Abstract
OBJECTIVE:
To assess if there is a relationship between use of combined antiretroviral therapy among pregnant women living with HIV and hypertensive disorders of pregnancy (HDP)
DESIGN:
Due to the heterogeneity of study designs in the literature and the utilization of different outcome measures in regards to assessing the presence of HDP, a systematic review was performed.
METHODS:
ClinicalTrials.gov and MEDLINE, via PubMed, EMBASE, Scopus, CINAHL, ProQuest Dissertations & Theses Global, EBSCOHost, DARE, and the Cochrane Library, were queried from January 1997 to October 2017. Studies were included if they reported HDP and focused on pregnant women living with HIV who used combined antiretroviral therapy. The Cochrane Collaboration’s tool for assessment of risk of bias and the U.S. Preventive Services Task Force grading scale were used to assess the studies.
RESULTS:
Of 1055 abstracts, 28 articles met inclusion criteria. The data are marked by multiple biases and poor study design. All studies demonstrate an increased risk of HDP among pregnant women living with HIV who used combined antiretroviral therapy when compared to seropositive pregnant women not using antiretroviral therapy. Three studies suggest protease inhibitors may be associated with a higher risk of HDP.
CONCLUSION:
Despite all studies indicating a higher frequency of HDP among pregnant women living with HIV using combined antiretroviral therapy when compared with seropositive pregnant women not using antiretroviral therapy, the quality of the studies is mixed, necessitating further research.
Keywords: hypertensive disorders of pregnancy, preeclampsia, cART, antiretroviral therapy, HIV, pregnancy
INTRODUCTION
Hypertensive disorders of pregnancy (HDP) – a family of conditions that include gestational hypertension, preeclampsia, eclampsia, and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome – are a major source of maternal morbidity and mortality worldwide (1). While there are multiple theories as to the etiology of preeclampsia, one major hypothesis posits that the disease stems from a dysregulated immune response to the pregnancy (2). In situations of immunocompromise – such as among women living with HIV (WLHIV) – researchers have theorized that a differential risk of HDP may exist. However, with the use of combined antiretroviral therapy (cART), the immune function of WLHIV may be reconstituted, though there may be a chronic inflammatory state that remains (3, 4). With the global successes of universal treatment, a growing number of women are exposed to cART during the preconception, antenatal, and peripartum periods (5, 6). Elucidating the relationship among HIV, cART, and HDP is crucial to further understanding the cause of HDP as well as providing appropriate clinical surveillance and counseling to WLHIV who are either attempting pregnancy or who are pregnant.
Initial reports suggested women with untreated HIV had lower frequency of preeclampsia compared to those who had received any form of antiretroviral therapy, suggesting that immunosuppressed women lack the ability to mount the exacerbated immune response that likely occurs in HDP (3). However, other data suggest rates of HDP are similar when comparing pregnant WLHIV using cART and those who did not receive treatment (7–9). There have been multiple hypothesized mechanisms underlying this relationship between cART use and HDP, including misattribution of cART toxicity or side effects as HDP, such as the known role of protease inhibitors (PI) in increasing blood pressure, and an immune reconstitution effect that renders pregnant WLHIV to have similar immune function as women without HIV (4, 10).
Four meta-analyses over the past ten years have evaluated the relationship among HIV, cART and HDP with conflicting results (11–14). Adams et al, Conde-Agudelo et al, and Brown et al. found that the majority of data do not support the relationship between preeclampsia and HIV, with or without the use of cART (11–13). In contrast, one meta-analysis of 17 studies notes that pregnant WLHIV had a higher risk for developing pregnancy-induced hypertension when compared with pregnant, HIV-uninfected women (OR 1.46, 95% CI 1.03–2.05) (14). A major limitation of existing literature has been the comparison of WLHIV to women without HIV. The more clinically relevant issue for WLHIV is the risk of HDP while using cART compared to WLHIV who are not using cART, as well as the impact of specific regimens or timing of cART initiation on this risk. Comprehensive reviews of these issues are limited (15).
Given the heterogeneity of the data surrounding HIV, cART, and HDP, as well as the recent contributions to the literature, we performed a systematic review to assess the relationship between cART use and HDP among WLHIV. Secondary objectives included an evaluation of whether the use of certain types of antiretroviral medications in a cART regimen increase the risk of HDP.
MATERIALS AND METHODS
Search strategy
Studies were eligible if they included pregnant WLHIV and reported HDP. Results were restricted to English language literature, due to the authors’ lack of proficiency in other languages, published from 1 January 1997 until 31 October 2017. This period of time was chosen to reflect the era of perinatal HIV treatment in which cART was the standard of care. A systematic literature search was performed with the support of a research librarian in ClinicalTrials.gov and MEDLINE via PubMed, EMBASE, Scopus, CINAHL, Proquest Dissertations & Theses Global, EBSCOHost, DARE, and the Cochrane Library. The study protocol was uploaded to PROSPERO, the international prospective register of systematic reviews, prior to performing the search (CRD #42017078838).(16) The study protocol adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (17).
Two authors (A.P. & A.M.D.) reviewed all abstracts for inclusion using Abstrackr Beta (Brown University, Providence, RI). Once relevant abstracts were agreed upon, full-text analysis of included abstracts was then performed by the same authors. Relevant articles meeting final inclusion criteria were then abstracted for bias, study quality, and overall findings. The Cochrane Collaboration’s tool for assessment of risk of bias was used for further analysis (18). In order to rate studies as “good,” “fair,” and “poor,” authors adhered to the U.S. Preventive Services Task Force grading scale (19). Both assessment of risk of bias and study rating were made independently by two authors (A.P. & A.M.D.); any disagreements were discussed with the other researchers (L.M.Y. & L.H.) for a final consensus.
Study selection
Meta-analyses, systematic reviews, randomized controlled trials (RCTs), including randomized crossover studies and cluster randomized trials, non-experimental observational studies (e.g. case-control, cohort, and cross-sectional) and abstracts/presentations available in the above databases were included for initial evaluation. The final required inclusion criteria were limited to full-manuscript randomized controlled trials and observational studies of pregnant WLHIV. Studies were excluded if they were of non-pregnant adults, did not address WLHIV, or did not measure HDP as an outcome.
Additional inclusion criteria were that at least one or more groups in the observational study or randomized trial must be on cART. cART includes any regimen with 3 or more medications, such as 3-class regimens, integrase inhibitor-based regimens, PI-based regimens, and non-nucleoside reverse transcriptase inhibitor (NNRTI) regimens. For the secondary objectives, we assessed outcomes based on the backbone drug class.
The primary outcome for this systematic review is a diagnosis of HDP, which includes the following: gestational hypertension, preeclampsia (with or without severe features), superimposed preeclampsia (with or without severe features), eclampsia, or HELLP syndrome. Due to the historical nature of the review, we chose to include definitions of HDP that were extant at the time of the study, but may fall outside the current recommendations set forth by the American College of Obstetricians and Gynecologists (ACOG) in 2013 (20). Given the lack of individual patient level data, there may be additional heterogeneity in definitions of HDP as they were determined locally within each study protocol. Similarly, we chose to look at all diagnoses together as HDP, recognizing the continuum of HDP and the inconsistencies among study definitions. The heterogeneity of the reported results and the variety of definitions employed regarding HDP precluded performance of a meta-analysis. Instead, the results are presented in the form of a systematic review.
RESULTS
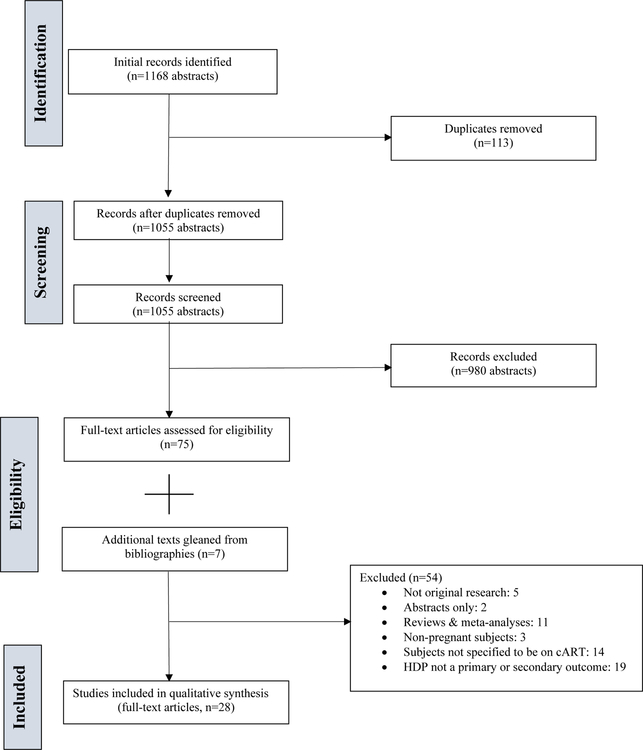
An electronic search retrieved 1168 documents. After EndNote X7.2 (Clarivate Analytics, Philadelphia, PA) was used to remove 113 duplicate records, 1055 abstracts were available for review. Ultimately, 75 articles were included for full text-analysis. After review, 54 articles were subsequently excluded. Seven additional articles were added after review of the bibliographies of all included articles. In total, 28 full-text articles were available for qualitative analysis (Figure 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram
All included studies
Of the 28 articles included, a majority of studies (n=22, 78.5%) had data from non-U.S. settings, with a significant proportion from sub-Saharan Africa (n=11, 39.2%) (Table 1). None were RCTs; studies were either retrospective cohort studies (n=10), case-control studies (n=6), secondary analyses of RCTs (n=1), case series (n=1), cross-sectional observational studies (n=1), or prospective observational cohort analyses (n=9). Of a total of 19 cohort studies included, 42.1% (n=8) did not include any control group for comparison. The remainder of cohort studies offered a matched, HIV-uninfected comparison group (n=11, 68.9%), while one study created a seronegative control group additionally matched for age and parity.(21) No studies matched for CD4 count.
TABLE 1.
Included Studies
| Study No. | Author, year, country | Study Design | Population | Exposure | Outcome | Results | Quality |
|---|---|---|---|---|---|---|---|
| A. cART v. no cART | |||||||
| 1 | Suy et al. (2006) (32) Spain | Prospective, matched observational cohort, 2001–03 (HIV-negative pregnant women), 1985–2003 (HIV-positive pregnant women) | HIV-positive and negative pregnant women | HIV | Preeclampsia and stillbirth | 472 HIV-positive women available for analysis. 76% had no ARV exposure prior to pregnancy, and 59% had no ARV exposure during pregnancy. Of those using ARVs during pregnancy, 25% used ARVs at an optimal dose. HIV-positive women had a higher incidence of preeclampsia than HIV-negative women (109.8/1000 deliveries versus 28.6/1000 deliveries, OR 4.3, 95% CI 1.9–9.0). After adjustment, use of cART was independently associated with development of preeclampsia among 9 women with HIV (aOR 8.9, 95% CI 1.7–45.5). |
Fair |
| 2 | Machado et al. (2014) (25) Latin America and Caribbean | Prospective observational cohort analysis (2002–2009) | HIV-positive pregnant women | cART, including PI v. single/dual/triple ARV therapy | HDP diagnosed after 20 weeks’ of gestation | 1513 pregnancies available, 73 cases of HDP (4.8%, 95% CI: 3.8–6%), 2.3% had preeclampsia/eclampsia (95% CI 1.7–3.2%) On bivariate analysis, use of cART or any PI regimen at conception was associated with HDP (7.1% v. 4.3%, p = 0.04), including preeclampsia/eclampsia (4.4% v. 1.8, p < 0.01); this trend did not persist with ARV initiation in 1st trimester (6.6 v. 4.2 % for gestational hypertension, p = 0.07; 3.6% v. 1.9% for preeclampsia/eclampsia, p = 0.07) |
Good |
| 3 | Sansone et al. (2016)(15) Italy | Retrospective cohort analysis, single site (1989–2015) | HIV-positive and negative pregnant women | HIV, cART | Preeclampsia with severe features, early onset preeclampsia with severe features, and preterm birth | 453 HIV-positive pregnant women available for analysis. 66.4% received cART during pregnancy, while 22.0% used single (AZT) or dual ARV (AZT and 3TC) therapy When compared with HIV- negative women, HIV- positive women had higher incidences of preeclampsia (10.2% v 4.1%, aOR 2.03, 95% CI 1.26–3.28) and preeclampsia with severe features (4.0% v 2.0%, aOR 2.03, 95% CI 1.26–3.28). HIV-positive women using cART had a higher incidence of preeclampsia when compared to seronegative women (13.0% v 4.1%, aOR 3.52, 95% CI 2.51–4.94) and HIV-positive women not using cART (13.0% v. 4.6%, aOR 3.08, 95% CI 1.34–5.07). HIV-positive women not using cART had similar incidences of preeclampsia when compared with HIV- negative women (4.6% v. 4.1%, aOR 1.14, 95% CI 0.53–2.44) |
Good |
| 4 | Stoner et al. (2016) (27) Zambia | Multicenter retrospective case-control study (2006–2012) | HIV-positive and negative pregnant women | HIV, cART | Pregnancy-induced hypertension | 249,771 women included for analysis, of whom 5354 women met criteria for pregnancy-induced hypertension (701 had preeclampsia, 394 had eclampsia). HIV-positive women using cART had a higher adjusted odds for pregnancy-induced hypertension when compared with HIV- positive women not on cART (aOR 1.27, 95% CI 1.04–1.55). This finding was unrelated to timing of initiation of cART. HIV-positive women who were untreated had a lower adjusted odds for pregnancy-induced hypertension when compared with HIV- negative women (aOR 0.75, 95% CI 0.61–0.93). |
Good |
| 5 | Sebitloane, Moodley, & Sartorius (2017) (35) South Africa | Retrospective, case-control study (2011–2013) | HIV-positive and HIV-negative pregnant women who had a maternal death | HIV | Death due to HDP | 640 deaths due to HDP, 32.5% occurred among HIV-positive women. When compared with HIV- negative women, HIV- positive women had a lower risk of maternal death due to HDP (RR 0.57, 95% CI, 0.41–0.64). HIV-positive women not using cART had a lower risk of having HDP when compared with HIV- negative women (RR 0.68, 95% CI 0.57–0.82) HDP was more likely to be cause of death among patients with AIDS receiving cART when compared with patients with AIDS not receiving cART (RR 1.15, 95% CI 1.02–1.29). Patients with AIDS not receiving cART were less likely to have HDP as underlying cause of death compared to HIV- positive women who were not using cART (RR 0.67, 95% CI 0.57–0.79) |
Fair |
| B. Type of ARV used in cART | |||||||
| 1 | McGowan et al. (1999) (38) USA | Single-site retrospective cohort analysis (1996–1998) | HIV-positive pregnant women | cART | Pregnancy-induced hypertension | 30 women included, of whom 4 had pregnancy- induced hypertension (13%). Use of PI was noted in all cases of pregnancy-induced hypertension, though 3 subjects were non-adherent. | Poor |
| 2 | Wimalasundera et al. (2002) (3) UK | Retrospective case-control analysis of two hospitals (1990–2001) | Pregnant women | HIV | Preeclampsia | 9/214 (4.2%) HIV-positive women were diagnosed with preeclampsia v. 12/214 HIV-negative controls (6%). The incidence of preeclampsia was lower among HIV-positive women who were untreated (0%, OR 15.3, 95% CI 0.9–270) or in the mono-or-dual therapy group (1%, OR 15.3, 95% CI 1.1–73) when compared with women who took triple therapy ARVs (8/76, 11%). When compared to HIV- negative women, no difference in incidence of preeclampsia among HIV-positive women who were untreated (p = 0.07) or on ARVs (0.2). |
Fair |
| 3 | Boer et al. (2007) (28) Netherlands | Retrospective cohort Analysis (1997–2003) | HIV-positive pregnant women on cART and HIV- negative women | HIV, cART with or without use of PI | Preeclampsia | 143 HIV infected women- infant pairs and 196 matched controls available for analysis. No difference in incidence of preeclampsia among HIV-positive women using cART v. negative women (2.8 v 1%) Incidence of preeclampsia 3.2% among 93 women on cART with PI and 2.0% among 50 women on cART without PI (ns). |
Fair |
| 4 | Williams et al. (2009) (39) USA | Case series, single site (1997–2007) | Pregnant women with perinatally- acquired HIV using cART | N/A | Antepartum, intrapartum, and postpartum complications. | 10 women with 13 pregnancies. Total of 3 pregnancies complicated by preeclampsia (23%) Medications used included 3TC, ABC, LPV/r, DDI, AZT, IDV/r, and NFV. |
Poor |
| 5 | Boyajian et al. (2012) (8) Canada | Single-site retrospective matched cohort analysis at a single hospital in Toronto (2003–2010) | HIV-positive pregnant women using cART and HIV-negative pregnant women | HIV, cART | Preeclampsia | Total of 91 HIV-positive women available for analysis. Incidence of preeclampsia 3.3% v. 5.1% in HIV- positive on cART v. HIV- negative pregnant women (aOR 0.59, 95% CI 0.11–3.08). When compared with HIV-negative women, HIV-positive women had higher incidence of thrombocytopenia (5.5% v. 1.5%, OR 3.91, 95% CI 1.11–13.76) and elevated liver enzymes (13.2% v. 5.1%, OR 2.81, 95% CI 1.27–6.23) despite no difference in incidence of severe preeclampsia. Subgroup analysis demonstrated that women who began cART prior to conception or during pregnancy did not have a difference in incidence of preeclampsia (p = 0.56). There was no difference in risk of preeclampsia among women using PIs v. those on other ARV regimens (p > 0.99). |
Fair |
| 6 | Powis et al. (2013) (34) Botswana | Secondary analysis of a randomized controlled trial (2006–2008) | HIV-positive, cART-naïve, preeclamptic pregnant women | ABC/AZT/3TC or AZT/3TC + LPV/r or observation with initiation of AZT/3TC/NVP when CD4 < 200 cells/mm3 | Change in level of PlGF and sFLT-1 after cART initiation; preeclampsia | 722 women available for analysis. 11 developed preeclampsia VL > 100,000 was associated with preeclampsia (OR 5.8, 95% CI 1.8–19.4), even after adjusting for CD4 count, cART regimen, and gestational age at cART initiation. Among women who developed preeclampsia, there were lower PlGF and higher sFlT-1 levels prior to initiation of cART. VL > 100,000 and PlGF were associated with preeclampsia after adjusting for sFlt-1. |
Poor |
| 7 | Machado et al. (2014) (25) | See Part A, No. 2 for details | |||||
| C. Other studies | |||||||
| 1 | Mattar et al. (2004) (37) Brazil | Single-site retrospective cohort analysis (2000–2002) | HIV-positive pregnant women using ARVs, HIV- negative pregnant women. | HIV | Preeclampsia | 1/123 HIV-positive women (0.8%) had preeclampsia v 182/1708 (10.6%) women without HIV. Among HIV-positive women, 22/123 (17.9%) using ARV monotherapy, 78/123 using cART (63.4%). | Poor |
| 2 | Tuomala et al. (2005) (47) USA | Multicenter, prospective cohort (1990–2002) | HIV-positive pregnant women with singleton gestations | N/A | HELLP, gestational hypertension, preeclampsia, eclampsia | 2286 women included for analysis. Low prevalence of HDP, precluding analysis. | Poor |
| 3 | Bodkin, Klopper, & Langley (2006) (22) South Africa | Single-site retrospective cohort analysis (2003) | HIV-positive and negative pregnant women | HIV | Eclampsia and pregnancy-induced hypertension | 204 HIV-positive women and 1336 HIV-negative women available for analysis. No significant difference in incidence of eclampsia (2.83 v. 0.99%, p = 0.44). Higher, nonsignificant incidence of pregnancy- induced hypertension among HIV-positive women (16.98% v. 9.90%) |
Fair |
| 4 | Kourtis et al. (2006) (24) USA | Retrospective cohort Analysis (1994–2008) | HIV-positive and HIV-negative pregnant women | cART | Hospitalization for preeclampsia | In 1994, among 6143 HIV- positive women, 9.9% developed preeclampsia/hypertension v. 7.3% among HIV- negative women (aOR 1.13, 95% CI 0.82–1.56) In 2003, among 6235 HIV- positive women, 9.9% developed preeclampsia/hypertension v. 8.9% among HIV- negative women (aOR 1.00, 95% CI 0.82–1.21). |
Good |
| 5 | Bera (2009) (29) South Africa | Retrospective cohort analysis, single site (2006–2008) | HIV-positive pregnant women initiating cART | N/A | Maternal death, preeclampsia, eclampsia, and HELLP | 385 women included. 96.4% started EFV-based ART, 4.2% were on NVP- based regimen, and 2.6% of women were on AZT- based regimen. IRIS occurred in 7% women. 7.5% of women developed preeclampsia, 10.3% developed eclampsia and 3.4% developed HELLP. |
Poor |
| 6 | Haeri et al. (2009) (7) USA | Retrospective cohort analysis at two sites (2000–2007) | HIV-positive pregnant women using cART and HIV-negative pregnant women | HIV, cART | Gestational hypertension and preeclampsia | 47,126 deliveries, with 188 deliveries among HIV- positive women. Total of 151 HIV-positive pregnant women on cART included for analysis Gestational hypertension (1 v 4%, p = 0.04) and preeclampsia (6 v. 12%, p = 0.04) less common among HIV-positive women, when compared with HIV-negative women. However, when controlling for risk factors for preeclampsia, these findings become nonsignificant. |
Good |
| 7 | Aebi-Popp et al. (2010) (48) Switzerland | Prospective observational cohort (2003–2008) | HIV-positive pregnant women | HIV, cART | Preeclampsia or “arterial hypertension” | 266 HIV-positive women. 35.5% ARV naïve at conception, 1.9% at time of delivery. 62.9% had CD4 > 350 at time of delivery, with VL < 50 among 78.7% of population. 45–57% of population on PI-boosted regimen. Almost 20–40% of population stopped ARVs during pregnancy. Overall prevalence of preeclampsia was 2.6%. | Poor |
| 8 | Parekh et al. (2011) (33) Botswana | Multicenter retrospective case-control study (2007–2010) | HIV-positive and negative pregnant women who gave birth at greater than 26 weeks’ gestation | HIV, cART | Hypertension during pregnancy (i.e. one blood pressure > 140/90 mmHg measured at any antenatal visit) | 16,219 pregnancies included for analysis, of which 4,347 were affected by HIV. Among HIV- positive women, 67.8% on AZT monotherapy, 8% on cART, 18.1% “continued” HAART, and 16.1% did not use ARVs. For cART regimen, majority used AZT, 3TC, and NVP. After adjusting for maternal age, marital status, and nulliparity, HIV was associated with increased odds for hypertension when compared with HIV- negative women (aOR 1.19, 95% CI 1.02–1.39). When compared with HIV- positive women using other ARVs, cART use prior to conception was independently associated with hypertension during pregnancy (aOR 1.34, 95% CI 1.00–1.77). |
Fair |
| 9 | Shapiro et al. (2012) (36) Botswana | Prospective case-control, single-site study (2010) | HIV-positive and negative pregnant women with stillbirths > 19w | HIV, cART | Preeclampsia, hypertension in pregnancy | 66 HIV-positive women and 33 HIV-negative women. 42% of HIV positive women were on cART at time of stillbirth, 81% from prior to pregnancy and 19% who started during pregnancy. 23% started AZT at greater than 28 weeks’ gestation. Nonsignificant difference in incidence of both pregnancy-induced hypertension (54% v. 47% v. 51%) and preeclampsia/eclampsia (23% v. 19% v. 22%) among HIV-infected women using cART v. not using cART v. HIV- negative women. |
Poor |
| 10 | Ngene et al. (2013)(30) South Africa | Prospective cohort, single site (2010–2011) | Pregnant women or women up to 42 days post-partum, admitted to ICU | HIV | Hypoxic- ischaemic brain injury, death, or preeclampsia | 82 patients in ICU, 31 were HIV positive. 8 were on cART while 10 were receiving AZT monotherapy. HIV-positive women had lower incidence of eclampsia (9.7 v. 31.0%) and severe preeclampsia (3.2 v. 14.3%) when compared with HIV- negative women. |
Poor |
| 11 | Hall et al. (2014)(9) South Africa | Prospective, single-site cohort analysis (2007–2011) | HIV-positive and HIV- negative pregnant women | HIV | Preeclampsia, gestational hypertension | 1093 HIV-positive and 1173 HIV-negative pregnancies included for analysis. 200 women were using cART, 865 using AZT monotherapy with intrapartum NVP; 28 cases had no medication reported. Lower incidences of preeclampsia (3.2 v 4.9%, OR 0.65, 95% CI 0.42–0.99) and gestational hypertension (1.6 v. 3.1%, OR 0.53, 95% CI 0.30–0.94) among HIV-positive v. HIV-negative women. No significant differences noted when stratifying HIV-positive women by CD4 count. On multivariable regression modelling, HIV was associated with a reduced odds of preeclampsia (aOR 0.46, 95% CI 0.28–0.76) and gestational hypertension (aOR 0.42, 95% CI 0.22–0.80). |
Good |
| 12 | Landi et al. (2014) (21) Italy | Prospective observational cohort study, two sites (2004–2012) | HIV-positive and negative pregnant women with singleton gestations | HIV | Gestational hypertension and preeclampsia | 126 HIV-positive women, 140 HIV-negative women included in the analysis. 35.7% of cART initiation was before pregnancy, and 64.3% occurred after the first trimester. Lower incidences of gestational hypertension and preeclampsia among HIV-positive v. HIV- negative women (2.38% v. 10%, p = 0.01). Initiating cART before pregnancy was not associated with an increased incidence of gestational hypertension and preeclampsia (4.4 v 1.2%, p = 0.26). |
Fair |
| 13 | Reitter et al. (2014)(49) Germany | Prospective cohort study (2002–2012) | HIV-positive pregnant women | N/A | Preeclampsia or hypertension | 330 pregnant women enrolled, 5 women (1.5%) developed preeclampsia or hypertension. 33.3% of the total sample of HIV- positive women were using a PI, 26.6% were using a triple NRTI regimen, and 21.2% had NNRTI + NRTI regimen. | Poor |
| 14 | Ewing et al. (2016)(26) USA | Retrospective cross-sectional cohort using the Nationwide Inpatient Sample (2004–2011) | Pregnant HIV - positive and HIV - negative women hospitalized for antenatal care and delivery | HIV | Multiple outcomes, of which preeclampsia/HDP were included | In 2004, preeclampsia/HDP affected 13.6% of HIV- positive women (n = 7107) v. 9.5% of HIV-negative patients (n = 4,675,615) (aOR 1.08, 95% CI 0.64–1.88). In 2011, preeclampsia/HDP affected 18.9% of HIV- positive women (n = 4751) v. 11.4% of all HIV- negative women (4,180,200) (aOR 1.44, 95% CI 1.16–1.80). | Good |
| 15 | Maharaj, Moodley, & Chuturgoon (2016) (31) South Africa | Prospective, observational cohort, single site (2013–2014) | HIV-positive and negative pregnant women | HIV using cART | Preeclampsia | 193 women recruited; 98 women had preeclampsia with HIV using cART and 53 had preeclampsia without HIV. 95 women were normotensive (45 with HIV using cART and 50 without HIV). 1.9% of HIV-positive women had eclampsia v. 0% among HIV-negative women (ns). | Fair |
| 16 | Tooke et al. (2016)(23) South Africa | Single site retrospective case-control analysis (2011–2013) | Pregnant women delivering ELBW infants (<1000g) | HIV | Preeclampsia | 46 HIV-positive women out of 195 ELBW births. 69.6% pregnancies affected by preeclampsia. When compared to HIV-positive women using cART < 4 weeks, those using cART > 4 weeks had a higher incidence of preeclampsia (24/29 v. 8/17, p = 0.01). Among HIV-positive women starting cART in pregnancy, those using it for > 4 weeks had a significantly higher incidence of preeclampsia (18/21) v. those who used it for < 4 weeks (8/17, p = 0.016). |
Poor |
| 17 | Yudin et al. (2016)(50) Canada | Single-site retrospective cohort analysis (2002–2010) | HIV-positive pregnant women delivering at a single hospital in Toronto, ON | N/A | Chronic hypertension, gestational hypertension, and preeclampsia | 142 HIV-positive women available for analysis. 94% using cART. 7 women (5%) had “hypertension as a complication in pregnancy”, 1 had chronic hypertension, 6 had gestational hypertension. 2 developed preeclampsia. | Poor |
3TC: lamivudine; ABC: abacavir; AIDS: acquired immunodeficiency syndrome; ARV: antiretroviral medication; AZT: zidovudine; cART: combined antiretroviral therapy; DDI: didanosine; ELBW: extremely low birth weight; EFV: efavirenz; GGT: gamma-glutamyl transferase; HELLP: hemolysis, elevated liver enzymes, low platelet count syndrome; HDP: hypertensive disorders of pregnancy; IDV/r: indinavir/ritonavir; IRIS: immune reconstitution inflammatory syndrome; IUFD: intrauterine fetal demise; LPV/r: lopinavir/ritonavir; NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; ns: nonsignificant; NVP: nevirapine; PI: protease inhibitor; PlGF: placental growth factor; sFlT-1: soluble fms-like tyrosine kinase-1; VL: viral load
All studies exhibited moderate to high risks of bias, primarily driven by nonrandomization and nonblinding, as well as high rates of selection and reporting bias (Table 2). When performed, statistical analyses either did not control for important confounders for development of HDP or suffered from multiple comparisons without appropriate correction (3, 7, 22, 23). Despite these limitations, a total of seven studies were deemed to be of “good” quality, in that the design and the included populations were sufficient to draw conclusions regarding the relationship of cART and HDP among pregnant WLHIV (7, 9, 15, 24–27).
TABLE 2.
Risk of bias
| Bias Domains | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author (year) | Selection | Performance | Detection | Attrition | Reporting | Other | Authors’ Judgement of Risk of Bias | Support |
| McGowan et al. (1999) (38) | + | + | + | − | + | + | High | Small, nonrandomized observational sample without a comparison group available for analysis. Did not control for confounders. |
| Wimalasundera et al. (2002) (3) | + | + | + | − | − | + | High | Case-control study with small sample sizes and multiple subgroup analyses. Matched HIV-negative control group for age, parity, and ethnic origin. |
| Mattar et al. (2004) (37) | + | + | + | − | − | + | High | Small, nonrandomized, single-site retrospective cohort. Controls were only matched based on HIV status. Authors reported limited baseline demographics with no comparisons between HIV-positive and HIV-negative groups. |
| Tuomala et al. (2005)(47) | + | + | + | − | + | + | High | Large, multicenter, prospective cohort analysis. HDP outcomes are not reported in their entirety and confounders were not controlled. |
| Bodkin, Klopper, & Langley (2006) (22) | + | + | + | − | + | + | Moderate | Single-site retrospective cohort analysis which reported selective HDP outcomes (e.g. eclampsia and pregnancy-induced hypertension). Random sampling was employed to generate HIV- positive cohort with matched controls in a 1:2 fashion for HIV status. Analysis did not control for confounding variables. |
| Kourtis et al. (2006) (24) | + | − | + | − | + | − | Moderate | Large retrospective cohort analysis. Significant baseline differences among individuals with HIV and without. Detection of hospitalization based on ICD-9 coding. Does not document medical history of the cohort, which may have an impact on development of HDP. |
| Suy et al. (2006) (32) | + | + | + | − | + | + | Moderate | Prospective, single-site, non-randomized cohort analysis. One outcome focused on preeclampsia and did not discuss other HDPs. For matched controls, historical in nature and based on HIV status, rather than HIV status and cART exposure. Analysis only controlled for a few confounding variables. |
| Boer et al. (2007) (28) | + | + | + | + | + | Moderate | Retrospective, nonrandomized cohort analysis. Offered matched, HIV-negative controls in 1:2 ratio, but did not discuss nonmatched baseline characteristic differences between groups. | |
| Bera (2009) (29) | + | + | − | + | + | + | High | Single center, nonrandomized descriptive study. Did not provide any comparison group for analysis or control for confounders. |
| Haeri et al. (2009) (7) | + | + | − | + | − | + | Moderate | Retrospective, nonrandomized, observational cohort. Provided matched control group in 1:2 fashion based on multiple baseline characteristics, but unmatched characteristics demonstrated significant differences between groups. Performed multiple comparisons. |
| Williams et al. (2009) (39) | + | + | + | − | + | + | High | Retrospective, nonrandomized case series of a specific population (e.g. women with perinatally-acquired HIV). Small sample size, with only 3 pregnancies complicated by preeclampsia. Did not describe severity of preeclampsia or timing of onset. No comparison group available for analysis. |
| Aebi-Popp et al. (2010)(48) | + | + | + | − | + | + | High | Prospective, nonrandomized, observational descriptive study with no comparison group available for analysis. Did not discuss any risk factors associated with preeclampsia. |
| Parekh et al. (2011) (33) | + | + | + | − | − | + | Moderate | Retrospective, nonrandomized cohort analysis. Did not discuss adherence to cART or use of PI. Hypertension during pregnancy was a secondary outcome. |
| Boyajian et al. (2012) (8) | − | + | + | − | − | + | Moderate | Single-site, retrospective cohort analysis. Excluded HIV-positive women not using cART. Randomly assigned matched controls in 1:3 ratio. Did not report on other HDP outcomes. |
| Shapiro et al. (2012) (36) | + | + | + | − | + | + | High | Prospective, nonrandomized, case-control study. Did not control for confounding variables. Small sample size. Did not report medical risk factors for HDP. Dataset focuses on women with stillbirth. |
| Ngene et al. (2013)(30) | + | + | + | − | + | + | High | Prospective, nonrandomized, single-site cohort analysis. Small sample size with no multivariate analysis performed. Population focused on pregnant or postpartum women admitted to ICU, rather than all admissions. No definition provided for preeclampsia. |
| Powis et al. (2013) (34) | + | + | + | + | + | High | Secondary analysis of a randomized controlled trial. Noted baseline differences between women who developed preeclampsia and those who did not. However, no discussion of cART adherence. Small number of women who developed preeclampsia. | |
| Hall et al (2014) (9) | + | + | + | + | − | + | High | Prospective, nonrandomized, cohort analysis. Excluded women with risk factors for preeclampsia. Significant baseline differences between HIV-positive and negative women. Did not perform matching for the control group. 1/3 of women enrolled in study did not delivery at study site, and no data is available for them. |
| Landi et al. (2014) (21) | + | + | + | − | − | + | High | Prospective, nonrandomized, observational cohort analysis. Provided HIV-negative matched cohort for age and parity, while excluding women with medical risk factors for HDP. Did not control for confounders. Did not define criteria for either gestational hypertension or preeclampsia. |
| Machado et al. (2014) (25) | + | + | + | + | − | + | High | Prospective, nonrandomized, observational cohort analysis of two ongoing studies. Did not provide HIV-negative or HIV-positive cART-unexposed controls for comparison. Small number of women who developed HDP. 35 women were excluded from primary analysis and no data were provided on these subjects. |
| Reitter et al. (2014)(49) | + | + | + | + | − | + | High | Prospective, nonrandomized, cohort study. No comparison group provided. Did not control for several confounders. Low number of women who developed HDP. |
| Ewing et al. (2016)(26) | + | + | + | − | − | + | High | Large retrospective, cross-sectional cohort study. Authors did not report baseline co-morbidities which could predispose to HDP. Study relied on ICD-9 codes to make diagnosis. |
| Maharaj, Moodley, & Chuturgoon (2016) (31) | + | + | + | + | − | + | Moderate | Prospective, observational, nonrandomized cohort study. Study focused only on preeclampsia and did not include gestational hypertension or HELLP. |
| Sansone et al. (2016)(15) | + | + | + | − | − | + | Moderate | Retrospective, observational, nonrandomized cohort analysis. Unable to discuss adherence or type of cART regimen. |
| Stoner et al. (2016) (27) | + | + | + | + | + | High | Large number of individuals excluded either due to diagnosis of chronic hypertension, missing information about HDP, or lack of a reported due date. Reporting on HDP was limited due to laboratory access. | |
| Tooke et al. (2016) (23) | + | + | + | − | + | + | High | Single site, nonrandomized, case-control study. Inclusion criteria focused on women delivering extremely low birthweight neonates. Performed multiple comparisons. Did not control for confounding variables. |
| Yudin et al. (2016) (50) | + | + | + | − | + | + | High | Single site, retrospective, nonrandomized cohort analysis. Small of women who developed HDP. No matched cohort presented for analysis. |
| Sebitloane, Moodley, & Sartorius (2017) (35) | + | + | + | − | + | + | High | Nonrandomized, case-control study. Data is focused on maternal deaths and does not include baseline characteristics of the subjects in the sample. No regression modeling was performed. |
Studies also varied in uniform definitions of HDP, driven largely by study site, years of study recruitment, and access to laboratory testing. For example, Sansone et al. used the definitions of preeclampsia with or without severe features based on criteria proposed by ACOG, whereas Stoner et al. relied upon either a single elevated blood pressure (greater than or equal to systolic blood pressure of 140 and/or diastolic blood pressure of 90) or local practitioner’s assessment to diagnose eclampsia, preeclampsia, and gestational hypertension (15, 27). Similarly, reported outcomes differed among studies. For example, reported outcomes could be clinically defined as a single disease process (e.g. preeclampsia) or a composite outcome (e.g. eclampsia and pregnancy-induced hypertension), while other studies may use ICD-9 codes for antepartum hospitalization for preeclampsia (22, 24, 26, 28).
In order to broadly overview the risk of HDP with HIV, we first reviewed the studies that examined frequency of HDP among pregnant WLHIV or compared WLHIV to seronegative women. Overall rates of HDP among pregnant WLHIV varied between 0.8–54%, depending on the study design, stratification of outcome (e.g. reporting preeclampsia alone or creating a composite outcome of all HDP), and inclusion criteria. The rate of eclampsia was reported to be between 1.9 and 10.3% in four different studies, but also varied due to study design and inclusion criteria (22, 29–31). Of the studies that compared pregnant WLHIV using cART with seronegative women, a majority (9/19, 47.4%) noted an increased risk for HDP among pregnant WLHIV using cART, with adjusted odds ratios (aOR) ranging from 1.19 to 8.90 (3, 15, 23, 25, 27, 32–35). In contrast, seven studies (7/19, 36.8%) demonstrated no difference in rates of HDP between pregnant WLHIV using cART and seronegative women (7, 8, 22, 24, 28, 31, 36). However, three studies (3/19, 15.8%) specifically demonstrated a statistically significant decreased frequency of HDP among pregnant WLHIV using cART, when compared with seronegative women (9, 21, 37). For example, Hall et al. note that the odds of developing preeclampsia or gestational hypertension are lower among pregnant WLHIV using cART (aOR 0.44, 95% CI 0.29–0.66) compared to women without HIV (9).
cART v. no cART among pregnant WLHIV
To examine the more clinically relevant questions regarding cART use among WLHIV and risk of HDP, we examined studies that addressed only populations of pregnant WLHIV (Table 1A). Five studies addressed the relationship of cART use and development of HDP among pregnant WLHIV by comparing them to a cohort of WLHIV who were not exposed to cART (15, 25, 27, 32, 35). All of these studies suggested cART is associated with increased odds for the development of HDP among pregnant WLHIV, with aORs reported specifically for HDP ranging from 1.27 to 8.90 (25, 27, 32).
Sansone et al. performed a comparison of pregnant WLHIV on cART, pregnant WLHIV not on cART, and seronegative pregnant women. They determined that pregnant WLHIV on cART had greater odds for preeclampsia when compared with WLHIV who were not on cART (aOR 3.08, 95% CI 1.34–5.07). In this cohort, similar rates of preeclampsia were noted between pregnant WLHIV not using cART and seronegative pregnant women (4.6 v. 4.1%) (15). Sebitloane et al concentrated their study population on pregnant WLHIV who died; their analysis demonstrated a higher relative risk (RR) for death due to HDP among pregnant WLHIV with AIDS using cART when compared to pregnant WLHIV with AIDS not receiving cART (RR 1.15, 95% CI 1.02–1.29) (35). Despite the aforementioned findings, many of the studies had low numbers of pregnant WLHIV using cART who eventually developed HDP, such as Suy et al., who used a total of 9 cases for statistical analysis (32).
Type of ARV used in cART regimen and risk of HDP
Next, we examined the specific nature of cART and relationship to HDP among pregnant WLIHV (Table 1B). Seven studies commented on the pharmacological composition of cART regimens and the association with HDP, although findings were limited (3, 8, 25, 28, 34, 38, 39). One study performed by Wimsalundera et al., focused on the role of mono versus multi-drug regimens, and noted no significant differences in rates of preeclampsia among pregnant WLHIV on any treatment (monotherapy, dual therapy, or combination antiretroviral therapy) compared to HIV-uninfected women (3).
However, when limiting the study population to WLHIV, few studies investigated specific characteristics of drugs. Three studies examined the potential association of a PI with HDP. First, McGowan et al. noted in a retrospective cohort analysis that four of thirty pregnant WLHIV (13%) were diagnosed with gestational hypertension, and all were using a PI (40). In a case series, Williams et al. found that of the three pregnancies affected by preeclampsia, two WLHIV used a boosted PI regimen (lopinavir/ritonavir and indinavir/ritonavir) (39). Finally, Machado et al. described that using cART or any PI regimen at conception was associated with a significantly higher rate of HDP (7.1 v. 4.3%, p = 0.04), and preeclampsia/eclampsia (4.4 v. 1.8%, p < 0.01) when compared with women either exposed to monotherapy, dual therapy, triple therapy without a PI, or no ARVs, all of which combined served as the comparison group (41).
Other studies found no significant relationship between types of antiretroviral medications and diagnosis of HDP. For example, Boer et al., focusing on 143 pregnant WLHIV, noted nonsignificant differences between pregnant WLHIV using PI-containing cART and those using non-PI-containing regimens (3.2 v. 2.0%) (28). Overall, these findings suggest that PIs may be associated with HDP, though the current data are likely underpowered to evaluate this relationship.
DISCUSSION
In the era of universal treatment, more women than ever before are using cART before and during pregnancy (5). Investigating the association between the use of cART and adverse outcomes, such as the development of HDP, is crucial for practitioners in order to appropriately counsel and monitor women throughout pregnancy. Additionally, selection of appropriate cART regimens is an essential component of HIV care, and thus it is critical to understand if such selection choices influence the risk of maternal morbidity due to HDP. Furthermore, examining this relationship can help researchers uncover the etiologies of HDP through an immunological lens, thereby improving a global understanding of the disease process itself (2).
Our systematic review highlights the limited information regarding specific drug classes and the risk of HDP. PIs have been demonstrated to increase the production of signaling molecules that activate the renin-angiotensin system in adipose tissue in vitro; exposure to PIs remain significant predictors of hypertension even after controlling for multiple intermediate risk factors for cardiovascular dysfunction (42). The effects of PIs on both the trajectory of blood pressure and its effects at the placental level, based primarily on the pathophysiological role of proteases in appropriate placental development, warrant further investigation both to elucidate possible side effects of PIs and other ARVs and to the knowledge base regarding the role of HDPs in increasing future cardiovascular risk for all women (43, 44).
Preeclampsia has been theorized to be due to a dysregulated, hyperimmune response to paternal antigens (2). Initial studies noted lower, though nonsignificant, rates of preeclampsia among pregnant WLHIV not using cART, suggesting that the immunocompromised state created by uncontrolled HIV prohibits the immune response that results in HDP. While some authors have posited that the use of cART in pregnancy leads to an improved immunological state, thereby allowing the dysregulated immune response underlying HDP pathophysiology to occur, others have posited that some HDP diagnoses may be indicative of cART toxicity rather than HDP (3, 8, 10). This systematic review highlighted that these questions remain inadequately answered.
Limitations of our systematic review include insufficiencies in data, such as low reported rates of preeclampsia, variations in cART regimens, and confounding by indication. The lack of information on the timing of ARV initiation, specific cART regimen, and measures of disease burden (e.g. viral load, CD4 count) make it difficult to determine whether it is HIV itself and HIV-related therapy that increase risk of HDP, or whether there is only an incidental correlation between HDP and HIV burden. Most studies were also underpowered to detect small differences in incidence of HDP. Given that the majority of data is derived from non-U.S. settings, the generalizability of these findings is unknown. The data are largely observational by necessity. Due to the current recommendation for universal cART for any individual diagnosed with HIV, there is no ethical manner to randomize individuals to treatment versus no treatment in order to evaluate the associated risks (5, 6). Finally, the authors only included published data in this systematic review in order to benefit from the peer review process. Therefore, publication bias may be present.
Future investigations into the role of cART in development of HDP should focus on the best measures of immune (re)constitution among WLHIV. Clinical research studies have used a variety of markers, such as CD4 count and HIV viral load, to classify well-controlled or poorly-controlled HIV (34). However, viremia may be only a crude marker for immune status and the ideal marker for immune status among pregnant WLHIV that could predict development of HDP has yet to be elucidated. Second, further research should consider how to use translational methodology to disentangle a diagnosis of HDP from cART toxicity among pregnant WLHIV, with careful attention placed on placental pathology (36). The information gleaned from this type of research could improve an understanding not only of the pathophysiology of HDP, but also the unique pharmacological side effects of cART during the pregnant state. Third, given the postulated relationship between types of cART regimens, particularly those that include PIs, future inquiry should address the relationship between different cART regimens, especially ones that include newer drugs like integrase strand transfer inhibitors, and the diagnosis of HDP. Finally, aspirin is now recommended to decrease the risk of HDP among women at high risk of developing the disease; investigating the role of aspirin among pregnant WLHIV using cART would be an informative project in order to improve perinatal outcomes (45, 46).
CONCLUSIONS
In this systematic review, we note that a majority of studies demonstrate an increased risk for developing HDP among pregnant WLHIV using cART, and all studies demonstrated an increased risk for developing HDP when the comparison group was comprised of pregnant WLHIV not using cART. Furthermore, some studies postulate that PIs may be driving this relationship.
HIGHLIGHTS.
HIV-positive pregnant women using cART have a higher risk of hypertensive disorders.
Protease inhibitors may increase the risk of hypertensive disorders of pregnancy.
All studies are marked by moderate to high risk of bias.
ACKNOWLEDGEMENTS
We wish to thank Corinne Miller, MLIS at Galter Health Sciences Library & Learning Center at Feinberg School of Medicine, Northwestern University, for her assistance in creating and performing the literature search.
Sources of Support: Lynn M. Yee M.D., M.P.H. is supported by the NICHD K12 HD050121–11. Lisa B. Haddad M.D., M.S., M.P.H.is supported by the NICHD K23 5K23HD078153–04.
Footnotes
Disclosures: The authors report no conflict of interest.
This paper was presented at the Infectious Diseases Society for Obstetrics and Gynecology in Philadelphia, PA USA, August 2–4 2018
REFERENCES
- 1.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Preeclampsia. Lancet 2016;387(10022):999–1011. [DOI] [PubMed] [Google Scholar]
- 2.Need JA. Pre-eclampsia in pregnancies by different fathers: immunological studies. BMJ 1975;1(5957):548–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wimalasundera RC, Larbalestier N, Smith JH, de Ruiter A, GTSA Mc, Hughes AD, et al. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet 2002;360(9340):1152–4. [DOI] [PubMed] [Google Scholar]
- 4.Hall DR. Is pre-eclampsia less common in patients with HIV/AIDS? J Reprod Immunol 2007;76(1–2):75–7. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living With HIV: Department of Health and Human Services (HHS); 2017 [updated 17 October 2017. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 7.Haeri S, Shauer M, Dale M, Leslie J, Baker AM, Saddlemire S, et al. Obstetric and newborn infant outcomes in human immunodeficiency virus–infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol 2009;201(3):315.e1. [DOI] [PubMed] [Google Scholar]
- 8.Boyajian T, Shah PS, Murphy KE. Risk of preeclampsia in HIV-positive pregnant women receiving HAART: a matched cohort study. J Obstet Gynaecol Can 2012;34(2):136–41. [DOI] [PubMed] [Google Scholar]
- 9.Hall D, Gebhardt S, Theron G, Grove D. Pre-eclampsia and gestational hypertension are less common in HIV infected women. Pregnancy Hypertens 2014;4(1):91–6. [DOI] [PubMed] [Google Scholar]
- 10.Mawson AR. Effects of antiretroviral therapy on occurrence of pre-eclampsia. Lancet 2003;361(9354):347–8. [DOI] [PubMed] [Google Scholar]
- 11.Adams JW, Watts DH, Phelps BR. A systematic review of the effect of HIV infection and antiretroviral therapy on the risk of pre-eclampsia. Int J Gynaecol Obstet 2016;133(1):17–21. [DOI] [PubMed] [Google Scholar]
- 12.Browne JL, Schrier VJMM, Grobbee DE, Peters SAE, Klipstein-Grobusch K HIV, Antiretroviral Therapy, and Hypertensive Disorders in Pregnancy: A Systematic Review and Meta-analysis. J Acquir Immune Defic Syndr 2015;70(1):91–8. [DOI] [PubMed] [Google Scholar]
- 13.Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2008;198(1):7–22. [DOI] [PubMed] [Google Scholar]
- 14.Calvert C, Ronsmans C. HIV and the risk of direct obstetric complications: a systematic review and meta-analysis. PLoS One 2013;8(10):e74848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sansone M, Sarno L, Saccone G, Berghella V, Maruotti GM, Migliucci A, et al. Risk of Preeclampsia in Human Immunodeficiency Virus-Infected Pregnant Women. Obstet Gynecol 2016;127(6):1027–32. [DOI] [PubMed] [Google Scholar]
- 16.Premkumar A, Dude A, Haddad L, Yee LM. The risk of hypertensive disorders of pregnancy among HIV-positive women receiving anti-retroviral therapy: PROSPERO CRD42017078838; 2017. [Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017078838.
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. [PMC free article] [PubMed] [Google Scholar]
- 18.Cochrane Handbook for Systematic Reviews of Interventions Higgins J, Green S, editors: Cochrane Collaboration; 2011. [Google Scholar]
- 19.U.S. Preventative Task Force. U.S. Preventative Task Force Ratings 2013. [Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/us-preventive-services-task-force-ratings.
- 20.American College of Obstetricians and Gynecologists (ACOG) Task Force on Hypertension in Pregnancy Hypertension In Pregnancy Washington D.C.: American College of Obstetricians and Gyncologists; 2013. [DOI] [PubMed] [Google Scholar]
- 21.Landi B, Bezzeccheri V, Guerra B, Piemontese M, Cervi F, Cecchi L, et al. HIV Infection in Pregnancy and the Risk of Gestational Hypertension and Preeclampsia. World J Cardiovasc Dis 2014;4:257–67. [Google Scholar]
- 22.Bodkin C, Klopper H, Langley G. A comparison of HIV positive and negative pregnant women at a public sector hospital in South Africa. J Clin Nurs 2006;15(6):735–41. [DOI] [PubMed] [Google Scholar]
- 23.Tooke L, Riemer L, Matjila M, Harrison M. Antiretrovirals causing severe pre-eclampsia. Pregnancy Hypertens 2016;6(4):266–8. [DOI] [PubMed] [Google Scholar]
- 24.Kourtis AP, Bansil P, McPheeters M, Meikle SF, Posner SF, Jamieson DJ. Hospitalizations of pregnant HIV-infected women in the USA prior to and during the era of HAART, 1994–2003. AIDS 2006;20(14):1823–31. [DOI] [PubMed] [Google Scholar]
- 25.Machado ES, Krauss MR, Megazzini K, Coutinho CM, Kreitchmann R, Melo VH, et al. Hypertension, preeclampsia and eclampsia among HIV-infected pregnant women from Latin America and Caribbean countries. J Infect 2014;68(6):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewing AC, Datwani HM, Flowers LM, Ellington SR, Jamieson DJ, Kourtis AP. Trends in hospitalizations of pregnant HIV-infected women in the United States: 2004 through 2011. Am J Obstet Gynecol 2016;215(4):499.e1-.e8. [DOI] [PubMed] [Google Scholar]
- 27.Stoner MC, Vwalika B, Smid MC, George S, Chi BH, Stringer EM, et al. A retrospective study of HIV, antiretroviral therapy, and pregnancy-associated hypertension among women in Lusaka, Zambia. Int J Gynaecol Obstet 2016;134(3):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boer K, Nellen JF, Patel D, Timmermans S, Tempelman C, Wibaut M, et al. The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG 2007;114(2):148–55. [DOI] [PubMed] [Google Scholar]
- 29.Bera E Maternal outcomes following introduction of antiretroviral therapy in the public sector: A prospective study at a tertiary hospital in the Eastern Cape. S Afr J Obstet Gynaecol 2009;15(1). [Google Scholar]
- 30.Ngene NC, Moodley J, Songca P, von Rahden R, Paruk F, Onyia CO, et al. Maternal and fetal outcomes of HIV-infected and non-infected pregnant women admitted to two intensive care units in Pietermaritzburg, South Africa. S Afr Med J 2013;103(8). [DOI] [PubMed] [Google Scholar]
- 31.Maharaj NR, Moodley J, Chuturgoon A. Association of HIV and highly active antiretroviral therapy with clinical and biochemical indices among women with pre-eclampsia. Int J Gynaecol Obstet 2016;134(3):304–8. [DOI] [PubMed] [Google Scholar]
- 32.Suy A, Martínez E, Coll O, Lonca M, Palacio M, De Lazzari E, et al. Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS 2006;20(1):59. [DOI] [PubMed] [Google Scholar]
- 33.Parekh N, Ribaudo H, Souda S, Chen J, Mmalane M, Powis K, et al. Risk factors for very preterm delivery and delivery of very-small-for-gestational-age infants among HIV-exposed and HIV-unexposed infants in Botswana. Int J Gynaecol Obstet 2011;115(1):20. [DOI] [PubMed] [Google Scholar]
- 34.Powis KM, McElrath TF, Hughes MD, Ogwu A, Souda S, Datwyler SA, et al. High viral load and elevated angiogenic markers associated with increased risk of preeclampsia among women initiating highly active antiretroviral therapy in pregnancy in the Mma bana study, Botswana. J Acquir Immune Defic Syndr 2013;62(5):517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebitloane HM, Moodley J, Sartorius B. Associations between HIV, highly active anti-retroviral therapy, and hypertensive disorders of pregnancy among maternal deaths in South Africa 2011–2013. Int J Gynaecol Obstet 2017;136(2):195–9. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro RL, Souda S, Parekh N, Binda K, Kayembe M, Lockman S, et al. High prevalence of hypertension and placental insufficiency, but no in utero HIV transmission, among women on HAART with stillbirths in Botswana. PLoS One 2012;7(2):e31580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattar R, Amed AM, Lindsey PC, Sass N, Daher S. Preeclampsia and HIV infection. Eur J Obstet Gynecol Reprod Biol 2004;117(2):240–1. [DOI] [PubMed] [Google Scholar]
- 38.McGowan JP, Crane M, Wiznia AA, Blum S. Combination antiretroviral therapy in human immunodeficiency virus–infected pregnant women. Obstet Gynecol 1999;94(5):641. [DOI] [PubMed] [Google Scholar]
- 39.Williams SF, Keane-Tarchichi MH, Bettica L, Dieudonne A, Bardeguez AD. Pregnancy outcomes in young women with perinatally acquired human immunodeficiency virus-1. Am J Obstet Gynecol 2009;200(2):149.e1–5. [DOI] [PubMed] [Google Scholar]
- 40.McGowan JP, Crane M, Wiznia AA, Blum S. Combination antiretroviral therapy in human immunodeficiency virus-infected pregnant women. Obstet Gynecol 1999;94(5 Pt 1):641–6. [DOI] [PubMed] [Google Scholar]
- 41.Machado ES, Krauss MR, Megazzini K, Coutinho CM, Kreitchmann R, Melo VH, et al. Hypertension, preeclampsia and eclampsia among HIV-infected pregnant women from Latin America and Caribbean countries. J Infect 2014;68(6):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripathi A, Jerrell JM, Skelton TN, Nickels MA, Duffus WA. Incidence of primary hypertension in a population-based cohort of HIV-infected compared with non-HIV-infected persons and the effect of combined antiretroviral therapy. J Am Soc Hypertens 2015;9(5):351–7. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sFlt-1 release in the human placenta. Placenta 2010;31(6):512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaffer D, Hughes MD, Sawe F, Bao Y, Moses A, Hogg E, et al. Cardiovascular disease risk factors in HIV-infected women after initiation of lopinavir/ritonavir- and nevirapine-based antiretroviral therapy in Sub-Saharan Africa: A5208 (OCTANE). J Acquir Immune Defic Syndr 2014;66(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 216(2):110–20.e6. [DOI] [PubMed] [Google Scholar]
- 46.Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for Prevention of Preeclampsia. Drugs 2017;77(17):1819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]