Abstract
Obesity is a global epidemic, yet successful interventions are rare. Up to 60% of people fail to achieve clinically meaningful, short-term weight loss (5–10% of start weight), whereas up to 72% are unsuccessful at achieving long-term weight loss (5–10% loss for ≥ 5 years). Understanding how biological, cognitive, and self-regulatory factors work together to promote or to impede weight loss is clearly needed to optimize obesity treatment. This paper describes the methodology of the Cognitive and Self-regulatory Mechanisms of Obesity Study (the COSMOS trial). COSMOS is the first randomized controlled trial to investigate how changes in multiple biopsychosocial and cognitive factors relate to weight loss and one another across two weight loss treatments. The specific aims are to: 1) Confirm that baseline obesity-related physiological dysregulation is linked to cognitive deficits and poorer self-regulation, 2) Evaluate pre- to post-treatment change across time to assess individual differences in biomarkers, cognition, and self-regulation, and 3) Evaluate whether the acceptance-based treatment (ABT) group has greater improvements in outcomes (e.g., greater weight loss and less weight regain, improvements in biomarkers, cognition, and self-regulation), than the standard behavioral treatment group (SBT) from pre- to post-treatment and 1-year follow-up. The results of COSMOS will provide critical information about how dysregulation in biomarkers, cognition, and/or self-regulation is related to weight loss and whether weight loss treatments are differentially associated with these factors. This information will be used to identify promising treatment targets that are informed by biological, cognitive, and self-regulatory factors in order to advance obesity treatment.
1. Introduction
Obesity has been named a global epidemic by the World Health Organization.1 Nearly 40% of U.S. adults are obese2 and at risk for more than 250 comorbid medical conditions.3 Obesity-attributable U.S. medical spending has been estimated as high as $147 billion annually.4,5 Despite this global crisis, successful interventions are rare, with up to 72% of individuals unsuccessful at achieving weight loss for five years or more.6 Consequently, there is still an urgent need to identify the factors impeding successful weight loss and to intervene. These factors may include a complex array of obesity-related physiological dysregulation, cognitive deficits, and self-regulation failure, as described below.
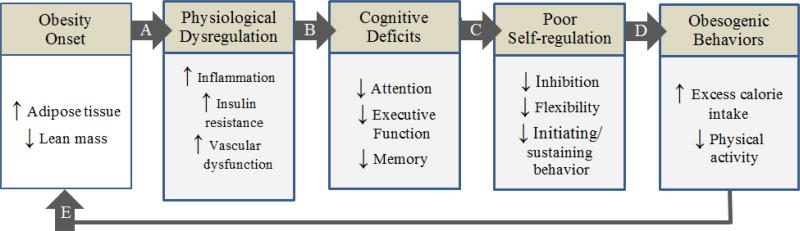
Obesity is a complex, cyclical disease in which excess adiposity causes physiological changes (see Figure 1, Path A)7 that impair cognitive functions (Path B).8–11 In turn, these cognitive deficits may contribute to reduced self-regulation (Path C), resulting in unhealthy behaviors (Path D) that promote or maintain obesity (Path E).7 Unfortunately, no studies to date have examined all of the above factors concurrently across different weight loss programs. Such a study is in order to identify potential differences in biopsychosocial and cognitive response patterns that may ultimately prove to be important predictors of weight loss or weight loss maintenance.
Figure 1.
The cyclical model of obesity and cognitive function. Adapted from “Body weight and neurocognitive function,” by M. A. W. Hawkins and J. Gunstad, 2016, in Eating Disorders and Obesity: A Comprehensive Handbook, 3rd edition. New York, NY: Guilford Press. Copyright 2017 by The Guilford Press. Adapted with permission.
Comparing the biopsychosocial and cognitive impacts of acceptance-based behavioral treatments (ABTs) versus standard behavioral treatments (SBTs) may be especially valuable given recent evidence that ABTs are superior to SBTs. Specifically, studies have previously shown that ABTs result in greater weight loss at both post-treatment and follow-up appointments than is typical for standard lifestyle interventions.12,13 The superior performance of ABTs may be due their ability to differentially improve biomarkers, cognitive function, and/or self-regulation. For instance, ABTs differ from SBTs in their promotion of self-regulation skills such as tolerating states of distress/discomfort, defusing thoughts from actions, committing to behaviors in line with defined values, and practicing mindful self-awareness of decisions.14–16 These focused skills are hallmarks of treatments like Acceptance and Commitment Therapy (ACT)17 and Dialectical Behavioral Therapy (DBT)18 but are not a focus of the cognitive restructuring commonly used in SBTs for weight loss.19–21 Thus, ABT may foster enhanced self-regulation skills, which have, in turn, been shown to reduce physiological reactivity22 and to enhance cognitive function and behavioral regulation.13,14 However, this possibility has yet to be empirically examined, and the current literature would greatly benefit from the explicit examination of ABT for weight loss and its potential impact on biomarkers and cognition in relation to SBT program outcomes.
Accordingly, Aim 1 of the proposed Cognitive and Self-regulatory Mechanisms of Obesity Study (COSMOS) trial is to examine the relationships between baseline obesity-related physiological dysregulation, cognitive deficits, and poor self-regulation. Aim 2 will extend these descriptive findings by evaluating change within individuals in biomarkers, cognitions, and self-regulation behaviors from pre- to post-treatment. Aim 3 will examine whether ABT yields greater improvements in outcomes (e.g., weight loss, biomarkers, cognition, etc.) than the SBT group across the duration of the study.
2. Methods
2.1 Conceptual Framework
The above aims are driven by the recent results that ABT may be superior to SBT for weight loss12,13 and the clear conceptual and theoretical differences between ABT and SBT16 that may be driving the different effects – though it is unknown by which biological, cognitive, or self-regulatory mechanisms. For instance, SBTs such as the Diabetes Prevention Program23 or LEARN program24 use cognitive restructuring as a core skill. In cognitive restructuring, an individual typically identifies a cognitive error or distorted thought (e.g., I need that candy bar), weighs evidence for and against the thought (e.g., I just ate a full lunch 20 minutes ago), and generates a more accurate or helpful thought to replace the old thought (e.g. I want the candy bar but eating it is not in line with my health goals). In direct contrast, ABTs focus on a nonjudgmental awareness of internal experiences (e.g., I’m having the thought that I want that candy bar), acceptance of these experiences (e.g., I am willing to accept this urge to eat), and mindful decisions to engage in committed actions (e.g., Even with this urge, I can put the candy back and go for a walk which is line with my value of being healthy for my grandchildren).25,26
Therefore, ABT strategies may promote weight loss via cognitive and emotional pathways, including: 1) enhanced awareness of physiological hunger and satiety cues,27 2) promotion of effective, flexible coping in response to non-nutritive cues (i.e., stress),28–30 and (3) inhibition of eating impulses.31 Indeed, mindfulness (a key ABT strategy) has been shown to promote cognitive function14,15,32 and distress-tolerance,29,30 broadly, and not just in relation to food.27,28,31 Studies support these findings by showing that those who have participated in ABTs are less likely to engage in overeating, especially if they have a greater responsivity to the food environment.12,33 Thus, ABTs may be especially efficacious for weight loss for several interrelated reasons: 1) they help combat the propensity for34 and high access to high calorie foods35 by increasing awareness of these states, 2) they may improve distress tolerance and therefore reduce stress and stress-related eating,28–30 3) they may enhance awareness of satiety cues, and 4) they remove the added mental effort of cognitive restructuring (i.e., changing thoughts) present within SBT approaches.
2.2 Trial Design Overview
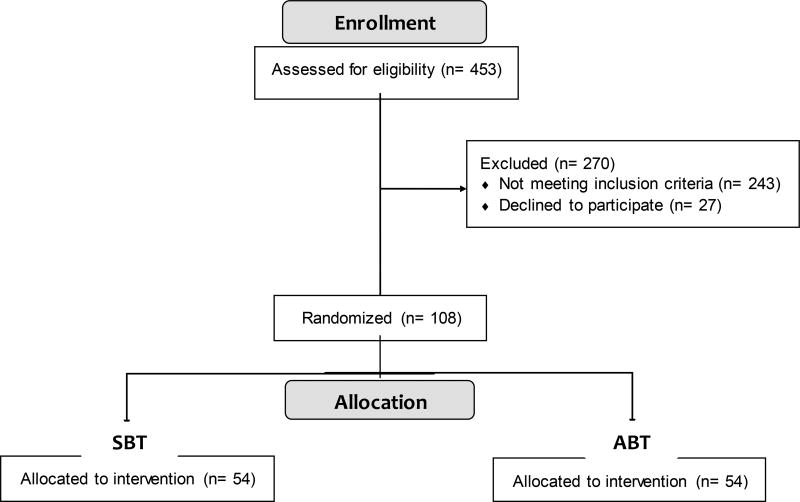
The COSMOS trial (Clinical Trials.gov Identifier: NCT02786238) is multi-year trial with multiple cohorts and will compare a maximum of 108 obese adults (21–65 years old) randomized to one of two treatment groups of interest – acceptance-based behavioral treatment (ABT) or standard behavioral treatment (SBT). The impact of the two interventions on physical, cognitive, and self-regulation factors will be assessed from baseline to post-treatment (6 months) and from baseline to follow-up (1 year). Given the complexity of assessments, we have included a summary table of the timing of assessments across the trial periods (see Table 1). Using this assessments, we propose to test whether a program like ABT promotes greater weight loss and less weight regain compared to SBT as well as directly examine whether treatment-related changes in physiological, cognitive, and self-regulatory factors differ by program and/or are associated with one another. Recruitment has been completed, so basic data on participant demographics are included (see Figure 2 for initial CONSORT flow diagram36 and Table 2 for participant characteristics). Importantly, randomization was effective, as there were no observed differences in any demographic or weight variable between SBT and ABT treatment arms.
Table 1.
Timing of Assessments
| Variable Category | Specific Measures | Baseline Time 0 |
Post-Tx Time 6 |
Follow-up Time 12 |
|---|---|---|---|---|
| Demographics or Medical History | Age, gender, race, education, WALI | • | -- | -- |
| Obesity Indicator | Weight (kg) | • | • | • |
| Fat mass (kg) | • | • | • | |
| Body fat % | • | • | • | |
| Waist circumference (cm) | • | • | • | |
| Biomarkers | Glucose | • | • | • |
| Insulin | • | • | • | |
| HOMA-IR | • | • | • | |
| Hemoglobin A1C | • | • | • | |
| SBP | • | • | • | |
| DBP | • | • | • | |
| High-sensitivity CRP | • | • | • | |
| TNF-α | • | -- | • | |
| IL-6 | • | -- | • | |
| Cognitive Function | NIH Toolbox Cognition Battery (NIHTB-CB) | • | • | • |
| Self-regulation | Brief Self-Control Scale | • | • | • |
| Effortful Control Scale | • | • | • | |
| Grip strength | • | • | • | |
| Unsolvable puzzle | • | -- | -- | |
| Psychosocial Factors | ACES, BDI-II, EES, IPAQ, PHMS, PFS | • | • | • |
Note. ACES = Adverse Childhood Experience Survey; BDI-II = Beck Depression Inventory-II; DBP = diastolic blood pressure; EES = Emotional Eating Questionnaire; HOMA-IR = homeostatic model of insulin resistance; IPAQ = International Physical Activity Questionnaire; PHMS = Philadelphia Mindfulness Survey; PFS = Power of Food Scale; SBP = systolic blood pressure; WALI = Weight and Lifestyle Inventory.
Figure 2.
CONSORT Flow Diagram
Table 2.
Baseline Demographics and Adiposity Indices of Enrolled COSMOS Participants
| Total Sample (N = 108) |
ABT (n = 54) |
SBT (n = 54) |
Group Difference p-value |
|
|---|---|---|---|---|
| Age (years) | 45.6 ± 11.4 | 45.6 ± 11.9 | 45.5 ± 11.0 | .987 |
| Gender (Female) | 78 (72.2) | 39 (72.2) | 39 (72.2) | 1.0 |
| Race (Non-white) | 23 (21.9) | 14 (25.9) | 9 (17.6) | .664 |
| Education (≥ Bachelor’s) | 81 (76.4) | 40 (74.1) | 41 (78.8) | .351 |
| BMI (kg/m2) | 35.7 ± 5.9 | 36.2 ± 6.2 | 35.2 ± 5.6 | .404 |
| Weight (kg) | 100.6 ± 20.8 | 101.8 ± 20.7 | 99.3 ± 21.0 | .545 |
| Fat Mass (kg) | 41.9 ± 12.5 | 42.8 ± 13.1 | 41.0 ± 11.9 | .659 |
| Percent Body Fat (%) | 41.5 ± 7.2 | 41.8 ± 7.7 | 41.2 ± 6.7 | .659 |
| Waist Circumference (cm) | 107.6 ± 13.1 | 108.4 ± 12.7 | 106.7 ±13.5 | .495 |
Note: Continuous variables are reported as mean ± standard deviation and were compared with t-test. Categorical variables are presented as n (%) and were compared with Chi-Square Test of Independence. BMI = Body Mass Index.
2.3 Eligibility and exclusions
COSMOS participants had to meet the following inclusion criteria: (a) aged 21–65 years, (b) English-speaking, (c) baseline BMI ≥ 27.0 and ≤ 52 kg/m2, and (d) attended a study information session. Exclusion criteria are: (a) age or BMI out of stated range, (b) non-English speaking, (c) currently pregnant or breastfeeding or planning to become pregnant in next 12 months, (d) history of bariatric surgery or planning to get surgery within next 12 months, (e) history of neurological disorder or injury (e.g. stroke, or seizures; loss of consciousness > 10 minutes), (f) current major medical condition (e.g., cancer, liver or kidney disease), (g) impaired sensory function (e.g., visually impaired), (h) history of or current serious psychological disorder (i.e., severe depression or anxiety, substance use, psychotic, bipolar, or eating disorder), (i) recent significant weight loss (> 10% of body weight), (j) taking medications that impact weight (e.g., mirtazapine, prednisone), (k) physical activity contraindicated, or (l) enrolled in another weight loss program (e.g., WeightWatchers®). Inclusion/exclusion were selected to maximize safety and/or to ensure validity of the treatment effects or cognitive testing. We aimed to recruit 60% (n = 61) female and 30% non-white (n = 31) participants, and our actual enrollment of 108 participants was 72% female (n = 78) and 24% non-white (n = 26). Equal numbers of participants were randomized to each group (SBT vs. ABT) (n = 54) (see Figure 2).
2.3 Recruitment
Participants were recruited from the local university and community in four cohorts. Multiple cohorts were used to keep the weekly treatment group sizes to 5–10 people. The primary recruitment strategies used were mailings, emails, and phone calls as well as promotional materials placed in high-traffic areas (e.g., restaurants, hospitals, YMCAs). These recruitment materials directed interested parties to an online screening form, which assessed the various inclusion/exclusion criteria as well as ability to engage in physical activity using the Physical Activity Readiness Questionnaire (PARQ+, see description below).37 Persons interested in enrolling in COSMOS were required to either to meet the appropriate threshold on the PARQ+ or to have written approval from their medical provider prior to beginning COSMOS in order to ensure their safety.
Once cleared per their PARQ+ score or by a signed clearance form from a medical provider as well as meeting all other screening criteria, eligible participants were invited to attend a 1-hour information session that was required for enrollment. In this session, study details were presented using a motivational interviewing technique to promote retention38 should the person decide to enroll. Participants attending the information session could enroll in COSMOS and, if they chose to do so, were asked to provide written informed consent and schedule a baseline fasting blood draw at the campus health center. Once our team received a participant’s blood specimen for analysis, a team member contacted him or her to schedule the 2-hour baseline visit for psychosocial assessments. After baseline blood and psychosocial assessments were complete, the participant was randomized to either SBT or ABT.
2.4 Randomization
The randomization to SBT or ABT was conducted using block randomization with random computer-generated block sizes stratified by sex to ensure equal representation of males and females between treatment arms.
2.5 Treatment Conditions
The two treatments offered (ABT and SBT) are modified versions of the protocols used in the previously successful Mind Your Health (MYH) trial.13 In the MYH protocol, 25 sessions were conducted over 1 year, with session 1–16 offered weekly, sessions 17–21 offered biweekly, sessions 22–23 offered monthly, and sessions 24–25 offered bimonthly. In the COSMOS trial, treatments will be condensed to 6 months, and we will drop sessions from the MYH protocol that involved transitioning participants from weekly sessions to longer duration between sessions. Thus, for COSMOS, 23 sessions will be delivered weekly over 6 months.
As summarized in the MYH results trial paper,13 both SBT and ABT share certain intervention components, including: 1) nutritional education (e.g., recommended servings across diverse food groups), 2) prescriptions for a balanced-deficit diet (~1200–2000 kcal/day depending on weight) and physical activity (i.e., gradual increase to 250 min/week of brisk walking or the equivalent by week 18), 3) expectations for daily self-monitoring of calorie intake and activity, 4) stimulus control, behavior shaping, behavior analysis, and relapse prevention strategies, and 5) social support. Key differences between SBT and ABT are detailed below.
Standard Behavioral Treatment
The SBT condition will utilize unique standard behavioral weight loss treatment strategies based on existing obesity treatments. These features include all of the shared features listed above as well as the following unique features: 1) changing the content of one’s thoughts, 2) cognitive restructuring, 3) building self-efficacy and positive self-esteem, and 4) learning to cope with food cravings by distracting from and psychologically confronting cravings.
Acceptance-Based Behavioral Treatment
ABT for weight loss is a manualized protocol that has been empirically tested in the MYH RCT (R01 DK095069)13 and has been recently published as a treatment manual for use by providers.26 It contains all of the shared features listed above as well as unique ABT training designed to help individuals increase awareness of their perceptual, cognitive, and affective experiences, and the following exercises: 1) identifying weight-related goals from personal life values (e.g., health) and connecting these values to day-to-day eating, 2) increasing awareness of moment-by-moment behavior choices, 3) tolerating aversive internal states that include eating-related states as well as affective states such as stress, sadness, and anxiety (i.e., “urge-surfing”). Details about these techniques can be found in the treatment manual.26
2.6 Participant Timeline and Reimbursement
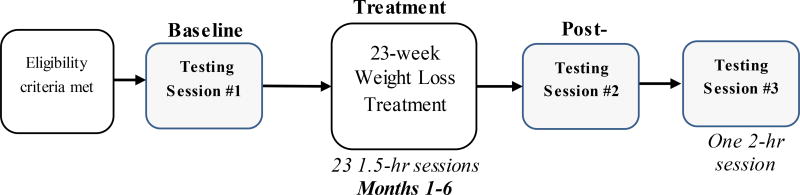
In addition to the 23 treatment groups over 6 months, participants attend three 1.5 to 2-hour assessment sessions: baseline, post-treatment (6 months), and 1-year follow-up (1 year after baseline). Participants receive $75 reimbursement for each of these assessment visits, totaling $225 if all sessions are attended. The timeline for specific study procedures is presented in Figure 3. Assessments are conducted by individuals blind to the participants’ treatment conditions.
Figure 3.
Participant timeline for procedures.
2.7 Primary Outcome
The primary outcome is change in measured total weight (kg) from baseline to post-treatment and from baseline to follow-up. We will also collect measured fat mass (kg) and waist circumference (cm). These adiposity indicators will be examined in sensitivity analyses to determine if the pattern of results observed for total weight is consistent when using different adiposity indicators (i.e., fat mass or waist circumference). Body weight and fat mass are both measured in the laboratory using a bioelectrical impedance device (Model TBF 310GS; Tanita Corporation: Arlington Heights, IL USA). Measurements are taken to the nearest 0.1 of the kg. Participants are weighed without shoes and wearing casual clothes. Waist circumference is assessed with measuring tape at a level parallel to the floor and placed snugly midway between the bottom of the ribcage and at the highest point of the iliac crest according to World Health Organization protocols.39
2.8 Other Key Measures
Secondary outcomes include multiple biomarkers, cognitive function tests, and self-regulation indices. Each of these outcomes is categorized and described below.
2.8.1 Biomarkers
Metabolic Indicators
At baseline, post-treatment, and follow-up, blood samples for the assessment of fasting glucose, hemoglobin A1C (HgA1C), and insulin are obtained and used to examine glucoregulation and insulin resistance. These samples are collected from whole blood or plasma specimens in sterile tubes and frozen until the time of assay. Samples are analyzed at the medical center laboratory. Fasting glucose and insulin values are used to calculate the homeostasis model assessment of insulin resistance (HOMA-IR).
Vascular Indicators
Following guidelines from the American Heart Association,40 five seated blood pressure readings are taken at 2-min intervals by a trained research assistant using a standard sphygmomanometer at baseline, post-treatment, and follow-up. Systolic (SBP) and diastolic (DBP) values are computed as the average of the last three readings.
Inflammatory Processes
At baseline and follow-up, three markers of inflammation (tumor necrosis factor; TNF-α, interleukin; IL-6 and high-sensitivity c-reactive protein; hsCRP) are measured using whole blood, serum, or plasma specimens. Samples are stored until the time of assay. Assays for serum TNF-α and IL-6 are performed using commercially available kits and conducted at the Oklahoma State Metabolic and Nutrition Phenotyping Core Facility while hsCRP is analyzed at the medical center laboratory.
2.8.2 Cognitive Function
At baseline, post-treatment, and follow-up, participants complete the NIH Toolbox Cognition Battery (NIHTB-CB);41 a comprehensive, computerized neuropsychological battery that assesses the following domains: attention, executive function, memory, processing speed, and language. The NIHTB-CB was created by the NIH for participants aged 3 to 85 years and can be administered in 30 minutes. This battery was chosen for the COSMOS trial because it is time-efficient, ensures that the trial results can be compared across existing NIHTB-CB studies, and allows us to measure cognitive indices over time in relation to the SBT and ABT interventions. The specific tests in the battery are: Flanker Inhibitory Control and Attention, Dimensional Change Card Sort, List Sorting, Picture Sequence Memory, Pattern Comparison Processing, Picture Vocabulary, Oral Reading Recognition. All tests in the battery have been validated against gold-standard instruments and normed for use in the age ranges for the COSMOS trial.41 As supplemental measures of executive function, participants also completed the Go/No-Go and Stroop from the Automated Neuropsychological Assessment Metrics-IV.42
2.8.3 Self-Regulation
At baseline, post-treatment, and follow-up, participants will complete two self-report questionnaires and two behavioral tasks assessing self-regulation, specifically, the ability to persist at and to refrain from certain behaviors. First, the Brief Self-Control Scale43 is a 10-item self-report questionnaire that assesses trait levels of self-control, with a focus on a person’s ability to override an inner urge and refrain from acting on it. It is rated from 1 (“not at all like me”) to 5 (“very much like me”) and has high internal consistency, test-retest reliability, and construct validity.43 Second, the Effortful Control Scale,44 which assesses both the ability to persist and the ability to inhibit impulses, also has good reliability and validity. In addition to these self-report measures of self-regulation, we also included two behavioral indices. The first behavioral task is the Handgrip Strength Test,45 in which participants grip a dynamometer and are timed. The second behavioral task is an unsolvable puzzle, in which participants are asked to solve a puzzle that has no solution and are timed;46 longer time (seconds) on both tasks is associated with greater persistence.
2.8.4 Demographic Factors and Key Covariates
Participants completed baseline self-report questionnaires assessing the following factors: (1) demographic: age (years), gender, race-ethnicity (Caucasian, African American, American Indian/Native American/Alaskan Native, Asian/Pacific Islander, Hispanic/Latino), education level (middle school, high school, some college, associate’s, bachelor’s, graduate or professional); (2) Weight and Lifestyle Inventory;47 and (3) psychosocial: Adverse Childhood Experiences Survey,48 Beck Depression Inventory-II,49 Emotional Eating Questionnaire Revised,50 International Physical Activity Questionnaire,51 Philadelphia Mindfulness Scale,52 and the Power of Food Scale.53
3. Data Analyses & Hypotheses
Before examining the primary aims of the study, requisite checks for missingness, outliers, skewness, kurtosis, and multicollinearity will be performed. Missing data will be imputed within person, within subscale when possible and using maximum likelihood estimation for mixed-effects model analysis. Transformations and corrections for non-linearity or multicollinearity will be performed as needed (e.g., square root or logarithmic transformations; removing or aggregating redundant items, etc.) Treatment groups will be compared on characteristics at baseline using t- tests or χ2 tests for continuous or categorical variables, respectively.
3.1 Aim 1 Analyses: Cross-sectional Relationships between Obesity, Biomarkers, Cognition, and Self-regulation
The first hypothesis for Aim 1 is that greater obesity will be associated with poorer glycemic control (higher fasting glucose, insulin, HbA1c and HOMA-IR levels), poorer cardiovascular function (elevated SBP and DBP), and elevated inflammatory markers (TNF-α, IL-6, CRP).
To determine the relationship between obesity level and biomarkers, a series of hierarchical regression analyses will be conducted for each biomarker (glucose, insulin, HbA1c, HOMA-IR, SBP, DBP, TNF-α, IL-6, and hs-CRP). Analyses will be identical and in the following form: Block one: Demographic variables and covariates, and Block two: BMI, waist circumference, or body fat mass.
The second hypothesis for Aim 1 is that higher levels of these physiological variables will be associated with relatively poorer performance on neuropsychological tests. Thus, a series of hierarchical regression analyses will be conducted for each domain of cognitive functioning (i.e., cognitive test scores), such that: Block one: Demographic and covariates and Block two: glucose, insulin, HbA1c, HOMA-IR, SBP, DBP, TNF-α, IL-6, or hs-CRP.
The final hypothesis for Aim 1 is that poorer performance on neuropsychological tests will predict lower scores on self-reported and behavioral indicators of self-regulation (e.g., Self-Control Scale, persistence at an unsolvable task). A series of hierarchical regressions will be conducted for each measure of self-regulation: Block one: Demographic variables and covariates, and Block two: Cognitive test scores.
3.2 Aim 2 & 3 Analyses: Within- and Between-Person Changes in Weight across Treatments
In order to evaluate within-person change and inter-individual differences across ABT and SBT in weight outcome variables, we will employ multilevel mixed-effects models of repeated adiposity measures (i.e., total weight, fat, or waist circumference) at baseline (Time 0), 3 months (Time 3), 6 months (Time 6), and 12 months (Time 12). Each adiposity indicator will be examined in a separate model. Advantages of mixed-effects models are their ability to simultaneously address within-person and between-person changes in weight while accounting for fixed and random effects and employing estimation techniques that avoid listwise deletion of cases with missing values.54 The level-1 submodel will consist of each participants’ weight (or waist circumference or fat mass) trajectory across time (i.e., [0, 3, 6, 12]) and corresponds to Aim 2. These repeated measures will be nested within the projected 96 participants (level 2 model). We expect to find substantial heterogeneity in the trajectories across individuals and will test whether treatment type (ABT vs. SBT) and other hypothesized predictors (biomarker levels, cognitive function, self-regulation) contribute meaningfully to the individual differences in the obesity indicator trajectories (Aim 3). We will include a first order autoregression component in order to account for the likelihood that obesity indicators at any two time points depend on the correlation between indicators at the previous time point. The hypothesis for Aim 2 is that – while most individuals will experience weight loss from baseline to post-treatment and follow-up – there will be variability in the slopes of weight loss and change in the secondary outcome variables. The hypothesis for Aim 3 is that the ABT group will show greater improvements outcomes variables (e.g., weight loss, biomarkers, cognition, etc.) than the SBT group.
3.3 Power Analysis & Sample Size Justification
Previous reports comparing ABT versus SBT effects on weight loss suggest a medium-to-strong effect of treatment type (d = .43–67).12,13 Sample size for a two-level mixed-effects model of repeated weight measures across two groups was estimated using the following input parameters: power = 0.80, alpha = 0.05 (two-tailed), four weight time points [Times 0, 3 6, 12], effect size = 0.5, expected minimum correlation between weight time point ρ = 0.7, and total attrition of 30%.55 According to Hedeker and colleagues,55 estimates using the above parameters indicated that a sample of 102 participants will be required at baseline (51 for each group) to test for a constant effect of group across time accounting for attrition.
4. Discussion
This paper describes the trial methodology for the COSMOS project and presents our screening, enrollment, and allocation to treatment rates as well as basic demographic data of enrolled participants. To our knowledge, COSMOS will be the first trial to examine concurrently how changes in physiological, cognitive, and self-regulatory factors are related to weight loss and also to one another before and after two different weight loss treatments: SBT vs. ABT. Understanding how these factors work together is clearly needed to optimize effective obesity treatment. In so doing, COSMOS will offer three significant innovations over previous work.
First, no study has examined multiple indices of biomarkers, cognitive function, and self-regulation in a comprehensive two-arm study (SBT vs. ABT). We seek to demonstrate that baseline obesity-related physiological dysregulation across multiple systems (higher glucose, insulin, HbA1c and HOMA-IR levels, elevated SBP and DBP, TNF-α, IL-6, and CRP) is linked to cognitive deficits and poor self-regulation by testing multiple physiologic indices, measures of cognitive function, and self-report and behavioral measures of self-regulation (Aim 1).
Second, no RCT has examined whether changes in these factors differ across individuals or whether the differential changes are related to one another. We will examine differential improvements in biomarkers, cognition, and self-regulation from pre- to post-treatment across individuals (Aim 2). These data will provide a better understanding of how physical, cognitive, and self-regulatory factors predict success and failure in obesity treatments across individuals.
Third, no study has comprehensively examined the potential physiological, cognitive, or self-regulatory mechanisms by which ABT promotes greater weight loss than standard behavioral therapies (i.e., SBT). Thus, we will evaluate whether the ABT group has greater improvements in biomarkers, cognition, self-regulation as well as greater weight loss and less weight regain than SBT from baseline to post-treatment and from baseline to follow-up (Aim 3).
As described above, the idea of accepting thoughts and urges about food and sedentary behaviors instead of using mental energy in trying to change or alter them is one of the fundamental differences between ABT and SBTs, particularly because ABTs operate under the explicit assumption that persistent efforts to get rid of or change certain internal experiences can exacerbate or create additional symptoms or maladaptive behaviors.56 This paradoxical effect is empirically supported and well-illustrated using the classic article57 of the “white bear effect,” in which participants’ attempts to suppress thoughts about a white bear ultimately led to the opposite outcome. This experimental finding has been replicated and expanded in the clinical literature, with treatment modalities such as ACT moving away from thought restructuring (which favors suppressing an old maladaptive thought in favor of a new one) and instead focusing on thought/urge acceptance. These different approaches to interacting with thoughts/urges may differentially impact cognitive function and, ultimately, self-control. Self-control has long been viewed as a limited resource43,55 that is related to glucose availability or allocation56 and cognitive load. However, recently an alternative view suggests that self-control acts more like an aversive emotion state,58,59 which emotional regulation strategies in ABTs are well-suited to target. Either view of self-control would predict that ABTs and SBTs may have different impacts on physiological, cognitive, and self-regulatory domains as they relate to weight loss.
In examining the above questions, COSMOS reflects an initiative to better understand the broader construct of behavioral dyscontrol (i.e., poor inhibition and/or behavioral initiation), particularly as it relates to eating and physical activity. A parallel literature from the substance abuse field suggests that behavioral dyscontrol results from poor regulation of the limbic system by the prefrontal cortex and/or potential impaired communication between these two regions.60,61 In the case of weight control, the brain’s reward circuitry (e.g., motivational drive to eat in the moment) tends to act independently of long-term, goal-oriented self-regulation (e.g., desire to lose weight and gain health).62 This dual process theory suggests that System 1 (automatic, limbic) often supersedes System 2 (rational, prefrontal).63
In obesity management, this behavioral dyscontrol and reduced inability of the prefrontal cortex to override limbic drives is likely related to the metabolic adaptation that occurs for individuals in weight-reduced state.64,65 Specifically, as individuals lose weight, their body begins to enact physiological changes to defend their weight set point, such as increases in subjective appetite and appetite stimulating hormones (e.g., ghrelin) as well as decreases in subjective and hormonal satiety signals (e.g., leptin), energy expenditure, and sympathetic tone.64 Thus, the individual who is losing weight is entering a biological state of trying to regain weight.65 These biological drives to consume food and conserve energy are occurring in an environment where calorie dense foods are still highly accessible and sedentary behavior is the norm rather than the exception. Despite this combination of factors that promote weight gain, some individuals are able to persist and succeed at long-term weight loss.6
One potential reason for long-term weight loss success may be a person’s ability to persistently engage in some healthful behaviors (e.g., exercise) and avoid unhealthful behavior (e.g., overeating) despite the acute experience of discomfort or reduction in pleasure and/or bodily urges/thoughts to do the opposite. As described above, these tenets of self-regulation are at the core of the theory underlying ABT for weight loss programs, which promote distress/discomfort tolerance, separating a person’s behaviors from automatic thoughts or urges, and mindfully focusing on long-term goals. Empirically, ABT’s superior performance to SBT on weight loss outcomes in some studies has been shown to be mediated through ABT’s promotion of psychological acceptance of food cravings and autonomous regulation of health behaviors.12 The COSMOS trial will address whether ABTs for weight loss also differentially impact physiological, cognitive, and other self-regulatory indicators.
In brief summary, our results will provide important data about how impaired physiology, cognitive function, and/or self-regulation promote obesity and how different weight loss treatments may differentially impact these factors. This information will be used to identify promising cognitive and self-regulatory treatment targets for preventing further obesity development and for maintaining weight loss.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease (Award Number K23DK103941-01A1). The views expressed in this article are those of the author(s) and do not necessarily represent the views of the National Institutes of Health. MH is supported under National Institute of Health (awards K23DK103941-01A1) administered by Oklahoma State University. This funding source had no role in the design or implementation of this study, and will have no role in data analysis, in of data, or the decision to submit results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declared no conflict of interest.
Study Status
Recruitment for this study is ongoing but is expected to be complete by October 2017. Misty Hawkins (misty.hawkins@okstate.edu) is the study contact.
The ClinicalTrials.gov identification number is NCT02786238.
Author’s contributions
MH is principle investigator of the study who conceptualized the research question/design and drafted and edited the manuscript.
JC is a postdoctoral fellow on the study who assists with data management and analyses and has reviewed and edited the manuscript.
JG is the neuropsychologist on the study who helped conceptualize the research plan and measures as MH’s K23 mentor, will assist with interpretation of the cognitive testing, and has reviewed and edited the manuscript.
JH helped conceptualize the research plan and patient-oriented assessments as MH’s K23 mentor and has reviewed and edited the manuscript.
LM provides clinical supervision of the weight loss groups and guidance on the implementation of the clinical trial as MH’s K23 mentor and reviewed the manuscript.
NB helped conceptualize the research plan and measures regarding nutritional assessment as MH’s K23 mentor and reviewed the manuscript.
NK and CS help conduct the weight loss treatment groups, are involved in data management, and have reviewed and edited the manuscript.
KV is the content expert in self-regulation, provided guidance of measures of self-regulation, and reviewed the manuscript.
SM provided consultation regarding appropriate research design of multidisciplinary clinical research and reviewed the manuscript.
EF is the creator/author of the acceptance-based weight loss protocol used in the current study and reviewed and edited the manuscript.
WL provided guidance on study measures and the development of the study protocol of biomarker assessment and has reviewed and edited the manuscript.
References
- 1.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. Jama. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Annals of Nutrition and Metabolism. 2015;66(Suppl. 2):7–12. doi: 10.1159/000375143. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health affairs. 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 5.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes, metabolic syndrome and obesity : targets and therapy. 2010;3:285–295. doi: 10.2147/DMSOTT.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. The American journal of clinical nutrition. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins M, Gustand J. Body weight and neurocognitive function. In: CG Fairburn, KD Brownell., editors. Eating disorders and obesity: A comprehensive handbook. 3. Guilford Press; 2016. pp. 84–88. [Google Scholar]
- 8.Cournot M, Marquie J, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 9.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Smith E, Hay P, Campbell L, Trollor J. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obesity Reviews. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 11.Stanek KM, Strain G, Devlin M, et al. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27(2):141. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman E, Hoffman KL, Juarascio AS, Butryn ML, Herbert JD. Comparison of acceptance-based and standard cognitive-based coping strategies for craving sweets in overweight and obese women. Eating behaviors. 2013;14(1):64–68. doi: 10.1016/j.eatbeh.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Forman EM, Butryn ML, Manasse SM, et al. Acceptance- based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity. 2016;24(10):2050–2056. doi: 10.1002/oby.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical psychology review. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and cognition. 2010;19(2):597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Forman EM, Butryn ML. A new look at the science of weight control: how acceptance and commitment strategies can address the challenge of self-regulation. Appetite. 2015;84:171–180. doi: 10.1016/j.appet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. Guilford Press; 1999. [Google Scholar]
- 18.Robins CJ, Ivanoff AM, Linehan MM. Dialectical behavior therapy. Handbook of personality disorders: Theory, research, and treatment. 2001:437–459. [Google Scholar]
- 19.The Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP) Diabetes Care. 2002;25(12):2165. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownell KD. LEARN program for weight control. American Health Pub. Co; 1994. [Google Scholar]
- 21.Kinsinger LS, Jones KR, Kahwati L, et al. Peer reviewed: design and dissemination of the MOVE! Weight-Management Program for veterans. Preventing chronic disease. 2009;6(3) [PMC free article] [PubMed] [Google Scholar]
- 22.Fogarty FA, Lu LM, Sollers JJ, Krivoschekov SG, Booth RJ, Consedine NS. Why it pays to be mindful: Trait mindfulness predicts physiological recovery from emotional stress and greater differentiation among negative emotions. Mindfulness. 2015;6(2):175–185. [Google Scholar]
- 23.Group DPPR. The diabetes prevention program (DPP) Diabetes care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brownell KD. LEARN program for weight management 2000. American Health; 2000. [Google Scholar]
- 25.Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eating disorders. 2010;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- 26.Forman E, Butryn ML. Effective Weight Loss: An Acceptance-Based Behavioral Approach. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 27.van de Veer E, van Herpen E, van Trijp H. Body and Mind: How Mindfulness Enhances Consumers Responsiveness to Physiological Cues in Food Consumption. Advances in Consumer Research. 2011;39:603. [Google Scholar]
- 28.Lillis J, Hayes SC, Bunting K, Masuda A. Teaching acceptance and mindfulness to improve the lives of the obese: a preliminary test of a theoretical model. Annals of Behavioral Medicine. 2009;37(1):58–69. doi: 10.1007/s12160-009-9083-x. [DOI] [PubMed] [Google Scholar]
- 29.Linehan MM. Skills training manual for treating borderline personality disorder. Guilford Press; 1993. [Google Scholar]
- 30.McKay M, Wood JC, Brantley J. The dialectical behavior therapy skills workbook: Practical DBT exercises for learning mindfulness, interpersonal effectiveness, emotion regulation and distress tolerance. 2010 ReadHowYouWant.com. [Google Scholar]
- 31.Hendrickson KL, Rasmussen EB. Effects of mindful eating training on delay and probability discounting for food and money in obese and healthy-weight individuals. Behaviour Research and Therapy. 2013;51(7):399–409. doi: 10.1016/j.brat.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Consciousness and cognition. 2009;18(1):176–186. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Forman EM, Butryn ML, Hoffman KL, Herbert JD. An open trial of an acceptance-based behavioral intervention for weight loss. Cognitive and Behavioral practice. 2009;16(2):223–235. [Google Scholar]
- 34.Schiffman SS, Graham BG, Sattely-Miller EA, Peterson-Dancy M. Elevated and sustained desire for sweet taste in African-Americans: a potential factor in the development of obesity. nutrition. 16(10):886–893. doi: 10.1016/s0899-9007(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 35.Levi J, Segal LM, St Laurent R, Rayburn J. Special Report: Racial and Ethnic Disparities in Obesity. Princeton, NJ: Trust for America’s Health; 2014. [Google Scholar]
- 36.Egger M, Jüni P, Bartlett C for the CG. Value of flow diagrams in reports of randomized controlled trials. Jama. 2001;285(15):1996–1999. doi: 10.1001/jama.285.15.1996. [DOI] [PubMed] [Google Scholar]
- 37.Warburton D, Jamnik VK, Bredin SS, Gledhill N. The 2014 physical activity readiness questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+) Health & Fitness Journal of Canada. 2014;7(1):80. [Google Scholar]
- 38.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health Education Research. 2005;20(4):439–447. doi: 10.1093/her/cyg139. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Waist circumference and waist-hip ratio: Report of a WHO expert consultation. Geneva: Dec 8–11, 2008. 2011. [Google Scholar]
- 40.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kane RL, Reeves DL. Computerized test batteries. The neuropsychology handbook. 1997;1:423–467. [Google Scholar]
- 43.Tangney JP, Baumeister RF, Boone AL. High self- control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 44.Lonigan CJ, Vasey MW, Phillips BM, Hazen RA. Temperament, anxiety, and the processing of threat-relevant stimuli. Journal of Clinical Child and Adolescent Psychology. 2004;33(1):8–20. doi: 10.1207/S15374424JCCP3301_2. [DOI] [PubMed] [Google Scholar]
- 45.Muraven M, Tice DM, Baumeister RF. Self-control as a limited resource: Regulatory depletion patterns. Journal of personality and social psychology. 1998;74(3):774. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- 46.McFarlin DB, Baumeister RF, Blascovich J. On knowing when to quit: Task failure, self- esteem, advice, and nonproductive persistence. Journal of Personality. 1984;52(2):138–155. [Google Scholar]
- 47.Wadden TA, Foster GD. Weight and lifestyle inventory (WALI) Obesity. 2006;14(S3) doi: 10.1038/oby.2006.289. [DOI] [PubMed] [Google Scholar]
- 48.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American journal of preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 49.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78(2):490–498. [Google Scholar]
- 50.Koball AM, Meers MR, Storfer-Isser A, Domoff SE, Musher-Eizenman DR. Eating when bored: revision of the emotional eating scale with a focus on boredom. Health psychology. 2012;31(4):521. doi: 10.1037/a0025893. [DOI] [PubMed] [Google Scholar]
- 51.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 52.Cardaciotto L, Herbert JD, Forman EM, Moitra E, Farrow V. The assessment of present-moment awareness and acceptance: The Philadelphia Mindfulness Scale. Assessment. 2008;15(2):204–223. doi: 10.1177/1073191107311467. [DOI] [PubMed] [Google Scholar]
- 53.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. 20032003 [Google Scholar]
- 55.Hedeker D, Gibbons RD, Waternaux C. Sample Size Estimation for Longitudinal Designs with Attrition: Comparing Time-Related Contrasts between Two Groups. Journal of Educational and Behavioral Statistics. 1999;24(1):70–93. [Google Scholar]
- 56.Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):70. [Google Scholar]
- 57.Wegner DM, Schneider DJ, Carter SR, White TL. Paradoxical effects of thought suppression. Journal of personality and social psychology. 1987;53(1):5. doi: 10.1037//0022-3514.53.1.5. [DOI] [PubMed] [Google Scholar]
- 58.Carter EC, Kofler LM, Forster DE, McCullough ME. A series of meta-analytic tests of the depletion effect: self-control does not seem to rely on a limited resource. American Psychological Association; 2015. [DOI] [PubMed] [Google Scholar]
- 59.Inzlicht M, Berkman E, Elkins-Brown N. The neuroscience of “ego depletion”. Social neuroscience: Biological approaches to social psychology. 2016:101–123. [Google Scholar]
- 60.Li C-sR, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal–limbic dysfunction in psycho-stimulant addiction. Neuroscience & Biobehavioral Reviews. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of P sychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng H, Lenard N, Shin A, Berthoud H-R. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. International journal of Obesity. 2009;33:S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans J. In two minds: dual-process accounts of reasoning. Trends in Cognitive Sciences. 7(10):454–459. doi: 10.1016/j.tics.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. New England Journal of Medicine. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 65.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clinical Science. 2013;124(4):231–241. doi: 10.1042/CS20120223. [DOI] [PubMed] [Google Scholar]