Abstract
In Argentina, NDM metallo-β-lactamase was first reported in 2013. By now, it has disseminated throughout the country in diverse Gram negative bacteria. Here, we report the case of a paediatric patient that underwent a 1-year hospitalisation due to erythrodermic psoriasis in 2014 and received multiple antimicrobial treatments. During his stay, five isolates were obtained from rectal swabs (rs) or blood culture (bc) suspicious of carbapenemase production: a K. quasipneumoniae subsp. quasipneumoniae (rs), Citrobacter freundii (rs), Escherichia coli (bc), Enterobacter cloacae (rs), and a Serratia marcescens (bc). The isolates were studied with broth microdilution, biparental conjugation and plasmid and whole genome sequencing (Illumina). All isolates harboured an 138,998-bp type 1 IncC plasmid that carried blaNDM-1, bleMBL, blaCMY-6, rmtC, aac(6’)-Ib, and sul1 resistance genes. Additionally, the blaNDM-plasmids contained ISKpn8 an insertion sequence previously described as associated only to blaKPC. One isolate, a colistin-resistant E. coli, also carried a mcr-1-containing an IncI2 plasmid, which did not harbour additional resistance. The whole genome of K. quasipneumoniae subsp. quasipneumoniae isolate was fully sequenced. This isolate harboured, additionally to blaNDM, three plasmid-mediated quinolone resistance genes: qnrB4, qnrB52 and aac(6’)-Ib-cr1. The E. cloacae isolate also harboured qnrA1. These findings alert to the underestimated horizontal dissemination of multidrug-resistant plasmids limiting treatment options with last resort antimicrobials.
Introduction
New Delhi metallo-β-lactamase (NDM) is a plasmid-borne carbapenemase that severely limits treatment options against gram-negative pathogens. The blaNDM-1 gene was initially identified in Klebsiella pneumoniae and Escherichia coli isolates but later it was reported in many Gram negative species like Klebsiella oxytoca, Proteus mirabilis, Enterobacter cloacae, Citrobacter freundii, Providencia spp., Acinetobacter spp., Pseudomonas aeruginosa and Vibrio choleae [1]. Some of these bacteria are carried as gut flora and are commonly found in the environment, providing reservoirs for future infections [2].
The blaNDM-1 gene is carried on plasmids usually carrying additional resistance genes that compromise antimicrobial treatment. Plasmids associated to the dissemination of blaNDM-1 vary greatly in attributes such as size, gene content, organization and incompatibility group or replicon type (e.g. IncA, IncC, IncF types, IncL/M, IncN, IncX, and IncH) [3, 4].
The plasmids harbouring mcr-1 have been identified in multidrug-resistant Enterobacteriaceae isolates, some of which co-produced carbapenemases such as KPC, VIM or NDM [5].
Here, we report five blaNDM-1-producing Enterobacteriaceae species isolated from the same patient, one of which co-produced MCR-1. We also characterised the genetic elements that harbour blaNDM-1 and mcr-1, and the high similarities found indicate the presence of one blaNDM-1 plasmid circulating among these different species.
Case study
In February 2014, a 4-year-old child suffering from erythrodermic psoriasis with previous hospitalisations was admitted to the hospital due to edematous-ascitic syndrome. After 124 days of hospitalisation the patient died as a result of septic shock. During the patient’s hospitalisation, five Enterobacteriaceae species suspicious of carbapenemase production due to imipenem inhibition halos ≤22mm were isolated. As a consequence, he was treated with standard doses of colistin and tigecycline. The timeline of isolation is as follows: at day 39 of hospitalisation, K. pneumoniae M17277 was isolated from a rectal swab, later identified as K. quasipneumoniae subsp. quasipneumoniae; at day 71, C. freundii M17394 from a rectal swab; at day 74, E. coli M17386 from a blood culture; at day 117, E. cloacae M17464 from a rectal swab and finally, at day 123, Serratia marcescens M17468 was obtained from a blood culture. Rectal swabs were periodically obtained as part of the hospital´s surveillance programme to detect KPC-producing K. pneumoniae. These isolates were sent to the National Reference Laboratory on Antimicrobial Resistance (NRLAR) for further characterisation.
In addition to the clinical situation described above, the patient was also infected and colonized with several microorganisms obtained from rectal swabs, blood cultures or catheter tips as follows: Staphylococcus aureus, Staphylococcus haemolyticus, Enterococcus faecalis, Enterococcus faecium, Acinetobacter baumanii, P. aeruginosa, and Candida parapsilosi. In addition, Candida glabrata, C. albicans and Toxocara spp. were isolated from urine cultures. The patient was treated according to the infecting microorganism with: clindamycin, ceftriaxone, vancomycin, cephalotin, trimethoprim-sulfamethoxazole, colistin, tigecycline, rifampicin, meropenem, minocycline, linezolid, amikacin, gentamicin, ampicillin-sulbactam, teicoplanin, and metronidazol (due to Giardia lambdia). In addition, the patient was treated with immunosupressors and antimycotics, as albendazol and fluconazol to treat the mycotic and parasitic infections. Unfortunately, exact dates of isolation of the mentioned isolates are missing in the hospital records.
Materials and methods
Microbiological identification, antimicrobial susceptibilities and PCR screening for resistance genes.
Isolates were identified using conventional biochemical tests. All Gram negatives, oxidase negative isolates were tested for Enterobacteriaceae species considering lactose fermentation in CLDE media and the following tests: TSI (Triple Sugar Iron), SIM (Sulfide Indole Motility), Citrate media, LIA (Lysine Iron Agar) and MIO (Motility, Indole, Ornithine). These media were purchased in Laboratorios Britania S.A, Argentina. Species identification were confirmed with matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (Bruker, Germany). Carbapenemase activity was screened with: Triton-Hodge Test, Blue-Carba Test and Carba-NP-Direct test (in house), [6–8]. Screening of metallo-ß-lactamase activity was done by synergy between a meropenem (10 μg) disc and Ethylenediaminetetraacetate acid/Sodium Mercaptoacetate (EDTA/SMA) (Laboratorios Britania S.A., Argentina). Antibiotic susceptibility profiles were determined by agar dilution according to the guidelines and interpretation criteria of the Clinical and Laboratory Standards Institute (CLSI 2018). MICs were determined for imipenem, meropenem, ceftazidime, cefotaxime, cefepime, aztreonam, ciprofloxacin, amikacin, gentamicin, fosfomycin, tigecycline, minocycline and colistin. PCRs were performed using the primers listed in S1A Table to detect the resistance genes: blaPER, blaCTX-M, blaKPC, blaCMY 2/7, blaVIM, blaIMP, blaNDM, mcr-1, rmtC, aac(6’)-Ib-cr and qnr-type (C, D, A, B, S and E) (S1A Table).
Biparental conjugation
The agar mating method was used to conjugate clinical isolates with E. coli J53 sodium azide-resistant (AzR) as recipient [9]. blaNDM-1 transconjugants were selected in meropenem (containing 0.2 μg/ml) Luria-Bertani plates plus sodium azide (200 μg/ml). E. coli M17386 harbouring blaNDM-1 and mcr-1 was conjugated to a susceptible Salmonella spp. strain M1744 (S1B Table). Transconjugants were identified using Salmonella-Shigella agar plates supplemented with meropenem (0.2 μg/ml) or colistin (2 μg/ml). Subsequently, Salmonella transconjugants producing NDM or MCR were separately conjugated with E. coli J53 AzR as explained above, and the retrotranscojugants were selected as explained above.
Plasmid characterisation
Plasmid content was analysed by S1 nuclease (Promega, Southampton, UK) digestion followed by PFGE, as described [10]. Plasmids were blotted onto a positively charged nylon membrane (Bio-Rad, Hercules, USA) and hybridised with a blaNDM-1 probe (ECL Direct Nucleic Acid Labelling And Detection, Buckinghamshire, England).
blaNDM-1- and mcr-1-harbouring plasmids were fully characterised as follows. Plasmids were extracted from transconjugants with Qiagen Large-Construct kit (Qiagen, Hilden, Germany) and sequenced on a MiSeq sequencer with MiSeq Reagent Kit v3 (Illumina, USA). Reads assembly was performed with the CLC Genomics Workbench software (CLC bio, Qiagen) and gaps were closed by PCR using the primers depicted in S1 Table, followed by Sanger sequencing. Open reading frames were predicted and annotated by Prokka v1.12 [11], and manual curation in Artemis [https://www.sanger.ac.uk/science/tools/artemis] and BLAST search [https://blast.ncbi.nlm.nih.gov/Blast.cgi]. ResFinder and PlasmidFinder were used to identify resistance genes and plasmid incompatibility groups, respectively [https://cge.cbs.dtu.dk/services/]. The insertion sequences were identified using ISfinder [https://www-is.biotoul.fr].
Whole genome sequencing (WGS) and phylogenetic analysis of M17277
Whole bacterial DNA was extracted with QIAcube, using the QIAamp® DNA Mini Kit (Qiagen) and sequenced on a MiSeq sequencer. Reads were de novo assembled using VelvetOptimiser v2.2.5 [12]. Automated annotation was done with Prokka [11].
Phylogenetic analysis of M17277 was done with a WGS dataset from 60 type-strains and isolates of K. pneumoniae, K. quasipneumoniae subsp. quasipneumoniae, K. quasipneumoniae subsp. similipneumoniae and Klebsiella variicola (namely, the Kleb-dataset). This dataset comprised: two draft genomes of the type-strains of K. quasipneumoniae subsp. quasipneumoniae and K. quasipneumoniae subsp. similipneumoniae, namely 01A030T and 07A044T, respectively [13], and genome assemblies from 58 isolates selected from a collection of 288 isolates previously characterised [14]. These 58 isolates comprised all K. quasipneumoniae subsp. quasipneumoniae, K. quasipneumoniae subsp. similipneumoniae and K. variicola available in the Holt’s database (1, 19 and 18 isolates, respectively), and 20 isolates randomly selected from the subset of K. pneumoniae isolates of the Holt´s database (S3 Table) [14]. The multifasta files of the type strains 01A030T and 07A044T were downloaded from NCBI accession numbers CCDF01000000 and CBZR010000000, respectively, and annotated with Prokka [11]. The annotated assemblies of M17277 and the Kleb-dataset were used in a pan-genome analysis with Roary [15] to generate a core-gene alignment (concatenated genes present in ≥99% of the genomes with ≥95% of nucleotide identity). This core-genome alignment was used to generate a single-nucleotide polymorphism (SNP) alignment with SNP-sites v2.3.2 [16] used to construct a maximum likelihood phylogenetic tree with RAxML v8.2.8 [17] under the generalised time reversible model (GTRCAT) and bootstrapping with 1,000 replicates. To support the re-classification of K. quasipneumoniae subsp. quasipneumoniae we used a genome-to-genome distance approach using the online tool at https://ggdc.dsmz.de/ggdc.php [18]
Nucleotide sequence accession number
Plasmid sequences were submitted to GenBank under accession no. MH995506 (TC-17394), MH995507 (TC17277), MH995508 (TC-17464), MK123267 (RT-17386), MK123268 (TC-17468). K. quasipneumoniae subsp. quasipneumoniae M17277 assembly was submitted under BioProject PRJNA499048 and BioSample: SAMN10340875, GenBank RZJN00000000,
Results
Species identification, antimicrobial susceptibilities and resistance genes
The five species were originally identified as K. pneumoniae M17277, E. coli M17386, C. freundii M17394. E. cloacae M17464 and S. marcescens M17468. M17277 was one of the first blaNDM-1-harbouring clinical isolates belonging to the genus Klebsiella that had been sent to the NRLAR (2014). K. pneumoniae M17277 was identified as K. pneumoniae by biochemical techniques and MALDI-TOF.
The screening tests for carbapenemase activity were positive for all isolates and showed synergy between a carbapenem disc and EDTA/SMA, indicating the production of a metallo-ß-lactamase.
Full resistance phenotype to aminoglycosides (gentamicin and amikacin) was observed in all isolates. Additionally, K. pneumoniae M17277, E. coli M17386, C. freundii M17394, E. cloacae M17464 were resistant to all β-lactams including carbapenems and aztreonam while S. marcescens M17468 was susceptible to this last drug (Table 1). E. coli M17386 was resistant to colistin. Three isolates were resistant to minocycline, two to ciprofloxacin, and two to tigecycline. All isolates remained susceptible to fosfomycin (Table 1).
Table 1. Antimicrobial susceptibilities of the clinical isolates anlysed in this study and their respective tranconjugants.
| Antimicrobial agent |
MIC (μg/ml) / interpretationa | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KQN-M17277 | TC-M17277 | ECO-M17386 | RT-NDM-M17386* | RT-MCR-M17386* | CFR-M17394 | TC-M17394 | ECL-M17464 | TC -M17464 | SMA-M17468b | TC-M17468 | E. co J53-AzR | ||||||||||||||
| Imipenem | 16 | R | 8 | R | 8 | R | 8 | R | 0.12 | S | 8 | R | 4 | R | 32 | R | 8 | R | 128 | R | 32 | R | 0.06 | S | |
| Meropenem | 64 | R | 32 | R | 32 | R | 8 | R | 0.03 | S | 32 | R | 16 | R | 64 | R | 16 | R | 64 | R | 16 | R | 0.015 | S | |
| Ceftazidime | >256 | R | >256 | R | >256 | R | >256 | R | 0.06 | S | >256 | R | >256 | R | >256 | R | >256 | R | >256 | R | >256 | R | 0.06 | S | |
| Cefotaxime | >256 | R | >256 | R | >256 | R | >256 | R | 0.03 | S | 256 | R | 256 | R | >256 | R | 256 | R | >256 | R | 64 | R | 0.015 | S | |
| Cefepime | 256 | R | 32 | R | 32 | R | 16 | R | 0.06 | S | 128 | R | 32 | R | 128 | R | 16 | R | 64 | R | 16 | R | 0.06 | S | |
| Aztreonam | 256 | R | 4 | S | 16 | R | 4 | S | 0.12 | S | 8 | I | 8 | I | 16 | R | 4 | S | 4 | S | 4 | S | 0.12 | S | |
| Ciprofloxacin | 32 | R | 0.015 | S | 0.015 | S | 0.06 | S | 0.03 | S | 0.06 | S | 0.03 | S | 2 | R | 0.015 | S | 0.06 | S | 0.008 | S | 0.015 | S | |
| Amikacin | >64 | R | >64 | R | >64 | R | 64 | R | 0.5 | S | >64 | R | >64 | R | >64 | R | >64 | R | >64 | R | >64 | R | 1 | S | |
| Gentamicin | >64 | R | >64 | R | >64 | R | >256 | R | 0.25 | S | >64 | R | >64 | R | >64 | R | >64 | R | >64 | R | >64 | R | 0.25 | S | |
| Fosmomycinc | 16 | S | ≤16 S | 32 | S | 32 | S | 32 | S | 8 | S | 4 | S | 32 | S | 2 | S | 32 | S | 2 | S | ≤ 16 | S | ||
| Tigecyclinec | 16 | R | 0.5 | S | 0.5 | S | 1 | S | 1 | S | 0.5 | S | 0.5 | S | 16 | R | 0.5 | S | 8 | R | 1 | S | ≤ 1 | S | |
| Minocycline | >64 | R | 0.5 | S | 1 | S | 1 | S | 0.5 | S | 0.5 | S | 0.5 | S | 16 | R | 0.12 | S | 1 | S | 0.5 | S | 1 | S | |
| Colistinc | 0.25 | S | ≤1 S | 8 | R | 0.25 | S | 8 | R | 0.5 | S | ≤1 | S | 0.5 | S | ≤1 | S | ND | N D | ≤ 1 | S | ||||
Abbreviations used: R, resistant; I, intermediate; S, susceptible; ND, Not determined; TC, transconjugant; RT, retrotransconjugant; KQN, K. quasipneumoniae subsp. quasipneumoniae; ECO, E. coli, CFR, C. freundii; ECL, E. cloacae; SMA, S. marcescens.
aMICs were interpreted according to CLSI 2018 guidelines.
bSerratia spp. are naturally resistant to colistin.
cFosfomycin, tigecycline and colistin MICs were interpreted following the breakpoints of EUCAST.
E. coli J53-AzR, biparental conjugation acceptor strain, azide resistant.
In line with those results, PCR assays showed that all the clinical isolates carried blaNDM-1 and rmtC while E. coli M17386 additionally carried mcr-1. The plasmid mediated quinolone resistance genes PMQRs detected in K. pneumoniae M17277 were qnrB4 and qnrB52 and are described in the text below. E. cloacae M17464 harboured qnrA1 which could explain the resistance to ciprofloxacin detected.
Horizontal transfer of blaNDM-1 and mcr-1-harbouring plasmids
All blaNDM-1-harbouring plasmids were transferred by conjugation and the susceptibility profiles of transconjugants are shown in Table 1. Together with carbapenem resistance mediated by NDM-1, high level resistance to aminoglycosides was co-transferred mediated by RmtC. Resistance to cephalosporins was transferred mediated by CMY-6. On the contrary, resistance to ciprofloxacin, aztreonam, tigecycline and minocycline was not transferred to transconjugant strains, demonstrating that these resistance determinants may be encoded in the chromosome, in a non-mobile plasmid or in another plasmid that was not transferred.
E. coli-M17386 harboured both blaNDM-1 and mcr-1. Therefore sequential conjugations were performed to demonstrate that both genes were located on separate conjugative plasmids. In consequence, we obtained two retrotransconjugants that each expressed NDM-1 (RT-NDM-M17386) or MCR-1 (RT-MCR-M17386) (Table 1).
Whole plasmid characterisation
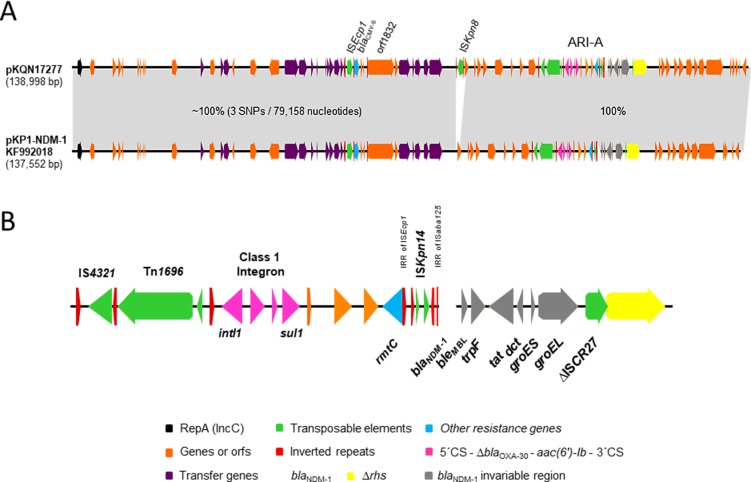
All isolates harboured 2 to 4 plasmids (48.5–470 kb) as observed by S1 nuclease-PFGE (S1A Fig). The blaNDM-1 probe hybridised with one band of ca. 138-kb in all clinical isolates and their respective transconjugants (S1B Fig). Full sequencing showed that all isolates harboured a plasmid of 138 kb carrying blaNDM-1. The blaNDM-1 plasmids from K. pneumoniae M17277, E. coli M17386 and C. freundii M17394, were 100% identical while those from E. cloacae M17464 and S. marcescens M17468 showed 3 and 10 SNPs, respectively, with the former three, and 7 SNPs between them. The plasmid of isolate K. pneumoniae M17277 (pKQN17277) was selected for the subsequent analysis. pKQN17277 showed maximal identity (99.0%) with pKP1-NDM-1 (acc. no. KF992018) a type 1 IncC obtained from a clinical isolate of K. pneumoniae in 2010 in Australia from a patient arrived from India [19]. Interestingly, besides 3 SNPs, the unique difference between both plasmids was the presence of the insertion sequence ISKpn8 (acc. no. EF382672) in pKQN17277 (Fig 1) not associated to any resistance gene [20]. Moreover, all the NCBI entries that showed 98% cover query and around 99% identity with pKQN17277 did not harbour ISKpn8. Given the high similarity with pKP1-NDM-1, the plasmids characterised here harboured the main hallmarks of type 1 IncC plasmids (Fig 1A and 1B) including rhs1 (unknown biological function) [21], orf1832 and ARI-A, which contained rmtC and blaNDM-1 [21] (Fig 1B). This last gene was located within a truncated Tn125 structure previously reported [4] (Fig 1B). Additionally, these plasmids harboured blaCMY-6 associated to ISEcp1, which was located outside ARI-A (Fig 1). On the other hand, pKQN17277 was not related to the blaNDM-1-harbouring plasmid of K. quasipneumoniae CCBH16302 strain reported in Brazil which was isolated in 2014 (ca. 346 kb, acc.no. MDCA00000000)[22]. Moreover, this last plasmid did not contain rmtC and harboured blaNDM-1 associated with Tn3000 [17].
Fig 1. Genetic map of pKQN17277.
Genes and orfs are denoted by arrows. Genes, mobile elements and other relevant features are colored as indicated in the key or specified in the figure. Shading denotes regions of identity. A, comparison of the sequenced plasmids and pKP1-NDM-1 (GenBank KF992018). B, main hallmarks of ARI-A resistance island containing blaNDM-1.
Full sequencing of the mcr-1-harbouring plasmid of E. coli M17386 (pMCR-M17386) showed that this gene was located in a ca. 61-kb IncI2 plasmid, which was 100% identical to pMCR-M15224 (acc. no. KY471309) isolated from a clinical isolate of C. amalonaticus in 2016 from the same paediatric hospital than the case reported here {Faccone, 2018 #4738}[23]. No other antimicrobial resistance gene was identified in pMCR-M17386.
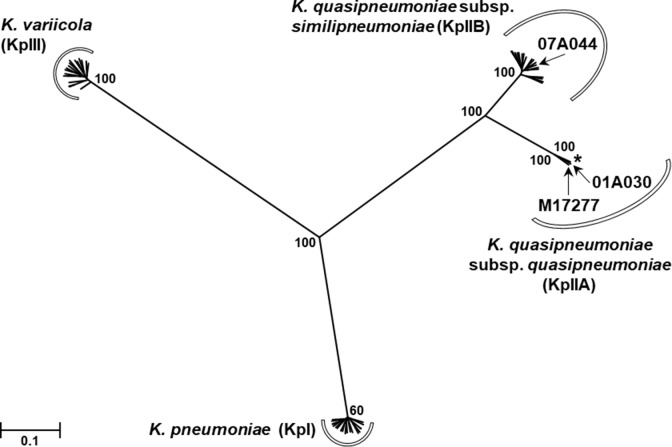
Phylogenetic analysis of the isolate M17277
M17277 was one of the first blaNDM-1-harbouring clinical isolates belonging to the genus Klebsiella that had been sent to the NRLAR (2014). M17277 was identified as K. pneumoniae by biochemical techniques and MALDI-TOF. However, these methodologies did not discriminate between K. pneumoniae, K. quasipneumoniae and K. variicola [13]. Therefore, M17277 was subjected to WGS and pan-genome analysis in the context of a global collection (S2 Table) [14]. The phylogenetic tree (S3 Table) showed that the isolate M17277 clustered with 100% bootstrap support with the type strain 01A030T and the unique isolate of K. quasipneumoniae subsp. quasipneumoniae (Fig 2). In order to validate the species identification, we determined the overall genomic similarity between K. quasipneumoniae subsp. quasipneumoniae M17277 and the type strains 01A030T and 07A044 with a genome-to-genome distance calculation. The results confirmed same species between M17277 and 01A030T (0.95%G+C) and distinct species between M17277 and 07A044 (1.19% G+C) (see S4 Table). We determined the overall genomic similarity by calculating the genome-to-genome distance between and the type strains 01A030 and 07A044. The results obtained confirmed that M17277 is the same species than the type strain 01A030 with a difference in % G+C: 0.95 and distinct species compared with 07A044 with a difference in % G+C: 1.19 (details in S4 Table).
Fig 2. Phylogenetic analysis of the isolate M17277.
A maximum likelihood phylogenetic tree was built based on the SNPs in the core-genome assemblies of the isolate M17277 and the Kleb-dataset, which comprised 20 isolates of K. pneumoniae; 1 isolate of K. quasipneumoniae subsp. quasipneumoniae, plus the type strain 01A030T for this species; 19 isolates of K. quasipneumoniae subsp. similipneumoniae, plus the type strain 07A044T for this species, and 18 isolates of K. variicola. The final SNP alignment had 61 taxa and 206,622 positions. For simplicity, the taxa names were excluded (arrows indicate the locations of M17277 and both type strains, and the asterisk shows the location of the unique isolate of K. quasipneumoniae subsp. quasipneumoniae of the Kleb-dataset) and the boostrap percentages (over 1,000 replicates) for only relevant nodes are shown. Branch lengths are expressed in units of changes/nucleotide position (scale bar).
M17277 showed ciprofloxacin resistance (Table 1). However, the analysis of gyrA, gyrB, parC and parE, compared to the corresponding figures of K. quasipneumoniae subsp. quasipneumoniae 01A030T, did not show quinolone resistance mutations. Interestingly, M17277 harboured three plasmid-mediated quinolone resistance genes. The qnrB alleles qnrB4 and qnrB52, were found in contigs of 14,325 and 2,947 nucleotides, respectively, that showed maximal identity with the plasmids pYNKP001-dfrA (99.98% identity) of Raoultella ornithinolytica YNKP001 and pQnrB52_020046 (100% identity) of K. pneumoniae SCKP020046, respectively (acc. no. KY270853 and CP028782, respectively). Both qnrB alleles were located in the variable region 2 of complex class 1 integrons (associated with ISCR1), showing the typical immediate genetic environment of qnrB alleles, i.e., bordered by the genes sapA and pspF [24]. In addition, aac(6’)-Ib-cr1 was found in a 3,542-nucleotide contig that encompassed the cassette region of complex class-1 integron [aac(6’)-Ib-cr1, arr-3, dfrA27 and aadA16], with 100% identity with 26 plasmids from nine enterobacterial species, including K. quasipneumoniae.
Discussion
Here, we report the case of a paediatric patient with erythrodermic psoriasis hospitalised due to edematous-ascitic syndrome. The patient was treated with multiple antibiotics including carbapenems and colistin. Notwithstanding the treatments provided, he was infected and colonised with multiple microorganisms including five NDM-1-producing Enterobacteriaceae, one co-producing MCR-1. Unfortunately, during 2014, parenteral fosfomycin (all five strains were susceptible to fosfomycin) was not available in that hospital.
The isolate M17277, originally typified as K. pneumoniae, was found to be K. quasipneumoniae subsp. quasipneumoniae. This species was first proposed in 2014 [13] and, therefore, the current epidemiological information is still scarce [14]. K. pneumoniae was divided through phylogenetic analysis into three species named K. pneumoniae, Klebsiella quasipneumoniae and K. variicola [13, 14]. In turn, K. quasipneumoniae was subdivided into two subspecies, namely, K. quasipneumoniae subsp. quasipneumoniae and K. quasipneumoniae subsp. similipneumoniae (phylogroups KpIIA and KpIIB, respectively) [13, 14]. In 2017, Aires et. al. reported the first observation of an NDM-1-producing K. quasipneumoniae, isolated from a rectal swab in Brazil [22]. M17277 was also isolated from a rectal swab and becomes the first report on NDM-1-producing K. quasipneumoniae subsp. quasipneumoniae from Argentina. Besides blaNDM-1, this isolate also harboured qnrB4 and qnrB52 that are epidemiologically relevant since they were not previously reported in Argentina [25]. The presence of these genes, in addition to aac(6’)-Ib-cr1, explains, at least in part, the high ciprofloxacin resistance level observed in M17277 (Table 1).
Up to date, there are four reports that involve seven cases of co-carriage of unrelated bacteria with fully sequenced blaNDM plasmids [4, 26–28]. In two of these cases, blaNDM was located in different plasmids associated with different mobile genetic elements [4, 26]. In other two cases, blaNDM was harboured by different plasmids but within the same mobile genetic element [4, 27]. Finally, in the remaining three cases, blaNDM was located in identical plasmids (IncC or IncN groups) [4, 28], a situation similar to that reported here. A/C plasmids are now considered IncA and IncC groups [21]. These were the earliest broad host range plasmids to be associated with antibiotic resistance [29]. IncC was divided into type 1 and type 2 plasmids defined by the backbone and resistance island features [29]. Most IncC type 1 plasmids are known to disseminate blaNDM within an antibiotic resistance island known as ARI-A [29, 30].
Plasmids highly identical to those found herein were only reported in Australia [19] and USA [31]. Interestingly, the Argentinian plasmids harboured ISKpn8, an insertion sequence that up to date has only been associated to blaKPC environment in Argentina and Asia [20]. Moreover, we recently characterised a C. amalonaticus-producing blaNDM-1 and mcr1.5 in plasmids highly similar to those reported here, isolated in 2016 and recovered from the same pediatric hospital than the case reported here [32]. Therefore, our analyses contribute to show that blaNDM was and continues to be disseminated through several mechanisms worldwide and across species barriers.
The first three blaNDM-1 plasmids isolated were identical, therefore, it is likely that K. quasipneumoniae-M17277 colonized the patient and passed pKQN17277 to a colonizing mcr-1-harbouring E. coli strain giving rise to E. coli-M17386, which later caused a systemic infection. Similarly, C. freundii-M17394 could have acquired pKQN17277 by conjugation from previously colonizing Enterobacteriaceae. The SNPs detected in the blaNDM-1 plasmids of E. cloacae-M17464 and S. marcescens-M17468 suggest independent acquisitions in these isolates, but more data is necessary to corroborate these assumptions.
Colistin is a last resort drugs that, in particular, has been reclassified by the World Health Organization as “Highest Priority Critically Important Antimicrobials” to human medicine, in an attempt to optimize its clinical use to treat serious human infections considering also the emergence of plasmid encoded colistin resistance gene mcr-1 [33]. In this study, we report an NDM-1-producing E. coli-M17386 that was additionally resistant to colistin due to the co-expression of MCR-1 [23]. The co-production of NDM-type and MCR-type enzymes has already been reported in E. coli and other Enterobacteriaceae species from hospitalized patients [5], healthy and sick food animals like broiler chickens and swine as well as from the farm environment like the slaughterhouse and sewage [34].
Conclusions
Health institutions worldwide have to affront the lack of effective antimicrobial treatments against multidrug-resistant bacteria and the spread of promiscuous plasmids that are able to disseminate resistance determinants into infecting or colonising unrelated bacterial species. In this case study, we showed the intra-patient dissemination of a blaNDM-1 harbouring plasmid among different Enterobacteriaceae species including the emerging pathogen K. quasipneumoniae subsp. quasipneumoniae [25]. As a consequence, active surveillance measures and strict institutional policies are imperative to determine the local prevalence and prevent further dissemination.
Supporting information
(a)Estimation of plasmid content and size of clinical isolates and transconjugants. Nuclease S1-PFGE of DNA plugs was perfomed to estimate plasmid content and size of the studied isolates. The five clinical isolates and transconjugants harboured between 2 to 4 plasmids (48.5–470 kb). (b) S1 nuclease-PFGE and Southern blot with blaNDM-1 probe of blaNDM-1 transcojugants
(PDF)
(a) List of primers used. (b) Salmonella spp. M1744 origin and MIC.
(XLSX)
The phylogroups KpI, KpIIA, KpIIB and KpIII correspond to K. pneumoniae, K. quasipneumoniae subsp. quasipneumoniae, K. quasipneumoniae subsp. similipneumoniae and K. variicola, respectively.
(XLSX)
The alignment of concatenated core genes (highlighted in yellow) resulted in a dataset of 61 taxa with 1,702,827 positions. The subsequent selection of the SNP sites from that core-genome alignment resulted in a SNP alignment of 61 taxa with 206,622 positions, which was used to construct the maximum likelihood phylogenetic tree.
(XLSX)
(XLSX)
Acknowledgments
We would like to thank Juan Manuel de Mendieta for his assistance in the annotation of the sequences, Josefina Campos of the Genomics and Bioinformatics Platform for the technical assistance in the WGS of K. quasipneumoniae M17277, and Daniel Cisterna of Neurovirosis Service for Sanger sequencing assistance. DDB, DFand SAG are members of the Research Career at Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by Agencia Nacional de Promoción Científica y Tecnológica - Fondo para la Investigación Científica y Tecnológica, PICT-2012-0145 to SAG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends in microbiology. 2011;19(12):588–95. 10.1016/j.tim.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Wailan AM, Paterson DL. The spread and acquisition of NDM-1: a multifactorial problem. Expert Rev Anti Infect Ther. 2014;12(1):91–115. 10.1586/14787210.2014.856756 [DOI] [PubMed] [Google Scholar]
- 3.Marquez-Ortiz RA, Haggerty L, Olarte N, Duarte C, Garza-Ramos U, Silva-Sanchez J, et al. Genomic Epidemiology of NDM-1-Encoding Plasmids in Latin American Clinical Isolates Reveals Insights into the Evolution of Multidrug Resistance. Genome biology and evolution. 2017;9(6):1725–41. 10.1093/gbe/evx115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wailan AM, Sartor AL, Zowawi HM, Perry JD, Paterson DL, Sidjabat HE. Genetic contexts of blaNDM-1 in patients carrying multiple NDM-producing strains. Antimicrob Agents Chemother. 2015;59(12):7405–10. 10.1128/AAC.01319-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Mu X, Zhang P, Zhao D, Ji J, Quan J, et al. Detection and characterization of a clinical Escherichia coli ST3204 strain coproducing NDM-16 and MCR-1. Infect Drug Resist. 2018;11:1189–95. 10.2147/IDR.S175041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasteran F, Gonzalez LJ, Albornoz E, Bahr G, Vila AJ, Corso A. Triton Hodge Test: Improved Protocol for Modified Hodge Test for Enhanced Detection of NDM and Other Carbapenemase Producers. J Clin Microbiol. 2016;54(3):640–9. 10.1128/JCM.01298-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasteran F, Tijet N, Melano RG, Corso A. Simplified Protocol for Carba NP Test for Enhanced Detection of Carbapenemase Producers Directly from Bacterial Cultures. J Clin Microbiol. 2015;53(12):3908–11. 10.1128/JCM.02032-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012;18(9):1503–7. 10.3201/eid1809.120355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andres P, Lucero C, Soler-Bistue A, Guerriero L, Albornoz E, Tran T, et al. Differential distribution of plasmid-mediated quinolone resistance genes in clinical enterobacteria with unusual phenotypes of quinolone susceptibility from Argentina. Antimicrobial agents and chemotherapy. 2013;57(6):2467–75. 10.1128/AAC.01615-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton BM, Harding GP, Zuccarelli AJ. A general method for detecting and sizing large plasmids. Anal Biochem. 1995;226(2):235–40. 10.1006/abio.1995.1220 [DOI] [PubMed] [Google Scholar]
- 11.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–9. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 12.Page AJ, De Silva N, Hunt M, Quail MA, Parkhill J, Harris SR, et al. Robust high-throughput prokaryote de novo assembly and improvement pipeline for Illumina data. Microb Genom. 2016;2(8):e000083 10.1099/mgen.0.000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisse S, Passet V, Grimont PA. Description of Klebsiella quasipneumoniae sp. nov., isolated from human infections, with two subspecies, Klebsiella quasipneumoniae subsp. quasipneumoniae subsp. nov. and Klebsiella quasipneumoniae subsp. similipneumoniae subsp. nov., and demonstration that Klebsiella singaporensis is a junior heterotypic synonym of Klebsiella variicola. Int J Syst Evol Microbiol. 2014;64(9):3146–52. [DOI] [PubMed] [Google Scholar]
- 14.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A 2015;112(27):E3574–81. 10.1073/pnas.1501049112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics (Oxford, England). 2015;31(22):3691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom. 2016;2(4):e000056 10.1099/mgen.0.000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England). 2014;30(9):1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wailan AM, Sidjabat HE, Yam WK, Alikhan NF, Petty NK, Sartor AL, et al. Mechanisms involved in acquisition of blaNDM genes by IncA/C2 and IncFIIY plasmids. Antimicrob Agents Chemother. 2016;60(7):4082–8. 10.1128/AAC.00368-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Belder D, Lucero C, Rapoport M, Rosato A, Faccone D, Petroni A, et al. Genetic diversity of KPC-producing Escherichia coli, Klebsiella oxytoca, Serratia marcescens, and Citrobacter freundii isolates from Argentina. Microbial drug resistance. 2018;24(7):958–65. 10.1089/mdr.2017.0213 [DOI] [PubMed] [Google Scholar]
- 21.Ambrose SJ, Harmer CJ, Hall RM. Compatibility and entry exclusion of IncA and IncC plasmids revisited: IncA and IncC plasmids are compatible. Plasmid. 2018;96–97:7–12. 10.1016/j.plasmid.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 22.Aires CA, Pereira PS, de Araujo CF, Chagas TP, Oliveira JC, Buonora SN, et al. Multiclonal expansion of Klebsiella pneumoniae isolates producing NDM-1 in Rio de Janeiro, Brazil. Antimicrob Agents Chemother. 2017;61(4):e01048–16. 10.1128/AAC.01048-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tijet N, Faccone D, Rapoport M, Seah C, Pasteran F, Ceriana P, et al. Molecular characteristics of mcr-1-carrying plasmids and new mcr-1 variant recovered from polyclonal clinical Escherichia coli from Argentina and Canada. PLoS One. 2017;12(7):e0180347 10.1371/journal.pone.0180347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro TG, Novais A, Branquinho R, Machado E, Peixe L. Phylogeny and comparative genomics unveil independent diversification trajectories of qnrB and genetic platforms within particular Citrobacter species. Antimicr Agents Chemother. 2015;59(10):5951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albornoz E, Lucero C, Romero G, Quiroga MP, Rapoport M, Guerriero L, et al. Prevalence of plasmid-pediated quinolone resistance genes in clinical enterobacteria from Argentina. Microbial drug resistance. 2017;23(2):177–87. 10.1089/mdr.2016.0033 [DOI] [PubMed] [Google Scholar]
- 26.Mataseje LF, Peirano G, Church DL, Conly J, Mulvey M, Pitout JD. Colistin-Nonsusceptible Pseudomonas aeruginosa Sequence Type 654 with blaNDM-1 Arrives in North America. Antimicrob Agents Chemother. 2016;60(3):1794–800. 10.1128/AAC.02591-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Lan R, Xiong Y, Ye C, Yuan M, Liu X, et al. Sequential isolation in a patient of Raoultella planticola and Escherichia coli bearing a novel ISCR1 element carrying blaNDM-1. PLoS One. 2014;9(3):e89893 10.1371/journal.pone.0089893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tijet N, Richardson D, MacMullin G, Patel SN, Melano RG. Characterization of multiple NDM-1-producing Enterobacteriaceae isolates from the same patient. Antimicrob Agents Chemother. 2015;59(6):3648–51. 10.1128/AAC.04862-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmer CJ, Partridge SR, Hall RM. pDGO100, a type 1 IncC plasmid from 1981 carrying ARI-A and a Tn1696-like transposon in a novel integrating element. Plasmid. 2016;86:38–45. 10.1016/j.plasmid.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 30.Carattoli A. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother. 2009;53(6):2227–38. 10.1128/AAC.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson CM, Bent ZW, Meagher RJ, Williams KP. Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain. PLoS One. 2014;9(6):e99209 10.1371/journal.pone.0099209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faccone D, Albornoz E, Tijet N, Biondi E, Gomez S, Pasteran F, et al. Characterization of a multidrug resistant Citrobacter amalonaticus clinical isolate harboring blaNDM-1 and mcr-1.5 genes. Infect Genet Evol. 2018;67:51–4. 10.1016/j.meegid.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 33.Collignon PC, Conly JM, Andremont A, McEwen SA, Aidara-Kane A, Agerso Y, et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis. 2016;63(8):1087–93. 10.1093/cid/ciw475 [DOI] [PubMed] [Google Scholar]
- 34.Wang R, Liu Y, Zhang Q, Jin L, Wang Q, Zhang Y, et al. The prevalence of colistin resistance in Escherichia coli and Klebsiella pneumoniae isolated from food animals in China: coexistence of mcr-1 and blaNDM with low fitness cost. Int J Antimicrob Agents. 2018;51(5):739–44. 10.1016/j.ijantimicag.2018.01.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a)Estimation of plasmid content and size of clinical isolates and transconjugants. Nuclease S1-PFGE of DNA plugs was perfomed to estimate plasmid content and size of the studied isolates. The five clinical isolates and transconjugants harboured between 2 to 4 plasmids (48.5–470 kb). (b) S1 nuclease-PFGE and Southern blot with blaNDM-1 probe of blaNDM-1 transcojugants
(PDF)
(a) List of primers used. (b) Salmonella spp. M1744 origin and MIC.
(XLSX)
The phylogroups KpI, KpIIA, KpIIB and KpIII correspond to K. pneumoniae, K. quasipneumoniae subsp. quasipneumoniae, K. quasipneumoniae subsp. similipneumoniae and K. variicola, respectively.
(XLSX)
The alignment of concatenated core genes (highlighted in yellow) resulted in a dataset of 61 taxa with 1,702,827 positions. The subsequent selection of the SNP sites from that core-genome alignment resulted in a SNP alignment of 61 taxa with 206,622 positions, which was used to construct the maximum likelihood phylogenetic tree.
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.