Abstract
Purpose
To compare different metrics and acquisition modes of fixation stability as a new visual function biomarker in a large cohort of patients with ABCA4-related Stargardt disease from the multicenter prospective ProgStar study.
Methods
Fixation was tested during a separate fixation exam and also dynamically during a sensitivity exam, using fundus-tracking microperimetry (Nidek MP-1). Fixation data were analyzed using the bivariate contour ellipse area (BCEA), the 2/4 degree method, and the Fujii classification.
Results
In a total of 235 patients, the mean BCEA was larger when measured during the sensitivity exam (418 eyes; 12.5 vs. 4.6 deg2 during the fixation task in 427 eyes). Correlations between the two tests were generally weak. Fixation stability during the sensitivity test was significantly correlated with visual acuity. Comparing the BCEA values and the corresponding Fujii categories for these eyes revealed ranges of overlap where an eye with one defined BCEA value can fall into each of the three Fujii categories.
Conclusions
Patients may have limited ability to fixate over defined time periods, which leads to significant differences between shorter and longer measurements of fixation stability. The most appropriate way to use this functional biomarker appears to be using continuous metrics for fixation stability, such as the BCEA, during a macular sensitivity test.
Keywords: fixation, Fujii method, fundus-tracking microperimetry, BCEA, Stargardt
The most common type of juvenile macular degeneration is autosomal recessive Stargardt disease (STGD1) with an estimated prevalence of 1/8000 to 1/10,000.1 Although foveal-sparing cases with preserved visual acuity have been described,2–4 most cases show central involvement.5,6 As a result, bilateral symmetrical loss of visual acuity down to 20/200 or 20/400 is typically observed in STGD1.7,8 However, recent evidence suggests that visual acuity is not a sensitive biomarker for longitudinal change of visual function.8
In healthy observers, eye movements such as microsaccades and tremor keep the retina constantly in motion.9 The magnitude of these movements, however, is very small.9 Eyes with various forms of central macular disease show eccentric and unstable fixation with bivariate contour ellipse areas (BCEA) approximately 10 times as large as in normal eyes.10 Fixation stability is a functional biomarker introduced to more comprehensively assess the function of the macula.11 It positively correlates with reading speed,12–15 visual acuity,11,16–18 and visual search ability19 in patients with macular disease. Fixation stability testing using different devices has been shown to yield similar results in normal eyes and eyes with macular disease.20 It has been shown that the improvement in fixation stability is predictive of the visual outcome in patients with macular hole surgery.21
Given the emerging therapeutic approaches for retinal diseases,22,23 there is a need to identify the most appropriate outcome parameters and visual function biomarkers for clinical trials.24–27 This report compares different metrics and different modes of acquisition to report fixation values in a large cohort of patients with genetically confirmed STGD1. It tests the hypothesis that there is a strong correlation between the different metrics of fixation stability and that they are interchangeable. Furthermore, it tests the hypothesis that the fixation results obtained during a separate fixation exam11 and those obtained during a macular sensitivity26 test are interchangeable.
Methods
The ProgStar Study
In this study, research is based on the baseline data collected as part of the multicenter prospective ProgStar study, which is registered at www.clinicaltrials.gov (Identifier NCT01977846). The Guidelines of Good Clinical Practice of the International Council for Harmonisation of Technical Requirements for Phamaceuticals for Human Use, the applicable regulatory requirements, and the current Declaration of Helsinki were followed. The study is in compliance with the Health Insurance Portability and Accountability Act. Prior to enrollment of the first patient, the Western Institutional Review Board, the local institutional review boards (IRB), and the Human Research Protection Office of the U.S. Army Medical Research & Materiel Command granted ethics committee approval. Written informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study.
Inclusion Criteria
ProgStar Report No. 1 describes in detail the design, organization, baseline characteristics, and inclusion and exclusion criteria.28 The inclusion criteria were set along the needs of a natural history study that investigates novel biomarkers for retinal degenerative diseases; the following were critical for the presented subproject: Patients (aged ≥6 years) with ≥2 ABCA4 mutations or 1 ABCA4 mutation plus a phenotype typical of STGD1 were enrolled if fundus autofluorescence testing revealed ≥1 well-demarcated area of atrophy with a minimum diameter of 300 μm but a total lesion area ≤ 12 mm2. Best-corrected visual acuity (BCVA) had to be ≥20 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (20/400 Snellen equivalent). Clear ocular media and patient ability to perform all examinations were required.
Procedure
Microperimetric macular sensitivity and fixation exams were performed with the Nidek MP-1 microperimeter (Navis Software 1.7.0 or higher; Nidek Technologies Srl, Albignasego, PD, Italy) at eight out of nine participating sites, and only exams performed with the Nidek MP-1 were included. Real-time retinal imaging is rendered possible through an infrared fundus camera with an included liquid crystal display to display a single red cross (2° visual angle in extension and 1° visual angle in thickness as default, both increasable if necessary) on a white, monochromatic background.
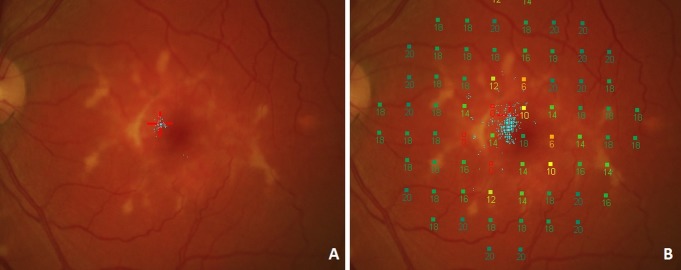
Fixation stability was assessed during two tasks (Fig. 1). For the fixation module, patients are asked to monocularly fixate a target during a short independent fixation exam. Alternatively, fixation results were also obtained during microperimetric macular sensitivity testing (dynamic testing). In both cases, the patient's retina is being tracked and a scatter plot of all fixation locations is generated. There are two fundamentally different methods to describe the cloud of fixation points. The Fujii method uses a circle of 2° or 4° in diameter centered on the barycenter of all fixation points,29 and the BCEA is an ellipse surrounding one, two, or three standard deviations (SD) of all fixation points.11
Figure 1.
Fixation was tested during a separate fixation task (A) where the patient was instructed to focus on the center of a red cross. Blue dots represent the recorded fixation events. Afterward, fixation was determined during a sensitivity exam (B). Here, a higher number of fixation points is available as a result of the longer test duration. Fixation events also seem to be more widely scattered during the sensitivity exam.
Monocular testing was performed with the contralateral eye patched. All patients were instructed to steadily fixate the center of the cross and to use peripheral fixation if necessary. After the subject had located the red fixation target, the fixation task for approximately 30 seconds was started (fixation test). Afterward, macular sensitivity was tested with simultaneous dynamic quantification of fixation (sensitivity test). During both exams, the Nidek MP-1 acquired real-time fundus images with a frequency of 25 Hz providing 25 x- and 25 y-coordinates per second. During the testing, if the MP-1 cannot track the eye, testing is not resumed until the eye can be tracked again, so that the total time of the procedure is often greater than the tracked time. Nidek Navis software generated raw fixation files (.mfd format), which were exported after the test.
Data Analysis
Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) was used to calculate the gravitational center as the mean of the x- and y-coordinates. The number of fixation events within 2° and 4° was calculated (i.e., the “2/4 degree method”) and the Fujii category determined.29 “Stable” meant that at least 75% of all fixation points were within the 2° circle. “Relatively unstable” meant that less than 75% of the points were within the 2° circle but at least 75% were within the 4° circle. “Unstable” meant that less than 75% of all fixation points were within the 4° circle. Additionally, the global BCEA for one, two, and three standard deviations was calculated using the following equation:
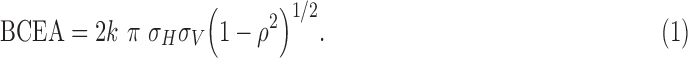 |
σH and σV are the standard deviations of horizontal and vertical eye movements, ρ is the Pearson product–moment correlation coefficient of fixation positions in the horizontal and vertical meridian, k is dependent on the chosen probability area:
 |
And e is the base of the natural logarithm. P is the chosen probability for the SD that the BCEA is based on and the equation is solved for k.
 |
Statistical Analyses
By definition, only this constant value k, which is based on the chosen probability area in the equation BCEA = 2k π σH σV (1 − ρ2)½, separates the 2SD- and 3SD-BCEA from the 1SD-BCEA. All correlation coefficients for 1SD-/2SD-/3SD-BCEA values with all other variables (tracked duration, percentage of points within 2° or 4°, and so on) are thus identical. Therefore, we present the correlations exclusively for the 1SD-BCEA.
The main outcome measures in this investigation were the cloud of fixation points with x- and y-coordinates for each point and the BCVA as measured with the ETDRS system.
Data Management
The central reading center was the Doheny Imaging Reading Center (DIRC) at the Doheny Eye Institute. The data coordinating and data management center (DCC) was the Dana Center for Preventive Ophthalmology, Wilmer Eye Institute, Johns Hopkins University School of Medicine and Johns Hopkins Bloomberg School of Public Health. REDCap databases were used to store the data.22 All Nidek MP-1 instruments and photographers were certified by the DIRC prior to enrollment.
Results
In the prospective ProgStar study, a total of 259 patients and 489 eyes were enrolled at nine centers between October 21, 2013, and January 30, 2015.28 One participating center did not perform microperimetry using the MP-1. Overall, MP-1 testing was graded in at least one eye for 235 patients. Fixation data were missing in several instances and were available from the separate fixation task in 427 eyes and for 418 eyes derived from the sensitivity test. Table 1 shows the demographic features of these patients. The median BCVA was approximately 20/125 Snellen equivalent or 42 ETDRS letters (mean ± SD, 46.3 ± 16.3; range, 20–88).
Table 1.
Patient Characteristics: Patients With at Least One MP-1 Grading
| Patient Characteristics |
Mean ± SD |
Range |
|
Patients With at Least One MP-1 Grading | ||
| Person level, N = 235 | ||
| Age, y | 33.7 ± 15.2 | 7 to 69 |
| Age of onset, y | 20.9 ± 13.9 | 2 to 64 |
| Disease duration, y | 12.7 ± 10.4 | 0 to 61 |
| Count female (%) | 130 (55.3) | |
| Race (%) | ||
| Asian/South Asian | 9 (3.8) | |
| African American | 19 (8.1) | |
| White | 202 (86) | |
| Other | 1 (0.4) | |
| Unknown ethnicity | 4 (1.7) | |
| Eye level, N = 440 | ||
| Best-corrected visual acuity, ETDRS letters | 46.3 ± 16.3 | 20 to 88 |
Overall Summary
The mean area of the BCEA encompassing 1 SD (68.3%) of all fixation events was 4.6 deg2 when measured during the fixation test and significantly larger (12.5 deg2) when measured during the macular sensitivity test, indicating more stable fixation during the fixation test (P < 0.0001, Table 2). This is also reflected by the lower percentages of points within the 2° and 4° circles during the sensitivity test compared with the fixation test (P < 0.0001, Table 2). Using the 2SD- or the 3SD-BCEA, which are distinct from the 1SD-BCEA only through a constant factor, led to proportionately larger areas but identical proportional differences between the fixation test and the sensitivity test. The tracked test duration was aimed at approximately 30 seconds and therefore has a low SD of 5.3 seconds. The percentage of examination time tracked by the Nidek MP-1 was significantly larger during the fixation test when compared to the sensitivity test.
Table 2.
Summary Fixation Stability All Eyes
|
Fixation Stability |
Fixation Test,
N
= 427 |
Sensitivity Test,
N
= 418 |
Estimate |
95% Confidence Interval |
P
Value |
||
|
Mean ± SD |
(Range) |
Mean ± SD |
(Range) |
||||
| 1SD-BCEA, deg2 | 4.6 ± 9.0 | (0.01–134.9) | 12.5 ± 15.7 | (0.04–163.9) | −8.1 | −9.9, −6.4 | <0.0001 |
| 2SD-BCEA, deg2 | 12.5 ± 24.4 | (0.03–365.6) | 33.8 ± 42.6 | (0.10–444.1) | −22.0 | −26.7, −17.4 | <0.0001 |
| 3SD-BCEA, deg2 | 24.0 ± 46.7 | (0.06–699.9) | 64.7 ± 81.6 | (0.19–850.1) | −42.2 | −51.1, −33.2 | <0.0001 |
| Percentage within 2° | 71% ± 28% | (0%–100%) | 48% ± 27% | (0%–100%) | 0.24 | 0.21, 0.26 | <0.0001 |
| Percentage within 4° | 91% ± 16% | (0%–100%) | 77% ± 21% | (3%–100%) | 0.14 | 0.12, 0.17 | <0.0001 |
|
Duration of Test |
Mean ± SD |
(Range) |
Mean ± SD |
(Range) |
|||
| Total duration, s | 36.2 ± 12.2 | (20–142) | 974.1 ± 293.9 | (278–2441) | −941.1 | −974.5, −907.7 | <0.0001 |
| Duration tracked, s | 31.3 ± 5.3 | (11–75) | 751.5 ± 152.8 | (237–1244) | −720.1 | −739.2, −701.0 | <0.0001 |
| Percentage tracked, s | 90% ± 14% | (14%–100%) | 81% ± 16% | (24%–100%) | 0.093 | 0.076, 0.11 | <0.0001 |
| Fujii Category |
Count |
Percent |
Count |
Percent |
|||
| Unstable | 47 | 11% | 158 | 38% | |||
| Relatively unstable | 145 | 34% | 180 | 43% | |||
| Stable | 235 | 55% | 80 | 19% | |||
| Missing | 13 | 22 | |||||
Correlations Among Different Metrics to Describe Fixation Stability
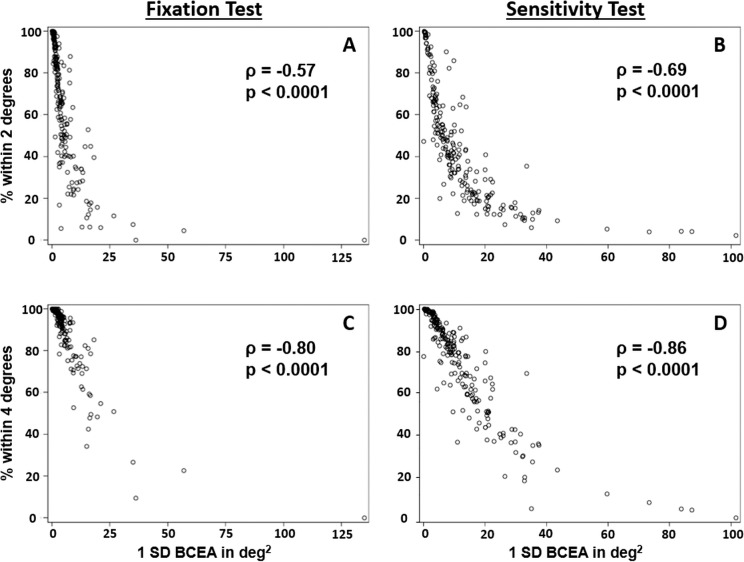
We correlated various measures of fixation stability. The two metrics of describing the cloud of fixation points, the BCEA and the 2/4 degree method, showed moderate to strong correlation when compared within the fixation test or within the sensitivity test (Tables 3, 4). The scatter plots (Fig. 2) demonstrate that 1SD-BCEAs less than 15 deg2 are associated with a wide range of different percentages of fixation events within the 2° circle. There was only a small number of cases with a 1SD-BCEA < 20 deg2 and very unstable fixation with less than 20% of points within 2°, especially in the sensitivity test.
Table 3.
Pearson Correlation Coefficient and P Values of the Correlation of Fixation Stability Parameters From the Separate Fixation Test With Other Fixation Parameters
| Parameters From the Fixation Test |
Data From Fixation Task, Effective Sample Size 228 |
Data From Macular Sensitivity Test, Effective Sample Size 215 |
||||||
| Percentage Within 2° |
Percentage Within 4° |
Percentage of Time Tracked |
Total Duration |
Duration Tracked |
1SD-BCEA |
Percentage Within 2° |
Percentage Within 4° |
|
| 1SD-BCEA | −0.57 | −0.80 | −0.25 | 0.13 | −0.09 | 0.34 | −0.31 | −0.35 |
| <0.0001 | <0.0001 | 0.0001 | 0.0547 | 0.1743 | <0.0001 | <0.0001 | <0.0001 | |
| Percentage within 2° | 1.000 | 0.80 | 0.31 | −0.11 | 0.15 | −0.45 | 0.55 | 0.52 |
| <0.0001 | <0.0001 | 0.0959 | 0.0250 | <0.0001 | <0.0001 | <0.0001 | ||
| Percentage within 4° | 0.80 | 1.000 | 0.34 | −0.14 | 0.15 | −0.45 | 0.41 | 0.48 |
| <0.0001 | <0.0001 | 0.0291 | 0.0259 | <0.0001 | <0.0001 | <0.0001 | ||
Table 4.
Pearson Correlation Coefficient and P Values of the Correlation of Fixation Stability Parameters From the Macular Sensitivity Test With Other Fixation Parameters
| Parameters From the Macular Sensitivity Test |
Data From Macular Sensitivity Test, Effective Sample Size 222 |
Data From Fixation Task, Effective Sample Size 215 |
||||||
| Percentage Within 2° |
Percentage Within 4° |
Percentage of Time Tracked |
Total Duration |
Duration Tracked |
1SD-BCEA |
Percentage Within 2° |
Percentage Within 4° |
|
| 1SD-BCEA | −0.69 | −0.86 | −0.29 | 0.10 | −0.11 | 0.34 | −0.45 | −0.45 |
| <0.0001 | <0.0001 | <0.0001 | 0.1337 | 0.1106 | <0.0001 | <0.0001 | <0.0001 | |
| Percentage within 2° | 1.000 | 0.87 | 0.37 | −0.20 | 0.07 | −0.31 | 0.55 | 0.41 |
| <0.0001 | <0.0001 | 0.0032 | 0.2722 | <0.0001 | <0.0001 | <0.0001 | ||
| Percentage within 4° | 0.87 | 1.000 | 0.35 | −0.18 | 0.09 | −0.35 | 0.52 | 0.48 |
| <0.0001 | <0.0001 | 0.0068 | 0.1841 | <0.0001 | <0.0001 | <0.0001 | ||
Figure 2.
Correlation of the bivariate contour ellipse area with the percentage of fixation points within 2° (A, B) and 4° (C, D) during a separate fixation test (A, C) and the macular sensitivity test (B, D).
The percentages of points within the 2° or 4° circles strongly correlate, especially within the sensitivity test. Correlations were generally weak when fixation measures from the fixation test were compared with the sensitivity test. In addition, correlations of fixation measures with the test duration were very weak if not absent. Weak correlations were found with the proportion of time the Nidek MP-1 was able to track the retina (Tables 3, 4).
Fujii Classification and the BCEA
All eyes were categorized in the corresponding Fujii category (“stable,” “relatively unstable,” or “unstable”) based on the 2° and 4° circles (Table 5). Eyes in the “stable” category had the smallest 1SD-BCEAs with a mean area of 1.3 ± 1.4 deg2 during the fixation test. The 1SD-BCEAs in the “relatively unstable” group (5.6 ± 4.4 deg2) and in the “unstable” group (18.5 ± 20.6 deg2) were larger. However, there was a significant amount of overlap, which is visualized by the box plots in Figure 3. For example, during the fixation test, an eye with a BCEA of 4 deg2 may fall into any of the three Fujii categories.
Table 5.
Fixation Stability as Measured Using the Bivariate Contour Ellipse Area Across Different Categories of the Fujii Classification. Certain Values of the BCEA May Correspond to All Three Fujii Categories (“Unstable,” “Relatively Unstable,” or “Stable”) at the Same Time
|
BCEA Probability Area |
Fujii Categories |
|||||
|
Unstable |
Relatively Unstable |
Stable |
||||
|
Mean ± SD |
Range |
Mean ± SD |
Range |
Mean ± SD |
Range |
|
| BCEA in deg2 during fixation task | N = 47 | N = 145 | N = 235 | |||
| 1SD | 18.5 ± 20.6 | 3.8–134.9 | 5.6 ± 4.4 | 1.4–32.6 | 1.3 ± 1.4 | 0.01–8.5 |
| 2SD | 50.1 ± 55.9 | 10.3–365.6 | 15.0 ± 11.9 | 3.7–88.5 | 3.5 ± 3.8 | 0.03–23.0 |
| 3SD | 95.8 ± 107.0 | 19.7–699.9 | 28.8 ± 22.8 | 7.0–169.3 | 6.7 ± 7.2 | 0.06–44.0 |
| BCEA in deg2 during macular sensitivity test | N = 158 | N = 180 | N = 80 | |||
| 1SD | 24.2 ± 20.3 | 4.4–163.9 | 6.9 ± 3.4 | 0.04–20.1 | 2.0 ± 2.0 | 0.2–9.8 |
| 2SD | 65.5 ± 55.1 | 11.8–444.1 | 18.6 ± 9.3 | 0.10–54.6 | 5.5 ± 5.4 | 0.4–26.6 |
| 3SD | 125.3 ± 105.4 | 22.6–850.1 | 35.5 ± 17.8 | 0.19–104.4 | 10.5 ± 10.4 | 0.8–50.9 |
Figure 3.
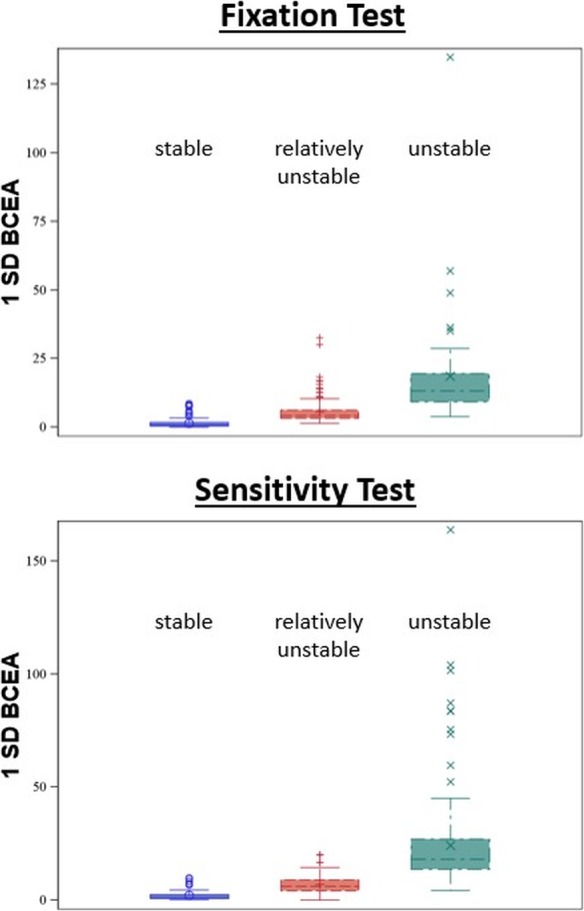
Box plots showing the distribution of different bivariate contour ellipse areas across different Fujii categories. 1SD-BCEA, bivariate contour ellipse area with one standard deviation.
Fixation Stability and Visual Acuity
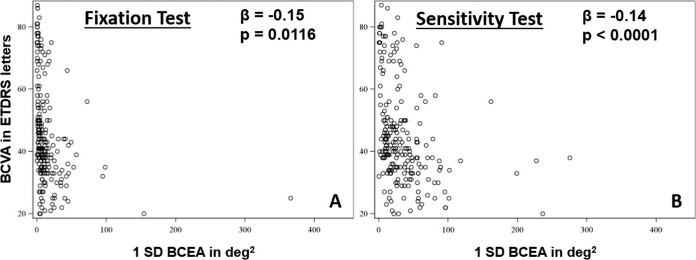
A single linear model best described the relationship of the BCVA with fixation stability during both the fixation and sensitivity test. An increase of 1 deg2 in BCEA during the fixation test was associated with a 0.15-letter (ETDRS) worse BCVA (95% confidence interval [CI], −0.33 to −0.026; P = 0.0116). And an increase of 1 deg2 in BCEA during the sensitivity test was associated with a 0.14-letter (ETDRS) worse BCVA (Fig. 4) (95% CI, −0.75 to −0.020; P < 0.0001).
Figure 4.
Correlation of the bivariate contour ellipse area as measured during the fixation (A) and sensitivity (B) test with visual acuity. BCVA, best-corrected visual acuity; 1SD-BCEA, bivariate contour ellipse area of one standard deviation.
We performed subanalyses of fixation stability based on the BCVA score of that eye (Table 6). For both the fixation test and the sensitivity test, the mean fixation stability is more unstable in poorer BCVA groups. Especially when looking at eyes with a BCVA worse than 20/70, fixation was more unstable during the sensitivity exam. The correlation of the 1SD-BCEA between the fixation test and the sensitivity test was very strong for the few eyes with a BCVA better than 20/25 (ρBCVA ≥ 20/25 = 0.84; P = 0.0167; N = 7). The correlation was weak to moderate for eyes with BCVA between 20/25 and 20/70 (ρBCVA 20/25–20/70 = 0.39; P = 0.0153; N = 39), for eyes with a BCVA between 20/70 and 20/200 (ρBCVA 20/70–20/200 = 0.51; P < 0.0001; N = 115), and for eyes with a BCVA worse than 20/200 (ρBCVA worse than 20/200 = 0.35; P = 0.0088; N = 56).
Table 6.
Summary of 1SD−BCEA, Percentage of Fixation Points Within 2 and 4 Degrees by Visual Acuity Level
| Applied Measure and Visual Acuity Group |
Data From Fixation Test |
Data From Sensitivity Test |
Estimate |
95% CI |
P Value |
||||
|
N |
Mean ± SD (Deg2) |
(Range) (Deg2) |
N |
Mean ± SD (Deg2) |
(Range) (Deg2) |
||||
| 1−SD BCEA | |||||||||
| BCVA ≥ 20/25 | 17 | 0.33 ± 0.28 | (0.04 to 1.22) | 14 | 4.16 ± 4.98 | (0.17 to 15.94) | −3.2 | (−5.5, −0.80) | 0.0087 |
| BCVA 20/25 – 20/70 | 75 | 2.3 ± 5.3 | (0.06 to 36.19) | 72 | 9.16 ± 22.12 | (0.16 to 163.85) | −6.9 | (−11.6, −2.20) | 0.004 |
| BCVA 20/70 – 20/200 | 234 | 4.17 ± 5.48 | (0.01 to 36.2) | 230 | 12.06 ± 13.97 | (0.25 to 103.93) | −8.4 | (−10.6, −6.16) | <.0001 |
| BCVA worse than 20/200 | 101 | 8.12 ± 15.32 | (0.49 to 134.89) | 102 | 16.84 ± 13.96 | (0.04 to 45) | −9.9 | (−13.4, −6.41) | <.0001 |
| Percentage of points within 2 deg | |||||||||
| BCVA ≥ 20/25 | 17 | 99% ± 2% | (94% to 100%) | 14 | 78% ± 28% | (13% to 100%) | 0.18 | (0.04, 0.31) | 0.0125 |
| BCVA 20/25 – 20/70 | 75 | 87% ± 25% | (9% to 100%) | 72 | 73% ± 29% | (10% to 100%) | 0.13 | (0.07, 0.19) | <.0001 |
| BCVA 20/70 – 20/200 | 234 | 71% ± 27% | (0% to 100%) | 230 | 45% ± 23% | (0% to 100%) | 0.26 | (0.23, 0.30) | <.0001 |
| BCVA worse than 20/200 | 101 | 57% ± 27% | (0% to 98%) | 102 | 32% ± 19% | (4% to 81%) | 0.25 | (0.20, 0.30) | <.0001 |
| Percentage of points within 4 deg | |||||||||
| BCVA ≥ 20/25 | 17 | 100% ± 0% | (99% to 100%) | 14 | 92% ± 16% | (43% to 100%) | 0.071 | (0.0004, 0.14) | 0.0488 |
| BCVA 20/25 – 20/70 | 75 | 95% ± 13% | (32% to 100%) | 72 | 88% ± 17% | (34% to 100%) | 0.071 | (0.036, 0.11) | <.0001 |
| BCVA 20/70 – 20/200 | 234 | 92% ± 14% | (10% to 100%) | 230 | 77% ± 20% | (3% to 100%) | 0.15 | (0.13, 0.18) | <.0001 |
| BCVA worse than 20/200 | 101 | 85% ± 21% | (0% to 100%) | 102 | 68% ± 21% | (13% to 99%) | 0.19 | (0.15, 0.24) | <.0001 |
Discussion
Fixation in STGD1 Using Different Metrics and Different Modes of Acquisition
We tested fixation stability, a relatively novel visual function biomarker, in a large cohort of patients with genetically confirmed STGD1 using the Nidek MP-1 both during a dedicated fixation test and during a macular sensitivity exam. Our group recently demonstrated the clinical relevance and the correlation of fixation stability with disease duration, age of onset, and visual acuity in Stargardt disease (Schönbach EM, IOVS 2016;57:ARVO E-Abstract 2694).11 We were also able to demonstrate that fixation parameters are able to detect longitudinal changes of visual function in selected cases where microperimetric sensitivity parameters failed to provide significant changes (Schönbach EM, IOVS 2017;58:ARVO E-Abstract 4635). Possibly, these fixation parameters may serve as secondary outcome measures in future clinical trials.11 It remains unclear whether the most appropriate way to measure fixation stability is during the macular sensitivity test, which is primarily conducted to test macular light sensitivity, or if a separate fixation test is necessary.
By definition, fixation is more stable when the fixation events recorded by the eye-tracking device are spatially closer together. There are different approaches to describe this distribution. The BCEA describes the smallest elliptical area that encompasses one, two, or three SDs of all fixation events while accounting for their horizontal and vertical spread. In the presented dataset, we measured a mean 1SD-BCEA of 4.6 deg2 during the fixation test. This is in line with previous articles that reported a mean fixation stability of 2.4 deg2 in 60 eyes of patients with STGD1.30 We report a mean BCEA of 12.5 deg2 during the sensitivity exam, substantially larger than during the fixation test. We are unaware of any previously published data on fixation stability measurements in STGD1 or any other macular disease during an exam primarily intended to determine macular light sensitivity.
Many research groups describe fixation stability by counting the points within 2° or 4° from the gravitational center.31,32 Using the MP-1 microperimeter and a separate fixation exam, one report on 15 patients with STGD1 found less stable fixation (mean within 2°, 53%; mean within 4°, 69%)30 than our report, which may be due to the older patient age and supposedly more advanced disease in their patient cohort.
Correlation Among the Different Metrics to Describe Fixation Stability
The 2/4 degree method and the BCEA are two different metrics to quantify the spatial proximity of fixation points within the cloud of recorded fixation events. Only the BCEA considers the elliptical distribution of the fixation points. Especially eyes with macular disease33 and amblyopic eyes34 have been shown to present with a more elliptical distribution than normal eyes. In addition, the BCEA can become infinitely small and allows an accurate quantification of fixation stability in the stable end of the spectrum, while the 2/4 degree method is limited by a ceiling effect. Fixation stability outcomes from both methods have been correlated with demographic features,11,32 BCVA,11,30 and fixation location,11,32 but only limited data are available on their correlation with each other. In a small group of eyes with age-related macular degeneration (AMD) undergoing a fixation test, Crossland et al.35 were able to demonstrate a weak linear correlation between the logBCEA and the 2/4 degree method. Our results on a cohort of STGD1 patients are consistent with the article by Crossland et al.35
Correlation Among the Different Acquisition Modes to Describe Fixation Stability
We compared fixation data from the fixation test and the sensitivity test and found significantly more stable fixation during the fixation exam. This result is not surprising because the patients are given different instructions in the two tests. During the short fixation exam, the patient makes an effort to look at the center of the fixation target as steadily as possible, whereas he or she focuses on not missing any displayed light stimuli during the sensitivity test. Furthermore, longer test duration has been shown to adversely affect the stability of fixation,36 possibly due to effects of fatigue. Another explanation is that if patients just keep their eye in one position during the short fixation task, they will be assessed as having stable fixation when they may in fact be attending to something else.
The design of this research introduces an important limitation to the interpretation of our correlations with test duration because the fixation test was aimed at 30 seconds and the sensitivity test was conducted until a threshold for all 68 retinal test locations was determined. However, we were able to find a correlation between fixation stability and the proportion of tracked to total test duration. We expected an association because the retina of unstable eyes is often harder to follow. We found a weak yet important association of the percentage of tracked time with fixation stability (Tables 3, 4).
Fujii Classification and the BCEA
The Fujii classification is a straightforward categorization of fixation stability results and has been extensively discussed in the literature. We add that there is a significant range of overlap between its different categories.17 Our analysis shows that a single BCEA value from the fixation test between 3.8 and 8.5 deg2 can correspond to each of the three Fujii categories, making the Fujii classification less useful. A similar range of overlap could also be found for the sensitivity test (4.4–9.8 deg2). Other disadvantages of this classification include that counting the fixation points within 2° or 4° from the center of gravity does not recognize the presence of two spatially distinct but stable PRLs (preferred retinal locus), which is common in macular diseases.35,37–39 In other words, this method will categorize both eyes with truly poor fixation as well as those with multiple distinct PRLs as unstable, whereas the BCEA has been reported for use in eyes with multiple PRLs. Moreover, the Fujii classification categorizes vastly different degrees of stability in the same group. Both 100% and 75% of points within 2° are considered “stable,” discarding much useful information. We showed in a previous analysis that small deteriorations of fixation stability are associated with a large effect on visual acuity.11 We therefore speculate that this is the reason why studies in AMD showed that the fixation report generated by the MP-1 is very poorly correlated to any parameters of reading.40 The BCEA is a less quantized measure of fixation and better correlated to reading speed.40 Additionally, functional analyses have shown that the BCEA is more closely linked to reading speed than the central 2/4 degree method.40
Association With Visual Acuity
BCVA and fixation stability correlate, and significant associations have been demonstrated in STGD1,11 AMD,17 and infantile nystagmus.41 In a previous article using fixation tests, we showed that an earlier onset of STGD1 is associated with more unstable fixation and that the association with BCVA is most pronounced when fixation is relatively central.11 Also when measured during the sensitivity test, we found significant associations of BCVA with fixation stability (Schönbach EM, IOVS 2016;57:ARVO E-Abstract 2694). However, we are unaware of any previous study comparing stability values from both the fixation test and the sensitivity test. We decided to present visual acuity as an outcome of the stability of fixation. The opposite direction also seems plausible but appears less applicable in clinical practice. Our results show that small BCEAs seem to be associated with a large range of different BCVA scores, regardless of whether the results are derived from a dedicated fixation exam or from the sensitivity test. Stable fixation (BCEA ≤ 50 deg2), regardless of its origin from a fixation test or sensitivity test, is almost universally associated with a good BCVA score (BCVA > 60 ETDRS letters), whereas less stable fixation tends to more often be associated with poor BCVA. Our results suggest that fixation data derived from either the fixation or the sensitivity test can reasonably describe the fixation aspect of vision and both tests correlate with BCVA. However, only the association of fixation stability from the sensitivity test was significantly associated with BCVA. One possible explanation is that patients may be able to keep their eyes straight during the short fixation task with a marked underestimate of the BCEA. The higher percentage of tracked test duration during the fixation test supports this theory. From this perspective, the fixation data from the sensitivity test seem more useful, and the continuous measures more than the categorical measures. Fixation data from the sensitivity test may be more unstable, but they give a rough idea of what the outcome of the fixation test will be. Since many similarities exist between STGD1 and other macular diseases, the presented principles may also apply to, for example, AMD.
Limitations
As mentioned earlier, important differences between the fixation test and the full microperimetry exam introduce limitations to this research. Patients pay attention to different aspects in both tests (fixating only the red cross versus fixating the red cross and focusing on displayed light stimuli at the same time).42,43 The analysis also assumes that fixation is not wandering and that a very high number of fixation events is comparable to a much lower number. Not surprisingly, the tests do not correlate very well, and stable fixation during a short test does not necessarily predict stable fixation during a light detection task. However, in light of the paucity of literature on this subject, the primary purpose of this article was to provide a comparison between the two tests and the different measures for the clinician, although there are limitations, from a physiological standpoint for the mentioned reasons.
Conclusions
Our results demonstrate that the continuous BCEA has certain advantages over both the 2/4 degree method and the Fujii classification. The latter two seem of little use when fixation is in a certain range. The BCEA from both the separate fixation and the sensitivity exam are functional biomarkers that correlate with each other and with BCVA. But only the data from the sensitivity exam showed a statistically significant association with BCVA. Data from both exams seem to describe the fixation dimension of vision reasonably well. BCEA values from the sensitivity test are larger, and one explanation is that a patient can keep his or her eye in one position during the short fixation task and will be assessed as having stable fixation when in fact he or she is attending to something else. We conclude that the separate fixation exam may be redundant when fixation is also tested during the sensitivity exam. Further steps to validate these biomarkers include investigation of their usefulness to monitor disease progression in longitudinal studies and to test if changes of fixation stability correlate with other outcome parameters.
Acknowledgments
Supported by the Foundation Fighting Blindness Clinical Research Institute (FFB CRI, The ProgStar studies) and a grant to FFB CRI by the U.S. Department of Defense U.S. Army Medical Research and Material Command: The Telemedicine and Advanced Technology Research Center, Fort Meade, Maryland, United States (Grants W81-XWH−07-1−0720 and W81XWH−09-2−0189); The Shulsky Foundation, New York, New York, United States; Ocular Albinism Research Fund (Clark Enterprises, Inc.); unrestricted grant to the Wilmer Eye Institute from Research to Prevent Blindness; Baylor-Johns Hopkins Center for Mendelian Genetics (National Human Genome Research Institute, NHGRI/NIH); the Leopoldina Fellowship Program, German National Academy of Sciences (Halle, Germany, Grant LPDS 2015-14) (EMS); the Austrian Science Fund (FWF; Project no. J 3383-B23) (RWS). The authors alone are responsible for the content and writing of this paper.
Disclosure: E.M. Schönbach, German National Academy of Sciences Leopoldina (F); M.A. Ibrahim, None; X. Kong, None; R.W. Strauss, None; B. Muñoz, None; D.G. Birch, None; J.S. Sunness, None; S.K. West, Alcon Research Institute (S), Research to Prevent Blindness (S); H.P.N. Scholl, Acucela, Inc. (F), QLT, Inc. (F), NightstaRx, Ltd. (F), Astellas Institute for Regenerative Medicine (C), Boehringer Ingelheim Pharma GmbH & Co. KG (C), Coleman Research Group, Inc. (C), Daiichi Sankyo (C), DeMatteo Monness Consulting (C), Gerson Lehrman Group (C), Guidepoint Global, LLC (C), Intellia Therapeutics, Inc. (C), Ocata Therapeutics, Inc. (C), Shire (C), F. Hoffmann-La Roche, Ltd. (R, S), Genentech, Inc. (R, S), Genzyme, Corp. (R, S), Ora, Inc. (R, S), ReNeuron Group, Plc. (R, S), Sanofi (R, S), Clinical Advisory Board of Gensight Biologics (S), Food and Drug Administration (S), Ophthalmic Devices Panel of the Medical Devices Advisory Committee (S), Scientific Advisory Board of Vision Medicines, Inc. (S)
References
- 1.Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30:63–68. doi: 10.1080/13816810802695550. [DOI] [PubMed] [Google Scholar]
- 2.van Huet RA, Bax NM, Westeneng-van Haaften SC, et al. Foveal sparing in Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55:7467–7478. doi: 10.1167/iovs.13-13825. [DOI] [PubMed] [Google Scholar]
- 3.Nakao T, Tsujikawa M, Sawa M, Gomi F, Nishida K. Foveal sparing in patients with Japanese Stargardt's disease and good visual acuity. Jpn J Ophthalmol. 2012;56:584–588. doi: 10.1007/s10384-012-0172-1. [DOI] [PubMed] [Google Scholar]
- 4.Westeneng-van Haaften SC, Boon CJ, Cremers FP, Hoefsloot LH, den Hollander AI, Hoyng CB. Clinical and genetic characteristics of late-onset Stargardt's disease. Ophthalmology. 2012;119:1199–1210. doi: 10.1016/j.ophtha.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101:25–30. doi: 10.1136/bjophthalmol-2016-308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feucht N, Schonbach EM, Lanzl I, Kotliar K, Lohmann CP, Maier M. Changes in the foveal microstructure after intravitreal bevacizumab application in patients with retinal vascular disease. Clin Ophthalmol. 2013;7:173–178. doi: 10.2147/OPTH.S37544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishman GA, Farber M, Patel BS, Derlacki DJ. Visual acuity loss in patients with Stargardt's macular dystrophy. Ophthalmology. 1987;94:809–814. doi: 10.1016/s0161-6420(87)33533-x. [DOI] [PubMed] [Google Scholar]
- 8.Kong X, Strauss RW, Michaelides M, et al. Visual acuity loss and associated risk factors in the retrospective Progression of Stargardt Disease Study (ProgStar Report No. 2) Ophthalmology. 2016;123:1887–1897. doi: 10.1016/j.ophtha.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Rigoni E, Cacciotti V, Trabucco P. Normal values and repeatability of bivariate contour ellipse area (BCEA) in microperimeter MP-1. Low Vision J. 2014;1:1. [Google Scholar]
- 10.Bellmann C, Feely M, Crossland MD, Kabanarou SA, Rubin GS. Fixation stability using central and pericentral fixation targets in patients with age-related macular degeneration. Ophthalmology. 2004;111:2265–2270. doi: 10.1016/j.ophtha.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Schönbach EM, Ibrahim MA, Strauss RW, et al. Fixation location and stability using the MP1 microperimeter in Stargardt disease: ProgStar Report No. 3. Ophthalmol Retina. 2016;1:68–76. doi: 10.1016/j.oret.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic Physiol Opt. 2004;24:327–333. doi: 10.1111/j.1475-1313.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 13.Sunness JS, Applegate CA, Haselwood D, Rubin GS. Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology. 1996;103:1458–1466. doi: 10.1016/s0161-6420(96)30483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson UL, Frennesson C, Nilsson SE. Location and stability of a newly established eccentric retinal locus suitable for reading, achieved through training of patients with a dense central scotoma. Optom Vis Sci. 1998;75:873–878. doi: 10.1097/00006324-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson UL, Frennesson C, Nilsson SE. Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vision Res. 2003;43:1777–1787. doi: 10.1016/s0042-6989(03)00219-0. [DOI] [PubMed] [Google Scholar]
- 16.Tarita-Nistor L, Gonzalez EG, Markowitz SN, Steinbach MJ. Plasticity of fixation in patients with central vision loss. Vis Neurosci. 2009;26:487–494. doi: 10.1017/S0952523809990265. [DOI] [PubMed] [Google Scholar]
- 17.Tarita-Nistor L, Gonzalez EG, Markowitz SN, Steinbach MJ. Fixation characteristics of patients with macular degeneration recorded with the mp-1 microperimeter. Retina. 2008;28:125–133. doi: 10.1097/IAE.0b013e3180ed4571. [DOI] [PubMed] [Google Scholar]
- 18.Macedo AF, Crossland MD, Rubin GS. Investigating unstable fixation in patients with macular disease. Invest Ophthalmol Vis Sci. 2011;52:1275–1280. doi: 10.1167/iovs.09-4334. [DOI] [PubMed] [Google Scholar]
- 19.Plank T, Frolo J, Brandl-Ruhle S, et al. Gray matter alterations in visual cortex of patients with loss of central vision due to hereditary retinal dystrophies. Neuroimage. 2011;56:1556–1565. doi: 10.1016/j.neuroimage.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Bittencourt MG, Sophie R, et al. Fixation stability measurement using two types of microperimetry devices. Trans Vis Sci Tech. 2015;4(2):3. doi: 10.1167/tvst.4.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarita-Nistor L, Gonzalez EG, Mandelcorn MS, Lillakas L, Steinbach MJ. Fixation stability, fixation location, and visual acuity after successful macular hole surgery. Invest Ophthalmol Vis Sci. 2009;50:84–89. doi: 10.1167/iovs.08-2342. [DOI] [PubMed] [Google Scholar]
- 22.Scholl HP, Strauss RW, Singh MS, et al. Emerging therapies for inherited retinal degeneration. Sci Transl Med. 2016;8:368rv6. doi: 10.1126/scitranslmed.aaf2838. [DOI] [PubMed] [Google Scholar]
- 23.Scholl HP, Sahel JA. Gene therapy arrives at the macula. Lancet. 2014;383:1105–1107. doi: 10.1016/S0140-6736(14)60033-7. [DOI] [PubMed] [Google Scholar]
- 24.Schonbach EM, Scholl HP. Fundus autofluorescence in a subclinical case of Best disease. Retinal Cases Brief Rep. 2016;11:S159–S162. doi: 10.1097/ICB.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 25.Kong X, West SK, Strauss RW, et al. Progression of visual acuity and fundus autofluorescence in recent-onset Stargardt disease: ProgStar Study Report. Ophthalmol Retina. 2017] doi: 10.1016/j.oret.2017.02.008. #4 [published online ahead of print April 28, [DOI] [PubMed]
- 26.Schonbach EM, Wolfson Y, Strauss RW, et al. Macular sensitivity measured with microperimetry in Stargardt disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: Report No. 7. JAMA Ophthalmol. 2017;135:696–703. doi: 10.1001/jamaophthalmol.2017.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schonbach EM, Chaikitmongkol V, Annam R, et al. 7-Hexagon multifocal electroretinography for an objective functional assessment of the macula in 14 seconds. Ophthalmic Res. 2017;58:117–124. doi: 10.1159/000475996. [DOI] [PubMed] [Google Scholar]
- 28.Strauss RW, Ho A, Munoz B, et al. The natural history of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Studies: design and baseline characteristics: ProgStar Report No. 1. Ophthalmology. 2016;123:817–828. doi: 10.1016/j.ophtha.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Fujii GY, de Juan E, Jr,, Sunness J, Humayun MS, Pieramici DJ, Chang TS. Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology. 2002;109:1737–1744. doi: 10.1016/s0161-6420(02)01120-x. [DOI] [PubMed] [Google Scholar]
- 30.Reinhard J, Messias A, Dietz K, et al. Quantifying fixation in patients with Stargardt disease. Vision Res. 2007;47:2076–2085. doi: 10.1016/j.visres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Plank T, Frolo J, Farzana F, Brandl-Ruhle S, Renner AB, Greenlee MW. Neural correlates of visual search in patients with hereditary retinal dystrophies. Hum Brain Mapp. 2013;34:2607–2623. doi: 10.1002/hbm.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Testa F, Melillo P, Di Iorio V, et al. Macular function and morphologic features in juvenile Stargardt disease: longitudinal study. Ophthalmology. 2014;121:2399–2405. doi: 10.1016/j.ophtha.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabanarou SA, Crossland MD, Bellmann C, Rees A, Culham LE, Rubin GS. Gaze changes with binocular versus monocular viewing in age-related macular degeneration. Ophthalmology. 2006;113:2251–2258. doi: 10.1016/j.ophtha.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci. 2013;54:1998–2003. doi: 10.1167/iovs.12-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crossland MD, Culham LE, Kabanarou SA, Rubin GS. Preferred retinal locus development in patients with macular disease. Ophthalmology. 2005;112:1579–1585. doi: 10.1016/j.ophtha.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Longhin E, Convento E, Pilotto E, et al. Static and dynamic retinal fixation stability in microperimetry. Can J Ophthalmol. 2013;48:375–380. doi: 10.1016/j.jcjo.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Invest Ophthalmol Vis Sci. 1988;29:268–278. [PubMed] [Google Scholar]
- 38.Deruaz A, Whatham AR, Mermoud C, Safran AB. Reading with multiple preferred retinal loci: implications for training a more efficient reading strategy. Vision Res. 2002;42:2947–2957. doi: 10.1016/s0042-6989(02)00354-1. [DOI] [PubMed] [Google Scholar]
- 39.Lei H, Schuchard RA. Using two preferred retinal loci for different lighting conditions in patients with central scotomas. Invest Ophthalmol Vis Sci. 1997;38:1812–1818. [PubMed] [Google Scholar]
- 40.Crossland MD, Dunbar HM, Rubin GS. Fixation stability measurement using the MP1 microperimeter. Retina. 2009;29:651–656. doi: 10.1097/IAE.0b013e318196bd65. [DOI] [PubMed] [Google Scholar]
- 41.Dell'Osso LF, Leigh RJ, Sheth NV, Daroff RB. Two types of foveation strategy in “latent” nystagmus: fixation, visual acuity and stability. Neuroophthalmology. 1995;15:167–186. doi: 10.3109/01658109509044600. [DOI] [PubMed] [Google Scholar]
- 42.Behrend C, Schonbach E, Coombs A, Coyne E, Prasarn M, Rechtine G. Smoking cessation related to improved patient-reported pain scores following spinal care in geriatric patients. Geriatr Orthop Surg Rehabil. 2014;5:191–194. doi: 10.1177/2151458514550479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behrend CJ, Schonbach EM, Vaccaro AR, Coyne E, Prasarn ML, Rechtine GR. Maximum pain on visual analog scales in spinal disorders. Spine J. 2016;17:1061–1065. doi: 10.1016/j.spinee.2016.11.017. [DOI] [PubMed] [Google Scholar]