Abstract
Aquaporin-3 (AQP3), a water and glycerol channel, plays an important role in epidermal function, with studies demonstrating its involvement in keratinocyte proliferation, differentiation and migration and epidermal wound healing and barrier repair. Increasing speculation about the use of histone deacetylase (HDAC) inhibitors to treat skin diseases led us to investigate HDAC’s role in the regulation of AQP3. The broad-spectrum HDAC inhibitor, suberolyanilide hydroxamic acid (SAHA) induced AQP3 mRNA and protein expression in a dose- and time- dependent manner in normal keratinocytes. The SAHA-induced increase in AQP3 levels resulted in enhanced [3H]glycerol uptake in normal but not in AQP3 knockout keratinocytes, confirming that the expressed AQP3 was functional. Utilization of HDAC inhibitors with different specificities limited our exploration of the responsible HDAC member to HDAC1, HDAC2 or HDAC3. Cre-recombinase-mediated knockdown and overexpression of HDAC3 suggested a role for HDAC3 in suppressing AQP3 expression basally. Further investigation implicated p53 as a transcription factor involved in regulating HDAC inhibitor-induced AQP3 expression. Thus, our study supports the regulation of AQP3 expression by HDAC3 and p53. Since SAHA is already approved to treat cutaneous T-cell lymphoma, it could potentially be used as a novel therapy for skin diseases like psoriasis, where AQP3 is abnormally expressed.
Keywords: acetylation, aquaporin-3 (AQP3), epidermis, glycerol, histone deacetylase, HDAC3, Keratinocytes, p53, psoriasis, SAHA, skin
INTRODUCTION
Aquaporin-3 (AQP3), a water and glycerol channel that also transports hydrogen peroxide (Hara- Chikuma et al., 2015, Verkman, 2008), is known to play a key role in many tissues and organs including the skin. In the dermis AQP3 is required for bleomycin-induced fibrosis (Luo et al., 2016), whereas in the epidermis it has been reported to contribute to keratinocyte proliferation and differentiation (Bollag et al., 2007, Choudhary et al., 2015, Dumas et al., 2002, Hara- Chikuma et al., 2009, Kim and Lee, 2010, Nakahigashi et al., 2011, Serna et al., 2014, Zheng et al., 2003). Studies characterizing AQP3 knockout mice suggest that this aquaglyceroporin is also required for normal skin hydration, barrier recovery, wound healing and elasticity (Hara et al., 2002, Hara and Verkman, 2003, Ma et al., 2002). Further, these reports suggest that it is the glycerol rather than the water transported by AQP3 that is important for these functions (Hara et al., 2002, Hara and Verkman, 2003). AQP3 has also been linked to various human skin diseases, such as atopic dermatitis (Boury-Jamot et al., 2006, Olsson et al., 2006), vitiligo (Kim and Lee, 2010), cutaneous pruritus (Ikarashi et al., 2012), and psoriasis (Qin et al., 2011). Indeed, in psoriatic lesions, AQP3 immunoreactivity is decreased (Lee et al., 2012) and/or mislocalized (Voss et al., 2011), suggesting that dysregulation of AQP3 might be involved in the etiology of psoriasis. It is speculated that induction of AQP3 expression may be a potential mechanism for treating dry skin conditions (Draelos, 2012), with various agents shown to increase AQP3 expression (Aburada et al., 2011, Dumas et al., 2007, Pereda Mdel et al., 2010). Due to the importance of AQP3 in the epidermis, it is critical to understand the mechanisms regulating its expression in keratinocytes.
Histone deacetylases (HDACs) are a class of enzymes that remove acetyl groups from lysine residues on core histones as well as a number of non-histone proteins including transcription factors and co-regulators, thereby regulating their activities (Grunstein, 1997). HDAC inhibitors block the action of HDACs to result in hyperacetylation of histones and other proteins thereby modulating gene expression (Dokmanovic et al., 2007). For example, hypoacetylated (via HDAC enzymes) chromatin is transcriptionally silent while hyperacetylated chromatin (via the action of histone acetyl transferases, HATs) is transcriptionally active. Various HDAC inhibitors are either Food and Drug Administration (FDA)-approved (e.g., Zolinza, Istodax, Valproate) or are in clinical trials for treating various diseases, mainly cancer (https://clinicaltrials.gov). Considering the important action of these HDAC inhibitors to suppress inflammation and angiogenesis and induce differentiation or apoptosis of hyperproliferative cancer cells, experts have proposed that HDAC inhibitors be considered for treating skin cancers and psoriasis (Dinarello et al., 2011, McLaughlin and La Thangue, 2004, Robertson et al., 2012, Shuttleworth et al., 2010).
Considering the likely involvement of AQP3 in various skin diseases and the possible therapeutic use of HDAC inhibitors for hyperproliferative disorders, our objective was to investigate the effect of HDACs and HDAC inhibitors on AQP3 levels. Using various HDAC inhibitors and genetic manipulations, we show that AQP3 is inhibited basally by HDAC3 and that p53 mediates, at least part, HDAC inhibition-induced increase in AQP3 levels.
RESULTS
HDAC inhibitors increase AQP3 expression
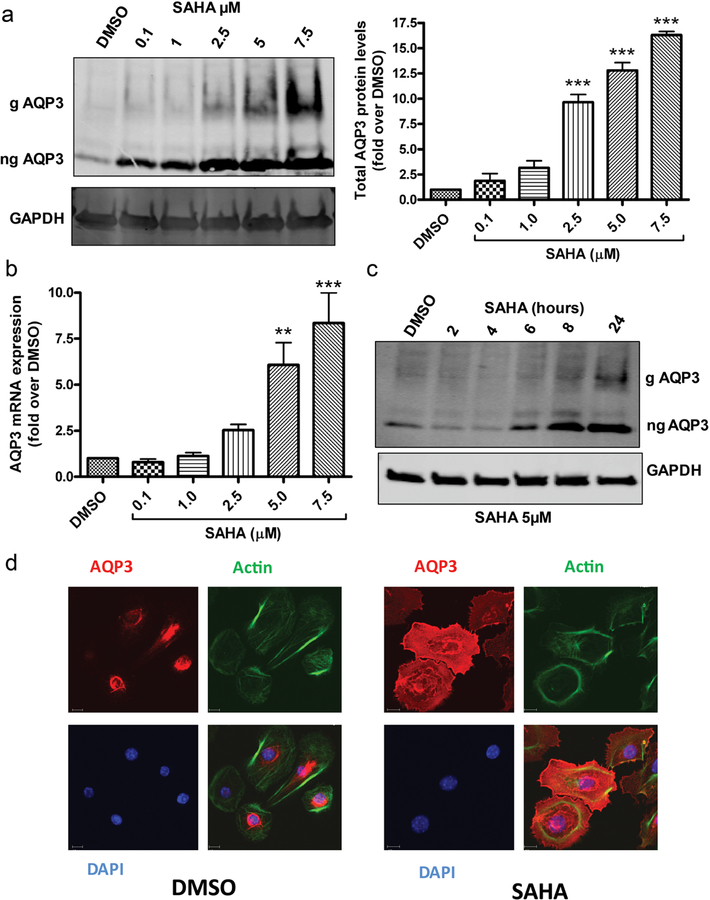
Primary cultures of keratinocytes from neonatal (1- to 3-day-old) mice were treated with various concentrations of the pan-HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA) for 24h. AQP3 is visualized as two bands; by analogy with aquaporin-2 (AQP2) (Hendriks et al., 2004), the lower 28-kDa protein represents non-glycosylated AQP3, whereas the upper 37–40 kDa diffuse band is glycosylated. As shown in Figure 1a (left panel), both forms of AQP3 are increased with SAHA treatment. Quantitation of Western blots from multiple experiments indicated that SAHA increased AQP3 protein levels in a concentration-dependent manner (Figure 1a, right panel). We then examined the mRNA expression of AQP3 in SAHA-treated cells and observed a significant increase in AQP3 mRNA expression with increasing concentrations of SAHA after 24h of treatment (Figure 1b). The increase in AQP3 protein levels occurred also in a time-dependent manner (Figure 1c). We further confirmed our results using immunocytochemistry showing increased staining of AQP3 in mouse keratinocytes treated for 24h with SAHA (5µM), which also induced actin cytoskeletal reorganization (Figure 1d), consistent with previous reports (Koppaka et al., 2015, Nikkhah et al., 2011). These results suggest that AQP3 expression is basally repressed by one or more HDACs in normal proliferating mouse keratinocytes such that treatment with the pan-HDAC inhibitor, SAHA, results in increased AQP3 mRNA and protein levels. Interestingly, SAHA was able to increase AQP3 levels in other epithelial cells as well, including MCF7 breast cancer cells, SW480 colon cancer cells and HeLa cervical cancer cells (Supplemental Figure 1). However, SAHA treatment did not increase AQP3 levels in DU145 or LNCaP prostate cancer cell lines (data not shown), suggesting tissue specificity in AQP3 regulation by the pan-HDAC inhibitor.
Figure 1. The pan-HDAC inhibitor, SAHA, dose- and time-dependently increases AQP3 expression in primary mouse epidermal keratinocytes.
Mouse keratinocytes were treated with vehicle (DMSO) or SAHA for 24h or as indicated. (a) Representative Western blot showing AQP3 and GAPDH levels. The right panel shows quantification and cumulative values (means±SEM; n=3). (b) Quantitative RT-PCR analysis for AQP3 from at least 3 independent experiments was performed using the ΔΔCt method with GAPDH as the endogenous control; results represent the means±SEM of groups presented relative to maximal response. (c) A representative (n=3) Western blot for AQP3 levels in keratinocytes treated with SAHA for the indicated time points (d) Keratinocytes were treated with 5µM SAHA or DMSO for 24h. Cells were fixed and immunostained using antibodies recognizing AQP3 (red) and β-actin (green) with nuclei counterstained with DAPI (blue). g= glycosylated; ng= non-glycosylated; **p<0.01, ***p<0.001 versus control.
SAHA increased AQP3 levels in mouse skin organ culture, in situ
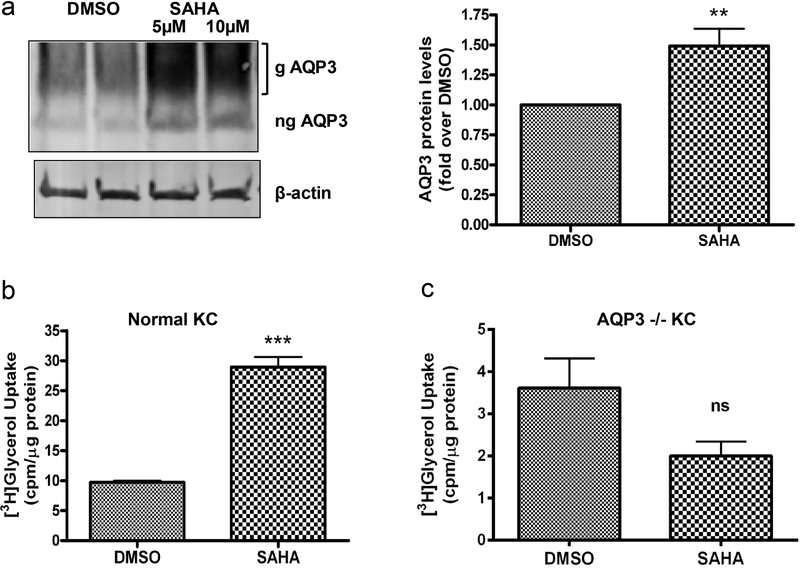
To examine the ability of SAHA to affect AQP3 levels in a more physiologically relevant three- dimensional system, we harvested skin from neonatal (1- to 3-day-old) mice, split the skin in half lengthwise and incubated one half with medium containing DMSO (the vehicle control) and one half with medium containing 5 or 10µM SAHA for 24h. We then homogenized the skin and determined AQP3 protein expression by Western analysis. As shown in Figure 2a, left panel, AQP3 protein (both unglycosylated and glycosylated) was expressed in skin (Voss et al., 2011), and SAHA was able to increase AQP3 levels in this skin organ culture system in situ.
Figure 2. SAHA increases AQP3 levels in situ and AQP3 activity in vitro.
(a) Neonatal mouse skin incubated with medium containing DMSO or SAHA for 24h. A representative (n=3) Western blot for AQP3 levels is shown (left panel). In the right panel, AQP3 levels were normalized to β-actin and expressed as fold over the DMSO-treated group; cumulative results from at least 3 separate skins using DMSO or 5µM SAHA are presented as means±SEM; **p<0.01 versus the DMSO group. (b, c) Keratinocytes from wild-type (b) and AQP3 knockout (c) mice were treated with DMSO or 5µM SAHA for 24h. AQP3 functionality was assessed by [3H]glycerol uptake assay. Please note the change in x-axis (panel b versus c). The data represent means±SEM from 3 independent experiments; ***p<0.001 versus DMSO-treated keratinocytes. g= glycosylated; ng= non-glycosylated.
Quantitation of multiple experiments confirmed a significant SAHA-induced increase in AQP3 protein levels in skin in situ (Figure 2a, right panel).
SAHA-induced AQP3 upregulation resulted in increased glycerol uptake in normal but not in AQP3 knockout keratinocytes
In order to determine if the AQP3 induced by SAHA is functional, we measured radiolabeled glycerol uptake by SAHA-exposed keratinocytes. SAHA treatment of normal keratinocytes resulted in a significant increase in [3H]glycerol uptake by the cells (Figure 2b); however, SAHA treatment of AQP3 knockout keratinocytes did not increase radiolabeled glycerol uptake (Figure 2c), demonstrating the involvement of AQP3 in the measured [3H]glycerol uptake. These results indicate that the AQP3 induced by SAHA in wild-type keratinocytes is functional. As expected, AQP3 knockout cells also exhibited reduced glycerol uptake under basal conditions (Figure 2b and c).
We have previously proposed a role for AQP3 in promoting keratinocyte differentiation, such that re-expression of AQP3 in AQP3 knockout keratinocytes enhanced mRNA and protein expression of both early and late differentiation markers, either alone or in conjunction with a differentiating agent (Choudhary et al., 2015). Indeed, the HDAC inhibitor trichostatin A has previously been shown to induce a marker of intermediate differentiation (involucrin) without affecting the expression of the late marker loricrin and even suppressing another late marker (profilaggrin) (Markova et al., 2007). To examine the potential role of AQP3 in SAHA’s effects, we examined the effect of the HDAC inhibitor on proliferation and differentiation in wild-type and AQP3 knockout keratinocytes. As shown in Supplemental Figure 2, we found that SAHA decreased expression of proliferation markers and increased the expression of differentiation markers. In each case SAHA was less potent in its action on some markers in AQP3 knockout keratinocytes. This result suggests that HDAC inhibition can promote keratinocyte differentiation with its potency on some aspects of differentiation determined, at least in part, by its ability to promote AQP3 expression.
The HDAC3-selective inhibitor, RGFP966, induced AQP3 expression
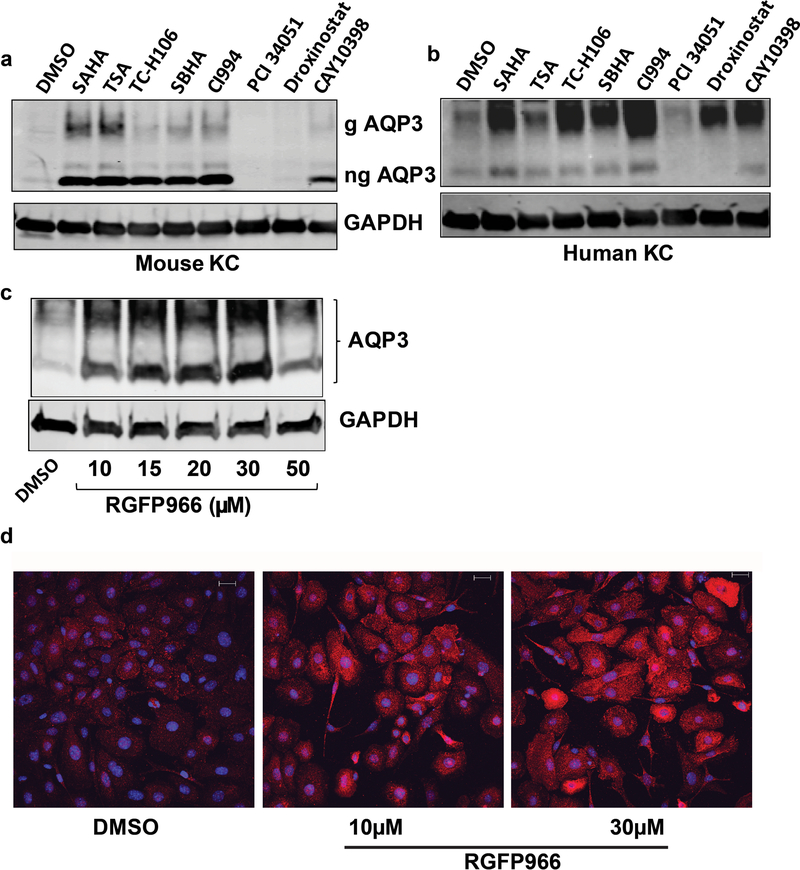
SAHA is a pan-HDAC inhibitor; therefore, it is not clear which HDAC represses basal AQP3 expression and is inhibited by SAHA to upregulate AQP3. To delineate the potentially relevant HDAC(s), we treated keratinocytes with additional inhibitors that selectively target different HDACs. Initially, we performed dose response experiments to determine the appropriate concentrations (Supplemental Figure 3). With these doses, and as shown in Figure 3a, we found that, like SAHA, trichostatin A (TSA), TC-H106, SBHA and CI994, which are reported to inhibit HDAC1 and HDAC3, also increased AQP3 protein expression, whereas PCI 34051, which does not inhibit either HDAC1 or HDAC3, and droxinostat, which is not thought to inhibit HDAC1 and only weakly inhibits HDAC3, did not. Together these results suggest that HDAC1, HDAC2 and/or HDAC3 is/are likely to be the HDAC(s) that normally repress(es) AQP3 expression in proliferating keratinocytes. Similar results were obtained in normal human epidermal keratinocytes (Figure 3b).
Figure 3. HDAC3 regulates AQP3 levels.
Mouse (a) or human (b) keratinocytes (KC) were treated for 24h with vehicle (DMSO) or HDAC inhibitors targeting different HDACs: 5µM SAHA, 300nM trichostatin A (TSA), 50µM TC-H106, 50µM suberoyl bis-hydroxamic acid (SBHA), 50µM CI994, 50µM PCI 34051, 50µM droxinostat, or 50µM CAY10398 as indicated. Glycosylated (g) and non-glycosylated (ng) AQP3 protein levels were analyzed by Western blotting. Blots shown are representative of 3 separate experiments. (c) Primary mouse keratinocytes were treated for 24h with the HDAC3 inhibitor RGFP966 or the control vehicle (DMSO) and analyzed by Western blotting for levels of AQP3 compared to the loading control, GAPDH. Results shown are representative of 3 separate experiments. (d) Primary mouse keratinocytes grown on cover slips were treated as above. At the end of 24h of treatment cells were fixed and immunostained using an antibody recognizing AQP3 (red) with nuclei counterstained with DAPI (blue).
To determine if inhibition of HDAC3 increased AQP3 levels, keratinocytes were treated with various concentrations of the HDAC3-selective inhibitor, RGFP966 (Leus et al., 2016), for 24h and AQP3 levels were determined by Western analysis and immunostaining. As shown in Figure 3c, RGFP966 treatment dose-dependently increased AQP3 levels. AQP3 immunostaining also intensified with RGFP966 treatment, further suggesting the involvement of HDAC3 in inhibition of AQP3 expression basally (Figure 3d).
Genetic manipulation of HDAC3 levels altered SAHA-induced AQP3 expression
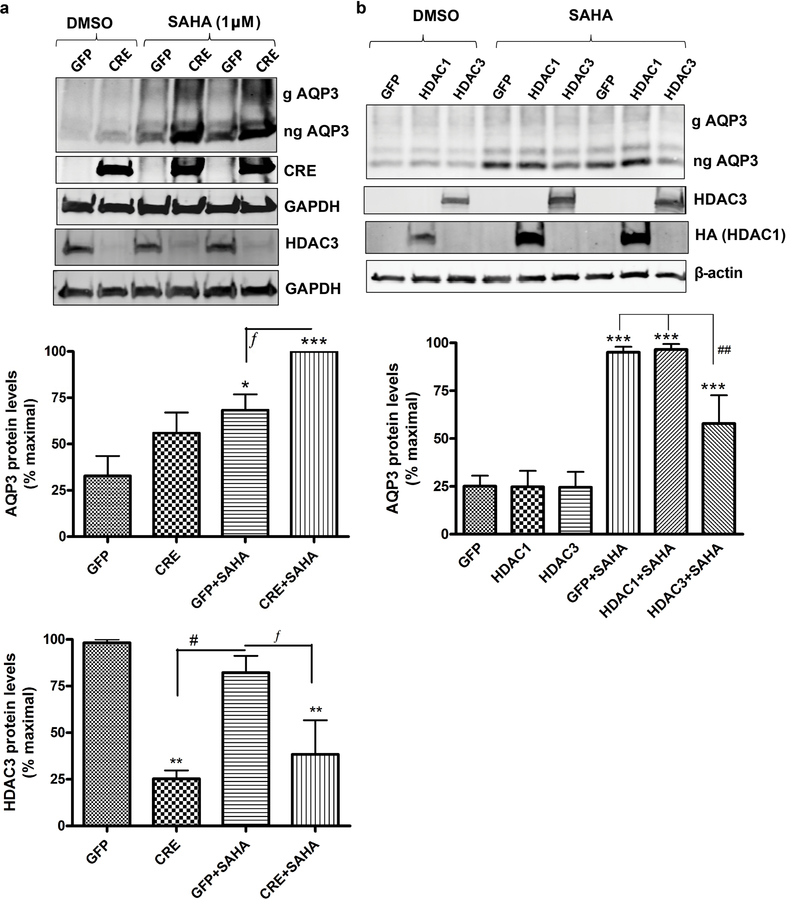
As inhibitors often have the disadvantage of a lack of specificity/selectivity, another approach to decrease HDAC3 activity in keratinocytes was used. Keratinocytes were prepared from transgenic neonatal mice in which the HDAC3 gene is flanked by loxP sites (McGee-Lawrence et al., 2015). These cells were then infected with GFP-expressing (control) adenovirus and adenovirus expressing Cre-recombinase (to delete the HDAC3 gene), as in (Choudhary et al., 2014). Infection with Cre-expressing adenovirus significantly decreased HDAC3 protein expression in the floxed keratinocytes, reducing protein levels to about 25–30% of the GFP- expressing control cells (Figure 4a, upper and lower panels). However, approximately a third of the protein remained (and there may have been even more activity persisting depending on whether or not cells attempted to compensate for the protein loss). Indeed, inhibition of the remaining HDAC3 activity with SAHA resulted in an enhancement of AQP3 levels (upper and middle panels). q-RT-PCR results also confirmed the increase in AQP3 mRNA expression induced by SAHA treatment in keratinocytes in which HDAC3 mRNA levels were decreased (Supplemental Figure 4).
Figure 4. HDAC3 knockdown increases and HDAC3 overexpression decreases AQP3 expression.
Keratinocytes from neonatal floxed HDAC3 mice were infected with Cre- recombinase (CRE)- or control (GFP)-expressing adenovirus for 24h. 57h post-infection the cells were treated with DMSO or 1µM SAHA for 15h. (a) Representative Western blots (upper panel) and quantitation (lower panels), with cumulative values expressed relative to the maximum response as means±SEM from 3 separate experiments. (b) Keratinocytes (wild-type) were infected with adenovirus expressing GFP (control), HDAC3 or HA-tagged HDAC1 for 12h and treated with DMSO or 2.5µM SAHA for 10h. Representative Western blots (upper panel) and quantitation of AQP3 levels (lower panel; n=3) as in (a) is shown. ***p<0.001 versus the control (GFP); fp<0.05, ##p<0.01, #p<0.05 versus the indicated groups. g= glycosylated; ng= non- glycosylated.
We also examined the effect of adenovirus-mediated overexpression of HDAC3 (or HDAC1) on SAHA-stimulated AQP3 levels. As shown in Figure 4b, we verified the overexpression of the two HDACs. Basal AQP3 expression is low, making it difficult to observe further reductions with HDAC3 overexpression. However, overexpression of HDAC3, but not HDAC1, reduced the ability of SAHA to increase AQP3 levels in mouse keratinocytes, with a significant reduction in SAHA-induced AQP3 levels in HDAC3-overexpressing, but not HDAC1-overexpressing, keratinocytes (Figure 4b lower panel). This result suggests that HDAC3 is the HDAC that basally represses AQP3 levels in normal proliferating keratinocytes.
Increasing or decreasing p53 activity/levels increased or decreased, respectively, SAHA- induced AQP3 expression in keratinocytes
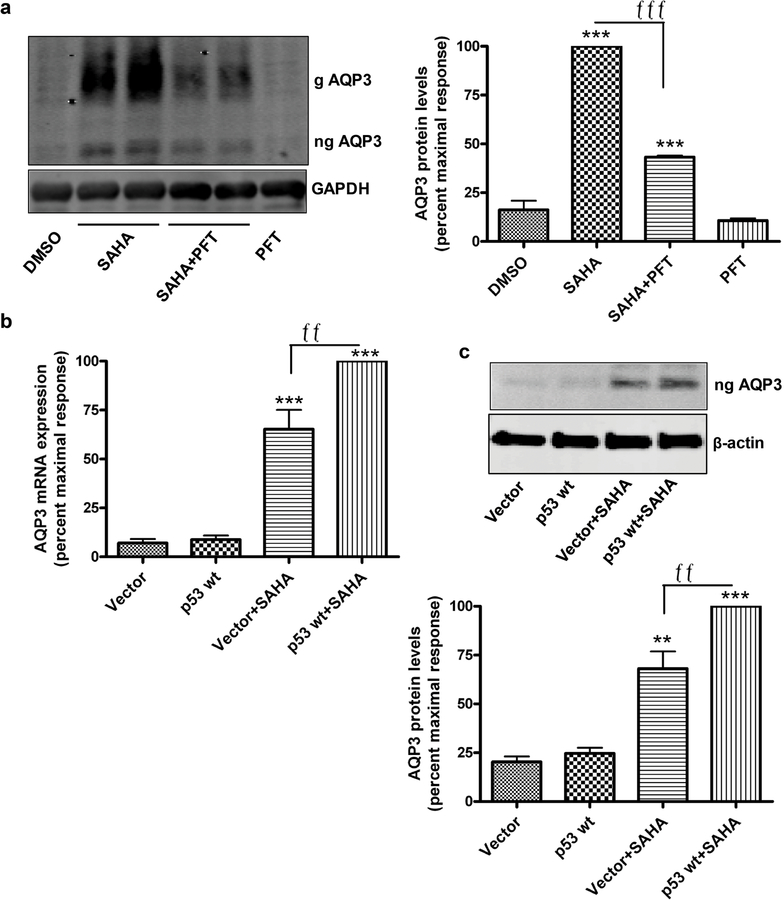
A previous report has demonstrated the presence of a p53 response element in the AQP3 promoter such that members of the p53 family, including also p63 and p73, can induce AQP3 expression (Gu et al., 2008, Ratovitski, 2013, Zheng and Chen, 2001). In addition, p53 is known to be acetylated, with this post-translational modification enhancing its transcriptional activity (Li et al., 2002, Luo et al., 2004). Therefore, we hypothesized the potential involvement of p53 in the ability of HDAC inhibition to induce AQP3 expression. To examine this idea, mouse keratinocytes were treated with or without SAHA (2.5µM) in the presence or absence of an inhibitor of p53/p73 (Davidson et al., 2008), pifithrin, for 24h and harvested for Western analysis. As shown in Figure 5a, pifithrin (30µM) significantly inhibited SAHA-induced AQP3 protein expression, while exerting little or no effect on AQP3 levels alone, suggesting the possibility that HDACs regulate AQP3 levels through their ability to modulate the transcriptional activity of one or more p53 family members. A similar effect was observed with 10µM pifithrin (data not shown).
Figure 5. p53 mediates SAHA-increased AQP3 levels in keratinocytes.
(a) Mouse keratinocytes were treated with DMSO or 5µM SAHA in the presence and absence of 30µM pifithrin (PFT) for 24h. Representative Western blot for AQP3 (left panel) and quantitation of 3 experiments (right panel) are shown. AQP3 levels normalized to GAPDH and expressed relative to the maximal response are presented as means±SEM; ***p<0.001 versus DMSO and fffp<0.001 as indicated. (b,c) Mouse keratinocytes were infected with adenovirus expressing wild-type p53 or vector for 12h and treated with 5µM SAHA for 12h. AQP3 mRNA expression analyzed by qPCR (b) and protein levels by Western blots (c, upper panel) with quantitation of 3 experiments are shown (c, lower panel). Results presented as means±SEM are expressed relative to the maximal response. **p<0.01 versus vector, ffp<0.01 as indicated.
Since pifithrin inhibits both p53 and p73, we used an overexpression approach to dissect the role of p53 versus p73, determining the effect of adenoviral infection with wild-type p53 on the induction of AQP3 by SAHA in keratinocytes. Cells were infected with empty virus or virus expressing wild-type p53 for 12h, and after treatment for 12h with or without SAHA, AQP3 levels were determined by Western analysis. As shown in Figure 5b and c, although wild-type p53 overexpression had no effect alone on AQP3 levels, p53 enhanced the ability of SAHA to increase AQP3 mRNA and protein levels. The ability of acetylation to increase p53’s transcriptional activity (Luo et al., 2004) may explain this result, with SAHA inducing overexpressed p53 activation by enhancing acetylation. These data suggest that acetylated p53, or a p53 family member, is a regulator of AQP3 levels.
DISCUSSION
AQP3 is a protein involved in the normal processes of keratinocyte proliferation and differentiation and is thus an important regulator of skin homeostasis, with gene deletion in transgenic mice resulting in, for example, delayed wound healing and barrier repair (Hara et al., 2002). Our recent study, in which AQP3 was re-expressed in AQP3 knockout keratinocytes to result in an increase in differentiation markers, suggests a pro-differentiation role for AQP3 in mouse keratinocytes (Choudhary et al., 2015). In addition, ours and others’ previous studies have suggested that AQP3 is reduced (Lee et al., 2012) and/or mislocalized (Voss et al., 2011) in psoriatic lesions, suggesting that a dysregulation of AQP3 might be involved in the etiology of psoriasis. Using various research techniques, we have determined that HDAC3 represses AQP3 expression basally such that treatment of keratinocytes with a pan-HDAC inhibitor like SAHA, or an HDAC3 inhibitor specifically, increased AQP3 expression. Further, we identified p53 as an important mediator of this HDAC inhibitor-induced AQP3 expression.
HDAC inhibitors represent a potential new class of therapeutic agents initially developed for the treatment of cancers, because they can inhibit cell proliferation and induce differentiation (Tang et al., 2013). In fact, Vorinostat (suberoylanilide hydroxamic acid or SAHA) is already approved by the FDA for the treatment of cutaneous T-cell lymphoma. For the last decade HDAC inhibitors have been proposed as potential therapeutic agents for treating psoriasis and other skin conditions due to their anti-proliferative and anti-inflammatory activities (McLaughlin and La Thangue, 2004, Shuttleworth et al., 2010). Indeed, HDAC1 was found to be overexpressed in psoriatic lesions, suggesting the possibility of direct implementation of HDAC inhibitors in psoriasis treatment (Tovar-Castillo et al., 2007). Recently, resveratrol, via inhibition of the class III HDAC, Sirt1, has been shown to have beneficial effects on the psoriasiform phenotype induced by imiquimod in a mouse model of psoriasis (Xie et al., 2015). However, few studies have investigated the utility of HDAC inhibitors in the treatment of psoriasis (Ekman and Enerback, 2016). Our data demonstrating that HDACs regulate the expression of AQP3, an important protein for normal skin function, suggest that further studies are warranted.
SAHA inhibits a large number of class I and class II HDACs. These individual HDACs have different functions in different tissues. It was therefore important to identify which HDAC is involved in regulating AQP3 levels. We utilized various HDAC inhibitors that have been reported to inhibit one or more class I or II HDACs. These inhibitor studies narrowed our search to three HDACs, HDAC1, HDAC2 and/or HDAC3. Utilizing a gain-of-function approach we discovered that overexpression of HDAC3, but not HDAC1, inhibited the SAHA-induced increase in AQP3 levels. Surprisingly, CAY10398, an inhibitor that is reported to be selective for HDAC1 (https://www.caymanchem.com/Product.vm/catalog/89740),) increased AQP3 levels, although overexpression of HDAC1 did not affect SAHA-induced AQP3 expression. This result highlights one of the caveats of chemical inhibitors, that is, their lack of specificity/selectivity, and we postulate that this compound may also inhibit other HDACs (likely HDAC3) at concentrations of 1µM or above. Further, utilizing a more selective inhibitor for HDAC3, RGFP966, and a loss-of-function approach using Cre-LoxP technology in floxed HDAC3 keratinocytes, we confirmed HDAC3 as the likely HDAC that regulates AQP3. Interestingly, knockdown of HDAC3 alone produced only a moderate increase in AQP3 expression, which did not reach statistical significance; however, combining SAHA treatment with reduced HDAC3 levels resulted in a significant enhancement of AQP3 mRNA and protein levels in keratinocytes. This result is consistent with the fact that infection with Cre-recombinase-expressing adenovirus did not completely ablate HDAC3, such that inhibition of the remaining HDAC3 with a lower 2.5µM concentration of SAHA was required for a maximal response. Studies on the role of HDAC3 in skin are limited although one study identified HDAC3 as a target for the development of therapies for allergic skin inflammation disorders (Kim et al., 2012), suggesting the possibility of a beneficial role for AQP3 in this particular condition.
HDAC inhibitors act not only to deacetylate histone proteins to inhibit transcription of various genes but also affect the acetylation of several transcription factors. In fact, SAHA has been shown to promote the acetylation of various transcription factors, such as p21WAF1, p53 and TBP- 2, as well as other proteins like gelsolin, metallothionein 1L, and others (Glaser et al., 2003, Grunstein, 1997). Since previous reports indicated the presence of p53 response elements in the AQP3 promoter (Zheng and Chen, 2001), we examined p53 as a possible transcription factor involved in AQP3 expression in keratinocytes. Our results using two different approaches supported the involvement of p53 in SAHA-increased AQP3 levels. The p53 inhibitor used in one approach, pifithrin, can also inhibit p73 at higher concentrations (Codelia et al., 2010, Davidson et al., 2008). However, using two doses of pifithrin, with the lower dose (10µM) expected to inhibit only p53 and not p73, we observed similar inhibition of SAHA-induced AQP3 protein expression. Thus, our study suggested p53, rather than p73, as the p53 family member regulating AQP3 expression. Interestingly, we observed no effect of p53 overexpression alone to induce AQP3 protein expression; this result is consistent with the idea that acetylation of p53 stabilizes and promotes the transcriptional activity of the protein (Brooks and Gu, 2011, Lavin and Gueven, 2006, Luo et al., 2004), such that overexpression of p53 alone was not sufficient for AQP3 induction but required inhibition of HDAC activity for manifestation. Alternatively, enhanced acetylation of other proteins, for example, histones or proteins of the transcriptional complex, may be needed for p53 to effectively promote AQP3 expression.
In addition to the p53 family of transcription factors, there are several other factors that have been implicated in AQP3 regulation, including PPARgamma (Jiang et al., 2011), TNFalpha (Horie et al., 2009), Notch1 (Guo et al., 2013), and CLOCK/BMAL1 (Takase et al., 2011), among others. Micro-RNAs are also reported to regulate AQP3 expression in various tissues. In larynx-derived SCC-11 cells, phosphorylated-deltaNp63alpha was shown to upregulate miR- 185–5p and downregulate let7–5p, which subsequently modulated AQP3 through its 3’- untranslated region (Ratovitski, 2013). In other studies, miR-874 decreased AQP3 expression in Caco-2 and gastric cancer cells by targeting its 3’ untranslated region (Jiang et al., 2014, Zhi et al., 2014).
We and others have previously reported a pro-differentiative role for AQP3 in keratinocytes and skin (Bollag et al., 2007, Choudhary et al., 2015, Dumas et al., 2002, Kim and Lee, 2010, Zheng and Bollag, 2003). Further, we suggested a signaling partner, phospholipase D2 (PLD2), as an important mediator of AQP3’s pro-differentiative role (Choudhary et al., 2015), with the association of AQP3 and PLD2 thought to be important for induction of keratinocyte differentiation. In addition, for another aquaporin, AQP2, glycosylation is required for its surface localization (Hendriks et al., 2004); by analogy with AQP2, glycosylated AQP3 likely represents mature, active AQP3 at the plasma membrane. Our immunocytochemistry experiments confirmed that SAHA can increase both membranous (presumably glycosylated) and cytoplasmic (thought to be non-glycosylated) AQP3, and treatment with SAHA resulted in increased glycerol uptake by keratinocytes from wild-type but not from AQP3 knockout mice. These results thus confirmed the functional transport activity of the SAHA-induced AQP3. It should be noted that glycosylated, but not non-glycosylated, AQP3 levels were increased in droxinostat-treated human keratinocytes whereas droxinostat had no effect on AQP3 levels (non- glycosylated or glycosylated) in mouse keratinocytes. This result suggests that droxinostat promotes AQP3 glycosylation, presumably increasing plasma membrane-localized, mature AQP3, suggesting its possible utility for therapy of diseases in which AQP3 is mislocalized. Further, this result suggests that one or more histone acetyl transferases and/or HDACs may regulate AQP3 glycosylation, with different HDAC profiles likely responsible for the (few) disparities observed between mouse and human keratinocytes. Although beyond the scope of this manuscript, further research is necessary to address this possible mechanism of post-translational regulation of AQP3 activity.
Our present results provide evidence that HDAC3 represses AQP3 expression basally, suggesting the possibility that HDAC inhibition could be useful in treating various skin diseases in which AQP3 levels are decreased, such as psoriasis (Lee et al., 2012). The HDAC inhibitor SAHA has known anti-proliferative properties further supporting its use in psoriasis therapy. Indeed, we observed an ability of SAHA to inhibit the expression of some markers of keratinocyte proliferation and increase the expression of some differentiation-associated genes, and this effect was partially determined by its ability to increase AQP3 levels, since SAHA exhibited lower potency to modulate expression of some of these markers in AQP3 knockout cells. Nevertheless, lack of AQP3 did not completely prevent the SAHA-induced changes in expression in keratinocyte markers. This fact, in addition to the disparity between the differentiation induced by SAHA-mediated HDAC inhibition and that elicited by AQP3 overexpression (Choudhary et al., 2015), suggests additional effects of HDACs in regulating keratinocyte function. This result is not unexpected given the large number of protein substrates targeted by HDACs, including histones that control chromatin accessibility and gene transcription. Our results therefore suggest that SAHA, already in clinical use to treat cutaneous T-cell lymphoma, might be quickly repurposed as a psoriasis therapy. Nevertheless, SAHA’s ability to influence AQP3 levels in other epithelial cells (Supplemental Figure 1), as well as data in the literature supporting a pro-proliferative role of this aquaglyceroporin in various epithelia (e.g., (Chen et al., 2014, Hou et al., 2016, Nakahigashi et al., 2011) suggest that caution should be exercised with SAHA’s application to the treatment of skin disorders.
MATERIALS AND METHODS
For detailed Materials and Methods, please see Supplemental Materials.
Cell culture and experimental design
All animal protocols were approved by the Augusta University or Charlie Norwood VA Medical Center Institutional Animal Care and Use Committees and were conducted according to NIH guidelines for the Care and Use of Laboratory Animals. Primary epidermal mouse keratinocytes were prepared from neonatal (1–3 day old) CD1, AQP3 knockout (Ma et al., 2002) and/or floxed HDAC3 mice (McGee-Lawrence et al., 2015) and cultured in K-SFM containing 50µM CaCl2 as described previously (Choudhary et al., 2015). Adult normal human keratinocytes (NHEK #192627) were obtained from Lonza, Inc. and were sub-cultured in KBMGold as in (Helwa et al., 2015). Experiments were performed on 70–80% confluent keratinocytes.
Adenoviral infection
The GFP-tagged adenovirus expressing Cre-recombinase was purchased from Vector Biolabs (Philadelphia, PA), the HDAC3-expressing adenovirus from SignaGen Laboratories (Gaithersburg, MD, USA) and the HA-tagged HDAC1 expressing adenovirus (Ad-hHDAC1- HA) from Applied Biological Materials (Canada). The wild-type p53-expressing adenovirus was kindly provided by Dr. Shuang Huang (Augusta University, Augusta, GA). Amplification and purification of viruses were performed as described earlier (Arun et al., 2011, Shapiro et al., 2010).
For adenoviral infection, keratinocytes from floxed HDAC3 neonatal mice were infected with adenoviruses expressing either Cre-recombinase or GFP as described earlier (Choudhary et al., 2015). Virus-containing media was removed 24h post-infection and replaced with fresh K-SFM. For overexpression studies, keratinocytes were infected with adenovirus expressing GFP, HDAC3, HA-tagged HDAC1 or wild-type p53 for 12h.
Western blot analysis
Western analysis was performed as described previously (Choudhary et al., 2015).
Immunocytochemistry
Immunocytochemistry was performed as described previously (Qin et al., 2010).
In-situ culture
Skin was harvested from newborn wild-type CD1 mice, treated as described in legends and processed for Western analysis.
Quantitative RT-PCR (qRT-PCR) analysis
qRT-PCR reaction was performed and analyzed by the delta-delta Ct method as previously described (Choudhary et al., 2015).
[3H]Glycerol uptake assay
Radiolabeled glycerol uptake assays were performed as in (Choudhary et al., 2015).
Statistical Analysis
Data from at least three independent experiments are presented as means±SEM. Unpaired, two- tailed t-tests were used to analyze differences between two groups; for more than two groups, one-way analysis of variance with Newman-Keuls multiple comparison post-hoc tests was used (GraphPad Prism, La Jolla, CA).
Supplementary Material
ACKNOWLEDGEMENTS:
This work was supported in part by VA Merit Award #I01CX001357 to WBB and in part by an intramural Early Success Award from Augusta University to WBB. WBB was supported by a VA Research Career Scientist Award. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. Generation and maintenance of Hdac3 floxed mice was supported by the American Diabetes Association Award (1–16-JDF-062) to MML. Both KK and AC were supported by Alpha Omega Alpha Carolyn L. Kuckein Student Research fellowships. LM was a fellow of the Medical College of Georgia’s Medical Scholars Program.
Footnotes
CONFLICT OF INTEREST: The authors state no conflict of interest.
REFERENCES
- Aburada T, Ikarashi N, Kagami M, Ichikawa Y, Sugitani M, Maniwa A, et al. Byakkokaninjinto prevents body water loss by increasing the expression of kidney aquaporin-2 and skin aquaporin-3 in KKAy mice. Phytother Res 2011;25(6):897–903. [DOI] [PubMed] [Google Scholar]
- Arun SN, Kaddour-Djebbar I, Shapiro BA, Bollag WB. Ultraviolet B irradiation and activation of protein kinase D in primary mouse epidermal keratinocytes. Oncogene 2011;30:1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag WB, Xie D, Zheng X, Zhong X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol 2007;127(12):2823–2831. [DOI] [PubMed] [Google Scholar]
- Boury-Jamot M, Sougrat R, Tailhardat M, Le Varlet B, Bonte F, Dumas M, et al. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim Biophys Acta 2006;1758:1034–1042. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell 2011;2(6):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang T, Zhou YC, Gao F, Zhang ZH, Xu H, et al. Aquaporin 3 promotes epithelial- mesenchymal transition in gastric cancer. J Exper Clin Cancer Res 2014;33:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Olala LO, Kaddour-Djebbar I, Helwa I, Bollag WB. Protein kinase D1 deficiency promotes differentiation in epidermal keratinocytes. J Dermatol Sci 2014;76:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Olala LO, Qin H, Helwa I, Pan ZQ, Tsai YY, et al. Aquaporin-3 re-expression induces differentiation in a phospholipase D2-dependent manner in aquaporin-3- knockout mouse keratinocytes. J Invest Dermatol 2015;135(2):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codelia VA, Cisterna M, Alvarez AR, Moreno RD. p73 participates in male germ cells apoptosis induced by etoposide. Mol Hum Reprod 2010;16(10):734–742. [DOI] [PubMed] [Google Scholar]
- Davidson W, Ren Q, Kari G, Kashi O, Dicker AP, Rodeck U. Inhibition of p73 function by Pifithrin-alpha as revealed by studies in zebrafish embryos. Cell Cycle 2008;7(9):1224–1230. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Fossati G, Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med 2011;17(5–6):333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 2007;5(10):981–989. [DOI] [PubMed] [Google Scholar]
- Draelos Z Aquaporins: an introduction to a key factor in the mechanism of skin hydration. J Clin Aesthet Dermatol 2012;5(7):53–56. [PMC free article] [PubMed] [Google Scholar]
- Dumas M, Gondran C, Barre P, Sougrat R, Verbavatz JM, Heusele C, et al. Effect of an Ajuga turkestanica extract on aquaporin 3 expression, water flux, differentiation and barrier parameters of the human epidermis. Eur J Dermatol 2002;12(6):XXV–XXVI. [PubMed] [Google Scholar]
- Dumas M, Sadick NS, Noblesse E, Juan M, Lachmann-Weber N, Boury-Jamot M, et al. Hydrating skin by stimulating biosynthesis of aquaporins. J Drugs Dermatol 2007;6(6 Suppl):s20–s24. [PubMed] [Google Scholar]
- Ekman AK, Enerback C. Lack of preclinical support for the efficacy of histone deacetylase inhibitors in the treatment of psoriasis. Br J Dermatol 2016;174(2):424–426. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther 2003;2(2):151–163. [PubMed] [Google Scholar]
- Grunstein M Histone acetylation in chromatin structure and transcription. Nature 1997;389(6649):349–352. [DOI] [PubMed] [Google Scholar]
- Gu X, Coates PJ, Boldrup L, Nylander K. p63 contributes to cell invasion and migration in squamous cell carcinoma of the head and neck. Cancer Lett 2008;263(1):26–34. [DOI] [PubMed] [Google Scholar]
- Guo L, Chen H, Li Y, Zhou Q, Sui Y. An aquaporin 3-notch1 axis in keratinocyte differentiation and inflammation. PLoS One 2013;8(11):e80179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3 deficient mice may account for impaired skin hydration, elasticity and barrier recovery. J Biol Chem 2002;277:46616–46621. [DOI] [PubMed] [Google Scholar]
- Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci USA 2003;100:7360–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Satooka H, Watanabe S, Honda T, Miyachi Y, Watanabe T, et al. Aquaporin- 3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nature Commun 2015;6:7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Takahashi K, Chikuma S, Verkman AS, Miyachi Y. The expression of differentiation markers in aquaporin-3 deficient epidermis. Arch Dermatol Res 2009;301:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwa I, Patel R, Karempelis P, Kaddour-Djebbar I, Choudhary V, Bollag WB. The antipsoriatic agent monomethylfumarate has antiproliferative, prodifferentiative, and anti- inflammatory effects on keratinocytes. J Pharmacol Exp Ther 2015;352(1):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks G, Koudijs M, van Balkom BW, Oorschot V, Klumperman J, Deen PM, et al. Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J Biol Chem 2004;279(4):2975–2983. [DOI] [PubMed] [Google Scholar]
- Horie I, Maeda M, Yokoyama S, Hisatsune A, Katsuki H, Miyata T, et al. Tumor necrosis factor- alpha decreases aquaporin-3 expression in DJM-1 keratinocytes. Biochem Biophys Res Comm 2009;387(3):564–568. [DOI] [PubMed] [Google Scholar]
- Hou SY, Li YP, Wang JH, Yang SL, Wang Y, Wang Y, et al. Aquaporin-3 inhibition reduces the growth of NSCLC cells induced by hypoxia. Cell Physiol Biochem 2016;38(1):129–140. [DOI] [PubMed] [Google Scholar]
- Ikarashi N, Ogiue N, Toyoda E, Kon R, Ishii M, Toda T, et al. Gypsum fibrosum and its major component CaSO4 increase cutaneous aquaporin-3 expression levels. J Ethnopharmacol 2012;139(2):409–413. [DOI] [PubMed] [Google Scholar]
- Jiang B, Li Z, Zhang W, Wang H, Zhi X, Feng J, et al. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J Gastroenterol 2014;49(6):1011–1025. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Kim P, Lu YF, Feingold KR. PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp Dermatol 2011;20:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N-H, Lee A-Y. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol 2010;130:2231–2239. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim K, Park D, Lee E, Lee H, Lee YS, et al. Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein. J Biol Chem 2012;287(31):25844–25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppaka V, Lakshman N, Petroll WM. Effect of HDAC inhibitors on corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Mol Vis 2015;21:502–514. [PMC free article] [PubMed] [Google Scholar]
- Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ 2006;13(6):941–950. [DOI] [PubMed] [Google Scholar]
- Lee Y, Je YJ, Lee SS, Li ZJ, Choi DK, Kwon YB, et al. Changes in transepidermal water loss and skin hydration according to expression of aquaporin-3 in psoriasis. Ann Dermatol 2012;24(2):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leus NG, van der Wouden PE, van den Bosch T, Hooghiemstra WT, Ourailidou ME, Kistemaker LE, et al. HDAC 3-selective inhibitor RGFP966 demonstrates anti- inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-kappaB p65 transcriptional activity. Biochem Pharmacol 2016;108:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. The Journal of biological chemistry 2002;277(52):50607–50611. [DOI] [PubMed] [Google Scholar]
- Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W. Acetylation of p53 augments its site- specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci USA 2004;101(8):2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Liu X, Liu J, Jiang M, Luo M, Zhao J. Activation of TGF-beta1 by AQP3-mediated H2O2 transport into fibroblasts of a bleomycin-induced mouse model of scleroderma. J Invest Dermatol 2016; 136(12):2372–2379. [DOI] [PubMed] [Google Scholar]
- Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem 2002;277:17147–17153. [DOI] [PubMed] [Google Scholar]
- Markova NG, Karaman-Jurukovska N, Pinkas-Sarafova A, Marekov LN, Simon M. Inhibition of histone deacetylation promotes abnormal epidermal differentiation and specifically suppresses the expression of the late differentiation marker profilaggrin. J Invest Dermatol 2007;127(5):1126–39. [DOI] [PubMed] [Google Scholar]
- McGee-Lawrence ME, White TA, LeBrasseur NK, Westendorf JJ. Conditional deletion of Hdac3 in osteoprogenitor cells attenuates diet-induced systemic metabolic dysfunction. Mol Cell Endocrinol 2015;410:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin F, La Thangue NB. Histone deacetylase inhibitors in psoriasis therapy. Curr Drug Targets Inflamm Allergy 2004;3(2):213–219. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K, Kabashima K, Ikoma A, Verkman AS, Miyachi Y, Hara-Chikuma M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J Invest Dermatol 2011;131:865–873. [DOI] [PubMed] [Google Scholar]
- Nikkhah M, Strobl JS, Schmelz EM, Roberts PC, Zhou H, Agah M. MCF10A and MDA-MB- 231 human breast basal epithelial cell co-culture in silicon micro-arrays. Biomaterials 2011;32(30):7625–7632. [DOI] [PubMed] [Google Scholar]
- Olsson M, Broberg A, Jernas M, Carlsson L, Rudemo M, Suurküla M, et al. Increased expression of aquaporin 3 in atopic eczema. Allergy 2006;61:1132–1137. [DOI] [PubMed] [Google Scholar]
- Pereda Mdel C, Dieamant Gde C, Eberlin S, Werka RM, Colombi D, Queiroz ML, et al. Expression of differential genes involved in the maintenance of water balance in human skin by Piptadenia colubrina extract. J Cosmet Dermatol 2010;9(1):35–43. [DOI] [PubMed] [Google Scholar]
- Qin H, Frohman MA, Bollag WB. Phospholipase D2 mediates acute aldosterone secretion in response to angiotensin II in adrenal glomerulosa cells. Endocrinology 2010;151:2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Zheng X, Zhong X, Shetty AK, Elias PM, Bollag WB. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch Biochem Biophys 2011;508:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratovitski EA. Phospho-DeltaNp63alpha regulates AQP3, ALOX12B, CASP14 and CLDN1 expression through transcription and microRNA modulation. FEBS Lett 2013;587(21):3581–3586. [DOI] [PubMed] [Google Scholar]
- Robertson ED, Weir L, Romanowska M, Leigh IM, Panteleyev AA. ARNT controls the expression of epidermal differentiation genes through HDAC- and EGFR-dependent pathways. J Cell Sci 2012;125(Pt 14):3320–3332. [DOI] [PubMed] [Google Scholar]
- Serna A, Galan-Cobo A, Rodrigues C, Sanchez-Gomar I, Toledo-Aral JJ, Moura TF, et al. Functional inhibition of aquaporin-3 with a gold-based compound induces blockage of cell proliferation. J Cell Physiol 2014; 229(11):1787–1801. [DOI] [PubMed] [Google Scholar]
- Shapiro BA, Olala L, Arun SN, Parker PM, George MV, Bollag WB. Angiotensin II-activated protein kinase D mediates acute aldosterone secretion. Mol Cell Endocrinol 2010;317:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth SJ, Bailey SG, Townsend PA. Histone deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Current Drug Targets 2010;11(11):1430–1438. [DOI] [PubMed] [Google Scholar]
- Takase T, Ishikawa H, Murakami H, Kikuchi J, Sato-Nara K, Suzuki H. The circadian clock modulates water dynamics and aquaporin expression in Arabidopsis roots. Plant Cell Physiol 2011;52(2):373–383. [DOI] [PubMed] [Google Scholar]
- Tang J, Yan H, Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clin Sci (Lond) 2013;124(11):651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Castillo LE, Cancino-Diaz JC, Garcia-Vazquez F, Cancino-Gomez FG, Leon-Dorantes G, Blancas-Gonzalez F, et al. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol 2007;46(3):239–246. [DOI] [PubMed] [Google Scholar]
- Verkman AS. A cautionary note on cosmetics containing ingredients that increase aquaporin-3 expression. Experimental dermatology 2008;17(10):871–872. [DOI] [PubMed] [Google Scholar]
- Voss KE, Bollag RJ, Fussell N, By C, Sheehan DJ, Bollag WB. Abnormal aquaporin-3 protein expression in hyperproliferative skin disorders. Arch Dermatol Res 2011;303:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Su Z, Zhang B, Ge J, Song S, Sun G, et al. SIRT1 Activation Ameliorates Aldara-Induced Psoriasiform Phenotype and Histology in Mice. J Invest Dermatol 2015;135(7):1915–1918. [DOI] [PubMed] [Google Scholar]
- Zheng X, Bollag WB. Aquaporin 3 colocates with phospholipase D2 in caveolin-rich membrane microdomains and is regulated by keratinocyte differentiation. J Invest Dermatol 2003;121:1487–1495. [DOI] [PubMed] [Google Scholar]
- Zheng X, Chen X. Aquaporin 3, a glycerol and water transporter, is regulated by p73 of the p53 family. FEBS Lett 2001;489:4–7. [DOI] [PubMed] [Google Scholar]
- Zheng X, Ray S, Bollag WB. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim Biophys Acta 2003;1643:25–36. [DOI] [PubMed] [Google Scholar]
- Zhi X, Tao J, Li Z, Jiang B, Feng J, Yang L, et al. MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS Lett 2014;588(5):757–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.