Abstract
We previously found that 3- and 6-month-old male mice with conditional ablation of protein kinase D1 (PRKD1) in osteoprogenitor cells (expressing Osterix) exhibited reduced bone mass. Others have demonstrated similar effects in young female PRKD1-deficient mice. Here we examined the bone resorptive response of adult female floxed control and conditional knockout (cKO) mice undergoing sham surgery or OVX. Femoral and tibial bone mineral density (BMD) values were significantly reduced upon OVX in control, but not cKO, females compared to the respective sham-operated mice. Micro-CT analysis showed that OVX significantly increased trabecular number and decreased trabecular spacing in cKO but not control mice. Finally, in control mice serum levels of a marker of bone resorption (pyridinoline crosslinks) and the osteoclast activator RANKL significantly increased upon OVX; however, no such OVX-induced increase was observed in cKO mice. Our results suggest the potential importance of PRKD1 in response to estrogen loss in bone.
Keywords: bone mass, osteopenia, osteoporosis, protein kinase D1 (PRKD1), pyridinoline crosslinks (PYD), receptor activator of nuclear factor kappa-B ligand (RANKL)
1. INTRODUCTION
Osteoporosis is estimated to affect more than 200 million women worldwide and will result in osteoporotic fractures in about 1 in 3 women over the age of 50 (Melton et al., 1992). Women lose bone mass rapidly in the first 4–8 years after menopause and then subsequently continue to lose bone at a steady, gradual rate, comparable to that of men, with continued aging. Thus, post-menopausal bone loss is a significant determinant of osteopenia (low bone mass), osteoporosis, and risk of skeletal fracture, which can negatively impact quality of life. Nevertheless, the exact mechanisms by which menopause and estradiol deficiency result in bone loss is not completely understood, although new insights and therapies to treat these conditions are emerging through the study of novel regulators of bone formation (Manolagas, 2010,Ucer et al., 2017).
Protein kinase D1 (PRKD1) is a serine/threonine protein kinase with similarities to both the protein kinase C (PKC) and calcium/calmodulin-dependent protein kinases of the AGC superfamily of kinases. PRKD1 (first named PKCμ) is the first member of the PRKD family that also includes PRKD2 and PRKD3 (also known as PKCυ) [reviewed in (Guha et al., 2010,Lavalle et al., 2010)]. PRKD isoforms mediate a number of key cellular processes, including proliferation, migration, Golgi trafficking and secretion and angiogenesis [reviewed in (Guha et al., 2010,Lavalle et al., 2010)]. However, the role of PRKD in mesenchymal stem cell and bone function has been relatively understudied.
In previous studies the effects of PRKD1 in bone in vivo were examined in young male and female mice pooled for analysis of bone parameters; the results showed an important role of PRKD1 in bone biology (Ford et al., 2013,Li et al., 2017). Our study, in which we demonstrated that PRKD1 ablation resulted in decreased bone formation (Bollag et al., 2017), was confined to mature males to limit any potential confounding effects from skeletal growth or estrogen-related biology. However, since these initial studies were completed, a potentially exciting interaction between estrogen and PRKD1 has emerged: in breast cancer cells overexpression of PRKD1 upregulates estrogen receptor-alpha (ERα) and sensitizes these cells to the proliferative action of estradiol (Karam et al., 2014). Conversely, siRNA-mediated knockdown of PRKD1 decreases ERα and decreases the proliferative potency of estrogen (Karam et al., 2014). In addition, estrogens are known to have anti-oxidant properties (Almeida et al., 2007); in other systems PRKD1 also protects against oxidative stress by inducing the expression of superoxide dismutase-2 (Storz et al., 2005,Storz, 2007). Thus, we hypothesized that a lack of estrogen and of PRKD1 might act through similar mechanisms. Given these potentially important interactions among PRKD1, estrogens and ERα, we sought to expand upon our earlier investigations and interrogate the role of PRKD1 in estrogen deficiency-induced bone loss by employing an ovariectomized (OVX) model to elucidate the effects of estrogen depletion in PRKD1 conditional knockout (cKO) mice versus floxed control littermates. Analysis of bone mass, microarchitecture and serum markers of bone resorption in mice experiencing sham surgery versus OVX demonstrated that the ablation of PRKD1 in osteoprogenitor cells resulted in minimal bone loss upon OVX in comparison with floxed control animals, which demonstrated significant decreases in bone mineral density (BMD) and increases in markers of bone resorption with OVX.
2. MATERIALS AND METHODS
2.1. Generation of PRKD1 Conditional Knockout Mice
A transgenic mouse model in which a portion of the PRKD1 gene was flanked by loxP sites was created (Flelitz et al., 2008) and generously provided by Dr. Eric Olson (University of Texas Southwestern Medical Center, Dallas, TX). These mice were crossed with transgenic mice in which Cre recombinase expression is expressed under the control of the Osterix promoter as described previously (Bollag et al., 2017). The heterozygous offspring were intercrossed to generate PRKD1 cKO mice, which were backcrossed to floxed PRKD1 mice, and the floxed and cKO littermates used for experimentation.
2.2. Ovariectomy
Female mice approximately 6 months of age were anesthetized and a surgical incision made in the back. The ovaries were then removed, and the incision was closed by Michel wound clips (Roboz Surgical, Gaithersburg, MD). Sham mice were surgically operated upon without removal of the ovaries. The mice were sacrificed after a further 4 weeks and various bone parameters analyzed. All experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals under a protocol approved by the Augusta University Institutional Animal Care and Use Committee.
2.3. DXA
Bone mineral content (BMC) and bone mineral density (BMD) were determined by dual-energy X-ray absorptiometry using a Lunar Piximus DXA instrument (General Electric Healthcare, Barrington, IL) as in (Xie et al., 2005). Briefly, mice were sacrificed and femora and tibiae isolated and cleaned of soft tissue. Bones were then scanned to determine BMD and BMC.
2.4. μCT
Bone microarchitectural parameters were determined using microcomputed tomography (μCT) with a Skyscan 1174 μCT system (Micro Photonics Inc.; PA, USA) as previously described (Refaey et al., 2017). Femora were scanned with a voxel size of 15.9 μm and a 0.25 mm aluminum filter and their three-dimensional (3D) structure recreated using a single reconstruction log file and Skyscan NRecon/NRecon server software (Micro Photonics Inc.; PA, USA) with standardized parameters. Datasets were used to determine 3D bone morphometric parameters with the Skyscan CTanalyzer software (Micro Photonics Inc.; Allentown, PA). Parameters determined included femoral head bone mineral density (BMD), bone volume fraction (%BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp), after adjusting the normal control side to a grey scale range from 77 to 255. Analysis was performed according to recommended procedures (Bouxsein et al., 2010). 3D models of trabecular bone in femoral heads were performed using CTvol software.
2.5. ELISA
Mice were sacrificed, blood samples collected from each mouse by cardiac puncture and serum samples prepared, aliquoted and stored at −80°C. The levels of pyridinoline crosslinks (PYD), a marker of bone breakdown, in these samples were measured with an ELISA kit from Quidel Corp. (San Diego, CA) according to the manufacturer’s directions. The levels of receptor activator of nuclear factor-kB ligand (RANKL), which stimulates osteoclast function, and osteoprotegerin (OPG), a negative regulator of RANKL, were also determined using ELISA kits from R&D Systems (Minneapolis, MN), again based on the supplier’s protocol.
2.6. Tartrate-resistant Acid Phosphatase (TRAP) Staining
Paraffin sections were stained with an acid phosphatase kit (Sigma #386A) and counterstained with Fast Green to highlight bone surfaces, as described in (Refaey et al., 2017).
2.7. Statistical Analysis
A log transformation was used prior to analysis to stabilize the within group variance for PYD, RANKL and RANKL/OPG levels. A one-way ANOVA was used to test for overall differences among the genotype-ovariectomy groups (WT-Sham, WT-OVX, KO-Sham, KO-OVX). The tests of interest were determined using contrasts to compare OVX vs. Sham for floxed (“WT”) and cKO (“KO”) mice and WT versus KO under the Sham condition. Statistical significance was determined at alpha=0.05, and SAS 9.4 (SAS Institute, Inc., Cary, NC) was used for all analyses.
3. RESULTS
3.1. Osteoprogenitor-specific PKD1 conditional knockout (cKO) female mice exhibit a minimal effect of ovariectomy on mineral content and mass whereas wild-type females exhibit a significant decrease in these parameters upon ovariectomy
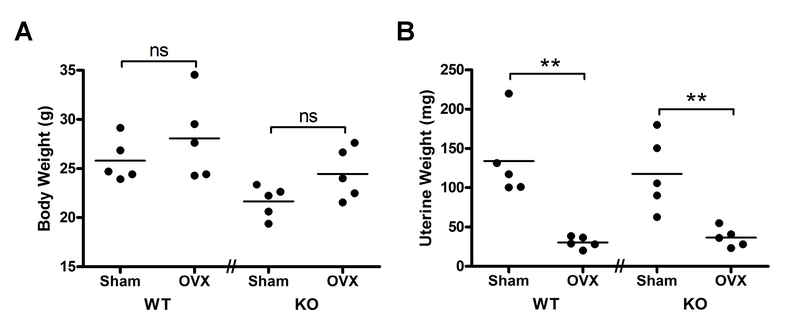
Female PRKD1 cKO mice of approximately 6 months of age and floxed littermates were subjected to sham surgery or were ovariectomized and maintained for 4 weeks before sacrifice. We have previously demonstrated that the PRKD1 conditional knockout (cKO) mice exhibit reduced PRKD1 expression in bone marrow stromal cells and bone shafts (Bollag et al., 2017). We also verified the success of the ovariectomy (OVX) procedure by measuring uterine weight. There was an effect of surgery on uterine weight (p<0.001), with both sham surgical groups demonstrating significantly greater values than the respective OVX mice (Figure 1B), with no effect of the surgical manipulation on body weight (Figure 1A).
Figure 1. Ovariectomy (OVX) resulted in significant decreases in uterine weight in both control and PRKD1 cKO mice.
Floxed (“WT”) or cKO (“KO”) mice were sham-operated or ovariectomized as described in Methods. (A) After 4 weeks, each mouse was weighed, with individual symbols representing each mouse and the lines representing the means of 5 mice per group. (B) After sacrifice, the uterus of each mouse was collected and weighed. Each symbol represents an individual mouse with the lines representing the means of 5 mice per group; **p<0.01 as indicated.
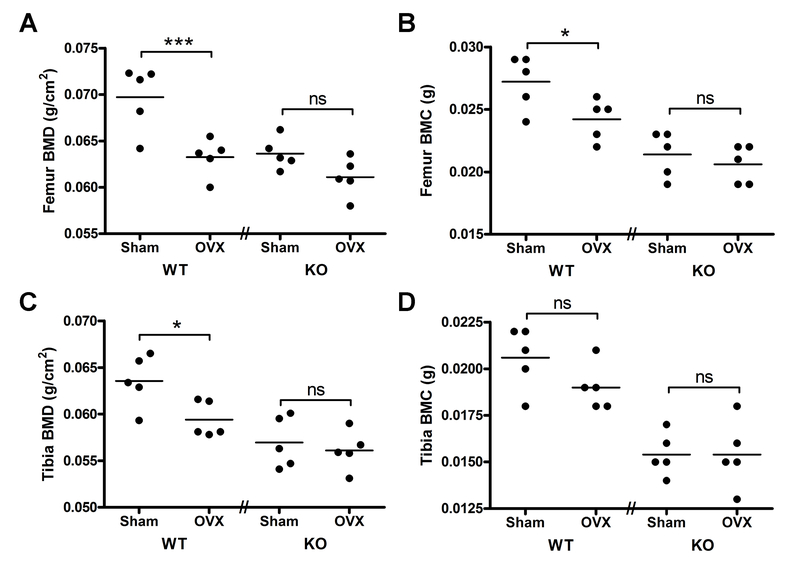
Isolated femora or tibiae were then analyzed for their bone mineral content (BMC) and BMD. In the control mice significant differences in both BMD and BMC were detected for the femora, and for the tibia only the BMD showed a significant difference with surgical manipulation, with OVX less than sham surgery (p<0.05) (Figure 2). Thus, OVX resulted in a 9% decrease in femoral BMD and a 7% reduction in tibial BMD in floxed controls. On the other hand, in the PRKD1 cKO mice, neither BMD nor BMC were significantly different between the mice subjected to sham surgery or OVX in either the femora or tibiae.
Figure 2. Control mice exhibit reduced bone mineral content and bone mineral density with OVX, whereas PRKD1 conditional knockout mice exhibit no significant OVX-induced changes.
Femora and tibiae were collected from mice treated as in Figure 1. Bone mineral density (A and C) and bone mineral content (B and D) of each femur (A and B) and tibia (C and D) were then determined using a Piximus instrument. Each symbol represents an individual mouse with the lines representing the means of 4–5 mice per group; *p<0.05, ***p<0.001 as indicated.
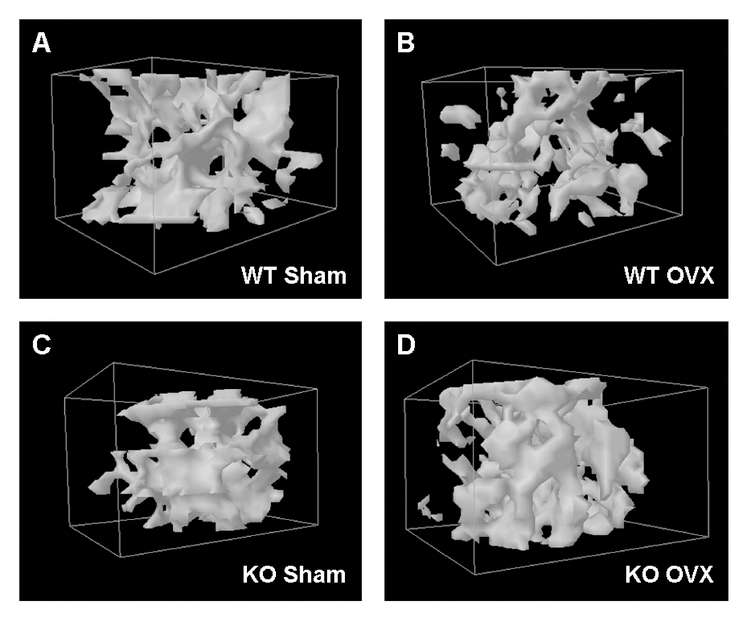
Three-dimensional reconstruction of femoral heads was performed using μCT data for mice representative of each group, as described in Methods. Representative animals were selected to exhibit femoral BMD values, as determined by DXA, closest to the mean value determined for that group. As shown, in control mice OVX resulted in less bone relative to the sham surgery manipulation (comparing panels A and B), whereas there appeared to be no effect of OVX in the cKO mice (comparing panels C and D).
3.2. OVX results in altered bone microarchitecture in PRKD1 cKO female mice relative to the floxed control animals
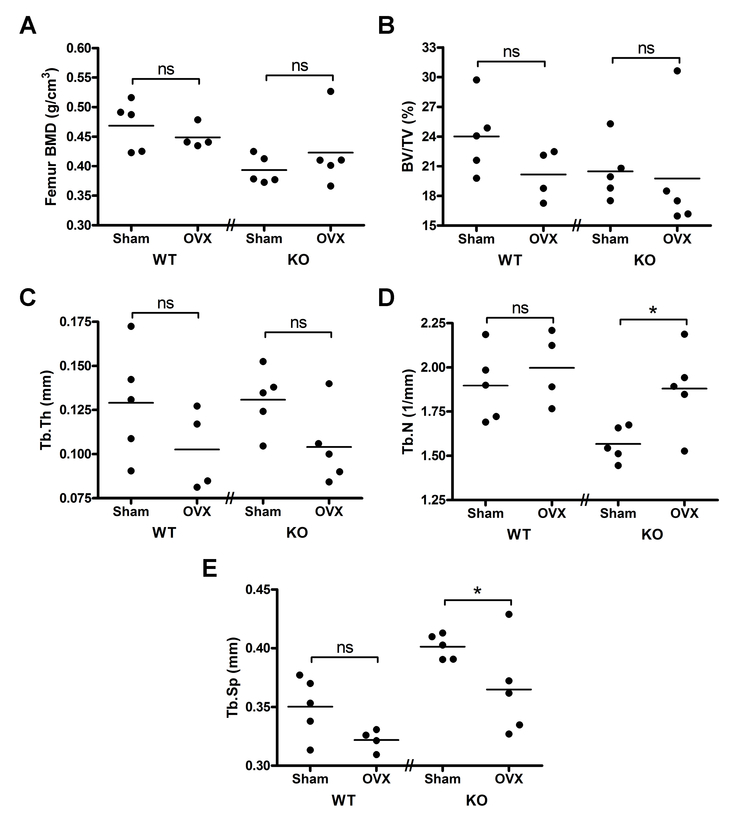
μCT analysis of sham-operated PRKD1 cKO female animals in comparison to OVX cKO mice demonstrated altered bone microarchitecture (Figure 4). Thus, the OVX PRKD1 cKO mice display a significantly increased trabecular number (p<0.05) and decreased trabecular separation (p<0.05). In the PRKD1 cKO mice there were no significant differences observed in femoral BMD, fractional bone volume or trabecular thickness with OVX. In contrast, there was no significant effect of OVX on any of these parameters in the floxed control mice.
Figure 4. PRKD1 conditional knockout mice exhibit altered trabecular microarchitectural parameters upon OVX with no changes observed in control mice.
Femora isolated as in Figure 2 were analyzed by microCT as described in Methods. Shown are (A) femoral head bone mineral density (BMD), (B) bone volume fraction (BV/TV, %), (C) trabecular thickness (Tb.Th, mm), (D) trabecular number (Tb.N, 1/mm) and (E) trabecular spacing (Tb.Sp, mm). Each symbol represents an individual mouse with the lines representing the means of 4–5 mice per group; *p<0.05 as indicated.
3.3. Ovariectomy induced significant increases in the serum levels of PYD and RANKL and the RANKL/OPG ratio in floxed control mice but not in the PRKD1 conditional knockout mice
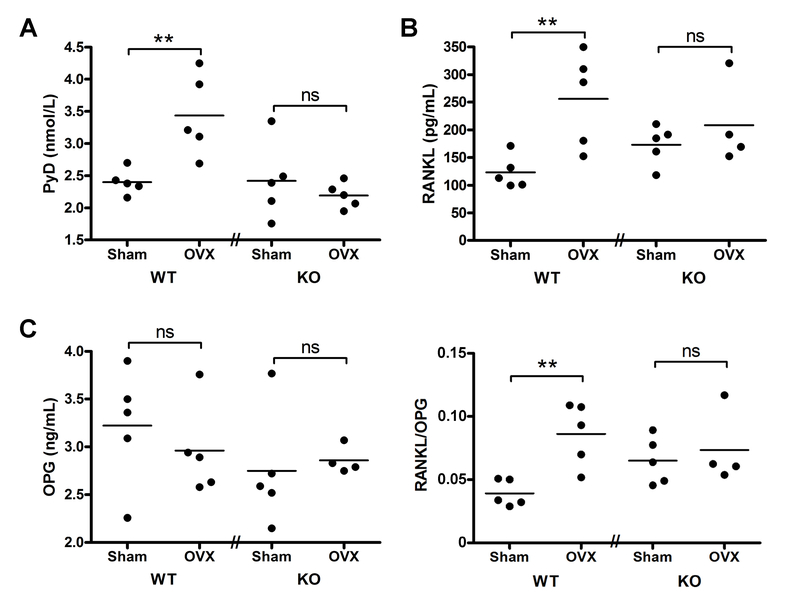
The serum levels of PYD, a marker of bone resorption, were also measured in the four groups of mice. As shown in Figure 5A, ovariectomy resulted in significantly increased serum PYD levels in the floxed control animals, as expected since estrogen deficiency promotes osteoclast action (Riggs, 2000). In comparison, the cKO mice did not exhibit increased PYD levels with ovariectomy. To determine whether the lack of effect of ovariectomy on serum PYD levels in the PRKD1 cKO mice resulted from changes in agents that regulate activation of osteoclasts, the amounts of RANKL, which promotes osteoclast generation and activation, and of OPG, which binds RANKL to inhibit its action on osteoclasts, were measured in serum. There were no significant differences observed in OPG levels with OVX in either control or PRKD1 cKO mice (Figure 5C). In control floxed mice OVX induced a significant approximately 2-fold increase in serum RANKL levels (Figure 5B). On the other hand, serum RANKL levels exhibited no significant elevation with OVX in the PRKD1 cKO mice. Similar results were obtained when the ratio of RANKL/OPG was analyzed (Figure 5D). The impaired increase in RANKL and/or the RANKL/OPG ratio upon OVX may explain, at least in part, the lack of an increase in serum PYD levels in the OVX PRKD1 cKO mice in panel A.
Figure 5. Serum PYD and RANKL levels increased in control ovariectomized mice but not in ovariectomized PRKD1 conditional knockout mice.
Blood was collected by cardiac puncture from mice subjected to sham surgery or OVX after sacrifice and serum prepared. (A) PYD, (B) RANKL or (C) OPG levels were assayed using appropriate ELISA kits as described in Methods. RANKL data were log-transformed prior to analysis. Each symbol represents an individual mouse with the lines representing the means of 4–5 mice per group; **p<0.01 as indicated.
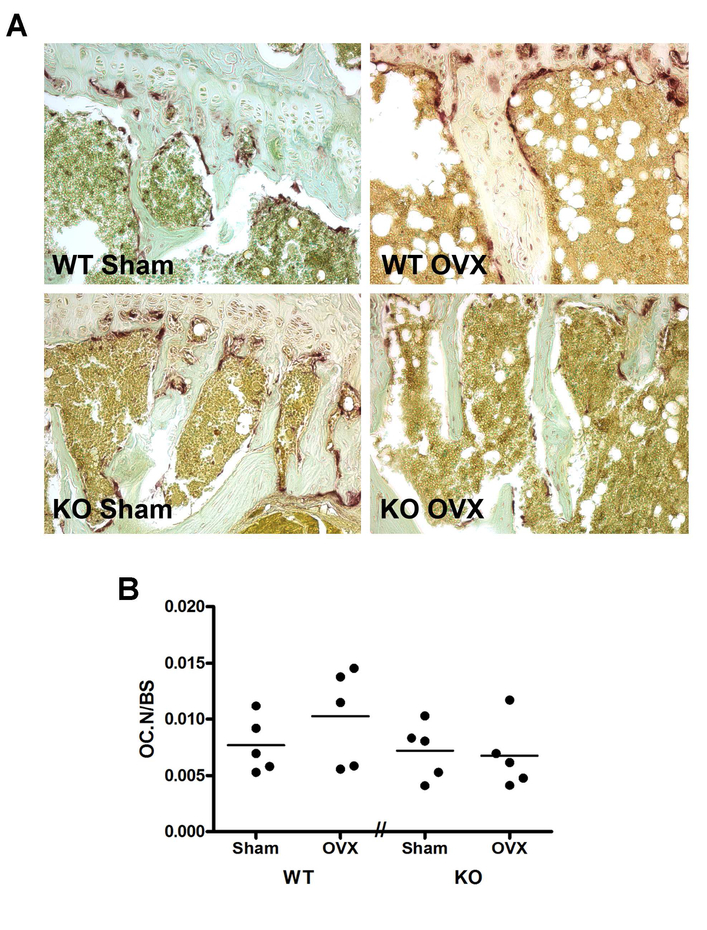
To determine whether the impaired RANKL response to OVX resulted in changes in osteoclast number, we performed histomorphometry for tartrate-resistant acid phosphatase (TRAP)-positive cells. Representative photomicrographs are shown in Figure 6A and the number of osteoclasts (TRAP-positive cells) relative to bone surface are shown in Figure 6B. In support of the results obtained with RANKL, OVX tended to increase the mean value of the number of osteoclasts relative to bone surface in the floxed control mice compared to the sham-operated animals, and this effect seemed to be blunted in the PRKD1 cKO mice; however, the differences did not achieve statistical significance.
Figure 6. TRAP-positive osteoclast number tended to increase in control ovariectomized mice but not in ovariectomized PRKD1 conditional knockout mice.
Collected tibiae were fixed for paraffin embedding, sectioning and staining. (A) Representative micrographs from each group of mice are illustrated and show TRAP-positive osteoclasts in red. (B) The number of osteoclasts was determined as described in Methods. Each symbol represents an individual mouse with the lines representing the means of 4–5 mice per group.
3.4. Several measured parameters were different between floxed control and PRKD1 conditional knockout mice under sham conditions.
A previous study examined the effect of PRKD1 deficiency on bone parameters in male versus female mice transgenic for a dominant-negative mutant PRKD1 allele (Ford et al., 2013). These authors found that PRKD1 deficiency similarly decreased BMD in both sexes as well as the mRNA levels of osteoblast and osteoclast differentiation markers (Ford et al., 2013); however, they did not determine the serum levels of markers of bone resorption. Similarly, our earlier study reported the effects of PRKD1 in mature male mice with no additional interventions (Bollag et al., 2017). Therefore, it was of interest to determine whether any baseline, statistically significant differences existed in bone properties or circulating serum markers between female PRKD1-deficient mice and floxed controls. Accordingly, we performed an additional statistical analysis to determine whether there were any significant differences in bone mass or serum markers of bone resorption between the control and the PRKD1 cKO mice under the sham surgical condition. Femoral and tibial BMD and BMC were significantly reduced in the sham-operated PRKD1 cKO mice compared to the sham control floxed animals (p<0.01), as was femoral BMD determined by μCT analysis (P<0.01). In addition, we found that serum RANKL levels, as well as the RANKL/OPG ratio were significantly greater in the conditional PRKD1 knockout mice (p<0.05), but there were no differences in PyD or OPG levels between the control and PRKD1 cKO mice under sham conditions.
4. DISCUSSION
Effects of PRKD1 deficiency in bone in vivo have been examined previously (Ford et al., 2013,Li et al., 2017,Bollag et al., 2017). The first two studies reported on young animals (4 to 10 weeks old) and found a similar reduction in femoral and whole-body BMD in males and females (Ford et al., 2013) or analyzed a pooled population of both males and females for determination of bone parameters (Li et al., 2017). A differential effect of PRKD1 deficiency on the expression of certain genes was also observed in bone marrow stromal cells derived from males versus females ex vivo (Ford et al., 2013). In our previous study we investigated the effects of PRKD1 ablation (Bollag et al., 2017), but focused on older ages to exclude the Cre-related developmental effects that have been observed in younger Osterix-Cre transgenic mice (Wang et al., 2015,Davey et al., 2012) (please see below) and on male animals to negate potential effects of the estrus cycle. In the present study we sought to determine whether PRKD1 affects the bone loss response to OVX in adult female mice.
Whereas OVX significantly decreased BMD in the femora and tibiae of floxed control mice, it had no effect on this parameter in PRKD1 cKO mice. This result suggests that some of estrogen’s effects to maintain bone formation may be related to activation of PRKD1 in osteoprogenitor cells. In this study floxed PRKD1 littermates served as the control. Concern has been raised with the Osterix promoter-Cre recombinase transgenic mouse model as a result of reports that these animals can display cranial defects (Wang et al., 2015) and decreased size/weight (Davey et al., 2012) due to the transgene itself, in the absence of a floxed gene. However, it seems unlikely that these non-specific effects of the transgene are responsible for the observed phenotype, since bone effects in Osx-Cre transgenic mice resolve by two to three months of age (Davey et al., 2012), and the mice in the current study were approximately six months of age. Thus, it seems unlikely that the Cre transgene would affect the bone turnover associated with estrogen deficiency.
Serum PYD levels did not increase with OVX in the osteoprogenitor-specific PRKD1 cKO female mice, whereas the levels were elevated in OVX floxed control animals. It is not entirely clear why PRKD1 deficiency in osteoprogenitor cells should influence bone breakdown by osteoclasts. However, it is known that osteoblasts secrete factors, such as RANKL, that can influence osteoclast function. Indeed, the PRKD1 cKO OVX mice showed no signficant increase in serum RANKL levels whereas OVX elevated serum RANKL levels in the floxed control mice by approximately two-fold. The mechanism underlying this reduction is unknown but may be related to the fact that in some cell types PRKD1 has been shown to be involved in trafficking and secretion of proteins from the trans-Golgi network. Thus, Malhotra and colleagues have shown a key role for PRKD in transport and secretion of a vesicular stomatitis virus-protein G from the trans-Golgi network to the plasma membrane [(Baron and Malhotra, 2002, Liljedahl et al., 2001,Yeaman et al., 2004) and reviewed in (Van Lint et al., 2002)]. Therefore, it seems possible that secretion of osteoclast-regulating factors might be defective in PRKD1 knockout osteoprogenitor cells. In addition, bone formation and resorption are known to be coupled (Matsuo and Irie, 2008), with osteoblasts secreting agents that recruit osteoclasts for bone resorption. Thus, an impairment in osteoblast differentiation and/or bone formation in the PRKD1 cKO mice, as reported previously (Li et al., 2017,Bollag et al., 2017), could presumably also result in a defect in osteoclast-mediated bone breakdown, with a subsequent lack of increase in serum PYD levels concomitant with a failure to enhance serum RANKL levels in the PRKD1 cKO OVX mice.
We have previously observed that male PRKD1 cKO mice exhibit significantly lower BMD values at 6 months but not at 9 or 12 months and proposed the initiation of potential compensatory mechanisms between 6 and 9 months of age (Bollag et al., 2017). The female PRKD1 cKO mice in the current study, although slightly older than 6 months, continued to exhibit a significantly reduced femoral and tibial BMD compared to the control floxed mice under sham conditions, indicating that any compensatory changes induced with age have not yet completely counterbalanced the loss of PRKD1. Nevertheless, it seems possible that inhibition of osteoclast activity through a reduced ability to enhance RANKL secretion in response to a challenge may be one process by which BMD is corrected as the mice age. On the other hand, under sham conditions serum RANKL levels and the RANKL/OPG ratio were greater in the PRKD1 cKO than in floxed control mice, arguing against an impairment in RANKL secretion and inhibited osteoclast activity as a likely compensatory mechanism under basal conditions. Although the baseline enhancement of serum RANKL levels could explain in part the reduced BMD observed in PRKD1 osteoprogenitor-specific knockout mice (Li et al., 2017,Bollag et al., 2017), this result contrasts with a previous study showing that RANKL expression is decreased in PRKD1-deficient bone marrow cells (Ford et al., 2013). Nevertheless, in this prior report the PRKD deficiency was neither restricted to osteoprogenitor-lineage cells nor to PRKD1 alone, since the dominant-negative PRKD1 transgene used by Ford et al. can also impair PRKD2 activity (Yin et al., 2008), possibly explaining the disparate results.
Another cellular response known to be impacted by PRKD1 promotes protection against mitochondrially generated reactive oxygen species (ROS): thus, in cervical cancer cells PRKD1 has been shown to induce superoxide dismutase-2 (SOD2) expression through activation of nuclear factor-kappaB (Storz et al., 2005,Storz, 2007,Storz and Toker, 2003). Differentiation of bone marrow mesenchymal stem cells (BMMSCs) results in their increased use of oxidative phosphorylation for ATP production, and this change in cell metabolism would be expected to enhance the mitochondrial generation of ROS (Guntur et al., 2014). Excessive ROS, in turn, can inhibit BMMSC differentiation into osteoblasts as well as bone formation [e.g., (Kim et al., 2010,Bai et al., 2004,Hamada et al., 2009)]. Therefore, an impairment in endogenous anti-oxidant systems, such as could occur with a reduction in SOD2 expression, could result in greater oxidative stress during osteoblast differentiation and a resulting reduction in bone formation, as is seen in the PRKD1-deficient mice (Ford et al., 2013,Li et al., 2017,Bollag et al., 2017). In this regard, oxidative stress is reported to enhance RANKL mRNA and protein expression in osteoblasts (Bai et al., 2005), potentially explaining the elevated serum RANKL levels in the PRKD1 conditional knockout mice basally. Clearly, the effects of PRKD1 deficiency are complex and require further investigation.
In conclusion, then, our results indicate an important role for osteoprogenitor cell PRKD1 in the bone loss induced by OVX. Thus, our data suggest that PRKD1 may mediate some of the osteogenic and anti-resorptive effects of estrogen in bone, based on our finding that OVX was able to induce few additional changes in bone in PRKD1 cKO female mice, in which reduced bone mass has already been reported (Ford et al., 2013). Clearly, future studies are warranted in order to define the mechanisms underlying these important effects of PRKD1 in bone.
Figure 3. OVX resulted in bone loss in control mice but not PRKD1 conditional knockout mice.
Three-dimensional reconstruction of the femoral heads of (A) sham-operated control and (B) OVX control mice as well as (C) sham-operated and (D) OVX cKO mice, was performed as described in Methods.
Highlights.
Floxed control and protein kinase D1 conditional knockout (cKO) mice were examined
Ovariectomy (OVX) decreased bone mineral density in control but not cKO mice
OVX elevated serum levels of a bone resorption marker in control but not cKO mice
OVX increased serum levels of an osteoclast activator in control but not cKO mice
Protein kinase D1 likely plays a role in bone responses to estrogen and its loss
Acknowledgments
Funding: This work was supported by the National Institutes of Health/National Institute of Aging [P01 AG036675 (to CMI)]; WBB was supported by a VA Research Career Scientist Award. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Declaration of Interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW and Riggs BL, 1992. Perspective. How many women have osteoporosis?, J Bone Miner Res. 7, 1005–10. [DOI] [PubMed] [Google Scholar]
- [2].Manolagas SC, 2010. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis, Endocr Rev. 31, 266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ucer S, Iyer S, Kim HN, Han L, Rutlen C, Allison K, Thostenson JD, de Cabo R, Jilka RL, O’Brien C, Almeida M and Manolagas SC, 2017. The Effects of Aging and Sex Steroid Deficiency on the Murine Skeleton Are Independent and Mechanistically Distinct, J Bone Miner Res. 32, 560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guha S, Tanasanvimon S, Sinnett-Smith J and Rozengurt E, 2010. Role of protein kinase D signaling in pancreatic cancer, Biochem. Pharmacol. 80, 1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lavalle CR, George KM, Sharlow ER, Lazo JS, Wipf P and Wang QJ, 2010. Protein kinase D as a potential new target for cancer therapy, Biochim. Biophys. Acta. May 24, e-published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ford JJ, Yeh LC, Schmidgal EC, Thompson JF, Adamo ML and Lee JC, 2013. Protein kinase D1 is essential for bone acquisition during pubertal growth, Endocrinology. 154, 4182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li S, Xu W, Xing Z, Qian J, Chen L, Gu R, Guo W, Lai X, Zhao W, Li S, Wang Y, Wang QJ and Deng F, 2017. A Conditional Knockout Mouse Model Reveals a Critical Role of PKD1 in Osteoblast Differentiation and Bone Development, Sci Rep. 7, 40505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bollag WB, Choudhary V, Zhong Q, Ding KH, Xu J, Elsayed R, Yu K, Su Y, Bailey LJ, Shi XM, Elsalanty M, Johnson MH, McGee-Lawrence ME and Isales CM, 2017. Deletion of protein kinase D1 in osteoprogenitor cells results in decreased osteogenesis in vitro and reduced bone mineral density in vivo, Mol Cell Endocrinol. 461, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karam M, Bieche I, Legay C, Vacher S, Auclair C and Ricort JM, 2014. Protein kinase D1 regulates ERalpha-positive breast cancer cell growth response to 17beta-estradiol and contributes to poor prognosis in patients, J Cell Mol Med. 18, 2536–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL and Manolagas SC, 2007. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids, J Biol Chem. 282, 27285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Storz P, Doppler H and Toker A, 2005. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species, Mol Cell Biol. 25, 8520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Storz P, 2007. Mitochondrial ROS--radical detoxification, mediated by protein kinase D, Trends Cell Biol. 17, 13–8. [DOI] [PubMed] [Google Scholar]
- [13].Flelitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, Bassek-Duby R and Olson EN, 2008. Requirement of protein kinase D1 for pathological cardiac remodeling, Proc. Natl. Acad. Sci. USA 105, 3059–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xie D, Cheng H, Hamrick M, Zhong Q, Ding KH, Correa D, Williams S, Mulloy A, Bollag W, Bollag RJ, Runner RR, McPherson JC, Insogna K and Isales CM, 2005. Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover, Bone. 37, 759–69. [DOI] [PubMed] [Google Scholar]
- [15].Refaey ME, McGee-Lawrence ME, Fulzele S, Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG, Shi XM, Xu J, Hill WD, Johnson MH, Hunter M, Pierce JL, Yu K, Hamrick MW and Isales CM, 2017. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss, J Bone Miner Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ and Muller R, 2010. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography, J Bone Miner Res. 25, 1468–86. [DOI] [PubMed] [Google Scholar]
- [17].Riggs BL, 2000. The mechanisms of estrogen regulation of bone resorption, J Clin Invest. 106, 1203–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang L, Mishina Y and Liu F, 2015. Osterix-Cre transgene causes craniofacial bone development defect, Calcif Tissue Int. 96, 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, Anderson PH and Zajac JD, 2012. Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual, Transgenic Res. 21, 885–93. [DOI] [PubMed] [Google Scholar]
- [20].Baron CL and Malhotra V, 2002. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane, Science. 295, 325–328. [DOI] [PubMed] [Google Scholar]
- [21].Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J and Malhotra V, 2001. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network, Cell. 104, 409–420. [DOI] [PubMed] [Google Scholar]
- [22].Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ and Malhotra V, 2004. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network, Nat. Cell Biol. 6, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, Vandenheede JR and Seufferlein T, 2002. Protein kinase D: an intracellular traffic regulator on the move, Trends Cell Biol. 12, 193–200. [DOI] [PubMed] [Google Scholar]
- [24].Matsuo K and Irie N, 2008. Osteoclast-osteoblast communication, Arch Biochem Biophys. 473, 201–9. [DOI] [PubMed] [Google Scholar]
- [25].Yin DM, Huang YH, Zhu YB and Wang Y, 2008. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus, J Neurosci. 28, 8832–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Storz P and Toker A, 2003. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway, EMBO J. 22, 109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guntur AR, Le PT, Farber CR and Rosen CJ, 2014. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass, Endocrinology. 155, 1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim WK, Meliton V, Bourquard N, Hahn TJ and Parhami F, 2010. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress, J Cell Biochem. 111, 1199–209. [DOI] [PubMed] [Google Scholar]
- [29].Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM and Luo SQ, 2004. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB, Biochem Biophys Res Commun. 314, 197–207. [DOI] [PubMed] [Google Scholar]
- [30].Hamada Y, Fujii H and Fukagawa M, 2009. Role of oxidative stress in diabetic bone disorder, Bone. 45 Suppl 1, S35–8. [DOI] [PubMed] [Google Scholar]
- [31].Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL and Luo SQ, 2005. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast, J Biol Chem. 280, 17497–506. [DOI] [PubMed] [Google Scholar]