changes with a one-unit change in the independent variable. Values >1 indicate a positive relationship and values <1 indicate a negative relationship. P-values are not reported as a test statistic for lasso models is still under development.38 To be selected, predictors had to be related to all five psychiatric symptom outcomes, though directionality of the relationships could differ.
Thirty-nine predictor variables were entered into the lasso regression model (Table 2); including offspring baseline demographics, psychiatric disorders, and psychotropic medication use; parental lifetime psychiatric disorders; and offspring-reported SSHS change scores (Follow-up minus Baseline). All continuous variables were standardized. Table S4 (available online) describes ranges of Baseline, Follow-up, and absolute change for continuous predictors.
Table 2:
List of the 39 Predictor Variables Included in the Multivariate Lasso Regression Models
| Offspring Demographics | Parent Diagnoses |
| - Baseline Age (Years) | - Bipolar Disorder |
| - Sex (Female) | - Unipolar Depression |
| - Race (White) | - Anxiety Disorder |
| - Socioeconomic Statusa | - ADHD |
| - Lives with Biological Parents | - Disruptive Behavior Disorders |
| - Duration of Follow-up (Years) | - Substance Use Disorders |
| - Pubertal Status Change Codeb | |
| Offspring Change (Δ) in Sleep (Follow-up – Baseline) | |
| Offspring Medications | - Δ Averages: |
| - Antidepressants | ○ Bedtime (turned off lights to go to sleep) |
| - Antipsychotics | ○ Risetime (time woke up) |
| - Mood Stabilizer | ○ Sleep Duration |
| - Stimulant/Non-Stimulant ADHD Medication | ○ Sleep Latency |
| ○ Sleep Midpoint | |
| - Sedatives/Hypnotics | - Δ Weekend-School Day Differences: |
| ○ Bedtime | |
| Offspring Diagnoses | ○ Risetime |
| - Bipolar Spectrum Disorder | ○ Sleep Duration |
| - Unipolar Depression | ○ Sleep Latency |
| - Anxiety Disorder | ○ Sleep Midpoint |
| - ADHD | - Δ Eveningness |
| - Disruptive Behavior Disorders | - Δ Nighttime Awakening Frequency |
| - Substance Use Disorders | - Δ Daytime Sleepiness |
| - Psychotic Disorders | - Napping at last follow-up |
Note: ADHD= attention-deficit/hyperactivity disorder; Δ= Residualized change from baseline through last follow-up.
SES was assessed using the Hollingshead Four-Factor Index54
Pubertal status was measured using the self-report Petersen Pubertal Development Scale55 and categorized as pre/early versus mid/late/post-pubertal.
To assess variance explained by the lasso-selected predictors, pseudo-r-squared was computed using the loglikelihood from leave-one-out MANOVA regression model analyses in R.35 A fixed-effects MANOVA model was used for the main analyses, as a multivariate mixed-effect model accounting for within-family correlation indicated that family membership accounted for a small portion of the total random effect variance and yielded no improvement in model fit relative to the fixed effects MANOVA in a loglikelihood ratio test (p>0.1). See Supplemental Methods (available online) for more detailed rationale. Using the leave-one-out method, the loglikelihood for the full MANOVA model was compared with models containing fewer predictor variables; the difference in these models describes the variance explained by the left-out variables.
Supplemental Analyses
Four supplemental analyses were conducted (Supplemental Methods and Results, available online). First, a cross-sectional lasso regression model was used to identify demographic, clinical, and sleep predictors of psychiatric symptoms at baseline (Table S8, Figure S2, available online). This model was intended to evaluate the extent to which cross-sectional associations between sleep and psychiatric symptom severity overlapped with the main analysis. Three additional lasso regression models evaluated predictors of change in psychiatric symptom severity, as in the main analysis. The first model aimed to identify baseline sleep predictors of subsequent change in psychiatric symptom severity (Table S9, Figure S3, available online). The second model aimed to maximize the use of available follow-up data by examining associations between change in offspring-reported sleep and psychiatric symptoms from baseline to the mean across all available follow-up assessments (Mean Follow-Up minus Baseline) (Tables S5–S7 and S10, Figure S4, available online). The third model replicated the main analysis using parent-reported sleep variables, rather than-offspring-reported sleep, to assess consistency of sleep predictor selection based on the reporter (Table S11, Figure S5, available online).
Results
Lasso regression
Lasso regression identified 2 demographic features, 3 clinical variables, and 5 sleep patterns with non-zero coefficients predicting change in the five psychiatric symptoms from baseline through follow-up (Table 3, Figure 1). These predictors explained 16.0% of the variance in the multivariate psychiatric symptom change score outcome.
Table 3:
Exponentiated Non-Zero Coefficients of Predictors Identified with a Multivariate Lasso Regression Model among 484 Pittsburgh Bipolar Offspring Study Youth
| Δ KMRS (mania) | Δ KDRS (depression) | Δ CALS (mood lability) | Δ SCARED (anxiety) | Δ DBDRS (inattention/externalizing) | |
|---|---|---|---|---|---|
| Non-Zero predictors | Exp(B) | Exp(B) | Exp(B) | Exp(B) | Exp(B) |
| Demographic | |||||
| Baseline SES | 0.9752 | 0.9615 | 0.9439 | 0.9309 | 0.9790 |
| Follow-Up Duration | 1.0309 | 1.0479 | 1.0392 | 1.0838 | 1.1069 |
| Clinical | |||||
| Parental History of BP | 1.0049 | 1.0117 | 1.0069 | 1.0036 | 1.0113 |
| Offspring ADHD | 1.3232 | 1.4253 | 1.1895 | 1.1593 | 1.3914 |
| Offspring DBD | 1.1232 | 1.1664 | 1.1474 | 1.0799 | 1.1850 |
| Sleep | |||||
| Δ Sleep Duration | 0.9966 | 0.9890 | 0.9464 | 0.9900 | 0.9642 |
| Δ Sleep Latency | 1.0030 | 1.0004 | 1.0031 | 1.0011 | 1.0006 |
| Δ Nighttime Awakenings | 1.0106 | 1.0470 | 1.0801 | 1.1025 | 1.0257 |
| Δ Daytime Sleepiness | 1.0564 | 1.0560 | 1.2317 | 1.1286 | 1.0337 |
| Δ Eveningness | 0.9949 | 0.9932 | 0.9794 | 0.9927 | 0.9876 |
Note: The model evaluated demographics, clinical features, and change in sleep variables as predictors of change in five psychiatric symptom severity outcome measures. KMRS = KSADS Mania Rating Scale; KDRS = KSADS Depression Rating Scale; CALS = Child Affective Lability Scale; SCARED = Screen for Childhood Anxiety-Related Disorders; DBDRS=Disruptive Behavior Disorders Rating Scale; Δ = Residualized change from baseline to last follow-up; Exp(B) = exponentiated coefficients. Exponentiated coefficients tell us, similar to a rate ratio (OR), how much the dependent variable changes with a one-unit change in the independent variable. Values >1 indicate a positive relationship and values <1 indicate a negative relationship; SES=Socioeconomic status; BP = Bipolar Disorder; ADHD = Attention Deficit Hyperactivity Disorder; DBD = Disruptive Behavior Disorder.
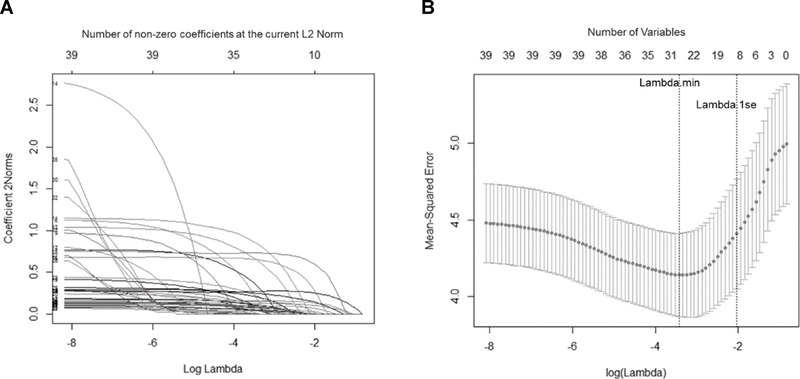
Figure 1.
Lasso Plots Generated using GLMNET in R for (A) Variable Fit and (B) Non-Zero Variable Fit After Cross-Validation for the Change in the Multivariate Psychiatric Symptom Outcome Over Follow-Up among 484 Pittsburgh Bipolar Offspring Study Youth
Note: (A) Plot of variable fit for each standardized psychiatric symptom outcome, where each curve corresponds to a predictor variable in the full model before optimization. Curves indicate the path of each variable coefficient as λ varies. (A) Plot of non-zero variable fit after cross-validation for the multivariate psychiatric symptom outcome. This represents the 10-fold cross-validation performed in lasso that chooses the optimal λ. Lambda.min corresponds to the λ that minimizes the mean-squared error of the regression model. Lambda.1se corresponds to the λ that is 1 standard error from the lambda.min and provides the most regularized (parsimonious) regression model. Lambda.1se was used for predictor selection.
Sociodemographic and Clinical
Longer follow-up duration, lower baseline SES, offspring ADHD and DBD, and parental history of BP predicted worsening across all symptom measures. These predictors accounted for 69.3% of the explained variance (11.1% total variance) in the multivariate psychiatric symptom outcome.
Sleep
Increasing severity across all psychiatric symptom measures was associated with decreasing sleep duration, later sleep timing preference (eveningness), increasing sleep latency, more nighttime awakenings, and increasing sleepiness. Sleep predictors accounted for 33.1% of the explained variance (5.3% total variance) in the multivariate psychiatric symptom outcome.
Supplemental Analyses (see Supplemental Results, available online, for further detail)
Cross-sectional model
A lasso model at baseline identified cross-sectional associations between psychiatric symptom severity and several demographic, clinical, and sleep measures. Baseline age; sex; lower SES; parental history of BP and DBD; and offspring BPSD, MDD, anxiety disorder, ADHD, and DBD were associated with greater baseline psychiatric symptom severity. Lastly, baseline sleep predictors overlapped with the main analyses, with the addition of a sleep variability predictor (weekend-school day bedtime difference) (Table S8, Figure S2, available online).
Follow-up models
The demographic, clinical, and sleep variables selected into the three supplemental lasso models evaluating predictors of psychiatric symptom change over follow-up were largely consistent with the main analysis. In the baseline sleep predictor model (Table S9, Figure S3, available online), an additional sleep timing variable was selected (bedtime). In the mean follow-up model (Table S10, Figure S4, available online), parental history of BP and sleep latency were not selected. In the parent-reported sleep model (Table S11, Figure S5, available online), an additional sleep timing variable was selected (risetime).
Discussion
Lasso regression identified associations between sleep patterns and longitudinal changes in five psychiatric symptom severity measures (mania, depression, anxiety, mood lability, and inattention/ externalizing) in BIOS youth. Over an average of 3.8 years, changes in these psychiatric symptom severities paralleled changes across follow-up in a combination of shorter sleep duration, later sleep timing preference, poorer sleep continuity, and worsening daytime sleepiness. Sleep patterns accounted for nearly a third (33.1%) of the explained variance in psychiatric symptom change over the follow-up period. These findings are the first, to our knowledge, to demonstrate that a constellation of sleep patterns are associated with change in psychiatric symptoms over time in a transdiagnostic sample of youth.
From childhood into adolescence, insufficient and delayed sleep become more prevalent;11 both of these sleep patterns were associated with worsening psychiatric symptom severity in BIOS youth. Over follow-up, shorter sleep duration and later sleep timing preference predicted worsening across all measured psychiatric outcomes. These findings align with frameworks proposing that changing sleep patterns across development may coincide with the progression of psychopathology in youth.12 However, because circadian rhythm abnormalities are strongly implicated in the genetic and behavioral basis of mood disorders,42,43 further work is needed to disentangle the extent to which delays in circadian timing in at-risk youth reflect a normative developmental process versus an endogenous circadian vulnerability for mood disorders. Though baseline weekend bedtime delay was related to greater baseline psychiatric symptom severity, we did not observe associations between changes in sleep pattern variability and symptom severity in our sample. It is possible that changes in sleep pattern variability are secondary to changes in the timing and amount of sleep, though more nuanced prospective analyses are needed to test this hypothesis. Developmentally-driven shifts in sleep duration, timing, and stability are proposed to result from a confluence of biopsychosocial factors, including delays in endogenous circadian phase, school start times, and peer socializing.44 Interventions addressing sufficient sleep duration, stable sleep timing, and biopsychosocial barriers to sleep may help mitigate worsening of psychiatric symptom severity in psychiatrically unwell youth.
Sleep latency and frequency of nighttime awakenings also increased in tandem with worsening psychiatric symptom severity over follow-up. Sleep continuity complaints are a core feature of insomnia, and the relationship between sleep continuity disturbances and worsening psychopathology observed here is consistent with models emphasizing insomnia as a transdiagnostic mechanism across psychiatric conditions.6 There is promising evidence that insomnia in otherwise healthy youth can be effectively treated with behavioral interventions45 and that improving insomnia mediates improvements in psychopathology.46 Though fewer studies have examined behavioral insomnia interventions in psychiatrically unwell youth,47 this may be a promising component for future transdiagnostic interventions.
Daytime sleepiness also worsened in conjunction with psychiatric symptom outcome measures. Excessive daytime sleepiness has been linked to poorer health48 and higher rates of psychiatric disorders in youth.49 While sleepiness may stem from disrupted sleep patterns, such complaints could also be related to medication side effects, underlying sleep disorders (e.g., sleep apnea), or other medical conditions.50 It will be necessary for future research to disambiguate whether problematic daytime sleepiness due to one (or more) of these factors is most relevant to worsening psychopathology.
Several demographic and clinical measures were also predictors of increasing psychiatric symptom severity over follow-up. A longer follow-up duration was associated with greater psychiatric symptom severity change, which may reflect that a longer follow-up period captured transition from childhood into adolescence, the height psychiatric disorder onset risk.1,3 Lower SES was also associated with symptom worsening over follow-up, consistent with a large body of work linking SES with poorer pediatric mental health outcomes.51 Regarding clinical features, parental history of BP and offspring diagnoses of ADHD and DBD were selected as predictors of change in psychiatric symptom severity over follow-up. Elevated symptom severities among offspring of parents with BP is consistent with a prior report examining baseline symptom severity in the BIOS sample.19 Likewise, offspring ADHD and DBD diagnoses have previously been associated with poorer clinical prognosis in the BIOS sample.18,52
While key strengths of this study include the large longitudinal sample and validated sleep and psychopathology measures, several limitations merit consideration. Our analyses identified longitudinal sleep-psychopathology associations, but causal interpretations cannot be made. Though our analyses were designed to ascertain specific sleep patterns associated with psychiatric symptom change, there may have been fluctuation in symptoms and sleep between assessments that was not captured here. Future longitudinal analyses should examine the directionality of these relationships and parse out the extent to which sleep and psychiatric symptoms may drive changes in one another. It will also be important to better understand what factors are causing changes in sleep to best tailor behavioral sleep interventions for youth. Data missingness is another limitation. However, BIOS participants with versus without complete sleep and psychiatric symptom measures did not differ on demographic or clinical variables. Sleep was assessed using self-report; accurate report of sleep patterns can be affected by the presence of psychiatric symptoms.53 On the other hand, the SSHS shows good reliability with sleep behaviorally-assessed with actigraphy in youth.31 Analysis of parent-reported sleep corroborated findings from offspring-reported sleep analyses (Table S10, available online). Overall, these results underscore the importance of including a combination of sleep measures, including objective, youth-report, and parent-report measures in future prospective studies.
The present findings indicate that a combination of sleep features – namely, sleep duration, sleep timing, poor sleep continuity, and daytime sleepiness – change in conjunction with psychiatric symptom severity in youth. As behavioral sleep treatment improves psychopathology in adults,10 the sleep features identified here may represent promising transdiagnostic intervention targets for the improvement of psychopathology in youth. Sleep intervention frameworks bridging these domains have been proposed for children and adolescents.13 Our results highlight a need for additional longitudinal and experimental research examining sleep as a transdiagnostic process across development to clarify causal relationships between sleep and psychiatric symptom severity.
Supplementary Material
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 2.Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Archives of general psychiatry. 2010;67(8):822–829. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin KA, King K. Developmental trajectories of anxiety and depression in early adolescence. J Abnorm Child Psychol. 2015;43(2):311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weeks M, Ploubidis GB, Cairney J, Wild TC, Naicker K, Colman I. Developmental pathways linking childhood and adolescent internalizing, externalizing, academic competence, and adolescent depression. J Adolesc. 2016;51:30–40. [DOI] [PubMed] [Google Scholar]
- 5.Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31(2):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolsen MR, Asarnow LD, Harvey AG. Insomnia as a transdiagnostic process in psychiatric disorders. Curr Psychiatry Rep. 2014;16(9):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robillard R, Hermens DF, Naismith SL, et al. Ambulatory sleep-wake patterns and variability in young people with emerging mental disorders. J Psychiatry Neurosci. 2015;40(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott EM, Robillard R, Hermens DF, et al. Dysregulated sleep-wake cycles in young people are associated with emerging stages of major mental disorders. Early Interv Psychiatry. 2016;10(1):63–70. [DOI] [PubMed] [Google Scholar]
- 9.Gregory AM, Sadeh A. Annual Research Review: Sleep problems in childhood psychiatric disorders--a review of the latest science. J Child Psychol Psychiatry. 2016;57(3):296–317. [DOI] [PubMed] [Google Scholar]
- 10.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive Behavioral Therapy for Insomnia Comorbid With Psychiatric and Medical Conditions: A Meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. [DOI] [PubMed] [Google Scholar]
- 11.Crowley SJ, Van Reen E, LeBourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: Physiology, cognition and mental health. Neurosci Biobehav Rev. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey AG. A transdiagnostic intervention for youth sleep and circadian problems. Cognitive Behavioral Therapy and Practice 2016;23(3):341–355. [Google Scholar]
- 14.Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev. 2012;16(2):129–136. [DOI] [PubMed] [Google Scholar]
- 15.Tesler N, Gerstenberg M, Huber R. Developmental changes in sleep and their relationships to psychiatric illnesses. Curr Opin Psychiatry. 2013;26(6):572–579. [DOI] [PubMed] [Google Scholar]
- 16.Levenson JC, Axelson DA, Merranko J, et al. Differences in sleep disturbances among offspring of parents with and without bipolar disorder: association with conversion to bipolar disorder. Bipolar Disord. 2015;17(8):836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levenson JC, Soehner A, Rooks B, et al. Longitudinal sleep phenotypes among offspring of bipolar parents and community controls. J Affect Disord. 2017;215:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axelson D, Goldstein B, Goldstein T, et al. Diagnostic Precursors to Bipolar Disorder in Offspring of Parents With Bipolar Disorder: A Longitudinal Study. Am J Psychiatry. 2015;172(7):638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafeman DM, Merranko J, Axelson D, et al. Toward the Definition of a Bipolar Prodrome: Dimensional Predictors of Bipolar Spectrum Disorders in At-Risk Youths. Am J Psychiatry. 2016;173(7):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 22.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Archives of general psychiatry. 1977;34(10):1229–1235. [DOI] [PubMed] [Google Scholar]
- 24.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of child and adolescent psychopharmacology. 2003;13(4):463–470. [DOI] [PubMed] [Google Scholar]
- 25.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. [DOI] [PubMed] [Google Scholar]
- 26.Gerson AC, Gerring JP, Freund L, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry research. 1996;65(3):189–198. [DOI] [PubMed] [Google Scholar]
- 27.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):545–553. [DOI] [PubMed] [Google Scholar]
- 28.Silva RR, Alpert M, Pouget E, et al. A rating scale for disruptive behavior disorders, based on the DSM-IV item pool. The Psychiatric quarterly. 2005;76(4):327–339. [DOI] [PubMed] [Google Scholar]
- 29.Russo PM, Bruni O, Lucidi F, Ferri R, Violani C. Sleep habits and circadian preference in Italian children and adolescents. J Sleep Res. 2007;16(2):163–169. [DOI] [PubMed] [Google Scholar]
- 30.Short MA, Gradisar M, Lack LC, Wright HR, Dohnt H. The sleep patterns and well-being of Australian adolescents. J Adolesc. 2013;36(1):103–110. [DOI] [PubMed] [Google Scholar]
- 31.Short MA, Gradisar M, Lack LC, Wright HR, Chatburn A. Estimating adolescent sleep patterns: parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. Nat Sci Sleep. 2013;5:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26(2):213–216. [DOI] [PubMed] [Google Scholar]
- 33.Multivariate Imputation by Chained Equations (MICE) [computer program]. Version 2.252015.
- 34.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertocci MA, Bebko G, Dwojak A, et al. Longitudinal relationships among activity in attention redirection neural circuitry and symptom severity in youth. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(4):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro-Schilo L, Grimm KJ. Using residualized change versus difference scores for longitudinal research. Journal of Social and Personal Relationships. 2018;35(1):32–58. [Google Scholar]
- 37.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3 ed. Mahwah NJ: Lawrence Erlbaum Associates, Inc.; 2003. [Google Scholar]
- 38.GLMNET [computer program]. Version 2.0–22014.
- 39.Tibshirani R Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological). 1996;58(1):267–288. [Google Scholar]
- 40.Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2005;67(2):301–320. [Google Scholar]
- 41.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 42.McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74(4):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etain B, Milhiet V, Bellivier F, Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. Eur Neuropsychopharmacol. 2011;21 Suppl 4:S676–682. [DOI] [PubMed] [Google Scholar]
- 44.Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115(1 Suppl):250–256. [DOI] [PubMed] [Google Scholar]
- 45.de Bruin EJ, Bogels SM, Oort FJ, Meijer AM. Efficacy of Cognitive Behavioral Therapy for Insomnia in Adolescents: A Randomized Controlled Trial with Internet Therapy, Group Therapy and A Waiting List Condition. Sleep. 2015;38(12):1913–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Bruin EJ, Bogels SM, Oort FJ, Meijer AM. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psychiatry. 2017. [DOI] [PubMed] [Google Scholar]
- 47.Clarke G, McGlinchey EL, Hein K, et al. Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behav Res Ther. 2015;69:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafsson ML, Laaksonen C, Aromaa M, et al. Association between amount of sleep, daytime sleepiness and health-related quality of life in schoolchildren. J Adv Nurs. 2016;72(6):1263–1272. [DOI] [PubMed] [Google Scholar]
- 49.Rose D, Gelaye B, Sanchez S, et al. Morningness/eveningness chronotype, poor sleep quality, and daytime sleepiness in relation to common mental disorders among Peruvian college students. Psychol Health Med. 2015;20(3):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millman RP, Working Group on Sleepiness in Adolescents/Young A, Adolescence AAPCo. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774–1786. [DOI] [PubMed] [Google Scholar]
- 51.Reiss F Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc Sci Med. 2013;90:24–31. [DOI] [PubMed] [Google Scholar]
- 52.Kim JW, Yu H, Ryan ND, et al. Longitudinal trajectories of ADHD symptomatology in offspring of parents with bipolar disorder and community controls. J Clin Psychiatry. 2015;76(5):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertocci MA, Dahl RE, Williamson DE, et al. Subjective sleep complaints in pediatric depression: a controlled study and comparison with EEG measures of sleep and waking. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1158–1166. [DOI] [PubMed] [Google Scholar]
- 54.Hollingshead AB. Four-Factor Index of Social Status. New Haven: Yale University Department of Sociology; 1975. [Google Scholar]
- 55.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.