Abstract
AMD is a major cause of legal blindness in older adults approachable through multidisciplinary research involving human tissues and patients. AMD is a vascular-metabolic-inflammatory disease, in which two sets of extracellular deposits, soft drusen/basal linear deposit (BLinD) and subretinal drusenoid deposit (SDD), confer risk for end-stages of atrophy and neovascularization. Understanding how deposits form can lead to insights for new preventions and therapy. The topographic correspondence of BLinD and SDD with cones and rods, respectively, suggest newly realized exchange pathways among outer retinal cells and across Bruch's membrane and the subretinal space, in service of highly evolved, eye-specific physiology. This review focuses on soft drusen/BLinD, summarizing evidence that a major ultrastructural component is large apolipoprotein B,E-containing, cholesterol-rich lipoproteins secreted by the retinal pigment epithelium (RPE) that offload unneeded lipids of dietary and outer segment origin to create an atherosclerosis-like progression in the subRPE-basal lamina space. Clinical observations and an RPE cell culture system combine to suggest that soft drusen/BLinD form when secretions of functional RPE back up in the subRPE-basal lamina space by impaired egress across aged Bruch's membrane-choriocapillary endothelium. The soft drusen lifecycle includes growth, anterior migration of RPE atop drusen, then collapse, and atrophy. Proof-of-concept studies in humans and animal models suggest that targeting the “Oil Spill in Bruch's membrane” offers promise of treating a process in early AMD that underlies progression to both end-stages. A companion article addresses the antecedents of soft drusen within the biology of the macula.
Keywords: age-related macular degeneration, drusen, atrophy, lipoproteins, cholesterol, retinal pigment epithelium, Bruch's membrane, apolipoprotein mimetic, statin, non-human primate, mouse models
Introduction and Synopsis
AMD is a major cause of legal blindness in older adults approachable through multidisciplinary research involving human tissues and patients via clinical imaging and genetics. The central theses of this review are as follows:
Soft drusen and basal linear deposit (BLinD) are two forms of the same extracellular lipid rich material that together make up an Oil Spill on Bruch's membrane (BrM). Drusen are defined in reference to a three-layer BrM and in distinction to other entities that are not drusen;
AMD is a vascular-metabolic-inflammatory disease in which soft drusen/BLinD and subretinal drusenoid deposit (SDD; also called reticular pseudodrusen) are major risk factors for progression to end-stages of atrophy and neovascularization that involve substantial loss of retinal pigment epithelium (RPE) and photoreceptors1;
The topographic relation of soft drusen/BLinD to cones and SDD to rods strongly suggests that deposit biogenesis reflects newly realized exchange pathways among cones, rods, RPE, Müller cells, and choriocapillary endothelium, across BrM and the subretinal space, in service of highly evolved, eye-specific physiology;
A major component of soft drusen/BLinD is lipoprotein particles containing apolipoproteins B and E, secreted by RPE in a physiologic lipid-recycling program. The composition suggests a dual origin of lipids (fatty acids from diet, cholesterol from diet and photoreceptor outer segments);
Clinical imaging and an RPE cell culture system together define a druse lifecycle to which RPE demise can be linked. Soft drusen/BLinD form when secretions of functional RPE back up in the subRPE-basal lamina space, because egress across aged BrM-choriocapillary endothelium is impaired. Drusen can expand in volume, RPE migrate off the top into the retina, leading to disintegration of the RPE layer, druse collapse, and atrophy;
The Oil Spill strategies for druse abatement to forestall type 1 neovascularization and geographic atrophy have supportive preclinical and clinical data; and
Understanding outer retinal physiology driving lipoprotein production has potential to advance treatments as impactful for AMD as statins have been for atherosclerotic cardiovascular disease; relevant model systems exist.
This conceptual framework directs attention to understanding the formation and clearing of drusen as a basis for targeting precursors pharmacologically to delay end-stages. The overall hypothesis is limited to discussion of soft drusen (and their differential diagnoses) and should be contextualized among other known contributors to AMD pathobiology. These include aging in the choroidal vasculature,2 inflammation, and activity of resident/transient immune cells,3 among others. Many mechanisms operating simultaneously give rise to AMD's complexity. Validated multimodal clinical imaging offers bright prospects for connecting disparate pieces in a coherent timeline to clarify therapeutic strategies. Despite knowledge gaps, enough is known about soft drusen/BLinD biology to launch new approaches. A companion article considers what aspects of macular biology drive soft drusen biogenesis.4
Neurobiology and Aging of the Macula
A neurovascular unit5,6 comprises microvessels, neurons, glia, pericytes, and extracellular matrix that link blood flow to the metabolic demands of neurons. The cells and tissues most prominently affected by AMD pathology are those of the outer retinal neurovascular unit7 (i.e., photoreceptors, RPE, Müller cells [in neurosensory retina], and the choriocapillaris [ChC] endothelium [in the choroidal vasculature]). The choroid has the highest blood flow in the body, and the choriocapillaris is sinusoidal and fenestrated. Between RPE and ChC is a laminated subendothelial extracellular matrix called Bruch's membrane (BrM), which functions as a vessel wall laid out flat, paralleling vascular lumens.8 The RPE is a monolayer of cuboidal polygonal cells embedded between photoreceptors and BrM. Strong apical to basolateral polarization makes the RPE a key player in the homeostasis of photoreceptors and the pathology of SDD apically and choriocapillaris and the pathology of drusen basally. The macular neurosensory retina consists of a 0.8-mm diameter all-cone fovea surrounded by a rod-dominated annulus of 6-mm outer diameter. The Henle fiber layer contains inner fibers of photoreceptors and Müller glia that form junctions at the external limiting membrane. Among numerous Müller cell functions9 are recently recognized roles in delivering to cones for phototransduction vitamin A derivatives of dietary origin.10,11 Xanthophyll pigments lutein and zeaxanthin are prominent in the foveal center, and lutein, in the Henle fiber and inner plexiform layers.12,13 A hypothesis that Müller cells are major xanthophyll reservoirs is explored separately.4
Of major age-related tissues changes detailed separately,4 we focus on BrM, where AMD pathology is prominent, including cross-linking,14 thickening,15 and lipidization,16–18 and loss of ChC density and apposition to BrM.19 The lipidization of BrM provides a straightforward path to lipids in soft drusen, arguably the first druse component described.20–24 Lipid accumulation in vessel walls connects to both the pathophysiology of atherosclerotic cardiovascular disease25 and the clinical success in reducing its public health burden.26
Defining the Layers of AMD
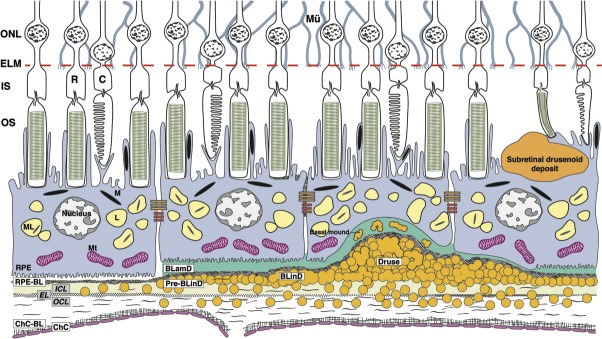
A cellular- and molecular-level understanding of drusen begins with delicate tissue layers in the RPE-BrM-ChC complex and adjoining potential spaces (Fig. 1). The anatomic definition of BrM27 is five layers (from inner to outer), RPE-basal lamina (BL) and inner collagenous, elastic, and outer collagenous layers (inner collagenous layer [ICL], elastic layer [EL], outer collagenous layer [OCL]). Pathology may be best understood with the Sarks-Gass concept of a three-layer BrM (ICL+EL+OCL) that does not include the RPE and ChC basal laminas, thereby defining the subRPE-BL space between the RPE-BL and the ICL. Drusen are focal deposits located between the RPE-BL and the ICL of BrM, in the subRPE-BL space. BLinD is a thin layer of soft druse material, in the same compartment. This framework facilitates explaining the participation of basal laminar deposit (BLamD) in clinical AMD, the trajectory of type 1 (subRPE-BL) neovascularization, the differing embryologic origins of RPE-BL versus ICL+EL+OCL, and Mendelian disorders preferentially affecting the RPE-BL28–30 versus structural elastin and collagen.31,32 By this definition, the Oil Spill in aging BrM33 becomes the Oil Spill on BrM.
Figure 1.
AMD by the layers. BrM consists of the ICL, EL, and OCL. Soft drusen and BLinD are two forms (lump and layer) of the same AMD-specific extracellular deposit. BLamD is a thickening of the RPE-BL. Basal mound is soft druse material within BLamD. Subretinal drusenoid deposit localizes to the subretinal space (between photoreceptors and RPE). RPE cells contain melanosomes, lipofuscin and melanolipofuscin, and mitochondria that provide signals for color fundus photography, fundus autofluorescence, and OCTs. Abbreviations from inner to outer: ONL, outer nuclear layer; ELM, external limiting membrane; IS, inner segments of photoreceptors; OS, outer segments of photoreceptors; R, rods; C, cones; L, lipofuscin; M, melanosome; ML, melanolipofuscin; Mt, mitochondria; Mu, Müller glia; circles, lipoprotein particles.
Drusen are focal and can be recognized clinically. In contrast BLinD is thin and diffusely distributed, poorly visible in paraffin histology, and invisible clinically, leading to a common misperception that BrM thickens in AMD when in fact new layers are interposed (Fig. 1).
Imaging, Epidemiology, and the Expanding Spectrum of Drusen
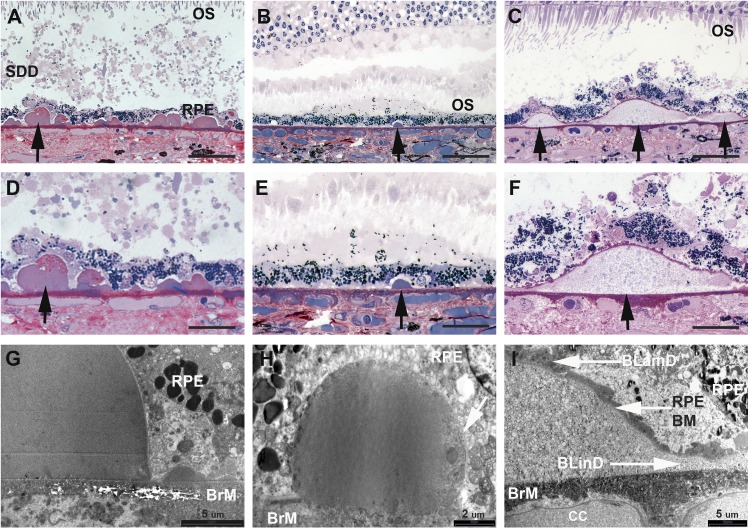
Drusen are the major intraocular risk factor for progression, and how they are detected clinically impacts theories of their formation and significance.34 Drusen were linked to end-stages of geographic atrophy (GA) and neovascularization on a time course of years by Gass using color fundus photography (CFP) and fluorescein angiography,35 as repeated in large samples.36,37 Major epidemiologic studies of European-derived populations since 1991 are based on standardized CFP-based grading systems.38 Soft drusen are yellow-white elevations ranging from 30 μm to more than 1000 μm in diameter with an indistinct border due to sloping sides (Figs. 2C, 2F, 2I).39 Numerous hard drusen (Figs. 2B, 2E, 2H) and cuticular drusen (originally called basal laminar drusen [Figs. 2A, 2D, 2G]) increase risk in the aggregate and over the long term (15 years), in part by increasing risk for soft drusen.40–42 East and South Asian populations have low prevalence of typical drusen but progress to neovascularization.43,44
Figure 2.
Drusen types in AMD macula have distinct geometry and ultrastructure. Cuticular, small hard and soft drusen in (A–F) high-resolution light microscopy and (G–I) transmission electron microscopy. (A–C) Drusen are found between the RPE-BL and the inner collagenous layer of BrM). Hard drusen and cuticular drusen are small with steep sides and contain dense hyalinized contents; cuticular drusen are numerous. Soft drusen are large and have sloping sides. BLamD and BLinD associate only with soft drusen (F) and not (D) cuticular drusen or (E) small, hard drusen. (G, H) Cuticular and small hard drusen are homogenous and electron dense, with small vacuoles attributed to extracted lipids distributed throughout. (I) Soft drusen are packed with ‘membranous debris' (considered partially preserved lipoproteins) and are continuous with BLinD, giving rise to a ‘soft' appearance in the fundus. Detachment of the retina from RPE is a postmortem artifact. Black arrows denote individual drusen. Images (A, D, G) taken from macular sections of the left eye of the patient with cuticular drusen. Scale bars: (A–C) 60, (D–F) 30, (G) 5, (H) 2, and (I) 5 μm. BM, basement membrane; CC, choriocapillaris. Reprinted with permission from Balaratnasingam C, Cherepanoff S, Dolz-Marco R, et al. Cuticular drusen: Clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology. 2018;125:100–118. © 2017 by the American Academy of Ophthalmology.
Spectral-domain optical coherence tomography (OCT), commercialized in 2007, is an interferometry technique using low-coherence light to achieve depth-resolved, comprehensive, and noninvasive cross-sectional views of chorioretinal structure. Advancements, such as eye tracking and signal averaging, combine to make cross-sectional structural OCT the base modality for AMD clinical trials going forward.45 By OCT, soft drusen are dome-shaped RPE elevations with homogenous and moderately reflective “ground-glass” interiors internal to BrM, which appears at the druse base as a fine reflective line. In small cohorts examined so far, soft drusen are the most common among macular druse types.46 Internal structure in soft drusen visible on OCT signify risk for progression,47–51 and approximately 10% of soft drusen may have subclinical (nonexudative) neovascularization.52 A spectrum of RPE elevations now exists.53–59 By the gold standard of histology of clinically documented cases (Fig. 2),40,60–63 hard and cuticular drusen are ultrastructurally similar, small, globular deposits 30 to 60 μm in diameter. Cuticular drusen are numerous in generally younger patients, exhibiting imaging signs of RPE attenuation at the apices.64
A major limitation to current estimates of progression risk is the recent recognition of extracellular deposits in the subretinal space, between photoreceptors and RPE, first called reticular pseudodrusen65 and recently, SDD.66,67 SDD is biologically distinct and not just drusen in the wrong place34 (see the Subretinal Drusenoid Deposits: Extracellular, Space-Filling, Distinct From Drusen section). SDD were in part misclassified as soft drusen or omitted altogether from five CFP-based grading systems36–38,68–71 that underlie prevalence estimates, risk models, and genetic associations. Thus, risk attributed to soft drusen in some CFP-based grading systems is aggregate risk of soft drusen plus SDD. All literature must therefore be interpreted anew—do authors mean subretinal or subRPE? Did study eyes have SDD? Consequently, experimental studies must include highly polarized RPE cells for greatest AMD relevance (see the Model Systems for Mechanistic and Translational Drusen Research section).
Introduction to Cholesterol and Lipoproteins
Because ample multidisciplinary evidence supports lipoprotein particles as a major component of soft drusen, we introduce the chemistry and biology of cholesterol and lipoproteins (Fig. 3); comprehensive reviews are available.72,73 Cholesterol is a lipid with a hydrophobic four-ring system. A 3β-hydroxyl group binds long-chain fatty acids to form esters. We refer to unesterified and esterified cholesterol (UC and EC), respectively. EC, accounting for approximately 70% of total cholesterol in humans, is used for storage and transport. UC is essential to all animal cells in roles of membrane integrity, fluidity, and permeability. Membrane UC is intercalated among phospholipids (PL) and concentrated in lipid rafts to influence many cellular activities, include gene transcription, nerve conduction, and synaptogenesis. Three physical forms—oily droplets, lamellar membranes, and monohydrate crystals—differ in the relative proportions of EC, UC, and PL. For transport through plasma and interstitial fluid, UC and EC form with apolipoproteins, PL, and triglycerides (TG) spherical multimolecular complexes called lipoproteins. Plasma lipoprotein classes identified by ultracentrifugation include (from large to small) chylomicrons (CM), very low-density (VLDL), low-density (LDL), and high-density (HDL) lipoproteins. Apolipoprotein B-100 (apoB-100) is the principal protein of LDL and is present with apoE in VLDL of hepatic origin, which is the parent particle of LDL. Apolipoprotein A-I (apoA-I) is the principal protein of plasma HDL. Brain HDL lipoproteins are rich in apoE.74 Cross talk between plasma lipoproteins and complement components is under investigation.73
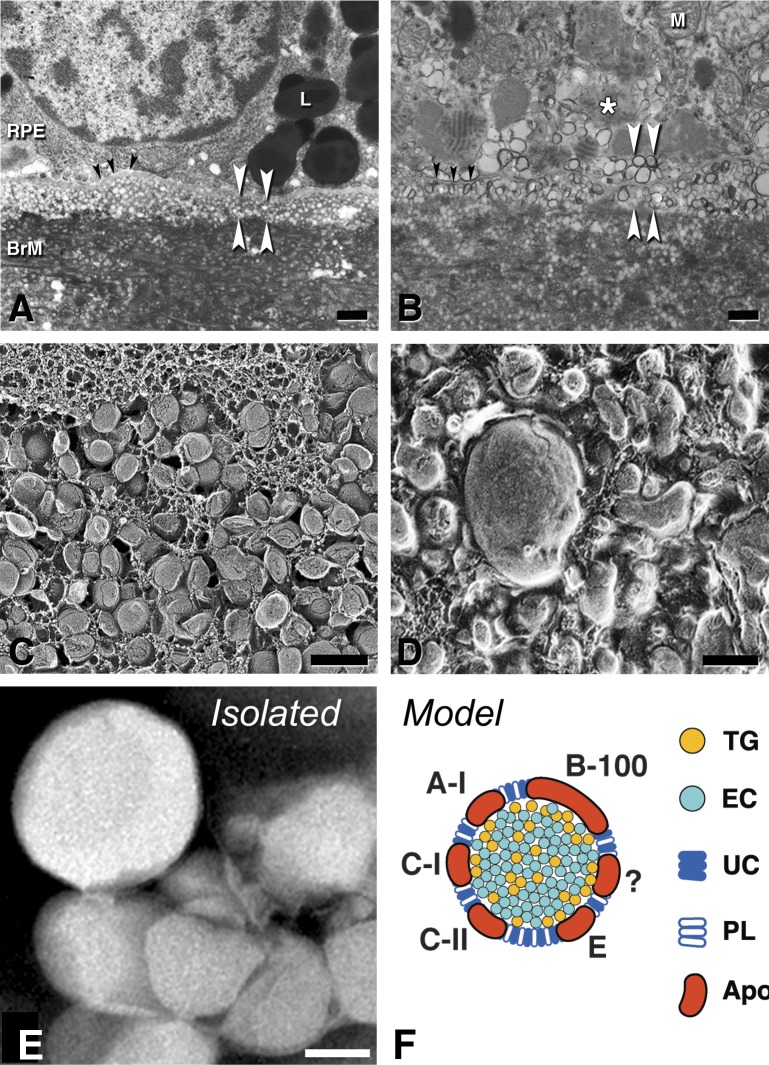
Figure 3.
A major component of BLinD and pre-BLinD is lipoproteins. Black arrowheads, RPE-BL; white arrowhead, subRPE-basal laminar space. (A, B) Thin-section transmission electron microscopy; osmium postfixation, vertical cross-section, scale bars: 1 μm. (C, D) Quick freeze deep etch microscopy; en face fracture plane of subRPE-basal laminar space; scale bars: 200 nm. (A, C) Pre-BLinD is a layer of lipoproteins 3 to 4 deep in the subRPE-BL space of many older eyes. Lipoproteins are spherical particles of uniform diameter with surface-and-core morphology. They were originally described as vesicles in osmium postfixed specimens (A). RPE, (B, D) BLinD in an eye with geographic atrophy is a mixture of native and fused lipoprotein particles, with lipid pools, in the same plane, originally described as membranous debris in osmium postfixed specimens (B). (E) Lipoprotein particles isolated from BrM are large and spherical; negative stain,144 scale bar: 50 nm. (F) BrM lipoprotein composition inferred from direct assay,97,144 druse composition, and RPE gene expression.135,358 Apo, apolipoproteins. ?, as-yet-unknown apolipoproteins.
Cells have many ways to efflux UC, and RPE may be capable of all, because some evidence currently exists for many. These include transfer to circulating HDL,75 complexing with endogenously synthesized apolipoproteins, conversion to an oxysterol capable of passing through cellular membranes,76 and release as microvesicles (budding of plasma membrane) or exosomes (trafficked from endosomes).7 Conversely, lipoprotein particles and milk fat represent the only known ways by which cells release EC.
Genetics and Gene Expression Studies Relevant to Lipids
AMD's major genetic associations are complement factor H (CFH)78 and ARMS2, a gene with an uncertain function, now separated statistically from HTRA1, also on chromosome 10.79 Among pathways, lipids are the most highly implicated after complement.80 Candidate gene studies reported an association with AMD of single nucleotide polymorphisms (SNP) in APOE.81,82 Genome-wide association studies (GWAS) later also identified SNPs associated with advanced AMD in CETP, ABCA1, and LIPC, best known from plasma HDL homeostasis.83,84 The International Age-related Macular Degeneration Genomics Consortium found associations of these genes with AMD (n = 16,144 cases and 17,832 controls) but with not elevated levels of plasma HDL85 (see Refs. 86–88). The Consortium dataset was probed via Mendelian randomization,89 which showed that three variants of genes associated with plasma lipid levels (LIPC, 2; CETP, 1) reached genome-level significance, placing AMD between cardiovascular disease and Alzheimer disease in the strength of lipid gene associations. SNPs in LIPC and ABCA1 are associated with intermediate and large drusen, and CFH, C3, C2, and ARMS2/HTRA1, large drusen.90 A rare CFH variant is associated with abundant soft drusen,91 and two CFH SNPs, with greater drusen area in central macula.92
These studies and others72,93 suggest that lipid genes impact AMD risk significantly, yet independent of, or even reverse to, plasma lipoprotein profiles from cardiovascular disease, a paradox likely related to the existence of intraocular regulatory mechanisms. In normal human donor eyes, microarray93 and comprehensive RNA-sequencing94 analysis demonstrated that scores of genes controlling all aspects of cholesterol and lipoprotein homeostasis are expressed in both neurosensory retina and RPE. Immunolocalization using validated antibodies and polarized RPE (in vivo or high-fidelity culture, Refs. in 34) include APOE (photoreceptor outer segments, RPE, Müller cells, drusen, and SDD); ABCA1 (diffuse labeling of RPE cell bodies); CETP (photoreceptor outer segments and outer plexiform layer (OPL), with some labeling in the choroid); LIPC (all retinal neurons including photoreceptors and ganglion cells plus RPE, and not in Müller cells). Thus, theories of AMD pathogenesis based on genes well studied in liver, intestine, adipose tissue, and brain must also incorporate chorioretinal expression.94
Human retina expresses two hallmark genes of hepatic and intestinal lipoprotein secretion, microsomal TG transfer protein (MTTP) and apoB (APOB) (for expert review see Ref. 95). Localization of both proteins in RPE and in retinal ganglion cells appears consistent with endoplasmic reticulum.96 Secretion of full-length apoB-100 was demonstrated in rat-97 and human-derived RPE cell lines98 and in mouse RPE-choroid explants.99 MTTP is a soluble heterodimer100,101 that co-translationally transfers lipid to apoB to ensure correct folding.102,103 Cells expressing apoB without MTTP cannot secrete lipoproteins.104–107 ApoB production is regulated via co- and posttranslational degradation by the ubiquitin-proteasome system, which is in turn regulated by lipid availability.108 ApoB's classic function is delivering exogenous and endogenous TG, cholesterol, and lipophilic vitamins throughout the body as part of VLDL/LDL and chylomicrons. ApoB is also expressed in kidney, placenta, and heart,109,110 apparently to regulate TG content and forestall lipotoxicity.111 In mice, absence of apoB is lethal in utero, and reduced apoB causes neural tube defects.112,113 Lack of functional MTTP and apoB results in abetalipoproteinemia (ABL, OMIM 200100) and hypobetalipoproteinemia (HBL, OMIM 615558), rare Mendelian disorders that include a pigmentary retinopathy and ataxic neuropathy. Attributed to impaired delivery of lipophilic vitamins, ABL/HBL are partly alleviated by long-term dietary supplementation.114 Intraocular apoB and MTTP expression indicates that ABL/HBL are intrinsic degenerations and that lipoprotein assembly and secretion are required for retinal health and good vision. It also means abundant research on hepatic and intestinal lipoproteins are relevant to AMD.
Soft Drusen, BLinD: Lifelong Physiology, Uncovered by Aging
In the 19th century Donders,115 Wedl,116 and Müller117 discovered drusen; Wedl116 described them as lipid globules. Long-standing theories for druse formation117 are transformation of the overlying RPE and deposition of materials onto BrM. The latter is now accepted.118
S.H. and J.P. Sarks, two ophthalmologists in Australia, together and in collaboration with pathologist M.C. Killingsworth, contributed foundational AMD pathology, including the heterogeneity of drusen within a heterogeneously presenting disease.23,40,60,65,119–122 Studies using panoramic electron microscopy of affected macular tissue from clinically documented eyes of S.H. Sarks' patients40,60,119–121,123,124 definitively localized drusen in the subRPE-BL space, distinct from the overlying RPE-BL/BLamD and underlying ICL, and proved that clinical druse phenotypes differed in ultrastructure and thus in composition.60
Soft drusen are dome-shaped with sloping sides125 and filled with ‘membranous debris'60 (Figs. 2, 4), implying lipids, and considered by the Sarks to set the disease course. Soft drusen and BLinD are two physical forms (lump and layer, respectively), often continuous,126 of the same material; BLinD was also called “diffuse drusen” by paraffin histology.127 When soft drusen/BLinD are processed for conventional thin-section electron microscopy using osmium postfixation, biomechanical fragility23,128,129 and partial extraction of lipid combine to produce curvilinear elements resembling coiled membranes (Figs. 4C, 4D). Thus, the principal soft druse component was initially called membranous debris,120,123 influencing mechanistic hypotheses and development of model systems exhibiting cellular membrane release.130,131 Also, descriptions of aging BrM using conventional osmium postfixed tissue mentioned vesicles (i.e., membranous coils with aqueous interiors).132,133
Figure 4.
Soft drusen/basal linear deposit: lipid lakes, no structural collagen/elastin. (A) Ex vivo OCT imaging of a short postmortem donor eye with large soft drusen (arrow, shown in [B]) and central GA, 79-year-old male. R, retina, (C) choroid; ONH, optic nerve head; (B) Large soft druse shown in (A) has numerous lipid pools (arrow) containing esterified cholesterol (refer to Fig. 3C,135 overlaid with dysmorphic RPE and BLamD. The underlying BrM has refractile patches of hydroxyapatite (light blue). Choriocapillaris endothelium ranges from normal to ghosts. Submicrometer section, osmium tannic acid paraphenylenediamine postfixation, toluidine blue stain. (C, D) Soft druse (C) and BLinD ([D] above the yellow arrowheads) from different donors126 contain membranous profiles with electron-dense exteriors and homogeneous and moderately electron-dense interiors, thought to represent partly preserved lipoprotein particles. The same material is found internal (basal mound,8 X) and external to the RPE-BL. Scale bar: 1 μm. Osmium postfixation and transmission electron microscopy. White arrowheads, RPE-BL. Asterisks, lipid lake.
Lipid-preserving histochemical and ultrastructural techniques united “vesicles” and “membranous debris” as manifestations of lipoprotein particles at different levels of preservation and disintegrity. Evidence for lipoprotein involvement is best for pre-BLinD (Fig. 3C) and hard drusen, where electron-dense spherical particles are visible, and more than 40% of druse volume is Folch-extractable lipid.134 Soft drusen/BLinD are biomechanically fragile (called “localized detachments of BLamD”)127 so evidence for their composition rests on consistent ultrastructural and histochemical results across studies. Soft drusen/BLinD exhibit polygonal regions of homogeneously and moderately electron-dense material (Fig. 4), originally called “hard drusen breaking up.” When prepared by sterol-specific filipin histochemistry, these shapes are EC-rich lakes.135,136 Similar processes occur in the lipid-rich cores of atherosclerotic plaques, where plasma LDL insudates137,138 and binds to extracellular matrix, followed by particle surface degradation, fusion, and pooling of core lipids to create UC-rich liposomes139,140; these processes can be mimicked in vitro by physically disrupting LDL.141
A natural history of aging BrM (17–92 years) using osmium tannic acid post-fixation showed that “vesicles” were solid, spherical particles approximately 80 nm in diameter. Further, quick-freeze deep-etch analysis of BrM (27–86 years; Fig. 3C)17,142 revealed that particles had a surface-and-core morphology consistent with lipoproteins.143,144 Lipoprotein particles also appear in multivesicular bodies in BrM,17,144–146 and in lines crossing BLamD.126,127,147,148 Both studies demonstrated three to four rows of densely packed lipoproteins in the subRPE-BL space, logically the direct precursors of BLinD. This formation, first called Lipid Wall, represents preBLinD (Fig. 3C).143,144 The nondescript fluid phase surrounding particles contain proteins and other components not discernible at these magnifications. We proposed the name “lipoprotein-derived debris”149 for masses of modified lipoproteins in soft drusen/BLinD (Figs. 3D, 4C, 4D). This debris also appears in basal BLamD (basal mounds)121,123,150,151 and rarely, within large vacuoles in RPE.123,126,152 Similar material said to occupy the subretinal space66,123 is really SDD (see the Subretinal Drusenoid Deposits: Extracellular, Space-Filling, Distinct From Drusen section).
A lipophilic barrier in aged BrM blocking normal, choroid-directed fluid efflux from the RPE was postulated by Bird and Marshall153 to explain RPE detachments in older adults. A seminal study by Pauleikhoff et al.16 demonstrated that oil red O binding lipids localized exclusively to BrM of healthy human eyes. This staining was abundant in adults 61 years and older, variably present in midlife adults, and absent in young adults. Direct assay confirmed the age-related increase (although not the initially reported composition).154,155 Marshall employed BrM explants to explore transport across this tissue.156–158 Later analysis showed excellent correlation of an age-related increase in resistivity (inverse of hydraulic conductance) with content of hydrophobic EC.159
Specific histochemistry and analytic biochemistry combine with gene expression (see the Genetics and Gene Expression Studies Relevant to Lipids section) to support the concept of EC- and linoleate-rich, apoB, apoE-containing, large lipoprotein particles secreted by RPE (Figs. 3E, 3F). The oil red O-binding material is EC, verified by multiple direct assays.17,97,144,160 EC accumulates markedly in BrM, in 7-fold higher quantities in macula than periphery.17,18 EC localizes exclusively to BrM whereas UC and PL, also present, additionally localize to nearby cellular membranes.161 Particles 60 to 80 nm in diameter and with flotation properties and spherical shapes indicating neutral lipid cores are isolable from healthy human BrM.97,144 In the same fractions are also apolipoproteins B, A-I, and E. BrM lipoproteins are highly EC-enriched relative to TG,17,97,144,160 unlike hepatic VLDL, of similar diameter. Thus, BrM lipoproteins are large like VLDL and EC-rich like atherogenic LDL. In contrast, the neurosensory retina contains little EC.17,160
Lipoproteins are assembled from multiple lipid sources, and fatty acid profiling of EC and other lipid classes in BrM lipoproteins and extracts allowed inferences about the source of this component. Docosahexaenoate (22:n6) is distinctively high in PL of outer segment membranes162 and neural tissue in general. Yet, high-performance liquid chromatography in two laboratories showed that all lipid classes in BrM are overwhelmingly dominated by the fatty acid linoleate (18:2, most abundant in plasma) with little docosahexaenoate.97,160 This result suggests that RPE recycles docosahexaenoate back to photoreceptors efficiently, as postulated,163 and that plasma lipoproteins are the major fatty acid sources to BrM lipids. On the basis of fatty acid composition alone, it is not possible to distinguish BrM lipoproteins from those of plasma origin, in transit to RPE from choriocapillaris. However, BrM lipoprotein composition and gene expression support a local source, because enrichment with EC over TG differs sharply from plasma VLDL, and intracellular gene and protein data (see the Genetics and Gene Expression Studies Relevant to Lipids section) indicate RPE capacity for lipoprotein assembly and secretion.
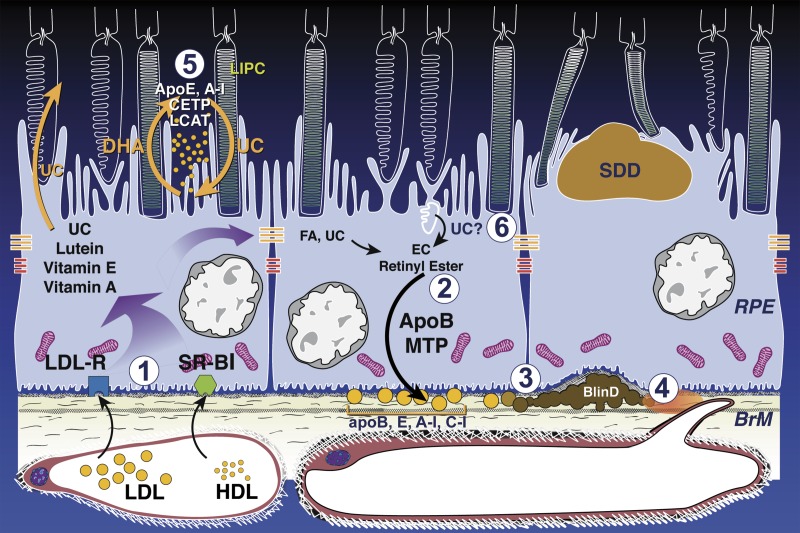
A long-standing hypothesis164 states that debris in aging BrM represents outer segment membranes phagocytosed and processed by RPE.165 Outer segment UC content is notably low93,166,167 but could be concentrated in bulk phagocytosis by RPE.150 Figure 5 expands this model by postulating that the fatty acids in this material come largely from diet. BrM lipid deposition (steps 1–2, Fig. 5) is proposed as a recycling system in which plasma lipoproteins delivering dietary essentials are stripped of cargo destined for photoreceptors. Unneeded fatty acids and UC are repackaged with outer segment UC for secretion to BrM and eventual choroidal clearance. One appeal of this model is the specificity for BrM, unlike models involving by-products of other lipids.168,169 Soft drusen/BLinD form (steps 3–4, Fig. 5) when egress is blocked through aging BrM/ChC, either due to abnormal amounts or types of BrM proteins, loss/dysfunction of ChC, loss of VEGF sustenance to ChC, or all. Aged BrM and subsequent soft drusen/BLinD could act as a transport barrier to large molecular complexes,170 a source of peroxidizable proinflammatory lipids,171,172 and part of an increased diffusion distance impeding oxygen exchange.173
Figure 5.
Lipid cycling pathways leading to soft drusen and an atherosclerosis-like progression in the sub-retinal pigment epithelium basal lamina space. BLinD/soft drusen and SDD are localized external and internal to the RPE, respectively. Normal-aging RPE is at the left and center. AMD is at right. Shown are RPE-based lipid recycling pathways for rods and cones that could drive formation of AMD extracellular lesions. (1) Plasma LDL and HDL delivering lipophilic essentials, including vitamins E, A, lutein, and cholesterol (UC), enter basolateral RPE via LDL receptor and scavenger receptors BI and BII, respectively. (2) ApoB, E lipoproteins secreted basolaterally by RPE (gold circles) are assembled from multiple lipid sources. Fatty acids are dominated by linoleate, implicating internalized plasma lipoproteins (from step 1) as a major source, plus UC from all sources esterified to EC. (3) Lipoproteins retained by binding to BrM extracellular matrix accumulate throughout adulthood (perhaps in concert with less efficient transport by aged choriocapillaries), creating pre-BLinD between the RPE-BL and the inner collagenous layer of BrM. (4) Lipoproteins degrade, fuse, and form lipid pools within BLinD/soft drusen, making them biomechanically fragile, proinflammatory, and cytotoxic. (5) Disks in rod OS lose UC and gain docosahexaenoate in transit from OS base to tip (shown as loss of white). OS-derived docosahexaenoate stored as triacylglycerol in RPE after phagocytosis return to OS. The mechanism of transfer is unknown but could be familiar proteins like interphotoreceptor retinoid-binding protein or hypothetical HDL particles cycling between RPE and photoreceptors, especially under rod-rich perifovea, where subretinal drusenoid deposit forms. (6) Cone OS maintain high-UC content along their length, because their disks are comb-like projections of plasma membrane. Cone OS UC enters RPE via disk shedding, lysosomal uptake, and acid lipase activity. UC is released for intracellular transfer, esterification, and assembly into basolaterally secreted lipoproteins, especially under cone-rich fovea. Reprinted with permission from Pikuleva IA, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Ret Eye Res. 2014;41:64–89. Copyright © 2014 Elsevier Ltd.
The Calcific End-Stage of Soft Drusen and Differentiation From Amyloid β
One end-stage of soft drusen is calcification, inferred from glistening fundus appearance35 and in tissues, refractility,62,123 von Kossa staining,128,174,175 and microanalysis.176 Concentric shells within spherules impart the glistening appearance and a punctate reflectivity on OCT.175 Spherules less than 1-μm diameter177 show strong hydroxyapatite signal via microprobe synchrotron x-ray fluorescence and specific dyes,178,179 and they may enclose other druse components and promote deposit expansion.179 Nonreflective multilobular nodules calcific (5–100 μm) within drusen are associated with reduced autofluorescence signal of overlying RPE.49,177 Hydroxyapatite is also abundant in subRPE deposits created by well-differentiated cultured RPE,151 emphasizing the importance of basolaterally directed physiologic mineral regulation.
An alternate interpretation stems from the finding of colocalized and spherically distributed activation fragments of complement C3 with amyloid β peptide, a major constituent of Alzheimer disease neuritic plaques180 in some drusen of some AMD eyes,181 with staining correlated to overall drusen load per eye.182 Light and electron microscopy showed concentric shells,129,182 which were not labeled by antibodies to other amyloids.183,184 Many proteins bind to hydroxyapatite,185 which is used in chromatography, raising the possibility that amyloid binding to spherules is nonspecific. Amyloid β peptide was recently found in inner retina of Alzheimer patients, signifying a separate neurodegeneration, distant from drusen.186
Other Components of Soft Drusen
Understanding druse composition is considered an important route to discern pathways perturbed in AMD187 (Supplementary Table S1). ApoE was an early, consistent, and abundant component.81,135,187,188 Proteomics and immunohistochemistry also revealed vitronectin, complement components, clusterin, ATP synthase subunit beta, scavenger receptor B2, and retinol dehydrogenase.134,189–191 Oxidatively modified proteins including tissue metalloproteinase inhibitor 3 and vitronectin, and carboxyethyl pyrrole protein adducts also191 supporting oxidative damage as important in AMD progression.192
Many proteins, minerals such as zinc, and carbohydrates can be confidently placed in macular drusen that confer progression risk (Supplementary Table S1). However, it is unclear if these signals are specific to macula, a question of biologic importance.4 Data comparing macular and peripheral drusen in the same eyes are sparse.129,193 The macula is 3% of total retinal area,194 requiring specific measures for its analysis. Many studies assayed peripheral drusen,134,182 combined macular and peripheral drusen,191 or did not specify regional source.189 The apparent synergy of immunohistochemistry with genetic associations implicating complement was largely based on labeling that cannot be definitively placed in the macula. Neither membrane attack complex (terminal element of the complement cascade)195 nor CD59196 localized to macular soft drusen. Experimental studies suggest that BrM lipoprotein binding can be modulated by plasma CFH factor H,197 and genetics implicate a role for CFH in soft drusen biogenesis (see the Genetics and Gene Expression Studies Relevant to Lipids section). Continued investigation is warranted.
RPE Lipofuscin – Distinct From Drusen
RPE lipofuscin comprises abundant and long-lasting intracellular inclusion bodies, related to lysosomes, which are rich in bisretinoids (vitamin A derivatives).198 Appearing in humans in childhood and increasing throughout adulthood, RPE lipofuscin is the principal signal source of fundus autofluorescence imaging. Lipofuscin has been proposed as a source of intermediates in the pathway to age-related glycation products in drusen.199,200 Evidence included in vitro studies201 exposing cells to a lipofuscin fluorophore recently found to be less abundant in macula than in periphery.202–207 Histopathology of human AMD eyes indicate that lipofuscin is present in RPE40,60,121,123 and rarely in drusen.129 Further evidence that lipofuscin is not a major source of druse components includes different topographies of lipofuscin (high in perifovea208,209) and soft drusen (high in central macula) and different emission spectra of fluorophores in lipofuscin versus soft drusen.210 Because lipofuscin-attributable autofluorescence is a superb reporter of RPE metabolism that can be combined with OCT for subcellular-level insight in vivo,211 the biology and role in AMD pathophysiology of RPE lipofuscin remains a research priority.
BLamD - Distinct From Drusen, Important in Druse Biogenesis
BLamD is a distinct deposit meriting its own study (Fig. 1). Continuous subfoveal BLamD is considered diagnostic for AMD, and continuous BLamD in the presence of BLinD is an early AMD threshold.119,212 BLamD's role besides association with drusen can now be explored in clinical OCT. If RPE is present, BLamD is shadowed and appears hyporeflective.213 If RPE is absent, BLamD is a moderately reflective line across the atrophic macula.214,215
In many older healthy eyes BLamD forms small patches (∼5-μm wide) between the basolateral RPE plasma membrane and the RPE-BL. Early (palisade) BLamD is discontinuous, thin, and fibrous. In AMD, continuous BLamD is 15-μm thick or more.121,150,216 Late BLamD is thick, multilayered, and scalloped on the inner aspect.123,126,147,214 BLamD ultrastructure resembles basement membrane, containing laminin, fibronectin, type IV, and type VI collagen with 120-nm periodicity,217–220 as well as vitronectin, matrix metalloproteinase (MMP), metalloproteinase inhibitor 3 (TIMP-3), C3, and C5b-9.216 Eyes with BLamD also tend to have high drusen loads. BLamD contains lipid-rich particles transiting to BrM147,150 that aggregate as basal mounds121 (Fig. 9B151; Figs. 3E–H150). By retaining lipoproteins en route from RPE to BrM, BLamD may increase exposure time to oxidizing agents that result in proinflammatory, cytotoxic lipids.221 Some inherited retinopathies exhibit BLamD containing lipid and associate with drusen30,222 and/or type 1 (subRPE) neovascularization.147 Other retinopathies lacking drusen also have BLamD223 suggesting it is a nonspecific RPE stress response with a specific role in AMD.
BLamD and BLinD are often jointly named “basal deposits.” This imprecise term (to which this author added148) comes from low resolution paraffin and cryosection histology and is unwarranted if epoxy-resin histology or transmission electron microscopy is available. The commendable goal of “determining the origin and pathogenesis of BLamD and BLinD as a route to preventive measures”127 is best served by high-resolution visualization techniques and precise terminology.
Subretinal Drusenoid Deposits: Extracellular, Space-Filling, Distinct From Drusen
As reviewed,34 “drusen seen in blue light” reported in 1990224 were called various names depending on detection technology and patient population,225 finally settling on reticular pseudodrusen (viewed en face)65 and SDD (viewed cross-sectionally).66,67 In 1988 Sarks et al.123 described by electron microscopy “focal collections of membranous debris”123 in the subretinal space (see the Soft Drusen, BLinD: Lifelong Physiology, Uncovered by Aging section).65 In a donor eye, Rudolf et al.66 described regularly spaced deposits, distinct from photoreceptors and RPE. Definitive histology of clinical cases122,226 established the presence of extracellular deposits. The association of SDD with atrophy,227 intraretinal neovascularization,228 and photoreceptor degeneration229,230 indicates a place for SDD in the AMD spectrum.1 Beyond location, SDD differs from soft drusen/BLinD (Table 1) in lipid, protein, and mineral content, specificity for AMD, and association with neovascular subtypes.34 A histologic survey of AMD donor eyes225 showing that SDD was thickest in the perifovea, and that soft drusen/BLinD was thickest under the fovea, leading to a novel suggestion that deposits reflect differential physiology of rod and cone photoreceptors, respectively. Hypothesized driving pathways include lipid transport via lipoproteins (Fig. 5) and/or interphotoreceptor retinoid binding protein.34 A comprehensive understanding of SDD molecular composition is urgently needed.
Table 1.
Differentiating Soft Drusen From Subretinal Drusenoid Deposit
|
Soft Drusen/BLinD |
SDD |
Reference |
|
| Location | Between the RPE-BL and ICL of BrM* (sub-RPE-BL space) | Between RPE and photoreceptors (subretinal space) | 66, 122, 123, 225, 226 |
| Proteins | ApoE, vitronectin, CFH; CD59− | ApoE, vitronectin, CFH; CD59+ | 66, 226 |
| Lipids | Unesterified and esterified cholesterol; oil red O-binding | Unesterified cholesterol; oil red O binding | 150, 226, 359 |
| Minerals | Hydroxyapatite | Undetected to date | 151, 175, 179, 254 |
| Topography | Follows cones (BLinD) | Follows rods | 225, 227, 360 |
| Specificity for AMD | AMD | AMD; inherited diseases of BrM, retinoid transport | 60, 123, 126, 361–365 |
| Associated neovascular subtype | Type 1 (subRPE), 2 (subRPE, subretinal) | Type 3 (intraretinal) | 228, 366–368 |
RPE-BL, basal lamina of the RPE.
Proof-of-Concept Via Drusen-in-a-Dish Culture Systems
The BrM lipoproteins that make up soft drusen are thus postulated as dual-source, with fatty acids coming from uptake of plasma lipoproteins and cholesterol coming from outer segments as well (Fig. 5). If diet is an important driver of constitutive lipid cycling pathways, then cultured RPE cells might generate deposits in vitro with only culture media containing serum (and plasma lipoproteins) and lacking outer segments. Amin et al.131 demonstrated membranous material between the ARPE-19 cell line and a solid surface in 11 weeks of supplementation with a retinal extract. Recent advances include the use of commercially available culture medium231 over custom formulations232 and culture well inserts that allow independent monitoring of apical and basal chambers of polarized cells, essential for parsing druse- and SDD-relevant pathways. In a proof-of-principle study by Johnson et al.,146 cultured fetal human RPE on 100-μm thick porous supports in a standard medium without retinal supplementation produced particulate deposition of apoE-immunoreactive material within the insert (replicated in Ref. 151).
Using a 10-μm thick polyester membrane that restricted access to the basal compartment to pores crossing the insert, Pilgrim et al.151 found that beginning at 8-weeks polarized and highly differentiated primary porcine RPE lay down extensive deposits on the insert surface. Deposits were approximately 2-μm thick, extracellular, electron-dense, continuous, and sometime focal, with similar material filling the insert pores. Deposits exhibited histochemical and spectroscopic signatures of soft drusen, including lipid, apoE, and hydroxyapatite. Because cells were polygonal, had good transepithelial resistance, and expressed RPE-specific genes, including MTTP, cells appeared functional. Thus, deposits formed, because egress through the insert was blocked as the pores filled. These data strongly suggest that dietary input is required for druse initiation. Outer segments are not required, although they clearly shape deposit composition in vivo, nor were exogenous stressors. Learning what aspects of culture medium are essential will require selective depletion experiments.
Similarly, Galloway et al.233 showed that induced pluripotent stem cells (iPS) from patients with inherited retinopathies (see the BLamD - Distinct From Drusen, Important in Druse Biogenesis section) also generate electron-dense deposits between the RPE-BL and culture dish inserts. Deposit was generally sparse and discontinuous and varied according to genotype. Layers of collagen IV and apoE immunoreactivity resembled the RPE-BL and subRPE-BL space in vivo, respectively. Contrary to previous findings,146 exposure to human serum was not required to initiate deposits but did enrich them with C5b-9 immunoreactivity. Deposit sparseness relative to Pilgrim et al.151 may be due to withdrawal of fetal bovine serum and/or use of iPS cells at passage 3. The authors concluded that RPE dysfunction leads to deposits in these iPS cells that express mutant genes.233 However, deposits can be made by wild-type, differentiated porcine RPE.151
Culture systems are valuable when functional RPE can be studied in isolation and can demonstrate the minimum requirements for deposit. They offer limited mechanistic insights beyond that, in the absence of choroid and retina. In vivo, the “insert” to which RPE-BL attaches is ICL of BrM. Data suggest that aged BrM/ChC acts as a physical barrier to retain constitutively secreted material that accumulates (as it does in vivo) under the RPE.142 Neither cell culture studies nor other approaches have addressed whether BrM protein composition, BrM molecular sieving properties, inefficient translocation by aging ChC endothelium, or other factors initiate the binding of lipid in situ.234–236 Atherosclerosis research may be a source of ideas.237,238
How Soft Drusen Lead to Atrophy
AMD natural history is now visualizable at the cellular level with optimized structural eye-tracked OCT imaging.239 A pathway from soft drusen to subRPE neovascularization, includes gradients of VEGF secretion by stressed RPE, macrophage activity in breaching BrM, invading capillaries that remove or replace friable deposits, and damage to surrounding cells by peroxidized lipids.192,221,240 Recent data now also strongly implicate drusen as a causative factor in GA, further stimulating interest in targeting drusen to prevent or delay atrophy.
Clinicopathologic correlation,124 epidemiology,41,241,242 and clinical observation243 show that hyperpigmentation is the largest intraocular risk factor for progression after drusen abundance. In OCT, intraretinal hyperreflective foci found overlying drusen244–247 and appearing frequently in photoreceptor layers47,248 are correlated with hyperpigmentation on CFP248,249 and are now attributed to anteriorly migrated RPE.250 Reflective foci seen by either in vivo or ex vivo OCT could be directly linked to intraretinal RPE by histology251–256 and distinguished from cells with lipid droplets (presumed microglia or macrophages) in neovascular AMD.252,255 A high-resolution histology survey of RPE morphology suggested two main pathways of RPE fate.239 One pathway, apparently apoptotic, comprised the shedding of RPE organelles into underlying BLamD. A second pathway comprised rounding and sloughing of cells into the subretinal space, followed by anterior migration into the neurosensory retina, in coordination with Müller cells and photoreceptors at the external limiting membrane.
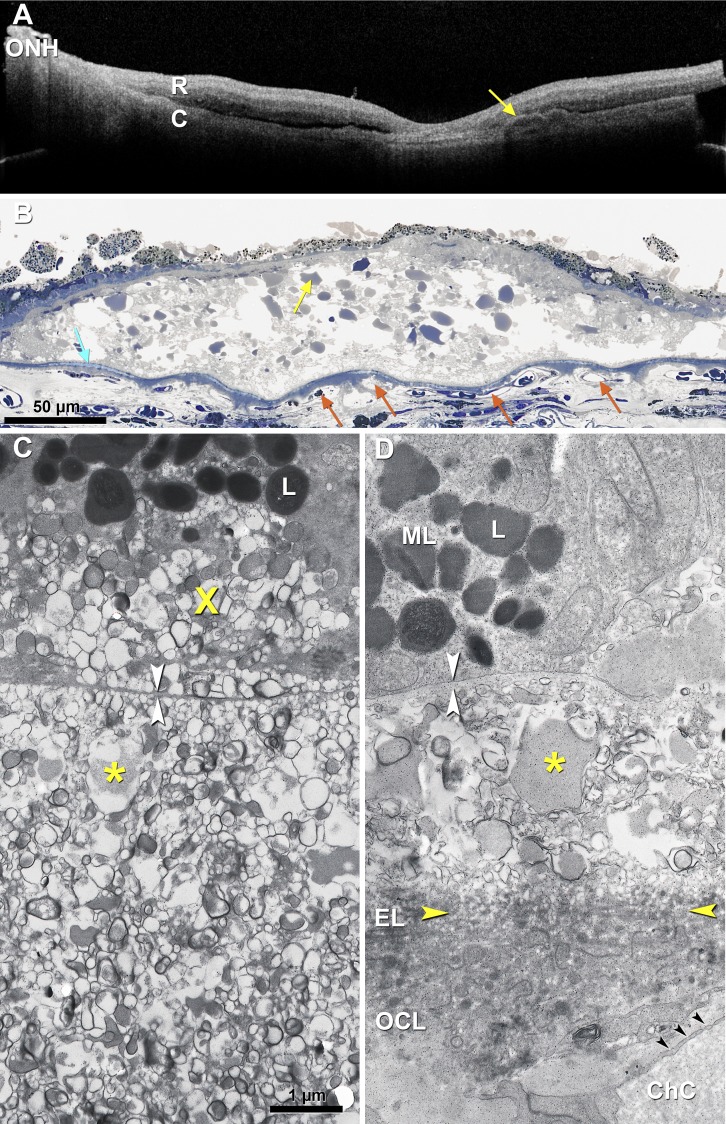
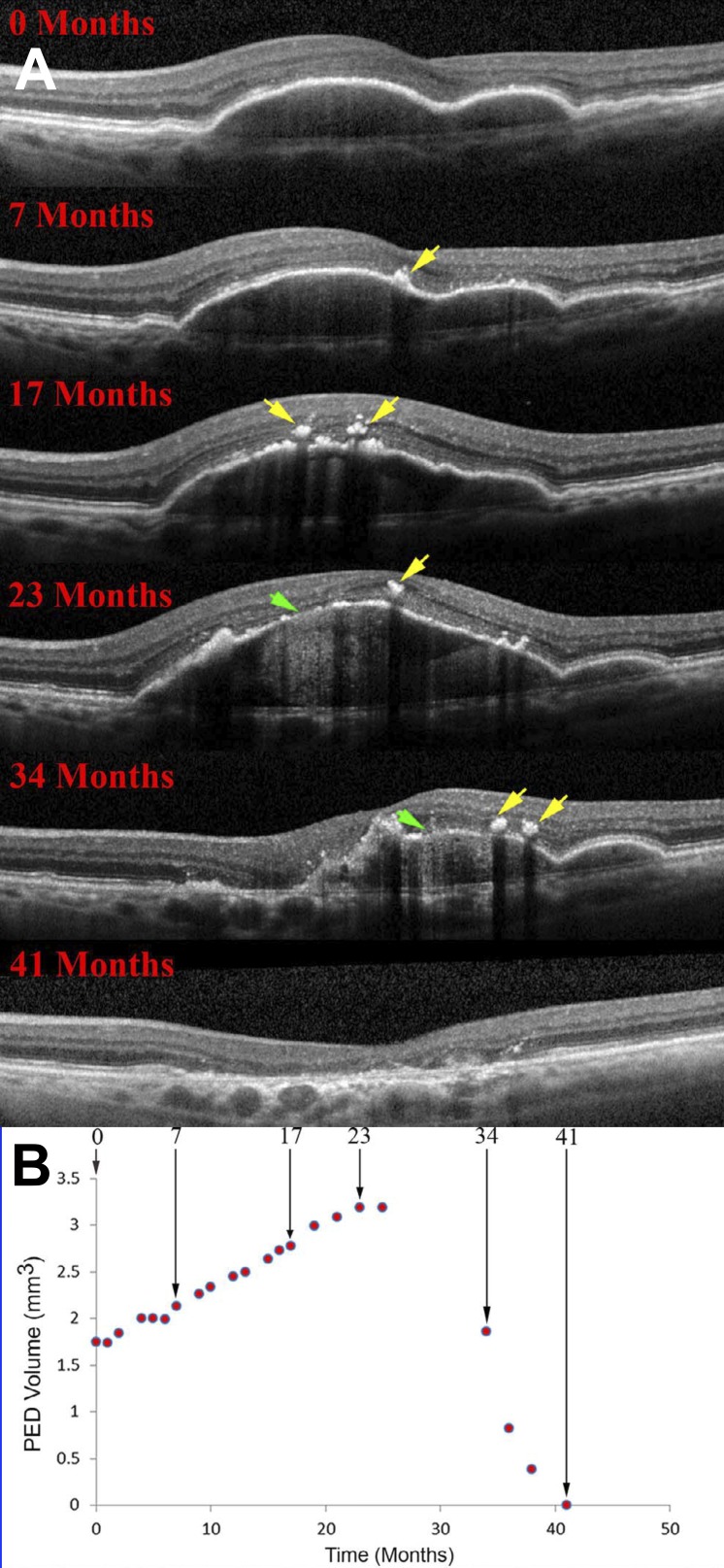
Drusen are dynamic, coalescing and disappearing in a manner suggestive of regulated processes.60,257–260 Over 5 to 7 years, 20% to 34% spontaneously disappear.261–263 Others disappear after retinal detachment.91 Drusenoid pigment epithelial detachment (PED; i.e., drusen with >350 μm base diameter) is a defined route to atrophy.123,264 A PED lifecycle was determined by measuring deposit volume in OCT scans for periods up to 6.6 years.265 Deposits grew slowly and collapsed quickly, with a legacy of complete RPE and outer retinal atrophy (Fig. 6). Before collapse, the RPE layer thickened at the druse apex, hyperreflective foci appeared vertically above in the retina, and the RPE-BL disintegrated.254 A similar lifecycle was demonstrated independently for more than 6000 RPE elevations of varying sizes.266 In some eyes, RPE death/migration leaves a raised line of reflective persistent BLamD across the atrophic area.215
Figure 6.
RPE demise linked to the life cycle of drusenoid pigment epithelial detachment (DPED). (A) Eye-tracked, spectral-domain OCT, in a 72-year-old patient. Intraretinal hyperreflective foci are first noted at 7 months as localized hyperreflective lesions arising from the RPE-BL band (yellow arrows). At 23 months, disruptions to the RPE-BL band (green arrow) with increased light transmission (hypertransmission) to the choroid are evident, followed by reduction in DPED volume until 41 months. (B) DPED volume increased slowly and declined rapidly in this patient. Modified from Balaratnasingam C, Yannuzzi LA, Curcio CA, et al. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Invest Ophthalmol Vis Sci. 2016;57:5479–5489.
These spatiotemporal characteristics together with cell culture studies (see the Proof-of-Concept Via Drusen-in-a-Dish Culture Systems section) clarify how RPE cells die over drusen. If a druse is growing, the RPE is functional enough to secrete druse components, which then back up against the BrM-ChC complex due to slowed clearance.151 When the druse gets large enough, RPE cells on the apex either migrate or die. Then the druse collapses, because druse component production is discontinued, and clearing processes catch up. It has been thought that as drusen collapse, the RPE dies, but the contrary is true. Further, because drusenoid PED are the largest deposits on a continuum leading to GA, we can judiciously extrapolate to drusen-associated atrophy overall.267 Interestingly, other clinical studies support this overall model, including drusen over choroidal nevi that compress ChC59 and diminished ChC flow signal under drusen by OCT angiography.268
Anterior migration of RPE suggests attractants from the retina, repellents in the druse, or both. Oxygen tension is reduced by 30% to 50% at druse apices, depending on height.173,269 RPE atop drusen are maximally distant from the ChC and may migrate to seek oxygen from retinal capillaries. In intraretinal neovascularization (retinal angiomatous proliferation), ectopic RPE cells positive for VEGF immunoreactivity are found immediately adjacent to capillaries.255,270,271 Further, druse volume is a strong predictor of which individual deposits proceed to atrophy.272 Data can support a model of RPE cell death related to distance from the ChC, in concert with local lipotoxicity,172 driven by hypoxia, micronutrient deficiency, and bioenergetic failure, with drusen the common underlying mechanism.
Continuing to Learn From Atherosclerotic Cardiovascular Disease
Atherosclerotic cardiovascular disease has been a rich source of molecules, mechanisms, techniques, and inspiration for approaching the biology of soft drusen and new treatments and preventions. Arguably, the several biologic pathways for AMD risk outlined by meta-analyses of GWAS80,273 align along an atherosclerotic progression.72
Table 2 keeps this comparison in perspective by showing that despite many similarities at the level of the vessel wall, from calcific end-stages to extracellular matrix regulation to lipoprotein sources of cholesterol, the top-level biologic risk factors of AMD and cardiovascular disease (e.g., plasma LDL and apoE4 genotype) are dissociated. The evidence that lipid deposition in aging BrM is dictated by needs of outer retinal cells and not an ocular manifestation of systemic perifibrous lipid in human connective tissues and atherosclerosis is compelling. Nevertheless, the commonality of lipoprotein-instigated vascular disease suggests that the many antidyslipidemic agents developed for cardiovascular disease may be intelligently probed for their utility in AMD.
Table 2.
Learning About AMD From Atherosclerotic Cardiovascular Disease
|
Compare and Contrast* at the Level of the Vessel Wall | ||
|
CVD (Arterial Intima, Liver/Intestine) |
AMD (BrM, RPE) |
|
| Calcific, inflammatory, neovascular complications in a vessel wall | Calcification of drusen and BrM, neovascularization types 1-2-3 | |
| Toxically modified lipoprotein components | Linoleate hydroperoxide, 7-ketocholesterol | |
| Lipid-rich and biomechanically unstable lesions (necrotic core of plaque) | Soft drusen and basal linear deposit | |
| Stereotypic locations in vasculature | Central macula | |
| Perifibrous lipid – lipoprotein binding to extracellular matrix in sub-endothelial space | Age-related deposition of lipoproteins in BrM | |
| Age-related thickening of sub-endothelial space | Age-related thickening of BrM | |
| Esterified, unesterified, crystalline cholesterol | Esterified, unesterified cholesterol | |
| LDL (VLDL remnant) as cholesterol source | BrM lipoprotein as cholesterol source | |
| ApoB,E lipoprotein particles | ApoB,E lipoprotein particles | |
| Lipoproteins of hepatocyte, enterocyte origin | Lipoproteins of RPE origin | |
| Macrophages are source of foam cells | Macrophages active in neovascularization, druse clearance (with Müller cells) | |
| Physiological needs driving lipoprotein production (delivery of fuel, cholesterol, lipophilic vitamins) | Physiological needs driving lipoprotein protein (recycling of unneeded lipids from diet-delivery and outer segment phagocytosis to plasma) | |
| Evolutionary selection of fitness | Evolutionary selection of acute vision | |
|
Contrast* at the Level of Persons and Populations | ||
|
CVD |
AMD |
|
| ApoE4 genotype | Increase risk | Decrease risk |
| Elevated plasma cholesterol or LDL | Increase risk | Not associated |
| Elevated plasma HDL | Decrease risk | ± risk |
| Diabetes (type 2) | Increase risk | Not associated |
| Statin therapy | Standard of care | Under investigation |
| Antioxidant therapy | ± effect | Standard of care |
Table outlines the limitations of analogizing AMD and CVD.
Contrast shown in italic.
Therapeutic Approaches to Soft Drusen
Our hypotheses motivate the pharmacologic targeting of soft drusen components and antecedent processes to prevent downstream sequelae, a strategy similar to that used for stroke: target vessel walls, so neurons and supporting cells will benefit. We elaborated the Oil Spill strategies (Fig. 7)33,72: detoxifying or removing drusen (“Skimmers and Dispersants”), retarding drusen formation by preventing RPE lipoprotein outflow (“Top Kill”), and preventing drusen formation by modulating dietary input (“Bottom Kill”).
Figure 7.
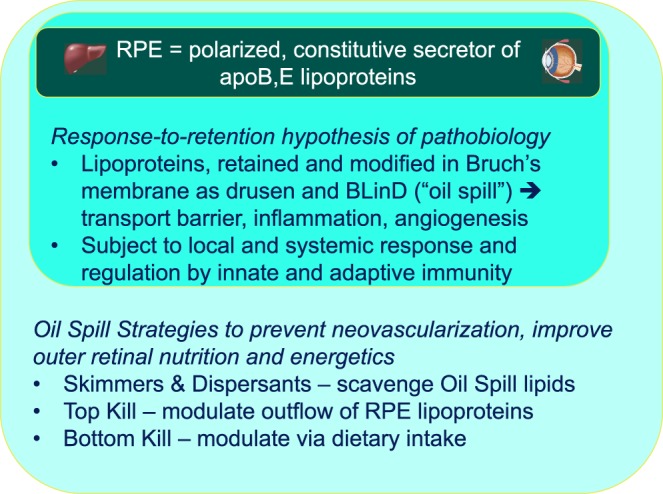
Interlocking Oil Spill strategies for AMD. The RPE is a polarized and constitutive secretor of lipoproteins bearing apolipoproteins B and E (and likely others). As such it fills a role like liver in atherosclerosis, with the role of arterial intima played by BrM.
Statins are widely used inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme for cholesterol synthesis, that reduce plasma LDL by upregulating LDL receptors throughout the body, especially the liver. Because statins also directly reduce apoB secretion,274 they are dual-action Top Kill and Bottom Kill, because RPE and RPE-derived cells expresses LDL receptors,275 secrete apoB, and respond differentially to various statins. 98 Clinical evidence regarding statin efficacy for AMD has been equivocal.276–278 Retrospective and population-based studies included patients at varying AMD severity levels, used statins of varying lipophilicity, and predated concepts of intraocular cholesterol and lipoprotein homeostasis. Several authors have advocated revisiting statins.98,279,280
In a double-masked randomized placebo-controlled proof-of-concept trial by Guymer and associates,279 114 normolipemic AMD patients received either simvastatin 40 mg/day or placebo. Patients with bilateral intermediate AMD receiving simvastatin experienced a significant 2-fold decrease in the risk of progression, with no effect seen in unilateral intermediate AMD (advanced AMD in the fellow eye). Further, a single-arm, two-center trial of 80 mg/day atorvastatin reported by Vavvas and associates281 showed that over 1 year, 10 of 23 patients exhibited marked reduction of large drusen, lack of progression to atrophy and neovascularization, and quiet RPE over druse domes. Three patients dropped out due to side-effects not uncommon at this dose. Although this study lacked quantification of druse volume and a comparison group to account for the natural history of druse dynamism, results were singular and corroborated the previously seen lack of progression.279
A “Skimmers and Dispersants” approach is exemplified by a recent preclinical study of a lipid scavenger.282 Apolipoprotein (apo) A-I mimetics are short (18 amino acids) synthetic amphipathic helical peptides that emulate the antiatherogenic properties of apoA-I (243 amino acids).283,284 Amphipathicity allows peptides to sequester lipids and travel through an aqueous environment. Peptide 4F has four phenylalanine residues on the nonpolar face of the helix.283–289 It is anti-inflammatory, avidly binding oxidized phospholipids and fatty acid hydroperoxides290 and reducing large-artery atherosclerosis in animal models.283,291–293 In phase II trials for cardiovascular disease, systemic 4F was tolerated,294,295 but not advanced, due to uneven absorption after oral administration. 4F's small size and the commonality of lipoprotein-instigated vascular disease in atherosclerosis and AMD made it an excellent candidate for targeting drusen and/or druse precursors. A popular model of atherosclerosis, ApoE−/− mice also exhibit BrM disintegrity, thickening, and EC accumulation at 10 to 11 months.282 One eye was injected with 4F or a scrambled peptide (0.6, 1.2, 2.4 μg), and the fellow eye served as a control. Transmission electron microscopy and perfringolysin-green fluorescent protein histochemistry showed at all doses that BrM ultrastructure improved and EC was reduced.296 Animals receiving 4F tagged with a fluorescent tracer exhibited fluorescence at 1 day postinjection in BrM, remaining for at least 14 days, while replenished from neurosensory retina. Many questions remain, including effects on plasma inflammatory markers, precise lipids removed, safety profile, and effects on retinal function.17,143 Despite limitations, this study demonstrated a tolerated and effective pharmacologic reduction of BrM lipids from mice.126,129 Because soft drusen are extracellular and loosely packed,126,129 surface-active agents like 4F offer advantages.
Unlike targeting extracellular drusen, other lipid-based approaches involve intracellular RPE lipid. For example, the offloading of cellular cholesterol to circulating HDL via LXR agonists297 may reduce substrate available for apoB lipidation and druse biogenesis. Another approach is to stimulating RPE uptake of lipids, presumably to clear drusen (e.g., via the CD36 scavenger receptor).298 It is instructive to recall that among agents modulating VLDL for cardiovascular disease, the lipid content of source cells was a less fruitful target than impacting the vessel wall through plasma lipid-lowering. Indeed, inhibitors of hepatic microsomal triglyceride transfer protein (MTTP) and acyl cholesterol acyltransferase-1 caused steatosis (fatty liver).108,299–302 Investigators should thus check for RPE lipoidal degeneration, a steatosis-like intracellular accumulation of lipid droplets associated with depressed electroretinograms.303–306
A major roadblock to clinical trials of drugs targeting drusen is nonavailability of approved and appropriate endpoints, although candidates exist. Visual acuity can remain good until late AMD. Rod-mediated dark adaptation and low-luminance visual acuity are sensitive to early disease stages but take time to administer.307–309 The only imaging endpoint currently approved by regulatory authorities is slowed expansion of GA viewed with fundus autofluorescence, a bar which several agents failed to meet.310–313 It is possible that GA is too late for intervention, because photoreceptor degeneration and gliosis are already severe.123,314–318 A earlier-stage surrogate endpoint is druse volume,261 in the causal pathway to progression and readily calculable from OCT scans.319 Another potential surrogate is hyperreflective foci over drusen (migrating RPE254), a risk factor for atrophy47,51,320 that can be quantified.272
Model Systems for Mechanistic and Translational Drusen Research
Monkeys: Drusen Without Progression
Monkeys in closed colonies have strong matrilines that vary in the degree of AMD pathology.321 They share with humans AMD susceptibility genes322 and plasma hyperlipidemias.323 To date, monkeys have not exhibited neovascularization, GA, BLamD, SDD, migratory RPE, or drusen with internal structure visible on OCT,306 all typical for human AMD; this possibly reflects a controlled environment and diet. Yet some monkeys appear to have soft drusen and requisite Oil Spill biology. Drusen with “membranous debris” and lipoprotein-like particles in BrM were demonstrated by electron microscopy.303 BrM exhibits both oil red O and filipin staining for EC.305 A large study (n = 60 eyes, 2- to 26-years old) showed an age-related increase in immunoreactivity for 7-ketocholesterol, an oxidation product of UC,172 that was selective for RPE-choroid. Like humans, monkey drusen contain apoE324 and carbohydrates,325 and BrM has entrapment sites (i.e., upward swellings of the ICL). Unlike humans, monkeys have cellular processes evaginated from RPE.40,326
Mouse Models Capture Some Pathways Well
Mice are experimentally advantageous yet exhibit only some AMD-relevant biology. Relative to humans and nonhuman primates, mice differ in the following ways: they are nocturnal; lack an all-cone fovea, foveal pit, centrifugal displacement of photoreceptor terminals, and long Henle fibers; have small cones and densely packed outer segments; naturally lack xanthophyll pigment; exhibit subretinal microglia in aging and in retinal degenerations; have a uniform distribution of bisretinoid A2E in RPE; have panretinal multinucleate RPE (versus in Ref. 327); frequently have vacuolated RPE in retinopathy (versus in Ref. 251); and do not express CETP and thus transport cholesterol in plasma HDL rather than in LDL. Nevertheless, components of AMD can be studied to great effect in genetically engineered mice. Several models exhibit BLamD, sometimes containing lipid,223,328–332 suggesting that mice have apolipoprotein pathways but normally lack a retentive matrix. Other mouse strains have EC in BrM,99,296,333 activated RPE,334,335 spontaneous intraretinal neovascularization,336,337 and xanthophyll accumulation.338 Because AMD-risk genes like APOE are expressed in several outer retinal cell types, technologies for cell-specific knockouts will be especially informative.339,340
It is agreed that mice lack drusen (hard or soft).197,341 Several studies claimed drusen in mice342–346 but did not meet the Sarks standard of ultrastructurally confirmed focal, extracellular, subRPE-BL material, correlated to fundus appearance. Regularly spaced microglia appear in the subretinal space of several aging mouse strains,347–349 and correlate to neither human drusen nor SDD.226,350 Precision in specifying layer and ultrastructural findings in animal models and benchmarking against human pathology will accelerate progress on AMD.
Cell Culture Systems are Standardizing
Several RPE culture systems produce druse-relevant deposits,146,151,233 with one recreating a continuous deposit.151 Confluent and polygonal with sharp vertices, healthy RPE in vivo maintains the physiologic blood–retina barrier and exercises distinct roles vis à vis photoreceptors and the choroid. Due to AMD deposits of differing composition in subretinal and subRPE-BL compartments, polarity is more essential than ever for RPE culture systems. Many protocols exist for high-fidelity native and engineered RPE in culture, emphasizing properties of the intact layer, particularly high transepithelial resistance (≥250 MΩ).77,146,231,232,351–355 Of current interest, cell-based therapies are raising expectations for all RPE culture systems.356 Studies using nonconfluent cells, cells with low transepithelial resistance, and high-passage cell lines, such as human-derived ARPE-19, should be interpreted cautiously. Ideally cultured RPE should be characterized for polarity, barrier function, cytoskeletal precision, and expression of RPE-specific genes.
Final Thoughts
Soft drusen are a very prominent intraocular risk factor that are seen routinely in vivo. Yet the true impact of soft drusen on AMD progression will be better understood when all the layers in Figure 1 can be followed clinically and their contribution to risk assessed. In less than decade thanks to OCT, SDD went from invisible to a major contributor to retinal dysfunction. Now the participation of BLamD is also becoming known. We anticipate a day when BLinD is visible clinically and its risk assessed along with drusen. In 2007 the Sarks et al.121 staged eyes by the presence of ‘membranous debris'. Arguably some of AMD's infamous heterogeneity is because this specific pathology cannot be directly followed in the clinic. Thus, presentations currently attributed to individual variability may be consequences of invisible BLinD (or other invisible deposits). For example, Asian populations prone to neovascularization without many drusen may have BLinD that escapes detection. Fortunately, imaging technologies with promise of revealing BLinD are emerging.210,357 There is still much to learn about the biology of soft drusen, in the clinic and in the laboratory. Nevertheless, current knowledge can motivate targeting these deposits and contributory biologic processes, to delay or avoid AMD's sight-robbing late stages.
Limitations to this analysis are sparse experimental confirmation of hypotheses largely generated from human tissues and patients. Our hypotheses, while speculative, bring together many evidence lines and do not exclude other major extant hypotheses for AMD biology and may in fact occur in parallel.
Supplementary Material
Acknowledgments
Currently supported by grants from the National Eye Institute (R01EY027948, EY021470), Heidelberg Engineering, Hoffman LaRoche with institutional support from the EyeSight Foundation of Alabama, Research to Prevent Blindness. Original research was supported by grants from the National Eye Institute (R01EY06109), Macula Vision Research Foundation, and International Retinal Research Foundation.
Disclosure: C.A. Curcio, Macregen Inc. (I)
References
- 1.Spaide RF. Improving the age-related macular degeneration construct: a new classification system. Retina. 2017;38:891–899. doi: 10.1097/IAE.0000000000001732. [DOI] [PubMed] [Google Scholar]
- 2.Seddon JM, McLeod DS, Bhutto IA, et al. Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol. 2016;134:1272–1280. doi: 10.1001/jamaophthalmol.2016.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copland DA, Theodoropoulou S, Liu J, Dick AD. A perspective of AMD through the eyes of immunology. Invest Ophthalmol Vis Sci. 2018;59:AMD83–AMD92. doi: 10.1167/iovs.18-23893. [DOI] [PubMed] [Google Scholar]
- 4.Curcio CA. Antecedents of soft drusen, the specific deposit of age-related macular degeneration, in the biology of human macula. Invest Ophthalmol Vis Sci. 2018;59:AMD182–AMD194. doi: 10.1167/iovs.18-24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 6.Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140195. doi: 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Wang Z, Zhou X, Li B, Zhang H. Choroidal and photoreceptor layer thickness in myopic population. Eur J Ophthalmol. 2012;22:590–597. doi: 10.5301/ejo.5000092. [DOI] [PubMed] [Google Scholar]
- 8.Zouache MA, Eames I, Klettner CA, Luthert PJ. Form, shape and function: segmented blood flow in the choriocapillaris. Sci Rep. 2016;6:35754. doi: 10.1038/srep35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 10.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue Y, Shen SQ, Jui J, et al. CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest. 2015;125:727–738. doi: 10.1172/JCI79651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 13.Nolan JM, Power R, Stringham J, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central Retinal Enrichment Supplementation Trials - Report 1. Invest Ophthalmol Vis Sci. 2016;57:3429–3439. doi: 10.1167/iovs.16-19520. [DOI] [PubMed] [Google Scholar]
- 14.Karwatowski WSS, Jeffried TE, Duance VC, Albon J, Bailey AJ, Easty DL. Preparation of Bruch's membrane and analysis of the age-related changes in the structural collagens. Br J Ophthalmol. 1995;79:944–952. doi: 10.1136/bjo.79.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newsome DA, Huh W, Green WR. Bruch's membrane age-related changes vary by region. Curr Eye Res. 1987;6:1211–1221. doi: 10.3109/02713688709025231. [DOI] [PubMed] [Google Scholar]
- 16.Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane: a histochemical and morphological study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- 17.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- 18.Haimovici R, Gantz DL, Rumelt S, Freddo TF, Small DM. The lipid composition of drusen, Bruch's membrane, and sclera by hot stage polarizing microscopy. Invest Ophthalmol Vis Sci. 2001;42:1592–1599. [PubMed] [Google Scholar]
- 19.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PGH, de Jong PTVM. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 20.Streeten BW. The sudanophilic granules of the human retinal pigment epithelium. Arch Ophthalmol. 1961;66:391–398. [Google Scholar]
- 21.Wolter JR, Falls HF. Bilateral confluent drusen. Arch Ophthalmol. 1962;68:219–226. doi: 10.1001/archopht.1962.00960030223013. [DOI] [PubMed] [Google Scholar]
- 22.Farkas TG, Sylvester V, Archer D, Altona M. The histochemistry of drusen. Am J Ophthalmol. 1971;71:1206–1215. doi: 10.1016/0002-9394(71)90964-0. [DOI] [PubMed] [Google Scholar]
- 23.Sarks SH. Council lecture: drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980;8:117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 24.Pauleikhoff D, Zuels S, Sheraidah GS, Marshall J, Wessing A, Bird AC. Correlation between biochemical composition and fluorescein binding of deposits in Bruch's membrane. Ophthalmology. 1992;99:1548–1553. doi: 10.1016/s0161-6420(92)31768-3. [DOI] [PubMed] [Google Scholar]
- 25.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curcio CA, Johnson M. Structure, function, and pathology of Bruch's membrane. In: Ryan SJ, Schachat AP, Wilkinson CP, Hinton DR, Sadda S, Wiedemann P, editors. Retina. London: Elsevier;; 2017. pp. 466–481. [Google Scholar]
- 28.Weber BHF, Vogt G, Pruett RC, Stöhr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994;8:352–365. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- 29.Hayward C, Shu X, Cideciyan AV, et al. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum Mol Genet. 2003;12:2657–2667. doi: 10.1093/hmg/ddg289. [DOI] [PubMed] [Google Scholar]
- 30.Stone E, Lotery A, Munier F, et al. A single EFEMP1 mutation associated with both Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- 31.Bergen AA, Plomp AS, Schuurman EJ, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 32.Gorin MB, Paul TO, Rader DJ. Angioid streaks associated with abetalipoproteinemia. Ophthalmic Genet. 1994;15:151–159. doi: 10.3109/13816819409057843. [DOI] [PubMed] [Google Scholar]
- 33.Curcio CA, Johnson M, Rudolf M, Huang J-D. The oil spill in ageing Bruch's membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018 doi: 10.1016/j.survophthal.2018.05.005. published online ahead of print May 31. [DOI] [PubMed]
- 35.Gass JDM. Drusen and disciform macular detachment and degeneration. Arch Ophthalmol. 1973;90:206–217. doi: 10.1001/archopht.1973.01000050208006. [DOI] [PubMed] [Google Scholar]
- 36.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferris FL, III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein R, Davis MD, Magli YL, Segal P, Klein BEK, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 39.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age-related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83:358–368. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The incidence and progression of age-related macular degeneration over 15 years: the Blue Mountains Eye Study. Ophthalmology. 2015;122:2482–2489. doi: 10.1016/j.ophtha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan T, Ravindran RD, Murthy GV, et al. Prevalence of early and late age-related macular degeneration in India: the INDEYE study. Invest Ophthalmol Vis Sci. 2010;51:701–707. doi: 10.1167/iovs.09-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19–29. doi: 10.1016/j.preteyeres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Holz FG, Sadda S, Staurenghi G, et al. Imaging protocols for clinical studies in age-related macular degeneration – recommendations from Classification of Atrophy (CAM) Consensus Meeting. Ophthalmology. 2017;124:464–478. doi: 10.1016/j.ophtha.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Khanifar AA, Koreishi AF, Izatt JA, Toth CA. Drusen ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. Ophthalmology. 2008;115:1883–1890. doi: 10.1016/j.ophtha.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang Y, Heussen FM, Hariri A, Keane PA, Sadda SR. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology. 2013;120:2656–2665. doi: 10.1016/j.ophtha.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonnet C, Querques G, Zerbib J, et al. Hyperreflective pyramidal structures on optical coherence tomography in geographic atrophy areas. Retina. 2014;34:1524–1530. doi: 10.1097/IAE.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 49.Oishi A, Thiele S, Nadal J, et al. Prevalence, natural course, and prognostic role of refractile drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:2198–2206. doi: 10.1167/iovs.16-20781. [DOI] [PubMed] [Google Scholar]
- 50.Veerappan M, El-Hage-Sleiman AM, Tai V, et al. Optical coherence tomography reflective drusen substructures predict progression to geographic atrophy in age-related macular degeneration. Ophthalmology. 2016;123:2554–2570. doi: 10.1016/j.ophtha.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lei J, Balasubramanian S, Abdelfattah NS, Nittala M, Sadda SR. Proposal of a simple optical coherence tomography-based scoring system for progression of age related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255:1551–1558. doi: 10.1007/s00417-017-3693-y. [DOI] [PubMed] [Google Scholar]
- 52.Roisman L, Zhang Q, Wang RK, et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology. 2016;123:1309–1319. doi: 10.1016/j.ophtha.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaide RF. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina. 2017;38:708–716. doi: 10.1097/IAE.0000000000001689. [DOI] [PubMed] [Google Scholar]
- 54.Guigui B, Querques G, Leveziel N, et al. Spectral-domain optical coherence tomography of early onset large colloid drusen. Retina. 2013;33:1346–1350. doi: 10.1097/IAE.0b013e318283127d. [DOI] [PubMed] [Google Scholar]
- 55.Carnevali A, Sacconi R, Querques L, et al. Abnormal quiescent neovascularization in a patient with large colloid drusen visualized by optical coherence tomography angiography. Retin Cases Brief Rep. 2017 doi: 10.1097/ICB.0000000000000648. published online ahead of print October 23. [DOI] [PubMed]
- 56.Veronese C, Maiolo C, Mora LD, Morara M, Armstrong GW, Ciardella AP. Bilateral large colloid drusen in a young adult. Retina. 2017;37:e132–e134. doi: 10.1097/IAE.0000000000001868. [DOI] [PubMed] [Google Scholar]
- 57.Invernizzi A, dell'Arti L, Leone G, et al. Drusen-like deposits in young adults diagnosed with systemic lupus erythematosus. Am J Ophthalmol. 2017;175:68–76. doi: 10.1016/j.ajo.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Deutsch TA, Jampol LM. Large druse-like lesions on the surface of choroidal nevi. Ophthalmology. 1985;92:73–76. doi: 10.1016/s0161-6420(85)34066-6. [DOI] [PubMed] [Google Scholar]
- 59.Francis JH, Pang CE, Abramson DH, et al. Swept-source optical coherence tomography features of choroidal nevi. Am J Ophthalmol. 2015;159:169–176.e1. doi: 10.1016/j.ajo.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Sarks JP, Sarks SH, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye. 1994;8:269–283. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- 61.Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathological correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142. [PubMed] [Google Scholar]
- 62.Curcio CA, Medeiros NE, Millican CL. The Alabama Age-related Macular Degeneration Grading System for donor eyes. Invest Ophthalmol Vis Sci. 1998;39:1085–1096. [PubMed] [Google Scholar]
- 63.Curcio CA. Imaging maculopathy in the post-mortem human retina. Vision Res. 2005;45:3496–3503. doi: 10.1016/j.visres.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 64.Balaratnasingam C, Cherepanoff S, Dolz-Marco R, et al. Cuticular drusen: clinical phenotypes and natural course defined using multimodal imaging. Ophthalmology. 2018;125:100–118. doi: 10.1016/j.ophtha.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 65.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15:183–191. [PubMed] [Google Scholar]
- 66.Rudolf M, Malek G, Messinger JD, Wang L, Clark ME, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008;87:402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312.e1. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–210. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 69.Bird AC, Bressler NM, Bressler SB, et al. ; The International ARM Epidemiological Study Group An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 70.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113:260–266. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Pikuleva I, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Ret Eye Res. 2014;41:64–89. doi: 10.1016/j.preteyeres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Leeuwen EM, Emri E, Merle BMJ, et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res. 2018 doi: 10.1016/j.preteyeres.2018.04.006. published online ahead of print May 4. [DOI] [PubMed]
- 74.Chang TY, Yamauchi Y, Hasan M, Chang CC. Cellular cholesterol homeostasis in Alzheimer's disease. J Lipid Res. 2017;58:2239–2254. doi: 10.1194/jlr.R075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishida BY, Duncan KG, Bailey KR, Kane JP, Schwartz DM. High density lipoprotein mediated lipid efflux from retinal pigment epithelial cells in culture. Br J Ophthalmol. 2006;90:616–620. doi: 10.1136/bjo.2005.085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mast N, Reem R, Bederman I, et al. Cholestenoic acid is an important elimination product of cholesterol in the retina: comparison of retinal cholesterol metabolism to that in the brain. Invest Ophthalmol Vis Sci. 2011;52:594–603. doi: 10.1167/iovs.10-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes Rickman C. Directional exosome proteomes reflect polarity-specific functions in retinal pigmented epithelium monolayers. Sci Rep. 2017;7:4901. doi: 10.1038/s41598-017-05102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toomey CB, Johnson LV, Bowes Rickman C. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog Retin Eye Res. 2018;62:38–57. doi: 10.1016/j.preteyeres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grassmann F, Heid IM, Weber BH;, International AMDGC Recombinant haplotypes narrow the ARMS2/HTRA1 association signal for age-related macular degeneration. Genetics. 2017;205:919–924. doi: 10.1534/genetics.116.195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klaver CC, Kliffen M, van Duijn CM, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Souied EH, Benlian P, Amouyel P, et al. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- 83.Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu DJ, Peloso GM, Yu H, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49:1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein R, Klein BE, Jensen SC, Mares-Perlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–1065. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 87.Delcourt C, Michel F, Colvez A, Lacroux A, Delage M, Vernet MH. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237–249. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- 88.Dashti N, McGwin G, Jr, Owsley C, Curcio CA. Plasma apolipoproteins and risk for age-related maculopathy. Br J Ophthalmol. 2006;90:1028–1033. doi: 10.1136/bjo.2006.093856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology. 2017;124:1165–1174. doi: 10.1016/j.ophtha.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu Y, Reynolds R, Fagerness J, Rosner B, Daly MJ, Seddon JM. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4663–4670. doi: 10.1167/iovs.10-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrara D, Seddon JM. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015;133:785–791. doi: 10.1001/jamaophthalmol.2015.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Asten F, Simmons M, Singhal A, et al. A deep phenotype association study reveals specific phenotype associations with genetic variants in age-related macular degeneration: Age-Related Eye Disease Study 2 (AREDS2) Report No. 14. Ophthalmology. 2017;125:559–568. doi: 10.1016/j.ophtha.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng W, Reem R, Omarova S, et al. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One. 2012;7:e37926. doi: 10.1371/journal.pone.0037926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li M, Jia C, Kazmierkiewicz KL, et al. Comprehensive analysis of gene expression in human retina and supporting tissues. Hum Mol Genet. 2014;23:4001–4014. doi: 10.1093/hmg/ddu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walsh MT, Hussain MM. Targeting microsomal triglyceride transfer protein and lipoprotein assembly to treat homozygous familial hypercholesterolemia. Crit Rev Clin Lab Sci. 2017;54:26–48. doi: 10.1080/10408363.2016.1221883. [DOI] [PubMed] [Google Scholar]
- 96.Li CM, Presley JB, Zhang X, et al. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J Lipid Res. 2005;46:628–640. doi: 10.1194/jlr.M400428-JLR200. [DOI] [PubMed] [Google Scholar]
- 97.Wang L, Li C-M, Rudolf M, et al. Lipoprotein particles of intra-ocular origin in human Bruch membrane: an unusual lipid profile. Invest Ophthalmol Vis Sci. 2009;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu T, Fujihara M, Tian J, et al. Apolipoprotein B100 secretion by cultured ARPE-19 cells is modulated by alteration of cholesterol levels. J Neurochem. 2010;114:1734–1744. doi: 10.1111/j.1471-4159.2010.06884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujihara M, Cano K, Handa JT. Mice that produce ApoB100 lipoproteins in the RPE do not develop drusen yet are still a valuable experimental system. Invest Ophthalmol Vis Sci. 2014;55:7285–7295. doi: 10.1167/iovs.14-15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 101.Gordon DA, Jamil H. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim Biophys Acta. 2000;1486:72–83. doi: 10.1016/s1388-1981(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 102.Jamil H, Dickson JK, Jr, Chu CH, et al. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J Biol Chem. 1995;270:6549–6554. doi: 10.1074/jbc.270.12.6549. [DOI] [PubMed] [Google Scholar]
- 103.Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004;45:764–772. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Wetterau JR, Aggerbeck LP, Bouma ME, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 105.Gordon DA, Jamil H, Sharp D, et al. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc Natl Acad Sci U S A. 1994;91:7628–7632. doi: 10.1073/pnas.91.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leiper JM, Bayliss JD, Pease RJ, Brett DJ, Scott J, Shoulders CC. Microsomal triglyceride transfer protein, the abetalipoproteinemia gene product, mediates the secretion of apolipoprotein B-containing lipoproteins from heterologous cells. J Biol Chem. 1994;269:21951–21954. [PubMed] [Google Scholar]
- 107.Sellers JA, Shelness GS. Lipoprotein assembly capacity of the mammary tumor-derived cell line C127 is due to the expression of functional microsomal triglyceride transfer protein. J Lipid Res. 2001;42:1897–1904. [PubMed] [Google Scholar]
- 108.Zhou L, Irani S, Sirwi A, Hussain MM. MicroRNAs regulating apolipoprotein B-containing lipoprotein production. Biochim Biophys Acta. 2016;1861:2062–2068. doi: 10.1016/j.bbalip.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 109.Nielsen LB, Veniant M, Borén J, et al. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation. 1998;98:13–16. doi: 10.1161/01.cir.98.1.13. [DOI] [PubMed] [Google Scholar]
- 110.Madsen EM, Lindegaard ML, Andersen CB, Damm P, Nielsen LB. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J Biol Chem. 2004;279:55271–55276. doi: 10.1074/jbc.M411404200. [DOI] [PubMed] [Google Scholar]
- 111.Björkegren J, Veniant M, Kim SK, et al. Lipoprotein secretion and triglyceride stores in the heart. J Biol Chem. 2001;276:38511–38517. doi: 10.1074/jbc.M106839200. [DOI] [PubMed] [Google Scholar]
- 112.Raabe M, Flynn LM, Zlot CH, et al. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci U S A. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Farese RV, Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc Natl Acad Sci U S A. 1995;92:1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chowers I, Banin E, Merin S, Cooper M, Granot E. Long-term assessment of combined vitamin A and E treatment for the prevention of retinal degeneration in abetalipoproteinaemia and hypobetalipoproteinaemia patients. Eye. 2001;15:525–530. doi: 10.1038/eye.2001.167. [DOI] [PubMed] [Google Scholar]
- 115.Donders F. Beitrage zur pathologischen anatomie des auges [in German] Archiv Fr Ophthalmologie. 1854;1:106–118. [Google Scholar]
- 116.Wedl C. Grundzüge der Pathologischen Histologie (Translated by G. Busk) Vienna: Carl Gerold & Sohn;; 1854. [Google Scholar]
- 117.Müller H. Anatomische beiträge zur ophthalmologie [in German] Graefes Arch Clin Exp Ophthalmol. 1856;2:1–69. [Google Scholar]
- 118.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999;5:28–37. [PubMed] [Google Scholar]
- 119.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarks SH, van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc U K. 1980;100:414–422. [PubMed] [Google Scholar]
- 121.Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- 122.Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011;95:979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- 123.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 124.Sarks SH, Arnold JJ, Sarks JP, Gilles MC, Walter CJ. Prophylactic perifoveal laser treatment of soft drusen. Aust N Z J Ophthalmol. 1996;24:15–26. doi: 10.1111/j.1442-9071.1996.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 125.van der Schaft TL, Mooy CM, de Bruijn WC, Oron FG, Mulder PGH, de Jong PTVM. Histologic features of the early stages of age-related macular degeneration. Ophthalmology. 1992;99:278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- 126.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 127.Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 128.Green WR, Key SN., III Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 129.Rudolf M, Clark ME, Chimento M, Li C-M, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Strunnikova N, Baffi J, Gonzalez A, Silk W, Cousins SW, Csaky KG. Regulated heat shock protein 27 expression in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:2130–2138. [PubMed] [Google Scholar]
- 131.Amin S, Chong NH, Bailey TA, et al. Modulation of sub-RPE deposits in vitro: a potential model for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1281–1288. doi: 10.1167/iovs.03-0671. [DOI] [PubMed] [Google Scholar]
- 132.Bairaiti A, Orzalesi N. The ultrastructure of the pigment epithelium and of the photoreceptor-pigment epithelium interface. J Ultrastruct Res. 1963;9:484–496. doi: 10.1016/s0022-5320(63)80080-5. [DOI] [PubMed] [Google Scholar]
- 133.Nakaisumi Y, Hogan MJ, Feeney L. The ultrastructure of Bruch's membrane. III. The macular area of the human eye. Arch Ophthalmol. 1964;72:395–400. doi: 10.1001/archopht.1964.00970020395018. [DOI] [PubMed] [Google Scholar]
- 134.Wang L, Clark ME, Crossman DK, et al. Abundant lipid and protein components of drusen. PLoS One. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malek G, Li C-M, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits in eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C-M, Clark M, Rudolf M, Curcio CA. Distribution and composition of esterified and unesterified cholesterol in extra-macular drusen. Exp Eye Res. 2007;85:192–201. doi: 10.1016/j.exer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 137.Guyton JR, Klemp KF. The lipid-rich core region of human atherosclerotic fibrous plaques. Prevalence of small lipid droplets and vesicles by electron microscopy. Am J Pathol. 1989;134:705–717. [PMC free article] [PubMed] [Google Scholar]
- 138.Guyton JR, Klemp KF. Transitional features in human atherosclerosis. Intimal thickening, cholesterol clefts, and cell loss in human aortic fatty streaks. Am J Pathol. 1993;143:1444–1457. [PMC free article] [PubMed] [Google Scholar]
- 139.Kruth HS, Shekhonin B. Evidence for loss of apo B from LDL in human atherosclerotic lesions: extracellular cholesteryl ester lipid particles lacking apo B. Atherosclerosis. 1994;105:227–234. doi: 10.1016/0021-9150(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 140.Kruth HS. The fate of lipoprotein cholesterol entering the arterial wall. Curr Opin Lipidol. 1997;8:246–252. doi: 10.1097/00041433-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 141.Guyton JR, Klemp KF, Mims MP. Altered ultrastructural morphology of self-aggregated low density lipoproteins: coalescence of lipid domains forming droplets and vesicles. J Lipid Res. 1991;32:953–962. [PubMed] [Google Scholar]
- 142.Huang J-D, Presley JB, Chimento MF, Curcio CA, Johnson M. Age-related changes in human macular Bruch's membrane as seen by quick-freeze/deep-etch. Exp Eye Res. 2007;85:202–218. doi: 10.1016/j.exer.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ruberti JW, Curcio CA, Millican CL, Menco BP, Huang JD, Johnson M. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch's membrane. Invest Ophthalmol Vis Sci. 2003;44:1753–1759. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- 144.Li C-M, Chung BH, Presley JB, et al. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 145.Killingsworth MC. Age-related components of Bruch's membrane. Graefes Arch Clin Exp Ophthalmol. 1987;225:406–412. doi: 10.1007/BF02334166. [DOI] [PubMed] [Google Scholar]
- 146.Johnson LV, Forest DL, Banna CD, et al. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Milam AH, Curcio CA, Cideciyan AV, et al. Dominant late-onset retinal degeneration with regional variation of sub-RPE deposits, retinal function, and photoreceptor degeneration. Ophthalmology. 2000;107:2256–2266. doi: 10.1016/s0161-6420(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 148.Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005;80:761–775. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 149.Curcio CA, Johnson M, Huang J-D, Rudolf M. Aging, age-related macular degeneration, and the Response-to-Retention of apolipoprotein B-containing lipoproteins. Prog Ret Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 151.Pilgrim MG, Lengyel I, Lanzirotti A, et al. Sub-retinal pigment epithelial deposition of drusen components including hydroxyapatite in a primary cell culture model. Invest Ophthalmol Vis Sci. 2017;58:708–719. doi: 10.1167/iovs.16-21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Schaft TL, de Bruijn WC, Mooy CM, de Jong PTVM. Basal laminar deposit in the aging peripheral human retina. Graefes Arch Clin Exp Ophthalmol. 1993;231:470–475. doi: 10.1007/BF02044234. [DOI] [PubMed] [Google Scholar]
- 153.Bird AC, Marshall J. Retinal pigment epithelial detachments in the elderly. Trans Ophthalmol Soc U K. 1986;105:674–682. [PubMed] [Google Scholar]
- 154.Sheraidah G, Steinmetz R, Maguire J, Pauleikhoff D, Marshall J, Bird AC. Correlation between lipids extracted from Bruch's membrane and age. Ophthalmology. 1993;100:47–51. doi: 10.1016/s0161-6420(13)31712-6. [DOI] [PubMed] [Google Scholar]
- 155.Holz FG, Sheraidah G, Pauleikhoff D, Bird AC. Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol. 1994;112:402–406. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- 156.Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- 157.Starita C, Hussain AA, Marshall J. Decreasing hydraulic conductivity of Bruch's membrane: relevance to photoreceptor survival and lipofuscinoses. Am J Med Genet. 1995;57:235–237. doi: 10.1002/ajmg.1320570224. [DOI] [PubMed] [Google Scholar]
- 158.Starita C, Hussain AA, Patmore A, Marshall J. Localization of the site of major resistance to fluid transport in Bruch's membrane. Invest Ophthalmol Vis Sci. 1997;38:762–767. [PubMed] [Google Scholar]
- 159.Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Ann Rev Biomed Eng. 2004;6:249–273. doi: 10.1146/annurev.bioeng.6.040803.140055. [DOI] [PubMed] [Google Scholar]
- 160.Bretillon L, Thuret G, Gregoire S, et al. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp Eye Res. 2008;87:521–528. doi: 10.1016/j.exer.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 161.Rudolf M, Curcio CA. Esterified cholesterol is highly localized to Bruch's membrane, as revealed by lipid histochemistry in wholemounts of human choroid. J Histochem Cytochem. 2009;57:731–739. doi: 10.1369/jhc.2009.953448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Progr Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 163.Bazan NG, Gordon WC, Rodriguez de Turco EB. Docosahexaenoic acid uptake and metabolism in photoreceptors: retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Adv Exp Med Biol. 1992;318:295–306. doi: 10.1007/978-1-4615-3426-6_26. [DOI] [PubMed] [Google Scholar]
- 164.Grindle CFJ, Marshall J. Ageing changes in Bruch's membrane and their functional implications. Trans Ophthalmol Soc U K. 1978;98:172–175. [PubMed] [Google Scholar]
- 165.Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:64–80. [PubMed] [Google Scholar]
- 166.Zheng W, Mast N, Saadane A, Pikuleva IA. Pathways of cholesterol homeostasis in mouse retina responsive to dietary and pharmacologic treatments. J Lipid Res. 2015;56:81–97. doi: 10.1194/jlr.M053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saadane A, Mast N, Dao T, Ahmad B, Pikuleva IA. Retinal hypercholesterolemia triggers cholesterol accumulation and esterification in photoreceptor cells. J Biol Chem. 2016;291:20427–20439. doi: 10.1074/jbc.M116.744656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Suzuki M, Kamei M, Itabe H, et al. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- 170.Cankova Z, Huang J-D, Kruth H, Johnson M. Passage of low-density lipoproteins through Bruch's membrane and choroid. Exp Eye Res. 2011;93:947–955. doi: 10.1016/j.exer.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spaide R, Ho-Spaide W, Browne R, Armstrong D. Characterization of peroxidized lipids in Bruch's membrane. Retina. 1999;19:141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 172.Rodriguez IR, Clark ME, Lee JW, Curcio CA. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp Eye Res. 2014;128:151–155. doi: 10.1016/j.exer.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Prog Retin Eye Res. 2017;58:115–151. doi: 10.1016/j.preteyeres.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klien BA. The heredodegeneration of the macula lutea: diagnostic and differential diagnostic considerations and a histopathologic report. Am J Ophthalmol. 1950;33:371–379. [Google Scholar]
- 175.Suzuki M, Curcio CA, Mullins RF, Spaide RF. Refractile drusen: clinical imaging and candidate histology. Retina. 2015;35:859–865. doi: 10.1097/IAE.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 176.van der Schaft TL, de Bruijn WC, Mooy CM, Ketelaars DAM, de Jong PTVM. Element analysis of the early stages of age-related macular degeneration. Arch Ophthalmol. 1992;110:389–394. doi: 10.1001/archopht.1992.01080150087034. [DOI] [PubMed] [Google Scholar]
- 177.Pilgrim M, Tan ACS, Fearn S, et al. A mineralomic study of the retinal pigment epithelium-Bruch's membrane complex in human eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:2266–2266. [Google Scholar]
- 178.Flinn JM, Kakalec P, Tappero R, Jones B, Lengyel I. Correlations in distribution and concentration of calcium, copper and iron with zinc in isolated extracellular deposits associated with age-related macular degeneration. Metallomics. 2014;6:1223–1228. doi: 10.1039/c4mt00058g. [DOI] [PubMed] [Google Scholar]
- 179.Thompson RB, Reffatto V, Bundy JG, et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc Natl Acad Sci U S A. 2015;112:1565–1570. doi: 10.1073/pnas.1413347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's Aβ-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003;9:184–190. [PubMed] [Google Scholar]
- 182.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 183.Luibl V, Isas JM, Kayed R, Glabe CG, Langen R, Chen J. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J Clin Invest. 2006;116:378–385. doi: 10.1172/JCI25843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Isas JM, Luibl V, Johnson LV, et al. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010;51:1304–1310. doi: 10.1167/iovs.09-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thompson RB, Reffatto V, Bundy J, et al. A novel mechanism for initiation of sub-RPE deposits. Invest Ophthalmol Vis Sci. 2014;55:623. [Google Scholar]
- 186.Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer's disease. JCI Insight. 2017;2:93621. doi: 10.1172/jci.insight.93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 188.Anderson DH, Ozaki S, Nealon M, et al. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 189.Hageman GS, Luthert PJ, Chong NHC, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Ret Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 190.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 191.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Spaide RF, Armstrong D, Browne R. Continuing medical education review: choroidal neovascularization in age-related macular degeneration–what is the cause? Retina. 2003;23:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 193.Lewis H, Straatsma BR, Foos RY. Chorioretinal juncture. Multiple extramacular drusen. Ophthalmology. 1986;93:1098–1112. doi: 10.1016/s0161-6420(86)33615-7. [DOI] [PubMed] [Google Scholar]
- 194.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 195.Mullins RF, Schoo DP, Sohn EH, et al. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2012;229:729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Natl Acad Sci U S A. 2015;112:E3040–E3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans-retinal: the making of RPE bisretinoids. J Lipid Res. 2010;51:247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Handa JT, Verzijl N, Matsunaga H, et al. Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci. 1999;40:775–779. [PubMed] [Google Scholar]
- 200.Ishibashi T, Murata T, Hangai M, et al. Advanced glycation end products in age-related macular degeneration. Arch Ophthalmol. 1998;116:1629–1629. doi: 10.1001/archopht.116.12.1629. [DOI] [PubMed] [Google Scholar]
- 201.Yoon KD, Yamamoto K, Ueda K, Zhou J, Sparrow JR. A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PLoS One. 2012;7:e41309. doi: 10.1371/journal.pone.0041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bhosale P, Serban B, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys. 2009;483:175–181. doi: 10.1016/j.abb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ablonczy Z, Higbee D, Anderson DM, et al. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013;54:5535–5542. doi: 10.1167/iovs.13-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zemski Berry KA, Gordon WC, Murphy RC, Bazan NG. Spatial organization of lipids in the human retina and optic nerve by MALDI imaging mass spectrometry. J Lipid Res. 2014;55:504–515. doi: 10.1194/jlr.M044990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Adler L IV, Boyer NP, Anderson DM, et al. Determination of N-retinylidene-N-retinylethanolamine (A2E) levels in central and peripheral areas of human retinal pigment epithelium. Photochem Photobiol Sci. 2015;14:1983–1990. doi: 10.1039/c5pp00156k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pallitto P, Ablonczy Z, Jones EE, et al. A2E and lipofuscin distributions in macaque retinal pigment epithelium are similar to human. Photochem Photobiol Sci. 2015;14:1888–1895. doi: 10.1039/c5pp00170f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Anderson DMG, Ablonczy Z, Koutalos Y, et al. Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas. Sci Rep. 2017;7:17352. doi: 10.1038/s41598-017-17402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wing GL, Blanchard GC, Weiter JL. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalm Vis Sci. 1978;17:601–617. [PubMed] [Google Scholar]
- 209.Ach T, Huisingh C, McGwin G, Jr, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:4832–4841. doi: 10.1167/iovs.14-14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tong Y, Ben Ami T, Hong S, et al. Hyperspectral autofluorescence imaging of drusen and retinal pigment epithelium in donor eyes with age-related macular degeneration. Retina. 2016;36(suppl 1):S127–S136. doi: 10.1097/IAE.0000000000001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rudolf M, Vogt SD, Curcio CA, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013;120:821–828. doi: 10.1016/j.ophtha.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yamada Y, Ishibashi K, Ishibashi K, et al. The expression of advanced glycation endproduct receptors in RPE cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840–848. doi: 10.1016/j.exer.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fleckenstein M, Schmitz-Valckenberg S, Martens C, et al. Fundus autofluorescence and spectral domain optical coherence tomography characteristics in a rapidly progressing form of geographic atrophy. Invest Ophthalmol Vis Sci. 2011;52:3761–3766. doi: 10.1167/iovs.10-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ooto S, Vongkulsiri S, Sato T, Suzuki M, Curcio CA, Spaide RF. Outer retinal corrugations in age-related macular degeneration. JAMA Ophthalmol. 2014;132:806–813. doi: 10.1001/jamaophthalmol.2014.1871. [DOI] [PubMed] [Google Scholar]
- 215.Tan ACS, Astroz P, Dansingani KK, et al. The plateau, an optical coherence tomographic signature of geographic atrophy: evolution, multimodal imaging, and candidate histology. Invest Ophthalmol Vis Sci. 2017;58:2349–2358. doi: 10.1167/iovs.16-21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lommatzsch A, Hermans P, Muller KD, Bornfeld N, Bird AC, Pauleikhoff D. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes Arch Clin Exp Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- 217.Löffler KU, Lee WR. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224:493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- 218.Marshall GE, Konstas AGP, Reid GG, Edwards JG, Lee WR. Type IV collagen and laminin in Bruch's membrane and basal linear deposit in the human macula. Br J Ophthalmol. 1992;76:607–614. doi: 10.1136/bjo.76.10.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Knupp C, Munro PM, Luther PK, Ezra E, Squire JM. Structure of abnormal molecular assemblies (collagen VI) associated with human full thickness macular holes. J Struct Biol. 2000;129:38–47. doi: 10.1006/jsbi.1999.4202. [DOI] [PubMed] [Google Scholar]
- 220.Reale E, Groos S, Eckardt U, Eckardt C, Luciano L. New components of ‘basal laminar deposits' in age-related macular degeneration. Cells Tissues Organs. 2008;190:170–181. doi: 10.1159/000187632. [DOI] [PubMed] [Google Scholar]
- 221.Curcio CA, Johnson M. Structure, function, and pathology of Bruch's membrane. In: Ryan SJ, Schachat AP, Wilkinson CP, Hinton DR, Sadda S, Wiedemann P, editors. Retina. London: Elsevier;; 2013. pp. 466–481. [Google Scholar]
- 222.Marmorstein LY, Munier FL, Arsenijevic Y, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wavre-Shapton ST, Tolmachova T, da Silva ML, Futter CE, Seabra MC. Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PLoS One. 2013;8:e57769. doi: 10.1371/journal.pone.0057769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mimoun G, Soubrane G, Coscas G. Macular drusen [in French] J Fr Ophtalmol. 1990;13:511–530. [PubMed] [Google Scholar]
- 225.Curcio CA, Messinger JD, Sloan KR, McGwin G, Jr, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Greferath U, Guymer RH, Vessey KA, Brassington K, Fletcher EL. Correlation of histologic features with in vivo imaging of reticular pseudodrusen. Ophthalmology. 2016;123:1320–1331. doi: 10.1016/j.ophtha.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 227.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- 228.Nagiel A, Sarraf D, Sadda SR, et al. Type 3 neovascularization: evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina. 2015;35:638–647. doi: 10.1097/IAE.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 229.Flamendorf J, Agron E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122:2053–2062. doi: 10.1016/j.ophtha.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33:1800–1808. doi: 10.1097/IAE.0b013e31829c3765. [DOI] [PubMed] [Google Scholar]
- 231.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- 233.Galloway CA, Dalvi S, Hung SSC, et al. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. Proc Natl Acad Sci U S A. 2017;114:E8214–E8223. doi: 10.1073/pnas.1710430114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chirco KR, Tucker BA, Stone EM, Mullins RF. Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Exp Eye Res. 2015;146:393–397. doi: 10.1016/j.exer.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Whitmore SS, Sohn EH, Chirco KR, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 238.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Curcio CA, Zanzottera EC, Ach T, Balaratnasingam C, Freund KB. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:BIO211–BIO226. doi: 10.1167/iovs.17-21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang H, Hartnett ME. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis. 2016;22:189–202. [PMC free article] [PubMed] [Google Scholar]
- 241.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye study. Ophthalmology. 2002;109:1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 242.Joachim ND, Mitchell P, Kifley A, Wang JJ. Incidence, progression, and associated risk factors of medium drusen in age-related macular degeneration: findings from the 15-year follow-up of an Australian cohort. JAMA Ophthalmol. 2015;133:698–705. doi: 10.1001/jamaophthalmol.2015.0498. [DOI] [PubMed] [Google Scholar]
- 243.Klein ML, Ferris FL, III, Armstrong J, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1026–1031. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 244.Pieroni CG, Witkin AJ, Ko TH, et al. Ultrahigh resolution optical coherence tomography in non-exudative age related macular degeneration. Br J Ophthalmol. 2006;90:191–197. doi: 10.1136/bjo.2005.076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fleckenstein M, Charbel Issa P, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:4137–4144. doi: 10.1167/iovs.08-1967. [DOI] [PubMed] [Google Scholar]
- 246.Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009;116:488–496.e2. doi: 10.1016/j.ophtha.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Keane PA, Patel PJ, Liakopoulos S, Heussen FM, Sadda SR, Tufail A. Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol. 2012;57:389–414. doi: 10.1016/j.survophthal.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 248.Folgar FA, Chow JH, Farsiu S, et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012;53:4626–4633. doi: 10.1167/iovs.12-9813. [DOI] [PubMed] [Google Scholar]
- 249.Ho J, Witkin AJ, Liu J, et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118:687–693. doi: 10.1016/j.ophtha.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zacks DN, Johnson MW. Transretinal pigment migration: an optical coherence tomographic study. Arch Ophthalmol. 2004;122:406–408. doi: 10.1001/archopht.122.3.406. [DOI] [PubMed] [Google Scholar]
- 251.Zanzottera EC, Messinger JD, Ach T, Smith RT, Freund KB, Curcio CA. The project MACULA retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3253–3268. doi: 10.1167/iovs.15-16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pang C, Messinger JD, Zanzottera EC, Freund KB, Curcio CA. The onion sign in neovascular age-related macular degeneration represents cholesterol crystals. Ophthalmology. 2015;122:2316–2326. doi: 10.1016/j.ophtha.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen KC, Jung JJ, Curcio CA, et al. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: clinical and histologic study. Am J Ophthalmol. 2016;164:89–98. doi: 10.1016/j.ajo.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 254.Balaratnasingam C, Messinger JD, Sloan KR, Yannuzzi LA, Freund KB, Curcio CA. Histologic and optical coherence tomographic correlations in drusenoid pigment epithelium detachment in age-related macular degeneration. Ophthalmology. 2017;124:644–656. doi: 10.1016/j.ophtha.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li M, Dolz-Marco R, Messinger JD, et al. Clinicopathologic correlation of anti-vascular endothelial growth factor-treated type 3 neovascularization in age-related macular degeneration. Ophthalmology. 2018;125:276–287. doi: 10.1016/j.ophtha.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 256.Dolz-Marco R, Balaratnasingam C, Messinger JD, et al. The border of macular atrophy in age-related macular degeneration: a clinicopathologic correlation. Am J Ophthalmol. 2018;193:166–177. doi: 10.1016/j.ajo.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 257.Bressler NM, Munoz B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities. Arch Ophthalmol. 1995;113:301–308. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- 258.Sebag M, Peli E, Lahav M. Image analysis of changes in drusen area. Acta Ophthalmol. 1991;69:603–610. doi: 10.1111/j.1755-3768.1991.tb04847.x. [DOI] [PubMed] [Google Scholar]
- 259.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118:2434–2441. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schlanitz FG, Baumann B, Kundi M, et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br J Ophthalmol. 2016;101:198–203. doi: 10.1136/bjophthalmol-2016-308422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Schaal KB, Rosenfeld PJ, Gregori G, Yehoshua Z, Feuer WJ. Anatomic clinical trial endpoints for nonexudative age-related macular degeneration. Ophthalmology. 2016;123:1060–1079. doi: 10.1016/j.ophtha.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 262.Bressler NM, Munoz B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities. Waterman study. Arch Ophthalmol. 1995;113:301–308. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- 263.Sparrow JM, Dickinson AJ, Duke AM, Thompson JR, Gibson JM, Rosenthal AR. Seven year follow-up of age-related maculopathy in an elderly British population. Eye. 1997;11:315–324. doi: 10.1038/eye.1997.67. [DOI] [PubMed] [Google Scholar]
- 264.Cukras C, Agron E, Klein ML, et al. Natural history of drusenoid pigment epithelial detachment in age-related macular degeneration: Age-Related Eye Disease Study Report No. 28. Ophthalmology. 2010;117:489–499. doi: 10.1016/j.ophtha.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Balaratnasingam C, Yannuzzi LA, Curcio CA, et al. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Invest Ophthalmol Vis Sci. 2016;57:5479–5489. doi: 10.1167/iovs.16-19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schlanitz FG, Sacu S, Baumann B, et al. Identification of drusen characteristics in age-related macular degeneration by polarization-sensitive optical coherence tomography. Am J Ophthalmol. 2015;160:335–344.e1. doi: 10.1016/j.ajo.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121:2415–2422. doi: 10.1016/j.ophtha.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 268.Zhang Q, Zheng F, Motulsky EH, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Invest Ophthalmol Vis Sci. 2018;59:203–211. doi: 10.1167/iovs.17-22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stefansson E, Geirsdottir A, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011;30:72–80. doi: 10.1016/j.preteyeres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 270.Tan AC, Dansingani KK, Yannuzzi LA, Sarraf D, Freund KB. Type 3 neovascularization imaged with cross-sectional and en face optical coherence tomography angiography. Retina. 2016;37:234–246. doi: 10.1097/IAE.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 271.Monson DM, Smith JR, Klein ML, Wilson DJ. Clinicopathologic correlation of retinal angiomatous proliferation. Arch Ophthalmol. 2008;126:1664–1668. doi: 10.1001/archopht.126.12.1664. [DOI] [PubMed] [Google Scholar]
- 272.Bogunovic H, Montuoro A, Baratsits M, et al. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest Ophthalmol Vis Sci. 2017;58:BIO141–BIO150. doi: 10.1167/iovs.17-21789. [DOI] [PubMed] [Google Scholar]
- 273.Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–439. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Funatsu T, Suzuki K, Goto M, et al. Prolonged inhibition of cholesterol synthesis by atorvastatin inhibits apo B-100 and triglyceride secretion from HepG2 cells. Atherosclerosis. 2001;157:107–115. doi: 10.1016/s0021-9150(00)00714-0. [DOI] [PubMed] [Google Scholar]
- 275.Hayes KC, Lindsey S, Stephan ZF, Brecker D. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest Ophthalmol Vis Sci. 1989;30:225–232. [PubMed] [Google Scholar]
- 276.Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst Rev. 2012;3:CD006927. doi: 10.1002/14651858.CD006927.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tsao SW, Fong DS. Do statins have a role in the prevention of age-related macular degeneration? Drugs Aging. 2013;30:205–213. doi: 10.1007/s40266-013-0061-4. [DOI] [PubMed] [Google Scholar]
- 278.Ma L, Wang Y, Du J, Wang M, Zhang R, Fu Y. The association between statin use and risk of age-related macular degeneration. Sci Rep. 2015;5:18280. doi: 10.1038/srep18280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Guymer RH, Baird PN, Varsamidis M, et al. Proof of concept, randomized, placebo-controlled study of the effect of simvastatin on the course of age-related macular degeneration. PLoS One. 2013;8:e83759. doi: 10.1371/journal.pone.0083759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Barathi VA, Yeo SW, Guymer RH, Wong TY, Luu CD. Effects of simvastatin on retinal structure and function of a high-fat atherogenic mouse model of thickened Bruch's membrane. Invest Ophthalmol Vis Sci. 2014;55:460–468. doi: 10.1167/iovs.13-11636. [DOI] [PubMed] [Google Scholar]
- 281.Vavvas DG, Daniels AB, Kapsala ZG, et al. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBioMed. 2016;5:198–203. doi: 10.1016/j.ebiom.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rudolf M, Mir Mohi Sefat A, Miura Y, et al. ApoA-I mimetic peptide 4F reduces age-related lipid deposition in murine Bruch's membrane and causes its structural remodeling. Curr Eye Res. 2018;43:135–146. doi: 10.1080/02713683.2017.1370118. [DOI] [PubMed] [Google Scholar]
- 283.Navab M, Anantharamaiah GM, Reddy ST, Fogelman AM. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat Clin Pract Cardiovasc Med. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- 284.Navab M, Anantharamaiah GM, Reddy ST, et al. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol. 2005;25:1325–1331. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 285.Datta G, Chaddha M, Hama S, et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- 286.Buga GM, Frank JS, Mottino GA, et al. D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J Lipid Res. 2008;49:192–205. doi: 10.1194/jlr.M700433-JLR200. [DOI] [PubMed] [Google Scholar]
- 287.Gupta H, Dai L, Datta G, et al. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ Res. 2005;97:236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 288.Anantharamaiah GM, Mishra VK, Garber DW, et al. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J Lipid Res. 2007;48:1915–1923. doi: 10.1194/jlr.R700010-JLR200. [DOI] [PubMed] [Google Scholar]
- 289.Leman LJ, Maryanoff BE, Ghadiri MR. Molecules that mimic apolipoprotein A-I: potential agents for treating atherosclerosis. J Med Chem. 2014;57:2169–2196. doi: 10.1021/jm4005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Van Lenten BJ, Wagner AC, Jung C-L, et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Reddy ST, Navab M, Anantharamaiah GM, Fogelman AM. Apolipoprotein A-I mimetics. Curr Opin Lipidol. 2014;25:304–308. doi: 10.1097/MOL.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Smith JD, Apolipoprotein A-I. and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2010;11:989–996. [PMC free article] [PubMed] [Google Scholar]
- 293.Navab M, Anantharamaiah GM, Reddy ST, et al. Potential clinical utility of high-density lipoprotein-mimetic peptides. Curr Opin Lipidol. 2006;17:440–444. doi: 10.1097/01.mol.0000236371.27508.d4. [DOI] [PubMed] [Google Scholar]
- 294.Watson CE, Weissbach N, Kjems L, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Rudolf M, Mohi A, Dettbarn MC, et al. Detection of esterified cholesterol in murine Bruch's membrane wholemounts with a perfringolysin O-based cholesterol marker. Invest Ophthalmol Vis Sci. 2014;55:4759–4767. doi: 10.1167/iovs.14-14311. [DOI] [PubMed] [Google Scholar]
- 297.Apte RS. Targeting tissue lipids in age-related macular degeneration. EBioMedicine. 2016;5:26–27. doi: 10.1016/j.ebiom.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Picard E, Houssier M, Bujold K, et al. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging. 2010;2:981–989. doi: 10.18632/aging.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nissen SE, Tuzcu EM, Brewer HB, et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med. 2006;354:1253–1263. doi: 10.1056/NEJMoa054699. [DOI] [PubMed] [Google Scholar]
- 300.Rudel LL, Farese RV., Jr ACAT inhibition and the progression of coronary atherosclerosis. N Engl J Med. 2006;354:2616–2617. doi: 10.1056/NEJMc061094. author reply 2616–2617. [DOI] [PubMed] [Google Scholar]
- 301.Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cuchel M, Blom DJ, Averna MR. Clinical experience of lomitapide therapy in patients with homozygous familial hypercholesterolaemia. Atheroscler Suppl. 2014;15:33–45. doi: 10.1016/j.atherosclerosissup.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 303.Fine BS, Kwapien RP. Pigment epithelial windows and drusen: an animal model. Invest Ophthalmol Vis Sci. 1978;17:1059–1068. [PubMed] [Google Scholar]
- 304.Fine BS. Lipoidal degeneration of the retinal pigment epithelium. Am J Ophthalmol. 1981;91:469–473. doi: 10.1016/0002-9394(81)90234-8. [DOI] [PubMed] [Google Scholar]
- 305.Anderson MD, Dawson WW, Martinez-Gonzalez J, Curcio CA. Drusenoid lesions and lipid-filled retinal pigment epithelium cells in a rhesus macula. Vet Ophthalmol. 2006;9:201–207. doi: 10.1111/j.1463-5224.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 306.Yiu G, Tieu E, Munevar C, et al. In vivo multimodal imaging of drusenoid lesions in rhesus macaques. Sci Rep. 2017;7:15013. doi: 10.1038/s41598-017-14715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Owsley C, Clark ME, Huisingh CE, Curcio CA, McGwin G., Jr Visual function in older eyes in normal macular health: association with incident early age-related macular degeneration 3 years later. Invest Ophthalmol Vis Sci. 2016;57:1782–1789. doi: 10.1167/iovs.15-18962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Owsley C, McGwin G, Jr, Clark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123:344–351. doi: 10.1016/j.ophtha.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Owsley C, Clark M, McGwin G., Jr Natural history of rod-mediated dark adaptation over two years in intermediate age-related macular degeneration. Trans Vis Sci Tech. 2017;6(3):15. doi: 10.1167/tvst.6.3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, Singerman LJ. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33:498–507. doi: 10.1097/IAE.0b013e318265801d. [DOI] [PubMed] [Google Scholar]
- 311.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Jaffe GJ, Schmitz-Valckenberg S, Boyer D, et al. Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. Am J Ophthalmol. 2015;160:1226–1234. doi: 10.1016/j.ajo.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 313.Rosenfeld PJ, Dugel PU, Holz FG, et al. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2018 doi: 10.1016/j.ophtha.2018.03.059. published online ahead of print April 28. [DOI] [PubMed]
- 314.Guidry C, Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:267–273. [PubMed] [Google Scholar]
- 315.Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br J Ophthalmol. 2003;87:1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bird AC, Phillips RL, Hageman GS. Geographic atrophy: a histopathological assessment. JAMA Ophthalmol. 2014;132:338–345. doi: 10.1001/jamaophthalmol.2013.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Edwards MM, McLeod DS, Bhutto IA, Grebe R, Duffy M, Lutty GA. Subretinal glial membranes in eyes with geographic atrophy. Invest Ophthalmol Vis Sci. 2017;58:1352–1367. doi: 10.1167/iovs.16-21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Li M, Huisingh C, Messinger JD, et al. Histology of geographic atrophy secondary to age-related macular degeneration: a multilayer approach. Retina. 2018 doi: 10.1097/IAE.0000000000002182. published online ahead of print May 3. [DOI] [PMC free article] [PubMed]
- 319.Garcia Filho CA, Yehoshua Z, Gregori G, et al. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:18–31. doi: 10.3928/23258160-20131217-01. [DOI] [PubMed] [Google Scholar]
- 320.Christenbury JG, Folgar FA, O'Connell RV, Chiu SJ, Farsiu S, Toth CA. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120:1038–1045. doi: 10.1016/j.ophtha.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Hope GM, Dawson WW, Engel HM, Ulshafer RJ, Kessler MJ, Sherwood MB. A primate model for age related macular drusen. Br J Ophthalmol. 1992;76:11–16. doi: 10.1136/bjo.76.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Francis PJ, Appukuttan B, Simmons E, et al. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Human Mol Genet. 2008;17:2673–2680. doi: 10.1093/hmg/ddn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yin W, Carballo-Jane E, McLaren DG, et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. 2012;53:51–65. doi: 10.1194/jlr.M019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Umeda S, Ayyagari R, Allikmets R, et al. Early-onset macular degeneration with drusen in a cynomolgus monkey (Macaca fascicularis) pedigree: exclusion of 13 candidate genes and loci. Invest Ophthalmol Vis Sci. 2005;46:683–691. doi: 10.1167/iovs.04-1031. [DOI] [PubMed] [Google Scholar]
- 325.Mullins RF, Hageman GS. Histochemical comparison of “ocular drusen” in monkey and human. In: Lavail M, Hollyfield J, Anderson RE, editors. Degenerative Retinal Diseases. NY: Plenum;; 1997. pp. 1–10. [Google Scholar]
- 326.Ishibashi T, Sorgente N, Patterson R, Ryan SJ. Pathogenesis of drusen in the primate. Invest Ophthalm Vis Sci. 1986;27:184–193. [PubMed] [Google Scholar]
- 327.Starnes AC, Huisingh C, McGwin G, et al. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis Neurosci. 2016;33:E001. doi: 10.1017/S0952523815000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Marmorstein LY, McLaughlin PJ, Peachey NS, Sasaki T, Marmorstein AD. Formation and progression of sub-retinal pigment epithelium deposits in Efemp1 mutation knock-in mice: a model for the early pathogenic course of macular degeneration. Human Mol Genet. 2007;16:2423–2432. doi: 10.1093/hmg/ddm199. [DOI] [PubMed] [Google Scholar]
- 329.Fu L, Garland D, Yang Z, et al. The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Human Mol Genet. 2007;16:2411–2422. doi: 10.1093/hmg/ddm198. [DOI] [PubMed] [Google Scholar]
- 330.Garland DL, Fernandez-Godino R, Kaur I, et al. Mouse genetics and proteomic analyses demonstrate a critical role for complement in a model of DHRD/ML, an inherited macular degeneration. Hum Mol Genet. 2014;23:52–68. doi: 10.1093/hmg/ddt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Jiang M, Esteve-Rudd J, Lopes VS, et al. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J Cell Biol. 2015;210:595–611. doi: 10.1083/jcb.201410112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Dinculescu A, Min SH, Dyka FM, et al. Pathological effects of mutant C1QTNF5 (S163R) expression in murine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:6971–6980. doi: 10.1167/iovs.15-17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bretillon L, Acar N, Seeliger MW, et al. ApoB100,LDLR−/− mice exhibit reduced electroretinographic response and cholesteryl esters deposits in the retina. Invest Ophthalmol Vis Sci. 2008;49:1307–1314. doi: 10.1167/iovs.07-0808. [DOI] [PubMed] [Google Scholar]
- 334.Collin GB, Hubmacher D, Charette JR, et al. Disruption of murine Adamtsl4 results in zonular fiber detachment from the lens and in retinal pigment epithelium dedifferentiation. Hum Mol Genet. 2015;24:6958–6974. doi: 10.1093/hmg/ddv399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Saksens NT, Krebs MP, Schoenmaker-Koller FE, et al. Mutations in CTNNA1 cause butterfly-shaped pigment dystrophy and perturbed retinal pigment epithelium integrity. Nat Genet. 2016;48:144–151. doi: 10.1038/ng.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hu W, Jiang A, Liang J, et al. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2008;49:407–415. doi: 10.1167/iovs.07-0870. [DOI] [PubMed] [Google Scholar]
- 337.Kumar S, Berriochoa Z, Ambati BK, Fu Y. Angiographic features of transgenic mice with increased expression of human serine protease HTRA1 in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:3842–3850. doi: 10.1167/iovs.13-13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li B, Vachali PP, Gorusupudi A, et al. Inactivity of human beta,beta-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci U S A. 2014;111:10173–10178. doi: 10.1073/pnas.1402526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Le YZ, Zheng W, Rao PC, et al. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2008;49:1248–1253. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ueki Y, Ash JD, Zhu M, Zheng L, Le YZ. Expression of Cre recombinase in retinal Muller cells. Vision Res. 2009;49:615–621. doi: 10.1016/j.visres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res. 2002;75:543–553. doi: 10.1006/exer.2002.2047. [DOI] [PubMed] [Google Scholar]
- 342.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 343.Imamura Y, Noda S, Hashizume K, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lee WH, Higuchi H, Ikeda S, et al. Mouse Tmem135 mutation reveals a mechanism involving mitochondrial dynamics that leads to age-dependent retinal pathologies. Elife. 2016;5:e19264. doi: 10.7554/eLife.19264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 348.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 349.Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiol Aging. 2013;34:943–960. doi: 10.1016/j.neurobiolaging.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lad EM, Cousins SW, Van Arnam JS, Proia AD. Abundance of infiltrating CD163+ cells in the retina of postmortem eyes with dry and neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253:1941–1945. doi: 10.1007/s00417-015-3094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ablonczy Z, Dahrouj M, Tang PH, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011;52:8614–8620. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Peng S, Rao VS, Adelman RA, Rizzolo LJ. Claudin-19 and the barrier properties of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:1392–1403. doi: 10.1167/iovs.10-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Gamm DM, Melvan JN, Shearer RL, et al. A novel serum-free method for culturing human prenatal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:788–799. doi: 10.1167/iovs.07-0777. [DOI] [PubMed] [Google Scholar]
- 355.Miyagishima KJ, Wan Q, Corneo B, et al. In pursuit of authenticity: induced pluripotent stem cell-derived retinal pigment epithelium for clinical applications. Stem Cells Transl Med. 2016;5:1562–1574. doi: 10.5966/sctm.2016-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Miyagishima KJ, Wan Q, Miller SS, Bharti K. A basis for comparison: sensitive authentication of stem cell derived RPE using physiological responses of intact RPE monolayers. Stem Cell Transl Investig. 2017;4:e1497. [PMC free article] [PubMed] [Google Scholar]
- 357.Chen L, Zhang X, Liu B, Mi L, Wen F. Age-related scattered hypofluorescent spots on late-phase indocyanine green angiography: the multimodal imaging and relevant factors. Clin Exp Ophthalmol. 2018 doi: 10.1111/ceo.13306. published online ahead of print April 19. [DOI] [PMC free article] [PubMed]
- 358.Li C-M, Clark ME, Chimento MF, Curcio CA. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Invest Ophthalmol Vis Sci. 2006;47:3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- 359.Oak ASW, Messinger JD, Curcio CA. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014;34:825–826. doi: 10.1097/IAE.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 360.Zarubina AV, Neely DC, Clark ME, et al. Prevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imaging. Ophthalmology. 2016;123:1090–1100. doi: 10.1016/j.ophtha.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Genead MA, Fishman GA, Lindeman M. Spectral-domain optical coherence tomography and fundus autofluorescence characteristics in patients with fundus albipunctatus and retinitis punctata albescens. Ophthalmic Genet. 2010;31:66–72. doi: 10.3109/13816810903584971. [DOI] [PubMed] [Google Scholar]
- 362.Zweifel SA, Imamura Y, Freund KB, Spaide RF. Multimodal fundus imaging of pseudoxanthoma elasticum. Retina. 2011;31:482–491. doi: 10.1097/IAE.0b013e3181f056ce. [DOI] [PubMed] [Google Scholar]
- 363.Aleman TS, Garrity ST, Brucker AJ. Retinal structure in vitamin A deficiency as explored with multimodal imaging. Doc Ophthalmol. 2013;127:239–243. doi: 10.1007/s10633-013-9403-0. [DOI] [PubMed] [Google Scholar]
- 364.Gliem M, Hendig D, Finger RP, Holz FG, Charbel Issa P. Reticular pseudodrusen associated with a diseased Bruch membrane in pseudoxanthoma elasticum. JAMA Ophthalmol. 2015;133:581–588. doi: 10.1001/jamaophthalmol.2015.117. [DOI] [PubMed] [Google Scholar]
- 365.Gliem M, Muller PL, Mangold E, et al. Reticular pseudodrusen in Sorsby fundus dystrophy. Ophthalmology. 2015;122:1555–1562. doi: 10.1016/j.ophtha.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 366.Freund KB, Zweifel SA, Englebert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30:1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- 367.Dansingani KK, Gal-Or O, Sadda SR, Yannuzzi LA, Freund KB. Understanding aneurysmal type 1 neovascularisation (polypoidal choroidal vasculopathy): a lesson in the taxonomy of “expanded spectra”. Clin Exp Ophthalmol. 2017;46:189–200. doi: 10.1111/ceo.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–1506. doi: 10.1097/IAE.0000000000000627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.