Abstract
Human epidemiological and animal-model studies suggest that separate exposure to stress or serotonin-selective reuptake inhibitor (SSRI) antidepressants during pregnancy increases risks for neurodevelopmental disorders in offspring. Yet, little is known about the combined effects of maternal stress and SSRIs with regard to brain development in utero. We found that the placenta is highly permeable to the commonly prescribed SSRI (±)-citalopram (CIT) in humans and mice, allowing rapid exposure of the fetal brain to this drug. We investigated the effects of maternal chronic unpredictable stress in mice with or without maternal oral administration of CIT from embryonic day (E)8 to E17. We assessed fetal brain development using magnetic resonance imaging and quantified changes in serotonergic, thalamocortical, and cortical development. In utero exposure to maternal stress did not affect overall fetal brain growth. However, serotonin tissue content in the fetal forebrain was increased in association with maternal stress; this increase was reversed by maternal CIT. In utero exposure to stress increased the numbers of deep-layer neurons in specific cortical regions, whereas CIT increased overall cell numbers without changing the proportions of layer-specific neurons to offset the effects of stress on deep-layer cortical development. These findings suggest that stress and SSRI exposure in utero differentially impact serotonin-dependent fetal neurodevelopment such that CIT reverses key effects of maternal gestational stress on offspring brain development.
Keywords: Serotonin, serotonin-selective reuptake inhibitor, antidepressant, pregnancy, thalamocortical axons, cortex, behavior
Graphical Abstract
INTRODUCTION
Increasing numbers of pregnant women are prescribed SSRIs to treat clinically significant depression and anxiety symptoms.1,2 Some epidemiological studies suggest an association between in utero SSRI exposure and increased risks for adverse outcomes for offspring, including the development of autism spectrum disorder (ASD), attention-deficit hyperactivity disorder, depression and anxiety disorders, and other sequelae that span broad developmental domains.3–11 Other studies find no increased risk for offspring associated with maternal SSRI treatment.8,11–13 Forgoing treatment to avoid potential risks associated with fetal SSRI exposure places offspring at risk for the well-documented effects of maternal depressive and anxiety disorders, which include developmental delays and increased propensity for psychiatric disorders later in life.4,14–16 Together, these observations highlight key uncertainties as to whether SSRIs aggravate or ameliorate the impact of maternal affective disorders on the developing fetal brain.
The SSRIs indirectly impact serotonin transmission through inhibition of the plasma membrane serotonin transporter (SERT, Slc6a4).17,18 Because serotonin plays important trophic roles during development,19 it is assumed that pharmacologic treatment with SSRIs during pregnancy negatively impacts serotonin-dependent neurogenic processes in the developing fetal brain.20,21 Studies in animal models show that pre- or postnatal SSRI exposures reduce adult serotonergic innervation and function and, depending on the SSRI and time of exposure, elicit or reduce depressive- and anxiety-like behaviors during adulthood.22,23 Little is known about the effects of SSRI exposure in utero on fetal brain development.
The underlying causes of depression and anxiety disorders are poorly understood and thus are challenging to model in experimental animals. Chronic and/or severe stress are, however, the most commonly identified risk factors for affective disorders, particularly in susceptible individuals. Here, we used chronic unpredictable stress (CUS), a form of stress that is difficult to adapt to, in pregnant mice to delineate the effects of maternal stress from those produced by a commonly prescribed SSRI on fetal brain development. Pregnant mice were exposed to CUS from embryonic day (E)8 to E17. This period of development in mice corresponds to the late first and second trimesters of human pregnancy, wherein serotonin neurons are extending axons to forebrain regions and nonserotonergic neurons transiently express SERT.19
We found that gestational CUS produced changes in maternal behavior during the late stage of pregnancy indicative of stress susceptibility. We assessed whether the SSRI (±)-citalopram (CIT) administered orally to pregnant mice reaches the fetal brain. We determined the developmental impact of CIT exposure alone and in combination with maternal CUS. Our findings revealed that in utero exposure to the SSRI CIT largely reverses the effects of chronic maternal stress on fetal serotonin-dependent neurogenic processes and cortical brain development.
RESULTS AND DISCUSSION
Citalopram Accesses the Fetal Brain.
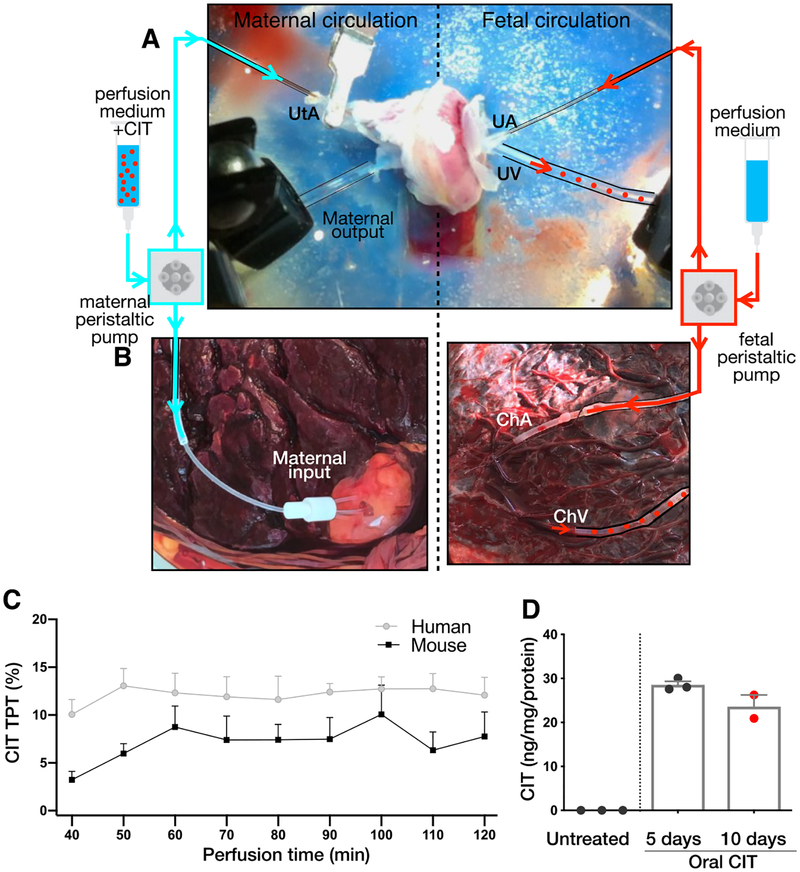
To determine whether CIT administered to mothers crosses the placenta, maternal–fetal CIT transfer was studied using ex vivo perfusion systems in isolated mouse and human placentas. Citalopram (500 ng/mL) was perfused through the maternal circulatory input of E18 mouse placentas (N = 4 placentas; Figure 1A)24 or human term placentas (N = 3 placentas; Figure 1B). While higher than human therapeutic blood serum levels (30–200 ng/mL),25 the CIT concentration used is informative regarding short-term, rapid maternal–fetal drug transfer such as that occurring directly after per oral doses in humans. Steady maternal–fetal transfer of CIT ex vivo was observed in both species (Figure 1C).
Figure 1.
Citalopram crosses the placental barrier in mice and humans. (A, B) Ex vivo perfusion systems for isolated mouse and human placentas. The maternal input (left) consisted of a peristaltic perfusion system connected to the uterine artery (UtA) on the maternal side of E18 mouse placentas (A) or the decidual surface of intact cotyledons in human term placentas (B). On the fetal side (right), a peristaltic pump was connected to the umbilical artery (UA, mouse) or the chorionic artery (UhA, human). Samples collected through the fetal outputs (mouse umbilical vein, UV and human chorionic vein, ChV; red dotted lines) were analyzed by high-pressure liquid chromatography. (C) The CIT (500 ng/mL) was perfused through the maternal input of human term placentas and mouse E18 placentas. Next, 10 min fractions were collected over 120 min through the corresponding fetal outputs. After a 40 min stabilization period, steady maternal–fetal transfer of CIT was observed in both species (N = 3 human placentas; N = 4 mouse placentas). (D) Maternal, orally administered CIT reaches the fetal mouse brain. Pregnant dams received CIT via drinking water from E8 to E17. The CIT was measured in fetal brain at E13 (5 days) and E17 (10 days) by HPLC (N = 3 dams, 3 fetal brains per dam). The CIT was not detected in fetal brains from untreated dams (N = 3 per time point, 3 fetal brains per dam).
Consistent with placental permeability to CIT measured ex vivo and previous pharmacokinetic analyses,26 CIT accumulated in the fetal mouse brain following 5 and 10 days of maternal oral administration via drinking water (N = 2–3 fetal brains per data point; Figure 1D). Direct actions of SSRIs on fetal brain development require that these drugs cross the maternal–fetal placental barrier and accumulate in the fetal brain. Nonetheless, the potential developmental effects of maternal SSRIs are more aptly considered in the context of factors hypothesized to contribute to maternal affective disorders such as gestational stress. Therefore, we next determined if CUS, commonly used to evoke increased anxiety-like behavior in rodents,27,28 can be employed in pregnant mice to disambiguate the effects of gestational stress from those of SSRI exposure on fetal brain development.
Changes in Maternal Behavior Associated with Gestational CUS Are Reversed by CIT.
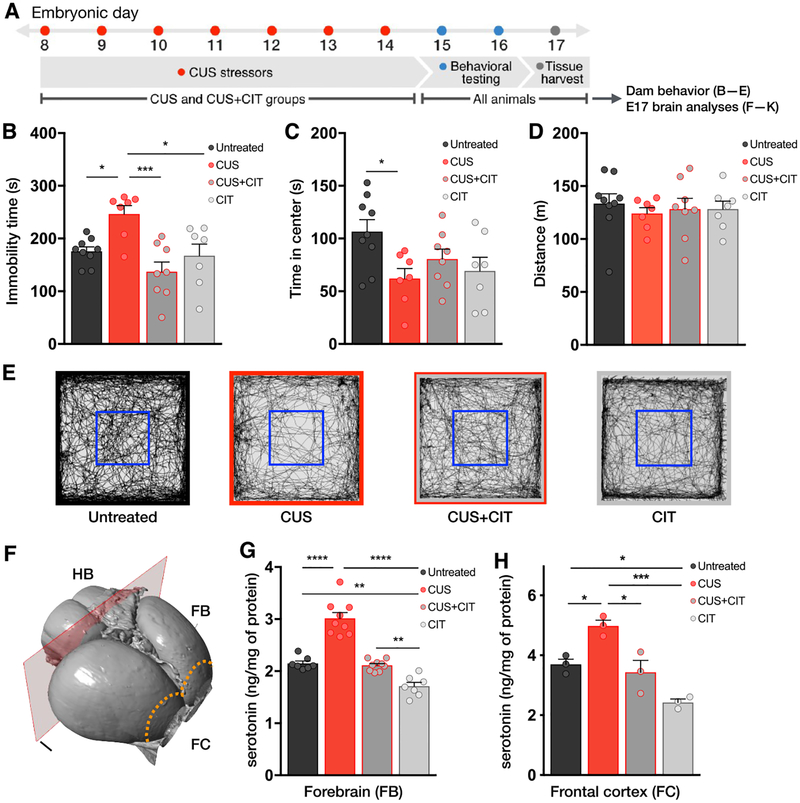
Pregnant mice were exposed to a sequence of daily, nonhabituating mild stressors29 from E8 to E17 (N = 7 dams; Figure 2A; Table S1). A separate group of dams received CIT via drinking water while also undergoing CUS (N = 8 dams; CUS+CIT group). We evaluated the effects of CUS and treatment with CIT on anxiety-like behavior in the open field test (OFT) at E15 and behavioral immobility in the forced swim test (FST) at E16 (Figure 2B and C). In the OFT, CUS-exposed dams showed greater avoidance of the anxiogenic central region of the open field, as indicated by reduced center times compared to untreated controls (untreated = 107 ± 11 vs CUS = 62.1 ± 9.5 s; N = 9 dams; Figure 2C; treatment: F(3,27) = 3.4, P < 0.05; see Table S2B for individual group comparisons). There were no differences in center times between the untreated control, CUS+CIT, and CIT groups (N = 7 dams). There were no group differences in locomotor activity in the OFT (Figure 2D and E; treatment: F(3,27) = 0.20, P =0.89; see Table S2C for individual group comparisons).
Figure 2.
Citalopram normalizes the effects of prenatal stress on dam behavior and tissue serotonin levels in fetal forebrain and frontal cortex. (A) Timed-pregnant mice were subjected to a sequence of CUS from E8 to E17 (details in Table S1). Dams in the CIT and CUS+CIT group received CIT through drinking water from E8 to E17. Only dams in the CUS and CUS+CIT groups were exposed to stressors. Mice were tested in the OFT on E15 followed by the FST on E16. (B) Immobility times in the FST were significantly greater in CUS-exposed dams compared to the other study groups. This effect was reversed by exposure to CIT during gestational CUS (CUS+CIT group). Immobility times in the untreated control and CIT groups were not significantly different. (C) The CUS paradigm induced a significant decrease in time spent in the center of the OFT when compared to untreated controls. This effect was reversed in the CUS+CIT group. (D) There were no differences in total distance traveled in the OFT between groups. (B–D) N = 7–9 dams per condition. (E) Representative activity traces acquired during OFT sessions. The blue squares denote the boundary of the center area quantified in panel B. (F) Serotonin and 5-HIAA levels were quantified in the fetal forebrain (FB), hindbrain (HB), and frontal cortex (FC, dotted line) at E17 by HPLC (N = 7–9 dams per condition, 3 fetal fore/hindbrain samples per dam). (G, H) A significant increase in forebrain (G) and frontal cortex (H) serotonin tissue levels was observed following maternal CUS, whereas CIT alone induced a significant decrease compared to untreated controls at E17. Forebrain serotonin levels following exposure to CUS and CIT (CUS+CIT group) were not different from untreated controls in both regions. Data are means ± SEMs. *P < 0.05, **P < 0.01, or ***P < 0.001.
In the FST, dams exposed to CUS had longer immobility times (untreated = 176 ± 8.4 vs CUS = 246 ± 16 s), an effect that was reversed by treatment with CIT (137 ± 19 s) (Figure 2B; treatment: F(3,27) = 7.47, P < 0.01; see Table S2A for individual group comparisons). There were no significant differences in immobility times between the CIT-alone and untreated control groups (P > 0.99). Together, these results indicate that 10 days of gestational CUS in pregnant mice altered maternal behavior indicative of the effects of stress. These stress-related behavioral changes in dams were reversed by concomitant oral CIT administration. The findings are consistent with previously published observations in non-pregnant rodents,27,30–35 validating the use of CUS and/or CIT administration during a critical prenatal period.
Notably, animals from all groups, including untreated controls, experienced shipping-related and behavioral testing stressors. Therefore, the effects of CUS in dams and fetuses may be somewhat underestimated if compared to mice naïve to stress. We limited stress and drug exposure to the E8-E17 time frame during mouse gestation (roughly equivalent to the late first and second trimesters in humans36) to confine effects to a period of active neurogenesis, neuronal migration, and axonal pathway formation in the fetal mouse brain. These processes are modulated by serotonin19,37,38 and could therefore be impacted not only by maternal–fetal transfer of CIT but also by maternal stress.
Fetal Brain Volumes Are Not Impacted by Maternal Stress or CIT.
To assess the effects of stress and/or CIT exposure generally on fetal brain development, we used magnetic resonance imaging (MRI) to measure total brain and specific brain-structure volumes in post mortem embryos (N = 3–4 dams per group, volumes averaged from 2 males and 2 female brains per dam). There were no significant sex by treatment interactions (whole brain, treatment: F(3,16) = 2.1, P = 0.13, sex: F(1,16) = 8.81, P < 0.01, interaction: F(3,16) = 0.50, P = 0.68; see Table S4A–D for individual group comparisons). The effects of effects of sex were significant such that fetal brain volumes and the volumes of individual brain structures were smaller in females. Exposure to maternal CUS and/or CIT did not significantly alter total fetal brain volumes at E17 nor the volumes of individual brain structures that could be delineated at this stage of development (Figure S1; treatment: F(3,40) = 0.89, P = 0.45, region: F(4,40) = 678.8, P < 0.01, interaction: F(12,40) = 0.49, P = 0.90; see Tables S3 and S4A–D for individual group comparisons). Thus, detailed brain morphological analyses obtained using MRI T2-weighted images showed that neither CUS, CIT, nor their combination significantly altered overall fetal brain growth or the volumes of individual fetal brain structures. These observations indicated that CUS or CIT are not overtly teratogenic and led us to investigate more specific effects related to serotonin-mediated development.
Maternal CIT Reverses CUS Effects on Fetal Forebrain Serotonin Levels.
At E17, tissue serotonin levels in the rostral portion of the fetal brain, i.e., cortex and midbrain structures (Figure 2F), are mainly provided by serotonin axons, which run through the medial forebrain bundle (mfb) and begin to ramify in cortical and subcortical structures. Serotonin content in the hindbrain derives largely from serotonergic neuron cell bodies and their proximal axons and dendrites.39 We investigated whether maternal CUS and/or CIT administration affects fetal serotonin brain tissue content and distribution.
Fetal forebrain serotonin levels in embryos exposed to maternal CUS were significantly higher than levels in all other study groups (N = 7–9 dams per condition, 3 pooled fetal samples per dam; Figure 2G; treatment: F(3,28) = 53.04, P <0.01; see Table S5A for individual group comparisons). In contrast, maternal CIT exposure led to a significant decrease in mean forebrain serotonin content compared to CUS, CUS+CIT, and untreated control groups (statistics in Table S5A). In the forebrains of embryos exposed to maternal CUS+CIT, serotonin tissue levels were not different from untreated controls (P > 0.99). To look more closely at the regionally specific forebrain effects, we measured serotonin levels in fetal frontal cortex (Figure 2H), which is sparsely innervated by serotonin axons emanating from hindbrain serotonin neurons at E17.39,40 Similar to forebrain measures, frontal cortex serotonin tissue levels in embryos exposed to maternal CUS were significantly higher than in all other groups (Figure 2H; treatment: F(3,8) =18.36, P < 0.01; see Table S5C for comparisons between groups). Maternal CIT exposure led to a significant decrease in frontal cortical serotonin content compared to CUS and untreated control groups (untreated vs CIT P = 0.03; statistics in Table S5C). In embryos exposed to maternal CUS+CIT, frontal cortical serotonin tissue levels were not different from CIT-exposed or untreated controls (statistics in Table S5C).
In the hindbrain, while serotonin levels in the treatment groups were not significantly different from the untreated control group, there was a significant increase in serotonin in the CUS group compared to the CUS+CIT and CIT groups (Figure S2A; treatment: F(3,28) = 6.45, P < 0.01; see Table S6A for individual group comparisons). Tissue levels of the serotonin metabolite 5-HIAA in the fetal hindbrain (Figure S2B) and forebrain (Figure S2C) in the exposure groups did not differ significantly from untreated controls at E17 (hindbrain, treatment: F(3,28) = 0.03, P = 0.98; forebrain, treatment: F(3,28) = 6.27, P < 0.01). However, fetal forebrain 5-HIAA tissue levels in embryos exposed to maternal CUS or CUS+CIT were significantly greater than levels in the CIT-alone group (Figure S2B–C; see statistics in Table S5B). The patterns of change in frontal cortex 5-HIAA tissue levels were similar to those observed for 5-HIAA in the forebrain at large (Figure S2D; treatment: F(3,8) = 13.03, P < 0.01; see Table S5D for individual comparisons between groups).
Maternal CUS and CIT Affect Fetal Thalamocortical Neuron Development.
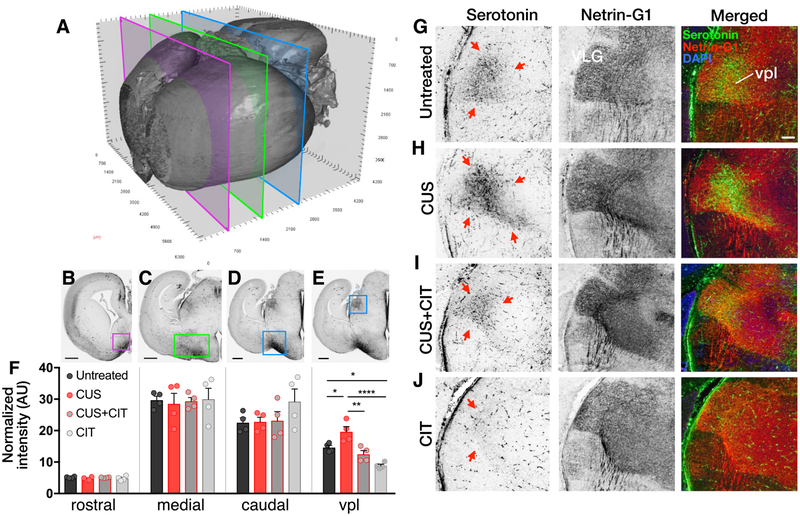
We next investigated whether differences in fetal forebrain serotonin tissue levels measured among the various maternal exposure groups were associated with alterations in serotonin axon densities by examining three different rostro-caudal levels of E17 forebrain from untreated, CUS, CUS+CIT, and CIT exposed mice (N = 4 dams per condition, 1 fetal brain per dam; Figures 3A–E). Densitometric analyses of serotonin immunolabeled structures throughout the rostro-caudal extent of the medial forebrain bundle (mfb) did not reveal differences between groups (Figure 3F; treatment, rostral: F(3,12) = 0.69, P = 0.57; medial: F(3,12) = 0.06, P =0.97; caudal: F(3,12) = 1.42, P = 0.28; see Table S7A–C for individual comparisons between groups). These findings suggest that the development of axons projecting to the forebrain from serotonergic neurons in the hindbrain are not affected by CUS, CIT, or their combination at E17.
Figure 3.
Gestational stress and CIT exposure differentially affect serotonin immunoreactivity in the fetal forebrain. (A) A 3D reconstruction of an iDISCO-cleared E17 brain showing rostral-to-caudal levels (clipping planes) used for analyses of serotonin immunofluorescence distributions. (B–D) Serotonin immunoreactivity was analyzed in coronal sections at three levels of the medial forebrain bundle (mfb; boxed areas: B, rostral, magenta; C, medial, green; D, caudal, light blue; scale bars = 500 jam). (F) Quantification of serotonin-immunopositive axon densities did not reveal differences between treatment groups throughout the mfb (corresponding images above). (E, G–J) The serotonin immunostaining was observed in neuronal cell bodies located in the ventroposteriolateral thalamic nucleus (vpl) in the thalamus (arrows; scale bar = 100 μm). Thalamic neurons and their axons are immunolabeled with the specific marker netrin-G1. The densities of serotonin immunoreactivity were significantly increased in thalamic neurons from embryos exposed to gestational stress (CUS) compared to untreated controls (F, right bar graph). In contrast, serotonin immunoreactivity densities were significantly decreased in thalamic neurons from embryos exposed to CIT compared to controls and in embryos exposed to CUS+CIT compared to CUS. In panel F, data are means ± SEMs for normalized pixel intensities in each region of interest (N = 4 dams per condition, 1 fetal brain per dam). *P < 0.05, **P < 0.01, or ***P < 0.001.
In contrast, we noted large differences between exposure groups in the presumptive ventroposteriolateral nucleus (vpl) of the thalamus (Figure 3E, G–J). Quantification of fetal serotonin immunopositive cell bodies in the vpl indicated that CUS led to a significant increase in serotonin-containing vpl neurons compared to all other treatment groups (Figure 3F; treatment: F(3,12) = 17.0, P < 0.01; see Table S7D for individual group comparisons). Furthermore, consistent with forebrain and frontal cortex serotonin tissue measures (Figure 2H), CIT exposure significantly decreased serotonin immunolabeling in the vpl compared to untreated controls (P = 0.01), whereas maternal CUS+CIT exposure had no significant effect on vpl serotonin immunopositive cell bodies (P > 0.99).
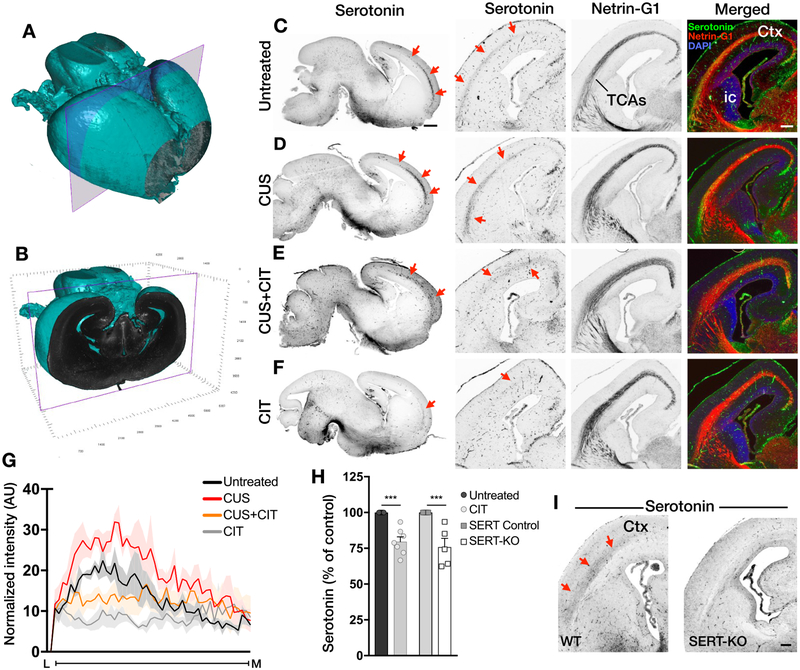
Thalamic neurons, which are not serotonergic per se, transiently express SERT beginning at E15.5.41–43 Thalamocortical axons (TCAs) take up and temporarily contain serotonin exclusively during the late fetal and early postnatal periods.44,45 Here, TCAs were identified using netrin-G1 as a specific marker. Although the overall projection pathway of netrin-G1+ TCAs to the medial cortex was grossly similar across all groups (N = 4 dams per condition, 1 fetal brain per dam; Figure 4A–F), we found that serotonin-containing TCA axons that also expressed netrin-G1 were significantly increased in the CUS group compared to all other groups (Figure 4D, G; treatment: F(3,12) = 51.36, P < 0.01; see Table S8A, B for comparisons between groups). A significant decrease in serotonin immunopositive TCA-netrin-G1+ axons was measured in the CIT group (P < 0.01), whereas there was no significant difference in the CUS+CIT group compared to the untreated control group (P > 0.99; Figure 4E–G).
Figure 4.
Serotonin-positive immunolabeling along TCAs is differentially affected by gestational exposure to CUS and/or CIT. (A, B) Coronal planes that bound the rostro-caudal extent of serotonin immunopositive TCAs. In A, a 3D rendering of an iDISCO-cleared E17 brain. The rostral boundary is the cut face in A. The purple plane in A denotes the caudal boundary (cut face in B). C–F, The serotonin-positive TCAs running through the E17 cortex visualized in iDISCO-cleared, sagittal views (left column) and coronal cryo-sections (right three columns) at the level depicted in panel B. Immunostaining reveals alterations in serotonin distributions along TCAs (arrows) in the forebrain from CUS- (D), CUS+CIT- (E), and CIT-exposed(F) brains compared to untreated embryonic brains (C); sagittal scale bar = 500 pm, N = 2 dams per condition, 1 fetal brain per dam; coronal = 250 μm, N = 4 dams per condition, 1 fetal brain per dam. Netrin-G1 immunostaining labeled TCAs running through the internal capsule (ic) and the cortex (ctx). (G) The CUS exposure induces a significant increase in serotonin immunofluorescence intensity (quantified as AUC along the lateral (L) to medial (M) extent of TCAs (Figure S3) compared to the control condition. In contrast, a significant decrease in serotonin immunofluorescence was measured in CIT exposed embryos. There were no significant differences in the CUS+CIT group compared to the untreated control group (N = 4 dams per condition, 1 fetal brain per dam). H, The magnitude of the serotonin tissue level decrease measured in the fetal forebrain after CIT exposure in utero in wild-type mice (CIT group) was similar to that observed in SERT-KO embryos at E17 compared to respective controls, N = 5–8 dams per condition, 2–4 fetal brains per dam, data are means ± SEMs. (I) The serotonin staining in TCAs (arrows) of wild-type mice (left) is visibly present compared to its absence in SERT-KO mice at E17 (right, scale bar = 250 μn, N = 3 dams per condition, 1 fetal brain per dam).
The magnitude of the reduction in forebrain serotonin tissue levels at E17 following in utero CIT exposure (N = 5–8 dams per condition, 2–4 pooled fetal brains per dam; Figure 4H; 20.5 ± 3.4% reduction; t(12) = 5.05; P < 0.01) was similar to that measured in a separate cohort of drug-naïve mice genetically lacking SERT expression (SERT-KO), implicating loss of SERT function in the serotonin deficits observed in CIT-exposed embryos (24.1 ± 5.9% reduction; t(11) = 4.59; P < 0.01). Moreover, the absence of serotonin immunolabeled TCAs in SERT-KO embryos at E17 (Figure 4I) was comparable to that observed in in utero CIT-exposed wild-type mice.
In the forebrain at E17, a number of sources contribute to total tissue serotonin content, i.e., intracellular serotonin in axons from hindbrain serotonergic neurons that extend to forebrain39 and thalamic neurons and TCAs that transiently uptake serotonin44 as well as an extraneuronal pool that includes serotonin synthesized peripherally, which is transported to the brain in fetal blood.40,46,47 Changes in serotonin tissue levels in the fetal forebrain do not appear to have been caused by overt alterations in serotonin system development. Although we did not measure serotonin terminal axon densities in every forebrain structure, unaltered development of medial forebrain bundle serotonin fibers suggests that the sparse infiltration of serotonin terminals in the forebrain at E17 is likely to be normal. By contrast, we observed increases in serotonin immunolabeled fetal thalamic neurons (Figure 3E, H) and TCAs (Figure 4D, G) following CUS.
Serotonin immunopositive axon densities projecting from the hindbrain to the forebrain of E17 embryos were not altered by maternal exposure to CIT (Figure 3F, see ANOVA statistics in page 11 and Table S7A–C). Presumably, CIT inhibited serotonin uptake into these serotonergic axons. In addition to taking up serotonin, these axons and the neurons they originate from in the hindbrain synthesize serotonin. By contrast, CIT abolished serotonin immunolabeling in thalamic neurons and TCAs (thalamic neurons: Figure 3E, J and TCAs: Figure 4F, G; see ANOVA statistics in page 12–13 and Table S8A, B). While thalamic neurons transiently express SERT, they do not synthesize serotonin. Thus, the only source of intracellular serotonin in thalamic neurons is that which is taken up from the extracellular space. These findings suggest that in the absence of the ability to synthesize serotonin, inhibition of serotonin uptake differentially impacts the development of thalamic neurons and their projections. The SSRI CIT has alternately been shown to affect TCA guidance in a SERT-independent manner.48 However, the CIT concentrations needed to achieve SERT-independent effects in vitro (μM) are much higher than the CIT levels detected in the fetal forebrain in vivo after maternal oral administration of CIT (nM, Figure 1).
In contrast to CIT-mediated inhibition of SERT, increased serotonin immunolabeling associated with CUS may result from elevated SERT-mediated uptake of serotonin into thalamic neurons and/or TCAs, where serotonin is protected from degradation,44 and/or more exuberant proliferation of these structures. A reversal of CUS effects by CIT is implicated in the CUS+CIT group where forebrain serotonin tissue levels (Figure 2G, H) and serotonin immunolabeling of thalamic neurons and TCAs (Figures 3F, H, I and 4D, E, G) were similar to those in untreated controls. Contradicting the common oversimplification that SSRI exposure “increases serotonin” in the fetal brain, in utero exposure to CIT decreases total tissue serotonin levels in the forebrain mainly by blocking serotonin uptake into TCAs.
A possible means by which maternal CUS may increase tissue serotonin in the fetal forebrain is that elevated plasma concentrations of tryptophan in stressed dams49 may lead to increased serotonin synthesis in fetal compartments.40,47 Increased serotonin synthesis throughout the stress period could lead to elevated serotonin accumulation, particularly in thalamic neurons and their axons projecting to cortex E17. Because vpl thalamic neurons do not express the enzymes need to synthesize and break down serotonin, they may be particularly sensitive to factors that alter serotonin levels in other fetal compartments.
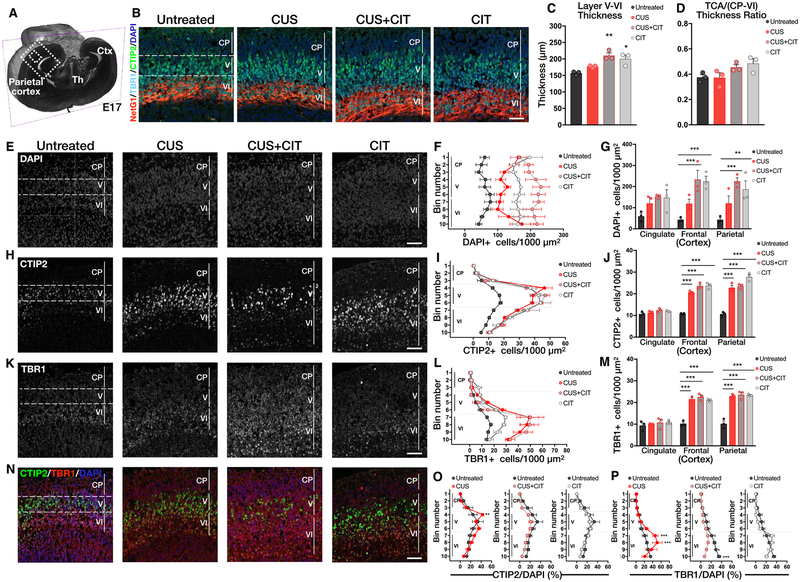
Interestingly, CUS, CIT, and CUS+CIT exposures did not affect the thickness of netrin-G1+ TCA bundles relative to the overall thickness of the parietal (somatosensory) cortex (Figures 5A, B, D; N = 3 dams per condition, 3 fetal brains per dam; treatment: F(3,8) = 3.35, P = 0.07; see Table S9B for individual group comparisons). However, parietal cortex thickness itself (from the cortical plate to layer V–VI) was significantly increased in the CIT and CUS+CIT groups but not the CUS alone group compared to untreated controls (Figure 5C; treatment: F(3,8) = 11.26, P < 0.01; see Table S9A for individual group comparisons), suggesting an effect of CIT exposure from E8 to E17 on cortical cell proliferation.
Figure 5.
Exposure to CIT mitigates maternal CUS effects on deep-layer cortical development at E17. (A) The dashed box indicates the parietal cortex region-of-interest depicted throughout the figure. Ctx, cortex; Th, thalamus. (B) Representative coronal sections of E17 brains immunostained for CTIP2 (cortical layer V), TBR1 (cortical layer VI), and netrin-G1 (NetG1;TCA). Scale bar = 50 μm. (C) Cortical layer V–VI thickness was significantly increased in the CUS+CIT and CIT groups. (D) The TCA/(CP to layer VI) thickness ratios were similar in all groups. (E) Representative images of DAPI+ cells in the CP to layers V–VI of the parietal cortex. (F, G) The numbers of DAPI+ cells were increased in the CP to layer VI in CUS +CIT and CIT groups in the frontal and parietal cortex but not the cingulate cortex compared to untreated controls; CUS had no significant effect. (H) Representative images of CTIP2+ cells in cortical layers V–VI. (I, J) The numbers of CTIP2+ cells were significantly increased in layer V in CUS, CUS +CIT, and CIT groups compared to untreated controls in the frontal and parietal but not cingulate cortex. (K) Representative images of TBR1+ cells in cortical layers VI. (L, M) The numbers of TBR1 + cells were significantly increased in cortical layer V–VI in CUS, CUS+CIT, and CIT groups compared to untreated controls in frontal and parietal cortex but not cingulate cortex. (N) Colocalization of CTIP2+, TBR1+, and DAPI+ cells in layers V–VI of the parietal cortex. (O, P) The numbers of CTIP2+ and TBR1+ cells normalized to total cell numbers (CTIP2/DAPI% and TBR1/DAPI%, respectively) in CP to layer VI were increased in CUS but not in CUS+CIT and CIT groups when compared to the untreated control group. Data are means ± SEMs; N = 3 dams per condition, 3 fetal brains per dam for quantification. *P < 0.05, **P < 0.01, or ***P < 0.001. Scale bars = 50 μm.
Exposure to Maternal CUS Alters Cortical Development.
Because in utero CUS and/or CIT exposure alters serotonin levels in the fetal cortex where serotonin modulates neurogenic events such as cellular proliferation and migration,38,50 we next quantified overall cortical cell densities (DAPI + cells) and the laminar distributions of specific neuron subtypes at E17 using deep-layer molecular markers, i.e., corticofugal (CTIP2+) and cortico-striatal or cortico-thalamic (TBR1+) neurons (N = 3 dams per condition, 3 fetal brains analyzed per dam). We found that the number of DAPI+ cells spanning the cortical plate (CP) to layer VI was significantly increased in parietal and frontal but not cingulate cortex in the CIT and CUS+CIT groups compared to untreated or CUS groups (Figure 5E–G; Figure 5F: bin distribution: F(9,80) = 0.55, P = 0.82, treatment: F(3,80) = 108.9, P < 0.01, interaction: F(27,80) = 1.08, P = 0.38; see Tables S10,11 for individual group comparisons). The numbers of CTIP2+ cells in layer V (Figure 5H–J; Figure 5I: bin distribution: F(9,80) = 108.9, P < 0.01, treatment: F(3,80) = 59.78, P < 0.01, interaction: F(27,80) = 4.852, P < 0.01; Figure S4A; see Tables S12, 13, 18, and 20 for individual group comparisons) and TBR1+ cells in layer VI (Figure 5K–M; Figure 5L, bin distribution: F(9,80) = 69.85, P <0.01, treatment: F(3,80) = 34.15, P < 0.01, interaction: F(27,80) = 5.05, P < 0.01; Figure 5M, region: F(2,24) = 144, P < 0.01, treatment: F(3,24) = 70.31, P < 0.01, interaction: F(6,24) =13.49, P < 0.01; Figure S4B, D; see Tables S14, 15, 19, and 21 for individual group comparisons) were significantly increased in parietal and frontal but not cingulate cortex of CUS, CUS+CIT, and CIT-exposed fetal brains when compared with untreated controls.
Changes in progenitor cell proliferation, differentiation, and/or survival can ultimately alter the numbers of CTIP2+ or TBR1+ neurons present in layers V–VI at E17. Because CIT and CUS+CIT but not CUS exposure increased the total numbers of cells in the parietal cortex, we normalized CTIP2+ and TBR1+ cell counts to DAPI+ cell numbers from the cortical plate to layer VI in each exposure group. We found that CUS induced a significant increase in CTIP2+/DAPI+ cell ratio in layer V of parietal cortex compared to the control group (Figure 5N, O; bin distribution: F(9,80) = 34.33, P < 0.01, treatment: F(3,80) = 12.28, P < 0.01, interaction: F(27,80) = 1.38, P = 0.13; see Table S16 for individual group comparisons). In contrast, normalized CTIP2+/DAPI+ cell ratios in layer V in the CIT and CUS+CIT groups were not different from untreated controls (Figure 5O; see statistics in Table S16). Similarly, normalized TBR1+/DAPI+ cell ratio was significantly increased in layer VI of the parietal cortex in the CUS group compared to untreated controls (Figure 5N, P; bin distribution: F(9,80) = 33.44, P <0.01, treatment: F(3,80) = 17.32, P < 0.01, interaction: F(27,80) = 3.02, P < 0.01; see Table S17 for individual group comparisons). In the CUS+CIT group, the TBR1+/DAPI+ cell ratio was significantly decreased in layer VI compared to the untreated group (Figure 5P; statistics in Table S17). In the CIT group, the TBR1+/DAPI+ cell ratio in layer VI was not different from untreated controls (Figure 5P; statistics in Table S17).
These results suggest that the CUS-mediated increase in cortical serotonin levels increased CTIP2+ and TBR1+ cell numbers in layer V and VI of parietal and frontal cortex. Because overall cortical cell numbers (DAPI+) were not affected by CUS, these effects more likely result from serotonin-dependent effects on neuronal migration and/or differentiation and not proliferation from E8 to E17. Although these possibilities remain to be more deeply investigated, they are consistent with studies demonstrating a direct effect of serotonin signaling on neuronal migration in the developing cortex.51 In contrast, in utero CIT exposure for 10 days, in the absence of maternal stress, considerably increased total cortical cell numbers without altering specific cell proportions, i.e., CTIP2+/DAPI+ and TBR1+/DAPI+ cell numbers in layers V–VI (Figure 5). These findings suggest that CIT either directly or via alterations in serotonin levels in the cortical microenvironment, e.g., around growing TCAs (Figure 5B), alters cortical progenitor cell proliferation and/or survival.
In fact, both SSRIs and serotonin signaling have been shown to affect neurogenesis during development and in adulthood.38,52,53 Importantly, our results show that when combined with CUS, the potent effect of CIT on neurogenesis normalized the density of CTIP2+ cells in layer V and TBR1+ cells in layer VI of parietal and frontal cortex (Figure 5) suggesting that, to an extent, CIT counteracts the deleterious effects of maternal stress on cortical development. Corticofugal (CTIP2+) and cortico-striatal or cortico-thalamic (TBR1+) projection neurons in the fetal frontal cortex of humans are believed to be a point of convergence of autism-risk gene expression;54 therefore, it will be important to evaluate the long-term impact of prenatal CUS and/or CIT exposure on the formation and function of these cortical networks later in life.
CONCLUSIONS
We delineated the consequences of maternal stress from those of the SSRI citalopram for maternal behavior and fetal brain development. We found that maternal gestational stress increased fetal forebrain serotonin levels, a change associated with an abnormal distribution of serotonin in developing thalamocortical neurons and axons. Furthermore, gestational stress increased the number of defined deep-layer neuron subtypes in the fetal cortex compared to embryos from unstressed mothers. Importantly, maternal CIT administration reversed most of the effects of stress, not only in terms of maternal depressive-like behavior but also with regard to fetal forebrain serotonin levels and fetal cortical formation during a critical time in development.
We focused on the acute effects of maternal stress and SSRI exposure on fetal brain development, yet many studies have shown that similar types of exposures or a genetically driven excess of brain serotonin levels have long-term consequences for offspring brain function.23,55 Although the long-term effects of the specific exposures that we studied are currently under investigation, it is important to mention that SSRI-induced changes in early serotonergic signaling do not necessarily lead to negative outcomes for offspring, particularly in the context of maternal stress effects.56,57 In fact, recent studies in rodents indicates that in the context of prenatal stress, maternal treatment with the SSRI fluoxetine had a potentially beneficial effect on spatial memory in female offspring and aggressive behavior in male offspring.58–60 There is also substantial evidence that SSRIs reduce depression-like behaviors in adult rodents following a single dose (usually delivered intraperitoneally) and only 30 min to 1 h prior to behavioral testing. Although we cannot know exactly when during the 10 days of exposure to SSRI the effect is becoming beneficial, the fetal brain is in a very active and rapid state of developmental flux in term of structure and function; therefore, CIT directly targeting brain SERT could affect baseline serotonin levels and signaling, thereby impacting multiple phases of brain development that converge to rapidly counteract stress effects. Our findings emphasize the importance of further investigating the fetal effects of SSRI exposure in the context of maternal stress and genetic variations in animal models to inform future therapeutic decision-making in humans.
METHODS
Animals.
Timed-pregnant CD-1 mice were obtained from Charles River Laboratories (Wilmington, MA) at E7. Serotonin transporter knockout (SERT-KO; B6.129(Cg)-Slc6a4tm1Kpl/J) female mice and C57BL/6J male mice were used to generate nonsibling SERT-heterozygous breeding pairs to generate the wild-type and SERT-KO fetal mice shown in Figure 4I. Plug date was considered E0, and the age of individual embryos was confirmed by measuring the crown–rump length and checking for developmental landmarks such as digits and eye formation. Dams at 8–10 weeks of age were housed in groups of 2–4 in standard cages with ad libitum access to food and water. Animals were maintained under 12 h:12 h light–dark cycles (6:00–18:00 h) unless otherwise noted (see Table S1). All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Timed-pregnant CD-1 mice at E7 were assigned to one of the following groups: untreated, CUS, CUS+CIT, or CIT. The CUS protocol was initiated at E8 in the CUS and CUS+CIT groups wherein subjects were exposed to a series of different daily stressors until late gestation (E17; Figure 2A, Table S1). The CUS paradigm is nonhabituating, not physically painful, and does not affect maternal food intake.29
Detailed descriptions of the experimental methods are provided in the Supporting Information.
Statistical Analysis.
Dams were assigned to specific treatment groups by simple randomization. To avoid potential litter effects, the number of dams (not embryos) in each group (“N”) was used for statistical calculations throughout. Therefore, reported N values refer to dams/litters and not individual animals. Investigators were blind to experimental conditions when analyzing data. Fetal samples were genotyped for SRY; because statistical analyses did not show sex by treatment interactions in any of the reported measures throughout the study, an equal number of male and female embryos were analyzed per dam in every experiment.
All data are expressed as means ± standard errors of the means (SEMs). Data were analyzed for significant differences using one-way or two-way analysis of variance (ANOVA), as appropriate, followed by Bonferroni’s post hoc tests corrected for multiple comparisons. Placental perfusion data were analyzed using unpaired two-tailed Student’s t-tests. Statistical results are detailed in the Supplementary Tables. Throughout, P < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism7 (La Jolla, CA).
Supplementary Material
ACKNOWLEDGMENTS
We thank Jonathan Wang and Clarissa James for technical assistance, Drs. Juli Wu and Ryan Kast for helpful discussions and comments on the manuscript, and Dr. Fernandez of the CHLA Cellular Imaging Core for helping with light sheet imaging.
Funding: This work was supported by a Chateaubriand Fellowship (J.C.V.), funding from the National Institute of Mental Health (R01MH106806, A.B.), and a NARSAD Independent Investigator Grant (#25717) from the Brain and Behavior Research Foundation (A.B.).
ABBREVIATIONS
- SSRI
serotonin-selective reuptake inhibitor
- CIT
citalopram
- CUS
chronic unpredictable stress
- TCAs
thalamocortical axons
- mfb
medial forebrain bundle
- vpl
ventroposteriolateral nucleus
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneuro.9b00180.
Supporting information, methods, and references, volumes of fetal brain structures, fetal hindbrain serotonin tissue levels, fetal 5-HIAA levels, TCA serotonin immunofluorescence, cortical layer formation data, chronic unpredictable stress schedule, and detailed statistical analyses (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Cooper WO, Willy ME, Pont SJ, and Ray WA (2007) Increasing use of antidepressants in pregnancy. Am. J. Obstet Gynecol 196, 544–e1.. [DOI] [PubMed] [Google Scholar]
- (2).Toohey J (2012) Depression during pregnancy and postpartum. Clin. Obstet. Gynecol 55, 788–797. [DOI] [PubMed] [Google Scholar]
- (3).Rai D, Lee BK, Dalman C, Golding J, Lewis G, and Magnusson C (2013) Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. Bmj 346, No. f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Velasquez JC, Goeden N, and Bonnin A (2013) Placental serotonin: implications for the developmental effects of SSRIs and maternal depression. Front Cell Neurosci 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Harrington RA, Lee L-C, Crum RM, Zimmerman AW, and Hertz-Picciotto I (2014) Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 133, No. e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kaplan YC, Keskin-Arslan E, Acar S, and Sozmen K (2016) Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: A systematic review and meta-analysis. Reprod. Toxicol 66, 31–43. [DOI] [PubMed] [Google Scholar]
- (7).Brown AS, Gyllenberg D, Malm H, McKeague IW, Hinkka-Yli-Salomäki S, Artama M, Gissler M, Cheslack-Postava K, Weissman MM, Gingrich JA, and Sourander A (2016) Association of Selective Serotonin Reuptake Inhibitor Exposure During Pregnancy With Speech, Scholastic, and Motor Disorders in Offspring. JAMA Psychiatry 73, 1163–1170. [DOI] [PubMed] [Google Scholar]
- (8).Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, and Vigod SN (2017) Association Between Serotonergic Antidepressant Use During Pregnancy and Autism Spectrum Disorder in Children. JAMA 317, 1544–1552. [DOI] [PubMed] [Google Scholar]
- (9).Brown HK, Hussain-Shamsy N, Lunsky Y, Dennis CE, and Vigod SN (2017) The Association Between Antenatal Exposure to Selective Serotonin Reuptake Inhibitors and Autism: A Systematic Review and Meta-Analysis. J. Clin Psychiatry 78, No. e48. [DOI] [PubMed] [Google Scholar]
- (10).Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, Lau WCY, Schuemie MJ, Sturkenboom MCJM, and Wong ICK (2017) Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. Bmj, No. j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sujan AC, Rickert ME,Öberg AS, Quinn PD, Hernandez-Diaz S, Almqvist C, Lichtenstein P, Larsson H, and D’Onofrio BM (2017) Associations of Maternal Antidepressant Use During the First Trimester of Pregnancy With Preterm Birth, Small for Gestational Age, Autism Spectrum Disorder, and Attention-Deficit/Hyperactivity Disorder in Offspring. JAMA 317, 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sorensen MJ, Grønborg TK, Christensen J, Thorlund E, Vestergaard M, Schendel D, and Pedersen LH (2013) Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 5, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, Erb JL, Churchill SE, Kaimal AJ, Doyle AE, Robinson EB, Smoller JW, Kohane IS, and Perlis RH (2015) Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol. Psychiatry 20, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, and Kuhn C (2004) Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry 67, 63–80. [DOI] [PubMed] [Google Scholar]
- (15).Oberlander TF, Warburton W, Misri S, Aghajanian J, and Hertzman C (2006) Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch. Gen. Psychiatry 63, 898–906. [DOI] [PubMed] [Google Scholar]
- (16).Oberlander TF, and Vigod SN (2016) Developmental Effects of Prenatal Selective Serotonin Reuptake Inhibitor Exposure in Perspective: Are We Comparing Apples to Apples. J. Am. Acad. Child Adolesc Psychiatry 55 (5), 351–352. [DOI] [PubMed] [Google Scholar]
- (17).Blakely RD, DeFelice LJ, and Galli A (2005) Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology 20, 225–231. [DOI] [PubMed] [Google Scholar]
- (18).Yang H, Sampson MM, Senturk D, and Andrews AM (2015) Sex- and SERT-mediated differences in stimulated serotonin revealed by fast microdialysis. ACS Chem. Neurosci 6, 1487–1501. [DOI] [PubMed] [Google Scholar]
- (19).Gaspar P, Cases O, and Maroteaux L (2003) The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci 4, 1002–1012. [DOI] [PubMed] [Google Scholar]
- (20).St-Pierre J, Laurent L, King S, and Vaillancourt C (2016) Effects of prenatal maternal stress on serotonin and fetal development. Placenta 48, S66–S71. [DOI] [PubMed] [Google Scholar]
- (21).Brummelte S, Mc Glanaghy E, Bonnin A, and Oberlander TF (2017) Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 342, 212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ansorge MS, Morelli E, and Gingrich JA (2008) Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci 28, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Altieri SC, Yang H, O’Brien HJ, Redwine HM, Senturk D, Hensler JG, and Andrews AM (2015) Perinatal vs genetic programming of serotonin states associated with anxiety. Neuropsychopharmacology 40, 1456–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Goeden N, and Bonnin A (2013) Ex vivo perfusion of mid-to-late-gestation mouse placenta for maternal–fetal interaction studies during pregnancy. Nat. Protoc 8, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Meng QH, and Gauthier D (2005) Simultaneous analysis of citalopram and desmethylcitalopram by liquid chromatography with fluorescence detection after solid-phase extraction. Clin. Biochem 38, 282–285. [DOI] [PubMed] [Google Scholar]
- (26).Velasquez JC, Goeden N, Herod SM, and Bonnin A (2016) Maternal Pharmacokinetics and Fetal Disposition of (±)-Citalopram during Mouse Pregnancy. ACS Chem. Neurosci 7, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bourke CH, Capello CF, Rogers SM, Yu ML, Boss-Williams KA, Weiss JM, Stowe ZN, and Owens MJ (2013) Prenatal exposure to escitalopram and/or stress in rats: a prenatal stress model of maternal depression and its treatment. Psychopharmacology 228, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Champagne FA, and Meaney MJ (2006) Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatry 59, 1227–1235. [DOI] [PubMed] [Google Scholar]
- (29).Mueller BR, and Bale TL (2008) Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci 28, 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rygula R, Abumaria N, Flügge G, Hiemke C, Fuchs E, Rüther E, and Havemann-Reinecke U (2006) Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav. Pharmacol 17, 19–29. [DOI] [PubMed] [Google Scholar]
- (31).Sarro EC, Sullivan RM, and Barr G (2014) Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience 258, 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yang J, Han H, Cao J, Li L, and Xu L (2006) Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus 16, 431–436. [DOI] [PubMed] [Google Scholar]
- (33).Bourke CH, Stowe ZN, and Owens MJ (2014) Prenatal antidepressant exposure: clinical and preclinical findings. Pharmacol. Rev 66, 435–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bourke CH, Stowe ZN, Neigh GN, Olson DE, and Owens MJ (2013) Prenatal exposure to escitalopram and/or stress in rats produces limited effects on endocrine, behavioral, or gene expression measures in adult male rats. Neurotoxicol. Teratol 39, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Van den Hove DLA, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HWM, Schruers KRJ, and Prickaerts J (2014) Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur. Neuropsychopharmacol 24, 595–607. [DOI] [PubMed] [Google Scholar]
- (36).Cox B, Kotlyar M, Evangelou AI, Ignatchenko V, Ignatchenko A, Whiteley K, Jurisica I, Adamson SL, Rossant J, and Kislinger T (2009) Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol. Syst. Biol 5, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Bonnin A, Torii M, Wang L, Rakic P, and Levitt P (2007) Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat. Neurosci 10, 588–597. [DOI] [PubMed] [Google Scholar]
- (38).Vitalis T, Ansorge MS, and Dayer AG (2013) Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front Cell Neurosci 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lidov HG, and Molliver ME (1982) An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res. Bull 8, 389–430. [DOI] [PubMed] [Google Scholar]
- (40).Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, and Levitt P (2011) A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Narboux-Nême N, Pavone LM, Avallone L, Zhuang X, and Gaspar P (2008) Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology 55, 994–1005. [DOI] [PubMed] [Google Scholar]
- (42).Hansson SR, Mezey E, and Hoffman BJ (1998) Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience 83, 1185–1201. [DOI] [PubMed] [Google Scholar]
- (43).Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, and Gaspar P (1998) Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol 401, 506–524. [PubMed] [Google Scholar]
- (44).Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P, and Mestikawy SE (1996) Transient uptake and storage of serotonin in developing thalamic neurons. Neuron 17, 823–835. [DOI] [PubMed] [Google Scholar]
- (45).Brüning G, Liangos O, and Baumgarten HG (1997) Prenatal development of the serotonin transporter in mouse brain. Cell Tissue Res. 289, 211–221. [DOI] [PubMed] [Google Scholar]
- (46).Côte F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, and Vodjdani G (2007) Maternal serotonin is crucial for murine embryonic development. Proc. Natl. Acad. Sci. U. S. A 104, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Branchek TA, and Gershon MD (1989) Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enter-ochromaffin cells. J. Comp. Neurol 285, 262–273. [DOI] [PubMed] [Google Scholar]
- (48).Bonnin A, Zhang L, Blakely RD, and Levitt P (2012) The SSRI citalopram affects fetal thalamic axon responsiveness to netrin-1 in vitro independently of SERT antagonism. Neuropsychopharmacology 37, 1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Peters DA (1990) Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: a possible mechanism by which stress influences brain development. Pharmacol., Biochem. Behav 35, 943–947. [DOI] [PubMed] [Google Scholar]
- (50).Riccio O, Jacobshagen M, Golding B, Vutskits L, Jabaudon D, Hornung JP, and Dayer AG (2011) Excess of serotonin affects neocortical pyramidal neuron migration. Transl. Psychiatry 1, No. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Frazer S, Otomo K, and Dayer A (2015) Early-life serotonin dysregulation affects the migration and positioning of cortical interneuron subtypes. Transl Psychiatry 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, and Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (Washington, DC, U. S.) 301, 805–809. [DOI] [PubMed] [Google Scholar]
- (53).Cheng A, Scott AL, Ladenheim B, Chen K, Ouyang X, Lathia JD, Mughal M, Cadet JL, Mattson MP, and Shih JC (2010) Monoamine oxidases regulate telencephalic neural progenitors in late embryonic and early postnatal development. J. Neurosci 30, 10752–10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, Murtha MT, Bichsel C, Niu W, Cotney J, Ercan-Sencicek AG, Gockley J, Gupta AR, Han W, He X, Hoffman EJ, Klei L, Lei J, Liu W, Liu L, Lu C, Xu X, Zhu Y, Mane SM, Lein ES, Wei L, Noonan JP, Roeder K, Devlin B, Sestan N, and State MW (2013) Coexpression Networks Implicate Human Midfetal Deep Cortical Projection Neurons in the Pathogenesis of Autism. Cell 155, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, and Gaspar P (1996) Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: Role of a serotonin excess during the critical period. Neuron 16, 297–307. [DOI] [PubMed] [Google Scholar]
- (56).Brummelte S, Galea LAM, Devlin AM, and Oberlander TF (2013) Antidepressant use during pregnancy and serotonin transporter genotype (SLC6A4) Affect newborn serum reelin levels. Dev Psychobiol 55, 518. [DOI] [PubMed] [Google Scholar]
- (57).Rayen I, Gemmel M, Pauley G, Steinbusch HWM, and Pawluski JL (2015) Developmental exposure to SSRIs, in addition to maternal stress, has long-term sex-dependent effects on hippocampal plasticity. Psychopharmacology 232, 1231–1244. [DOI] [PubMed] [Google Scholar]
- (58).Salari A-A, Fatehi-Gharehlar L, Motayagheni N, and Homberg JR (2016) Fluoxetine normalizes the effects of prenatal maternal stress on depression- and anxiety-like behaviors in mouse dams and male offspring. Behav. Brain Res. 311, 354–367. [DOI] [PubMed] [Google Scholar]
- (59).Kiryanova V, Meunier SJ, Vecchiarelli HA, Hill MN, and Dyck RH (2016) Effects of maternal stress and perinatal fluoxetine exposure on behavioral outcomes of adult male offspring. Neuroscience 320, 281–296. [DOI] [PubMed] [Google Scholar]
- (60).Kiryanova V, Meunier SJ, and Dyck RH (2017) Behavioural outcomes of adult female offspring following maternal stress and perinatal fluoxetine exposure. Behav. Brain Res 331, 84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.