Abstract
Context
In end-stage renal disease (ESRD), serum high-density lipoprotein cholesterol (HDL-C) level is not an accurate predictor of mortality, partly because it does not necessarily correlate with indices of HDL function. Paraoxonase (PON) is a major enzyme constituent of HDL and a key component of HDL antioxidant activity. Apolipoprotein A-I (Apo A-1) is the core HDL structural protein that plays a major role in various aspects of HDL function.
Objective
We sought to examine PON activity and Apo A-I levels in patients with ESRD vs healthy controls.
Design and Setting
PON/arylesterase activity was measured in 499 patients with maintenance hemodialysis (MHD) and 24 healthy controls with similar distributions of age, sex, and race/ethnicity. Serum acrolein-modified Apo A-I was measured in 30 patients with MHD and 10 healthy controls.
Main Outcome Measures
Multilevel Cox models were used to assess associations among PON activity, Apo A-I, and HDL-C levels with 12-month all-cause mortality.
Results
PON activity was significantly lower in patients with MHD vs controls. Furthermore, acrolein-modified Apo A-I levels were higher in patients with MHD vs controls. In fully adjusted models, high PON activity was associated with lower 12-month mortality, whereas no difference of mortality risk was observed across HDL-C levels. The combination of high PON and low Apo A-I compared with low PON and low Apo A-I was associated with lower mortality risk.
Conclusions
In patients with MHD, PON activity had a stronger association with 12-month mortality than HDL-C. Future studies are needed to examine the role of these markers as potential diagnostic and therapeutic tools in ESRD.
End-stage renal disease (ESRD) is associated with a significantly higher risk of all-cause and cardiovascular (CV) mortality (1). Nearly one-half of all deaths in ESRD are attributed to CV disease (2). Although increasing serum concentrations of high-density lipoprotein (HDL) cholesterol (HDL-C) are associated with reduced risk of all-cause and CV mortality in the general population (3, 4), in certain subsets of patients, including those with ESRD being treated with hemodialysis, elevated HDL-C levels are paradoxically associated with worse outcomes (5–7).
It is well-known that HDL can play an important role in prevention of atherosclerosis and CV disease via mechanisms such as reverse cholesterol transport as well as antioxidant, antiapoptotic, vasoprotective, and anti-inflammatory properties (8–11). Apolipoprotein A-I (Apo A-I) is the major apolipoprotein component of HDL and also plays a key role in these functions (12). However, there is accumulating evidence that, under certain conditions, including those associated with inflammation such as ESRD, HDL becomes dysfunctional and loses these protective characteristics (13). Oxidant/chemical modification of Apo A-I or enrichment of HDL with proinflammatory proteins (such as serum amyloid A) can lead to this substantial impairment of HDL function and potentially result in a deleterious proinflammatory HDL, which can be associated with worse outcomes (14–16).
More recently, there has been increasing interest in determining whether measures of HDL function can be better predictors of HDL-related outcomes. However, determining which index of function to measure can be difficult (17–19), given the complexities involved in isolation of the HDL particle and the assays used to evaluate its function (17). Thus, serum measurements that are related to HDL function and that can be easily adopted in the laboratory setting would be of substantial value. Paraoxonase (PON) is a major antioxidant enzyme which is mostly associated with the HDL particle in serum and is thought to play an important role in HDL-mediated antioxidant activity (20, 21). Therefore, increasing serum PON activity can be associated with improved HDL function and several studies have found low PON activity to be a predictor of atherosclerosis and CV disease (22–24). In patients with ESRD, serum PON activity has been consistently shown to be decreased compared with healthy controls (25, 26), especially in those on long-term hemodialysis therapy (27). Furthermore, recent studies have found that low serum and HDL PON function is associated with significantly worse outcomes in patients with advanced (predialysis) chronic kidney disease (28, 29).
In light of these observations, we sought to determine the relationship between serum PON activity alone as well as in consideration of serum HDL-C and Apo A-I levels with ESRD-related mortality. Given the previous studies indicating increased oxidative modification of Apo A-I in patients with MHD, we also determined and compared the concentration of acrolein-modified Apo A-I in a subgroup of ESRD patients and healthy controls (30–32).
Materials and Methods
Study population
In this study, a cohort of healthy controls (n = 24) was recruited from the University of California (UC), Irvine Institute for Clinical and Translational Science. Eligible control subjects were at least 18 years old; not diagnosed with hypertension, diabetes, or other major CV comorbidities; and not taking medications. We then identified a subset of 500 patients with maintenance hemodialysis (MHD) enrolled in the prospective Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study (NCT01415570) between June 2014 and May 2017 with a similar distribution of age, sex, and race/ethnicity to that of the healthy control cohort. In brief, MADRAD is a prospective cohort study that examines the differences in dietary factors and nutritional status across racial/ethnic groups of patients with MHD recruited from large dialysis outpatient (LDO) facilities in the Los Angeles-Orange County, California, areas. A detailed description of the MADRAD study design and enrollment criteria have been previously reported (33). From the MADRAD MHD cohort, one patient was excluded on the basis of not having a serum PON measurement.
Clinical characteristics of patients with MHD
Baseline data on patient demographic and clinical characteristics and medication use were collected at study entry by MADRAD study coordinators. A combination of patient self-reports and International Classification of Diseases-9 codes from LDO records were used to identify the presence of diabetes as a preexisting condition. During regular hemodialysis sessions, body composition surrogates were measured by MADRAD study coordinators. Additional details about the collection of body anthropometric data have been previously described (33). Information on body mass index (BMI) (determined from postdialysis weight) was obtained from LDO electronic records and MADRAD study coordinators. We used the nearest body anthropometric data and BMI values collected within 90 days before or after the measurement of serum PON activity. Given that data on residual kidney function were unavailable, we used patient self-reported urine output and frequency (as measured by a validated questionnaire) closest to PON measurement as an estimate of residual kidney function. We defined dialysis vintage for MHD patients as the time interval between the dates of serum PON activity measurement and first hemodialysis treatment.
Medication ascertainment
Information on medication use was obtained from LDO records and entries by MADRAD study coordinators. Given limited data on medication use for MHD patients in our study cohort, we defined medication ever-use as having received a prescription within 1 year before or after the measurement of serum PON activity.
Serum samples and laboratory tests
Serum samples in MHD patients were collected predialysis during routine hemodialysis sessions, coinciding chronologically with blood tests performed at the LDOs, and frozen at −80°C until analysis. Laboratory tests obtained from patients with MHD at the LDOs were drawn using standardized techniques and measured using automated and standardized methods at a central laboratory in Deland, Florida, typically within 24 hours. Serum from healthy controls was purchased from Innovative Research (Novi, MI) and obtained via the assistance of UC Irvine Institute for Clinical and Translational Science. In all analyses, we used laboratory values measured closest to the time of serum PON activity measurement.
This study was approved by the institutional review committees of the Los Angeles Biomedical Research Institute at Harbor-UC Los Angeles (Torrance, CA) and the UC Irvine Medical Center, Orange, CA (MADRAD study institutional review board protocol #2012-9045).
Laboratory measures
Serum Apo A-I was checked using Apolipoprotein A-I Human SimpleStep ELISA Kit purchased from Abcam (Cambridge, MA). Serum PON activity was measured using an arylesterase/paraoxonase assay kit purchased from ZeptoMetrix, Inc. (Buffalo, NY). Serum concentrations of IL-6 were determined using ELISA assay kits from R&D systems (Minneapolis, MN) and Affymetrix ThermoFisher Scientific. All measurements were performed according to the manufacturer’s specifications and provided protocols.
Acrolein-modified Apo A-I
In additional analyses, we randomly selected 30 nonsmoking patients with MHD and 10 healthy controls from the study cohort. Only nonsmokers were selected (given that smoking can increase serum acrolein levels) via measurement of serum cotinine levels using a cotinine ELISA kit purchased from MyBioSource, Inc. (San Diego, CA) following the manufacturer’s protocol. Serum acrolein-modified Apo A-I adduct was measured with a sandwich ELISA assay using the ELISA kit purchased from Abcam, commercial antibody (GeneTex, Irvine, CA) against acrolein and acrolein-modified Apo A-I as a positive control (obtained from V.N.’s laboratory). Serum (100 μL) was inoculated in duplicate manner in the wells precoated with antihuman Apo A-I and incubated for 2.5 hours at room temperature with gentle shaking. After incubation, the wells were decanted and washed four times. A total of 100 μL acrolein antibody (GeneTex) was added to each well and incubated for 2 hours. After four washes, 100 μL antirabbit IgG horseradish peroxidase preadsorbed (Abcam, Cambridge, MA) was added to each well and incubated for 1 hour. After four washes, we added 3,3′,5,5′-tetramethylbenzidine ELISA substrate and stop solution (Abcam) and performed colorimetric detection at 450 nm in a microplate reader. Measurement was corrected by both negative controls and blanks.
Exposure and outcome ascertainment
The main exposure of interest was serum PON activity, which we categorized into quartiles (<44.9, 44.9 to <76.0, 76.0 to <104.4, and ≥104.4 kU/L). Other exposures of interest included HDL-C (n = 498) and Apo A-I (n = 493), which were divided into quartiles as follows: <29, 29 to <39, 39 to <50, and ≥50 mg/dL, and <79.7, 79.7 to <101.3, 101.3 to <126.3, and ≥126.3 mg/dL, respectively.
We also estimated rank scores of PON, HDL-C ,and Apo A-I separately based on their distribution in our cohort. We defined a low and high threshold for scores as <50th and ≥50th percentiles, respectively. We then created a 2 × 2 matrix of high and low categories for exposure groups based on PON with HDL-C or PON with Apo A-I levels.
The primary end point was 12-month all-cause mortality. Follow-up began at the date of measured serum PON activity to death, transplantation, loss-to-follow-up, end of study period (7 April 2018) or 12-month follow-up, whichever occurred first. Information on mortality and censored events were collected every 6 months by MADRAD study coordinators and reviewed by MADRAD study nephrologists (C.M.R. and K.K.-Z.).
Statistical analysis
Values are reported as mean (± SD) or median (interquartile range) for continuous variables, and as percentages for categorical variables. Parametric and nonparametric tests for trend were used, as appropriate. Baseline patient characteristics of demographics, comorbidities, and laboratory tests were compared between included and excluded patients. Using the Shapiro-Wilk test, we determined that PON activity was nonnormally distributed. We therefore used the nonparametric Wilcoxon-Mann-Whitney test to compare PON activity in MHD patients and healthy controls. Spearman’s rank correlations were used to assess the relationship between PON and laboratory, clinical, and body anthropometric data. Extreme outliers <0.5 or >99.5 percentiles were removed and replaced with lower or upper threshold values, respectively, for laboratory tests, time on hemodialysis, dialysis efficiency, BMI, and body anthropometric measurements.
Cox proportional hazards models were used to assess the association between exposure groups and 12-month all-cause mortality with hierarchical adjustment for covariates in the following three models: (i) model 1, unadjusted; (ii) model 2, adjusted for case-mix variables (age, sex, race, and ethnicity); and (iii) model 3, adjusted for covariates in model 2 plus diabetes and dialysis vintage.
Additionally, we conducted subgroup analyses examining the association of high (≥76) vs low (<76 kU/L, reference) PON and 12-month all-cause mortality in the fully adjusted model. We tested for potential effect modification by sex and race (white or nonwhite and black or nonblack) on the PON-mortality relationship by using the Wald test and including interaction terms for PON-gender and PON-race, respectively, in separate Cox models.
To identify other possible confounders on the PON quartile-mortality association, we also conducted sensitivity analyses of expanded models composed of covariates in model 3 plus either of the following: albumin, BMI, albumin and BMI, hypertension, myocardial infarction, other CV disease, cerebrovascular accident, heart failure, polycystic kidney disease, peripheral vascular disease, and current smoking status.
All patients had complete data on all covariates used in the primary analysis. In sensitivity analyses, we used imputation by mean for missing data (7% and 16% for BMI and albumin, respectively). A two-sided P < 0.05 was considered significant for all analyses in this study. All statistical analyses were conducted with SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Characteristics of patients with MHD
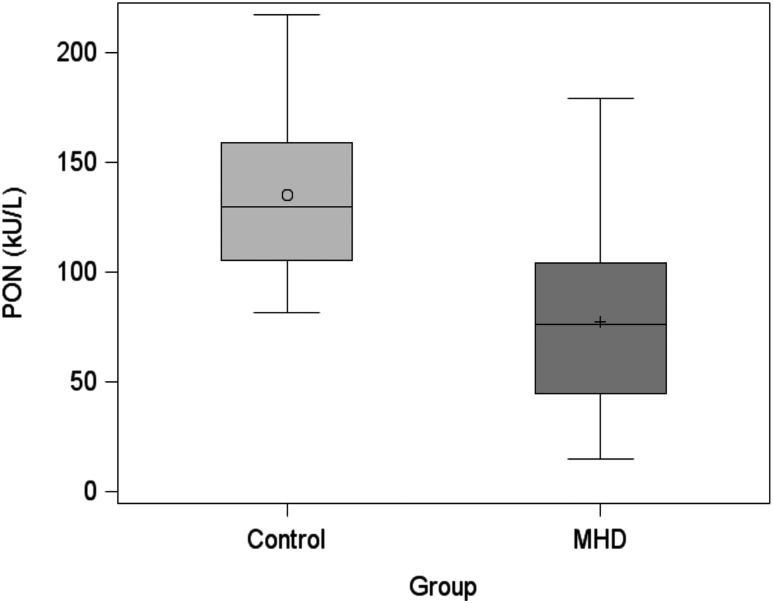
There were 499 patients included in our analytical cohort [comparisons between selected patients and 662 excluded patients are summarized in an online repository (34)]. Baseline characteristics of demographic, clinical, and medication use data for the 499 patients with MHD according to quartiles of PON levels are shown in Table 1. The mean (± SD) age of the cohort was 55 ± 15 years with 44% female, 34% black, and 49% Hispanic patients; 53% had diabetes. Baseline values of laboratory tests for the cohort are reported in Table 2. Patients in the highest PON quartile were more likely to have higher albumin and serum Apo A-I levels vs patients in the lowest PON quartile. Additionally, serum PON activity was significantly lower in patients with MHD than in healthy controls (mean ± SD, 77.2 ± 35.8 kU/L and 135.2±38.7 kU/L, respectively; P < 0.0001; Fig. 1).
Table 1.
Baseline Patient Characteristics of 499 Maintenance Hemodialysis Patients According to Serum PON Activity Quartiles
| Variables | Total | Serum PON Activity, kU/L | P for Trend | |||
|---|---|---|---|---|---|---|
| <44.9 | 44.9 to <76.0 | 76.0 to <104.4 | ≥104.4 | |||
| N, % | 499 | 122 (24) | 126 (25) | 128 (26) | 123 (25) | |
| Age, y | 55 ± 15 | 56 ± 15 | 55 ± 16 | 54 ± 14 | 55 ± 13 | 0.45 |
| Female, % | 44 | 35 | 47 | 44 | 50 | 0.05 |
| Race, % | ||||||
| Caucasian | 56 | 53 | 44 | 63 | 63 | 0.01 |
| Black | 34 | 33 | 39 | 31 | 33 | 0.65 |
| Asian | 7 | 7 | 12 | 5 | 4 | 0.17 |
| Other | 3 | 7 | 6 | 0.78 | 0 | 0.0002 |
| Hispanic ethnicity, % | 49 | 47 | 39 | 55 | 55 | 0.04 |
| BMI, kg/m2 | 27.8 ± 6.5 | 27.8 ± 6.8 | 27.1 ± 6.4 | 27.6 ± 5.9 | 28.9 ± 7.0 | 0.18 |
| Current smoking status, % | 11 | 3 | 8 | 17 | 16 | 0.0001 |
| Vintage, % | ||||||
| 12 mo | 11 | 18 | 15 | 6 | 5 | 0.0001 |
| 12-<36 mo | 27 | 34 | 29 | 27 | 19 | 0.006 |
| 36-<72 mo | 29 | 20 | 21 | 33 | 41 | <0.0001 |
| ≥72 mo | 33 | 28 | 35 | 34 | 35 | 0.28 |
| Comorbidities, % | ||||||
| Hypertension | 28 | 25 | 25 | 30 | 32 | 0.14 |
| Diabetes | 53 | 50 | 52 | 55 | 54 | 0.41 |
| Dyslipidemia | 18 | 12 | 19 | 19 | 24 | 0.03 |
| Cardiovascular disease | 19 | 20 | 17 | 20 | 19 | 0.96 |
| Myocardial infarction | 7 | 7 | 6 | 10 | 7 | 0.72 |
| Cerebrovascular incident | 0.20 | 0 | 0 | 0 | 0.81 | 0.18 |
| Heart failure | 11 | 13 | 11 | 11 | 8 | 0.23 |
| Polycystic kidney disease | 0.40 | 0 | 0 | 0.78 | 0.81 | 0.20 |
| Peripheral vascular disease | 3 | 2 | 2 | 3 | 7 | 0.02 |
| Medication use, % | ||||||
| Statin | 23 | 24 | 27 | 23 | 20 | 0.34 |
| ACEI/ARBs | 38 | 37 | 34 | 44 | 37 | 0.56 |
| Urine output, % | 73 | 69 | 74 | 77 | 71 | 0.66 |
| Urination frequency, % | 0.66 | |||||
| More than once a day | 66 | 64 | 66 | 69 | 64 | |
| Approximately once a day | 25 | 27 | 26 | 24 | 23 | |
| Every 2 to 3 d | 10 | 8 | 9 | 8 | 13 | |
Values are reported as mean (±SD) for continuous variables and as percentages for categorical variables. Percentages may not add up to 100 as a result of rounding. Urine output (%) represents percentage of self-reported “yes” responses to the MADRAD study question, “Do you still urinate?”
Abbreviations: ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 2.
Baseline Characteristics of Laboratory Data in 499 Maintenance Hemodialysis Patients According to Serum PON Activity Quartiles
| Variables | Total | Serum PON Activity (kU/L) | P for Trend | |||
|---|---|---|---|---|---|---|
| <44.9 | 44.9 to <76.0 | 76.0 to <104.4 | ≥104.4 | |||
| Albumin, g/dL | 4.0 ± 0.3 | 3.9 ± 0.4 | 4.0 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.3 | 0.01 |
| Hemoglobin, g/dL | 10.7 ± 1.1 | 10.7 ± 1.1 | 10.6 ± 0.9 | 10.6 ± 1.0 | 10.8 ± 1.2 | 0.50 |
| Phosphorus, mg/dL | 5.2 ± 1.5 | 5.1 ± 1.4 | 5.1 ± 1.6 | 5.5 ± 1.6 | 5.1 ± 1.3 | 0.70 |
| HDL-C, mg/dL | 41.3 ± 16.3 | 39.6 ± 13.2 | 43.8 ± 16.3 | 40.0 ± 16.9 | 41.6 ± 18.2 | 0.75 |
| IL-6, pg/mL | 2 (1, 4) | 2 (1, 5) | 3 (1, 5) | 2 (1, 4) | 2 (1, 4) | 0.12 |
| Apo A-I, mg/dL | 105.7 ± 35.3 | 92.7 ± 27.9 | 110.5 ± 33.1 | 106.6 ± 39.0 | 112.9 ± 36.8 | <0.0001 |
Values are reported as mean (±SD) or median (interquartile range) for continuous variables, where appropriate.
Figure 1.
Distribution of serum PON activity in controls and patients with MHD. Serum PON activity in healthy control subjects (n = 24) and patients with MHD (n = 499) are shown. P < 0.0001.
Spearman correlation coefficients between serum PON activity and laboratory data are presented in Table 3. Serum PON positively correlated with levels of albumin (rho = 0.13, P = 0.009), Apo A-I (rho = 0.20, P < 0.0001), and BMI (rho = 0.11, P = 0.02), but negatively correlated with IL-6 (rho = −0.13, P = 0.01) after adjustment for case-mix, diabetes, and dialysis vintage covariates.
Table 3.
Correlations of Serum PON Activity With Laboratory, Clinical, and Body Anthropometric Data in 499 Maintenance Hemodialysis Patients
| Variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Rho | P Value | Rho | P Value | |
| Laboratory tests | ||||
| Albumin, g/dL | 0.12 | 0.01 | 0.13 | 0.009 |
| Hemoglobin, g/dL | 0.02 | 0.68 | 0.03 | 0.60 |
| Phosphorus, mg/dL | 0.04 | 0.46 | 0.01 | 0.76 |
| HDL-C, mg/dL | −0.02 | 0.61 | −0.03 | 0.45 |
| IL-6, pg/mL | −0.11 | 0.05 | −0.13 | 0.01 |
| Apo A-I, mg/dL | 0.20 | <0.0001 | 0.20 | <0.0001 |
| Time on hemodialysis, min | −0.005 | 0.91 | 0.03 | 0.52 |
| Kt/V | 0.03 | 0.60 | −0.07 | 0.15 |
| BMI, kg/m2 | 0.10 | 0.03 | 0.11 | 0.02 |
| Body anthropometric measurements | ||||
| Biceps average, mm | 0.12 | 0.03 | 0.09 | 0.11 |
| Triceps average, mm | 0.14 | 0.009 | 0.11 | 0.05 |
| Midarm muscle circumference, cm | −0.04 | 0.44 | 0.04 | 0.46 |
| Midarm circumference, cm | 0.07 | 0.23 | 0.12 | 0.03 |
| Near-IR body fat, % | 0.11 | 0.04 | 0.06 | 0.28 |
Spearman correlation coefficients (Rho) shown for all variables. Correlation coefficients were adjusted for covariates in the following models: (i) unadjusted; and (ii) adjusted (age, sex, race, ethnicity, diabetes, and dialysis vintage).
Abbreviations: IR, infrared; Kt/V, dialysis efficiency.
Associations of PON activity, serum HDL-C, and Apo A-I with 12-month all-cause mortality
Among 499 patients, there were 61 deaths during a total 12-month follow-up of 459 patient-years, and the incidence rate of 12-month mortality was 13.3 (95% CI, 10.0 to 16.6) per 100 person-years.
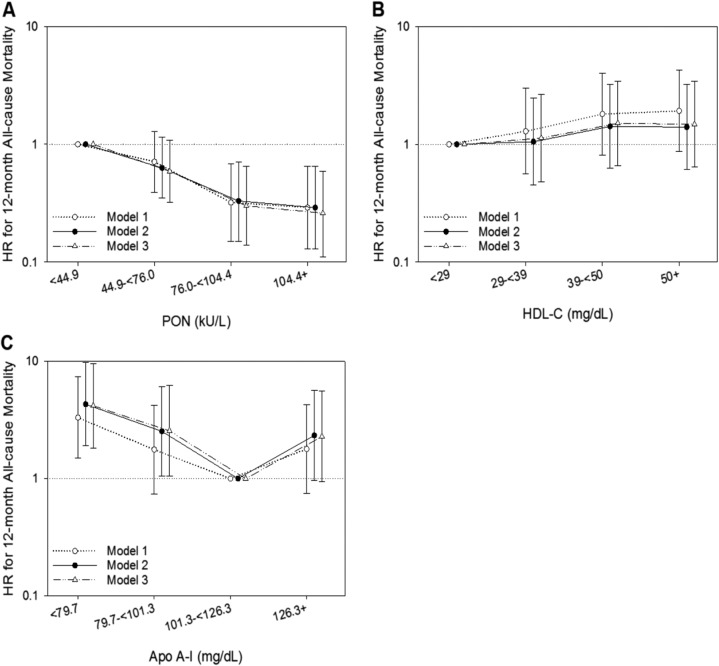
Patients in the highest serum PON quartile (≥104.4 kU/L) had the lowest risk of 12-month all-cause mortality compared with patients in the first quartile (<44.9 kU/L, reference) across all levels of adjustment, with an adjusted hazard ratio of 0.26 (95% CI, 0.11 to 0.59) in model 3 [Fig. 2A (34)].
Figure 2.
Twelve-mo all-cause mortality and PON, HDL-C, and Apo A-I quartiles. Associations among (A) PON quartiles, (B) HDL-C quartiles, and (C) serum Apo A-I quartiles and 12-mo all-cause mortality with adjustments for covariates in the following models: (i) model 1 (unadjusted); (ii) model 2 (case-mix variables: age, sex, race, and ethnicity); and (iii) model 3 (model 2 covariates plus diabetes and dialysis vintage). For visual purposes, the plots have log-transformed y-axes. HR, hazard ratio.
In analyses evaluating the association between HDL-C quartiles and 12-month all-cause mortality, no important association was observed [Fig. 2B (34)].
The lowest serum Apo A-I quartile (<79.7 mg/dL) was associated with 3.3-, 4.3-, and 4.2-fold higher risks of 12-month all-cause mortality in models 1, 2, and 3, respectively, when compared with the third quartile (101.3 to <126.3 mg/dL, reference) [Fig. 2C (34)]. [Results of the Apo A-I mortality associations using quartile 1 (<79.7 mg/dL) as the reference category are presented elsewhere (34)].
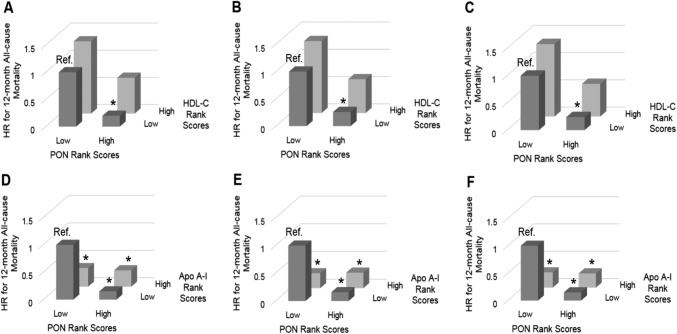
In analyses using rank scores, compared with a reference of low PON/low HDL-C, we observed that high PON/low HDL-C was significantly associated with lower 12-month all-cause mortality risk across all adjustment models [adjusted hazard ratios; 95% CI, 0.26 (0.09 to 0.80), and 0.24 (0.08 to 0.74) for models 2 and 3, respectively; Fig. 3B and 3C (34)]. However, there were no differences in mortality risk for either high PON/high HDL-C or low PON/high HDL-C vs low PON/low HDL-C. Compared with low PON/low Apo A-I, all other PON with Apo A-I levels rank score combinations had lower 12-month all-cause mortality risk in all adjustment models [Fig. 3D and 3F (34)].
Figure 3.
Twelve-mo all-cause mortality with rank scores of PON and HDL-C, and PON and Apo A-I. Associations between (A-C) low or high PON and low or high HDL-C rank scores and (D-F) low or high PON and low or high Apo A-I with 12-mo all-cause mortality with adjustment for covariates in the following models: (A, D) model 1 (unadjusted); (B, E) model 2 (case-mix variables: age, sex, race and ethnicity); and (C, F) model 3 (model 2 covariates plus diabetes and hemodialysis vintage). *P < 0.05. Ref., reference.
In subgroup analyses, we analyzed the association between high PON (≥76 vs <76 kU/L) and 12-month mortality risk according to sex (male, female), and race (white, nonwhite, black, and nonblack). A significantly lower mortality risk was observed for high PON across all subgroups, except in black patients, who also had a lower mortality risk but did not reach statistical significance (34). Furthermore, we did not find a significant interaction between sex and PON (Pinteraction = 0.68) or between race and PON (Pinteraction = 0.36 for black and 0.63 for white) for the association with 12-month all-cause mortality.
As sensitivity analyses, adjustment for additional covariates in expanded models did not change the association between higher PON and lower 12-month mortality risk (34).
Serum concentration of acrolein-modified Apo A-I
Of note, in a subset of 10 healthy controls and 30 patients with MHD and negative serum cotinine tests, we found that the MHD patients had significantly higher (+60%) levels of acrolein-modified Apo A-I adduct compared with healthy controls (P = 0.047) (34).
Discussion
ESRD is associated with abnormal HDL metabolism and function, with evidence indicating that in subgroups of patients HDL can become proinflammatory in nature (35–37). Furthermore, it has been postulated that this HDL dysfunction may underlie the paradoxical lack of relationship observed between higher concentrations of serum HDL-C levels and mortality in patients with MHD (5). In this study, we found that although HDL-C concentration was not significantly associated with 12-month all-cause mortality risk, higher PON was associated with lower mortality. Compared with patients with low PON/low Apo A-I, all other combinations of PON and Apo A-I in the 2 × 2 analysis had a lower risk of death. Similarly, patients with high PON/low HDL-C also had a significantly lower risk of death vs the reference group of patients with low PON/low HDL-C. Last, we did not find an important correlation between serum PON activity and HDL-C concentrations, but did find a modest correlation between serum PON activity and serum Apo A-I levels. The latter findings highlight the important point that serum HDL-C levels are not a good marker for indices of HDL function.
Although decreased serum and HDL activity of PON has been extensively studied and reported in MHD patients, our study examines the role of serum PON activity in ESRD-related mortality (28, 38, 39). We did not find serum HDL-C concentrations to be associated with any change in outcomes in this small cohort, whereas previously we had found a U-shaped relationship between HDL-C and mortality (5). This is most likely from the small number of patients in this investigation compared with previous studies. In our study, however, high PON combined with either low or high Apo A-I or low HDL-C was associated with lower mortality risk, possibly indicating the potential for a strong role of PON in predicting mortality outcomes in ESRD patients.
A previous study reported that increased oxidized Apo A-I levels in hemodialysis patients is associated with worse CV outcomes (16). We also found that acrolein-modified/content of Apo A-I is significantly increased in patients with ESRD on MHD when compared with healthy controls. This is consistent with previous reports which demonstrated that serum protein acrolein adducts are increased in ESRD patients (40). Increased acrolein content/modification of Apo A-I impairs ATP-binding cassette transporter A1 mediated cholesterol efflux and HDL function (41, 42). It is also important to note that free serum acrolein levels have been found to be inversely correlated with serum PON activity in ESRD patients (43), and increased acrolein-modified Apo A-I in MHD patients may be contributing to reduced PON activity and impaired HDL antioxidant activity in this patient population. Therefore, our findings provide another potential mechanism by which Apo A-I may become dysfunctional in ESRD setting. Further studies investigating the association of acrolein-modified Apo A-I with mortality in ESRD patients are needed.
Several limitations need to be mentioned. First, this is an observational study, and future mechanistic studies are needed to confirm our hypotheses. In addition, the relatively small sample size of our cohort limits our ability to adjust for all possible confounders. Therefore, large validation studies are needed to further elucidate the relationship between serum PON activity, HDL-C, Apo A-I levels, and outcomes in ESRD. Moreover, in this study, we did not have access to genetic information and hence could not assess PON genetic variants that may play a role in the activity of this enzyme and its association with outcomes. We were also unable to consider high-sensitivity C-reactive protein as a covariate in the adjusted models because this inflammatory marker was not measured regularly in MHD patients. However, additional adjustment for albumin as a marker of nutritional status and inflammation did not alter observed associations. Future investigations will be needed to evaluate the role of acrolein-modified Apo A-I in reduced PON activity and the association of increased Apo A-I levels and mortality.
In conclusion, we found a stronger relationship between serum PON activity as measured by its arylesterase function with 12-month all-cause mortality, compared with exposure measurements of serum HDL-C and Apo A-I. Furthermore, lower serum Apo A-I levels were associated with a significantly higher risk of death at 12 months and acrolein content/modified Apo A-I was increased in patients on MHD. Further studies are needed to determine the potential utility of these markers as diagnostic and therapeutic tools in the ESRD population.
Acknowledgments
The authors thank Ms. Amy You and Ms. Tracy Nakata for their assistance.
The content in this manuscript is the sole responsibility of the authors and in no way should be seen as official policy or interpretation by the US Department of Veterans Affairs or the United States government.
Financial Support: The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant UL1 TR001414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. H.M. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs 1 IK CX 001043-01A2. Y.S. and M.G. are supported overseas research scholarship by Sumitomo Life Welfare and Culture Foundation and Fukuoka University, School of Medicine Alumni, Eboshikai. A.C.F.N. is supported CNPq - Science Without Borders (201385/2012-0). WH.J. is partly supported by the International Postdoctoral Exchange Fellowship Program (No. 20150050). V.N. is supported by a grant from the NIH (GM105561).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Apo A-I
apolipoprotein A-I
- BMI
body mass index
- CV
cardiovascular
- ESRD
end-stage renal disease
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- LDO
large dialysis outpatient
- MADRAD
Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease
- MHD
maintenance hemodialysis
- PON
paraoxonase
- UC
University of California
References and Notes
- 1. Chang TI, Streja E, Moradi H. Could high-density lipoprotein cholesterol predict increased cardiovascular risk? Curr Opin Endocrinol Diabetes Obes. 2017;24(2):140–147. [DOI] [PubMed] [Google Scholar]
- 2. United States Renal Data System. 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 3. Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC; Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–1310. [DOI] [PubMed] [Google Scholar]
- 4. Gordon DJ, Rifkind BM. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med. 1989;321(19):1311–1316. [DOI] [PubMed] [Google Scholar]
- 5. Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. 2014;29(8):1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al-Aly Z. High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. veterans. Clin J Am Soc Nephrol. 2016;11(10):1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–2486. [DOI] [PubMed] [Google Scholar]
- 8. Miller NE, Thelle DS, Forde OH, Mjos OD. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977;1(8019):965–968. [DOI] [PubMed] [Google Scholar]
- 9. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J; Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32(12):2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Lüscher TF, Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127(8):891–904. [DOI] [PubMed] [Google Scholar]
- 12. Walldius G, Jungner I. Apolipoprotein A-I versus HDL cholesterol in the prediction of risk for myocardial infarction and stroke. Curr Opin Cardiol. 2007;22(4):359–367. [DOI] [PubMed] [Google Scholar]
- 13. Nicholls SJ, Zheng L, Hazen SL. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med. 2005;15(6):212–219. [DOI] [PubMed] [Google Scholar]
- 14. Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012;23(5):934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, Lehmann M, Kopecky C, Säemann MD, Lepper PM, Silbernagel G, Scharnagl H, Ritsch A, Thorand B, de las Heras Gala T, Wagenpfeil S, Koenig W, Peters A, Laufs U, Wanner C, Fliser D, Speer T, März W. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J. 2015;36(43):3007–3016. [DOI] [PubMed] [Google Scholar]
- 16. Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, Shishido K, Akizawa T. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis. 2012;220(2):493–501. [DOI] [PubMed] [Google Scholar]
- 17. Kronenberg F. HDL in CKD—the devil is in the detail. J Am Soc Nephrol. 2018;29(5):1356–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kopecky C, Genser B, Drechsler C, Krane V, Kaltenecker CC, Hengstschläger M, März W, Wanner C, Säemann MD, Weichhart T. Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol. 2015;10(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopecky C, Ebtehaj S, Genser B, Drechsler C, Krane V, Antlanger M, Kovarik JJ, Kaltenecker CC, Parvizi M, Wanner C, Weichhart T, Säemann MD, Tietge UJ. HDL cholesterol efflux does not predict cardiovascular risk in hemodialysis patients. J Am Soc Nephrol. 2017;28(3):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White CR, Anantharamaiah GM. Cholesterol reduction and macrophage function: role of paraoxonases. Curr Opin Lipidol. 2017;28(5):397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenblat M, Aviram M. Paraoxonases role in the prevention of cardiovascular diseases. Biofactors. 2009;35(1):98–104. [DOI] [PubMed] [Google Scholar]
- 22. Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(4):473–480. [DOI] [PubMed] [Google Scholar]
- 23. Shih DM, Lusis AJ. The roles of PON1 and PON2 in cardiovascular disease and innate immunity. Curr Opin Lipidol. 2009;20(4):288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299(11):1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samouilidou E, Kostopoulos V, Liaouri A, Kioussi E, Vassiliou K, Bountou E, Grapsa E. Association of lipid profile with serum PON1 concentration in patients with chronic kidney disease. Ren Fail. 2016;38(10):1601–1606. [DOI] [PubMed] [Google Scholar]
- 26. Gugliucci A, Mehlhaff K, Kinugasa E, Ogata H, Hermo R, Schulze J, Kimura S.. Paraoxonase-1 concentrations in end-stage renal disease patients increase after hemodialysis: correlation with low molecular AGE adduct clearance. Clin Chim Acta. 2007;377(1-2):213–220. [DOI] [PubMed] [Google Scholar]
- 27. Ribeiro S, do Sameiro Faria M, Mascarenhas-Melo F, Freitas I, Mendonça MI, Nascimento H, Rocha-Pereira P, Miranda V, Mendonça D, Quintanilha A, Belo L, Costa E, Reis F, Santos-Silva A. Main determinants of PON1 activity in hemodialysis patients. Am J Nephrol. 2012;36(4):317–323. [DOI] [PubMed] [Google Scholar]
- 28. Kennedy DJ, Tang WH, Fan Y, Wu Y, Mann S, Pepoy M, Hazen SL. Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc. 2013;2(2):e000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Untersteller K, Meissl S, Trieb M, Emrich IE, Zawada AM, Holzer M, Knuplez E, Fliser D, Heine GH, Marsche G. HDL functionality and cardiovascular outcome among nondialysis chronic kidney disease patients. J Lipid Res. 2018;59(7):1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tran TN, Kosaraju MG, Tamamizu-Kato S, Akintunde O, Zheng Y, Bielicki JK, Pinkerton K, Uchida K, Lee YY, Narayanaswami V. Acrolein modification impairs key functional features of rat apolipoprotein E: identification of modified sites by mass spectrometry. Biochemistry. 2014;53(2):361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeJarnett N, Conklin DJ, Riggs DW, Myers JA, O’Toole TE, Hamzeh I, Wagner S, Chugh A, Ramos KS, Srivastava S, Higdon D, Tollerud DJ, DeFilippis A, Becher C, Wyatt B, McCracken J, Abplanalp W, Rai SN, Ciszewski T, Xie Z, Yeager R, Prabhu SD, Bhatnagar A. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shao B, O’brien KD, McDonald TO, Fu X, Oram JF, Uchida K, Heinecke JW. Acrolein modifies apolipoprotein A-I in the human artery wall. Ann N Y Acad Sci. 2005;1043(1):396–403. [DOI] [PubMed] [Google Scholar]
- 33. Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suematsu Y, Goto M, Park C, Nunes ACF, Jing W, Streja E, Rhee CM, Cruz S, Kashyap ML, Vaziri ND, Narayanaswami V, Kalantar-Zadeh K, Moradi H. Data from: Association of serum paraoxonase/arylesterase activity with all-cause mortality in maintenance hemodialysis patients. UC Irvine Dash 2019. Deposited 17 April 2019. 10.7280/D1FT10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76(4):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaziri ND, Navab K, Gollapudi P, Moradi H, Pahl MV, Barton CH, Fogelman AM, Navab M. Salutary effects of hemodialysis on low-density lipoprotein proinflammatory and high-density lipoprotein anti-inflammatory properties in patient with end-stage renal disease. J Natl Med Assoc. 2011;103(6):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamamoto S, Kon V. Chronic kidney disease induced dysfunction of high density lipoprotein. Clin Exp Nephrol. 2014;18(2):251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kopecky C, Haidinger M, Birner-Grünberger R, Darnhofer B, Kaltenecker CC, Marsche G, Holzer M, Weichhart T, Antlanger M, Kovarik JJ, Werzowa J, Hecking M, Säemann MD. Restoration of renal function does not correct impairment of uremic HDL properties. J Am Soc Nephrol. 2015;26(3):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holzer M, Schilcher G, Curcic S, Trieb M, Ljubojevic S, Stojakovic T, Scharnagl H, Kopecky CM, Rosenkranz AR, Heinemann A, Marsche G. Dialysis modalities and HDL composition and function. J Am Soc Nephrol. 2015;26(9):2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noiri E, Yamada S, Nakao A, Tsuchiya M, Masaki I, Fujino K, Nosaka K, Ozawa T, Fujita T, Uchida K. Serum protein acrolein adducts: utility in detecting oxidant stress in hemodialysis patients and reversal using a vitamin E-bonded hemodialyzer. Free Radic Biol Med. 2002;33(12):1651–1656. [DOI] [PubMed] [Google Scholar]
- 41. Chadwick AC, Holme RL, Chen Y, Thomas MJ, Sorci-Thomas MG, Silverstein RL, Pritchard KA Jr, Sahoo D. Acrolein impairs the cholesterol transport functions of high density lipoproteins. PLoS One. 2015;10(4):e0123138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shao B, Fu X, McDonald TO, Green PS, Uchida K, O’Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem. 2005;280(43):36386–36396. [DOI] [PubMed] [Google Scholar]
- 43. Gugliucci A, Lunceford N, Kinugasa E, Ogata H, Schulze J, Kimura S.. Acrolein inactivates paraoxonase 1: changes in free acrolein levels after hemodialysis correlate with increases in paraoxonase 1 activity in chronic renal failure patients. Clin Chim Acta. 2007;384(1-2):105–12. [DOI] [PubMed] [Google Scholar]