Abstract
Introduction:
The Val allele of the Val158Met single-nucleotide polymorphism of the catechol-o-methyltransferase gene (COMT) confers greater catabolism of dopamine (DA) in the prefrontal cortex (PFC) than the Met allele. Met/Met homozygotes typically outperform Val-carriers on tests of executive function (EF), perhaps resulting from increased DA bioavailability. Methamphetamine (METH) causes large releases of DA, which is associated with neurotoxicity and executive dysfunction in chronic METH users. We hypothesized that, contrary to its effect in non-METH-using populations, slower DA clearance conferred by Met/Met will relate to worse EF in METH users.
Methods:
149 non-Hispanic White men, stratified by METH dependence (METH+/−) and COMT (Val/Val, Val/Met, Met/Met), completed three tests of EF: Wisconsin Card Sorting Test (WCST), Stroop Color-Word Test (Stroop), and Trail Making Test Part B (Trails B). Demographically-adjusted test scores were averaged to create an EF composite T-score. We examined the interaction of METH and COMT on the EF composite and individual test T-scores, controlling for premorbid functioning and alcohol use.
Results:
METH group differences in EF were evident only among Met/Met carriers (beta = −9.36, p <.001) but not among Val carriers: Val/Met (beta = −1.38, p=.44) and Val/Val (beta = −4.34, p= .10). These effects were most salient on the WCST.
Conclusions:
In the pre-frontal hyperdopaminergic state triggered by methamphetamine, greater DA inactivation conferred by the Val allele may protect against METH-related executive dysfunction, suggesting genetically-driven differences in vulnerability to METH.
Keywords: COMT Val158Met, methamphetamine, executive function, dopamine, cognition
1. Introduction
Heavy, chronic methamphetamine (METH) exposure is associated with central nervous system (CNS) injury (Davidson, Gow, Lee, & Ellinwood, 2001) and neurocognitive deficits (Scott et al., 2007). Human studies in abstinent users have described deficits in executive function, attention, learning and memory, information processing speed, and motor skills (Dean, Groman, Morales, & London, 2013; Scott et al., 2007). Functions localized to the prefrontal cortex (PFC) and frontostriatal connections may be especially vulnerable to METH effects (Chang et al., 2007; Bemacer et al 2013). The PFC plays a critical role in decision-making and inhibitory control (Sakagami, Pan, & Uttl, 2006), with DA as the major neurotransmitter implicated in the evaluation of rewards, maintenance of addictive behaviors, and differences cognitive function (Starr, Fox, Harris, Deary, & Whalley, 2007; Volkow, Fowler, Wang, & Goldstein, 2002).
Although METH-associated CNS injury is evident, METH exposure parameters (e.g., age at first use, total years of use, lifetime amount consumed, route of consumption, and post-acute length of abstinence) often do not inform the degree of impairment seen among people with a history of METH dependence (Cherner et al., 2010; McCann et al., 2008). This suggests individual differences invulnerability to the effects of METH, which may result from a combination of environmental and genetic factors. Examining genetic variability may offer insight as to how individual differences contribute to risk for cognitive dysfunction in chronic METH use.
Mechanisms of METH-related injury include alterations in dopamine (DA), serotonin, GABA and glutamate systems (Halpin, Collins, & Yamamoto, 2014; McCann et al., 2008). METH principally modulates DA neurotransmission and increases extracellular DA concentrations by a number of means, which include stimulating DA release and inhibiting reuptake via the DA transporter (Lin et al 2016). In addition to dopaminergic activity in the synapse, an important mechanism of DA-related METH neurotoxicity may occur at the receptor level; for example, a recent study shows that phasic METH-indueed DA release impacts D1 DA receptor availability which is negatively associated with cortical thickness (Okita et al., 2017). While DA is critical for cognitive function, overexposure to DA in the synapse caused by stimulant exposure likely plays a role in neural compromise, including damage to DA terminals, microvascular injury, and structural and functional abnormalities on neuroimaging (Nordahl, Salo, & Leamon, 2003; Schmidt, Ritter, Sonsalla, Hanson, & Gibb, 1985). Thus, regulatory mechanisms that assist in removing DA from the synapse, play an important role in DA homeostasis in the brain (Meyer-Lindenberg etal.,2006). Catechol-O-methyltransferase (COMT), COMT accounts for more than 60% of the metabolic degradation of released DA in the PFC (Carboni & Silvagni, 2004; Li et al., 2004; Westerink & Spaan, 1982).
A single nucleotide polymorphism (SNP) of COMT involves a Val to Met amino acid substitution at codon 158 in the membrane-bound COMT (COMT Val158Met). Due to 40% higher enzymatic activity of the Val compared to Met allele (Chen et al., 2004), homozygote carriers of the Val allele (Val/Val genotype) metabolize PFC DA at a more efficient rate, resulting in lower levels of DA in the synapse, whereas those with Met/Met genotype have the lowest rate of DA clearance, resulting in higher level of DA in the synapse. As METH substantially augments the concentration of extracellular DA, we hypothesized that COMT genotype would be a relevant predictor of brain consequences of METH exposure.
COMT Val158Met has been examined in many contexts relevant to catecholamine function. With regard to cognition, it has been linked most consistently to differences in executive function (Bruder et al., 2005; Wishart, 2011), although some controversy remains about the replicability of findings (Barnett, Scoriels, & Munafo, 2008; Goldman, Weinberger, Malhotra, & Goldberg, 2009). In healthy adults, the Val allele has been linked to executive dysfunction (Barnett, Jones, Robbins, & Muller, 2007), whereas the Met allele is associated with enhanced executive function (Barnett et al., 2007; Egan et al., 2001). Some evidence suggests this effect may be specific to men (Egan et al., 2001; Solis-Grtiz, Perez-Luque, Morado-Crespo, & Gutierrez-Munoz, 2010). The Met-associated cognitive advantage is likely due to higher DA bioavailability in the PFC resulting from slower clearance coded by Met. Other findings point to an inverted U-shape relationship between DA activity in the PFC and cognitive performance (Mattay et al., 2003; Tunbridge, Harrison, & Weinberger, 2006) such that the relationship between COMT and PFC function is likely to be context dependent and more complex than a simple dichotomy in which a Val allele is harmful and a Met allele is protective. For example, under conditions of DA excess, such as after METH administration, the greater metabolic activity conferred by Val alleles may be more advantageous in restoring the brain to homeostasis. In an earlier study of COMT Val158Met and executive dysfunction in the context of HIV disease and METH dependence, we found that, regardless of HIV status, individuals with Met/Met genotype had better executive function compared (Wallace, Gudelsky, & Vorhees, 1999) to Val carriers, except if they were METH users, and this effect did not generalize to other cognitive domains (Bousman, Chemer, Glatt, et al., 2010). Although increased bioavailability of cortical DA associated with the Met/Met genotype is thought to enhance executive function under physiologically normal conditions, in the hyperdopaminergic state induced by METH, slow DA clearance can result in neurotoxicity, possibly via DA auto-oxidation (Moszezynska & Callan, 2017; Riddle, Fleckenstein, & Hanson, 2006; Wallace et al., 1999), thus attenuating any advantage, or posing a liability for executive function in METH-using Met/Met individuals.
Here, we aim to examine whether variability in COMT Val158Met contributes to individual differences in executive deficits reported after heavy chronic METH exposure, with the goal to potentially identify genotype groups that are at higher risk of METH-associated executive dysfunction. In this investigation, we are focusing on a more homogenous sample than in our prior work, reducing variability associated with sex and racial background, as well as HIV status, since HIV can also affect dopaminergic circuitry. Our analyses will examine the main and interactive effects of COMT genotype and METH dependence on a three-test composite of executive function (Wisconsin Card Sorting Test, Stroop Color-Word Test, and Trail Making Test Part B). Follow-up analyses will examine the effects of COMT genotype and METH dependence on each test of executive function. We hypothesize that, contrary to its effect in the general population, among individuals with METH dependence, slower DA clearance in the PFC conferred by the Met/Met genotype, in conjunction with METH-induced dopaminergic excess, will be associated with worse executive function, while Val carriers will show comparatively better executive function.
2. Materials and Method
2.1. Participants
Participants were 85 METH dependent and 64 non-drug dependent comparison research volunteers evaluated at the University of California, San Diego All were HIV- non-Hispanic White men. We limited our sample to a demographically narrow group for the purpose of genetic analyses, as some sex and race differences in COMT effects and allele frequencies have been reported (e.g., Barnett et al., 2007; González-Castro et al., 2013), and we did not have sufficient numbers of women or non-White participants to conduct separate analyses.
Participants were excluded if: (l)they met DSM-IV criteria for lifetime dependence on any drugs other than METH or cannabis within the last 5 years, or alcohol dependence within the last 12 months; (2) they reported abuse of any substances other than METH within the last 12 months, with the exception of cannabis, alcohol and nicotine, given their high prevalence in this population; or (3) they had a history of neurologic, psychiatric, or developmental disorders of sufficient severity to confound neuropsychological test results. The Wide Range Achievement Test (WRAT) version 3 or 4 reading subtest was used as an estimate of preexisting cognitive ability. Participants who had WRAT reading scores below 80 were excluded to limit the confounding contribution of preexisting low intellectual functioning.
2.2. Procedure
Participants gave written informed consent prior to enrollment and collection of neuropsychological, neuropsychiatric, medical and genetic information. HIV status was determined using enzyme linked immunosorbent assays (ELISA) with a confirmatory test. Hepatitis C status was also determined and, while slightly more frequent in METH+, hepatitis C seropositivity did not differ significantly among the COMT genotypes. All procedures were approved by the Human Research Protection Program at UCSD.
2.2.1. Methamphetamine Status
Participants were evaluated for methamphetamine dependence and other substance use diagnoses using the Structured Clinical Interview for DSM-IV (SCID-IV) (Spitzer, Williams, Gibbon, & First, 1995) or the Composite International Diagnostic Interview (CIDI) (Robins etal., 1988), as the study was developed prior to the DSM-5. Lifetime exposure to METH and other commonly used substances was obtained with a timeline follow-back interview.
METH+ group status was based on: (1) a DSM-IV lifetime diagnosis of METH dependence; (2) METH dependence or abuse within the past 18 months; and (3) abstinence from METH for at least 10 days based on history and supported by urine toxicology screening conducted at the time of evaluation. Non drug-dependent (METH-) participants were allowed no more than 9 lifetime instances of METH use.
2.2.2. COMT Genotyping
DNA for genotyping was isolated from 0.2 ml whole blood stored at −70°C using the Qiagen QIAamp DNA Mini Kit (Qiagen, Valencia, CA) and QiaCube Robotic workstation for automated DNA purification. The COMT Val158Met (rs4680)SNP was assayed using an addiction-relevant gene array (Hodgkinson et al., 2008).
All participants were genotyped for COMT Val158Met by standard procedures. Genotyping involved hybridization of a locus-specific oligonucleotide and two allele-specific oligonucleotides to target genomic DNA, extension and ligation reactions, followed by PCR with common dye-labeled PCR primers (the dyes corresponding to the two allele-specific oligonucleotides, respectively). The PCR products were hybridized to the universal array, and imaged using a high-resolution scanner. Finally, the images were analyzed using software for automated genotype clustering and calling within BeadStudio software.
2.2.3. Executive Function
Participants completed three tests of executive function: Wisconsin Card Sorting Test 64-item-computerized version (WCST) (Kongs SK, 2000), Stroop Color-Word Test (Stroop), and Trail Making Test Part B (Trails B). The executive function (EF) composite consisted of (1) number of perseverative responses on the WCST, reflecting untimed ability to perceive complex pattern set-shifting; (2) score obtained in 45 seconds on the Stroop Color-Word interference condition, reflecting timed ability to selectively inhibit information and manage cognitive interference; and (3) time to complete Trails B, reflecting timed ability to switch and maintain attention between ongoing sequences. Raw scores from the component tests were converted to T-scores (M = 50, SD =10) adjusted for age, education, and gender according to published test norms, and then averaged across tests to form the EF composite T-score.
2.3. Statistical Analysts
To examine the conditional effects of METH dependence and COMT genotype on executive function, participants were classified into one of the following six groups: METH-Val/Val (n=19), METH- Val/Met (n=29), METH- Met/Met (n=16), METH+ Val/Val (n=16), METH+ Val/Met (n=49), METH+ Met/Met (n=20). COMT genotype distribution was consistent with Hardy-Weinberg equilibrium in the full sample (χ 2 = 0.33, p = 0.57) and within each METH group (METH−: χ2 = 0.54, p = 0.46; METH+: χ 2 = 2.06,p = 0.15). To compare background characteristics across the six groups, univariable comparisons were performed using one-way analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables. Variables that differed significantly across the six groups were included as covariates in primary analyses. Groups differed significantly by years of education, reading level (WRAT), days since last alcohol use, and lifetime average drinks per day. Thus, these variables (except for years of education) were included as covariates in subsequent models for executive function. Years of education was not included as a covariate given that the outcome variables of executive function are demo graphically-adjusted for years of education.
We used multivariable linear regression analyses to examine the effects of COMT genotype, METH dependence, and their interaction on the executive function composite, controlling for significant covariates. COMT genotype was coded with Val/Met as the reference group, given that it is the largest genotype group in our analyses and the most common in the general population, including White populations (Gonzalez-Castro et al., 2013). In order to probe significant COMT*METH interactions and assess the differential influence of each predictor on executive function, we conducted follow-up analyses stratified by COMT genotype and separately, stratified by METH dependence. Similar analyses examined the same predictors in multivariable linear regression analyses with the individual tests of executive function (WCST, Stroop, Trails B) as outcomes. For these three individual tests, stratified analyses that followed-up on COMT*METH interaction effects were interpreted using a Bonferroni-adjusted α-threshold of .0167 (i.e., .05/3).
3. Results
3.1. Participant Characteristics
Participants were all non-Hispanic White men, ranged in age from 18 to 66 years old (M=38.7, SD=10.9), and had an average of 12.6 years of formal education (SD=2.3). Table 1 provides sample demographic and lifetime substance use characteristics by METH status and COMT genotype (Val/Val, Val/Met, Met/Met) group. Across the six groups, METH+ participants had significantly fewer years of formal education, lower WRAT reading scores, more days since last alcohol use and higher average lifetime alcohol drinks per day compared to METH- participants. Importantly, within each METH group, COMT genotype groups had comparable background characteristics (age, years of education, WRAT reading scores), substance use histories, and proportion of hepatitis C seropositivity (Table 1).
Table 1:
Participant Characteristics by METH and COMT Status
| METH− N=64 |
METH+ N=85 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable, M(SD) or Median(IQR) | Val/Val n=19 |
Val/Met n=29 |
Met/Met n=16 |
Val/Val n=16 |
Val/Met n=49 |
Met/Met n=20 |
pa | METH− vs. METH+b |
|
| Age | 31.8 (10.7) | 39.6 (11.8) | 39.9 (15.7) | 38.6 (9.5) | 40.4 (8.0) | 38.9 (11.1) | .143 | ||
| Education | 13.4 (2.4) | 13.7 (1.9) | 13.2 (1.8) | 11.6 (1.9) | 12.2 (2.4) | 11.5 (2.5) | .012 | METH− > METH+ | |
| Hepatitis C+, n (%) | 0 (0%) | 1 (3%) | 1 (6%) | 3 (19%) | 9 (18%) | 3 (15%) | .186 | ||
| WRAT Reading | 106.3 (8.4) | 104.3 (8.6) | 108.1 (8.5) | 95.5 (7.3) | 99.6 (8.9) | 99.5 (8.3) | <.001 | METH− > METH+ | |
| Substance Use (lifetime use) | |||||||||
| Total Meth Exposure (lifetime grams) | – | – | – | 3462 [977–11201] | 3156 [1472–8406] | 2840 [975–7632] | .937 | ||
| Meth Use (grams/day) | – | – | – | 1.0 [0.3–1.5] | 0.8 [0.6–1.7] | 0.8 [0.4–1.7] | .912 | ||
| Meth Days Abstinent | – | – | – | 75 [30–244] | 91 [45–183] | 114 [38–167] | .935 | ||
| Cannabis Use (grams/day) | 0.2 [0.2–0.6] | 0.2 [0.1–0.5] | 0.2 [0.1–0.8] | 0.5 [0.1–2] | 0.4 [0.2–1] | 0.4 [0.2–1.1] | .084 | ||
| Cannabis Days Abstinent | 731 [35–2465] | 1461 [3–4018] | 548 [206–8309] | 213 [82–731] | 457 [114–2420] | 97 [6–913] | .483 | ||
| Alcohol Use (drinks/day) | 3.4 [2.3–5.6] | 3.8 [2.4–5] | 2.1 [1–5.3] | 6.6 [4.6–8.3] | 6.3 [3.3–8.8] | 8.3 [4.9–16.6] | <.001 | METH+ > METH− | |
| Alcohol Days Abstinent | 5 [2–93] | 22 [5–639] | 15 [2–221] | 122 [61–548] | 213 [49–875] | 91 [8–183] | .003 | METH+ > METH− | |
| Tobacco Use (cig/day) | 6.3 [4.9–15.8] | 6.5 [5.3–16] | 9.2 [4.8–16] | 11.3 [5.4–18.4] | 5.4 [4.8–16] | 6.4 [5.3–12.5] | .665 | ||
| Tobacco Days Abstinent | 22 [0–1674] | 46 [0–2922] | 365 [1–1826] | 0 [0–7] | 0 [0–7] | 0 [0–0] | .262 | ||
Omnibus p-value for six-group comparison.
Significant difference between METH− and METH+ across the entire sample.
Note. Within each METH group, there were no significant main effects of COMT genotype.
3.2. Effects of METH and COMT genotype on Executive Function
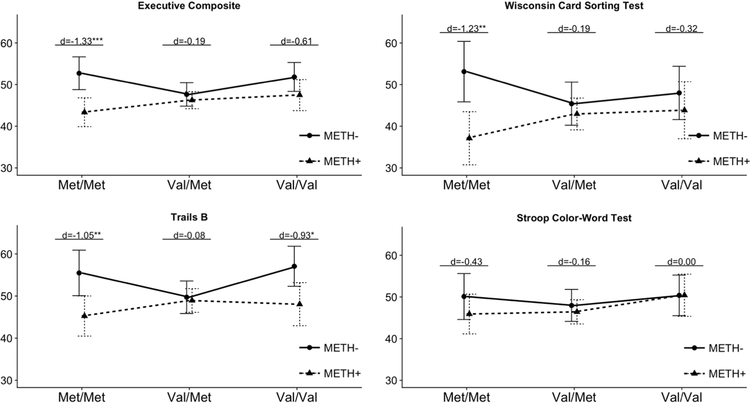
Table 2 presents model estimates for the multiple regression analysis predicting the executive function composite. Controlling for WRAT reading score, days since last alcohol use, and lifetime average alcohol consumption per day of use, a significant Met/Met*METH interaction (p = .01) was detected such that the deleterious effect of METH on executive function was only significant in the Met/Met genotype group (beta = −9.36, p <.001) compared to the Val/Met (beta = −1.38,p = .44). There was no significant Val/Val*METH interaction (p = .34), indicating no significant effect of METH in Val/Val (beta = −4.34, p = . 10), comparable to the lack of METH effect in Val/Met (Figure 1). Among METH- individuals, those with Met/Met genotype [M(SD) = 53.0 (8.0)] significantly outperformed the Val/Met group [M(SD) = 47.5 (6.2); p = .03] and non-significantly outperformed Val/Val group [M(SD) = 50.8 (10.4); p = .08]. Among METH+ individuals, Met/Met [M(SD) = 44.5(6.3)] displayed non-significantly poorer performance compared to those with Val/Met [M(SD) = 45.9(8.7), p = .63] and Val/Val genotypes [M(SD) = 47.1(5.3), p = .19]. Higher WRAT scores and greater lifetime alcohol use also significantly predicted higher executive function T-scores (ps<.01).
Table 2.
Full Multivariable Regression Model for Executive Function Composite
| Variable | beta (SE) | 95% CI | p |
|---|---|---|---|
| Met/Meta | 5.08 (2.37) | [0.39, 9.77] | 0.034 |
| Val/Vala | 4.19 (2.22) | [−0.19, 8.58] | 0.061 |
| METH+b | −1.38 (1.77) | [−4.88, 2.13] | 0.439 |
| Met/Met*METH+ | −7.98 (3.13) | [−14.18, −1.78] | 0.012 |
| Val/Val *METH+ | −2.96 (3.07) | [−9.04, 3.11] | 0.336 |
| WRATc | 0.27 (0.07) | [0.12, 0.41] | <0.001 |
| Days Since Last Drink | 0.00 (0.00) | [0.00, 0.00] | 0.300 |
| Alcohol Used | 0.36 (0.13) | [0.10, 0.62] | 0.007 |
Comparedto Val/Met
Compared to METH−
Wide-Range Achievement Test reading subtest
Lifetime average drinks per day
Figure 1. Adjusted means for executivefunctionT-scores by COMT x METH group.
Cohen’s d estimates reflect the difference between METH+ and METH- participants within each COMT genotype. ***p<.001, **p<01, *p<.05.
3.3. Effects of METH and COMT genotype across Executive Function Tests
Table 3 presents model estimates for each individual executive function test. Results demonstrated that the effect of METH on WCST performance differed significantly between Met/Met and Val/Val groups (Met/Met*METH interaction: p = .02). Follow-up analyses indicated that the deleterious effect of METH on WCST performance was only significant in the Met/Met genotype group (beta = −9.01, p = .002) compared to the effects of METH in Val/Met (beta = −2.47, p = .45) and Val/Val groups (beta = −4.14,p = .40). A similar interaction effect was detected for Trails B performance (Met/Met*METH interaction: p=.03) such that the deleterious effect of METH was significantly larger in the Met/Met group (beta = −10.23, p = .008) compared to Val/Met (beta = −0.77,p = .75). Although the effect of METH on Trails B performance did not significantly differ between Val/Met and Val/Val groups (ValVal*METH interaction: p= .053); the deleterious effect of METH also reached statistical significance in Val/Val (beta = −9.01, p = .014). For Stroop, no significant differences in executive function emerged by COMT genotype, METH status, nor their interaction (Met/Met*METH interaction: p = .54).
Table 3.
Model Estimates for Individual Executive Function Test T-scores
| Wisconsin Card Sorting Test | Trails B | Stroop Color-Word Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | beta (SE) | 95% CI | p | beta (SE) | 95% CI | p | beta (SE) | 95% CI | p |
| Met/Meta | 7.71 (4.37) | [−0.94, 16.36] | 0.080 | 5.76 (3.26) | [−0.69, 12.21] | 0.079 | 2.14 (3.3) | [−4.39, 8.68] | 0.518 |
| Val/Vala | 2.58 (4.08) | [−5.5, 10.66] | 0.529 | 7.35 (3.04) | [1.33, 13.37] | 0.017 | 2.42 (3.08) | [−3.68, 8.52] | 0.434 |
| METH+b | −2.47 (3.29) | [−8.98, 4.03] | 0.453 | −0.77 (2.44) | [−5.59, 4.04] | 0.751 | −1.53 (2.44) | [−6.36, 3.31] | 0.533 |
| Met/Met*METH+ | −13.53 (5.77) | [−24.96, −2.1] | 0.021 | −9.46 (4.30) | [−17.97, −0.94] | 0.030 | −2.67 (4.36) | [−11.3, 5.96] | 0.542 |
| Val/Val*METH+ | −1.67 (5.66) | [−12.87, 9.52] | 0.768 | −8.24 (4.22) | [−16.58, 0.11] | 0.053 | 1.56 (4.25) | [−6.85, 9.97] | 0.714 |
| WRATc | 0.19 (0.14) | [−0.08, 0.45] | 0.174 | 0.24 (0.1) | [0.04, 0.44] | 0.018 | 0.38 (0.1) | [0.18, 0.58] | <0.001 |
| Days Since Last Drink | 0.00 (0.00) | [0.00, 0.00] | 0.868 | 0.00 (0.00) | [0.00, 0.00] | 0.723 | 0.00 (0.00) | [0.00, 0.00] | 0.111 |
| Alcohol Used | 0.60 (0.24) | [0.11, 1.08] | 0.016 | 0.41 (0.18) | [0.05, 0.77] | 0.027 | 0.21 (0.19) | [−0.18, 0.59] | 0.284 |
Compared to Val/Met
Compared to METH−
Wide-Range Achievement Test reading subtest
Lifetime average drinks per day
Figure 1 depicts these relationships by plotting least squares means estimates across the six groups for the executive function composite and individual test T-scores.
4. Discussion
Our results suggest genetically influenced differences in vulnerability to METH effects on executive dysfunction. Consistent with literature, healthy participants with Met/Met genotype had better executive function performance than Val carriers, while among METH dependent individuals, the reverse was true. Moreover, the performance of METH+ Val carriers was generally indistinguishable from that of METH− Val carriers. That is, the negative effect of methamphetamine on executive dysfunction was salient only in the Met/Met group, and Val carriers tended to perform similarly irrespective of METH dependence. Under ordinary conditions, Met/Met genotype is thought to be advantageous for cognition because it results in more optimal levels of brain DA. However, among METH users, whose prefrontal cortex is serially exposed to larger than normal concentrations of DA, the Met/Met genotype may confer risk for executive dysfunction as a result of less efficient DA clearance compared to Val carriers.
In our sample, the differential influence of COMT genotype between METH+ and METH− was most salient in the WCST. The WCST functions as an index of abstract reasoning, concept formation, and response to changes in context and may be the only executive function test of our composite that is complex enough to be affected by nuanced differences in dopamine bioavailability in the PFC. For example, WCST perseverative errors have strong associations with PFC volume (Eling, Derckx, & Maes, 2008; Yuan & Raz, 2014). Kim and colleagues reported a significant decrease in the grey matter density in the PFC of abstinent METH users compared to controls, which was correlated with poor performance on the WCST (Kim et al., 2006). Chung and colleagues observed decreases in frontal white matter integrity in chronic METH users compared to healthy comparison subjects using diffusion tensor imaging (DTI) (Chung et al., 2007). They noted that these structural changes were associated with greater perseveration on the WCST. A reduction of WCST errors has also been correlated with recovery in DA transporter binding after METH abstinence (Chou et al., 2007). Beyond WCST and executive functioning deficits, a recent study found that adult METH users had deficits in attention and memory compared to controls, relative to the cognitive performance predicted by their childhood grade point averages; furthermore, these memory deficits were associated with lower whole-brain cortical thickness on structural magnetic resonance imaging (MRI) (Dean, Morales, Hellemann, & London, 2018).
Our findings confirm and extend previous work on the relationship between COMT Val158Met and executive abilities in METH-dependent individuals (Bousman, Cherner, Glatt, et al., 2010). In that sample of adult men, an interaction between COMT genotype, METH use, and HIV status indicated that Met/Met improved executive function among non-METH using individuals, but the effect was attenuated among those with history of METH dependence. We have also reported a complex association of the Met allele with increased sexual risk-taking behavior, seen among individuals with executive dysfunction (Bousman, Cherner, Atkinson, et al., 2010).
Mattay and colleagues were the first to report that COMT interacts with amphetamine acutely to produce harmful effects on cognitive performance among individuals with a Met allele (Mattay et al., 2003). More recently, COMT Val158Met was shown to modify the response of healthy participants to acute amphetamine administration, such that presence of the Val allele was associated with poorer baseline performance on measures of attention and processing speed, and greater improvement in performance post d-amphetamine exposure (Hamidovic, Dlugos, Palmer, & de Wit, 2010). The presence of the Val allele has also been associated with better response to modafinil, when the drug has been explored in the treatment of MA dependence (Heinzerling, McCracken, Swanson, Ray, & Shoptaw, 2012). While those studies highlight the relevance of COMT Val158Met in the acute response to stimulants, our studies in currently abstinent methamphetamine-dependent individuals inform our understanding of the role of this SNP in the long-term sequelae of chronic methamphetamine exposure.
Examination of covariates showed that greater lifetime alcohol use predicted better executive performance, independent of the interactive effects of COMT and METH. Although unexpected, our observations align with prior studies demonstrating that singly addicted stimulant abusers are at increased risk for executive dysfunction compared to individuals who simultaneously abuse stimulants and alcohol (Lawton-Craddock, Nixon, & Tivis, 2003; Robinson, Heaton, & O’Malley, 1999). From a neurophysiological perspective, alcohol’s vasodilatory properties (Bau, Bau, Naujorks, & Rosito, 2005; Lee et al., 1990) may be beneficial in attenuating METH-related neurovascular dysfunction (i.e., vasoconstriction, brain thermotoxicity) (Kiyatkin & Sharma, 2009). Given the known adverse consequences of heavy alcohol use (Grant, 1987), such results should be interpreted with caution until they are confirmed by studies using experimental, rather than observational, design.
This study has several limitations. For a genotype-phenotype investigation, our sample was rather small and may impact the reliability of our effect size estimates. Analyses examined a single SNP in association to executive function, which while interesting and significant, is likely one of many pathways and downstream interactions, including pharmacokinetic and pharmacodynamic mechanisms, as well as METH metabolic pathways (Cherner et al., 2010) that may impact cognition. Additionally, the results are limited to a demographically homogeneous group and many other environmental influences were not considered. Furthermore, racial/ethnic background was assessed via self-report and we did not access ancestry-informative genetic markers. Future research will need to determine whether the results are generalizable to diverse groups. Finally, examination of DA function markers via PET imaging has been helpful in documenting brain changes in METH-related injury (McCann et al., 2008; Volkow et al., 2001). Future studies might investigate the role of COMT genotype in the relationship between METH use, DA imaging markers, and executive dysfunction.
4.1. Conclusions
Our findings point to a context-dependent relationship between COMT Val158Met and executive function, such that Met/Met is advantageous for healthy individuals but a liability for long-term METH users. Results support the notion that in the context of supranormal exposure to dopamine associated with methamphetamine, greater dopamine clearance conferred by Val may be protective against neural injury. If this effect is replicated and generalizable, COMT Val158Met genotyping could inform personalized approaches to mitigate neurocognitive sequelae in chronic METH users and help to identify populations that may be especially vulnerable to METH effects on executive function.
Highlights.
Executive dysfunction in methamphetamine users varies by COMT Val158Met genotype
Methamphetamine effects on executive function are seen only in Met/Met carriers
Val carriers have similar executive function irrespective of methamphetamine use
Slower dopamine clearance conferred by Met is a liability in methamphetamine use
COMT-controlled prefrontal dopamine bioavailability impacts methamphetamine injury
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Discovery Institute (SBMDI), and the University of California, Irvine (UCI). The TMARC comprises: Administrative Coordinating Core (ACC) – Executive Unit: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager –Mariana Chemer, Ph.D.; Associate Center Managers – Erin E. Morgan, Ph.D. and Jared Young, Ph.D.; Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Behavioral Assessment and Medical (BAM) Core -Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core – Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph. D., Assawin Gongvatana, Ph. D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director).; Project 5: Marcus Kaul, Ph.D. (Project Director).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Supported by NIH R01DA26334: COMT Genotype and Risky Decision Making in HIV and Methamphetamine Dependence (M. Cherner) & P50DA26306: Translational Methamphetamine AIDS Research Center (I. Grant)
Sources of Funding: This research was supported by grants from the National Institute on Drug Abuse R01 DA26334: COMT Genotype and Risky Decision Making in HIV and Methamphetamine Dependence (M. Cherner) & P50DA26306: Translational Methamphetamine AIDS Research Center (I. Grant)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest were declared.
References
- Barnett JH, Jones PB, Robbins TW, & Muller U (2007). Effects of the catechol-O- methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry, 72(5), 502–509. doi: 10.1038/sj.mp.4001973 [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Sc Munafo MR (2008). Meta-,Analysis of the Cognitive Effects of the Catechol-O-Methyltransferase Gene Val158/108Met Polymorphism. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Bau PF, Bau CH, Naujorks AA, Sc Rosito GA (2005). Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol, 57(1), 53–58. doi: 10.1016/j.alcohol.2005.10.034 [DOI] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Atkinson JH, Heaton RK, Grant I, Everall IP, & Hnre Group T (2010). COMT Val158Met Polymorphism, Executive Dysfunction, and Sexual Risk Behavior in the Context of HIV Infection and Methamphetamine Dependence. Interdiscip Perspect Infect Dis, 2010, 678648. doi: 10.1155/2010/678648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Glatt SJ, Atkinson JH, Grant I, Tsuang MT, Sc Everall IP (2010). Impact of COMT Val158Met on executive functioning in the context of HIV and methamphetamine. Neurobehavi oral HIV Medicine. doi: 10.2147/NBHIV.S8245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Sc Gilliam TC (2005). Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry, 55(11), 901–907. [DOI] [PubMed] [Google Scholar]
- Carboni E, Sc Silvagni A (2004). Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol, 16( 1–2), 121–128. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T and Volkow N (2007), Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction, 102: 16–32. doi: 10.1111/i.1360-0443.2006.01782.X [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M,, Melhem S,… Weinberger DR (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects onmRNA, protein, and enzyme activity in postmortem human brain. Am JHum Genet, 75(5), 807–821. doi: 10.1086/425589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Bousman C, Everall I, Barron D, Letendre S, Vaida F,… Grant I (2010). Cytochrome P450–2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. Journal of the International Neuropsychological Society, 16(05), 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T,… Group H (2010). Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend, 106(2–3), 154–163. doi: 10.1016/j.drugalcdep.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Huang WS, Su TP, Lu RB, Wan FJ, Sc Fu YK (2007). Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: ASPECT study. Eur Neuropsychopharmacol, 17(1), 46–52. doi: 10.1016/j.euroneuro.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH,… Renshaw PF (2007). Decreased frontal white-matter integrity in abstinent methamphetamine abusers. IntJNeuropsychopharmacol, 10(6), 765–775. doi: 10.1017/S1461145706007395 [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, & Ellinwood EH (2001). Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Research Reviews, 36(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, Sc London ED (2013). An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology, 38(2), 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC,, Morales AM, Hellemann G, London ED (2018). Cognitive deficit in methamphetamine users relative to childhood academic performance: link to cortical thickness. Neuropsychopharmacology, 43(%), 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE,…Weinberger DR (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA, 98(12), 6917–6922. doi: 10.1073/pnas.l11134598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling P, Derckx K, Sc Maes R (2008). On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain Cogn, 67(3), 247–253. doi: 10.1016/j.bandc.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Goldman D, Weinberger DR, Malhotra AK, Sc Goldberg TE (2009). The role of COMT Val158Met in cognition. Biological psychiatry, 65(1), e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Castro TB, Tovilla-Zárate C, Juárez-Rojop I, García SP, Genis A, Nicolini H, Sc Narváez LL (2013). Distribution of the Vall08/158Met polymorphism of the COMT gene in healthy Mexican population. Gene, 526(2), 454–458. [DOI] [PubMed] [Google Scholar]
- Grant I (1987). Alcohol and the brain: Neuropsychological correlates. Journal of Consulting and Clinical Psychology, 55(3), 310–324. doi: 10.1037/0022-006X.55.3.310 [DOI] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Sc Yamamoto BK (2014). Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci, 97(1), 37–44. doi: 10.1016/j.lfs.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Palmer AA, Sc de Wit H (2010). Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet, 20(3), 85–92. doi: 10.1097/YPG.0b013e32833alf3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, McCracken JT, Swanson AN, Ray LA, Sc Shoptaw SJ (2012). COMT Val158Met, BDNF Val66Met, and OPRM1 Asn40Asp and methamphetamine dependence treatment response: preliminary investigation. J Clin Psychopharmacol, 32(1),135–137. doi: 10.1097/JCP.ObO13e318240a48e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, … Goldman D (2008). Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol, 43(5), 505–515. doi: 10.1093/alcale/agn032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J,… Renshaw PF (2006). Prefrontal grey-matter changes in short-term and long-term abstinent methamphe tami ne abusers. IntJ Neuropsychopharmacol, 9(2), 221–228. doi: 10.1017/S1461145705005699 [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Sc Sharma HS (2009). Acute methamphetamine intoxication: brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int Rev Neurobiol, 88, 65–100. doi: 10.1016/s0074-7742(09)88004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongs SK TL, Iverson GL, Heaton RK (2000). Wisconsin card sorting test-64 card computerized version. Psychological Assessment Resources, Odessa. [Google Scholar]
- Lawton-Craddock A, Nixon SJ, Sc Tivis R (2003). Cognitive efficiency in stimulant abusers with and without alcohol dependence. Alcohol Clin Exp Res, 27(3), 457–464. doi: 10.1097/01.ALC.0000056620.98842.E6 [DOI] [PubMed] [Google Scholar]
- Lee JA, Schoener EP, Nielsen DW, Kelly AR, Lin WN, & Berman RF (1990). Alcohol and the auditory brain-stem response, brain temperature, and blood alcohol curves: explanation of a paradox. Electroencephalogr Clin Neurophysiol, 77(5), 362–375. [DOI] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W, … Collier DA (2004). Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet, 129B( 1), 120–124. doi: 10.1002/ajmg.b.30024 [DOI] [PubMed] [Google Scholar]
- Lin M, Sambo D and Khoshbouei H. (2016). Methamphetamine Regulation of Firing Activity of Dopamine Neurons. Journal of Neuroscience, 36 (40) 10376–10391; DOI: 10.1523/JNEUROSCI.1392-16.2016.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, … Weinberger DR (2003). Catechol O-methyltransferase vall58-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA, 100( 10), 6186–6191. doi: 10.1073/pnas.0931309100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, … Wong DF (2008). Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse, 62(2), 91–100. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, … Weinberger DR (2006). Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry, 11(9), 867–877, 797. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Sc Callan SP (2017). Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. J Pharmacol Exp Ther, 362(3), 474–488. doi: 10.1124/jpet.116.238501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Sc Leamon M (2003). Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. The Journal of neuropsychiatry and clinical neurosciences, 15(3), 317–325. [DOI] [PubMed] [Google Scholar]
- Okita K, Morales AM, Dean AC, Johnson MC, Lu V, Farahi J, … London ED (2017). Striatal dopamine Dl-type receptor availability: no difference from control but association with cortical thickness in methamphetamine users. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleekenstein AE, Sc Hanson GR (2006). Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J, 8(2), E413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J,. … et al. (1988). The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and indifferent cultures. Arch Gen Psychiatry, 45(12), 1069–1077. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Heaton RK, Sc O’Malley SS (1999). Neuropsychological functioning in cocaine abusers with and without alcohol dependence. J Int Neuropsychol Soc, 5(1), 10–19. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Pan X, Sc Uttl B (2006). Behavioral inhibition and prefrontal cortex in decision-making. NeuralNetw, iP(8), 1255–1265. doi: 10.1016/j.neunet.2006.05.040 [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Sc Gibb JW (1985). Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther, 233(3), 539–544. [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Sc Grant I (2007). Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev, 17(3), 275–297. [DOI] [PubMed] [Google Scholar]
- Solis-Ortiz S, Perez-Luque E, Morado-Crespo L, Sc Gutierrez-Munoz M (2010). Executive functions and selective attention are favored in middle-aged healthy women carriers of the Val/Val genotype of the catechol-o-methyltransferase gene: a behavioral genetic study. Behav Brain Funct, 6, 67. doi: 10.1186/1744-9081-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, Sc First M (1995). Structured Clinical Interview forDSM-IV. Washington, DC: .American Psychiatric Press. [Google Scholar]
- Starr JM, Fox H, Harris SE, Deary IJ, Sc Whalley LJ (2007). COMT genotype and cognitive ability: a longitudinal aging study. Neuroscience letters, 421(1), 57. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, & Weinberger DR (2006). Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry, 60(2), 141–151. doi: 10.1016/j.biopsych.2005.10.024 [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, … Miller EN (2001). Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry, 158(3), 377–382. doi: 10.1176/appi.ajp.158.3.377 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Sc Goldstein RZ (2002). Role of Dopamine, the Frontal Cortex and Memory Circuits in Drug Addiction: Insight from Imaging Studies. Neurobiology of Learning and Memory, 78(3), 610–624. doi: 10.1006/nlme.2002.4099 [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Sc Vorhees CV (1999). Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. JNeurosci, 19(20), 9141–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, Sc Spaan SJ (1982). Simultaneous determination of the formation rate of dopamine and its metabolite 3,4-dihydroxyphenylacetie acid (DOPAC) in various rat brain areas. Brain Res, 252(2), 239–245. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, Moore JH, McAllister TW (2011). COMT Val158Met Genotype and Individual Differences in Executive Function in Healthy Adults. Journal of the International Neuropsychological Society, 17(1), 174–180. doi: 10.1017/S1355617710001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Sc Raz N (2014). Prefrontal cortex and executive functions in healthy adults: a metaanalysis of structural neuroimaging studies. Neurosci Biobehav Rev, 42, 180–192. doi: 10.1016/j.neubiorev.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]