Abstract
Chronic kidney disease (CKD) is a major public health issue. At the histological level, renal fibrosis is the final common pathway of progressive kidney disease irrespective of the initial injury. Considerable evidence now indicates that renal inflammation plays a central role in the initiation and progression of CKD. Some of the inflammatory signaling molecules involved in CKD include: monocyte chemoattractant protein-1 (MCP-1), bradykinin B1 receptor (B1R), nuclear factor κB (NF-κB), tumor necrosis factor-α (TNFα), transforming growth factor β (TGF-β), and platelet-derived growth factor (PDGF). Multiple antifibrotic factors, such as interleukin-10 (IL-10), interferon-γ (IFN-γ), bone morphogenetic protein-7 (BMP-7), hepatocyte growth factor (HGF) are also downregulated in CKD. Therefore, restoration of the proper balance between pro- and antifibrotic signaling pathways could serve as a guiding principle for the design of new antifibrotic strategies that simultaneously target many pathways. The purpose of this review is to summarize the existing body of knowledge regarding activation of cytokine pathways and infiltration of inflammatory cells as a starting point for developing novel antifibrotic therapies to prevent progression of CKD.
Keywords: Chronic kidney disease, inflammation, renal fibrosis, antifibrotic therapy, cytokines
1. Introduction
Chronic kidney disease (CKD has emerged as a world-wide public health issue (Hallan et al., 2016; Murphy et al., 2016; Shin and Kang, 2016). The prevalence of CKD is estimated to be 8–16% worldwide, and CKD is the third highest cause of premature mortality (82%), behind AIDS and diabetes mellitus (Decleves and Sharma, 2014; Jha et al., 2013). Renal fibrosis is the final common outcome of progressive kidney disease regardless of initial injury (Khwaja et al., 2007; Liu, 2011; Yu et al., 2016). Understanding the mechanisms behind renal fibrosis is essential for developing therapies to prevent or reverse this process and slow the progression of CKD. Considerable evidence now indicates that inflammation plays a critical role in the initiation and progression of renal fibrosis. In the case of acute kidney injury (AKI), transient renal ischemia may trigger similar responses to those in CKD, including increased release of cytokines, infiltration of inflammatory cells, epithelial to mesenchymal transformation (EMT), and fibroblast activation. The initial tubular injury is eventually repaired via tubular regeneration and matrix remodeling. However, if the initial fibrogenic processes are not fully resolved, they are often reactivated later in life by hypertension, diabetes, or activation of the immune system. The fibrogenic signal not only stimulates activation of fibroblasts but also initiates tubular EMT, a critical event that leads to renal fibrosis and CKD (Liu, 2006). The purpose of this review is to highlight recent studies on the role of immune cells or system and cytokines in renal fibrosis as the basis for the development of novel therapies (Table 1).
Table 1.
Anti-fibrotic agents that target inflammation in chronic kidney disease models and status of drug trials and clinical development
| Agent/Drug | Target | Kidney Disease | Clinical Trial |
|---|---|---|---|
| CX140-B | Inhibits CCR2 (CCL2 signaling) | Type 2 diabetic nephropathy | Additional renal protective effects in diabetic patients. |
| BX471 | CCR1 inhibitor | Murine models of nephropathy | |
| ssR240612 | Bradykinin receptor B1 inhibitor | UUO and nephrotoxic serum-induced models of renal injury | |
| Infliximab or Entanercept | Inhibits TNF-α | Various animal CKD models | Approved for arthritis and inflammatory bowel disease. FSGS trial failed to enroll sufficient number of patients. Status undetermined. |
| Pegylated TNFR1 | Inhibits TNF-α | Remnant kidney model | Ab tolerated in humans |
| Anti-Fn14 & Anti-TWEAK antibodies | Inhibit Fn14/TWEAK | UUO and aGBM FSGS | Trial of BIIB023 in Lupus nephritis terminated for lack of efficacy. |
| LY2382770 | Inhibits TGF-β1 | Diabetic nephropathy | No efficacy. |
| Suramin | Inhibits TGF-β/RTK signaling | Remnant kidney model | |
| FG-3019 | Inhibits CTGF | Steroid-resistant FSGS; Diabetic kidney disease | Terminated (not specified or poor study design). |
| Erlotinib or Gefitinib | Inhibits EGFR | Various pre-clinical models | Anticancer agents; no renal disease trials. |
| Anti-PDGF-C antibody | Inhibits PDGF-C | Murine UUO model | |
| Imatinib | Inhibits PDGFRs (various tyrosine kinases) | Various pre-clinical models | Anticancer agent; no renal disease trials. |
| Pirfenidone | Inhibits PDGF, TGF-β, TNFα | FSGS; Diabetic kidney disease; UUO, aGBM glomerulonephritis, remnant kidney, hypertension models | Slowed the decline in eGFR in FSGS and diabetic nephropathy. |
| Rapamycin/Sirolimus | Inhibits mTORC1 | Reduces fibrosis and loss of renal function in various pre-clinical models | Immunosuppressive agents for transplant. Improves long-term graft survival. Associated with nephrotoxicity in some FSGS patients. |
| Pyrrolidine dithiocarbamate (PDTC) | Inhibits NF-κB | Various pre-clinical CKD models | |
| BMS-566419 MPA/MMF | Inhibits IMPDH/NF-κB activation | Reduces renal fibrosis in UUO model andhypertension and diabetic nephropathy | Immunosuppressive agents for transplant trials. |
| HSc025 | Induces nuclear actions of YB-1 | Reduces renal fibrosis and injury in UUO and ischemia/reperfusion-induced inflammation models | |
| AA123 | ALK3 activator (BMP-7 mimetic) | Various pre-clinical models | Structural analog in phase II clinical testing. |
| Telmisartan | ARB and PPARγ agonist | Murine UUO model, IR-induced AKI; Type 2 diabetic nephropathy; rodent UUO, hypertension, and remnant kidney models | Widely used for renal protection in diabetic patients. |
| ONO-1301 | Prostacyclin analog | Murine UUO model | |
| Paricalcitol, Vitamin D analog | Negative regulation of RAAS; inhibition of NF-kB and TGF-β signaling | Various pre-clinical models | Still ongoing for diabetic kidney disease. Previous study showed lack of efficacy. Selective vitamin D receptor activation with paricalcitol reduced albuminuria in patients with type 2 diabetes (VITAL study). |
| Adipose tissue-derived MSCs | Inflammation, oxidative stress, and mitochondrial damage | Various models of AKI and CKD, including IR, adenine-induced, and cisplatin intake | Non-diabetic CKD, recruiting (NCT03321942). |
aGBM, anti-glomerular basement membrane; AKI, acute kidney injury; ARB, AngII type 1 receptor blocker; CAN, chronic allograft nephropathy; CKD. chronic kidney disease; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IMPDH, inosine monophosphate dehydrogenase; IR, ischemia/reperfusion; MMF, mycophenolate mofetil; MPA, mycophenolic acid; MSCs, mesenchymal stem cells; RAAS, renin–angiotensin–aldosterone system; RTK, receptor tyrosine kinase.
2. Chemokines and Inflammatory Cells
Sterile inflammation, which is defined as inflammation in the absence of infectious agents or specific immunogens, has a prominent role in initiating renal fibrosis (Anders et al., 2004; Duffield, 2014; Kurts et al., 2013; Lopez-de la Mora et al., 2015; Tampe and Zeisberg, 2014). During this process, leukocytes and fibrogenic cells are recruited to the glomerulus and renal interstitium. Together with activation of resident kidney immune cells, this recruitment leads to increased production of pro-inflammatory cytokines. The gradient of chemotactic cytokines, chemokines, further drives infiltration of monocytes/macrophages, T cells, and B cells to the injured site. Chemokines are also mediators of angiogenesis, fibroblast recruitment, and EMT. Of the currently known 47 chemokines and 20 chemokine receptors, the chemokine (C-C motif) ligand 2 (CCL2) – C-C chemokine receptor type 2 (CCR2) axis is the most studied therapeutic target in renal fibrosis. CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), is released by tubular epithelial cells after renal injury, prompting the influx of CCR2-positive monocytes, T cells, dendritic cells, and fibrocytes (Tampe and Zeisberg, 2014; Wang et al., 2000). Monocytes differentiate into M1 or M2 macrophages. M1 macrophages produce pro-inflammatory cytokines, such as TNFα, IL-1β, IL-6, and CCL2. M2 macrophages are involved in wound healing and tissue repair. They produce TGF-β and anti-inflammatory cytokines, such as IL-10. CCL2 has also been shown to induce TGF-β1 production by macrophages and mesangial cells, and TGF-β1 feedbacks to stimulate expression of CCL2 in mesangial cells (Cheng et al., 2005). Increased renal production of CCL2 is associated with glomerular macrophage infiltration and increased collagen expression in various models of diabetic nephropathy, including streptozotocin-induced nephropathy and type 2 diabetes in db/db mice (Lee et al., 2015). CCR2 knockout in mice or blockade of its actions with antagonists, reduced renal fibrosis, TGF-β expression, and macrophage accumulation in different models of renal injury, including the db/db mouse, transgenics overexpressing type 2 nitric-oxide synthase, diet-induced obesity, and the unilateral ureteral obstruction (UUO) mouse model (Chow et al., 2007; Kanamori et al., 2007; Kang et al., 2010; Kitagawa et al., 2004; Sullivan et al., 2013). A recent clinical trial found that CX140-B (Table 1), an antagonist of CCR2, has renal protective effects in patients with type 2 diabetic nephropathy when added on top of current standard of care (de Zeeuw et al., 2015). CCR1, whose ligands include CCL3 (MIP 1-alpha), CCL5 (RANTES), CCL7 (MCP-3), and CCL23, is also critical in recruiting leukocytes to sites of inflammation. Targeting CCR1 with the small-molecule antagonist, BX471 (Table 1) reduced renal inflammation and interstitial fibrosis in several murine models of nephropathy, including adriamycin nephropathy, lupus nephritis, and UUO (Anders et al., 2004; Anders et al., 2002; Vielhauer et al., 2004).
Klein and colleagues observed an association between inflammation and expression of bradykinin B1 receptor (B1R) in the glomerulus and renal interstitium of patients with various forms of glomerulonephritis, including ANCA-associated vasculitis and Henoch-Schönlein purpura nephropathy (Klein et al., 2010). B1R expression is also upregulated in the UUO and nephrotoxic serum-induced models of renal injury (Klein et al., 2010; Klein et al., 2009; Wang et al., 2009). Treatment with a B1R antagonist, ssR240612 (Table 1), was found to block macrophage infiltration, leading to a reversal of renal fibrosis in rodent models of UUO and serum nephritis. Bone marrow transplant studies, as well as in vitro studies on renal tubular cells, demonstrated that the antifibrotic effect of B1R blockade involves in part, a direct effect on resident renal immune cells by inhibiting chemokine CCL2 and CCL7 expression. These findings suggest that blocking B1R has potential as an antifibrotic target (Klein et al., 2010; Klein et al., 2009).
Han and colleagues investigated B cell function in tubulointerstitial fibrosis induced by UUO using both genetic B cell-deficient and CD20 antibody-mediated B cell-depleted mice (Han et al., 2016). They found that obstructed kidneys of both had less monocyte/macrophage infiltration and collagen deposition. Additionally, B cell depletion attenuated UUO-induced increases in the renal expression of genes involved in inflammation and monocyte recruitment, viz., TNFα, vascular cell adhesion molecule 1 (VCAM-1), and CCL2. B cells are a major source of CCL2 and the reduction in CCL2 attenuated monocyte/macrophage influx and fibrotic changes after UUO (Han et al., 2016). Thus, B cells also seem to be a potential target for preventing renal fibrosis.
Various proteins and autacoids help resolve inflammation by dampening the recruitment of leukocytes and causing a transition from the M1 to M2 immune cell phenotype. Among these factors, annexin A1 (AnxA1), aka lipocortin-1, has received much interest (Locatelli et al., 2014). Endogenous AnxA1 regulates epithelial cell repair and opposes lung fibrosis (Locatelli et al., 2014; Trentin et al., 2015). By interacting with the N-formyl peptide receptor 2/lipoxin A4 receptor (FPR2/ALX), AnxA1 downregulates the production of pro-inflammatory mediators, reduces neutrophil migration to inflammation sites, and promotes clearance of apoptotic granulocytes (Locatelli et al., 2014). Neymeyer and colleagues investigated the AnxA1/FPR2 axis in the ARAnp rat model of hypertensive nephropathy induced by treating rats with an angiotensin II (AngII) type 1 receptor blocker during the nephrogenic period (Neymeyer et al., 2015). AnxA1 and FPR2 levels increased in the renal interstitium of ARAnp rats along with fibroblasts and macrophages. Murine AnxA1 knockout mouse fibroblasts exhibited higher α-SMA and Col1a1 mRNA levels than controls. Treatment of murine wild-type fibroblasts with TGF-β increased expression of α-SMA and Col1a1, and these increases were reduced by AnxA1 overexpression. Treatment of human fibroblasts with the FPR2 inhibitor WRW4 further demonstrated the anti-fibrotic actions of AnxA1.
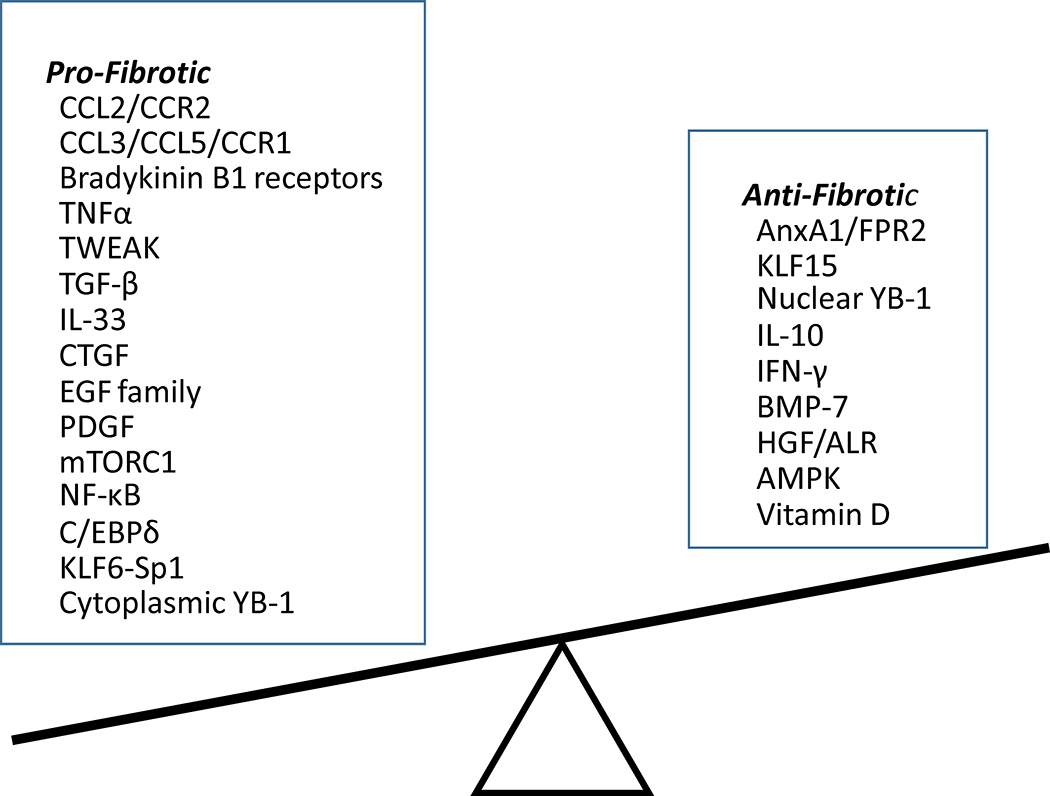
The process of renal fibrosis involves complex interactions between many intracellular signaling pathways. Myofibroblasts are activated by multiple means, including autocrine factors and paracrine signals from immune cells (Wynn, 2008). Besides CCL2, additional regulators of renal fibrosis have been identified. These factors have been divided into profibrotic and antifibrotic factors and are summarized in Fig. 1 and discussed below.
Fig. 1. Summary of pro- and anti-fibrotic factors involved in renal inflammation.
Renal inflammation plays a central role in the initiation and progression of chronic kidney disease (CKD) by causing fibrosis. Multiple inflammatory signaling molecules are activated that are potential targets for drug development. Multiple antifibrotic factors are also downregulated, thus contributing to the development of CKD indirectly. Therapies aimed at restoring levels of these factors or activating their signaling pathways are an alternative strategy for attenuating renal fibrosis. Restoration of the balance between pro- and antifibrotic signaling pathways could serve as a guiding principle for the design of new antifibrotic strategies to treat CKD.
3. Profibrotic Cytokines
3.1. Tumor necrosis factor-α
TNFα is produced by many cells, including macrophages, mesangial cells, and renal tubular epithelial cells (Lee et al., 2015). It stimulates the release of interleukin-1β, CCL2, and TGF-β1, and has a prominent role in glomerular inflammation and fibrosis (Idasiak-Piechocka et al., 2010). Elevated TNF-α levels have been reported in patients with various kidney disease (Carlos et al., 2014; Moriwaki et al., 2007; Omote et al., 2014; Santana et al., 2013). In addition, TNFα inhibition with infliximab or etanercept decreases albuminuria and slows progression of CKD in various animal models (Table 1) (Quiroga et al., 2015).
TNFα’s actions are mediated by TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2) (Oh et al., 2015). Expression of both receptors and their shedding from the cell membrane are increased in several kidney diseases (Idasiak-Piechocka et al., 2010). Soluble TNFRs may attenuate inflammation by neutralizing free TNFα and thus may be a potential antifibrotic therapy in CKD (Idasiak-Piechocka et al., 2010). The FONT (Novel Therapies for Resistant FSGS) Phase II clinical trial (NCT00814255) evaluated an anti-TNF-α monoclonal antibody as an anti-fibrotic agent in patients with primary focal segmental glomerulosclerosis (FSGS). Unfortunately, recruitment fell short of enrollment goals (Lee et al., 2015; Trachtman et al., 2015) (Table 1).
Pegylated forms of soluble TNFR1, which reduce blood pressure, albuminuria, and renal inflammation and fibrosis in rats with reduced renal mass CKD, appear to be another promising approach (Therrien et al., 2012) (Table 1). The beneficial effects in this model were associated with reduced renal NF-ĸB activation, and production of TGF-β1 and endothelin-1 (ET-1). Lastly, CD40, a member of the TNF receptor superfamily, is linked to the progression of ischemic renal injury (Haller et al., 2016). Haller and colleagues further reported that knockout of CD40 in Dahl S rats reduced proteinuria and renal fibrosis following the development of hypertension.
3.2. TWEAK
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes tubular cell injury and renal inflammation. TWEAK contributes to renal fibrosis in several ways, including stimulating fibroblast proliferation or their differentiation into ECM producing myofibroblasts, stimulating the formation of pro-inflammatory mediators by tubular cells, downregulating the antifibrotic molecule Klotho in tubular cells, and via actions on other cell types including, pericytes and podocytes (Gomez et al., 2016; Ruiz-Ortega et al., 2014; Sanchez-Nino et al., 2013). TWEAK activates the fibroblast growth factor-inducible molecule 14 (Fn14) receptor (Dhruv et al., 2013). Expression levels of TWEAK and Fn14 increased in animal models of renal fibrosis induced by UUO or administration of anti-glomerular basement membrane (anti-GBM) antibody (Poveda et al., 2013). TWEAK induces inflammatory chemokine production by renal fibroblasts via NF-κB and fibroblast proliferation via Ras/ERK signaling (Ucero et al., 2013). Overexpression of TWEAK induces kidney inflammation and fibrosis (Ucero et al., 2013). In contrast, TWEAK deficient mice exhibited decreased renal fibrosis after UUO. Experiments targeting the TWEAK-Fn14 pathway with anti-Fn14 blocking antibody or anti-TWEAK neutralizing antibody show renoprotective effects in different models of kidney disease (Table 1) (Gomez et al., 2016; Hotta et al., 2011; Izquierdo et al., 2012; Xia et al., 2012; Zhao et al., 2007). A trail of BIIB023, an anti-TWEAK neutralizing antibody, showed that it is well tolerated in humans (Michaelson et al., 2012). Unfortunately, a phase II trial (NCT01499355) testing whether BIIB023 has renoprotective effects in lupus nephritis patients failed to demonstrate sufficient efficacy and was terminated.
3.3. TGF-β signal pathway
TGF-β stimulates mesangial cells, interstitial fibroblasts, and tubular epithelial cells to become matrix-producing fibrogenic cells (Liu, 2006). TGF-β signaling is also a nodal point integrating the fibrogenic actions of many factors, such as AngII, high glucose, and connective tissue growth factor (CTGF). These factors act as either upstream inducers of TGF-β or as its downstream effectors (Liu, 2006; Trionfini et al., 2015). For example, perturbed glomerular hemodynamics activates both the renin-angiotensin-aldosterone system (RAAS) and TGF-β. RAAS induces further production of TGF-β and plasminogen activator inhibitor causing rapid accumulation of extracellular matrix (ECM). The protective effect of RAAS inhibition in various models of kidney disease also correlates with suppression of TGF-β production (Border and Noble, 1998).
The interactions between TGF-β and newly described protective RAAS members, such as ACE2, Ang(1-7), and Mas, has also been observed. In rat kidney epithelial cells, TGF-β1 decreases ACE2 and Ang(1-7) formation from AngII, as well as Mas (Chou et al., 2013). Ang-(1-7) via the Mas receptor, can attenuate TGF-β1-induced expression of fibronectin (Chou et al., 2013). Deletion of ACE2 increased the AngII/Ang(1-7) ratio in the kidney and enhanced renal fibrosis in a UUO model of nephropathy (Liu et al., 2012c). Liu and colleagues concluded that enhanced AngII-mediated TGF-β/Smad and NF-κB signaling might explain why the loss of ACE2 enhances renal fibrosis and inflammation (Liu et al., 2012c). TGF-β1 also can induce Fn14 expression in fibroblasts, and cooperate with the TWEAK-Fn14 pathway to promote fibroblast proliferation (Ucero et al., 2013).
TGF-β acts through its cell membrane type I and type II serine/threonine kinase receptors (TGFβR1 and TGFβR2) and intracellular Smad (similar to mothers against decapentaplegic) proteins. Inhibitory Smad (I-SMAD) Smad7 inhibits this pathway by blocking phosphorylation of the receptor-regulated Smads (R-SMAD), Smad2 and Smad3, promoting the degradation of the receptor complexes. Thus, Smad7 blocks TGF-β/Smad-dependent fibrosis (Trionfini et al., 2015). Smad signaling is also constrained by a family of proteins known as Smad transcriptional corepressors, which include SnoN, Ski, and TGIF. These factors limit Smad-mediated gene transcription (Liu, 2006). SnoN and Ski are reduced in the fibrotic kidney, suggesting that their loss amplifies TGF-β signaling (Liu, 2006).
Therapeutic approaches based on inhibiting TGF-β/Smad signaling have been reported to reduce renal injury and fibrosis in many disease models, whereas overexpressing TGF-β1 induces renal fibrosis (Ai et al., 2015; Border and Noble, 1998; Chuang et al., 2014; Decleves and Sharma, 2014; Delle et al., 2012; Doi et al., 2011; Hu et al., 2013; Hu et al., 2016; Kania et al., 2013; Lee et al., 2015; Liu et al., 2015; Malaga-Dieguez et al., 2015; Meng et al., 2016; Morinaga et al., 2013; Neyra and Hu, 2016; Soler et al., 2012; Tampe and Zeisberg, 2014; Wang et al., 2011; Williams et al., 2013; Zhang et al., 2016; Zununi Vahed et al., 2013). Results of a phase I clinical trial suggested that fresolimumab, an anti-TGF-β1 antibody, is well-tolerated in patients with primary resistant FSGS. However, a phase II study (NCT01113801) assessing the utility of the anti-TGF-β1 antibody, LY2382770, in treating diabetic nephropathy was terminated due to lack of efficacy (Table 1). Alternatively, a number of active components of traditional Chinese medicine have been demonstrated to have renoprotective effects by inhibiting TGF-β/Smad signaling, including amygdalin, curcumin, GQ5, lingzhilactones, rhein, and danshensu (Ai et al., 2015; Gaedeke et al., 2004; Guan et al., 2015; Guo et al., 2013; Soetikno et al., 2013; Tapia et al., 2016; Yan et al., 2015).
Excessive TGF-β1 activity leads to fibrosis, but blocking TGF-β1 may not be wise due to its pleiotropic actions (Lee et al., 2015). For example, while inhibiting TGF-β attenuates the progression of advanced-stage tumors and metastases, it may also induce the formation of new cancers (Tampe and Zeisberg, 2014). A growing body of evidence demonstrates that TGF-β1 enhances wound repair and tissue regeneration, and has anti-inflammatory actions. Thus, effects of TGF-β activation in renal injury may be protective or harmful depending on timing and disease context. Therapeutic strategies targeting the downstream effectors of TGF-β, such as Smad3, Smad4, Smad7, Yes-associated protein (YAP1), or the transcriptional coactivator with a PDZ-binding motif, TAZ (Decleves and Sharma, 2014; Kok et al., 2014; Lee et al., 2015; Nitta et al., 2016; Szeto et al., 2016) may be a better approach.
TGF-β1 can also induce renal fibrosis via non-canonical (non-Smad) signaling. Some drugs act on both canonical and noncanonical TGF-β signaling pathways. For example, suramin decreased TGFβR2 expression, activation of EGF and PDGF receptors, activation of STAT3 and ERK1/2, inflammation, fibrosis, and tissue damage in either the UUO or remnant kidney models (Liu et al., 2012b; Liu et al., 2011a; Liu et al., 2011b) (Table 1).
3.4. Interleukin-33
IL-33 is released from cells during necrosis or by various stresses. This “alarmin” then activates the ST2 receptor on neighboring cells and various immune cells to trigger innate and adaptive immune responses. A soluble form of ST2 (sST2) acts as a decoy receptor. For the most part, IL-33 is thought to sustain an inflammatory response, and emerging evidence indicates that IL-33 plays an important role in contributing to kidney injury and fibrosis. Serum IL-33 and sST2 levels were reported to be positively associated with cardiovascular events, vascular injury, and mortality in CKD patients (Gungor et al., 2017). Circulating IL-33 levels are also positively associated with greater risk of adverse cardiovascular events in renal transplant recipients (Mansell et al., 2015). In the mouse, expression levels of IL-33 were found to be increased with renal injury induced by UUO, principally in interstitial myofibroblasts (Chen et al., 2016). Genetic deletion of IL-33 attenuated UUO-induced renal fibrosis and increased the number of proliferating tubular epithelial cells, indicating that IL-33 promotes tubular cell injury and renal interstitial fibrosis in a UUO animal model. Similarly, IL-33 treatment exacerbated renal fibrosis in a mouse model of ischemia-reperfusion injury, whereas administrating sST2 reduced renal dysfunction and fibrosis, which was associated with reduced inflammatory cell infiltration, myeloid fibroblast accumulation, and expression of pro-inflammatory cytokines and chemokines (Liang et al., 2017). There are several reports that IL-33 contributes to early-stage renal injury in other models of acute renal injury (ovalbumin or cisplatin-induced) (Akcay et al., 2011; Park et al., 2016), as well as contrast-induced nephropathy in diabetic kidney disease (Demirtas et al., 2016). On the other hand, recent studies provide evidence that IL-33-mediated engagement of ST2 receptors selectively on CD4+Foxp3+ regulatory T cells (Tregs) or group 2 innate lymphoid cells (ILC2s) protects the kidney from ischemia/reperfusion injury (Riedel et al., 2017; Stremska et al., 2017).
4. Fibrotic Growth Factors and ECM Proteins
4.1. Connective tissue growth factor (CTGF)
Matricellular protein CTGF is a direct downstream early response gene of TGF-β (Lee et al., 2015). Its expression is increased in various human and animal models of kidney fibrosis, including diabetic nephropathy, crescentic glomerulonephritis, and hypertensive nephrosclerosis. CTGF is secreted by fibroblasts and binds TGF-β1, thereby potentiating its signaling. This interaction leads to activation of myofibroblasts and extracellular accumulation of fibronectin to promote tissue fibrosis. CTGF also enhances TGF-β1 signaling by binding tyrosine receptor kinase A (TrkA), leading to induction of the transcription factor TGF-β-inducible early gene (TIEG)-1, which in turn represses expression of inhibitory Smad7 (Wahab et al., 2005). CTGF also binds bone morphogenetic protein 7 (BMP-7), thereby inhibiting its renoprotective effects (Nguyen et al., 2008). CTGF binds other growth factors (e.g., IGF-1, EGF, VEGF) as well to modify their function (Lee et al., 2015).
Inhibiting CTGF with antisense oligonucleotides, small interfering RNA, or neutralizing antibodies has been reported to reduce fibrosis in experimental models of kidney disease (Lee et al., 2015). The results of a phase I trial of FG-3019, an anti-CTGF antibody, demonstrated that this agent reduced albuminuria in patients with diabetic nephropathy. A further phase I trial of FG-3019 in patients with steroid-resistant FSGS (NCT00782561) was terminated and a phase II trial in patients with diabetic kidney disease (NCT00913393) was also terminated due to suboptimal study design (Decleves and Sharma, 2014) (Table 1).
Chronic allograft nephropathy (CAN) remains a potential complication for transplant recipients. Liu and colleagues found that replacement of cyclosporine A with rapamycin improved long-term graft survival in human CAN (Liu et al., 2007). Protective effects of rapamycin were associated with reduced CTGF expression, which would explain its antifibrotic and antiproliferative actions (Liu et al., 2007). However, in a phase II, open-label clinical trial, Cho and colleagues reported that rapamycin is associated with nephrotoxicity in some patients with FSGS, particularly those with prolonged disease duration and prior cyclosporine therapy (Cho et al., 2007a).
4.2. Epidermal growth factor (EGF) family
EGFR (aka HER1 or erbB-1) is a member of a family of four transmembrane tyrosine kinase receptors also including erbB-2 (proto-oncogene Neu or HER2), erbB-3 (HER3), and erbB-4 (HER4) (Schlessinger, 2002). Ligand binding causes these receptors to heterodimerize, which causes autophosphorylation that increases kinase activity responsible for the phosphorylation of downstream mediators (Holbro and Hynes, 2004). EGFR’s major ligands are EGF, heparin-binding EGF-like growth factor (HB-EGF), and TGF-α. These factors are initially membrane-bound and inactive but are released by proteolytic cleavage by metalloproteinases (Huovila et al., 2005). Increased expression of both HB-EGF and TGF-α is associated with renal pathophysiology. EGFR can also be transactivated indirectly by AngII via Src kinase-mediated activation of the membrane sheddase ADAM17. The release of its ligands from the cell surface initiates fibrotic (collagen, CFGF, TGF-β), inflammatory (COX2, IL-6, IL-1β, TNF-α), apoptosis, and oxidative stress pathways (Qian et al., 2016; Rayego-Mateos et al., 2013; Skibba et al., 2016). AngII can increase renal expression of TGF-α, as well as its release from the membrane, thereby activating EGFR signaling, which is believed to be largely responsible for its fibrotic effects (Lautrette et al., 2005).
Preclinical studies on waved-2 (Wa-2) mice support the benefit of targeting EGFR for antifibrotic therapy in progressive CKD. These mice have a 90% reduction in EGFR signaling because of a point mutation that inhibits tyrosine kinase activity. The Wa-2 mutation reduced ECM deposition and myofibroblast proliferation in the UUO model of obstructive nephropathy (Liu et al., 2012a) and a model of chronic renal ischemia (Tang et al., 2013). In addition, limiting EGFR activation by inhibiting its tyrosine kinase activity using erlotinib or gefitinib, or by transgenic silencing, leads to reduced fibrosis in various animal models (Kok et al., 2014). Targeting EGFR ligands by HB-EGF knockdown or TGF-α deletion is also renoprotective in different mouse models of renal injury. Thus, targeting the EGFR axis in kidney disease may be achieved by multiple routes with comparable results (Kok et al., 2014). However, in acute kidney injuries, such as folic-acid-induced nephrotoxicity and ischemia-reperfusion, EGFR inhibition with a tyrosine kinase inhibitor prolonged recovery time (Chen et al., 2012; He et al., 2013). Regeneration was also impaired in Wa-2 mice in the acute post-ischemic phase (Tang et al., 2013; Wang et al., 2003). These observations suggest that EGFR activation is beneficial during the very early phase of renal injury (Kok et al., 2014), which is evidenced by a patient with lung cancer in whom gefitinib treatment was associated with development of interstitial fibrosis and impaired regeneration of renal epithelial cells (Masutani et al., 2008).
4.3. Platelet-derived growth factor (PDGF)
The PDGFs are formed by disulfide-linked homo- and heterodimeric glycoproteins (PDGF-AA, -AB, -BB, -CC, and -DD) and are key factors in driving renal fibrosis, independent of the underlying disease (Kok et al., 2014). PDGF promotes proliferation and recruitment of mesenchymal cells, including fibroblasts, mesangial cells, pericytes, and smooth muscle cells, and induces pericyte-myofibroblast transition (Boor et al., 2014). PDGF receptors (PDGFRs) are constituted by α or β transmembrane proteins. The α- and -β chains homodimerize upon ligand-binding and undergo transautophosphorylation and activation via tyrosine kinase activity. PDGFRα binds PDGF-AA, PDGF-BB, and PDGF-AB; PDGFRβ binds PDGF-BB and PDGF-AB. Increased renal expression of all PDGF isoforms and receptors occurs in nearly all rodent models of kidney disease and corresponding human renal disease (Ostendorf et al., 2014).
PDGF-A expression in mesangial cells is upregulated in models of mesangioproliferative glomerulonephritis (i.e., “glomerular fibrosis”), and PDGF-A chain antisense oligonucleotides attenuated kidney damage in stroke-prone spontaneously hypertensive rats. PDGF-B and -D are key in activating mesangial matrix expansion and development of glomerulosclerosis. PDGF-BB induces renal tubulointerstitial cell proliferation, myofibroblast formation, and fibrosis in the rat, while blockade of PDGFRβ signaling reduced mesangial cell proliferation and matrix accumulation in the rat anti-Thy 1.1 model of mesangioproliferative glomerulonephritis. PDGF-C is upregulated by interstitial cells and macrophages near areas of tubulointerstitial fibrosis with enhanced PDGFRα expression by smooth muscle cells and myofibroblasts. In the mouse model of UUO-induced renal fibrosis, antibodies against PDGF-C or genetic knockout of PDGF-C attenuated tubulointerstitial fibrosis and myofibroblast activation (Ostendorf et al., 2014). The available drug imatinib inhibits PDGFR tyrosine kinase activity and attenuates renal fibrosis in different animal models of renal disease (Table 1) (Kok et al., 2014). PDGF-related side-effects could involve defective wound-healing, myelosuppression, fluid retention and cardiovascular and bone toxicity (Boor et al., 2014). While PDGF-B blockade is beneficial in most models of renal disease (Ostendorf et al., 2014), the nonspecific PDGF antagonist trapidil increased acute kidney injury after ischemia/reperfusion injury (Takikita-Suzuki et al., 2003). Evidence indicated that PDGF-B/PDGFRβ is important for tubular regeneration in this situation.
Pirfenidone inhibits PDGF, as well as TGF-β and TNFα by unknown means (Cho and Kopp, 2010; Decleves and Sharma, 2014; Hewitson et al., 2001; Lopez-de la Mora et al., 2015). Pirfenidone reduces ECM accumulation and inflammatory cell infiltration in animal models of CKD (Chen et al., 2013). In a small clinical trial on patients with focal segmental glomerulosclerosis, pirfenidone slowed the decline in estimated glomerular filtration rate (eGFR) but did not affect proteinuria (Table 1) (Cho et al., 2007b). Pirfenidone also prevented the decline in eGFR in a randomized, double-blind, placebo-controlled study of 77 subjects with diabetic nephropathy, although baseline plasma biomarkers of inflammation and fibrosis that correlated with baseline eGFR, such as TNFα, soluble TNFα receptor, and fibroblast growth factor 23, did not predict responsiveness to pirfenidone treatment (Sharma et al., 2011). Larger scale clinical trials are needed to better understand long-term efficacy and safety of this medication in various patient populations.
5. Intracellular Signaling Molecules Linked to Fibrosis
5.1. Mammalian target of rapamycin (mTOR)
Serine-threonine protein kinase mammalian target of rapamycin (mTOR) serves as an intracellular nutrient sensor controlling protein synthesis, metabolism, and cell growth, and is activated in patients with diabetic nephropathy. There are two mTOR complexes, mTORC1 and mTORC2, and rapamycin exhibits some selectively for mTORC1. Rapamycin inhibits fibrosis, interstitial inflammation, and the loss of renal function observed in various animal models of CKD (Falke et al., 2015). Subcapsular delivery of rapamycin in the rat UUO model decreased renal fibrosis and accumulation of interstitial myofibroblasts, indicating a role for mTORC1 (Falke et al., 2015). However, rapamycin and other mTOR inhibitors have many adverse side effects, which likely explains the high attrition rate in clinical trials (Falke et al., 2015). Moreover, mTOR knockout in podocytes contributes to renal disease, and in some patients rapamycin worsens proteinuria, restricting its therapeutic potential for diabetic nephropathy (Decleves and Sharma, 2014). Targeted delivery of mTOR inhibitors to the kidney to avoid adverse events have focused on nanomedicines, microspheres, conjugates, and self-assembling hydrogels that accumulate in specific kidney cell types (Falke et al., 2015). Rapamycin, a hydrophobic compound that is formulated efficiently in polymeric devices, has proven utility in drug-eluting stents in preventing restenosis. Falke and colleagues demonstrated that subcapsular delivery of rapamycin-loaded microspheres could inhibit inflammatory and fibrotic responses in the kidney while limiting systemic adverse reactions (Falke et al., 2015).
5.2. NF-κB
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activates a large number of pro-inflammatory genes. Therefore, it is an attractive therapeutic target for attenuating the inflammatory processes involved in CKD (Poveda et al., 2013). Treatment with pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-κB, attenuated renal injury and renal inflammation in different animal models of CKD, such as gentamicin-treated rats (Volpini et al., 2004), 5/6 nephrectomy rats (Fujihara et al., 2007), and aldosterone and salt-induced renal disease in rats (Ding et al., 2012). These findings reinforce the conclusion that NF-κB has a pivotal role in the progression of chronic renal inflammation.
Mycophenolic acid (MPA) is a non-competitive inhibitor of inosine monophosphate dehydrogenase (IMPDH). Its ester prodrug mycophenolate mofetil (MMF) strongly inhibits proliferation of T- and B-lymphocytes and is used for preventing acute and chronic allograft rejection (Table 1). Recent evidence suggests that MMF also inhibits proliferation of non-immune cells, including fibroblasts. Djamali and colleagues reported that MPA inhibited activation of NF-κB, Nox-2, and Smad2, and expression of α-SMA during TGF-β1-induced EMT of NRK52E cells (Djamali et al., 2010). They suggested that MPA delays allograft fibrosis by inhibiting TGF-β1-induced activation of Nox-2 by NF-κB. Thus, MPA may be a potential inhibitor of NF-κB signaling in patients with renal fibrosis. Nakanishi and colleagues compared the antifibrotic effects of BMS-566419, a chemically synthesized IMPDH inhibitor, to MMF in the UUO rat model of renal fibrosis (Nakanishi et al., 2010). The results suggested that BMS-566419 and similar inhibitors have beneficial effects equivalent to MMF, and are potential therapeutic candidates for fibrotic renal disease (Mihovilovic et al., 2014; Morath et al., 2006; Nakanishi et al., 2010). Lastly, the active components of several traditional Chinese medicines with renal protective effects, such as celastrol, rhein, and danshensu, inhibit the NF-κB pathway (Guan et al., 2015; Kim et al., 2013).
5.3. Other transcription factors
Krüppel-like factor 6 (KLF6) is a transcription factor and tumor suppressor, and specificity protein 1 (Sp1) is a well-studied member of the Krüppel-like family (Botella et al., 2009). Regulation of gene expression often occurs through cooperation of KLF6 and Sp1 by their direct physical interaction. Example genes include TGF-β, its receptors (type I and II), endoglin (a TGF-β auxiliary receptor), and the ECM component collagen type I, as well as uPA (urokinase plasminogen activator), which activates latent TGF-β. In vascular injury and inflammation, TGF-β is induced through KLF6–Sp1 interaction. Intracellular signals of the TGF-β ligands are transduced by the Smad proteins, and KLF6-Sp1 co-operation in regulating transcription of endoglin and certain other TGF-β target genes is further enhanced by the formation of a tripartite KLF6-Sp1-Smad3 complex (Botella et al., 2009). Sung and colleagues investigated the effects of a Smad decoy oligodeoxynucleotides (ODN), a Sp1 decoy ODN, and a chimeric decoy ODN containing both Smad and Sp1 binding sequences in one molecule, in UUO-induced renal fibrosis in mice (Sung et al., 2013). Expression of inflammatory-related cytokines and products of fibrosis were ameliorated by the decoy ODNs compared to a scrambled ODN. EMT was also suppressed. The chimeric decoy ODN had a greater inhibitory effect on fibrosis and EMT compared with the use of a single decoy ODN (Sung et al., 2013).
CCAAT-enhancer-binding protein delta (C/EBPδ) is known for its role in inflammation and cellular apoptosis or proliferation. During early stages of anti-Thy1.1 glomerulonephritis, C/EBPδ is induced and contributes to induction of inflammatory genes and proliferation of mesangial cells (Miyoshi et al., 2007). Based on rodent models of mesangial proliferative glomerulonephritis, C/EBPδ also has a key role in myofibroblast transdifferentiation, while delaying renal functional deterioration (Takeji et al., 2004) Unexpectedly, C/EBPδ deficiency resulted in greater fibrosis in the UUO model with enhanced tubular injury and expression of TGF-β (Duitman et al., 2014). The authors proposed that C/EBPδ is important in preventing vascular barrier disruption, thereby limiting interstitial fibrosis.
The prototypic cold-shock protein family member, Y-box protein-1 (YB-1) is a key regulator of several fibrosis-related genes. With ureteral obstruction, Yb1+/− mice with reduced YB-1 expression showed attenuated tubular injury, immune cell infiltration, and renal fibrosis. Cytoplasmic and perinuclear YB-1 levels were increased following UUO especially in dilated tubules, while glomerular YB-1 remained localized to the nucleus. The increased renal YB-1 was not phosphorylated at Ser102, known to be important for regulating gene transcription, but was phosphorylated at Tyr99 within the highly conserved cold shock domain. The upregulated YB-1 that localized to the cytoplasm was found to stabilize Col1a1 mRNA, thereby promoting fibrosis (Wang et al., 2016). On the other hand, forced nuclear expression of phosphorylated YB-1 by the small molecule HSc025, which disrupts the interaction of YB-1 and cytoplasmic retention protein poly(A)-binding protein, together with endogenous Ser102 phosphorylation, attenuated fibrosis by repressing the Col1a1 promoter (Table 1). Notably, HSc025 reduced tubulointerstitial damage if applied during the period of maximum renal tubular injury following AKI (Wang et al., 2016). Recently, specific deletion of YB-1 in monocytes/macrophages was found to result in increased tissue damage, immune cell infiltrates, myofibroblast activation and fibrosis following UUO (Bernhardt et al., 2017). These studies support the conclusion that YB-1 is important for macrophage polarization and the temporal resolution of inflammation through upregulation of IL-10.
6. Endogenous Antifibrotic Factors
Recent studies have identified endogenous antifibrotic factors that antagonize the fibrogenic actions of TGF-β (Fig. 1). An important strategy for antifibrotic therapy would be to increase or restore the expression of antifibrotic factors in the diseased kidney (Liu, 2006). Thus, we will discuss the therapeutic potential of some endogenous antifibrotic factors.
6.1. Interleukin 10 (IL-10)
IL-10 functions as a general immunosuppressive cytokine and is produced by various immune cell types, including B cells, monocytes, macrophages, T-helper 2 (Th2) cells, and keratinocytes (Jin et al., 2013). IL-10 reduces inflammation and mesangial cell proliferation in acute glomerulonephritis induced by anti-Thy1 antibody and suppresses glomerulosclerosis formation in a spontaneous rat model of FSGS (Mu et al., 2005). Jin and colleagues found that IL-10 knockout enhanced renal fibrosis in the UUO model of renal injury, which was associated with greater tubular injury, collagen deposition, and expression of pro-fibrotic genes (Jin et al., 2013). They also found that IL-10 deficient mice had more renal inflammation with UUO, with increased inflammatory cell infiltration and upregulation of inflammatory chemokines (MCP-1 and RANTES) and cytokines (TNF-α, IL-6, IL-8, and M-CSF). The enhanced renal inflammation and fibrosis were associated with enhanced TGF-β/Smad3 and NF-κB signaling (Jin et al., 2013). Thus, IL-10 could be a potential anti-fibrotic therapy for patients with CKD (Jin et al., 2013; Rachmawati et al., 2011). Short-term treatment with recombinant human IL-10 (rhIL-10) attenuated inflammation and inhibited matrix deposition in the anti-Thy1 antibody-induced glomerulonephritis rat, but had no effect on proteinuria (Rachmawati et al., 2011). However, IL-10 has a very short plasma half-life in vivo. Soranno and colleagues employed self-assembling and injectable Dock-and-Lock hydrogels for local delivery of IL-10 in the UUO mouse model (Soranno et al., 2014). These hydrogels remained in the kidney for up to 30 days, and macrophage infiltration and apoptosis were reduced at days 21 and 35 following hydrogel delivery. Hydrogel-delivered IL-10 reduced macrophage infiltration and apoptosis on day 35 more than IL-10 injection alone. Fibrosis was decreased in all treatment groups. This work supports the use of hydrogel delivery of IL-10 to treat renal fibrosis and chronic kidney disease (Soranno et al., 2014).
6.2. Interferon-gamma (IFN-γ)
IFN-γ is produced by various activated immune cells including NK cells and T cells. Besides pro-inflammatory effects, IFN-γ also has antiproliferative and antifibrotic effects (Poosti et al., 2015). IFN-γ inhibits fibroblast activation and proliferation and reduces collagen synthesis; however, clinical use of IFN-γ is limited by its short half-life and side effects. Cell-specific delivery may improve the therapeutic actions of IFN-γ and reduce systemic side effects. In injured tissues, mesenchymal-derived fibroblasts have elevated levels of platelet-derived growth factor receptor β (PDGFRβ). Poosti and colleagues constructed PEGylated IFN-γ conjugated to a PDGFR-recognizing cyclic peptide (PPB), PPB-PEG-IFN-γ (Poosti et al., 2015). In TGF-β activated NIH3T3 fibroblasts, PPB-PEG-IFN-γ inhibited expression of col1a1, col1a2, and α-SMA. In vivo, delivery of PPB-PEG-IFN-γ preserved tubular morphology in the UUO mouse with reduced expression of renal collagen I, fibronectin, and α-SMA, lower interstitial T-cell infiltration, and less lymphangiogenesis (Poosti et al., 2015).
6.3. Bone morphogenetic protein 7 (BMP-7)
BMP-7 is a member of the TGF-β superfamily originally discovered as a morphogen in kidney development and bone formation. Re-expression of BMP-7 occurs in spontaneous kidney regeneration, while BMP-7 expression is reduced in chronic kidney disease (Decleves and Sharma, 2014; Liu, 2006; Tampe and Zeisberg, 2014). Renal fibrosis is attenuated by BMP-7, by activating Smad1, Smad5, and Smad8, and inducing Smad7 to inhibit TGF-β signaling (Decleves and Sharma, 2014; Zeisberg et al., 2003). Exogenous BMP-7 ameliorates renal fibrosis in experimental CKD models, but due to soft tissue calcification, BMP-7 has limited clinical use. However, the regulation of BMP7 activity in the kidney is complex and determined not only by BMP7 levels, but levels of Kielin/chordin-like protein (KCP) and BMP receptors, as well as antagonists that include gremlin 1, noggin, and uterine sensitization-associated gene-1 (USAG-1) (Zeisberg and Kalluri, 2008). BMP-7 receptor agonists or targeting its antagonists show promise for the treatment of renal fibrosis (Decleves and Sharma, 2014). A small molecule AA123 that mimics BMP-7 activity by activating ALK3 signaling reproduces the antifibrotic activity of recombinant BMP-7 in murine models of renal fibrosis (Tampe and Zeisberg, 2014). A structural analog of AA123 is in phase II clinical testing (Table 1)
6.4. Hepatocyte growth factor (HGF)
HGF is important for tubular repair and regeneration after AKI and has effects opposite of TGF-β (Liu, 2006). Anti-HGF antibodies enhance the progression of tubulointerstitial fibrosis in rodents with CKD, and HGF inhibits TGF-β expression and prevents progression of renal fibrosis in various animal models (Decleves and Sharma, 2014). In vitro, HGF blocks activation of interstitial fibroblasts and glomerular mesangial cells and inhibits tubular EMT. It interferes with Smad signaling by several means. It blocks nuclear translocation of activated Smad-2/3 in fibroblasts, upregulates expression of the Smad corepressor SnoN in tubular epithelial cells, and prevents degradation of Smad corepressor, TGIF in glomerular mesangial cells (Liu, 2004; Yang et al., 2005). By suppressing TGF-β1, HGF also activates metalloproteinases and induces apoptosis of myofibroblasts (Iekushi et al., 2010).
The AngII type 1 receptor blocker (ARB), telmisartan is more effective than the prototypical ARB, losartan in reducing proteinuria in hypertensive patients with diabetic nephropathy, in part because it is a partial agonist of PPARγ (Table 1). HGF is a downstream effector of PPARγ. Kusunoki and colleagues reported that telmisartan increased renal HGF expression in AngII type 1 receptor-deficient mice following UUO (Kusunoki et al., 2012). PPARγ/HGF activation was associated with decreased expression of TGF-β1 and other pro-inflammatory and profibrotic cytokine genes. The protective actions of telmisartan were attenuated by a neutralizing antibody against HGF. Nasu and colleagues recently demonstrated that ONO-1301, a novel sustained-release prostacyclin analog, also suppressed tubulointerstitial alterations in UUO by inducing HGF (Nasu et al., 2012).
The growth factor augmenter of liver regeneration (ALR) has actions similar to those of HGF (Chen et al., 2014). Chen and colleagues reported that endogenous ALR attenuates renal fibrosis in the UUO rat model (Chen et al., 2014). In vitro experiments showed that recombinant human ALR inhibits EMT by inhibiting TGFβR2 expression and TGF-β1-induced signaling (Liao et al., 2014).
The expression of CD44 cell surface glycoproteins on tubular epithelial cells (TEC) is increased following renal injury and contribute to renal fibrosis. The shortest and most common isoform is CD44 standard (CD44s), but CD44v3-v10 (CD44v3) binds HGF/BMP-7. Rampanelli and colleagues reported that overexpressing CD44v3 on primary TEC made them less responsive to TGF-β’s profibrotic actions and more sensitive to BMP-7 and HGF (Rampanelli et al., 2013). Compared to wild-type and CD44s transgenic mice, CD44v3 transgenic mice had less tubular damage and accumulation of myofibroblasts after UUO. This was associated with decreased TGF-β1 signaling and increased BMP-7 synthesis and signaling.
6.5. 5'-AMP-activated protein kinase (AMPK)
The adipokine, adiponectin likely has renoprotective actions based on its anti-inflammatory properties. A causal link between metabolic disease and development of CKD is suggested by studies of adiponectin and its downstream signal molecule, 5'-AMP-activated protein kinase (AMPK). In mouse models of diabetic nephropathy, AMPK activation reduces glomerular accumulation of TGF-β, collagen, and fibronectin, and inhibited myofibroblast transdifferentiation (Dugan et al., 2013; Mishra et al., 2008). AMPK regulates TGF-β1 production by inhibiting upstream stimulatory factor 1 (USF1), a transcription factor that mediates glucose-induced TGF-β1 gene transcription (Decleves and Sharma, 2014). AMPK activation also blocks palmitic acid-induced MCP-1 expression in mesangial cells and attenuates macrophage infiltration and activation (Decleves et al., 2011). With AMPK activation, kidneys of mice on a high-fat diet had reduced macrophage infiltrates with a lower CD11c to CD11b ratio, indicating less M1 macrophages.
6.6. Kruppel-like factor-15 (KLF15)
Transcription factor KLF15, which is associated with reduced cardiac fibrosis, is decreased in remnant kidneys (Gao et al., 2011; Gao et al., 2013). Expression of KLF15 in mesangial cells is repressed by oxidative stress, TGF-β, and TNF-α via the TNF receptor-1 and NF-κB. KLF15 overexpression in mesangial and HEK293 cells attenuated levels of type IV collagen and fibronectin mRNA. KLF15 overexpression also repressed basal and TGF-β1-induced ECM and CTGF in NRK-49F cells. Moreover, KLF15 knockout mice developed glomerulosclerosis following uninephrectomy. Gao and colleagues found that TGF-β1-mediated activation of ERK/MAPK and JNK/MAPK downregulated KLF15 expression and increased levels of ECM and CTGF, effects that were completely abolished by inhibitors of ERK1/2 and JNK in NRK-49F cells (Gao et al., 2013).
6.7. Vitamin D
Vitamin D has protective effects on the kidney and cardiovascular system beyond mineral metabolism. Vitamin D deficiency has been reported to contribute to the progression of chronic kidney disease. Vitamin D analogs exhibit impressive renal protective actions in several animal models of kidney injury, either when given as alone or combined with RAAS blockade. Vitamin D receptor knockout mice had more severe renal damage in a mouse model of diabetic nephropathy (Zhang et al., 2008). The interaction of 1,25-dihydroxy vitamin D3 [1,25(OH)2D3] with the vitamin D receptor (VDR) controls transcription of >200 genes. A good number of these genes suppress renal fibrosis. Also, vitamin D analogs are anti-inflammatory by inhibiting NF-κB-mediated gene transcription due to an interaction between VDR and the p65 NF-κB subunit. Because AngII is a potent inducer of NF-κB activity, the ability of the vitamin D2 analog, paricalcitol to downregulate the RAAS likely explains part of its anti-inflammatory actions (Mirkovic and de Borst, 2012). In adriamycin (ADR) nephropathy, paricalcitol prevented proteinuria and renal interstitial damage by inhibiting Wnt/β-catenin signaling (He et al., 2011). Ito and colleagues found that 1,25(OH)2D3-bound VDR reduced renal fibrosis by interacting with Smad3 and blocking TGF-β-Smad signaling (Ito et al., 2013). They generated two synthetic analogs of 1,25(OH)2D3 that interact with VDR and inhibit TGF-β-induced Smad signaling without inducing classical VDR-mediated genes so as to avoid development of hypercalcemia (Bonventre, 2013).
Overall, the mechanisms underlying the renoprotective actions of vitamin D include negative regulation of the RAAS, and inhibition of TGF-β-Smad, NF-κB, and Wnt/β-catenin pathways (Mirkovic and de Borst, 2012). A comparison of the renoprotective potential of different vitamin D analogs in CKD patients being treated with inhibitors of the RAAS is an important progression towards optimizing therapy. The available preclinical data suggests that vitamin D analogs might be used as add-on therapy to protect the kidney in combination with inhibition of RAAS (Mirkovic and de Borst, 2012). Addition of paricalcitol to RAAS inhibition was found to lower residual albuminuria in patients with diabetic nephropathy (de Zeeuw et al., 2010), but a recently completed study indicated a lack of add-on benefit of paricalcitol in patients with advanced CKD (Thethi et al., 2015) (Table 1).
7. Renal Fibrosis the Common Pathway in progressive CKD
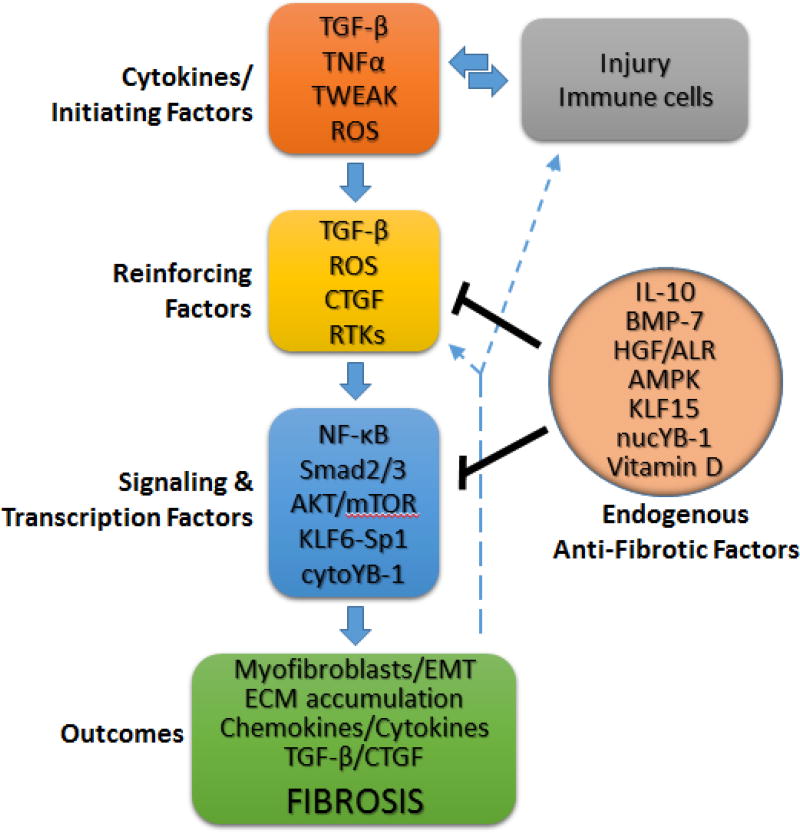
TGF-β plays a central role in inflammation/cytokine driven renal fibrosis (Fig. 2). Ultimately, regardless of the initiating factor, the process of renal fibrosis in CKD is sustained by the local production of TGF-β by resident kidney cells, infiltrating immune cells, and activated fibroblasts. TGF-β drives fibrosis by activating a number of signaling molecules that induce collagen production/turnover, form myofibroblasts from resident fibroblasts or epithelial/endothelial cells, and generate chemokines. The latter attract immune cells that sustain production of TGF-β and pro-inflammatory cytokines. The signaling molecules activated by TGF-β include AKT/mTOR, Smad2/3, NF-κB, KLF6, and Sp1 that induce protein synthesis or gene expression (including upregulation TGF-β). Moreover, the signaling actions of TGF-β are enhanced by CTGF, which is upregulated TGF-β. Pro-inflammatory cytokines TNF-α and TWEAK further enhance fibrosis and inflammation through NF-κB signaling. The resulting oxidative stress leads to sustained, ligand-dependent and -independent, activation of receptor tyrosine kinases EGFR and PDGFR, which fosters further oxidative stress that contributes to fibrosis, as well as cellular apoptosis. A number of endogenous signaling molecules oppose fibrosis, including IL-10 and vitamin D (targeting NF-κB), BMP-7, HGF, and vitamin D (targeting Smad2/3), and AMPK (targeting USF1/TGF-β and MCP-1 production).
Fig. 2. The common pathway in renal fibrosis.
In response to renal injury, resident renal cells and infiltrating immune cells produce a number of factors that initiate the processes of renal fibrosis, including TGF-β and ROS. These factors act in concert with various reinforcing factors, such as the sustained activation of receptor tyrosine kinases (RTKs), to activate signal transduction intermediates that drive ECM accumulation and renal fibrosis by multiple means, involving: the formation of fibroblasts with a synthetic phenotype (myofibroblasts) from resident fibroblasts or via the processes of epithelial-to-mesenchymal transition (EMT) or endothelial-to-mesenchymal transition (EndMT), cell death, and the production of pro-inflammatory or profibrotic cytokines (including TGF-β) that feedback to perpetuate the inflammatory and fibrotic cycle. Recent studies have defined a number of endogenous molecules that dampen the fibrotic process by targeting either the reinforcing factors or signaling intermediates. See text for additional details.
8. Conclusions and Future Perspectives
In summary, renal fibrosis involves complex interactions among multiple inflammatory cells and cytokine signaling pathways. Myofibroblasts are regulated by a variety of means, including paracrine signals derived from lymphocytes and macrophages, as well as autocrine factors. Critical profibrotic factors include MCP-1, NF-κB, TNFα, and TGF-β. Many endogenous antifibrotic factors have been identified, and others await discovery. However, there have been few clinical trials that have successfully targeted fibrosis in CKD, likely reflecting the fact that inflammation is multifactorial and self-sustaining. One complicating consideration is that the intracellular signaling pathways linked to fibrosis crosstalk with other signaling networks that influence critical cellular functions. Furthermore, a particular pathway may exhibit different effects depending upon the stage of renal fibrosis. Consequently, it may not be possible to rescue the disease by targeting only one particular pathway or molecule. Rather, a multi-pharmacological approach involving several anti-inflammatory and antifibrotic molecules may need to be employed. This conclusion is perhaps best illustrated by the greater efficacy of telmisartan, which not only blocks angiotensin II-induced TGF-β production and hypertension, but activates PPARγ and HGF production. The combination of telmisartan with a vitamin D analog may prove to be even more efficacious due to inhibition of AngII production and TGF-β signaling, along with minimal toxic actions that are a feature of single agents that are used in high doses. This combined treatment of telmisartan and vitamin D for CKD-related renal fibrosis awaits clinical trial. Finally, accumulating evidence shows that mesenchymal stem cells (MSCs) have renoprotective actions and reduce renal fibrosis in preclinical models of both AKI and CKD (Elseweidy et al., 2017; Roushandeh et al., 2017; Yokote et al., 2017; Zhu et al., 2017). Likely, their effectiveness is due to the release of multiple factors. A clinical trial (NCT03321942) is currently recruiting participants to assess the effficacy of adipose tissue-derived MSCs for treating CKD (Table 1).
Acknowledgments
This work was supported in part by grants HL36279 (Roman) and DK104184 (Roman), AG050049 (Fan), P20GM104357 (cores B and C-Roman; Pilot-Fan) from the National Institutes of Health; 16GRNT31200036 (Fan) from the American Heart Association and fellowship grants, 81270939, 81472983 and 81571625 from the National Natural Science Foundation of China. (Lv).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai J, Nie J, He J, Guo Q, Li M, Lei Y, Liu Y, Zhou Z, Zhu F, Liang M, Cheng Y, Hou FF. GQ5 Hinders Renal Fibrosis in Obstructive Nephropathy by Selectively Inhibiting TGF-beta-Induced Smad3 Phosphorylation. J Am Soc Nephrol. 2015;26:1827–1838. doi: 10.1681/ASN.2014040363. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, Altmann C, Toker A, Pacic A, Ljubanovic DG, Jani A, Faubel S, Edelstein CL. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol. 2011;22:2057–2067. doi: 10.1681/ASN.2010091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders HJ, Belemezova E, Eis V, Segerer S, Vielhauer V, Perez de Lema G, Kretzler M, Cohen CD, Frink M, Horuk R, Hudkins KL, Alpers CE, Mampaso F, Schlondorff D. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol. 2004;15:1504–1513. doi: 10.1097/01.asn.0000130082.67775.60. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Grone HJ, Onuffer J, Horuk R, Nelson PJ, Schlondorff D. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt A, Fehr A, Brandt S, Jerchel S, Ballhause TM, Philipsen L, Stolze S, Geffers R, Weng H, Fischer KD, Isermann B, Brunner-Weinzierl MC, Batra A, Siegmund B, Zhu C, Lindquist JA, Mertens PR. Inflammatory cell infiltration and resolution of kidney inflammation is orchestrated by the cold-shock protein Y-box binding protein-1. Kidney Int. 2017 doi: 10.1016/j.kint.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Antifibrotic vitamin D analogs. J Clin Invest. 2013;123:4570–4573. doi: 10.1172/JCI72748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol Dial Transplant. 2014;29(Suppl 1):i45–i54. doi: 10.1093/ndt/gft273. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- Botella LM, Sanz-Rodriguez F, Komi Y, Fernandez LA, Varela E, Garrido-Martin EM, Narla G, Friedman SL, Kojima S. TGF-beta regulates the expression of transcription factor KLF6 and its splice variants and promotes co-operative transactivation of common target genes through a Smad3-Sp1-KLF6 interaction. Biochem J. 2009;419:485–495. doi: 10.1042/BJ20081434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos CP, Sonehara NM, Oliani SM, Burdmann EA. Predictive usefulness of urinary biomarkers for the identification of cyclosporine A-induced nephrotoxicity in a rat model. PLoS One. 2014;9:e103660. doi: 10.1371/journal.pone.0103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GT, Zhang L, Liao XH, Yan RY, Li Y, Sun H, Guo H, Liu Q. Augmenter of liver regeneration ameliorates renal fibrosis in rats with obstructive nephropathy. Biosci Rep. 2014;34 doi: 10.1042/BSR20140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82:45–52. doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Ni HF, Pan MM, Liu H, Xu M, Zhang MH, Liu BC. Pirfenidone inhibits macrophage infiltration in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 2013;304:F676–685. doi: 10.1152/ajprenal.00507.2012. [DOI] [PubMed] [Google Scholar]
- Chen WY, Chang YJ, Su CH, Tsai TH, Chen SD, Hsing CH, Yang JL. Upregulation of Interleukin-33 in obstructive renal injury. Biochem Biophys Res Commun. 2016;473:1026–1032. doi: 10.1016/j.bbrc.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Cheng J, Diaz Encarnacion MM, Warner GM, Gray CE, Nath KA, Grande JP. TGF-beta1 stimulates monocyte chemoattractant protein-1 expression in mesangial cells through a phosphodiesterase isoenzyme 4-dependent process. Am J Physiol Cell Physiol. 2005;289:C959–970. doi: 10.1152/ajpcell.00153.2005. [DOI] [PubMed] [Google Scholar]
- Cho ME, Hurley JK, Kopp JB. Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis. 2007a;49:310–317. doi: 10.1053/j.ajkd.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Cho ME, Kopp JB. Pirfenidone: an anti-fibrotic therapy for progressive kidney disease. Expert Opin Investig Drugs. 2010;19:275–283. doi: 10.1517/13543780903501539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007b;2:906–913. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- Chou CH, Chuang LY, Lu CY, Guh JY. Interaction between TGF-beta and ACE2-Ang-(1–7)-Mas pathway in high glucose-cultured NRK-52E cells. Mol Cell Endocrinol. 2013;366:21–30. doi: 10.1016/j.mce.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- Chuang ST, Kuo YH, Su MJ. Antifibrotic effects of KS370G, a caffeamide derivative, in renal ischemia-reperfusion injured mice and renal tubular epithelial cells. Sci Rep. 2014;4:5814. doi: 10.1038/srep05814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, Potarca A, Tesar V, Heerspink HJ, Schall TJ, Group CBDNS. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3:687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22:1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decleves AE, Sharma K. Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol. 2014;10:257–267. doi: 10.1038/nrneph.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle H, Rocha JR, Cavaglieri RC, Vieira JM, Jr, Malheiros DM, Noronha IL. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012;23:37–48. doi: 10.1681/ASN.2011010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas L, Turkmen K, Kandemir FM, Ozkaraca M, Kucukler S, Gurbuzel M, Comakli S. The possible role of interleukin-33 as a new player in the pathogenesis of contrast-induced nephropathy in diabetic rats. Ren Fail. 2016;38:952–960. doi: 10.3109/0886022X.2016.1165034. [DOI] [PubMed] [Google Scholar]
- Dhruv H, Loftus JC, Narang P, Petit JL, Fameree M, Burton J, Tchegho G, Chow D, Yin H, Al-Abed Y, Berens ME, Tran NL, Meurice N. Structural basis and targeting of the interaction between fibroblast growth factor-inducible 14 and tumor necrosis factor-like weak inducer of apoptosis. J Biol Chem. 2013;288:32261–32276. doi: 10.1074/jbc.M113.493536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Yang L, Zhang M, Gu Y. Chronic inhibition of nuclear factor kappa B attenuates aldosterone/salt-induced renal injury. Life Sci. 2012;90:600–606. doi: 10.1016/j.lfs.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Djamali A, Vidyasagar A, Yagci G, Huang LJ, Reese S. Mycophenolic acid may delay allograft fibrosis by inhibiting transforming growth factor-beta1-induced activation of Nox-2 through the nuclear factor-kappaB pathway. Transplantation. 2010;90:387–393. doi: 10.1097/TP.0b013e3181e6ae0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duitman J, Borensztajn KS, Pulskens WP, Leemans JC, Florquin S, Spek CA. CCAAT-enhancer binding protein delta (C/EBPdelta) attenuates tubular injury and tubulointerstitial fibrogenesis during chronic obstructive nephropathy. Lab Invest. 2014;94:89–97. doi: 10.1038/labinvest.2013.127. [DOI] [PubMed] [Google Scholar]
- Elseweidy MM, Askar ME, Elswefy SE, Shawky M. Nephrotoxicity Induced by Cisplatin Intake in Experimental Rats and Therapeutic Approach of Using Mesenchymal Stem Cells and Spironolactone. Appl Biochem Biotechnol. 2017 doi: 10.1007/s12010-017-2631-0. [DOI] [PubMed] [Google Scholar]
- Falke LL, van Vuuren SH, Kazazi-Hyseni F, Ramazani F, Nguyen TQ, Veldhuis GJ, Maarseveen EM, Zandstra J, Zuidema J, Duque LF, Steendam R, Popa ER, Kok RJ, Goldschmeding R. Local therapeutic efficacy with reduced systemic side effects by rapamycin-loaded subcapsular microspheres. Biomaterials. 2015;42:151–160. doi: 10.1016/j.biomaterials.2014.11.042. [DOI] [PubMed] [Google Scholar]
- Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JM, Jr, Zatz R. Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol. 2007;292:F92–99. doi: 10.1152/ajprenal.00184.2006. [DOI] [PubMed] [Google Scholar]
- Gaedeke J, Noble NA, Border WA. Curcumin blocks multiple sites of the TGF-beta signaling cascade in renal cells. Kidney Int. 2004;66:112–120. doi: 10.1111/j.1523-1755.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Gao X, Huang L, Grosjean F, Esposito V, Wu J, Fu L, Hu H, Tan J, He C, Gray S, Jain MK, Zheng F, Mei C. Low-protein diet supplemented with ketoacids reduces the severity of renal disease in 5/6 nephrectomized rats: a role for KLF15. Kidney Int. 2011;79:987–996. doi: 10.1038/ki.2010.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wu G, Gu X, Fu L, Mei C. Kruppel-like factor 15 modulates renal interstitial fibrosis by ERK/MAPK and JNK/MAPK pathways regulation. Kidney Blood Press Res. 2013;37:631–640. doi: 10.1159/000355743. [DOI] [PubMed] [Google Scholar]
- Gomez IG, Roach AM, Nakagawa N, Amatucci A, Johnson BG, Dunn K, Kelly MC, Karaca G, Zheng TS, Szak S, Peppiatt-Wildman CM, Burkly LC, Duffield JS. TWEAK-Fn14 Signaling Activates Myofibroblasts to Drive Progression of Fibrotic Kidney Disease. J Am Soc Nephrol. 2016;27:3639–3652. doi: 10.1681/ASN.2015111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Wu XX, Duan JL, Yin Y, Guo C, Wei G, Wang YH, Zhu YR, Weng Y, Xi MM, Wen AD. Effects and Mechanism of Combination of Rhein and Danshensu in the Treatment of Chronic Kidney Disease. Am J Chin Med. 2015;43:1381–1400. doi: 10.1142/S0192415X15500780. [DOI] [PubMed] [Google Scholar]
- Gungor O, Unal HU, Guclu A, Gezer M, Eyileten T, Guzel FB, Altunoren O, Erken E, Oguz Y, Kocyigit I, Yilmaz MI. IL-33 and ST2 levels in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS One. 2017;12:e0178939. doi: 10.1371/journal.pone.0178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wu W, Sheng M, Yang S, Tan J. Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol Med Rep. 2013;7:1453–1457. doi: 10.3892/mmr.2013.1391. [DOI] [PubMed] [Google Scholar]
- Hallan SI, Ovrehus MA, Romundstad S, Rifkin D, Langhammer A, Stevens PE, Ix JH. Long-term trends in the prevalence of chronic kidney disease and the influence of cardiovascular risk factors in Norway. Kidney Int. 2016;90:665–673. doi: 10.1016/j.kint.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Haller ST, Kumarasamy S, Folt DA, Wuescher LM, Stepkowski S, Karamchandani M, Waghulde H, Mell B, Chaudhry M, Maxwell K, Upadhyaya S, Drummond CA, Tian J, Filipiak WE, Saunders TL, Shapiro JI, Joe B, Cooper CJ. Targeted disruption of Cd40 in a genetically hypertensive rat model attenuates renal fibrosis and proteinuria, independent of blood pressure. Kidney Int. 2016 doi: 10.1016/j.kint.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Zhu J, Wang Y, Zhu Z, Chen Y, Lu L, Jin W, Yan X, Zhang R. Renal Recruitment of B Lymphocytes Exacerbates Tubulointerstitial Fibrosis by Promoting Monocyte Mobilisation and Infiltration after Unilateral Ureteral Obstruction. J Pathol. 2016 doi: 10.1002/path.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F356–366. doi: 10.1152/ajprenal.00553.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson TD, Kelynack KJ, Tait MG, Martic M, Jones CL, Margolin SB, Becker GJ. Pirfenidone reduces in vitro rat renal fibroblast activation and mitogenesis. J Nephrol. 2001;14:453–460. [PubMed] [Google Scholar]
- Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, Nakamura S, Konishi N, Yagita H, Nonomura K, Nakajima Y. Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int. 2011;79:179–188. doi: 10.1038/ki.2010.379. [DOI] [PubMed] [Google Scholar]
- Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Mou L, Yang F, Tu H, Lin W. Curcumin attenuates cyclosporine A induced renal fibrosis by inhibiting hypermethylation of the klotho promoter. Mol Med Rep. 2016;14:3229–3236. doi: 10.3892/mmr.2016.5601. [DOI] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Idasiak-Piechocka I, Oko A, Pawliczak E, Kaczmarek E, Czekalski S. Urinary excretion of soluble tumour necrosis factor receptor 1 as a marker of increased risk of progressive kidney function deterioration in patients with primary chronic glomerulonephritis. Nephrol Dial Transplant. 2010;25:3948–3956. doi: 10.1093/ndt/gfq310. [DOI] [PubMed] [Google Scholar]
- Iekushi K, Taniyama Y, Azuma J, Sanada F, Kusunoki H, Yokoi T, Koibuchi N, Okayama K, Rakugi H, Morishita R. Hepatocyte growth factor attenuates renal fibrosis through TGF-beta1 suppression by apoptosis of myofibroblasts. J Hypertens. 2010;28:2454–2461. doi: 10.1097/HJH.0b013e32833e4149. [DOI] [PubMed] [Google Scholar]
- Ito I, Waku T, Aoki M, Abe R, Nagai Y, Watanabe T, Nakajima Y, Ohkido I, Yokoyama K, Miyachi H, Shimizu T, Murayama A, Kishimoto H, Nagasawa K, Yanagisawa J. A nonclassical vitamin D receptor pathway suppresses renal fibrosis. J Clin Invest. 2013;123:4579–4594. doi: 10.1172/JCI67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo MC, Sanz AB, Mezzano S, Blanco J, Carrasco S, Sanchez-Nino MD, Benito-Martin A, Ruiz-Ortega M, Egido J, Ortiz A. TWEAK (tumor necrosis factor-like weak inducer of apoptosis) activates CXCL16 expression during renal tubulointerstitial inflammation. Kidney Int. 2012;81:1098–1107. doi: 10.1038/ki.2011.475. [DOI] [PubMed] [Google Scholar]
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liu R, Xie J, Xiong H, He JC, Chen N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest. 2013;93:801–811. doi: 10.1038/labinvest.2013.64. [DOI] [PubMed] [Google Scholar]
- Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, Takahashi T, Abe H, Iehara N, Fukatsu A, Okamoto H, Kita T, Doi T, Arai H. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360:772–777. doi: 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- Kang YS, Lee MH, Song HK, Ko GJ, Kwon OS, Lim TK, Kim SH, Han SY, Han KH, Lee JE, Han JY, Kim HK, Cha DR. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 2010;78:883–894. doi: 10.1038/ki.2010.263. [DOI] [PubMed] [Google Scholar]
- Kania DS, Smith CT, Nash CL, Gonzalvo JD, Bittner A, Shepler BM. Potential new treatments for diabetic kidney disease. Med Clin North Am. 2013;97:115–134. doi: 10.1016/j.mcna.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Khwaja A, El Kossi M, Floege J, El Nahas M. The management of CKD: a look into the future. Kidney Int. 2007;72:1316–1323. doi: 10.1038/sj.ki.5002489. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lee MH, Nam DH, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Han SY, Han KH, Han JY, Cha DR. Celastrol, an NF-kappaB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One. 2013;8:e62068. doi: 10.1371/journal.pone.0062068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Gonzalez J, Decramer S, Bandin F, Neau E, Salant DJ, Heeringa P, Pesquero JB, Schanstra JP, Bascands JL. Blockade of the kinin B1 receptor ameloriates glomerulonephritis. J Am Soc Nephrol. 2010;21:1157–1164. doi: 10.1681/ASN.2009090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Gonzalez J, Duchene J, Esposito L, Pradere JP, Neau E, Delage C, Calise D, Ahluwalia A, Carayon P, Pesquero JB, Bader M, Schanstra JP, Bascands JL. Delayed blockade of the kinin B1 receptor reduces renal inflammation and fibrosis in obstructive nephropathy. FASEB J. 2009;23:134–142. doi: 10.1096/fj.08-115600. [DOI] [PubMed] [Google Scholar]
- Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol. 2014;10:700–711. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- Kusunoki H, Taniyama Y, Azuma J, Iekushi K, Sanada F, Otsu R, Iwabayashi M, Okayama K, Rakugi H, Morishita R. Telmisartan exerts renoprotective actions via peroxisome proliferator-activated receptor-gamma/hepatocyte growth factor pathway independent of angiotensin II type 1 receptor blockade. Hypertension. 2012;59:308–316. doi: 10.1161/HYPERTENSIONAHA.111.176263. [DOI] [PubMed] [Google Scholar]
- Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res. 2015;165:512–530. doi: 10.1016/j.trsl.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Xu F, Wen XJ, Liu HZ, Wang HB, Zhong JY, Yang CX, Zhang B. Interleukin-33 signaling contributes to renal fibrosis following ischemia reperfusion. Eur J Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Liao XH, Zhang L, Chen GT, Yan RY, Sun H, Guo H, Liu Q. Augmenter of liver regeneration inhibits TGF-beta1-induced renal tubular epithelial-to-mesenchymal transition via suppressing TbetaR II expression in vitro. Exp Cell Res. 2014;327:287–296. doi: 10.1016/j.yexcr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Liu M, Zhang W, Gu M, Yin C, Zhang WY, Lv Q, Xu D. Protective effects of sirolimus by attenuating connective tissue growth factor expression in human chronic allograft nephropathy. Transplant Proc. 2007;39:1410–1415. doi: 10.1016/j.transproceed.2007.03.072. [DOI] [PubMed] [Google Scholar]
- Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012a;23:854–867. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, He S, Tolbert E, Gong R, Bayliss G, Zhuang S. Suramin alleviates glomerular injury and inflammation in the remnant kidney. PLoS One. 2012b;7:e36194. doi: 10.1371/journal.pone.0036194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011a;22:1064–1075. doi: 10.1681/ASN.2010090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Ponnusamy M, Yan H, Zhuang S. Delayed administration of suramin attenuates the progression of renal fibrosis in obstructive nephropathy. J Pharmacol Exp Ther. 2011b;338:758–766. doi: 10.1124/jpet.111.181727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QF, Ye JM, Deng ZY, Yu LX, Sun Q, Li SS. Ameliorating effect of Klotho on endoplasmic reticulum stress and renal fibrosis induced by unilateral ureteral obstruction. Iran J Kidney Dis. 2015;9:291–297. [PubMed] [Google Scholar]
- Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Huang XR, Chen HY, Penninger JM, Lan HY. Loss of angiotensin-converting enzyme 2 enhances TGF-beta/Smad-mediated renal fibrosis and NF-kappaB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest. 2012c;92:650–661. doi: 10.1038/labinvest.2012.2. [DOI] [PubMed] [Google Scholar]
- Locatelli I, Sutti S, Jindal A, Vacchiano M, Bozzola C, Reutelingsperger C, Kusters D, Bena S, Parola M, Paternostro C, Bugianesi E, McArthur S, Albano E, Perretti M. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology. 2014;60:531–544. doi: 10.1002/hep.27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S, Macias-Barragan J, Armendariz-Borunda J. Role and New Insights of Pirfenidone in Fibrotic Diseases. Int J Med Sci. 2015;12:840–847. doi: 10.7150/ijms.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaga-Dieguez L, Bouhassira D, Gipson D, Trachtman H. Novel therapies for FSGS: preclinical and clinical studies. Adv Chronic Kidney Dis. 2015;22:e1–6. doi: 10.1053/j.ackd.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell H, Soliman M, Elmoselhi H, Shoker A. Elevated Circulating Interleukin 33 Levels in Stable Renal Transplant Recipients at High Risk for Cardiovascular Events. PLoS One. 2015;10:e0142141. doi: 10.1371/journal.pone.0142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani K, Fujisaki K, Maeda H, Toyonaga J, Inoshima I, Takayama K, Katafuchi R, Hirakata H, Tsuruya K, Iida M. Tubulointerstitial nephritis and IgA nephropathy in a patient with advanced lung cancer treated with long-term gefitinib. Clin Exp Nephrol. 2008;12:398–402. doi: 10.1007/s10157-008-0066-1. [DOI] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- Michaelson JS, Wisniacki N, Burkly LC, Putterman C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J Autoimmun. 2012;39:130–142. doi: 10.1016/j.jaut.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihovilovic K, Maksimovic B, Kocman B, Gustin D, Vidas Z, Bulimbasic S, Ljubanovic DG, Matovinovic MS, Knotek M. Effect of mycophenolate mofetil on progression of interstitial fibrosis and tubular atrophy after kidney transplantation: a retrospective study. BMJ Open. 2014;4:e005005. doi: 10.1136/bmjopen-2014-005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic K, de Borst MH. Beyond the RAAS: dissecting the antifibrotic effects of vitamin D analogues. Lab Invest. 2012;92:1666–1669. doi: 10.1038/labinvest.2012.150. [DOI] [PubMed] [Google Scholar]
- Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Okura T, Fukuoka T, Higaki J. CCAAT/enhancer-binding protein-delta is induced in mesangial area during the early stages of anti-Thy1.1 glomerulonephritis and regulates cell proliferation and inflammatory gene expression in cultured rat mesangial cells. Clin Exp Nephrol. 2007;11:26–33. doi: 10.1007/s10157-006-0445-4. [DOI] [PubMed] [Google Scholar]
- Morath C, Schwenger V, Beimler J, Mehrabi A, Schmidt J, Zeier M, Muranyi W. Antifibrotic actions of mycophenolic acid. Clin Transplant. 2006;20(Suppl 17):25–29. doi: 10.1111/j.1399-0012.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- Morinaga J, Kakizoe Y, Miyoshi T, Onoue T, Ueda M, Mizumoto T, Yamazoe R, Uchimura K, Hayata M, Shiraishi N, Adachi M, Sakai Y, Tomita K, Kitamura K. The antifibrotic effect of a serine protease inhibitor in the kidney. Am J Physiol Renal Physiol. 2013;305:F173–181. doi: 10.1152/ajprenal.00586.2012. [DOI] [PubMed] [Google Scholar]
- Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, Yamamoto T. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44:215–218. doi: 10.1007/s00592-007-0007-6. [DOI] [PubMed] [Google Scholar]
- Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651–3660. doi: 10.1681/ASN.2005030297. [DOI] [PubMed] [Google Scholar]
- Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY Centers for Disease, C., Prevention Chronic Kidney Disease Surveillance, T. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med. 2016 doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Morokata T, Noto T, Kubo K, Umeno H, Kinugasa F, Eikyu Y, Kozuki Y, Seki N. Effect of the inosine 5'-monophosphate dehydrogenase inhibitor BMS-566419 on renal fibrosis in unilateral ureteral obstruction in rats. Int Immunopharmacol. 2010;10:1434–1439. doi: 10.1016/j.intimp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Nasu T, Kinomura M, Tanabe K, Yamasaki H, Htay SL, Saito D, Hinamoto N, Watatani H, Ujike H, Suzuki Y, Sugaya T, Sugiyama H, Sakai Y, Matsumoto K, Maeshima Y, Makino H. Sustained-release prostacyclin analog ONO-1301 ameliorates tubulointerstitial alterations in a mouse obstructive nephropathy model. Am J Physiol Renal Physiol. 2012;302:F1616–1629. doi: 10.1152/ajprenal.00538.2011. [DOI] [PubMed] [Google Scholar]
- Neymeyer H, Labes R, Reverte V, Saez F, Stroh T, Dathe C, Hohberger S, Zeisberg M, Muller GA, Salazar J, Bachmann S, Paliege A. Activation of annexin A1 signalling in renal fibroblasts exerts antifibrotic effects. Acta Physiol (Oxf) 2015;215:144–158. doi: 10.1111/apha.12586. [DOI] [PubMed] [Google Scholar]
- Neyra JA, Hu MC. alphaKlotho and Chronic Kidney Disease. Vitam Horm. 2016;101:257–310. doi: 10.1016/bs.vh.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TQ, Roestenberg P, van Nieuwenhoven FA, Bovenschen N, Li Z, Xu L, Oliver N, Aten J, Joles JA, Vial C, Brandan E, Lyons KM, Goldschmeding R. CTGF inhibits BMP-7 signaling in diabetic nephropathy. J Am Soc Nephrol. 2008;19:2098–2107. doi: 10.1681/ASN.2007111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta K, Shi S, Nagai T, Kanasaki M, Kitada M, Srivastava SP, Haneda M, Kanasaki K, Koya D. Oral Administration of N-Acetyl-seryl-aspartyl-lysyl-proline Ameliorates Kidney Disease in Both Type 1 and Type 2 Diabetic Mice via a Therapeutic Regimen. Biomed Res Int. 2016;2016:9172157. doi: 10.1155/2016/9172157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YJ, An JN, Kim CT, Yang SH, Lee H, Kim DK, Joo KW, Paik JH, Kang SW, Park JT, Lim CS, Kim YS, Lee JP. Circulating Tumor Necrosis Factor alpha Receptors Predict the Outcomes of Human IgA Nephropathy: A Prospective Cohort Study. PLoS One. 2015;10:e0132826. doi: 10.1371/journal.pone.0132826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote K, Gohda T, Murakoshi M, Sasaki Y, Kazuno S, Fujimura T, Ishizaka M, Sonoda Y, Tomino Y. Role of the TNF pathway in the progression of diabetic nephropathy in KK-A(y) mice. Am J Physiol Renal Physiol. 2014;306:F1335–1347. doi: 10.1152/ajprenal.00509.2013. [DOI] [PubMed] [Google Scholar]
- Ostendorf T, Boor P, van Roeyen CR, Floege J. Platelet-derived growth factors (PDGFs) in glomerular and tubulointerstitial fibrosis. Kidney Int Suppl (2011) 2014;4:65–69. doi: 10.1038/kisup.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GH, Shinn HK, Kang JH, Na WJ, Kim YH, Park CS. Anti-interleukin-33 Reduces Ovalbumin-Induced Nephrotoxicity and Expression of Kidney Injury Molecule-1. Int Neurourol J. 2016;20:114–121. doi: 10.5213/inj.1632578.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poosti F, Bansal R, Yazdani S, Prakash J, Post E, Klok P, van den Born J, de Borst MH, van Goor H, Poelstra K, Hillebrands JL. Selective delivery of IFN-gamma to renal interstitial myofibroblasts: a novel strategy for the treatment of renal fibrosis. FASEB J. 2015;29:1029–1042. doi: 10.1096/fj.14-258459. [DOI] [PubMed] [Google Scholar]
- Poveda J, Tabara LC, Fernandez-Fernandez B, Martin-Cleary C, Sanz AB, Selgas R, Ortiz A, Sanchez-Nino MD. TWEAK/Fn14 and Non-Canonical NF-kappaB Signaling in Kidney Disease. Front Immunol. 2013;4:447. doi: 10.3389/fimmu.2013.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Peng K, Qiu C, Skibba M, Huang Y, Xu Z, Zhang Y, Hu J, Liang D, Zou C, Wang Y, Liang G. Novel Epidermal Growth Factor Receptor Inhibitor Attenuates Angiotensin II-Induced Kidney Fibrosis. J Pharmacol Exp Ther. 2016;356:32–42. doi: 10.1124/jpet.115.228080. [DOI] [PubMed] [Google Scholar]
- Quiroga B, Arroyo D, de Arriba G. Present and future in the treatment of diabetic kidney disease. J Diabetes Res. 2015;2015:801348. doi: 10.1155/2015/801348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmawati H, Beljaars L, Reker-Smit C, Bakker HI, Van Loenen-Weemaes AM, Lub-De Hooge MN, Poelstra K. Intravenous administration of recombinant human IL-10 suppresses the development of anti-thy 1-induced glomerulosclerosis in rats. PDA J Pharm Sci Technol. 2011;65:116–130. [PubMed] [Google Scholar]
- Rampanelli E, Rouschop K, Teske GJ, Claessen N, Leemans JC, Florquin S. CD44v3-v10 reduces the profibrotic effects of TGF-beta1 and attenuates tubular injury in the early stage of chronic obstructive nephropathy. Am J Physiol Renal Physiol. 2013;305:F1445–1454. doi: 10.1152/ajprenal.00340.2013. [DOI] [PubMed] [Google Scholar]
- Rayego-Mateos S, Rodrigues-Diez R, Morgado-Pascual JL, Rodrigues Diez RR, Mas S, Lavoz C, Alique M, Pato J, Keri G, Ortiz A, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J Mol Cell Biol. 2013;5:323–335. doi: 10.1093/jmcb/mjt030. [DOI] [PubMed] [Google Scholar]
- Riedel JH, Becker M, Kopp K, Duster M, Brix SR, Meyer-Schwesinger C, Kluth LA, Gnirck AC, Attar M, Krohn S, Fehse B, Stahl RA, Panzer U, Turner JE. IL-33-Mediated Expansion of Type 2 Innate Lymphoid Cells Protects from Progressive Glomerulosclerosis. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roushandeh AM, Bahadori M, Roudkenar MH. Mesenchymal Stem Cell-based Therapy as a New Horizon for Kidney Injuries. Arch Med Res. 2017;48:133–146. doi: 10.1016/j.arcmed.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Ortiz A, Ramos AM. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) and kidney disease. Curr Opin Nephrol Hypertens. 2014;23:93–100. doi: 10.1097/01.mnh.0000437331.23794.81. [DOI] [PubMed] [Google Scholar]
- Sanchez-Nino MD, Poveda J, Sanz AB, Mezzano S, Carrasco S, Fernandez-Fernandez B, Burkly LC, Nair V, Kretzler M, Hodgin JB, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A. Fn14 in podocytes and proteinuric kidney disease. Biochim Biophys Acta. 2013;1832:2232–2243. doi: 10.1016/j.bbadis.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Santana AC, Degaspari S, Catanozi S, Delle H, de Sa Lima L, Silva C, Blanco P, Solez K, Scavone C, Noronha IL. Thalidomide suppresses inflammation in adenine-induced CKD with uraemia in mice. Nephrol Dial Transplant. 2013;28:1140–1149. doi: 10.1093/ndt/gfs569. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Kang HT. Recent trends in the prevalence of chronic kidney disease in Korean adults: Korean National Health and Nutrition Examination Survey from 1998 to 2013. J Nephrol. 2016 doi: 10.1007/s40620-016-0280-y. [DOI] [PubMed] [Google Scholar]
- Skibba M, Qian Y, Bao Y, Lan J, Peng K, Zhao Y, Zhong P, Hu J, Li X, Liang G. New EGFR inhibitor, 453, prevents renal fibrosis in angiotensin II-stimulated mice. Eur J Pharmacol. 2016;789:421–430. doi: 10.1016/j.ejphar.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Soetikno V, Sari FR, Lakshmanan AP, Arumugam S, Harima M, Suzuki K, Kawachi H, Watanabe K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57:1649–1659. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- Soler MJ, Riera M, Gutierrez A, Pascual J. New options and perspectives for proteinuria management after kidney transplantation. Transplant Rev (Orlando) 2012;26:44–52. doi: 10.1016/j.trre.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Soranno DE, Lu HD, Weber HM, Rai R, Burdick JA. Immunotherapy with injectable hydrogels to treat obstructive nephropathy. J Biomed Mater Res A. 2014;102:2173–2180. doi: 10.1002/jbm.a.34902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremska ME, Jose S, Sabapathy V, Huang L, Bajwa A, Kinsey GR, Sharma PR, Mohammad S, Rosin DL, Okusa MD, Sharma R. IL233, A Novel IL-2 and IL-33 Hybrid Cytokine, Ameliorates Renal injury. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T, Miao Z, Dairaghi DJ, Krasinski A, Wang Y, Zhao BN, Baumgart T, Ertl LS, Pennell A, Seitz L, Powers J, Zhao R, Ungashe S, Wei Z, Boring L, Tsou CL, Charo I, Berahovich RD, Schall TJ, Jaen JC. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Renal Physiol. 2013;305:F1288–1297. doi: 10.1152/ajprenal.00316.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung WJ, Kim KH, Kim YJ, Chang YC, Lee IH, Park KK. Antifibrotic effect of synthetic Smad/Sp1 chimeric decoy oligodeoxynucleotide through the regulation of epithelial mesenchymal transition in unilateral ureteral obstruction model of mice. Exp Mol Pathol. 2013;95:136–143. doi: 10.1016/j.yexmp.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA. YAP/TAZ Are Mechanoregulators of TGF-beta-Smad Signaling and Renal Fibrogenesis. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeji M, Kawada N, Moriyama T, Nagatoya K, Oseto S, Akira S, Hori M, Imai E, Miwa T. CCAAT/Enhancer-binding protein delta contributes to myofibroblast transdifferentiation and renal disease progression. J Am Soc Nephrol. 2004;15:2383–2390. doi: 10.1097/01.ASN.0000136426.01160.2F. [DOI] [PubMed] [Google Scholar]
- Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol. 2003;163:277–286. doi: 10.1016/S0002-9440(10)63651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol. 2014;10:226–237. doi: 10.1038/nrneph.2014.14. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol. 2013;183:160–172. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia E, Garcia-Arroyo F, Silverio O, Rodriguez-Alcocer AN, Jimenez-Flores AB, Cristobal M, Arellano AS, Soto V, Osorio-Alonso H, Molina-Jijon E, Pedraza-Chaverri J, Sanchez-Lozada LG. Mycophenolate mofetil and curcumin provide comparable therapeutic benefit in experimental chronic kidney disease: role of Nrf2-Keap1 and renal dopamine pathways. Free Radic Res. 2016;50:781–792. doi: 10.1080/10715762.2016.1174776. [DOI] [PubMed] [Google Scholar]
- Therrien FJ, Agharazii M, Lebel M, Lariviere R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am J Nephrol. 2012;36:151–161. doi: 10.1159/000340033. [DOI] [PubMed] [Google Scholar]
- Thethi TK, Bajwa MA, Ghanim H, Jo C, Weir M, Goldfine AB, Umpierrez G, Desouza C, Dandona P, Fang-Hollingsworth Y, Raghavan V, Fonseca VA. Effect of paricalcitol on endothelial function and inflammation in type 2 diabetes and chronic kidney disease. J Diabetes Complications. 2015;29:433–437. doi: 10.1016/j.jdiacomp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Vento S, Herreshoff E, Radeva M, Gassman J, Stein DT, Savin VJ, Sharma M, Reiser J, Wei C, Somers M, Srivastava T, Gipson DS. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol. 2015;16:111. doi: 10.1186/s12882-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin PG, Ferreira TP, Arantes AC, Ciambarella BT, Cordeiro RS, Flower RJ, Perretti M, Martins MA, Silva PM. Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice. Br J Pharmacol. 2015;172:3058–3071. doi: 10.1111/bph.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol. 2015;11:23–33. doi: 10.1038/nrneph.2014.202. [DOI] [PubMed] [Google Scholar]
- Ucero AC, Benito-Martin A, Fuentes-Calvo I, Santamaria B, Blanco J, Lopez-Novoa JM, Ruiz-Ortega M, Egido J, Burkly LC, Martinez-Salgado C, Ortiz A. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta. 2013;1832:1744–1755. doi: 10.1016/j.bbadis.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Vielhauer V, Berning E, Eis V, Kretzler M, Segerer S, Strutz F, Horuk R, Grone HJ, Schlondorff D, Anders HJ. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004;66:2264–2278. doi: 10.1111/j.1523-1755.2004.66038.x. [DOI] [PubMed] [Google Scholar]
- Volpini RA, Costa RS, da Silva CG, Coimbra TM. Inhibition of nuclear factor-kappaB activation attenuates tubulointerstitial nephritis induced by gentamicin. Nephron Physiol. 2004;98:p97–106. doi: 10.1159/000081558. [DOI] [PubMed] [Google Scholar]
- Wahab NA, Weston BS, Mason RM. Modulation of the TGFbeta/Smad signaling pathway in mesangial cells by CTGF/CCN2. Exp Cell Res. 2005;307:305–314. doi: 10.1016/j.yexcr.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Wang J, Gibbert L, Djudjaj S, Alidousty C, Rauen T, Kunter U, Rembiak A, Enders D, Jankowski V, Braun GS, Floege J, Ostendorf T, Raffetseder U. Therapeutic nuclear shuttling of YB-1 reduces renal damage and fibrosis. Kidney Int. 2016 doi: 10.1016/j.kint.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Wang LH, Liu JS, Ning WB, Yuan QJ, Zhang FF, Peng ZZ, Lu MM, Luo RN, Fu X, Hu GY, Wang ZH, Tao LJ. Fluorofenidone attenuates diabetic nephropathy and kidney fibrosis in db/db mice. Pharmacology. 2011;88:88–99. doi: 10.1159/000329419. [DOI] [PubMed] [Google Scholar]
- Wang PH, Cenedeze MA, Campanholle G, Malheiros DM, Torres HA, Pesquero JB, Pacheco-Silva A, Camara NO. Deletion of bradykinin B1 receptor reduces renal fibrosis. Int Immunopharmacol. 2009;9:653–657. doi: 10.1016/j.intimp.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–1014. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14:3147–3154. doi: 10.1097/01.asn.0000098681.56240.1a. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Zammit SC, Cox AJ, Shackleford DM, Morizzi J, Zhang Y, Powell AK, Gilbert RE, Krum H, Kelly DJ. 3',4'-Bis-difluoromethoxycinnamoylanthranilate (FT061): an orally-active antifibrotic agent that reduces albuminuria in a rat model of progressive diabetic nephropathy. Bio org Med Chem Lett. 2013;23:6868–6873. doi: 10.1016/j.bmcl.2013.09.100. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Campbell SR, Broder A, Herlitz L, Abadi M, Wu P, Michaelson JS, Burkly LC, Putterman C. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol. 2012;145:108–121. doi: 10.1016/j.clim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YM, Wang XL, Zhou LL, Zhou FJ, Li R, Tian Y, Zuo ZL, Fang P, Chung AC, Hou FF, Cheng YX. Lingzhilactones from Ganoderma lingzhi ameliorate adriamycin-induced nephropathy in mice. J Ethnopharmacol. 2015;176:385–393. doi: 10.1016/j.jep.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- Yokote S, Katsuoka Y, Yamada A, Ohkido I, Yokoo T. Effect of adipose-derived mesenchymal stem cell transplantation on vascular calcification in rats with adenine-induced kidney disease. Sci Rep. 2017;7:14036. doi: 10.1038/s41598-017-14492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Mao S, Zhang Y, Gong W, Jia Z, Huang S, Zhang A. MnTBAP Therapy Attenuates Renal Fibrosis in Mice with 5/6 Nephrectomy. Oxid Med Cell Longev. 2016;2016:7496930. doi: 10.1155/2016/7496930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Kalluri R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr Nephrol. 2008;23:1395–1398. doi: 10.1007/s00467-008-0818-x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in Chronic Kidney Disease in China. N Engl J Med. 2016;375:905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73:163–171. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179:7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, Yang Q, Zhao Z, Xu H, Gao H, Yao W, Luo X, Liao W, Xu G, Zeng R, Yao Y. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8:70707–70726. doi: 10.18632/oncotarget.19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zununi Vahed S, Nikasa P, Ardalan M. Klotho and renal fibrosis. Nephrourol Mon. 2013;5:946–948. doi: 10.5812/numonthly.16179. [DOI] [PMC free article] [PubMed] [Google Scholar]