Abstract
Context
Microscopic measurement of adipocyte size is the gold standard for determining adipose tissue (AT) quality. AT density on CT may also reflect adipocyte quality (lower density = poorer quality).
Objective
We used abdominal subcutaneous AT (SAT) specimens and CT scans to validate CT SAT density as a marker of SAT quality in adults living with HIV.
Setting and Design
Secondary data analysis from completed trial of antiretroviral therapy (ART) initiation (ACTG A5224s). CT abdominal SAT density was measured in HU. SAT specimens were digitally scanned for calculation of mean adipocyte area.
Participants
Participants had SAT biopsy and CT data at baseline (n = 54) and HIV-1 RNA <50 copies per milliliter on ART and biopsy or CT data at week 96 (n = 30).
Outcome Measures
Spearman correlations and linear regression models adjusting for participant characteristics examined associations between SAT density and adipocyte area.
Results
Baseline median age was 40 years, CD4+ T lymphocyte count 219 cells per cubic millimeter, and body mass index 26.0 kg/m2; 89% were male and 67% white. Median SAT area and density were 199 cm2 and −100 HU. Over 96 weeks, SAT area increased (+18%) and SAT density decreased (−3%). Mean SAT adipocyte area correlated with SAT density (P < 0.01) off and on ART after adjustment for SAT area, age, race, sex, CD4+ T lymphocyte count, and HIV-1 RNA.
Conclusions
CT SAT density correlates with biopsy-quantified SAT adipocyte size in adults with HIV on and off ART, suggesting that CT is a useful tool for noninvasive assessment of SAT quality.
In adults living with HIV on and off antiretroviral therapy, CT subcutaneous fat density measurement reflects histologic adipocyte size and can be used as a noninvasive measure of adipocyte function.
Adipose tissue (AT) is an active immune and endocrine organ, and adipocyte dysfunction has been linked to proinflammatory cytokine expression and inflammatory diseases including insulin resistance and cardiovascular disease (CVD) (1). AT disturbances are common in people living with HIV (PLWH) and stem from traditional as well as HIV- and antiretroviral therapy (ART)–associated contributors (2). Most studies assessing relationships between AT and end-organ outcomes focus on AT quantity; however, AT function, or quality, may vary at any given quantity, and changes in function may independently have profound effects on comorbid disease risk. For example, adiponectin, an adipokine whose production declines with reduced AT function and a major mediator between AT and CVD, was lower in men with HIV than in men without HIV in the Multicenter AIDS Cohort Study independent of AT area and strongly correlated with subclinical CVD burden after adjustment for traditional risk factors (3).
AT quality has historically been characterized by adipocyte size and density; normal, healthy adipocytes are small, are well differentiated, and contain a modest lipid droplet. During weight gain, adipocytes become larger, engorged with lipids and less dense (4, 5), changes that have been associated with a proinflammatory tissue environment and the development of metabolic disease (2, 6, 7). With continued adipocyte expansion, compensatory mechanisms such as fibrosis (8) are eventually used, at which time AT density begins to increase again. AT fibrosis and inflammation may be triggered by processes other than lipid droplet expansion, including infection or tissue injury (9), and are associated with both increased AT density and altered adipocyte function.
AT density can be measured via CT, where AT is identified by a tissue density of −190 (less dense) to −30 (more dense) HU (10). Although CT-quantified AT density has been used to determine relationships between inflammatory biomarker levels and clinical outcomes in humans, the assumption that CT-quantified AT density accurately reflects AT histopathology is based primarily on female nonhuman primate data, in which denser visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were associated with smaller adipocytes, lower serum leptin levels, and (for VAT) higher adiponectin levels (10). Given that HIV infection and ART impart multiple nontraditional risk factors for perturbations of AT quality and quantity, we sought to determine whether CT-quantified SAT density adequately reflects biopsy-quantified SAT adipocyte size in PLWH before and during ART.
Materials and Methods
Study population
AIDS Clinical Trials Group Study A5224s was a metabolic substudy of A5202 (NCT00118898) in which ART-naive people ≥16 years of age with HIV-1 RNA >1000 copies per milliliter were randomly assigned to blinded, coformulated abacavir (ABC)/lamivudine (3TC) or tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) with open-label, ritonavir-boosted atazanavir (ATV) or efavirenz (EFV). Complete A5202/A5224s methods have previously been published (11–13). A preplanned substudy of A5224s enrolled participants at 13 sites who were willing to undergo lower abdominal SAT biopsies under local anesthesia at A5224s weeks 0 and 96 (14). Relevant A5224s exclusion criterion potentially affecting AT quality and quantity included hypogonadism or thyroid disease, Cushing syndrome, diabetes mellitus, and the use of growth hormone, anabolic steroids, or glucocorticoids. For this analysis, the population was limited for baseline analyses to people with available baseline biopsy and CT data and, for week 96 and 96-week change analyses, to people with HIV-1 RNA <50 copies per milliliter on their originally randomized ART regimen and biopsy or CT data at week 96. All participants provided written informed consent, which was approved by each participating site’s local institutional review board.
SAT biopsies
Paraffin-embedded SAT specimens were stained with hematoxylin and eosin and digitized on a ScanScope AT platform (Leica Biosystems, Inc., Vista, CA). Morphometric analysis was performed to determine mean adipocyte area with Definiens’ Tissue Studio software (Definiens Inc., Parsippany, NJ). Specifically, the Composer module was used to train the system to identify adipocyte membrane staining and adipocyte interior space. The data output generated was total number of adipocytes and internal adipocyte area. Scanning and analyses were performed through the Translational Pathology Core Laboratory, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA.
CT scans
Substudy participants underwent single-slice abdominal CT scanning at the L4 to L5 level at weeks 0 and 96. CTs were centrally interpreted for SAT and VAT quantity (in cm2) and quality (in HU) by a blinded reader at the Tufts University Body Composition Analysis Center using SliceOmatic© software (version 5.0; TomoVision, Quebec, Canada). AT was identified by a mean attenuation of −190 to −30 HU (more negative = lower density). VAT was manually distinguished from SAT by tracing along the fascial plane defining the internal abdominal wall.
Statistical analyses
Descriptive summaries included medians and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables. Changes over time were assessed with Wilcoxon sign-rank tests and differences between groups with Wilcoxon rank-sum or Kruskal-Wallis tests. Associations between continuous measures were assessed via Spearman correlations, and linear regression models adjusted for clinical and demographic characteristics. Significance was assessed with a two-sided α = 0.05, without adjustment for multiple testing for this exploratory proof-of-concept analysis.
Results
Study population
Clinical and demographic characteristics are presented in Table 1. Briefly, at baseline (n = 54), median age was 40 years, CD4+ T lymphocyte count 219 cells per cubic millimeter, body mass index (BMI) 26.0 kg/m2; 89% were male, 39% white non-Hispanic, 26% current smokers, and 35% had HIV-1 RNA >100,000 copies per milliliter. The subset of participants with paired weeks 0 and 96 data (n = 30) were demographically similar to the larger baseline study population. After 96 weeks, the median CD4+ T lymphocyte count was 456 cells per cubic millimeter, median BMI had increased to 27.0 kg/m2, and 20% of participants were still current smokers. Eight participants were on EFV/TDF/FTC, five were on EFV/ABC/3TC, 11 were on RTV/ATV/TDF/FTC, and six were treated with RTV/ATV/ABC/3TC.
Table 1.
Demographic and Clinical Characteristics
| Baseline Overall (n = 54) | Baseline for Those With Week 96 Data (n = 30) | Week 96 (n = 30) | |
|---|---|---|---|
| Age, y | 40 (31, 45) | 40 (36, 45) | 42 (38, 47) |
| White, non-Hispanic race | 39% | 43% | 43% |
| Male, sex | 89% | 93% | 93% |
| Current smoking | 26% | 23% | 20% |
| BMI, kg/m2 | 26.0 (22.0, 30.0) | 26.0 (24.0, 30.0) | 27.0 (25.0, 30.0) |
| Hypertension | 13% | 20% | 20% |
| Dyslipidemia | 11% | 13% | 20% |
| Viral hepatitis coinfection | 9% | 7% | 7% |
| CD4+ T lymphocyte count, cells per mm3 | 219 (70, 312) | 203 (70, 274) | 456 (342, 581) |
| HIV-1 RNA, log10 copies per mL | 4.8 (4.3, 5.3) | 4.8 (4.3, 5.3) | N/A |
| HIV-1 RNA >100,000 copies per mL | 35% | 37% | 0% |
| SAT area, cm2 | 199 (129, 303) | 192 (130, 335) | 223 (180, 361) |
| SAT density, HU | −100 (−106, −96) | −100 (−105, −97) | −104 (−107, −100) |
| VAT area, cm2 | 83 (54, 108) | 85 (69, 113) | 116 (65, 132) |
| VAT density, HU | −83 (−89, −78) | −84 (−90, −80) | −90 (−94, −84) |
| Adipocyte diameter, μm2 | 2156 (1909, 2439) | 2098 (1824, 2467) | 2759 (2304, 3029) |
Median (IQR) or percentage reported.
Abbreviations: BMI, body mass index; N/A, not applicable.
Baseline AT area, AT density, and adipocyte area
At baseline, median (IQR) SAT area and density were 199 (129, 303) cm2 and −100 (−106, −96) HU, respectively. Median VAT area and density were 83 (54, 108) cm2 and −83 (−89, −78) HU, respectively. The mean SAT adipocyte area was 2156 (1909, 2439) μm2. As expected, SAT and VAT were most dense for participants with BMI <25 kg/m2 and least dense for obese participants. SAT and VAT density also varied by sex at birth, race, and baseline CD4+ T lymphocyte count, with men having denser SAT than women and non-Hispanic blacks and participants with CD4+ T lymphocyte count ≤200 cells per cubic millimeter having denser VAT (Table 2). SAT and VAT density did not vary by smoking status or HIV-1 RNA. Mean adipocyte area was smallest for participants with normal BMI at study entry and largest for obese participants (although with overlapping distributions); these again varied by clinical and demographic characteristics.
Table 2.
Baseline SAT and VAT Area and Density and SAT Adipocyte Diameters
| SAT Density (HU) | VAT Density (HU) | Adipocyte Diameter (μm2) | |
|---|---|---|---|
| Overall (n = 54) | −100 (−106, −96) | −83 (−89, −78) | 2156 (1909, 2439) |
| BMI category, kg/m2 | |||
| 18.0–24.9 (n = 22) | −96 (−104, −87) | −81 (−84, −78) | 2082 (1919, 2392) |
| 25.0–30.0 (n = 18) | −100 (−102, −97) | −82 (−87, −75) | 2181 (1775, 2364) |
| >30.0 (n = 14) | −106 (−110, −102) | −90 (−95, −85) | 2448 (1909, 2707) |
| P | 0.001 | 0.001 | 0.16 |
| Race or ethnicity | |||
| White, non-Hispanic (n = 21) | −101 (−106, −96) | −89 (−91, −82) | 2306 (1922, 2551) |
| White, Hispanic (n = 15) | −101 (−107, −96) | −83 (−91, −80) | 2098 (1909, 2435) |
| Black (n = 13) | −100 (−105, −96) | −78 (−82, −75) | 2020 (1727, 2321) |
| Other (n = 5) | −97 (−97, −94) | −83 (−83, −78) | 2342 (2056, 2358) |
| P | 0.27 | 0.02 | 0.70 |
| Sex at birth | |||
| Male (n = 48) | −99 (−105, −95) | −83 (−89, −78) | 2156 (1909, 2435) |
| Female (n = 6) | −108 (−108, −105) | −87 (−91, −82) | 2182 (1909, 2524) |
| P | 0.004 | 0.39 | 0.72 |
| CD4+ T lymphocyte count, cells per mm3 | |||
| ≤200 (n = 25) | −100 (−106, −96) | −79 (−87, −75) | 2098 (1930, 2352) |
| >200 (n = 29) | −101 (−106, −95) | −85 (−91, −82) | 2342 (1853, 2664) |
| P | 0.87 | 0.003 | 0.28 |
| HIV-1 RNA, copies per mL | |||
| <100,000 (n = 35) | −101 (−106, −97) | −84 (−90, −76) | 2308 (1922, 2524) |
| ≥100,000 (n = 19) | −97 (−106, −95) | −82 (−87, −78) | 2098 (1824, 2321) |
| P | 0.22 | 0.34 | 0.28 |
Median (IQR) reported. P values based on Kruskal-Wallis or Wilcoxon rank-sum tests.
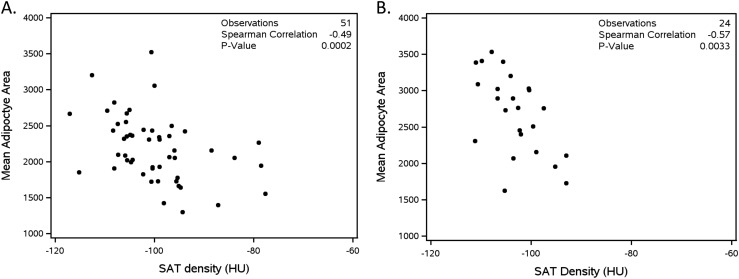
SAT adipocyte area correlated with SAT density (r = −0.49; 95% CI, −0.67 to −0.24; P = 0.002), indicating larger adipocytes had lower density, an association that was driven by participants with normal BMI at study entry (BMI 18.0 to 24.9 kg/m2, r = −0.57, P = 0.009; BMI 25.0 to 30.0 kg/m2, r = −0.21, P = 0.42; BMI >30.0 kg/m2, r = −0.24, P = 0.43; Fig. 1A). This relationship persisted in partial Spearman correlations (r = −0.41, P = 0.0003) adjusted for age, race, sex, SAT area, CD4+ T lymphocyte count, and HIV-1 RNA, and in regression models adjusting for age, race, sex, CD4+ T lymphocyte count, and SAT area (Table 3). Additionally, CT-quantified AT area and density correlated strongly with each other (SAT, r = −0.71, P < 0.0001; VAT, r = −0.59, P < 0.0001), as did SAT and VAT density (r = 0.58; 95% CI, 0.36 to 0.73; P < 0.0001).
Figure 1.
CT SAT density correlates strongly with SAT adipocyte area. (A) Week 0. (B) Week 96.
Table 3.
Multivariate Regression Estimates for Relationship With Mean SAT Adipocyte Area
| Baseline | Week 96 | |||
|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | |
| SAT density, HU | −26.1 (−44.9 to −7.3) | 0.008 | −63.7 (−115.3 to −12.1) | 0.02 |
| SAT area, cm2a | −0.9 (−2.5 to 0.7) | 0.26 | −0.3 (−2.6 to 2.0) | 0.80 |
| Age, y | −7.8 (−20.9 to 5.3) | 0.24 | −0.8 (−28.0 to 26.4) | 0.95 |
| Race or ethnicity | ||||
| Black | −43.6 (−395.6 to 308.5) | 0.80 | 23.3 (−830.1 to 876.7) | 0.95 |
| Hispanic white | 27.5 (−342.6 to 397.6) | 0.88 | 129.1 (−630.3 to 888.5) | 0.72 |
| Other | −93.4 (−551.7 to 365.0) | 0.68 | −90.1 (−1073.8 to 893.5) | 0.85 |
| Female sex at birth | 26.6 (−451.5 to 504.6) | 0.91 | 91.2 (−1194.5 to 1376.8) | 0.88 |
| CD4+ T lymphocyte count, cells per mm3 | 0.8 (−0.1 to 1.7) | 0.08 | 0.4 (−1.9 to 2.7) | 0.72 |
| HIV-1 RNA, log10 copies per mL | −37.9 (−285.7 to 210.1) | 0.76 | N/Ab | N/Ab |
Abbreviation: N/A, not applicable.
Area at baseline or week 96, respectively.
HIV-1 RNA <50 copies per milliliter for all participants at week 96.
Week 96 AT area, AT density, and adipocyte area
After 96 weeks of ART, median SAT area increased to 223 (180, 361) cm2, and SAT density decreased to −104 (−107, −100) HU. Median VAT area increased to 116 (65, 132) cm2, and VAT density decreased to −90 (−94, −84) HU. Mean SAT adipocyte area increased to 2759 (2304, 3029) μm2. For the 30 participants with paired baseline and week 96 data, these changes represent 18% (P = 0.002) and 35% (P < 0.001) median increases in SAT and VAT area, 3% (P = 0.01) and 6% (P < 0.001) decreases in median SAT and VAT density, and a 22% increase (P < 0.001) in mean SAT adipocyte area, respectively. Although analyses were exploratory and sample sizes were small, some variations in relationships by ART regimen were observed (Table 4).
Table 4.
Distribution of AT Outcomes by ART Regimen at Week 96
| NNRTI or PIa | NRTIa | ||||
|---|---|---|---|---|---|
| Week 96 | Overall (n = 30) | EFV (n = 13) | RTV/ATV (n = 17) | ABC/3TC (n = 11) | TDF/FTC (n = 19) |
| SAT density, HU | −104 (−107, −100) | −102 (108, −99) | −105 (−107, −102) | −99 (−104, −95) | −105 (−108, −103) |
| VAT density, HU | −90 (−94, −84) | −91 (−96, −86) | −88 (−93, −82) | −84 (−87, −81) | −91 (−94, −86) |
| Adipocyte area, μm2 | 2759 (2304, 3029) | 2455 (2158, 3007) | 2761 (2444, 3148) | 2488 (2072, 3029) | 2827 (2427, 3057) |
| SAT area-adipocyte size correlationb | −0.57 (0.003) | −0.60 (0.09) | −0.67 (0.005) | −0.73 (0.02) | −0.37 (0.18) |
| NNRTI or PIa | NRTIa | ||||
|---|---|---|---|---|---|
| 96-Week Change (%, P) | Overall (n = 30) | EFV (n = 13) | RTV/ATV (n = 17) | ABC/3TC (n = 11) | TDF/FTC (n = 19) |
| SAT density, HU | −3%, P = 0.01 | −1%, P = 0.70 | −3%, P = 0.003 | −1%, P = 0.50 | −3%, P = 0.02 |
| VAT density, HU | −6%, P < 0.001 | −4%, P = 0.08 | −8%, P = 0.004 | −5%, P = 0.25 | −7%, P = 0.002 |
| Adipocyte area, μm2 | 22%, P < 0.001 | 18%, P = 0.07 | 26%, P < 0.001 | 22%, P = 0.04 | 23%, P = 0.001 |
| 96-Week Change (%, P) | Overall (n = 30) | EFV + TDF/FTC (n = 8) | EFV + ABC/3TC (n = 5) | RTV/ATV + TDF/FTC (n = 11) | RTV/ATV + ABC/3TC (n = 6) |
|---|---|---|---|---|---|
| SAT density, HU | −3%, P = 0.01 | 1%, P = 1.00 | −2%, P = 0.63 | −3%, P = 0.004 | 1%, P = 0.81 |
| VAT density, HU | −6%, P < 0.001 | −4%, P = 0.30 | −6%, P = 0.25 | −8%, P = 0.004 | 2%, P = 0.81 |
| Adipocyte area, μm2 | 22%, P < 0.001 | 20%, P = 0.22 | 8%, P = 0.19 | 25%, P = 0.002 | 36%, P = 0.16 |
Median (IQR) reported. P values for percentage change overall or within treatment arms based on Wilcoxon signed-rank test.
Abbreviations: NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Participants are in the EFV or RTV/ATV group and in the ABC/3TC or TDF/FTC arms.
Spearman ρ (P value).
Similar to baseline, mean SAT adipocyte area on ART correlated with SAT density (r = −0.57, P = 0.003; Fig. 1B), with the strength of correlation decreasing with increasing BMI (data not shown). Again, this relationship persisted in partial Spearman correlations (r = −0.56, P = 0.012) adjusted for age, race, sex, CD4+ T lymphocyte count, and week 96 SAT area, and in regression models adjusting for age, race, sex, CD4+ T lymphocyte count, and SAT area (Table 3), with some differences observed by ART regimen (Table 4). Again, CT-quantified AT area and density correlated with each other (SAT, r = −0.35, P = 0.072; VAT, r = −0.55, P = 0.003), as did both week 96 SAT and VAT density (r = 0.55, P = 0.002) and percentage change in VAT and SAT density (r = 0.77, P < 0.001).
Discussion
In this analysis of abdominal SAT biopsy and CT scan data from a completed trial of ART initiation, we demonstrate that CT SAT density reflects SAT adipocyte size in PLWH before and during ART. This relationship persists after adjustment for important confounding factors such as AT quantity and lays the foundation for the use of CT as a noninvasive tool to assess AT quality or function in future studies. We also demonstrated that there were baseline differences in AT density by sex at birth, race, and CD4+ T lymphocyte count; in exploratory analyses, that changes in AT density on ART varied somewhat by ART regimen, but overall AT density decreased as AT area increased; that, as in the general population, physiologically expected relationships between AT density and BMI were observed (i.e., in general, AT density decreased as BMI increased) (15); and that SAT and VAT density tended to track together before and during ART, suggesting that redistribution of fat from the subcutaneous to the visceral spaces did not occur with initiation of these more modern ART regimens.
The theory that adipocyte density can reflect adipocyte function is based on the knowledge that healthy adipocytes are small and well differentiated and contain a modest lipid droplet. In the absence of tissue inflammation or fibrosis, during weight gain adipocytes become larger, less well differentiated, and lipid engorged, making them less dense (4, 5), whereas during weight loss adipocytes become smaller and contain fewer lipids, making them denser (16, 17). Although this theory has been the basis for the use of CT density in several large studies outside HIV, data confirming this hypothesis have previously been limited to nonhuman primate data, where denser VAT and SAT were associated with smaller adipocytes (VAT, r = −0.76, P < 0 0.001; SAT, r = −0.59, P = 0.003) (10). Given the complex relationships between HIV, ART, and traditional risk factors affecting AT quantity and function, it was important to document that CT SAT density reflected histologic adipocyte size, the gold standard for adipocyte quality assessment, before the use of CT AT density data as a surrogate noninvasive marker of AT quality in PLWH.
Regarding our observed baseline differences in AT density by sex, race, and CD4+ T lymphocyte count, because these relationships are novel, it is difficult to know whether they are physiologically expected. The Health, Aging and Body Composition and the Age, Gene and/or Environment Susceptibility–Reykjavik observational studies of older adults stated that “sex, race, adipose depot, and study site-specific quintiles of adipose density were created as the distribution of adipose density varied within these strata” (10). The Framingham Heart Study reported that women had SAT than was less dense but VAT that was more dense than men’s, without differential reporting by race (15). This finding of greater SAT density among men vs women parallels what we observed in our analysis of adults living with HIV. Interestingly, premenopausal, nonobese genetic women can modulate adipocyte size and function better than genetic men, in whom detrimental (hypertrophic) responses to fat gain predominate (18). Therefore, adipocyte hypertrophy in men on ART may be a maladaptive response influenced by genetic sex.
Although there are no previous studies specifically assessing the relationship with CD4+ T lymphocyte count and abdominal SAT or VAT quality, lower nadir CD4+ T lymphocyte count has been associated with greater gains in AT quantity on ART in some studies (19, 20). Given our observed relationship between decreasing SAT density and increasing SAT quantity, it may therefore be reasonable to expect greater changes in AT density in people with lower nadir CD4+ T lymphocyte counts. We observed denser VAT among participants with lower nadir CD4+ T lymphocyte counts in our analysis, which may reflect being in a more prolonged catabolic state or greater VAT inflammation among those participants, although VAT biopsies are not available to confirm this finding and current imaging techniques cannot distinguish between causes of density (smaller lipid droplets vs fibrosis vs inflammation).
Finally, changes in AT density over 96 weeks of ART varied somewhat by ART regimen in our cohort. It must be emphasized that these analyses were purely exploratory and hypothesis generating, and sample sizes were small. However, some provocative findings emerged. For nucleoside reverse transcriptase inhibitors (NRTIs), the correlation between SAT density and adipocyte size was much lower (and not statistically significant) among the subset of participants receiving TDF/FTC. SAT and VAT were less dense at week 96 among participants receiving TDF/FTC vs ABC/3TC, and the changes in AT density and adipocyte size were also greater for TDF/FTC. For the third agent, SAT density was lower and adipocyte size was larger for ATV/RTV-treated participants than for EFV-treated participants, and the changes were also larger for ATV/RTV vs EFV. Although NRTIs have been associated with adipocyte mitochondrial dysfunction (2, 14), ABC increases AT gene expression of cell adhesion molecules, which could lead to increased tissue AT density (21), as suggested by our data. NRTI penetration into AT is also limited (22), which could further affect the AT inflammatory milieu, given recent documentation of an AT HIV reservoir (23, 24).
Addressing the third agents, both EFV and ATV/RTV have numerous potentially adverse effects on AT (2), including proinflammatory effects (25–27), impaired adipogenesis, adipocyte function or differentiation (25, 27), preadipocyte apoptosis, and impaired mitochondrial function (25, 28, 29). There is also evidence that EFV shifts SAT toward anaerobic metabolism (30), which is conceivably proinflammatory. Although the ways in which these changes ultimately affect AT density in PLWH are unknown, tissue inflammation and subsequent fibrosis can result in increased tissue density, and poorly differentiated adipocytes are larger and less dense than well-differentiated adipocytes. Thus, it is reasonable to expect that individual ART regimens may affect AT density differently through their effects on AT function.
AT quality or function has important implications: AT is an active immune and endocrine organ, and AT dysfunction has been linked to proinflammatory cytokine expression and inflammatory diseases including insulin resistance and CVD (1). The ability to use noninvasive measures such as CT to measure AT quality may facilitate pathophysiologic understanding of metabolic disease states and the effectiveness of interventions to improve AT health in both research and clinical settings. To date, examples of the use of AT density to explore correlates of disease and clinical outcomes include the following. In the Health, Aging and Body Composition and the Age, Gene and/or Environment Susceptibility–Reykjavik observational studies of older adults, denser VAT was associated with lower leptin levels, and denser VAT and SAT were associated with higher adiponectin levels (10). In the Framingham Heart Study, less dense SAT and VAT were was associated with lower adiponectin and leptin receptor levels but higher leptin and fatty acid binding protein‐4 (a fatty acid transporter) levels in men and women after adjustment for confounding factors, including fat area (31). The Framingham Heart Study also observed a relationship between lower VAT and SAT densities and greater coronary artery calcium (32); lower VAT density and greater abdominal aortic calcium deposition (32) and higher serum triglyceride levels (33); and higher SAT density and diabetes and hypertension risk, which the authors hypothesized was secondary to greater AT fibrosis and dysfunction (34). Now that we have determined that CT SAT density accurately reflects SAT adipocyte size in PLWH, our next steps will be to use CT data from multiple longitudinal cohorts to investigate relationships between AT quality and cardiometabolic disease.
This analysis has several limitations. First, only a subset of A5224s participants had available biopsy specimen and CT data, and we were limited in our ability to pursue complete AT profiling, including adipocyte phenotypes, staining for fibrosis, and differential ART penetration into SAT vs VAT. However, the availability of 54 baseline CT and biopsy pairs and 30 longitudinal pairs is a unique and valuable resource that allowed us to achieve our main goal: assessment of the correlation between SAT CT density and SAT histopathologic adipocyte size. Second, this analysis contained a minority of women and racial or ethnic minorities, which may limit the generalizability of our results to other populations. Third, sample size limited exploratory subset analyses (including ART subset analyses); however, these data provided provocative, hypothesis-generating information for future work, which is the ultimate goal of a pilot study. Fourth, although we used the standard definition of CT fat density validated at our central reading center (−190 to −30 HU), reported cutoffs in the literature vary slightly, which limits comparability with studies using other cutoffs.
In conclusion, CT SAT density correlates strongly with biopsy-quantified SAT adipocyte size in PLWH before and during ART. CT may be a useful tool for noninvasive assessment of AT quality. Future, larger studies will better assess the contributions of clinical and demographic factors to AT density in PLWH, as well as the effects of therapeutic interventions on AT density.
Acknowledgments
The authors thank the study participants, sites, and staff for their participation.
Financial Support: This work was funded by National Institutes of Health Grants K23 AI110532 to J.E.L., R01 AI065348 to G.A.M., and UL1 TR000124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Disclosure Summary: J.E.L. has served as a consultant to Gilead Sciences and Merck and receives research support from Gilead Sciences. K.M.E. has served as a consultant to ViiV and Gilead Sciences and receives research support from Gilead Sciences. T.T.B. has served as a consultant to Gilead Sciences, Merck, BMS, Theratechnologies, and EMD-Serono. G.A.M. has served as a consultant for Merck, Gilead, and ViiV. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 3TC
lamivudine
- ABC
abacavir
- ART
antiretroviral therapy
- AT
adipose tissue
- ATV
atazanavir
- BMI
body mass index
- CVD
cardiovascular disease
- EFV
efavirenz
- FTC
emtricitabine
- IQR
interquartile range
- NRTI
nucleoside reverse transcriptase inhibitor
- PLWH
people living with HIV
- SAT
subcutaneous adipose tissue
- TDF
tenofovir disoproxil fumarate
- VAT
visceral adipose tissue
References and Notes
- 1. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. [DOI] [PubMed] [Google Scholar]
- 2. Erlandson KM, Lake JE. Fat matters: understanding the role of adipose tissue in health in HIV infection. Curr HIV/AIDS Rep. 2016;13(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ketlogetswe KS, Post WS, Li X, Palella FJ Jr, Jacobson LP, Margolick JB, Kingsley LA, Witt MD, Dobs AS, Budoff MJ, Brown TT. Lower adiponectin is associated with subclinical cardiovascular disease among HIV-infected men. AIDS. 2014;28(6):901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alligier M, Meugnier E, Debard C, Lambert-Porcheron S, Chanseaume E, Sothier M, Loizon E, Hssain AA, Brozek J, Scoazec JY, Morio B, Vidal H, Laville M. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97(2):E183–E192. [DOI] [PubMed] [Google Scholar]
- 5. Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94(12):5155–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161(1):146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okuno Y, Fukuhara A, Hashimoto E, Kobayashi H, Kobayashi S, Otsuki M, Shimomura I. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Diabetes. 2018;67(6):1113–1127. [DOI] [PubMed] [Google Scholar]
- 8. Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pastel E, Price E, Sjöholm K, McCulloch LJ, Rittig N, Liversedge N, Knight B, Møller N, Svensson PA, Kos K. Lysyl oxidase and adipose tissue dysfunction. Metabolism. 2018;78:118–127. [DOI] [PubMed] [Google Scholar]
- 10. Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, Cummings SR, Koster A, Nevitt MC, Satterfield S, Tylvasky FA, Strotmeyer ES, Newman AB, Simonsick EM, Scherzinger A, Goodpaster BH, Launer LJ, Eiriksdottir G, Sigurdsson S, Sigurdsson G, Gudnason V, Lang TF, Kritchevsky SB, Harris TB. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(1):109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, Godfrey C, Jahed NC, Myers L, Katzenstein D, Farajallah A, Rooney JF, Ha B, Woodward WC, Koletar SL, Johnson VA, Geiseler PJ, Daar ES; AIDS Clinical Trials Group Study A5202 Team. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361(23):2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, Myers L, Melbourne K, Ha B, Daar ES. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53(2):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Budhathoki C, Godfrey C, Jahed NC, Myers L, Katzenstein D, Farajallah A, Rooney JF, Pappa KA, Woodward WC, Patterson K, Bolivar H, Benson CA, Collier AC; AIDS Clinical Trials Group Study A5202 Team. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154(7):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McComsey GA, Daar ES, O’Riordan M, Collier AC, Kosmiski L, Santana JL, Fichtenbaum CJ, Fink H, Sax PE, Libutti DE, Gerschenson M. Changes in fat mitochondrial DNA and function in subjects randomized to abacavir-lamivudine or tenofovir DF-emtricitabine with atazanavir-ritonavir or efavirenz: AIDS Clinical Trials Group study A5224s, substudy of A5202. J Infect Dis. 2013;207(4):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6(7):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahlman I, Mejhert N, Linder K, Agustsson T, Mutch DM, Kulyte A, Isaksson B, Permert J, Petrovic N, Nedergaard J, Sjölin E, Brodin D, Clement K, Dahlman-Wright K, Rydén M, Arner P. Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer. 2010;102(10):1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mracek T, Stephens NA, Gao D, Bao Y, Ross JA, Rydén M, Arner P, Trayhurn P, Fearon KC, Bing C. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br J Cancer. 2011;104(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring). 2015;23(7):1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, Erlandson KM, Lake JE, Yin MT, Melbourne K, Ha B, Brown TT. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS. 2016;30(18):2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, Logeais M, Rimland D, Rodriguez-Barradas MC, Ruser C, Justice AC. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shahmanesh M, Phillips K, Boothby M, Tomlinson JW. Differential adipose tissue gene expression profiles in abacavir treated patients that may contribute to the understanding of cardiovascular risk: a microarray study. PLoS One. 2015;10(1):e0117164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Couturier J, Winchester LC, Suliburk JW, Wilkerson GK, Podany AT, Agarwal N, Xuan Chua CY, Nehete PN, Nehete BP, Grattoni A, Sastry KJ, Fletcher CV, Lake JE, Balasubramanyam A, Lewis DE. Adipocytes impair efficacy of antiretroviral therapy. Antiviral Res. 2018;154:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, Nguyen C, Iyer D, Kozinetz CA, Overbeek PA, Metzker ML, Balasubramanyam A, Lewis DE. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damouche A, Lazure T, Avettand-Fènoël V, Huot N, Dejucq-Rainsford N, Satie AP, Mélard A, David L, Gommet C, Ghosn J, Noel N, Pourcher G, Martinez V, Benoist S, Béréziat V, Cosma A, Favier B, Vaslin B, Rouzioux C, Capeau J, Müller-Trutwin M, Dereuddre-Bosquet N, Le Grand R, Lambotte O, Bourgeois C. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog. 2015;11(9):e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capel E, Auclair M, Caron-Debarle M, Capeau J. Effects of ritonavir-boosted darunavir, atazanavir and lopinavir on adipose functions and insulin sensitivity in murine and human adipocytes. Antivir Ther. 2012;17(3):549–556. [DOI] [PubMed] [Google Scholar]
- 26. Domingo P, Gutierrez MM, Gallego-Escuredo JM, Torres F, Mateo MG, Villarroya J, Lamarca K, Domingo JC, Vidal F, Villarroya F, Giralt M. A 48-week study of fat molecular alterations in HIV naive patients starting tenofovir/emtricitabine with lopinavir/ritonavir or efavirenz. J Acquir Immune Defic Syndr. 2014;66(5):457–465. [DOI] [PubMed] [Google Scholar]
- 27. Díaz-Delfín J, del Mar Gutiérrez M, Gallego-Escuredo JM, Domingo JC, Gracia Mateo M, Villarroya F, Domingo P, Giralt M. Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res. 2011;91(2):112–119. [DOI] [PubMed] [Google Scholar]
- 28. Gibellini L, De Biasi S, Pinti M, Nasi M, Riccio M, Carnevale G, Cavallini GM, Sala de Oyanguren FJ, O’Connor JE, Mussini C, De Pol A, Cossarizza A. The protease inhibitor atazanavir triggers autophagy and mitophagy in human preadipocytes. AIDS. 2012;26(16):2017–2026. [DOI] [PubMed] [Google Scholar]
- 29. Ganta KK, Mandal A, Chaubey B. Depolarization of mitochondrial membrane potential is the initial event in non-nucleoside reverse transcriptase inhibitor efavirenz induced cytotoxicity. Cell Biol Toxicol. 2017;33(1):69–82. [DOI] [PubMed] [Google Scholar]
- 30. McGee KC, Shahmanesh M, Boothby M, Nightingale P, Gathercole LL, Tripathi G, Harte AL, Shojaee-Moradie F, Umpleby AM, Das S, Al-Daghri NM, McTernan PG, Tomlinson JW. Evidence for a shift to anaerobic metabolism in adipose tissue in efavirenz-containing regimens for HIV with different nucleoside backbones. Antivir Ther. 2012;17(3):495–507. [DOI] [PubMed] [Google Scholar]
- 31. Lee JJ, Pedley A, Hoffmann U, Massaro JM, Keaney JF Jr, Vasan RS, Fox CS. Cross-sectional associations of computed tomography (CT)-derived adipose tissue density and adipokines: the Framingham Heart Study. J Am Heart Assoc. 2016;5(3):e002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alvey NJ, Pedley A, Rosenquist KJ, Massaro JM, O’Donnell CJ, Hoffmann U, Fox CS. Association of fat density with subclinical atherosclerosis. J Am Heart Assoc. 2014;3(4):e000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeoh AJ, Pedley A, Rosenquist KJ, Hoffmann U, Fox CS. The association between subcutaneous fat density and the propensity to store fat viscerally. J Clin Endocrinol Metab. 2015;100(8):E1056–E1064. [DOI] [PMC free article] [PubMed] [Google Scholar]