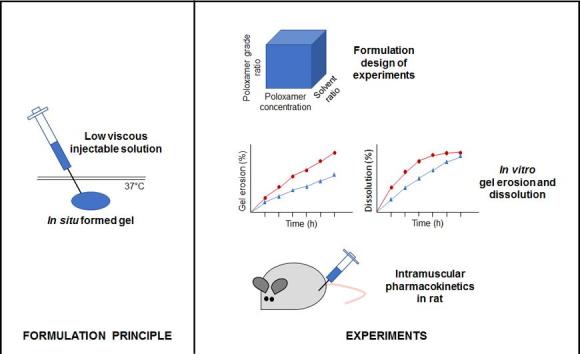
Graphical abstract
Abbreviations: CAN, Acetonitrile; ATP, Adenosine 5′ triphosphate; AUClast, Area under the analyte concentration versus time curve from time zero to the time of the last measurable (non-below quantification level) concentration; AUC80h, Area under the analyte concentration versus time curve from time zero to 80 h; AUC∞, Area under the analyte concentration vs time curve from time zero to infinite time; Cmax, Maximum observed analyte plasma concentration; CMC, Critical micellar concentration; CMT, Critical micellar temperature; C0, Analyte plasma concentration at time zero; DN, Dose normalized; DoE, Design of experiments; EO, Ethylene oxide; GPT, Gel point temperature; H&E, Hematoxylin and eosin; IM, Intramuscular; IV, Intravenous; K2.EDTA, Potassium ethylenediaminetetraacetic acid; LC–MS/MS, Liquid chromatography-tandem mass spectrometry; MDR-TB, Multi-drug resistant tuberculosis; MRM, Multiple reaction monitoring; n, Sample size; NMP, N-methyl-2-pyrrolidone; PLGA, Poly-(DL-lactic-co-glycolic acid); PO, Propylene oxide; P338, Poloxamer 338; P407, Poloxamer 407; SD, Standard deviation; TFA, Trifluoroacetic acid; tlast, Sampling time until the last measurable (non-below quantification level) analyte plasma concentration; tmax, Sampling time to reach the maximum observed analyte plasma concentration; t1/2, Apparent terminal elimination half-life; UHPLC, Ultra-high performance liquid chromatography; UV, Ultraviolet
Keywords: In situ forming gels, Poloxamer, Gel erosion, In vitro release, Pharmacokinetics, Sustained release
Abstract
The objective of this study was to evaluate in vitro and in vivo drug release from in situ forming gels prepared with poloxamer 338 (P338) and/or 407 (P407) in N-methyl-2-pyrrolidone (NMP)/water mixtures for the model compound bedaquiline fumarate salt. The impact of total poloxamer concentration (20%–25% (w/w)), P338/P407 ratio (100/0%–0/100% (w/w)) and NMP/water ratio (0/100%–25/75% (v/v)) on gel point temperature (GPT) was investigated via a design of experiments (DoE), showing that GPT decreased mainly with increasing poloxamer concentration and decreasing P338/P407 ratio, while the relation with NMP/water ratio was more complex resulting in a flexion. Based on the DoE, four formulations with 10 mg/g bedaquiline fumarate salt, a fixed NMP/water ratio of 25/75% (v/v) and varying total poloxamer concentration and P338/P407 ratio were selected for evaluation of gel erosion in vitro. The fastest eroding formulation had the lowest total poloxamer concentration (20% (w/w)) and the lowest P338/P407 ratio (20.4/79.6% (w/w)), while the formulation with the highest total poloxamer concentration (23.5% (w/w)) and highest P338/P407 ratio (100/0% (w/w)) showed the lowest gel erosion rate. These fast and slow eroding formulations showed a similar trend for in vitro drug release and in vivo pharmacokinetics after intramuscular (IM) injection in rats. In vivo tmax of the IM administered poloxamer in situ forming gels was about 6 h and a short-term sustained drug release was observed in vivo in rats up to 24 h after dosing, similar to a solution of bedaquiline fumarate salt in polyethylene glycol (PEG400)/water. In conclusion, IM administration of the evaluated poloxamer in situ forming gels may be useful for drugs that require a short-term sustained release, but is not able to extend drug release rates up to weeks or months.
1. Introduction
In drug development, scientists are often confronted with drugs having a short half-life or a low oral bioavailability due to poor solubility, degradation in the gastrointestinal tract or a high first pass effect, necessitating frequent administration. Drugs may also show high peak plasma concentrations, leading to side effects. This results in inconvenience for the patient and low adherence rates, especially for long-term therapies. Long acting injectables (LAIs), such as lipophilic solutions, polymeric microspheres, aqueous micro- or nanosuspensions and in situ forming gels, could help to overcome these challenges. They can provide drug coverage for days to months with a single administration and can be tailored to result in release profiles avoiding side effects or to deliver the drug to a specific target site (Kempe and Mäder, 2012). Compared to microparticulate systems, lipophilic solutions and in situ forming gels with dissolved drug may reduce manufacturing cost and complexity, since particle size control is not necessary and final sterilization may happen via sterile filtration.
Poloxamers are copolymers of hydrophilic ethylene oxide (EO) and hydrophobic propylene oxide (PO), arranged in a triblock structure EOx–POy–EOx with the chemical formula HO[CH2–CH2O]x[CH(CH3)–CH2O]y[CH2–CH2O]xH. The polymer blocks have varying lengths, resulting in different polymers covering a large range of liquids, pastes and solids (Dumortier et al., 2006, Raymond et al., 2006). Commonly used grades include P188, P237, P338 and P407, where “P” stands for paste, the first two digits multiplied by 100 represent the molecular mass of the PO core and the last digit multiplied by 10 corresponds to the weight percentage of EO (Raymond et al., 2006, Ramya Devi et al., 2013, Escobar-Chávez et al., 2006).
Poloxamers are widely used as solubilizers, emulsifiers, wetting and dispersing agents in oral, parenteral and topical drug products (Dumortier et al., 2006, Raymond et al., 2006, Ramya Devi et al., 2013, Escobar-Chávez et al., 2006). In addition, they have a reversible thermo-responsive gelling capacity when dissolved in water (Kempe and Mäder, 2012, Dumortier et al., 2006, Raymond et al., 2006, Ramya Devi et al., 2013, Escobar-Chávez et al., 2006). Above the critical micelle temperature (CMT) and critical micelle concentration, the polymer chains aggregate into micelles due to dehydration of the hydrophobic PO blocks. When temperature increases further above the gel point temperature (GPT), the physical entanglement and packing of the micelles results in gel formation for sufficiently concentrated poloxamer solutions (Dumortier et al., 2006, Ramya Devi et al., 2013, Escobar-Chávez et al., 2006). This characteristic is especially interesting when formulating thermally-induced in situ forming gel systems. At room temperature, the gelling systems are in a liquid state, while they form a gel at body temperature, after injection (Kempe and Mäder, 2012). Several parenteral short-term sustained release formulations have been explored using this technology. Compared to conventional injectable solutions, delayed and/or reduced peak plasma concentrations or increased half-lives were reported for poloxamer in situ forming gels, extending drug exposures up to a few days (Kempe and Mäder, 2012, Zhang et al., 2002, Yu et al., 2014, Shi et al., 2015, Jabarian et al., 2013, Bhardwaj and Blanchard, 1996, Zhang et al., 2015, Veyries et al., 1999). Release of drug substance from the gel matrix happens via gel erosion, i.e. dissolution of the polymers from the gel surface, and/or diffusion (Anderson et al., 2001). These processes are determined by the characteristics of the drug substance and formulation factors like type and concentration of the gel forming polymers and the presence of additives (e.g. co-solvents, hypromellose, carboxymethylcellulose, …) (Kempe and Mäder, 2012, Zhang et al., 2002). After dissolution from the in situ forming gel depot, poloxamers are cleared almost entirely by renal excretion, with limited metabolism (Grindel, 2002).
In this research work, in vitro and in vivo drug release from in situ forming gels prepared with poloxamer 338 (P338) and/or 407 (P407) in N-methyl-2-pyrrolidone (NMP)/water mixtures were evaluated for the model compound bedaquiline fumarate salt. Poloxamers were selected as gel formers based on their wide use in drug products, including parenterals, their low cost, high water solubility, low toxicity and known thermogelling behaviour (Kempe and Mäder, 2012, Dumortier et al., 2006, Raymond et al., 2006, Ramya Devi et al., 2013, Escobar-Chávez et al., 2006). NMP was added as co-solvent for its high solubilizing capacity and water miscibility (Sanghvi et al., 2008). It is also used as solvent in FDA approved parenteral drug products like Eligard® and Sublocade®, which are formulated as subcutaneous in situ forming gels with poly-(DL-lactic-co-glycolic acid) (PLGA) polymers (Drug label 2018, Drug label information 2019). Bedaquiline fumarate salt was chosen as model compound based on its physicochemical characteristics. The compound is highly lipophilic (logD = 5.1) and very slightly soluble to practically insoluble in aqueous media at pH 1.5 to 13, as many drugs in development. It inhibits the proton pump of M. tuberculosis adenosine 5′ triphosphate (ATP) synthase and is marketed for the treatment of multi drug resistant tuberculosis (MDR-TB). The commercial formulation is a 120.9 mg (equivalent to 100 mg bedaquiline free base) immediate release tablet, showing peak plasma concentrations (Cmax) about 5 h after dosing, followed by a tri-exponential decline in plasma concentrations with a terminal half-life of approximately 5 months, likely reflecting slow release from peripheral tissues (Summary of Product Characteristics Sirturo 100 mg tablets, 2018). Human pharmacokinetics are dose proportional up to doses of 700 mg. The advised treatment duration for MDR-TB is 24 weeks, which makes it interesting to explore LAIs. While bedaquiline would especially benefit from release rates covering weeks to months, poloxamer in situ forming gels have shown drug exposures up to a few days only, which makes them more suitable for drugs requiring a short-term sustained release (Kempe and Mäder, 2012, Zhang et al., 2002, Yu et al., 2014, Shi et al., 2015, Jabarian et al., 2013, Bhardwaj and Blanchard, 1996, Zhang et al., 2015, Veyries et al., 1999).
First, the impact of total poloxamer concentration, ratio of poloxamer grades P338/P407 and concentration of the co-solvent NMP on GPT of poloxamer formulations was investigated via a central composite design. In a next step, the gel erosion and in vitro release were determined for selected poloxamer formulations containing bedaquiline fumarate salt. Finally, the pharmacokinetics of bedaquiline were evaluated for two in situ forming gel formulations after intramuscular (IM) injection to the rat and compared to those of a solution in polyethylene glycol 400 (PEG400)/water after both IM and intravenous (IV) injection.
2. Materials and methods
2.1. Materials
Bedaquiline fumarate salt and purified water originated from Janssen Pharmaceutica NV (Beerse, Belgium). P338 and P407 were bought from BASF (Mannheim, Germany) and PEG400 from Clariant (Frankfurt am Main, Germany). NMP was obtained from Acros Organics (New Jersey, USA). Water for injections was sourced from Baxter (Lessines, Belgium) or Sterop Laboratoria (Brussels, Belgium). All other chemicals were of reagent grade and purchased from commercial sources.
2.2. Formulation preparations
Poloxamer placebo formulations were prepared applying the “cold method” (Schmolka and Artificial skin, 1972). Poloxamer was added to solvent (water or NMP/water mixtures) cooled to 5 °C. The mixture was stored in the refrigerator and periodically stirred until a clear solution was obtained. Poloxamer formulations containing 10 mg/g bedaquiline fumarate salt were prepared according to the same method, adding bedaquiline fumarate salt to the poloxamer solution as a stock solution of 100 mg/mL in NMP. For the 5 mg eq./mL bedaquiline fumarate salt formulation in PEG400/water 50/50% (v/v), the drug substance was dissolved in PEG400 and further diluted with water.
Formulations administered to rats were prepared with water for injections and filtered through a 0.22 µm Sterivex sterile filter. For other formulations, purified water was used.
2.2.1. Formulations for gel point temperature design of experiments
For the GPT design of experiments (DoE), eight placebo formulations were prepared as factorial points covering all combinations of a total poloxamer concentration of 20% or 25% (w/w), a P338/P407 ratio of 0/100% or 100/0% (w/w) and a NMP/water ratio of 0/100% or 25/75% (v/v). Six placebo formulations represented the face centered axial points with a total poloxamer concentration of 20% or 25% (w/w), a P338/P407 ratio of 50/50% (w/w) and a NMP/water ratio of 12.5/87.5% (v/v), with a total poloxamer concentration of 22.5% (w/w), a P338/P407 ratio of 0/100% or 100/0% (w/w) and a NMP/water ratio of 12.5/87.5% (v/v), and with a total poloxamer concentration of 22.5% (w/w), a P338/P407 ratio of 50/50% (w/w) and a NMP/water ratio of 0/100% or 25/75% (v/v). The center point placebo formulation contained a total poloxamer concentration of 22.5% (w/w), a P338/P407 ratio of 50/50% (w/w) and a NMP/water ratio of 12.5/87.5% (v/v). The ranges applied for the DoE were based on visual gel formation evaluations of a series of poloxamer placebo formulations.
2.2.2. Formulations for gel erosion study
Based on the GPT DoE, formulations 1, 2, 3 and 4 containing 10 mg/g bedaquiline fumarate salt were prepared in NMP/water 25/75% (v/v) with a total poloxamer content of 23.5%, 20.0%, 20.0% or 21.9% (w/w) and a P338/P407 ratio of 100/0%, 20.4/79.6%, 47.7/52.3% or 57.3/42.7% (w/w), respectively. The selected formulations were combinations of a fixed NMP/water ratio of 25/75% (v/v) to allow dissolution of bedaquiline fumarate salt and maximized or minimized total poloxamer concentration and P338/P407 ratio, resulting in a GPT of 26–30 °C, a range which was based on visual gel formation evaluations.
2.2.3. Formulations for in vitro release study
Based on the gel erosion study, formulations 1 and 2 containing 10 mg/g bedaquiline fumarate salt were prepared in NMP/water 25/75% (v/v) with a total poloxamer content of 23.5% or 20.0% (w/w) and a P338/407 ratio of 100/0% or 20.4/79.6% (w/w), respectively, as described in Section 2.2.2.
2.2.4. Formulations for in vivo pharmacokinetic study in rats
Formulations 1 and 2 described under Section 2.2.3 and their corresponding placebo formulations, vehicle 1 and 2, were prepared for the pharmacokinetic study in rats. In addition, formulation 5 of 5 mg eq./mL bedaquiline fumarate salt in PEG400/water 50/50% (v/v) was used.
2.3. In vitro evaluations
2.3.1. Gel point temperature design of experiments
The impact of NMP/water ratio (0/100%–25/75% (v/v)), total poloxamer concentration (20%–25% (w/w)), and P338/P407 ratio (0/100%–100/0% (w/w)) on GPT of the poloxamer placebo formulations was investigated through a central composite design with 8 factorial points, 6 axial points and 4 replicates of the center point. The GPT was determined on a HAAKE rheostress rheometer (Waltham, Massachusetts, USA) applying an oscillatory temperature-ramp measurement (Peltier system) at a frequency of f = 1 Hz and a temperature increase of ΔT/t = 0.06666 °C/s. The software Design expert 10 (Stat-Ease Inc., USA) was used to establish and analyze the design of experiments. A quadratic response surface model, with 9 degrees of freedom (model terms minus the intercept), was fitted to describe the GPT data. Backward selection was used for model reduction purposes based on a p-value <0.05 for significant terms.
2.3.2. Gel erosion study
The gel erosion study was performed in duplicate for in situ forming gel formulations 1–4. 5 g of the formulation was added to a 20 mL amber glass vial and equilibrated at 37 °C for at least 30 min to allow gel formation. Then, 1 mL of 0.133 M Sørensen’s phosphate buffer of pH 7.2 and 37 °C, was added to the vial to mimick muscle fluid (Juel, 2008, Recipe Sorensen’s phosphate buffer, 2010). After time intervals of 30 min (for time points up to 2 h) or 1 h (for time points from 2 to 7 h), the buffer was removed, the vial with remaining gel was weighed and 1 mL of fresh buffer was added to the vial. Gel weight loss was calculated to determine the gel erosion rate.
2.3.3. In vitro release study
The in vitro release study was performed in triplicate for in situ forming gel formulations 1 and 2 on a Distek USP paddle apparatus 2 with 6 dissolution vessels. 2.2 g of the formulation was added to a cylindric plastic cup and equilibrated at 37 °C to allow gel formation. The cup was then transferred to a dissolution vessel containing 900 mL 0.05 M phosphate buffer pH 7.4 with 1% (w/v) sodium lauryl sulphate at 37 °C and a paddle rotating at 50 rpm. Samples were collected at 5, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360 and 1440 min after introducing the formulation into the dissolution vessel by extracting 3.5 mL of dissolution medium through a Distek needle connected to a 5 mL syringe. The collected dissolution medium was filtered through a 30 mm × 0.2 µm Spartan Whatman filter and stored at room temperature until analysis (see Section 2.3.4). This set-up is commonly used for in vitro release testing of drug products and represents sink conditions.
2.3.4. Analytical method for the in vitro release study
The quantification of bedaquiline in the in vitro release samples was conducted on a Waters Acquity H-Class UHPLC system (Zellik, Belgium) with an ultraviolet (UV) absorbance detector set at 334 nm and a Waters Acquity CSH C18 1.7 µm 2.1 × 50 mm column kept at 45 °C. Sample injection volume was 3 µL. Gradient elution was applied for 3 min at a flow rate of 0.6 mL/min using a mobile phase of (A) 0.1% (v/v) trifluoroacetic acid (TFA) in water and (B) 0.05% (v/v) TFA in acetonitrile (ACN). The percentage of (B) TFA in ACN was increased from 10% at time zero to 90% at 1 min, kept stable at 90% until 1.5 min, reduced to 10% at 2 min and kept stable at 10% until 3 min. Data were processed via Waters Empower 3 software.
2.4. In vivo pharmacokinetic study in rats
2.4.1. Animals
Pharmacokinetic evaluation was performed in Sprague Dawley rats supplied by Charles River (Sulzfeld, Germany) with a body weight of 300 to 350 g and age of 9–11 weeks at the start of the study. The rats were group housed in polysulphon cages with corn cob bedding material, Rodent retreat (Bio-Serv, USA) and Aspen wood block (Datesand, UK) environmental enrichment in airconditioned (20–24 °C) rooms with a 12 h light cycle. They were acclimatized for at least 4 days before the study start and had free access to food and water during the entire experimental period. All experimental procedures involving animals were conducted following the guidelines of the Janssen Pharmaceutica (Beerse, Belgium) Animal Ethics Committee and the local Belgium laws controlling the use of experimental animals as well as EC Directive 2010/63/EU.
2.4.2. Pharmacokinetics
23 rats were allocated to five groups (4 animals for group 1 and 2, 6 animals for group 3 and 4 and 3 animals for group 5) based on body weight (Table 1). In group 1 and 2, rats were dosed twice 0.363 mL/kg of vehicle 1 and 2, respectively, via intramuscular (IM) injection in the left and right hind leg. Similar injections of formulation 1 and 2 were administered to rats of groups 3 and 4, respectively, corresponding to a dose of twice 3 mg eq./kg. Rats of group 5 received an IV injection of 1 mL/kg, or a dose of 5 mg eq./kg, of formulation 5 in the vena saphena, followed by two IM injections of 0.3 mL/kg of formulation 5 14 days later, each delivering a dose of 1.5 mg eq./kg in the left and right hind leg.
Table 1.
Pharmacokinetic study design in rats.
| Group | N | Formulation | Dosing route | Dose (mg eq. /kg) | Dosing volume (mL/kg) | Assessments |
|---|---|---|---|---|---|---|
| 1 | 4 | Vehicle in situ formulation 1 | Intramuscular (Day 1) | – | 2 * 0.363 | Histology: Day 1, 4, 8, 29a |
| 2 | 4 | Vehicle in situ formulation 2 | Intramuscular (Day 1) | – | 2 * 0.363 | |
| 3 | 6 | In situ formulation 1 | Intramuscular (Day 1) | 2 * 3 | 2 * 0.363 | Histology: Day 1, 4, 8, 29b Pharmacokinetics: 0-672hc |
| 4 | 6 | In situ formulation 2 | Intramuscular (Day 1) | 2 * 3 | 2 * 0.363 | |
| 5 | 3 | PEG400 solution formulation 5 | Intravenous (Day 1) Intramuscular (Day 15) |
5 2 * 1.5 |
1 2 * 0.3 |
Pharmacokinetics: 0–80 h |
For histology, 1 rat was analyzed per specified time point.
For histology, 1 rat was analyzed on days 1, 4 and 8 and 3 rats were analyzed on day 29.
For pharmacokinetics, 6, 5, 4 and 3 rats were analyzed for timepoints up to 2 h, 80 h, 168 h and 672 h, respectively.
32 µL blood samples were collected in Vitrex micro hematocrit tubes “soda lime glass” with potassium ethylenediaminetetraacetic acid (K2.EDTA) by puncture of the tail vein of the rats at specified timepoints ranging from 0.5 h to 28 days after IM dosing of groups 1 to 4 and from 0.25 to 80 h post IV and IM dosing of group 5. For groups 1 to 4, 1 rat was sacrificed at intermediate timepoints on days 1, 4 and 8 (after pharmacokinetic sampling at 2 h, 80 h and 168 h) for histological evaluation (see Section 2.4.3). Therefore, the number of rats with pharmacokinetic data in groups 3 and 4 was 6, 5, 4 and 3 up to timepoints of 2 h, 80 h, 168 h and 672 h, respectively. After sampling, blood samples were immediately placed on melting ice and centrifuged at 5 °C, 1500×g for approximately 10 min. Then, 10 µL plasma aliquots were collected with Vitrex end to end pipettes in FluidX tubes and stored in the freezer until analysis (see Section 2.4.4). Non-compartmental analysis was performed to calculate pharmacokinetic parameters using the validated computer program Phoenix™ WinNonlin® (Certara L.P., USA). Statistical comparisons between groups were performed using a two-tailed homoscedastic t-test.
2.4.3. Histology
Histological evaluation was performed for rats of groups 1 to 4. In each group, 1 rat was evaluated at intermediate timepoints on day 1, 4 and 8 (after pharmacokinetic sampling at 2 h, 80 h and 168 h). On day 29 (after pharmacokinetic sampling at 672 h), the remaining rats were examined, being 1 rat for groups 1 and 2 and 3 rats for groups 3 and 4. The entire left hind leg or limb muscle group containing the IM administration site of the rats was collected at necropsy and fixed in 10% buffered formalin. Since the gel was colourless, a standardized sampling of the administration site was performed. Sagittal cross sections of the formalin-fixed administration sites were embedded in paraffin wax, sectioned and stained with hematoxylin and eosin (H&E). All microscopic slides were examined histopathologically by a board-certified pathologist and the findings entered directly into a computerized database (Ascentos, Pathology Data Systems Limited, Pratteln, Basel, Switzerland).
2.4.4. Bioanalytical method for pharmacokinetics
Plasma levels of bedaquiline were determined for pharmacokinetic samples of groups 3 to 5 using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system consisting of a Shimadzu LC30AD HPLC equipment with an SIL-HTC autosampler (Shimadzu Scientific Instruments, MD, USA), coupled to an API4000™ triple quadrupole mass spectrometer (AB Sciex, Toronto, Canada) equipped with Turbo Ionspray source operated at 400 °C. Plasma samples were processed by adding subsequently 20 µL milliQ water, 20 µL methanol, 20 µL internal standard solution and 200 µL of ACN to the capillaries in the FluidX tubes. The internal standard solution consisted of 6-deuterium labeled bedaquiline at 100 ng/mL in methanol. After closing, the tubes were shaken horizontally for 10 min on an orbital shaker and centrifuged for 3 min at 2500g. The supernatant (150 µL) was transferred to a 96-deepwell plate and 1 µL of the sample extracts was injected onto a Waters BEH C18 50 × 2.1 mm, 1.7 µm column. Gradient elution was applied for 4 min at a flow rate of 0.6 mL/min using a mobile phase of (A) 0.01 M ammonium formate pH 4.0 and (B) methanol. The percentage of (B) methanol was increased from 65% at time zero to 85% at 1.25 min, to 98% at 1.30 min, kept stable at 98% until 2.49 min, reduced to 65% at 2.50 min and kept stable at 65% until 4 min. Multiple reaction monitoring (MRM) transitions were monitored for bedaquiline (555.2 → 58 m/z) and the internal standard (561.2 → 64 m/z) applying a collision energy of 71 eV.
3. Results
3.1. In vitro evaluations
3.1.1. Gel point temperature design of experiments
The GPT determined by rheology for the 18 DoE runs defined for the factors NMP/water ratio (factor A), total poloxamer content (factor B) and P338/P407 ratio (factor C) is shown in Table 2.
Table 2.
Gel point temperature (GPT) design of experiments (DoE) responses.
| Run | Point | Randomization | Factor A: NMP/water ratio % (v/v) | Factor B: total poloxamer conc % (w/w) | Factor C: P338/P407 ratio % (w/w) | Response: GPT °C |
|---|---|---|---|---|---|---|
| 1 | Factorial | 6 | 0/100 | 20 | 0/100 | 23.28 |
| 2 | Factorial | 10 | 25/75 | 20 | 0/100 | 22.32 |
| 3 | Factorial | 12 | 0/100 | 25 | 0/100 | 18.43 |
| 4 | Factorial | 2 | 25/75 | 25 | 0/100 | 10.06 |
| 5 | Factorial | 7 | 0/100 | 20 | 100/0 | 30.03 |
| 6 | Factorial | 3 | 25/75 | 20 | 100/0 | 36.88 |
| 7 | Factorial | 4 | 0/100 | 25 | 100/0 | 24.74 |
| 8 | Factorial | 9 | 25/75 | 25 | 100/0 | 21.12 |
| 9 | Axial | 13 | 0/100 | 22.5 | 50/50 | 24.91 |
| 10 | Axial | 15 | 25/75 | 22.5 | 50/50 | 23.53 |
| 11 | Axial | 14 | 12.5/87.5 | 20 | 50/50 | 30.64 |
| 12 | Axial | 18 | 12.5/87.5 | 25 | 50/50 | 22.27 |
| 13 | Axial | 17 | 12.5/87.5 | 22.5 | 0/100 | 21.11 |
| 14 | Axial | 16 | 12.5/87.5 | 22.5 | 100/0 | 29.93 |
| 15 | Center | 11 | 12.5/87.5 | 22.5 | 50/50 | 26.37 |
| 16 | Center | 8 | 12.5/87.5 | 22.5 | 50/50 | 25.74 |
| 17 | Center | 1 | 12.5/87.5 | 22.5 | 50/50 | 26.26 |
| 18 | Center | 5 | 12.5/87.5 | 22.5 | 50/50 | 26.30 |
The model equation built on significant terms is shown below:
GPT = 38.3 + (1.76 * NMP/water ratio) − (0.770 * Total poloxamer concentration) + (0.184 * P338/P407 ratio) − (0.0715 * NMP/water ratio * Total poloxamer concentration) + (2.51.10−3 * NMP/water ratio * P338/P407 ratio) − (3.94.10−3 * Total poloxamer concentration * P338/P407 ratio) − (0.0135 * NMP/water ratio2) − (3.22.10−4 * P338/P407 ratio2)
GPT was significantly positively correlated with NMP/water ratio, P338/P407 ratio, and the interaction between those two factors. GPT was significantly negatively correlated with total poloxamer concentration, with the quadratic terms of NMP/water ratio and P338/P407 ratio and with the interactions between total poloxamer concentration and NMP/water ratio or P338/P407 ratio.
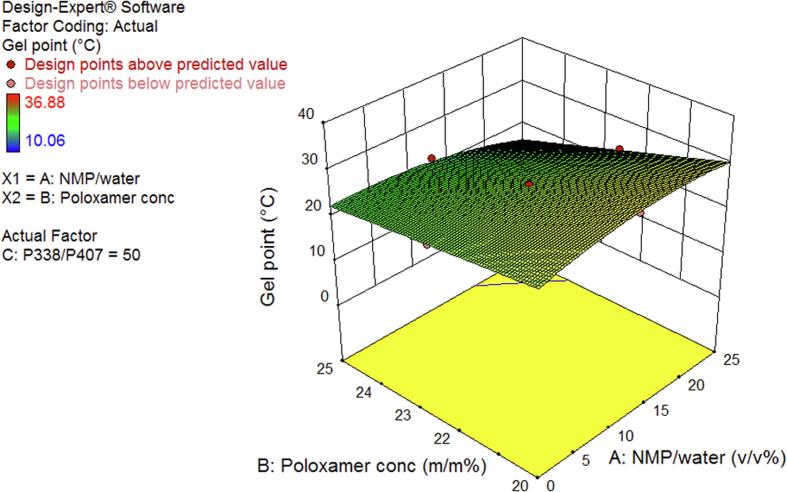
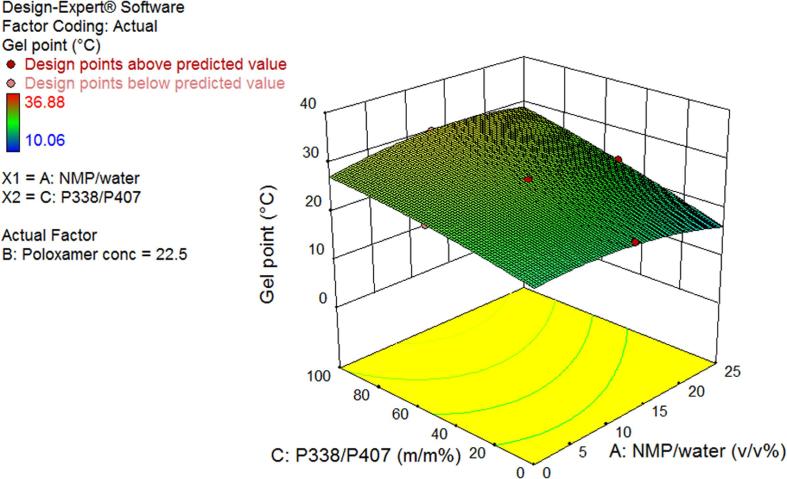
Fig. 1A, Fig. 1B show a 3-dimensional (3D) surface plot for a P338/P407 ratio of 50/50% (w/w) and total poloxamer concentration of 22.5% (w/w), respectively. In Fig. 1A, GPT decreased with increasing total poloxamer concentration, as all total poloxamer concentration terms in the DoE model equation showed a negative correlation with GPT. Fig. 1B shows an increase in GPT with increasing P338/P407 ratio. This trend was confirmed throughout the design space and was mainly driven by the positively correlated individual term P338/P407 ratio and its interaction with NMP/water ratio, while the interaction with total poloxamer concentration and the quadratic term were negatively correlated with GPT. In both Fig. 1A, Fig. 1B and within the design space, the relation between GPT and NMP/water ratio was rather complex, showing an inflection point.
Fig. 1A.
3D surface plot of gel point as a function of total poloxamer concentration and NMP/water ratio at a P338/P407 ratio of 50/50% (w/w).
Fig. 1B.
3D surface plot of gel point as a function of P338/P407 ratio and NMP/water ratio at a total poloxamer concentration of 22.5% (w/w).
3.1.2. Gel erosion study
The formulations evaluated for gel erosion with their corresponding GPT are described in Table 3. They were selected based on the DoE model equation described in Section 3.1.1 using Design expert 10 (Stat-Ease Inc., USA). While fixing the NMP/water ratio of 25/75% (v/v), 4 combinations of maximized or minimized total poloxamer concentration and P338/P407 ratio were defined, resulting in a GPT of 26–30 °C. The high NMP/water ratio was selected to allow solubility of 10 mg/g bedaquiline fumarate salt and the GPT range was defined based on visual gel formation tests, ensuring adequate gel formation at body temperature.
Table 3.
Formulations for gel erosion study.
| Formulation | Bedaquiline fumarate salt concentration mg/g | NMP/water ratio % (v/v) | Total poloxamer concentration % (w/w) | P338/P407 ratio % (w/w) | GPT °C |
|---|---|---|---|---|---|
| 1 | 10 | 25/75 | 23.5 | 100/0 | 26 |
| 2 | 10 | 25/75 | 20.0 | 20.4/79.6 | 26 |
| 3 | 10 | 25/75 | 20.0 | 47.7/52.3 | 30 |
| 4 | 10 | 25/75 | 21.9 | 57.3/42.7 | 26 |
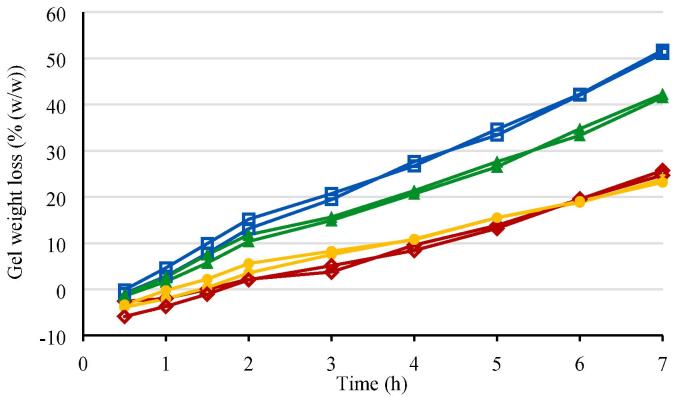
The gel erosion, expressed as percentage weight loss (% (w/w)) as a function of time, is shown in Fig. 2.
Fig. 2.
Gel erosion rate in situ forming gel formulations 1–4 (individual profiles for n = 2). Red diamond: in situ forming gel formulation 1 (10 mg/g bedaquiline fumarate salt in 23.5% (w/w) P338 in NMP/water 25/75% (v/v)); blue square: in situ forming gel formulation 2 (10 mg/g bedaquiline fumarate salt in 20.0% (w/w) P338/P407 20.4/79.6% (w/w) in NMP/water 25/75% (v/v)); green triangle: in situ forming gel formulation 3 (10 mg/g bedaquiline fumarate salt in 20.0% (w/w) P338/P407 47.7/52.3% (w/w) in NMP/water 25/75% (v/v)); yellow circle: in situ forming gel formulation 4 (10 mg/g bedaquiline fumarate salt in 21.9% (w/w) P338/P407 57.3/42.7% (w/w) in NMP/water 25/75% (v/v)).
The slowest gel erosion rates (24–25% after 7 h) were observed for formulation 1 with the highest total poloxamer concentration of 23.5% (w/w) and a P338/P407 ratio of 100/0% (w/w), and formulation 4 with a total poloxamer concentration of 21.9% (w/w) and a P338/P407 ratio of 57.3/42.7% (w/w). Formulations 2 and 3 with a lower total poloxamer concentration of 20% (w/w) eroded faster, with the fastest gel weight loss (51% after 7 h) observed for formulation 2 with the lower P338/P407 ratio and lower GPT as compared to formulation 3.
Up to 2 h, with buffer refreshments every 30 min, the weight loss curves were steeper than from 2 to 7 h, for which buffer refreshments happened hourly.
Upon or after collection of the buffer samples, a precipitation was observed in the buffer, corresponding to the Raman spectrum of bedaquiline fumarate salt. As the experimental set-up was designed to evaluate gel erosion rates and sink conditions for in vitro release of bedaquiline fumarate salt were not guaranteed, quantification of bedaquiline fumarate salt via UHPLC was not performed in the buffer samples. Instead, an in vitro release study was executed in sink conditions as described in Section 3.1.3.
3.1.3. In vitro release study
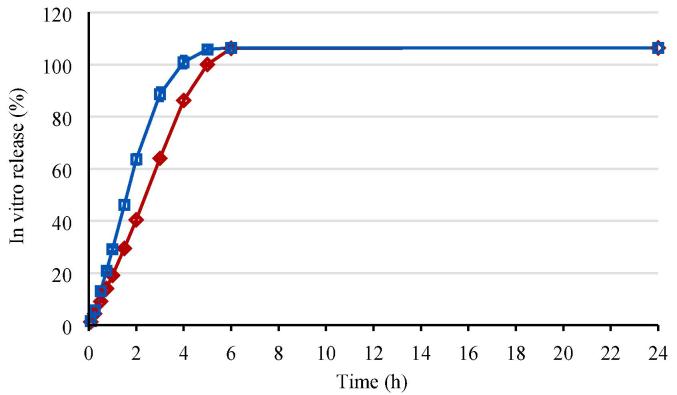
The in vitro release was determined for formulations 1 and 2 representing the slowest and fastest gel erosion rate, respectively, as described in Section 3.1.2. The in vitro release profiles are shown in Fig. 3.
Fig. 3.
In vitro release in situ forming gel formulations 1 and 2 (mean profile and standard deviation error bars for n = 3). Red diamond: in situ forming gel formulation 1 (10 mg/g bedaquiline fumarate salt in 23.5% (w/w) P338 in NMP/water 25/75% (v/v)); blue square: in situ forming gel formulation 2 (10 mg/g bedaquiline fumarate salt in 20.0% (w/w) P338/P407 20.4/79.6% (w/w) in NMP/water 25/75% (v/v)).
Formulation 1 with the slowest gel erosion rate in Section 3.1.2 corresponded to the slower in vitro release profile, while formulation 2 showed the higher gel erosion and in vitro release rate. Formulations 1 and 2 reached more than 100% in vitro release after 5 and 4 h, respectively. The 107% release at the end of the profiles was in line with the assay of the formulations, being 104%.
3.2. In vivo pharmacokinetic study in rats
3.2.1. Pharmacokinetics
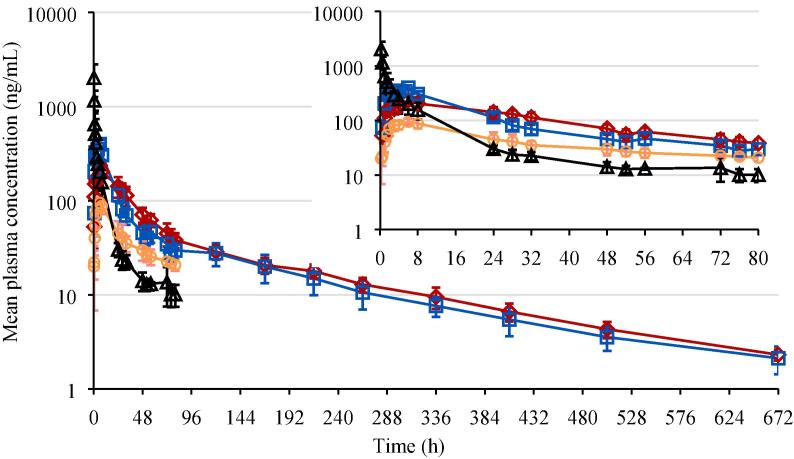
In situ forming gel formulations 1 and 2, evaluated for in vitro release in Section 3.1.3, were administered to rats via IM injection in the left and right hind legs, corresponding to a bedaquiline fumarate salt dose of 6 mg eq./kg. In addition, PEG400 solution formulation 5 was administered to rats via IV injection at a dose of 5 mg eq./kg and via IM injection in both hind legs delivering a dose of 3 mg eq./kg. The mean pharmacokinetic profiles are shown in Fig. 4. Mean pharmacokinetic parameters are summarized in Table 4.
Fig. 4.
Plasma concentration-time profiles in rat (mean profiles and standard deviation error bars for n = 3 to 5). Red diamond: Intramuscular (IM) in situ forming gel formulation 1 dosed at 6 mg eq./kg (10 mg/g bedaquiline fumarate salt in 23.5% (w/w) P338 in NMP/water 25/75% (v/v)); blue square: IM in situ forming gel formulation 2 dosed at 6 mg eq./kg (10 mg/g bedaquiline fumarate salt in 20.0% (w/w) P338/P407 20.4/79.6% (w/w) in NMP/water 25/75% (v/v)); orange circle: IM PEG400 solution formulation 5 dosed at 3 mg eq./kg (eq. 5 mg/mL bedaquiline fumarate salt in PEG400/water 50/50% (v/v)); black triangle: intravenous solution formulation 5 dosed at 5 mg eq./kg.
Table 4.
Pharmacokinetic parameters in rat following parenteral administration.
| Analyte | Bedaquiline |
|||
|---|---|---|---|---|
| In situ gel Formulation 1 | In situ gel Formulation 2 | PEG400 solution Formulation 5 | ||
| Dosing route | IM | IM | IM | IV |
| Dose (mg/kg) | 6 | 6 | 3 | 5 |
| n | 5a | 5a | 3b | 3c |
| C0 (ng/mL)d | – | – | – | 3589 (1779) |
| Cmax (ng/mL)d | 237 (34) | 410 (42) | 94.7 (12.5) | – |
| tmax (h)e | 6 (6–8) | 6 (4–6) | 6 (6–6) | – |
| tlast (h) | 672 | 672 | 80 | 80 |
| AUClast (ng.h/mL)d | 15,588 (425) | 14,295 (2274) | 3238 (631) | 5576 (1669) |
| AUC80h (ng.h/mL)d | 8262 (994) | 8393 (1044) | 3238 (631) | 5576 (1669) |
| AUC∞ (ng.h/mL)d | 16,117 (351) | 14,797 (2435) | NCf | 5512 (NCf) |
| t1/2 (h)d | 158 (17.9) | 164 (12.6) | 48.2 (12.1) | 32.1 (NCf) |
| C0/Dose (ng/mL)d | – | – | – | 718 (356) |
| Cmax/Dose (ng/mL)d | 39.5 (5.63) | 68.3 (7.03) | 31.6 (4.15) | – |
| AUClast/Dose (ng.h/mL)d | 2598 (71) | 2382 (379) | 1079 (210) | 1115 (334) |
| AUC80h/Dose (ng.h/mL)d | 1377 (166) | 1399 (174) | 1079 (210) | 1115 (334) |
| AUC∞/Dose (ng.h/mL)d | 2686 (58.5) | 2466 (406) | NCf | 1102 (NCf) |
n = 3 for AUClast, AUC∞ and t1/2 to include only profiles up to 672 h.
n = 0 for AUC∞ as values with R2adj < 0.90 and/or more than 20% extrapolation were omitted and n = 2 for t1/2 as values with R2adj < 0.90 were omitted.
n = 1 for AUC∞ as values with R2adj < 0.90 and/or more than 20% extrapolation were omitted and n = 1 for t1/2 as values with R2adj < 0.90 were omitted.
Mean (SD).
Median (min–max).
NC = not calculated as n = 0 or 1.
After single parenteral administration of bedaquiline fumarate salt to male rats, plasma concentrations of bedaquiline were quantifiable up to the last sampling points, i.e. 672 h post-dose at 6 mg eq./kg IM dosing of in situ forming gel formulations 1 and 2 and 80 h post-dose at 3 mg eq./kg IM and 5 mg eq./kg IV dosing of PEG400 solution formulation 5.
After IM dosing of the formulations 1, 2 and 5, peak concentrations were observed around 6 h post-dose. Formulation 2 showed a significantly higher Cmax (p value <0.001) and faster initial drop after reaching peak concentrations (p value <0.01 for slope of logarithmic plasma concentration-time profiles between tmax and 24 h) than formulation 1, suggesting a faster drug absorption or release rate. For all IM formulations, the slopes of the logarithmic plasma concentration-time profiles between tmax and 24 h were significantly lower than for formulation 5 (IV) between 6 and 24 h (p value < 0.001 for formulation 1, p value < 0.01 for formulations 2 and 5 (IM)). Terminal half-lives of formulations 1 and 2 were comparable (p value > 0.05). The half-life calculated for formulation 5 (IM and IV) was much shorter due to the earlier tlast and can therefore not be compared to the half-life of formulations 1 and 2. However, logarithmic plasma concentration-time profiles of formulations 1 and 2 (IM) declined parallel to the ones of formulation 5 (IM and IV) between 24 and 80 h after dosing, suggesting a similar half-life for all formulations within that timeframe. The intersubject variability for pharmacokinetic parameters of formulations 1, 2 and 5 (IM) was limited and varied from 10.3 to 14.3 %CV for Cmax (n = 5 for formulations 1 and 2 and n = 3 for formulation 5) and from 2.7 to 19.5 %CV for AUClast (n = 3 for formulations 1, 2 and 5). The intersubject variability for pharmacokinetic parameters of formulation 5 (IV) was higher and was 49.6 %CV for C0 (n = 3) and 29.9 %CV for AUClast (n = 3).
Cmax/Dose values of formulations 1 and 5 (IM) were comparable and lower than for formulation 2 (IM). Based on the AUC80h/Dose values, exposures of formulations 1 and 2 (IM) were comparable and slightly higher than for formulation 5 (IM or IV), which may (partially) be explained by the slightly higher assay of the formulations (104% for 1 and 2 versus 98% for 5).
3.2.2. Histology
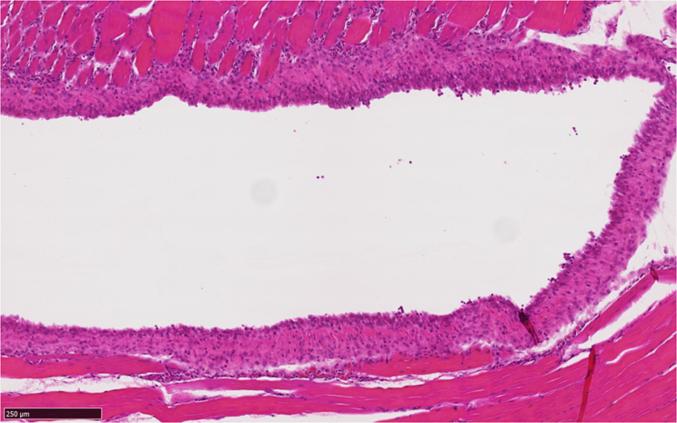
A single IM injection of in situ forming gel formulations 1 and 2 and their corresponding vehicles to rats, resulted in minimal to slight myofiber degeneration or necrosis, minimal to slight hemorrhage and minimal perivascular granulocytic infiltrates in all groups on day 1. A similar degree of myofiber degeneration or necrosis, microvacuolated histiocytic infiltrates and chronic inflammation was noted on day 4 and 8 after dosing. The only difference between the vehicle and the formulation-dosed groups was the palisading of macrophages around the presumed gel in the formulation-dosed groups on day 8 (Fig. 5). At day 29 recovery was almost complete, only a minimal myofiber regeneration, minimal histiocytic infiltrates or minimal chronic inflammation was noted for individual rats in both vehicle- and formulation- dosed groups.
Fig. 5.
IM administration site in rat (H&E staining) – Day 8 after intramuscular dosing in situ forming gel formulation 2 (10 mg/g bedaquiline fumarate salt in 20.0% (w/w) P338/P407 20.4/79.6% (w/w) in NMP/water 25/75% (v/v)): rim of palisading macrophages surrounding the presumed gel (clear space).
4. Discussion
The GPT DoE investigated the impact of total poloxamer concentration (20%–25% (w/w)), P338/P407 ratio (0/100%–100/0% (w/w)) and NMP/water ratio (0/100%–25/75% (v/v)) on the GPT of poloxamer placebo formulations. It was shown that GPT decreased with increasing total poloxamer concentration via the negatively correlated DoE model equation terms “total poloxamer concentration” and its interaction with P338/407 or NMP/water ratio. This is in line with the observations of A. Fakhari et al for P407 gels (Fakhari, 2017) and can be explained by the fact that the polymer chains are packed more densely at higher concentrations, lowering the energy needed to result in dehydration and consequent gelation of the polymer chains. Within the evaluated design space, GPT showed an increase with increasing P338/P407 ratio, mainly driven by the positively correlated individual term P338/P407 ratio and its interaction with NMP/water ratio, while the interaction with total poloxamer concentration and the quadratic term were negatively correlated with GPT. The overall positive correlation is in line with the shorter polyethylene oxide (PEO) polymer chains and lower GPT reported for poloxamer P407 (BASF and Grades, 2013, Bang et al., 2015). The relation between GPT and NMP/water ratio was rather complex, with positively and negatively correlated model equation terms, showing an inflection point. A similar observation was described by Phaechamud et al. (2012). At low concentrations, the NMP amide function undergoes mainly hydrophobic interactions, while it interferes strongly with hydrogen bonding at higher concentrations (Zaichikov, 2006, Jia et al., 2014). Both interactions may impact polymer micelle and gel formation, and lead to the complex relation between GPT and NMP/water ratio.
Based on the GPT DoE, 4 different formulations were selected with a GPT between 26 and 30 °C to ensure adequate gelation at body temperature and a fixed NMP/water ratio of 25/75% (v/v) to allow dissolution of 10 mg/g bedaquiline fumarate salt. Higher poloxamer concentrations slowed down gel erosion via an increase in number and size of poloxamer micelles, shorter inter-micellar distance and a greater number of cross-links between micelles, causing a greater tortuosity and rigidity of the gel structure (Zhang et al., 2002, Bhardwaj and Blanchard, 1996). A decreasing P338/P407 ratio led to an increased gel erosion rate, which may be attributed to the lower molecular weight of P407 compared to P338, requiring less time for hydration and dissolution of the poloxamer chains. More frequent buffer refreshments led to steeper gel erosion curves. According to the Noyes-Whitney equation (Smith, 2016), the poloxamer dissolution rate could be impacted by the poloxamer concentration gradient, which would be higher for 0.5 than 1 hourly buffer refreshments.
In vitro release profiles for the in situ forming gel formulations with lowest (formulation 1) and highest (formulation 2) gel erosion rate followed the same rank order as the gel erosion tests, indicating that gel erosion rate plays a significant role in the drug release. Diffusion of the drug throughout the gel matrix cannot be ruled out, but is expected to have a minimal effect on drug release based on available literature (Zhang et al., 2002, Anderson et al., 2001).
Based on the gel erosion and in vitro release profiles, only a short-term controlled release may be expected in vivo for the prepared poloxamer in situ forming gels. Gels eroded for 24% to 51% within 7 h and in vitro release profiles showed 100% release within 4–5 h. Similar gel erosion and in vitro drug release rates (about 100% after 6 h) were reported by L. Zhang et al for a ceftiofur in situ forming gel formulation containing 25% (w/v) P407 in water (Zhang et al., 2002).
Pharmacokinetic data were in line with the gel erosion and in vitro release tests. Both in situ forming gel formulations reached Cmax at about 6 h and formulation 1 showed a slower release than formulation 2. The slope of the logarithmic plasma concentration-time profiles between tmax and 24 h for the in situ gel formulations was lower than the one between 6 and 24 h for formulation 5 (IV), a solution of bedaquiline fumarate salt in PEG400/water, indicating a short-term sustained release. However, a similar short-term release was observed for formulation 5 (IM). The high viscosity of formulation 5 may have caused a slow diffusion of the drug at the injection site.
Based on the histological evaluation of the IM administration site, an IM injection of the bedaquiline in situ forming gels and their corresponding vehicles were well tolerated, only a transient inflammatory response was observed with almost complete recovery after 28 days. Johnston and Miller (1985) performed a toxicological evaluation of poloxamer vehicles after IM injection in rabbits, indicating that muscle toxicity was proportional to the lipophilicity of the poloxamer. P407 25% (w/w) showed gross lesions and elevations in creatinine phosphokinase comparable to normal saline and was considered an acceptable intramuscular vehicle (Johnston and Miller, 1985). P338 is not expected to be more toxic than P407, since it is less lipophilic.
5. Conclusions
In this study, the in vitro and in vivo drug release of in situ forming gels containing 10 mg/g bedaquiline fumarate salt, P338 and/or P407, NMP and purified water was evaluated. Keeping the NMP/water ratio fixed at 25/75% (v/v), the formulation with the lowest total poloxamer concentration (20% (w/w)) and the lowest P338/P407 ratio (20.4/79.6% (w/w)) showed the highest gel erosion rate; while the one with the highest total poloxamer concentration (23.5% (w/w)) and highest P338/P407 ratio (100/0% (w/w)) eroded the slowest. A similar trend for in vitro drug release and in vivo pharmacokinetics after IM injection in rats was observed. In vivo tmax of the poloxamer in situ forming gels was about 6 h and a short-term sustained drug release was observed in vivo in rats up to 24 h after dosing, similar to an IM administered solution of bedaquiline fumarate salt in PEG400/water. In conclusion, IM administration of the evaluated poloxamer in situ forming gels may be useful for drugs that require a short-term sustained release, but is not able to extend drug release rates up to weeks or months.
CRediT authorship contribution statement
Sandy Van Hemelryck: Conceptualization, Formal analysis, Methodology, Writing - original draft. Jonatan Dewulf: Formal analysis. Harm Niekus: Formal analysis. Marjolein van Heerden: Formal analysis, Writing - original draft. Benno Ingelse: Formal analysis, Writing - original draft. René Holm: Supervision, Writing - review & editing. Erik Mannaert: Supervision, Writing - review & editing. Peter Langguth: Supervision, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgements
The authors are grateful to Nicolas Darville (Drug Product Development, Janssen, Belgium) and An Vermeulen (Quantitative Sciences, Janssen, Belgium) for their scientific advice throughout the project, to Frank Snijkers (Analytical Development, Janssen, Beerse) for sharing his expertise in rheology, to Nancy Schoonvaere (Analytical Development, Janssen, Beerse) for her guidance during the in vitro release study, to Sophie Lachau-Durand (Pharmacokinetics, Dynamics and Metabolism, Janssen, Belgium), Ilham Smyej and Sandra De Jonghe (Non-Clinical Safety, Janssen, Belgium) for providing their input in the rat study protocol, to Kris Van Dijck (Drug Product Development, Janssen, Belgium) for developing the PEG400 solution formulation dosed to rats, to Chris Sas and Jan Van Dun (In vivo Rodents, Janssen, Belgium) for executing the in vivo study, to Ellen Broeckx, Greet Bertels and Peter Delille (In vivo rodents, Janssen, Belgium) for performing autopsy on rats and to Tom Verhaeghe and Dirk van Roosbroeck (Bioanalysis, Janssen, Belgium) for bioanalysis support.
References
- Anderson B.C. Understanding drug release from poly(ethylene oxide)-bpoly(propylene oxide)-b-poly(ethylene oxide) gels. J. Controlled Release. 2001;70:157–167. doi: 10.1016/s0168-3659(00)00341-2. [DOI] [PubMed] [Google Scholar]
- Bang F. Presented at 42nd CRS Annual Meeting & Exposition. 2015. Evaluating the impact of various additives upon the gel point temperature of aqueous poloxamer-based formulations. Edinburgh, Scotland. [Google Scholar]
- BASF, Kolliphor P Grades, 2013. Poloxamers Ph. Eur., Poloxamer USP/NF, Poloxamers for pharmaceutical use; Technical Information, 1–8.
- Bhardwaj R., Blanchard J. Controlled-release delivery system for the r-MSH analog melanotan-I using poloxamer 407. J. Pharm. Sci. 1996;85(9):915–919. doi: 10.1021/js960097g. [DOI] [PubMed] [Google Scholar]
- Drug label information Eligard-leuprolide acetate, US National Library of medicine, 2018, <https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d/>.
- Drug label information Sublocade-buprenorphine solution, US National Library of medicine, 2019, <https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d/>.
- Dumortier G. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- Escobar-Chávez J.J. Applications of thermos-reversible Pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Sci. 2006;9(3):339–358. [PubMed] [Google Scholar]
- Fakhari, A. et al., 2017. Thermogelling properties of purified poloxamer 407, Heliyon, 3: 1–26. [DOI] [PMC free article] [PubMed]
- Grindel J.M. Distribution, metabolism, and excretion of a novel surface-active agent, purified poloxamer 188, in rats, dogs, and humans. J. Pharm. Sci. 2002;90(9):1936–1947. doi: 10.1002/jps.10190. [DOI] [PubMed] [Google Scholar]
- Jabarian L.E. In vitro and in vivo evaluation of an in situ gel forming system for the delivery of PEGylated octreotide. Eur. J. Pharm. Sci. 2013;48:87–96. doi: 10.1016/j.ejps.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Jia G. The hydrogen bonding dynamics and cooperative interactions of NMP-water mixture studied by dielectric relaxation spectroscopy. J. Mol. Liquids. 2014;197:328–333. [Google Scholar]
- Johnston T.P., Miller S.C. Toxicological evaluation of poloxamer vehicles for intramuscular use. J. Parenteral Sci. Technol. 1985;39(2):83–88. [PubMed] [Google Scholar]
- Juel C. Regulation of pH in human skeletal muscle: adaptations to physical activity. Acta Physiol. 2008;193:17–24. doi: 10.1111/j.1748-1716.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- Kempe S., Mäder K. In situ forming implants – an attractive formulation principle for parenteral depot formulations. J. Controlled Release. 2012;161:668–679. doi: 10.1016/j.jconrel.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Phaechamud T. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J. Pharm. Sci. 2012;74(6):498–504. doi: 10.4103/0250-474X.110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya Devi D. Poloxamer: a novel functional molecule for drug delivery and gene therapy. J. Pharm. Sci. Res. 2013;5(8):159–165. [Google Scholar]
- Raymond C.R. fifth ed. Pharmaceutical Press and American Pharmacists Association; 2006. Handbook of Pharmaceutical Excipients; pp. 535–537. [Google Scholar]
- Recipe Sorensen’s phosphate buffer (0.133M, pH 7.2), Cold Spring Harbor Protocols, 2010, doi:10.1101/pdb.rec12327.
- Sanghvi R. Solubility improvement of drugs using N-methyl pyrrolidone, American Association of Pharmaceutical Scientists. PharmSciTech. 2008;9(2):366–376. doi: 10.1208/s12249-008-9050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolka I.R., Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972;6:571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- Shi X. Injectable long-acting in situ forming systems for Radix Ophiopogonis polysaccharide. Int. J. Biol. Macromolecules. 2015;72:553–559. doi: 10.1016/j.ijbiomac.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Smith B.T. Pharmaceutical Press; 2016. Remington Education: Physical Pharmacy; pp. 31–50. [Google Scholar]
- Summary of Product Characteristics Sirturo 100 mg tablets, 2018, <https://www.medicines.org.uk/emc/product/3560#PHARMACOKINETIC_PROPS/>.
- Veyries M.L. Controlled release of vancomycin from poloxamer gels. Int. J. Pharm. 1999;192:183–193. doi: 10.1016/s0378-5173(99)00307-5. [DOI] [PubMed] [Google Scholar]
- Yu Z.-G. In vitro and in vivo evaluation of an in situ forming gel system for sustained delivery of Florfenicol. J. Veterinary Pharmacol. Ther. 2014;38:271–277. doi: 10.1111/jvp.12171. [DOI] [PubMed] [Google Scholar]
- Zaichikov A.M. Thermodynamic characteristics of water-N-methylpyrrolidone mixtures and intermolecular interactions in Them. Russ. J. General Chem. 2006;76(4):626–633. [Google Scholar]
- Zhang L. Development and in vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J. Controlled Release. 2002;85(1–3):73–81. doi: 10.1016/s0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- Zhang K. Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv. 2015;22(3):375–382. doi: 10.3109/10717544.2014.891272. [DOI] [PubMed] [Google Scholar]