Abstract
Innate lymphocytes are selectively enriched in the liver where they have important roles in liver immunology. Murine studies have shown that type I NKT cells can promote liver inflammation whereas type II NKT cells have an anti-inflammatory role. In humans, type II NKT cells were found to accumulate in the gut during inflammation and IL13Rα2 was proposed as a marker for these cells. In the human liver, less is known about type I and II NKT cells. Here, we studied the phenotype and function of human liver T cells expressing IL13Rα2. We found that IL13Rα2 was expressed by around 1% of liver resident memory T cells but not on circulating T cells. In support of their innate-like T cell character, the IL13Rα2+ T cells had higher expression of PLZF compared to IL13Rα2- T cells and possessed the capacity to produce IL-22. However, the majority of human liver sulfatide-reactive type II NKT cells did not express IL13Rα2. Collectively, these findings suggest that IL13Rα2 identifies tissue-resident intrahepatic T cells with innate characteristics and the capacity to produce IL-22.
Introduction
The liver is the largest solid organ in the human body and receives the majority of its blood input from the gut through the portal vein. Thus, the liver is exposed to bacterial products from the gastrointestinal tract and acts as an organ barrier between the gut and the rest of the body(1). In physiological conditions the liver is considered to be an immune tolerant organ(2). However, chronic liver inflammation, in response to viral or environmental triggers or driven by auto-immune responses, often lead to the development of fibrosis (3).
Innate immune cells are selectively enriched within the liver and are important players in liver immunity. This includes innate lymphoid cells (ILC)(4), natural killer cells(5), mucosal associated invariant T (MAIT) cells(6), γδ T cells, and natural killer T (NKT) cells (1). NKT cells respond to glycolipid antigens presented on CD1d, an MHC class I-like molecule expressed on professional antigen presenting cells(7) but also by other specialized cells such as keratinocytes(8), cholangiocytes(9), and hepatocytes during hepatitis C virus infection(10). NKT cells are classified into two groups, type I NKT cells, expressing an invariant TCR (Vα24 paired with Vβ11 in humans) recognizing α-galactosylceramide (α-GalCer), typically referred to as invariant NKT (iNKT) cells and type II NKT cells displaying a diverse TCR repertoire that can recognize sulfatide(7, 11) or other glycolipids(12).
Studies in mice have shown that type I NKT cells promote inflammation and liver fibrosis while type II NKT cells have an anti-inflammatory role(13). In humans, type I NKT cells have been extensively studied in the context of infectious diseases, inflammatory disorders, and tumor immunity(14). In comparison, little is known about the tissue distribution and the role of type II NKT cells in health and disease. Recently, sulfatide-reactive type II NKT cells were found to accumulate in the lamina propria of patients with ulcerative colitis. These type II NKT cells co-expressed CD161 and IL13Rα2 and IL13Rα2 was suggested as a marker specifically identifying these cells in the human gut (15).
In this study, we characterized the phenotype and function of IL13Rα2 expressing human liver T cells. We found that IL13Rα2 was expressed by liver resident memory T cells but not by circulating T cells. The IL13Rα2+ T cells had a higher expression of PLZF compared to IL13Rα2- conventional T cells and were biased towards production of IL-22. However, only a fraction of the IL13Rα2+ liver T cells bound to sulfatide-loaded CD1d tetramer and the majority of human liver sulfatide-reactive type II NKT cells did not express IL13Rα2. Collectively, our results suggest that IL13Rα2 expression broadly associates with innate-like T cells in the human liver and that those cells could have an anti-inflammatory role by their production of IL-22.
Results and Discussion
IL13Rα2 together with CD161 have been proposed to be markers of sulfatide reactive type II NKT cells in the human gut(15). The portal vein directly connects the gut to the liver. Thus, we evaluated IL13Rα2 expression on peripheral blood and liver T cells. We found that very few, if any, circulating T cells expressed IL13Rα2 (Figure 1 A). In contrast, IL13Rα2 was detectable on a small population of intrahepatic T cells (Figure 1 A and B).
Figure 1. Human livers contain tissue-resident IL13Rα2+ innate T cells.
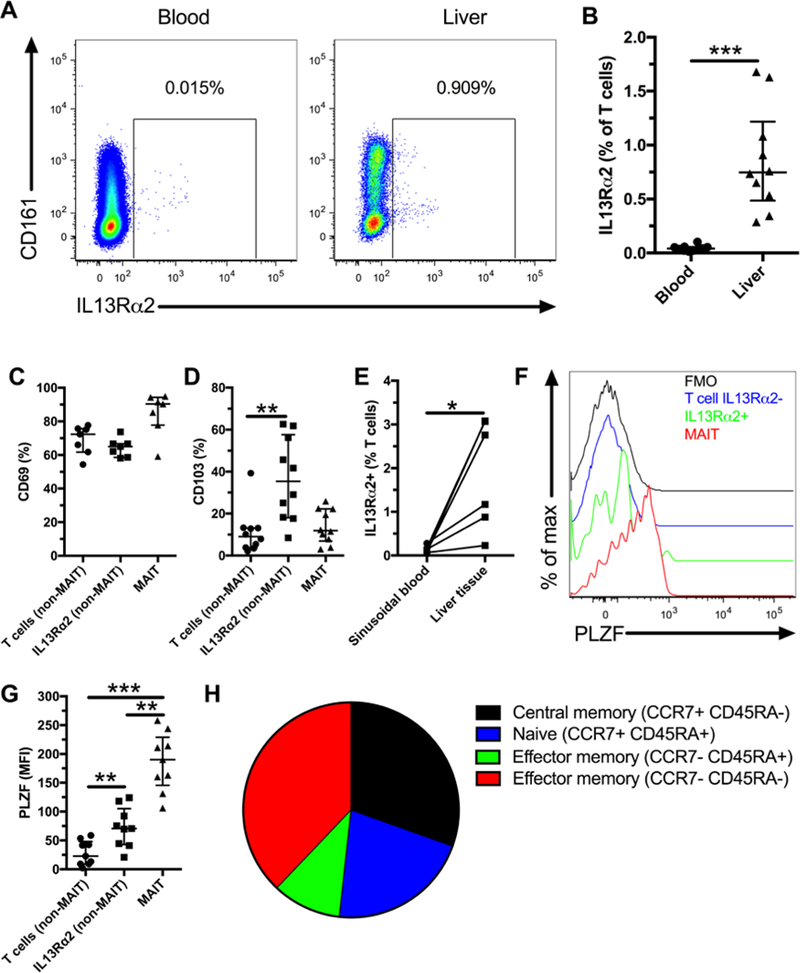
Representative flow plot showing expression of IL13Rα2 expression on total T cells in blood and liver (A). Frequency of IL13Rα2+ T cells in blood and liver (B, n=10). CD69 expression by T cell subsets in the liver (C, n=7). CD103 expression by T cell subsets in the liver (D, n=10). IL13Rα2 expression on T cells from matched flushed sinusoidal blood and liver tissue samples (E, n=6). Representative flow plots showing PLZF expression levels by MAIT cells, T cells, and IL13Rα2+ T cells (F). PLZF expression (MFI) by T cell subsets in the liver (G, n=9). Average % of IL13Rα2+ T cells with a central memory (CCR7+CD45RA-), naïve (CCR7+CD45RA+), and effector memory (CCR7-CD45RA+ or CCR7-CD45RA-) phenotype (H, n=6). *** indicates p < 0.001, ** indicates p < 0.01, and * indicates p < 0.05.
MAIT cells are innate T cells restricted by MR1 and that can be identified by the co-expression of CD161 together with Vα7.2(16, 17). Approximately half of the IL13Rα2+ T cells co-expressed CD161 (Figure 1 A and Sup. Figure 1 A) and a smaller fraction co-expressed CD161 and Vα7.2 (Sup. Figure 1 B) and would therefore be classified as MAIT cells. Thus, in further analysis we excluded MAIT cells from the bulk liver T cell population. The expression of CD4 and CD8 on IL13Rα2+ liver T cells ranged from below 10% to over 60% (Sup. Figure 1 C and D).
Since IL13Rα2+ T cells were selectively enriched in liver as compared to peripheral blood we next evaluated if these might be tissue resident T cells. CD69, a canonical marker for tissue resident lymphocytes(5) was highly expressed by the IL13Rα2+ liver T cells but expression levels did not differ as compared to IL13Rα2- T cells (Figure 1 C). Instead, a significantly larger fraction of the IL13Rα2+ liver T cells expressed CD103 as compared to other T cells (Figure 1D). These results would suggest that IL13Rα2+ T cells are resident to liver tissue. To further substantiate this, we found a higher frequency of IL13Rα2 expression by T cells in liver tissue as compared to sinusoidal blood (Figure 1 E). Thus, IL13Rα2+ T cells are another resident subset of lymphocytes in the human liver in addition to the recently described cells such as CD49a+ NK cells(18), IL2-high memory CD8 T cells(19), and MAIT cells(6).
One characteristic of innate T cells is their expression of the master transcription factor PLZF(20, 21). Therefore, we evaluated whether IL13Rα2+ T cells in the liver expressed PLZF. The IL13Rα2+ T cells had higher levels of PLZF than conventional liver T cells but lower than MAIT cells (Figure 1 F and G). We confirmed PLZF expression by performing qRT-PCR on sorted IL13Rα2+ liver T cells and conventional T cells (Supplementary Figure 1 E). Another characteristic of innate T cells is their memory cell phenotype. A majority of IL13Rα2+ liver T cells were effector memory (CCR7-) with significant central memory (CCR7+CD45RA-) and naïve (CCR7+CD45RA+) phenotypes (Figure 1 H and Supplementary Figure 1 F). Our results show that IL13Rα2 expression identifies a subset of tissue resident memory cells in the liver with an intermediate level of PLZF expression. These results are further consistent with an innate-like T cell phenotype of the IL13Rα2+ cells.
Innate-like T cells have been shown to display a different cytokine production profile in mucosal tissues compared to blood, with reduced production of IFNγ and an increased production of IL-22(22, 23). We next evaluated cytokine production by liver IL13Rα2+ T cells following stimulation with PMA and ionomycin (Figure 2 A). We found that IL13Rα2+ T cells produced IFNγ at similar levels to the IL13Rα2- T cells (Figure 2 B) whereas they produced more IL-22 (Figure 2 C and D). Intriguingly, IL-22 has been shown to ameliorate liver fibrosis(24, 25) and to promote survival of hepatocytes(26, 27), thus, IL13Rα2+ T cell could play a protective role during liver injury. In this regard, IL-22 producing cells are accumulating in the liver during viral hepatitis and stimulate proliferation of liver progenitor cells(28). However, we did not observe an increase in IL13Rα2+ T cells in the blood of patients with chronic hepatitis C virus (HCV) infection as compared to controls (data not shown). It is currently unknown what is the function of IL13Rα2 in the liver. The secreted form of IL13Rα2 has been shown to function has a decoy receptor(29). In the gut, cells bearing IL13Rα2 responded to TCR stimulation by producing IL-13(15). In the lung, signaling through IL13Rα2 has been shown to promote fibrosis by the induction of TGF-β1(30). Signaling through IL13Rα2 in myeloid cells has been shown to stimulate production of TGF-β1, reducing anti-tumor immunity in mice models(31). However, future studies are needed to confirm a potential protective role of intrahepatic IL13Rα2+ T cells in human liver fibrosis development.
Figure 2. Intrahepatic IL13Rα2+ T cells produce IL-22 upon stimulation.
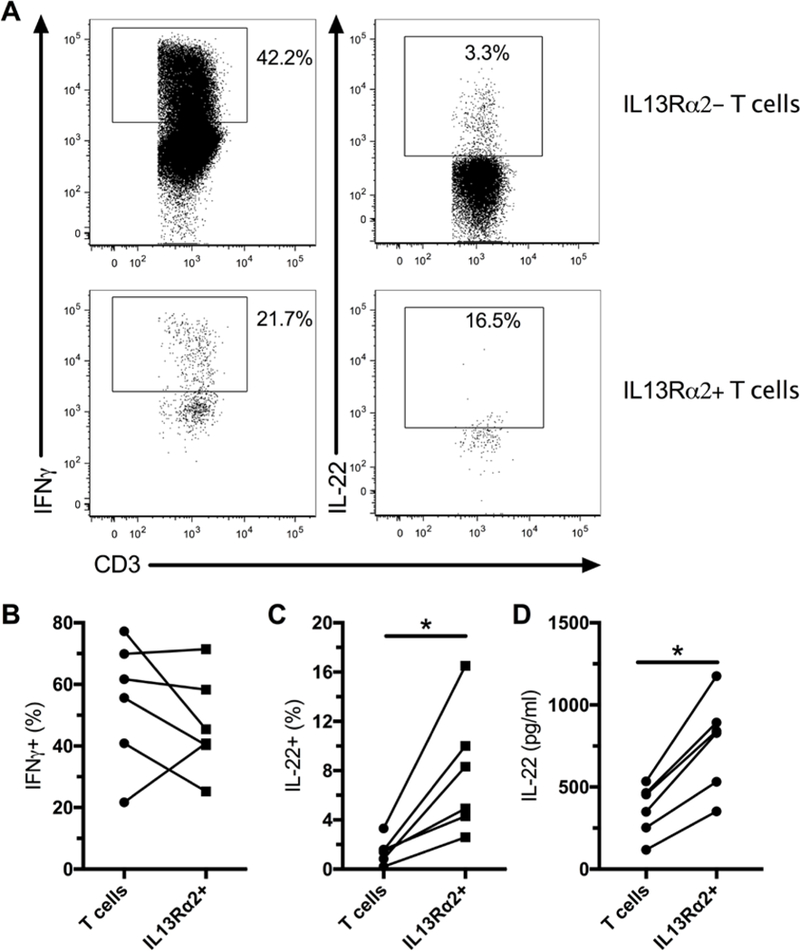
LMCs were stimulated with PMA and ionomycin for 6 hours and production of IFNγ and IL-22 was evaluated by flow cytometry. Representative flow plots for production of IFNγ and IL-22 (A). Production of IFNγ by liver IL13Rα2- T cells and IL13Rα2+ T cells (B, n=6). Production of IL-22 by liver IL13Rα2- T cells and IL13Rα2+ T cells (C, n=6). IL-22 was measured in the supernatant of sorted IL13Rα2+ and IL13Rα2- T cells following stimulation with PMA and ionomycin (D, n=6). * indicates p < 0.05.
Finally, we used CD1d tetramers loaded with α-Gal-Cer or sulfatide to identify type I and type II NKT cells in the human liver (Sup. Figure 2 A). We found that only a minority of liver IL13Rα2+ T cells bound to sulfatide-loaded CD1d tetramer (Figure 3 A). However, IL13Rα2 expression was higher for T cells that bound to the sulfatide-loaded CD1d tetramer (Figure 3 B). It is possible that some of the IL13Rα2+ T cells are type II NKT cells that recognize glycolipids other than sulfatide(12). Although IL13Rα2 expression was enriched within the sulfatide-reactive type II NKT cell population, this suggest that IL13Rα2 is not a specific marker for sulfatide-reactive type II NKT cell in the human liver. Furthermore, IL13Rα2 in combination with CD161 is also not a reliable marker of sulfatide-reactive type II NKT cells in the liver, as a fraction of the cells identified by this combination are MAIT cells.
Figure 3. A minority of intrahepatic IL13Rα2+ T cells are type II NKT cells.
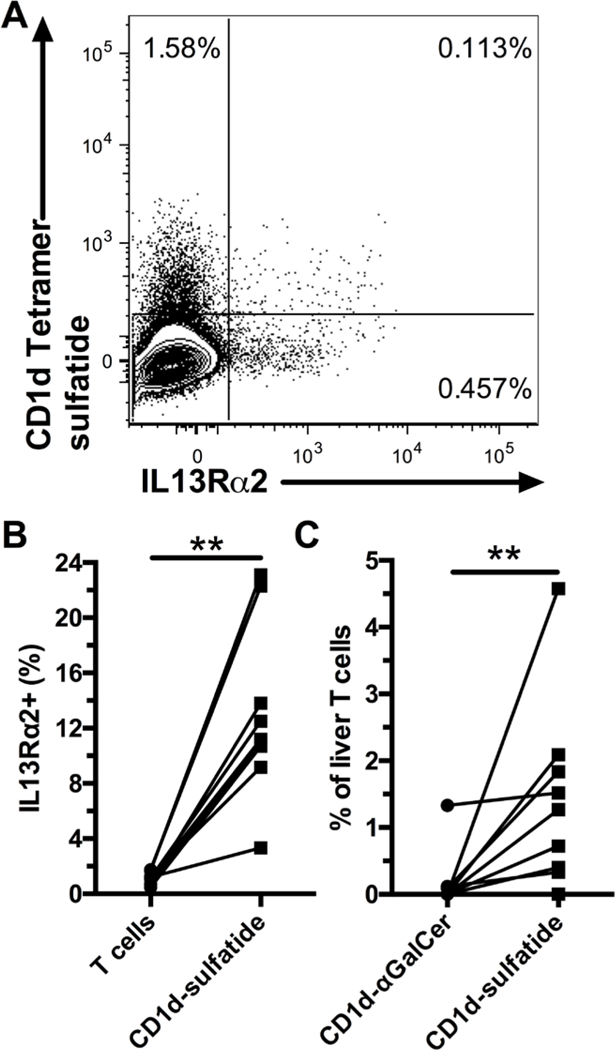
Representative staining of liver T cells for IL13Rα2 and CD1d tetramer loaded with sulfatide (A). Frequency of IL13Rα2+ cells within liver T cells and CD1d-sulfatide+ T cells (B, n=9). Frequency of CD1d-αGalCer+ and CD1d-sulfatide+ liver T cells (C, n=9), background staining with unloaded tetramer was subtracted. ** indicates p < 0.01.
When specifically analyzing type II NKT cells in the human liver using the sulfatide-loaded CD1d tetramer we found these cells to express higher levels of CD161 and CD103 than bulk liver T cells (Sup. Figure 2 B and C). This suggests that, similar to the IL13Rα2+ T cells, type II NKT cells found in the liver are tissue resident cells. With respect to type I iNKT cells, and with the exception of one liver sample, few, if any, of these cells could be identified in human liver samples (Figure 3 C), which is similar to previously reported rare detection of α-Gal-Cer reactivity in human livers(10). This is in contrast with the mouse liver where type I iNKT cells make up to 10–50% of the T cells(1, 13). Thus, type I NKT cells, as defined here as α-GalCer reactive, are less likely to play a key role in liver immunobiology in humans.
Concluding remarks
Overall, our results show that IL13Rα2 is expressed by tissue resident T cells in the liver that exhibit a memory phenotype and express PLZF. This is consistent with the phenotype usually associated with innate-like T cells. In accordance, IL13Rα2 was expressed by fractions of both MAIT and sulfatide-reactive type II NKT cells. However, the identity of the remaining IL13Rα2+ T cells is still to be determined. IL13Rα2+ T cells are biased towards production of IL-22, which may play a role in protection from liver fibrosis. Further studies are needed to determine the function of IL13Rα2+ T cells in liver pathologies.
Materials and Methods
Study cohort and samples
The regional Ethical Review Board in Stockholm, Sweden approved the study (approval numbers 2010/678–31/3 and 2013/2285–31/3). Oral and written informed consent was obtained from all subjects in accordance to the declaration of Helsinki. Livers were obtained during partial hepatectomy from living donors undergoing therapeutic tumor excision where only tumor-free non-affected tissue was used for isolation of immune cells. Immune cells were isolated using a previously described protocol(32). Buffy coats from healthy donors were obtained from healthy blood donors from the New York Blood Bank. Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation. Isolated PBMCs were washed twice in Hank’s balanced salt solution (Gibco, Grand Island, NY), and cryopreserved for subsequent analysis. Cryopreserved cells from all subjects were stored in liquid nitrogen until used in the assays. PBMC from ten patients with chronic HCV infection were obtained from the out-patient clinic at the Karolinska University Hospital, Stockholm, Sweden and results from these were compared to ten healthy controls. See supporting information for more details on sample preparation.
Flow cytometry and mAbs
Cryopreserved specimens were thawed and washed, and counts and viability were assessed using trypan blue. Cells were stained in Brilliant Violet Stain Buffer (BD Biosciences, San Jose, CA, USA) at room temperature for 15 min in 96-well V-bottom plates in the dark. Samples were then washed and fixed using Cytofix/Cytoperm (BD Biosciences) before flow cytometry data acquisition. Intracellular staining was performed in Perm/Wash (BD Biosciences). CD1d tetramers were prepared as previously described(33) and incubated for 30 minutes at 4°C in presence of Fc Bloc (BD bioscience) before staining for additional surface markers. Data were acquired on a BD LSRFortessa instrument (BD Biosciences) and analyzed using FlowJo Version 9.8.5 software (TreeStar, Ashland, OR, USA).
Cell sorting and qRT-PCR
Liver IL13Rα2+ T cells and conventional T cells (non-MAIT IL13Rα2-) were sorted on a SH800Z (Sony Biotechnology, San Jose, CA). For functional assay, an equal number of cells were sorted into culture media. For qRT-PCR, 100 cells per well were sorted into 96 well plates in duplicates directly into SuperScript™III Platinum™One-Step qRT-PCR mix (Invitrogen, Carlsbad, CA, USA) containing SUPERase RNase Inhibitor (Invitrogen) and primer and probes for ZBTB16 (PLZF) (Invitrogen). Real time qPCR was performed on an ABI ViiA 7 Real-Time PCR machine (Applied Biosystems).
Functional assay
LMCs were cultured for 18 hours at 37°C/5% CO2 in RPMI medium supplemented with 10% fetal bovine serum. Cells were then stimulated with 100 ng/ml PMA and 1 μg/ml ionomycin (both from Sigma-Aldrich, St Louis, MO, USA) in presence of Monensin (Golgi Stop, BD Biosciences) for 6 hours. Cells were then stained as described above. In some experiments, cells were incubated without Monensin and the supernatant was collected to measure IL-22 by ELISA following manufacturer’s instructions (Biolegend).
Statistical analysis
All statistical analysis was performed using Graph Pad Prism version 6.0f for Mac OSX (GraphPad Software, La Jolla, CA) using Mann-Whitney test or Wilcoxon matched-pairs signed rank test for paired samples. P values ≤ 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Lena Berglin, Karolinska Institutet, Stockholm, Sweden, for experimental assistance.
Funding
This work was funded by the Swedish Research Council, the Swedish Cancer Society, the Swedish Foundation for Strategic Research, the Swedish Society for Medical Research, the Cancer Research Foundations of Radiumhemmet, Knut and Alice Wallenberg Foundation, the Novo Nordisk Foundation, the Center for Innovative Medicine at Karolinska Institutet, the Stockholm County Council, and in part by an award from the District of Columbia Center for AIDS Research, an NIH funded program (AI117970), and the Karolinska Institutet. NCI Vaccine Branch scientists LP, MT, and JAB were funded by intramural NIH funds from the Center for Cancer Research, National Cancer Institute, Z1A-C-004020.
Abbreviations:
- MAIT
mucosal associated invariant T
- NKT
natural killer T
- α-GalCer
α-Galactosylceramide
- iNKT
invariant natural killer T
- LMC
liver mononuclear cells
Footnotes
Disclosure
The authors declare that no competing interests exist.
References
- 1.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47(2):729–36. [DOI] [PubMed] [Google Scholar]
- 2.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60(6):2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forkel M, Berglin L, Kekalainen E, Carlsson A, Svedin E, Michaelsson J, et al. Composition and functionality of the intrahepatic innate lymphoid cell-compartment in human nonfibrotic and fibrotic livers. Eur J Immunol. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16(5):310–20. [DOI] [PubMed] [Google Scholar]
- 6.Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunology. 2016;5(8):e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey DI, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Rossjohn J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin Immunol. 2010;22(2):61–7. [DOI] [PubMed] [Google Scholar]
- 8.Bosnjak L, Sahlstrom P, Paquin-Proulx D, Leeansyah E, Moll M, Sandberg JK. Contact-dependent interference with invariant NKT cell activation by herpes simplex virus-infected cells. J Immunol. 2012;188(12):6216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrumpf E, Tan C, Karlsen TH, Sponheim J, Bjorkstrom NK, Sundnes O, et al. The biliary epithelium presents antigens to and activates natural killer T cells. Hepatology. 2015;62(4):1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagisawa K, Yue S, van der Vliet HJ, Wang R, Alatrakchi N, Golden-Mason L, et al. Ex vivo analysis of resident hepatic pro-inflammatory CD1d-reactive T cells and hepatocyte surface CD1d expression in hepatitis C. J Viral Hepat. 2013;20(8):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar MV, Kumar V. Type II NKT Cells and Their Emerging Role in Health and Disease. J Immunol. 2017;198(3):1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol. 2016;13(3):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori L, Lepore M, De Libero G. The Immunology of CD1- and MR1-Restricted T Cells. Annu Rev Immunol. 2016;34:479–510. [DOI] [PubMed] [Google Scholar]
- 15.Fuss IJ, Joshi B, Yang Z, Degheidy H, Fichtner-Feigl S, de Souza H, et al. IL-13Ralpha2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. 2014;63(11):1728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–9. [DOI] [PubMed] [Google Scholar]
- 17.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–23. [DOI] [PubMed] [Google Scholar]
- 18.Marquardt N, Beziat V, Nystrom S, Hengst J, Ivarsson MA, Kekalainen E, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol. 2015;194(6):2467–71. [DOI] [PubMed] [Google Scholar]
- 19.Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, et al. IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med. 2017;214(6):1567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonzo ES, Sant’Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011;23(2):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, Andersson E, et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017;10(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paquin-Proulx D, Ching C, Vujkovic-Cvijin I, Fadrosh D, Loh L, Huang Y, et al. Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal Immunol. 2017;10(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmo RF, Cavalcanti MS, Moura P. Role of Interleukin-22 in chronic liver injury. Cytokine. 2016. [DOI] [PubMed] [Google Scholar]
- 25.Hu BL, Shi C, Lei RE, Lu DH, Luo W, Qin SY, et al. Interleukin-22 ameliorates liver fibrosis through miR-200a/beta-catenin. Sci Rep. 2016;6:36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39(5):1332–42. [DOI] [PubMed] [Google Scholar]
- 27.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27(4):647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143(1):188–98 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197(6):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99–106. [DOI] [PubMed] [Google Scholar]
- 31.Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68(9):3467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecluyse EL, Alexandre E. Isolation and culture of primary hepatocytes from resected human liver tissue. Methods Mol Biol. 2010;640:57–82. [DOI] [PubMed] [Google Scholar]
- 33.Izhak L, Ambrosino E, Kato S, Parish ST, O’Konek JJ, Weber H, et al. Delicate balance among three types of T cells in concurrent regulation of tumor immunity. Cancer Res. 2013;73(5):1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


