We read with interest the article by Zein et al.1 reporting on the results of the open-label Phase 1/2 trial to evaluate the safety and efficacy of ciliary neurotrophic factor (CNTF) on human cone function in patients with congenital achromatopsia (ACHM) caused by CNGB3 mutations. The authors report that, while there was evidence of CNTF delivery, there was no objectively measureable enhancement of cone function (assessed using visual acuity, mesopic increment sensitivity threshold, hue discrimination, photopic electroretinography [ERG]). In contrasting this with previous evidence of CNTF improving cone photoreceptor function in a CNGB3 canine model of ACHM, the authors conclude that there is a species difference between human and canine CNGB3 cones in their response to CNTF. While this certainly is plausible, we wish to propose an alternate possibility.
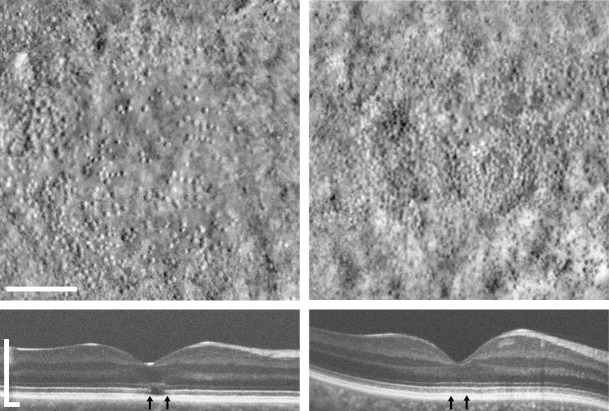
Two recent studies have shown considerable variability in cone structure using analysis of the photoreceptor bands on spectral-domain optical coherence tomography (SD-OCT) images and confocal adaptive optics scanning light ophthalmoscopy (AOSLO) imaging of the photoreceptor mosaic.2,3 Both studies showed considerable cone photoreceptor variation within and between genotypes, though there was no evidence of age-dependency. In addition, using a new split-detector AOSLO imaging approach, Scoles et al.4 recently demonstrated that patients with ACHM have highly variable degrees of remnant cone structure, with two examples of this shown in the Figure. These adaptive optics (AO) imaging studies allow more direct characterization of the number and placement of residual cone photoreceptors in molecularly proven subjects. Assessing the degree of residual cone structure in patients with ACHM may provide a way to assess the therapeutic potential for subsequent gene therapy efforts. However, the variability in residual cone structure also may be relevant for the current CNTF study. The patients in this CNTF study simply may have had too few cones to produce a detectable change in function using the methods employed here.
Figure.
Shown are split-detector AOSLO montages of foveal cone photoreceptor inner segments (top) and SD-OCT images through the fovea (bottom) of two individuals with congenital achromatopsia caused by CNGB3 mutations. Vertical arrows indicate the portion of the OCT scan represented by the AO montages. The subject on the left shows a hyporeflective zone on OCT and sparse cone structure on AOSLO imaging. The subject on the right shows no disruption in their EZ band on OCT and a more robust population of residual foveal cones. Scale bars: 100 μm (AOSLO); 300 μm (SD-OCT).
We believe that high-resolution quantitative retinal imaging techniques will not only have an important role in selecting patients for trials like these, but also will be valuable structural (and likely functional; e.g., AO-guided microperimetry) outcome measures to examine how the remaining cones (and rods) respond to a particular intervention.
References
- 1. Zein WM, Jeffrey BG, Wiley HE, et al. CNGB3-achromatopsia clinical trial with CNTF: diminished rod pathway responses with no evidence of improvement in cone function. Invest Ophthalmol Vis Sci. 2014; 55: 6301–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundaram V, Wilde C, Aboshiha J, et al. Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology. 2014; 121: 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubis AM, Cooper RF, Aboshiha J, et al. Genotype-dependent variability in residual cone structure in achromatopsia: toward developing metrics for assessing cone health. Invest Ophthalmol Vis Sci. 2014; 55: 7303–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scoles D, Sulai YN, Langlo CS, et al. In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci. 2014; 55: 4244–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]