Supplemental Digital Content is available in the text.
Abstract
Aortic stenosis is a heterogeneous disorder. Variations in the pathological and physiological responses to pressure overload are incompletely understood and generate a range of flow and pressure gradient patterns, which ultimately cause varying microvascular effects. The impact of cardiac-coronary coupling depends on these pressure and flow effects. In this article, we explore important concepts concerning cardiac physiology and the coronary microcirculation in aortic stenosis and their impact on myocardial remodeling, aortic valve flow patterns, and clinical progression.
“There is a form of cardiac lesion, not infrequent in occurrence, which has a clinical picture so characteristic that it deserves more frequent recognition than it commonly receives.”
Henry A Christian, 18th July 19311
Severe symptomatic aortic stenosis (AS) has a bleak prognosis2,3 and no medical treatment exists. As the population ages, the clinical importance and burden of AS are increasing, yet its diagnosis and management are multifaceted, especially in the era of percutaneous interventions. AS is characterized by progressive valve narrowing, which clinically manifests as dyspnea, syncope, and angina despite normal coronary arteries, and patients have a truncated life span of around 2 years without intervention. However, symptomatology is subjective and confounded by comorbidities (particularly in the aging population), and assessment of transvalvular pressures is heavily flow dependent. The clinician is therefore faced with the challenge of evaluating discordant parameters and balancing the potential risks and benefits of valve intervention.
In 1616, William Harvey was the first to propose that blood circulates because of pulsatile cardiac force.4 Interactions between the cardiac cycle and coronary circulatory flow were described in 1696 by Scaramucci who suggested that the coronary vasculature is filled in diastole and squeezed empty during systole.5 Cardiac-coronary coupling is pertinent in AS because alterations to the coronary microcirculation are synonymous with the pathophysiology of progressive disease. Disruption to the coronary circulation by ventricular hypertrophy, high left ventricular pressure, low coronary perfusion pressure, and extravascular forces (among many other factors) reduce physiological reserve. The ominous symptom of angina correlates with impaired myocardial perfusion reserve and is strongly associated with increased ventricular mass index.6 The fact that clinical symptoms occur at the end of the ischemic cascade (whereas perfusion abnormalities can be detected earlier) places great expectation on the physiological evaluation of AS.7
Patients with aortic stenosis and an aortic valve area (AVA) < 1 cm2 exhibit distinct pathophysiological responses to pressure overload. The ventricle remodels in response to pressure overload in different ways, generating a range of flow and pressure gradient patterns which ultimately cause varying microvascular effects. Detailed understanding of the pressure-flow relationship in this setting is important in fully understanding a patient’s symptoms and the complex relationship between disrupted coronary flow, left ventricular mechanics, and surrogate markers of ischemia.
Cardiac-Coronary Coupling in Health
Normal resting coronary blood flow comprises around 4% of total cardiac output,8 and both oxygen extraction and the myocardial metabolic rate are high when compared with skeletal muscle. During the cardiac cycle, cardiac contraction cyclically increases intramural tissue and microvascular pressures to impede systolic flow. This contraction induces greater subendocardial resistance and blood displacement in comparison with the subepicardium.9,10 Once the aortic valve closes and left ventricular (LV) relaxation ensues, the coronary vessels embedded in the myocardium recoil and blood flow accelerates. Coronary flow is dictated by this effect of cardiac contraction—the intramyocardial pump—which pushes blood backward and draws it in during systole and diastole, respectively,11 (Figure 1)12,13 but is also modulated by aortic and LV pressure, and inotropic state.
Figure 1.
Myocardial contraction results in muscle shortening and thickening to cause extravascular coronary compression. The mechanism of myocardium-vessel interaction is a collective effect of contraction-induced intramyocyte pressure and LV pressure-derived interstitial pressure.12 Adapted from Westerhof et al13 with permission. Copyright ©2006, The American Physiological Society.
The waterfall model14 proposes that external hydrostatic vascular pressure causes temporary partial collapse of the lumen. Distal luminal pressure therefore becomes similar to external (or intramyocardial) tissue pressure. This external pressure is presumed to result from intraventricular cavity pressure, creating a force against the myocardial walls that reduces from subendocardium to subepicardium. The intramyocardial pump model15 expands on this further to allow phase-lag between arterial and venous flows and the role of vascular compliance. Subendocardial vulnerability to ischemia in normal hearts therefore reflects changes in 2 main factors16:
Increased tension because of systolic compression and increased subendocardial wall stress, accompanied by increased myocardial oxygen requirements.17 Both invasive and noninvasive studies have demonstrated increasing intramyocardial pressure from the epicardial to the endocardial surface of the ventricular wall.18–20
- Decreased subendocardial perfusion, secondary to:
-
(a)Systolic backflow from endocardial to epicardial vessels causing preferential epicardial blood flow.21
- (b)
- (c)
-
(a)
Figure 2.
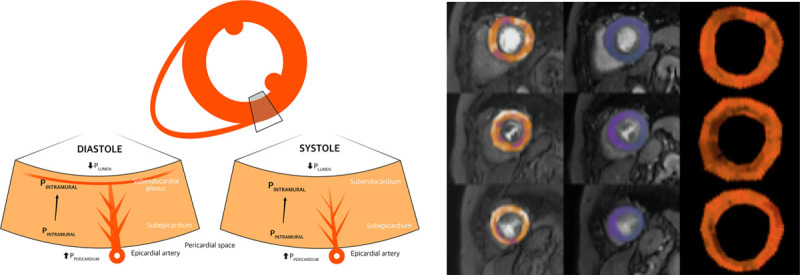
Structural and functional coronary and myocardial changes during the cardiac cycle and vasodilator stress. A, Diagrammatic representation of the extravascular forces and intraluminal pressures affecting myocardial layers, demonstrating greater subendocardial contraction during systole. B, Perfusion quantification map in a patient with AS (rows from top to bottom are basal, mid, and apical slices, respectively, with stress, rest, and myocardial perfusion reserve [MPR] in columns left to right). Global endocardial-epicardial gradient 0.9, MPR 2.0. PINTRAMURAL indicates intramural pressure; PLUMEN, pressure in the left ventricular lumen; and PPERICARDIUM, pressure in the pericardial space. Adapted from Duncker and Bache and Bell and Fox25,26 with permission. Copyright ©2008, The American Physiological Society.
According to Laplace law, circumferential wall tension is equal to the product of the vessel pressure and radius, divided by wall thickness (T=P.r/Th) meaning that the diameter-to-thickness ratio of the vessel or chamber plays an important role. Wall tension and extravascular compressive forces are therefore greatest in the innermost layers of the LV wall. Supporting intramyocardial pressure as a strong determinant of subendocardial blood flow, an early study on anesthetized dogs demonstrated a flow gradient favoring the subendocardium during hyperemia in cardiac arrest (thereby minimizing intramyocardial pressures). However, when tissue pressures were maximized by rapid pacing and coronary perfusion maintained through autoperfusion, the gradient of flow favored the subepicardium.27 At low preload, intramyocardial pressure shuts off systolic coronary blood flow across the entire LV wall.28 Conversely, there is preferential subepicardial blood flow at high preload.29 Coronary blood flow is therefore a balance between intravascular arterial and extravascular tissue pressure.30
Myocardial Blood Supply in Health
The coronary vascular bed acts as the primary gatekeeper to myocardial blood supply. Resting myocardial blood flow (MBF) is the greatest in the subendocardium (endocardial/epicardial flow ratio 1.29–1.3511,31), but subepicardial MBF is augmented during adenosine-induced hyperemia to a greater extent. During systole, there is significant subendocardial underperfusion because of the aforementioned physical determinants (transmural perfusion endocardial to epicardial ratio 0.3811). After a period of ischemia, reactive hyperemia is earliest in the subepicardium,9 and this delayed subendocardial response is thought to be because of sluggish reopening of the coronary vasculature embedded in ischemic, poorly compliant myocardium.
Among many other mechanisms, the gradient in coronary perfusion pressure (difference between aortic and LV end diastolic pressure) facilitates coronary perfusion, and flow is determined by the product of the net velocity-time integral and cross-sectional arterial area (Q=VA). The largest cross-sectional area exists in the microvasculature where reduced velocity allows adequate time for capillary bed gas transfer. In normal hearts, aortic and LV pressures are coupled during systolic ejection and higher perfusion pressure gradients enable coronary perfusion during diastole. There is a nonlinear connection between cross-sectional area and transmural pressure because vascular tone is influenced by metabolic/neurohormonal mediators and physical forces. According to Ohm’s law, flow through a vascular bed is equal to the perfusion pressure gradient divided by vessel resistance, 8ηl/πr4 (Hagen-Poiseuille equation, where η is blood viscosity, l is vessel length, and r is vessel radius). Microvascular resistance is therefore primarily determined by lumen diameter and vasodilatation is the principle means of microcirculatory autoregulation.
During maximal coronary vasodilatation, coronary flow depends on the relative duration of diastole.32 This diastolic time fraction (the length of diastole/length of cardiac cycle) has an inverse relationship with heart rate and is also determined by other modulators of systolic duration (such as altered myocyte contraction). Decreased coronary perfusion pressure induces an increase in diastolic time fraction, which in turn reduces the duration of intramyocardial vessel compression.
Coronary Wave Intensity Analysis
Studies of wave intensity analysis have identified 4 main coronary waves within the cardiac cycle in health and disease33 (Figure 3).
Figure 3.
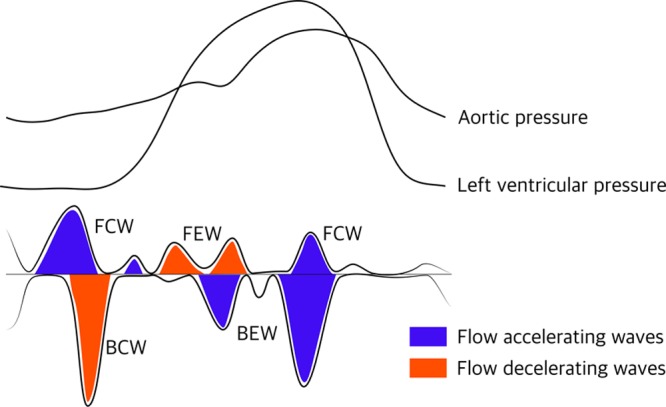
The 4 dominant coronary waves during the cardiac cycle in relation to hemodynamic indices (not to scale). BCW indicates backward compression wave; BEW, backward expansion wave; FCW, forward compression wave; and FEW, forward expansion wave.
Quantification of net wave intensity through the product of changes in pressure and flow velocity makes it possible to segregate components of coronary flow into forward or backward traveling waves from the aorta or microcirculation, and those caused by suction (expansion) or compression—blood can be pushed into or pulled out of the coronary circulation. Flow from the coronary circulation to the myocardium is largely determined by the prominent backward expansion wave (BEW), originating at the onset of LV relaxation. The decelerating backward compression wave and forward expansion wave impede coronary flow, while the BEW and forward compression wave are accelerating waves. Information concerning the size, direction, and duration of coronary waves throughout the cardiac cycle has helped us understand coronary flow in normal hearts, in AS,19 and transcatheter aortic valve implantation (TAVI),34,35 hypertrophic cardiomyopathy36 and several other settings.33,37–42
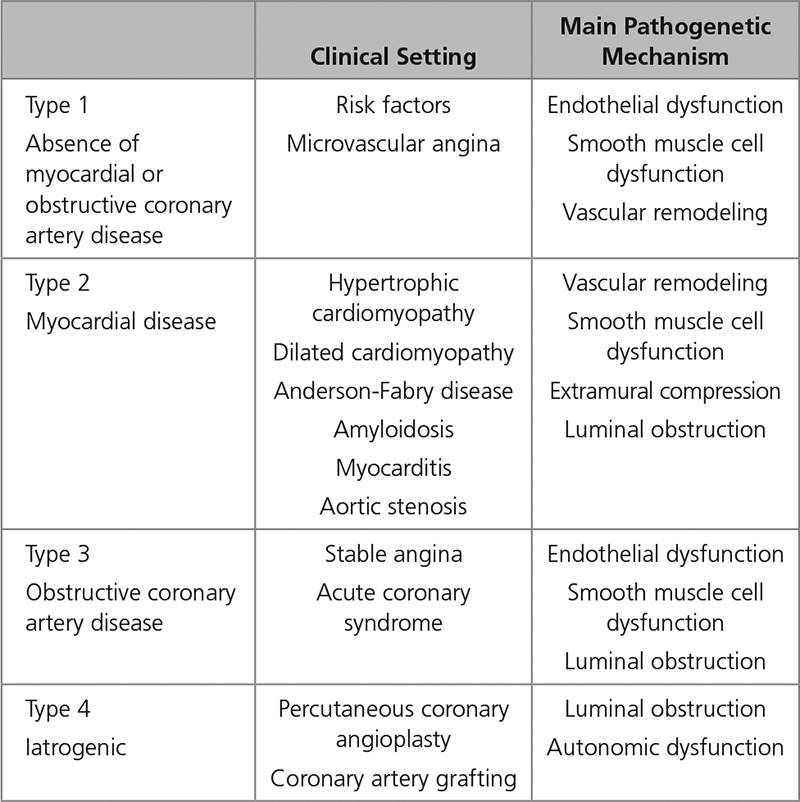
Cardiac-Coronary Coupling in AS
The pathophysiology of calcific degenerative AS has 2 distinct phases: initiation and propagation.43 The former overlaps with the development of atherosclerosis, centered around endothelial disruption and activation of inflammatory responses. Progressive AS induces left ventricular hypertrophy (LVH) to increase contractile force and reduce wall stress44 in response to progressive and eventually insurmountable afterload. Compressive forces resulting from rising intracavitary pressure determine coronary perfusion pressure and limit coronary circulatory response to increased myocardial demand—an association related to the extent of LVH.45 Oxygen requirements increase while perfusion through the small perforating coronary network is compromised by fixed elevated systolic wall stress46,47 and reduced relative capillary density,48,49 creating supply-demand mismatch. These structural changes of vascular rarefaction, compressive forces, and perivascular fibrosis and functional changes, such as reduced diastolic perfusion time (DPT, defined as [RR interval]−[S1-S2 interval]×heart rate) and endothelial and smooth muscle dysfunction, all exert adverse effects.
Preferential coronary flow shifts from the endocardium to epicardium resulting in a significant decrease in subendocardial (but not subepicardial) MBF.50 This reversal of normal endocardial-epicardial blood flow ratio51 at rest is fundamental to the pathophysiology of AS, resulting in subendocardial ischemia,52 apoptosis,47 and fibrosis—clinically manifest as angina despite normal epicardial coronary arteries. Noninvasive detection of this shift in resting endocardial-epicardial ratio could be used to guide timing of valve intervention.
Severe AS exhibits an array of flow parameters, but there is significant LV outflow tract obstruction in all forms, typically accompanied by LVH,53 which may cause dynamic obstruction in late systole with systolic anterior motion of the mitral valve. Unlike hypertrophic cardiomyopathy, where there is a strong linear relationship between peak-to-peak gradient and peak instantaneous gradients, significant scatter exists in AS patients.54
One study demonstrated that severity of AS and parameters of LV workload (but not LVH or diastolic indices) have important roles in determining coronary flow reserve (CFR).55 Another study, however, correlated impaired perfusion reserve with valve stenosis, myocardial fibrosis, and strongly with LVH.45 Cardiac amyloid is common in this population and may confound results.
There are strong similarities in the pathogenic manifestations of AS and hypertension, that is, interstitial and perivascular fibrosis, cardiomyocyte hypertrophy, reduced DPT, increased diastolic filling pressure (compressing the endocardium) and diastolic dysfunction, capillary rarefaction,51 and arteriolar remodeling.56 However, key differences exist. The BEW is the most important contributor to coronary blood flow and a measure of microcirculatory function—it is increased at rest in AS34,35 but reduced in isolated LVH,33 probably as a result of lower wall stress and slower isovolumetric LV relaxation (dP/dtmin). Furthermore, there is a direct relationship between systolic coronary velocity and systolic perfusion pressure in hypertensive patients with no AS—extravascular compressive forces which normally impede systolic coronary flow may be overcome in the setting of higher perfusion pressure.57
After TAVI or surgical aortic valve replacement, there is restoration of myocardial perfusion, oxygenation, energetics, and contractility, accompanied by improved microcirculatory function as a result of the relief of mechanical obstruction and wall stress, and eventual LVH regression.58,59 Indexed stroke volume drops sharply (41±8 to 33±10 mL/m2; P<0.001) as a result of increased systemic vascular resistance (P<0.0001), despite no clear difference in global afterload measured by valvulo-arterial impedance (Zva).60 Hyperemic microvascular resistance (hMR) decreases after TAVI, independent of resting hemodynamics.61 Remaining hypertrophy continues to influence coronary physiology with improved (but not normalized) CFR.
Disrupted Coronary Flow in AS
Microcirculatory autoregulation induces vasodilation to minimize microvascular resistance and increase total resting MBF, resulting in reduced CFR62,63 and MPR64 because of paired inability to further vasodilate (Figure 465). Low coronary perfusion pressure,66 extravascular compressive forces,67 and reduced DPT46,56,61 all seem to play a role. Reduced DPT because of prolonged systole in AS supports the maldistribution theory.68
Figure 4.
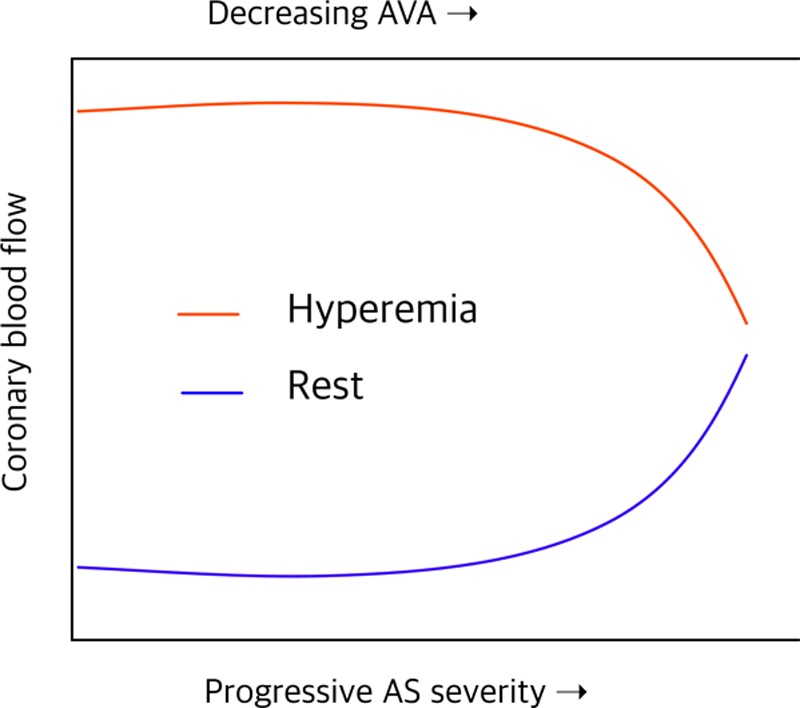
Impairment of coronary flow reserve in progressive aortic stenosis (AS): simulated resting and hyperemic mean coronary blood flow as a function of the severity of AS and estimated orifice area. Induced hyperemia is fundamentally important during circulatory assessment in AS because adaptive hyperemia is already established at baseline—several well-cited studies are flawed in this respect. AVA indicates aortic valve area. Adapted from Garcia et al65 with permission. Copyright ©2009, The American Physiological Society.
In contrast to normal physiology, the relative contribution of accelerating waves to total wave intensity decreases with exercise and hyperemia in AS.19 The contrary is true for decelerating waves: the backward compression wave increases with exercise and hyperemia, thereby hampering flow and driving ischemia. Davies et al34 analyzed wave intensity in the left main stem at programmed heart rates before and after TAVI (albeit without inducing hyperemia) and demonstrated progressive reduction (rather than the expected increase) in the BEW with increasing heart rate. This paradoxically blunted microvascular response normalized after TAVI where induced tachycardia caused the BEW to increase rather than decrease, probably because of a sharp reduction in afterload. A chronological summary of relevant coronary physiology and aortic stenosis studies is displayed in Table I in the Data Supplement.
Before valve intervention, forward flow is delayed, and peak systolic flow and velocity-time integral reduced.69 In comparison to normal hearts, the aortic-ventricular diastolic relationship impairs coronary perfusion.34,70 After TAVI, however, all coronary waves augment (apart from the backward compression wave35), inducing an immediate increase in coronary flow.71 In particular, the forward compression wave improves and its onset is shortened.35 Increased aortic diastolic pressure (with consequent forward pressure at the coronary ostia) accompanied by decreased LV end diastolic pressure and increased DPT causes an elevated driving pressure across the coronary bed. In part, improved forward flow may be because of the resolution of abnormal helical and eccentric vertical flow patterns seen in AS,72 which reduce high fluid pressure and the associated Venturi effect in the proximal aorta and coronary ostia.
LV systolic wall stress index and peak systolic flow velocity73 are tightly knit, suggesting that extravascular compressive forces change systolic flow, although these changes are independent of LV mass. This may explain why CFR may not respond immediately to relief of valve obstruction but improves after 1 year.74 Other studies have also demonstrated improved subendocardial blood flow at 2 weeks,50 CFR at 6 months,75 and indexed myocardial perfusion reserve at 8 months45 after valve replacement. The evidence is strong for structural and hemodynamic effects as the cause of myocardial ischemia in AS.
The pathophysiological and clinical manifestations of coronary microvascular dysfunction, described as heightened sensitivity to vasoconstrictor stimuli associated with limited vasodilator capacity, have been previously classified56 (Table).66
Table.
Classification of Coronary Microvascular Dysfunction66
Coronary physiological response to hyperemia can also be grouped into 4 categories, depending on the presence of normal or abnormal CFR (>2.0 and <2.0, respectively) and normal or abnormal hMR (<1.7 and >1.7 mmHg/cm per second, respectively).76 The reference standard of microvascular dysfunction is invasive measurement of coronary vascular resistance using pressure and flow during hyperemia,77 where hMR is calculated by dividing the mean distal coronary pressure (Pd) by the hyperemic average peak Doppler flow velocity. However, hMR does not determine global microvascular dysfunction but minimal static resistance which is strongly dictated by microcirculatory remodeling—either intrinsic (arteriolar remodeling or capillary rarefaction) or extrinsic to the vascular tree.
Two reasons for reduced CFR in AS have been proposed. The first hypothesis is that inherent microvascular dysfunction elaborates ischemia, as initially proposed by Ahn et al6 who demonstrated reduced myocardial perfusion reserve in patients with AS and angina using perfusion cardiac magnetic resonance imaging (without reporting hemodynamic or microvascular mechanisms).77 The second is that ischemic signs and symptoms result from high wall stress and mechanical effects in response to AS, supported by improvement of coronary physiological indices immediately after TAVI.
Transmural CFR and subendocardial-to-subepicardial perfusion ratio fall directly with decreased hyperemic DPT in AS (measured using positron emission tomography) and improve with increased hyperemic DPT and increased AVA after surgical aortic valve replacement,46,74 supporting a prominent role for hemodynamic conditions in determining CFR—microvascular disease would be expected to yield uniformly reduced transmural perfusion without a gradient.77 Equally, myocardial perfusion reserve may be low in AS patients6 because of the resting increase in perfusion (rather than reduced stress perfusion) because since myocardial perfusion reserve is a relative ratio of stress-to-rest of the magnetic resonance signal,77 and independently associated with exercise capacity.64 Intrinsic endothelial dysfunction does not correlate convincingly with hemodynamic factors that are promptly corrected after TAVI61—proposed mechanisms impacting disrupted microvascular function are illustrated in Figure 5.
Figure 5.
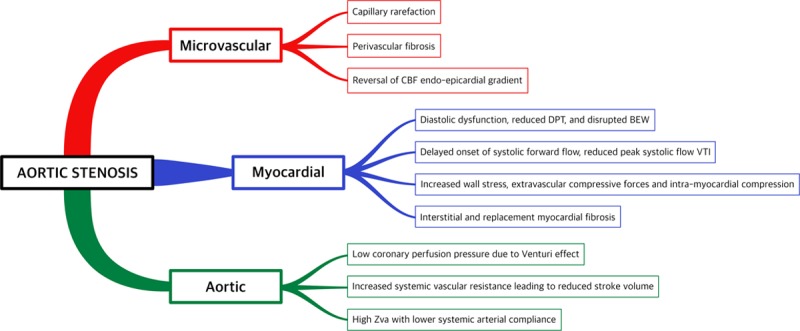
Factors implicated in disrupted coronary flow and reduced coronary flow reserve in aortic stenosis. Compensatory mechanisms fail because of structural and mechanical effects on the ventricle and coronary circulation. There is reduced physiological reserve as a result of inadequate myocardial oxygen supply and increased oxygen demand. BEW indicates backward expansion wave; CBF, coronary blood flow; DPT, diastolic perfusion time; and VTI, velocity-time integral.
Lumley et al19 found that perfusion efficiency during exercise in patients with AS was reduced when compared with normal patients, as a result of augmented early systolic deceleration waves (backward compression wave) and attenuated rise in systolic acceleration waves (forward compression wave). Importantly, further assessment found that AS patients and those with normal hearts are able to reduce microvascular resistance to the same extent.19 Decreased hMR after TAVI independent of resting hemodynamics has also been demonstrated in patients with severe AS (not differentiated into flow or pressure gradient status).61 Clearly, both intra- and extra-myocardial pressures dictate coronary supply and a combination of factors is likely to be responsible for the distortion of coronary flow and impaired CFR in AS.
Aortic Valve Flow and Pressure Gradients
The adaptive compensatory response to AS ultimately become maladaptive and results in cardiac decompensation, yet there are several guises with distinct anatomic and physiological characteristics (Figures 6 and 7).78 Normal-flow high-gradient AS usually provokes concentric hypertrophy, whereas paradoxical low-flow low-gradient (pLFLG) AS patients demonstrate concentric remodeling.79
Figure 6.
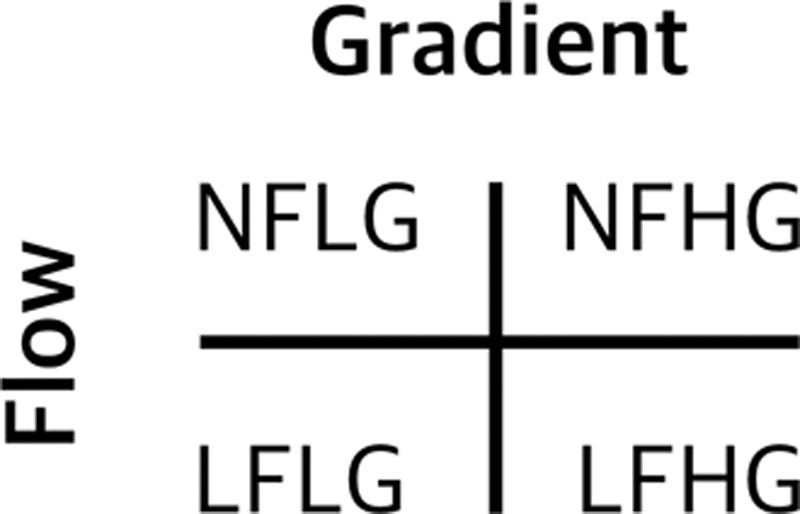
Classification of aortic stenosis according to flow (low-flow <35 mL/m2, normal-flow >35 mL/m2) and gradient (low-gradient mean pressure gradient [MPG] <40 mmHg, high-gradient MPG >40 mmHg). Low-flow low-gradient can be further subdivided into classical and paradoxical according to the presence or absence of impaired left ventricular function. LFHG indicates low flow-high gradient; LFLG, low flow-low gradient; NFHG, normal flow-high gradient; and NFLG, normal flow-low gradient.
Figure 7.
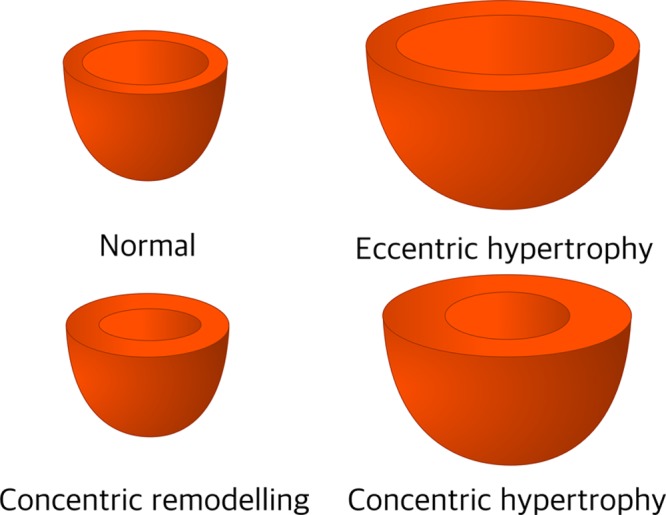
Patterns of cardiac remodeling based on normal or increased mass to volume ratio (concentric remodeling and concentric hypertrophy) and normal left ventricular wall thickness (concentric remodeling) or hypertrophy (concentric and eccentric). Adapted from Gjesdal et al78 with permission. Copyright ©2011, Springer Nature.
The ventricular adaptive response to high afterload in combination with valve obstruction is poorly understood and may be more varied than is currently appreciated. Flow and stroke volume can both be reduced or normal in patients with preserved and reduced LV ejection fraction (LVEF).80 While there is clear consensus that symptomatic AS with AVA <1cm2, peak velocity (Vmax) >4 m/s, and mean pressure gradient >40mmHg warrants intervention, diagnostic ambiguity exists in patients with a small AVA and lower pressure gradients (despite preserved LVEF) where lower stroke volumes contribute significantly to discrepancies.81 Aging, hypertension, diabetes mellitus, and dyslipidemia are associated with microvascular dysfunction and impaired CFR, and there is a higher proportion of diabetes mellitus and hypertension in pLFLG cohorts. These, in turn, are associated with an intrinsic likelihood of impaired CFR,82–84 arising as a consequence of nonendothelium-dependent disorders of nitric oxide metabolism, dysregulation of inflammatory cytokines, estrogen, or adrenergic receptors, and alterations in expression or production of local vasoactive substances such as angiotensin II and endothelin.66
Low-gradient groups may be more susceptible to microvascular disturbance, as evidenced by a higher burden of subendocardial fibrosis on cardiac magnetic resonance.85 Since the first description of pLFLG AS by Hachicha et al,86 there have been conflicting reports and evidence concerning the underlying pathophysiology. Accounting for up to 35% of severe AS cases (with a female preponderance), many are undiagnosed and surgical referral is frequently delayed or overlooked. The syndrome entails the perfect storm of valve, ventricular, and vascular abnormalities, with valve stenosis, concentric LV remodeling (culminating in restrictive physiology), and high Zva with markedly lower systemic arterial compliance and higher arterial resistance.85–90
A low-indexed stroke volume predicts mortality and risk increases sharply when it is <35 mL/m2.91–93 Although still controversial, the bulk of evidence suggests that patients with AS and SVi <35 mL/m2 have markedly worse outcomes.82,86–88,92,94–106 Some discrepant studies (which include a high proportion of asymptomatic patients or fail to account for stroke volume)107–110 have been criticized for imprecise data analysis and misclassification.111 The phenomenon of distinct remodeling is poorly understood, and there is a paucity of invasive data to characterize the cohort and understand factors that predict poor outcome and the response to valve intervention.
European112 and American113 guidelines provide a Class IIA indication for aortic valve intervention in symptomatic pLFLG AS but only after careful confirmation of clinical, hemodynamic, and anatomic data (in the normotensive setting), and exclusion of pseudo-stenosis, where the myopathic ventricle fails to generate adequate force. Although survival is improved when it is treated,80,99,105,106,114,115 these patients have adverse outcomes during and after valve intervention when compared with other AS cohorts,82,100,106 perhaps related to the burden of myocardial fibrosis.116,117 This fibrosis also impacts on myocardial perfusion reserve owing to reduced arteriolar and capillary density.
Structural Remodeling in Low-Gradient AS
The complex collagen weave is responsible for much of the ventricle’s passive diastolic stiffness,118 and remodeling in response to pressure overload causes fibroblast proliferation and collagen I accumulation.119 Myocardial collagen deposition is a common end point of many pathologies and accompanies advanced aging.120 Myocardial hypertrophy is detrimental to overall survival121–123 and correlates with fibrosis, impaired longitudinal shortening, and worsening diastolic function. This fibrosis associated with AS124–127 is a crucial determinant of cardiac dysfunction and prognosis,116,124,125,128,129 and replacement fibrosis may be the result of myocyte apoptosis accounting for progression to heart failure.130 Interstitial, subendocardial, and mid-wall patterns of fibrosis have been demonstrated in patients with AS and normal coronary arteries.85,116,117,123,131–137
While endomyocardial biopsy is the gold standard for confirming fibrosis,138 cardiac magnetic resonance imaging has been widely used in its detection, either using T1 mapping to calculate extracellular volume fraction or late gadolinium enhancement. Extracellular volume fraction can detect extracellular volume expansion with diffuse fibrosis, whereas late gadolinium enhancement only identifies replacement fibrosis.139
Patients with pLFLG AS typically have more profound impairment of LV longitudinal function98,114,140–142 and more florid myocardial fibrosis, predominantly located in the subendocardium.85 In comparison to circumferential fibers located in the mid-wall, longitudinal subendocardial fibers (responsible for long-axis function)143–146 are particularly vulnerable to microvascular ischemia and wall stress.85,131 Impaired longitudinal function as a consequence of subendocardial injury, small LV cavity size, and increased wall thickness lead to reduced stroke volume and lower flow-dependent valve gradients.147 Reduced stroke volume is primarily because of deficient LV filling (rather than emptying),95 and preserved LVEF should not be construed as normal systolic function. Consistent with this theme, a recent study demonstrated that indexed AVA, female sex, an abnormal exercise ECG and myocardial perfusion reserve (but not valve gradients or LV function) were independent predictors of event rates in moderate-severe AS.148
This distinct remodeling may be explained by decreased cardiac reserve resulting from chronic exposure to high afterload, eventually exceeding the limit of compensatory mechanisms with resulting LV impairment and reduced cardiac output.86 It is also possible that these patients have a coexisting or secondary heart failure syndrome, akin to heart failure with preserved ejection fraction,149 the cause of which is complex and poorly understood. Importantly, these 2 pathologies (which are both relatively common in older age) are not mutually exclusive and exhibit significant similarities, including impaired LV relaxation and microvascular abnormalities.46,73,150–153 Indeed, galactin-3, a novel marker of myocardial fibrosis, has prognostic value in heart failure with reduced or preserved ejection fraction154,155 and is associated with adverse outcomes after TAVI156—despite the lack of any association with AS severity.157 Patients with elevated galactin-3 before TAVI have lower valve gradients and reduced LVEF (although data were not divided into AS cohorts).156 Similarly, 1 study revealed that low flow (but not low LVEF or low gradient) is an independent predictor of early and late mortality after TAVI in high-risk AS patients.100 Comparable to patients with heart failure, LVEF does not correlate with outcomes.
Equally, the peril of low flow does not correlate with aortic valve calcification. There is less aortic valve calcification but higher global afterload in pLFLG than other types of AS,80 suggesting a coexistent ventricular disease entity that may explain why these patients have reduced survival benefit after valve intervention than other subgroups. This would support the theory that pLFLG AS is not end-stage normal-flow high-gradient AS158 but a distinct and separate entity.159–161 Furthermore, the concept of pLFLG AS as a transition stage from nonsevere to severe80 is undermined by a preponderance of myocardial injury and adverse outcomes.
Clinical Implications of Impaired Coronary Flow
Reduced capacity to augment myocardial oxygenation in response to stress is a physiological hallmark of AS and manifest by angina, dyspnea, and syncope. Up to 40% of patients with AS experience angina despite normal coronary arteries162 and are at increased risk of sudden death.163 These patients have reduced MBF, impaired CFR, and increased apoptosis47 and are more likely to have impaired reserve6,162 and diminished exercise capacity.64 One study found that low CFR was the only independent predictor of future cardiovascular events in AS patients.164 Exertion accentuates the imbalance between supply and demand, and rising LV end diastolic pressure blunts the pressure gradient required to achieve adequate coronary perfusion. Any rise in LV end diastolic pressure or fall in AVA has a deleterious effect on coronary supply,35,46 and there is a strong association between ventricular load (measured by LV rate-pressure product) and decreased CFR, particularly affecting the subendocardium.46 Stuttering ischemia yields subclinical LV dysfunction and apoptosis, which is linked with myocardial fibrosis165—an independent predictor of mortality.116
Biomarkers have an emerging role in the assessment of asymptomatic AS.166 High-sensitivity troponin I correlates with LVH, fibrosis, and clinical event rates,134 while cardiac myosin-binding protein C correlates closely with LV mass, fibrosis, and all-cause mortality (but not valve gradient).167 BNP (NT-pro B-natriuretic peptide) levels are significantly higher in paradoxical and classical low-flow low-gradient AS,85 and correlate with CFR ≤2.5 and parameters of diastolic function168—use of BNP in asymptomatic AS is endorsed by recent European guidelines.112
Conclusions
Patients with AS host a caustic environment where impaired microvascular responses are compounded by high wall stress and hemodynamic load; those with angina (and impaired CFR) are at increased risk of sudden death. Progression of AS is characterized by discrepancies between blood supply and metabolic demand. There is an array of abnormalities in myocardial remodeling, stroke volume, pressure gradients, and disordered coronary flow, which contribute to the signatures that determine varying AS phenotypes. These distinctions, which correlate with clinical outcomes, should prompt a directive path of physiological research. All patients with AS are not equal and the optimal timing and modality of treatment might differ according to phenotype. Relying on peak velocity to determine severity is now obsolete. Timing of intervention is crucial in avoiding irreversible myocardial fibrosis and a burnt out ventricle. Assessment of microcirculatory function may hold the key.
Sources of Funding
H. McConkey is supported by a Clinical Research Training Fellowship grant from the British Heart Foundation (FS/16/51/32365).
Disclosures
None.
Supplementary Material
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.118.007547.
References
- 1.Christian HA. Aortic stenosis with calcification of the cusps. JAMA. 1931;97:158. [Google Scholar]
- 2.Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. doi: 10.1161/01.cir.66.5.1105. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 3.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38(1)(suppl):61–67. doi: 10.1161/01.cir.38.1s5.v-61. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 4.Harvey W, Leake CD. Exercitatio anatomica de motu cordis et sanguinis in animalibus / by William Harvey; with an English Translation and Annotations by Chauncey D. Leake. Springfield, IL: Thomas; 1928. [Google Scholar]
- 5.Sabiston DC, Gregg DE. Effect of cardiac contraction on coronary blood flow. Circulation. 1957;15:14–20. doi: 10.1161/01.cir.15.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Ahn JH, Kim SM, Park SJ, Jeong DS, Woo MA, Jung SH, Lee SC, Park SW, Choe YH, Park PW, Oh JK. Coronary microvascular dysfunction as a mechanism of angina in severe AS: prospective adenosine-stress CMR study. J Am Coll Cardiol. 2016;67:1412–1422. doi: 10.1016/j.jacc.2016.01.013. doi: 10.1016/j.jacc.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Lancellotti P, Nchimi A. Coronary microvascular reserve and outcome in aortic stenosis: pathophysiological significance vs. clinical relevance. Eur Heart J. 2017;38:1230–1232. doi: 10.1093/eurheartj/ehw635. doi: 10.1093/eurheartj/ehw635. [DOI] [PubMed] [Google Scholar]
- 8.Laxson DD, Dai XZ, Homans DC, Bache RJ. Coronary vasodilator reserve in ischemic myocardium of the exercising dog. Circulation. 1992;85:313–322. doi: 10.1161/01.cir.85.1.313. doi: 10.1161/01.cir.85.1.313. [DOI] [PubMed] [Google Scholar]
- 9.Downey HF, Crystal GJ, Bashour FA. Asynchronous transmural perfusion during coronary reactive hyperaemia. Cardiovasc Res. 1983;17:200–206. doi: 10.1093/cvr/17.4.200. doi: 10.1093/cvr/17.4.200. [DOI] [PubMed] [Google Scholar]
- 10.Goto M, Flynn AE, Doucette JW, Jansen CM, Stork MM, Coggins DL, Muehrcke DD, Husseini WK, Hoffman JI. Cardiac contraction affects deep myocardial vessels predominantly. Am J Physiol. 1991;261(5)(pt 2):H1417–H1429. doi: 10.1152/ajpheart.1991.261.5.H1417. doi: 10.1152/ajpheart.1991.261.5.H1417. [DOI] [PubMed] [Google Scholar]
- 11.Hess DS, Bache RJ. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res. 1976;38:5–15. doi: 10.1161/01.res.38.1.5. doi: 10.1161/01.res.38.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Algranati D, Kassab GS, Lanir Y. Mechanisms of myocardium-coronary vessel interaction. Am J Physiol Heart Circ Physiol. 2010;298:H861–H873. doi: 10.1152/ajpheart.00925.2009. doi: 10.1152/ajpheart.00925.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006;86:1263–1308. doi: 10.1152/physrev.00029.2005. doi: 10.1152/physrev.00029.2005. [DOI] [PubMed] [Google Scholar]
- 14.Downey JM, Kirk ES. Inhibition of coronary blood flow by a vascular waterfall mechanism. Circ Res. 1975;36:753–760. doi: 10.1161/01.res.36.6.753. doi: 10.1161/01.res.36.6.753. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal MR, Wang HH, Wang SC. Effect of acute experimental aortic stenosis on coronary circulation. Circ Res. 1962;11:727–735. doi: 10.1161/01.res.11.4.727. doi: 10.1161/01.res.11.4.727. [DOI] [PubMed] [Google Scholar]
- 16.Komaru T, Kanatsuka H, Shirato K. Coronary microcirculation: physiology and pharmacology. Pharmacol Ther. 2000;86:217–261. doi: 10.1016/s0163-7258(00)00057-7. [DOI] [PubMed] [Google Scholar]
- 17.Heusch G. Alpha-adrenergic coronary vasoconstriction in humans. J Am Coll Cardiol. 2010;55:1278; author reply 1278, 1279. doi: 10.1016/j.jacc.2009.09.067. doi: 10.1016/j.jacc.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 18.Stanojevic D, Gunasekaran P, Tadros P, Wiley M, Earnest M, Mehta A, Lippmann M, Levine M, Dawn B, Gupta K. Intravenous adenosine infusion is safe and well tolerated during coronary fractional flow reserve assessment in elderly patients with severe aortic stenosis. J Invasive Cardiol. 2016;28:357–361. [PubMed] [Google Scholar]
- 19.Lumley M, Williams R, Asrress KN, Arri S, Briceno N, Ellis H, Rajani R, Siebes M, Piek JJ, Clapp B, Redwood SR, Marber MS, Chambers JB, Perera D. Coronary physiology during exercise and vasodilation in the healthy heart and in severe aortic stenosis. J Am Coll Cardiol. 2016;68:688–697. doi: 10.1016/j.jacc.2016.05.071. doi: 10.1016/j.jacc.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 20.Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Barbierato M, Caprioglio F, Vassanelli C, Ribichini F. Coronary physiology in patients with severe aortic stenosis: Comparison between fractional flow reserve and instantaneous wave-free ratio. Int J Cardiol. 2017;243:40–46. doi: 10.1016/j.ijcard.2017.05.117. doi: 10.1016/j.ijcard.2017.05.117. [DOI] [PubMed] [Google Scholar]
- 21.Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, ten Cate FJ, Serruys PW. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998;97:230–233. doi: 10.1161/01.cir.97.3.230. doi: 10.1161/01.cir.97.3.230. [DOI] [PubMed] [Google Scholar]
- 22.Blows LJ, Redwood SR. The pressure wire in practice. Heart. 2007;93:419–422. doi: 10.1136/hrt.2005.066837. doi: 10.1136/hrt.2005.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Gioia G, Pellicano M, Toth GG, Casselman F, Adjedj J, Van Praet F, Ferrara A, Stockman B, Degrieck I, Bartunek J, Trimarco B, Wijns W, De Bruyne B, Barbato E. Fractional flow reserve-guided revascularization in patients with aortic stenosis. Am J Cardiol. 2016;117:1511–1515. doi: 10.1016/j.amjcard.2016.02.023. doi: 10.1016/j.amjcard.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad Y, Götberg M, Cook C, Howard JP, Malik I, Mikhail G, Frame A, Petraco R, Rajkumar C, Demir O, Iglesias JF, Bhindi R, Koul S, Hadjiloizou N, Gerber R, Ramrakha P, Ruparelia N, Sutaria N, Kanaganayagam G, Ariff B, Fertleman M, Anderson J, Chukwuemeka A, Francis D, Mayet J, Serruys P, Davies J, Sen S. Coronary hemodynamics in patients with severe aortic stenosis and coronary artery disease undergoing transcatheter aortic valve replacement: implications for clinical indices of coronary stenosis severity. JACC Cardiovasc Interv. 2018;11:2019–2031. doi: 10.1016/j.jcin.2018.07.019. doi: 10.1016/j.jcin.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 26.Bell JR, Fox AC. Pathogenesis of subendocardial ischemia. Am J Med Sci. 1974;268:3–13. [PubMed] [Google Scholar]
- 27.Downey JM, Kirk ES. The transmural distribution of coronary blood flow during maximal vasodilation. Proc Soc Exp Biol Med. 1975;150:189–193. doi: 10.3181/00379727-150-39000. doi: 10.3181/00379727-150-39000. [DOI] [PubMed] [Google Scholar]
- 28.Archie JP., Jr Intramyocardial pressure: effect of preload on transmural distribution of systolic coronary blood flow. Am J Cardiol. 1975;35:904–911. doi: 10.1016/0002-9149(75)90127-7. doi: 10.1016/0002-9149(75)90127-7. [DOI] [PubMed] [Google Scholar]
- 29.Giezeman MJ, VanBavel E, Grimbergen CA, Spaan JA. Compliance of isolated porcine coronary small arteries and coronary pressure-flow relations. Am J Physiol. 1994;267(3)(pt 2):H1190–H1198. doi: 10.1152/ajpheart.1994.267.3.H1190. doi: 10.1152/ajpheart.1994.267.3.H1190. [DOI] [PubMed] [Google Scholar]
- 30.Wüsten B, Buss DD, Deist H, Schaper W. Dilatory capacity of the coronary circulation and its correlation to the arterial vasculature in the canine left ventricle. Basic Res Cardiol. 1977;72:636–650. doi: 10.1007/BF01907044. [DOI] [PubMed] [Google Scholar]
- 31.Vermeltfoort IA, Raijmakers PG, Lubberink M, Germans T, van Rossum AC, Lammertsma AA, Knaapen P. Feasibility of subendocardial and subepicardial myocardial perfusion measurements in healthy normals with (15)O-labeled water and positron emission tomography. J Nucl Cardiol. 2011;18:650–656. doi: 10.1007/s12350-011-9375-y. doi: 10.1007/s12350-011-9375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkus D, Kajiya F, Vink H, Vergroesen I, Dankelman J, Goto M, Spaan JA. Prolonged diastolic time fraction protects myocardial perfusion when coronary blood flow is reduced. Circulation. 1999;100:75–81. doi: 10.1161/01.cir.100.1.75. doi: 10.1161/01.cir.100.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Davies JE, Whinnett ZI, Francis DP, Manisty CH, Aguado-Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, Mayet J. Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation. 2006;113:1768–1778. doi: 10.1161/CIRCULATIONAHA.105.603050. doi: 10.1161/CIRCULATIONAHA.105.603050. [DOI] [PubMed] [Google Scholar]
- 34.Davies JE, Sen S, Broyd C, Hadjiloizou N, Baksi J, Francis DP, Foale RA, Parker KH, Hughes AD, Chukwuemeka A, Casula R, Malik IS, Mikhail GW, Mayet J. Arterial pulse wave dynamics after percutaneous aortic valve replacement: fall in coronary diastolic suction with increasing heart rate as a basis for angina symptoms in aortic stenosis. Circulation. 2011;124:1565–1572. doi: 10.1161/CIRCULATIONAHA.110.011916. doi: 10.1161/CIRCULATIONAHA.110.011916. [DOI] [PubMed] [Google Scholar]
- 35.Rolandi MC, Wiegerinck EM, Casadonte L, Yong ZY, Koch KT, Vis M, Piek JJ, Baan J, Jr, Spaan JA, Siebes M. Transcatheter replacement of stenotic aortic valve normalizes cardiac-coronary interaction by restoration of systolic coronary flow dynamics as assessed by wave intensity analysis. Circ Cardiovasc Interv. 2016;9:e002356. doi: 10.1161/CIRCINTERVENTIONS.114.002356. doi: 10.1161/CIRCINTERVENTIONS.114.002356. [DOI] [PubMed] [Google Scholar]
- 36.Raphael CE, Cooper R, Parker KH, Collinson J, Vassiliou V, Pennell DJ, de Silva R, Hsu LY, Greve AM, Nijjer S, Broyd C, Ali A, Keegan J, Francis DP, Davies JE, Hughes AD, Arai A, Frenneaux M, Stables RH, Di Mario C, Prasad SK. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: insights from wave intensity analysis and magnetic resonance. J Am Coll Cardiol. 2016;68:1651–1660. doi: 10.1016/j.jacc.2016.07.751. doi: 10.1016/j.jacc.2016.07.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva KD, Guilcher A, Lockie T, Marber M, Redwood S, Plein S, Perera D. Coronary wave intensity: a novel invasive tool for predicting myocardial viability following acute coronary syndromes. J Am Coll Cardiol. 2012;59:E421. DOI:10.1016/S0735-1097(12)60422-7. [Google Scholar]
- 38.Claridge S, Chen Z, Jackson T, De Silva K, Behar J, Sohal M, Webb J, Hyde E, Lumley M, Asrress K, Williams R, Bostock J, Ali M, Gill J, O'Neill M, Razavi R, Niederer S, Perera D, Rinaldi CA. Effects of epicardial and endocardial cardiac resynchronization therapy on coronary flow: insights from wave intensity analysis. J Am Heart Assoc. 2015;4:e002626. doi: 10.1161/JAHA.115.002626. doi:10.1161/JAHA.115.002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockie TP, Rolandi MC, Guilcher A, Perera D, De Silva K, Williams R, Asrress KN, Patel K, Plein S, Chowienczyk P, Siebes M, Redwood SR, Marber MS. Synergistic adaptations to exercise in the systemic and coronary circulations that underlie the warm-up angina phenomenon. Circulation. 2012;126:2565–2574. doi: 10.1161/CIRCULATIONAHA.112.094292. doi: 10.1161/CIRCULATIONAHA.112.094292. [DOI] [PubMed] [Google Scholar]
- 40.Asrress KN, Williams R, Lockie T, Khawaja MZ, De Silva K, Lumley M, Patterson T, Arri S, Ihsan S, Ellis H, Guilcher A, Clapp B, Chowienczyk PJ, Plein S, Perera D, Marber MS, Redwood SR. Physiology of angina and its alleviation with nitroglycerin: insights from invasive catheter laboratory measurements during exercise. Circulation. 2017;136:24–34. doi: 10.1161/CIRCULATIONAHA.116.025856. doi: 10.1161/CIRCULATIONAHA.116.025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arri S, Williams R, Asrress K, Lumley M, Ellis H, Patterson T, Khawaja M, Perera D, Clapp B, Marber M, et al. Unravelling the mechanisms of mental stress induced myocardial ischaemia: novel insights from intracoronary measurements during cardiac catheterisation. J Am Coll Cardiol. 2017;69:13. DOI:10.1016/S0735-1097(17)33402-2. [Google Scholar]
- 42.Williams R, Asrress K, Lumley M, Arri S, Patterson T, Ellis H, Manou-Stathopoulou V, Khawaja Z, Briceno N, Moschonas K, Clapp B, Perera D, Plein S, Marber M, Redwood S. Use of novel intracoronary technology to investigate the effect of cold air inhalation during exercise on coronary microvascular resistance and blood flow in coronary artery disease: a cross-sectional study. Lancet. 2016;387:S106. [Google Scholar]
- 43.Peeters FECM, Meex SJR, Dweck MR, Aikawa E, Crijns HJGM, Schurgers LJ, Kietselaer BLJH. Calcific aortic valve stenosis: hard disease in the heart: a biomolecular approach towards diagnosis and treatment. Eur Heart J. 2018;39:2618–2624. doi: 10.1093/eurheartj/ehx653. doi: 10.1093/eurheartj/ehx653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carabello BA. The relationship of left ventricular geometry and hypertrophy to left ventricular function in valvular heart disease. J Heart Valve Dis. 1995;4(suppl 2):S132–S138; discussion S138. [PubMed] [Google Scholar]
- 45.Mahmod M, Francis JM, Pal N, Lewis A, Dass S, De Silva R, Petrou M, Sayeed R, Westaby S, Robson MD, Ashrafian H, Neubauer S, Karamitsos TD. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J Cardiovasc Magn Reson. 2014;16:29. doi: 10.1186/1532-429X-16-29. doi: 10.1186/1532-429X-16-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, Camici PG. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470–476. doi: 10.1161/hc0402.102931. doi: 10.1161/hc0402.102931. [DOI] [PubMed] [Google Scholar]
- 47.Galiuto L, Lotrionte M, Crea F, Anselmi A, Biondi-Zoccai GG, De Giorgio F, Baldi A, Baldi F, Possati G, Gaudino M, Vetrovec GW, Abbate A. Impaired coronary and myocardial flow in severe aortic stenosis is associated with increased apoptosis: a transthoracic Doppler and myocardial contrast echocardiography study. Heart. 2006;92:208–212. doi: 10.1136/hrt.2005.062422. doi: 10.1136/hrt.2005.062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swinnen M, Hermans H, Gillijns H, Dymarkowski S, Herijgers P, Herregods M-C, Janssens S. Preoperative symptomatic status and outcome after valve replacement in severe aortic stenosis is correlated with capillary density, degree of fibrosis and cardiomyocyte hypertrophy [abstract 11057]. Circulation. 2012;126:A11057. [Google Scholar]
- 49.Breisch EA, Houser SR, Carey RA, Spann JF, Bove AA. Myocardial blood flow and capillary density in chronic pressure overload of the feline left ventricle. Cardiovasc Res. 1980;14:469–475. doi: 10.1093/cvr/14.8.469. doi: 10.1093/cvr/14.8.469. [DOI] [PubMed] [Google Scholar]
- 50.Miyagawa S, Masai T, Fukuda H, Yamauchi T, Iwakura K, Itoh H, Sawa Y. Coronary microcirculatory dysfunction in aortic stenosis: myocardial contrast echocardiography study. Ann Thorac Surg. 2009;87:715–719. doi: 10.1016/j.athoracsur.2008.11.078. doi: 10.1016/j.athoracsur.2008.11.078. [DOI] [PubMed] [Google Scholar]
- 51.Gould KL, Carabello BA. Why angina in aortic stenosis with normal coronary arteriograms? Circulation. 2003;107:3121–3123. doi: 10.1161/01.CIR.0000074243.02378.80. doi: 10.1161/01.CIR.0000074243.02378.80. [DOI] [PubMed] [Google Scholar]
- 52.Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. doi: 10.1136/hrt.2010.192997. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 53.Broyd CJ, Davies JE, Escaned JE, Hughes A, Parker K. Wave intensity analysis and its application to the coronary circulation. Glob Cardiol Sci Pract. 2015;2015:64. doi: 10.21542/gcsp.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geske JB, Cullen MW, Sorajja P, Ommen SR, Nishimura RA. Assessment of left ventricular outflow gradient: hypertrophic cardiomyopathy versus aortic valvular stenosis. JACC Cardiovasc Interv. 2012;5:675–681. doi: 10.1016/j.jcin.2012.01.026. doi: 10.1016/j.jcin.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Banovic MD, Vujisic-Tesic BD, Kujacic VG, Callahan MJ, Nedeljkovic IP, Trifunovic DD, Aleksandric SB, Petrovic MZ, Obradovic SD, Ostojic MC. Coronary flow reserve in patients with aortic stenosis and nonobstructed coronary arteries. Acta Cardiol. 2011;66:743–749. doi: 10.1080/ac.66.6.2136958. doi: 10.2143/AC.66.6.2136958. [DOI] [PubMed] [Google Scholar]
- 56.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 57.Kozàkovà M, Palombo C, Pratali L, Pittella G, Galetta F, L’Abbate A. Mechanisms of coronary flow reserve impairment in human hypertension. An integrated approach by transthoracic and transesophageal echocardiography. Hypertension. 1997;29:551–559. doi: 10.1161/01.hyp.29.2.551. doi: 10.1161/01.hyp.29.2.551. [DOI] [PubMed] [Google Scholar]
- 58.Beyerbacht HP, Lamb HJ, van Der Laarse A, Vliegen HW, Leujes F, Hazekamp MG, de Roos A, van Der Wall EE. Aortic valve replacement in patients with aortic valve stenosis improves myocardial metabolism and diastolic function. Radiology. 2001;219:637–643. doi: 10.1148/radiology.219.3.r01jn25637. doi: 10.1148/radiology.219.3.r01jn25637. [DOI] [PubMed] [Google Scholar]
- 59.Kenny A, Wisbey CR, Shapiro LM. Profiles of coronary blood flow velocity in patients with aortic stenosis and the effect of valve replacement: a transthoracic echocardiographic study. Br Heart J. 1994;71:57–62. doi: 10.1136/hrt.71.1.57. doi: 10.1136/hrt.71.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yotti R, Bermejo J, Gutiérrez-Ibañes E, Pérez del Villar C, Mombiela T, Elízaga J, Benito Y, González-Mansilla A, Barrio A, Rodríguez-Pérez D, Martínez-Legazpi P, Fernández-Avilés F. Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol. 2015;65:423–433. doi: 10.1016/j.jacc.2014.10.067. doi: 10.1016/j.jacc.2014.10.067. [DOI] [PubMed] [Google Scholar]
- 61.Wiegerinck EM, van de Hoef TP, Rolandi MC, Yong Z, van Kesteren F, Koch KT, Vis MM, de Mol BA, Piek JJ, Baan J., Jr Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8:e002443. doi: 10.1161/CIRCINTERVENTIONS.114.002443. doi: 10.1161/CIRCINTERVENTIONS.114.002443. [DOI] [PubMed] [Google Scholar]
- 62.Vassalli G, Kaufmann P, Villari B, Jakob M, Boj H, Kiowski W, Hess OM. Reduced epicardial coronary vasodilator capacity in patients with left ventricular hypertrophy. Circulation. 1995;91:2916–2923. doi: 10.1161/01.cir.91.12.2916. doi: 10.1161/01.cir.91.12.2916. [DOI] [PubMed] [Google Scholar]
- 63.Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982;307:1362–1366. doi: 10.1056/NEJM198211253072202. doi: 10.1056/NEJM198211253072202. [DOI] [PubMed] [Google Scholar]
- 64.Steadman CD, Jerosch-Herold M, Grundy B, Rafelt S, Ng LL, Squire IB, Samani NJ, McCann GP. Determinants and functional significance of myocardial perfusion reserve in severe aortic stenosis. JACC Cardiovasc Imaging. 2012;5:182–189. doi: 10.1016/j.jcmg.2011.09.022. doi: 10.1016/j.jcmg.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, Pibarot P. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985) 2009;106:113–121. doi: 10.1152/japplphysiol.00049.2008. doi: 10.1152/japplphysiol.00049.2008. [DOI] [PubMed] [Google Scholar]
- 66.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunn RB, Griggs DM., Jr Ventricular filling pressure as a determinant of coronary blood flow during ischemia. Am J Physiol. 1983;244:H429–H436. doi: 10.1152/ajpheart.1983.244.3.H429. doi: 10.1152/ajpheart.1983.244.3.H429. [DOI] [PubMed] [Google Scholar]
- 68.Gould KL, Johnson NP. Ischemia in aortic stenosis: new insights and potential clinical relevance. J Am Coll Cardiol. 2016;68:698–701. doi: 10.1016/j.jacc.2016.05.070. doi: 10.1016/j.jacc.2016.05.070. [DOI] [PubMed] [Google Scholar]
- 69.Hongo M, Goto T, Watanabe N, Nakatsuka T, Tanaka M, Kinoshita O, Yamada H, Okubo S, Sekiguchi M. Relation of phasic coronary flow velocity profile to clinical and hemodynamic characteristics of patients with aortic valve disease. Circulation. 1993;88:953–960. doi: 10.1161/01.cir.88.3.953. doi: 10.1161/01.cir.88.3.953. [DOI] [PubMed] [Google Scholar]
- 70.Kern MJ. Changing reflections of the coronary microcirculation after percutaneous aortic valve replacement: novel observations with arterial pulsed wave dynamics. Circulation. 2011;124:1505–1507. doi: 10.1161/CIRCULATIONAHA.111.058552. doi: 10.1161/CIRCULATIONAHA.111.058552. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Dor I, Malik R, Minha S, Goldstein SA, Wang Z, Magalhaes MA, Weissman G, Okubagzi PG, Torguson R, Lindsay J, Satler LF, Pichard AD, Waksman R. Coronary blood flow in patients with severe aortic stenosis before and after transcatheter aortic valve implantation. Am J Cardiol. 2014;114:1264–1268. doi: 10.1016/j.amjcard.2014.07.054. doi: 10.1016/j.amjcard.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 72.von Knobelsdorff-Brenkenhoff F, Karunaharamoorthy A, Trauzeddel RF, Barker AJ, Blaszczyk E, Markl M, Schulz-Menger J. Evaluation of aortic blood flow and wall shear stress in aortic stenosis and its association with left ventricular remodeling. Circ Cardiovasc Imaging. 2016;9:e004038. doi: 10.1161/CIRCIMAGING.115.004038. doi: 10.1161/CIRCIMAGING.115.004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Omran H, Fehske W, Rabahieh R, Hagendorff A, Lüderitz B. Relation between symptoms and profiles of coronary artery blood flow velocities in patients with aortic valve stenosis: a study using transoesophageal Doppler echocardiography. Heart. 1996;75:377–383. doi: 10.1136/hrt.75.4.377. doi: 10.1136/hrt.75.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. doi: 10.1161/01.CIR.0000074211.28917.31. doi: 10.1161/01.CIR.0000074211.28917.31. [DOI] [PubMed] [Google Scholar]
- 75.Hildick-Smith DJ, Shapiro LM. Coronary flow reserve improves after aortic valve replacement for aortic stenosis: an adenosine transthoracic echocardiography study. J Am Coll Cardiol. 2000;36:1889–1896. doi: 10.1016/s0735-1097(00)00947-5. doi: 10.1016/s0735-1097(00)00947-5. [DOI] [PubMed] [Google Scholar]
- 76.Yamanaga K, Tsujita K, Komura N, Kaikita K, Sakamoto K, Miyazaki T, Saito M, Ishii M, Tabata N, Akasaka T, Arima Y, Yamamoto E, Yamamuro M, Izumiya Y, Kojima S, Tayama S, Nakamura S, Hokimoto S, Ogawa H. Physiological basis of discordance between coronary flow velocity reserve and hyperemic microvascular resistance for evaluating coronary microvascular dysfunction in patients without atherosclerotic obstruction. Int J Cardiol. 2015;201:535–537. doi: 10.1016/j.ijcard.2015.08.102. doi: 10.1016/j.ijcard.2015.08.102. [DOI] [PubMed] [Google Scholar]
- 77.Gould KL, Johnson NP. Imaging coronary blood flow in AS: let the data talk, again. J Am Coll Cardiol. 2016;67:1423–1426. doi: 10.1016/j.jacc.2016.01.053. doi: 10.1016/j.jacc.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 78.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level–risk factors, screening, and outcomes. Nat Rev Cardiol. 2011;8:673–685. doi: 10.1038/nrcardio.2011.154. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 79.Pibarot P, Dumesnil JG. Paradoxical low-flow, low-gradient aortic stenosis adding new pieces to the puzzle. J Am Coll Cardiol. 2011;58:413–415. doi: 10.1016/j.jacc.2011.01.057. doi: 10.1016/j.jacc.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharyya S, Mittal T, Abayalingam M, Kabir T, Dalby M, Cleland JG, Baltabaeva A, Rahman Haley S. Classification of aortic stenosis by flow and gradient patterns provides insights into the pathophysiology of disease. Angiology. 2016;67:664–669. doi: 10.1177/0003319715611804. doi: 10.1177/0003319715611804. [DOI] [PubMed] [Google Scholar]
- 81.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 82.Dayan V, Vignolo G, Magne J, Clavel MA, Mohty D, Pibarot P. Outcome and impact of aortic valve replacement in patients with preserved LVEF and low-gradient aortic stenosis. J Am Coll Cardiol. 2015;66:2594–2603. doi: 10.1016/j.jacc.2015.09.076. doi: 10.1016/j.jacc.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 83.Rimoldi O, Rosen SD, Camici PG. The blunting of coronary flow reserve in hypertension with left ventricular hypertrophy is transmural and correlates with systolic blood pressure. J Hypertens. 2014;32:2465–2471; discussion 2471. doi: 10.1097/HJH.0000000000000338. doi: 10.1097/HJH.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 84.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrmann S, Störk S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G, Weidemann F. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 86.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 87.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. doi: 10.1016/j.jacc.2012.06.051. doi: 10.1016/j.jacc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 88.Barasch E, Fan D, Chukwu EO, Han J, Passick M, Petillo F, Norales A, Reichek N. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis. 2008;17:81–88. [PubMed] [Google Scholar]
- 89.Awtry E, Davidoff R. Low-flow/low-gradient aortic stenosis. Circulation. 2011;124:e739–e741. doi: 10.1161/CIRCULATIONAHA.111.075853. doi: 10.1161/CIRCULATIONAHA.111.075853. [DOI] [PubMed] [Google Scholar]
- 90.Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–1011. doi: 10.1016/j.jacc.2009.04.079. doi: 10.1016/j.jacc.2009.04.079. [DOI] [PubMed] [Google Scholar]
- 91.Rusinaru D, Bohbot Y, Ringle A, Maréchaux S, Diouf M, Tribouilloy C. Impact of low stroke volume on mortality in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Eur Heart J. 2018;63:e57. doi: 10.1093/eurheartj/ehy123. [DOI] [PubMed] [Google Scholar]
- 92.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Survival by stroke volume index in patients with low-gradient normal EF severe aortic stenosis. Heart. 2015;101:23–29. doi: 10.1136/heartjnl-2014-306151. doi: 10.1136/heartjnl-2014-306151. [DOI] [PubMed] [Google Scholar]
- 93.Pibarot P. Aortic stenosis: flow matters. Heart. 2015;101:5–6. doi: 10.1136/heartjnl-2014-306677. doi: 10.1136/heartjnl-2014-306677. [DOI] [PubMed] [Google Scholar]
- 94.Mohty D, Magne J, Deltreuil M, Aboyans V, Echahidi N, Cassat C, Pibarot P, Laskar M, Virot P. Outcome and impact of surgery in paradoxical low-flow, low-gradient severe aortic stenosis and preserved left ventricular ejection fraction: a cardiac catheterization study. Circulation. 2013;128(11)(suppl 1):S235–S242. doi: 10.1161/CIRCULATIONAHA.112.000031. doi: 10.1161/CIRCULATIONAHA.112.000031. [DOI] [PubMed] [Google Scholar]
- 95.Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. doi: 10.1093/eurheartj/ehp361. doi: 10.1093/eurheartj/ehp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lancellotti P, Magne J, Donal E, Davin L, O’Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–243. doi: 10.1016/j.jacc.2011.08.072. doi: 10.1016/j.jacc.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 97.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. doi: 10.1161/CIRCULATIONAHA.113.003695. doi: 10.1161/CIRCULATIONAHA.113.003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mehrotra P, Jansen K, Flynn AW, Tan TC, Elmariah S, Picard MH, Hung J. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur Heart J. 2013;34:1906–1914. doi: 10.1093/eurheartj/eht094. doi: 10.1093/eurheartj/eht094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–1267. doi: 10.1016/j.jacc.2011.12.054. doi: 10.1016/j.jacc.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 100.Le Ven F, Freeman M, Webb J, Clavel MA, Wheeler M, Dumont É, Thompson C, De Larochellière R, Moss R, Doyle D, Ribeiro HB, Urena M, Nombela-Franco L, Rodés-Cabau J, Pibarot P. Impact of low flow on the outcome of high-risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62:782–788. doi: 10.1016/j.jacc.2013.05.044. doi: 10.1016/j.jacc.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 101.Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani V, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation. 2013;127:2316–2326. doi: 10.1161/CIRCULATIONAHA.112.001290. doi: 10.1161/CIRCULATIONAHA.112.001290. [DOI] [PubMed] [Google Scholar]
- 102.Christensen KL, Ivarsen HR, Thuesen L, Kristensen BØ, Egeblad H. Aortic valve stenosis: fatal natural history despite normal left ventricular function and low invasive peak-to-peak pressure gradients. Cardiology. 2004;102:147–151. doi: 10.1159/000080482. doi: 10.1159/000080482. [DOI] [PubMed] [Google Scholar]
- 103.Mohty D, Boulogne C, Magne J, Pibarot P, Echahidi N, Cornu E, Dumesnil J, Laskar M, Virot P, Aboyans V. Prevalence and long-term outcome of aortic prosthesis-patient mismatch in patients with paradoxical low-flow severe aortic stenosis. Circulation. 2014;130(11)(suppl 1):S25–S31. doi: 10.1161/CIRCULATIONAHA.113.007819. doi: 10.1161/CIRCULATIONAHA.113.007819. [DOI] [PubMed] [Google Scholar]
- 104.Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Beanlands RS, Mathieu P, Magne J, Pibarot P. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation. 2008;118(14)(suppl):S234–S242. doi: 10.1161/CIRCULATIONAHA.107.757427. doi: 10.1161/CIRCULATIONAHA.107.757427. [DOI] [PubMed] [Google Scholar]
- 105.Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg. 2008;86:1781–1789. doi: 10.1016/j.athoracsur.2008.08.008. doi: 10.1016/j.athoracsur.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Debry N, Sudre A, Amr G, Delhaye C, Schurtz G, Montaigne D, Koussa M, Modine T. Transcatheter aortic valve implantation for paradoxical low-flow low-gradient aortic stenosis patients. Catheter Cardiovasc Interv. 2016;87:797–804. doi: 10.1002/ccd.26253. doi: 10.1002/ccd.26253. [DOI] [PubMed] [Google Scholar]
- 107.Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Lévy F. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. doi: 10.1016/j.jacc.2014.09.080. doi: 10.1016/j.jacc.2014.09.080. [DOI] [PubMed] [Google Scholar]
- 108.Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebø A, Pedersen TR, Skjærpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Bärwolf C. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 109.Maes F, Boulif J, Piérard S, de Meester C, Melchior J, Gerber B, Vancraeynest D, Pouleur AC, Lazam S, Pasquet A, Vanoverschelde JL. Natural history of paradoxical low-gradient severe aortic stenosis. Circ Cardiovasc Imaging. 2014;7:714–722. doi: 10.1161/CIRCIMAGING.113.001695. doi: 10.1161/CIRCIMAGING.113.001695. [DOI] [PubMed] [Google Scholar]
- 110.Malkin CJ, Long WR, Baxter PD, Gale CP, Wendler O, Monaghan M, Thomas MT, Ludman PF, de Belder MA, Cunningham AD, Moat NE, Blackman DJ National Institute for Cardiovascular Outcomes Research (NICOR) Impact of left ventricular function and transaortic gradient on outcomes from transcatheter aortic valve implantation: data from the UK TAVI Registry. EuroIntervention. 2016;11:1161–1169. doi: 10.4244/EIJY14M12_12. doi: 10.4244/EIJY14M12_12. [DOI] [PubMed] [Google Scholar]
- 111.Elmariah S. Patterns of left ventricular remodeling in aortic stenosis: therapeutic implications. Curr Treat Options Cardiovasc Med. 2015;17:391. doi: 10.1007/s11936-015-0391-0. doi: 10.1007/s11936-015-0391-0. [DOI] [PubMed] [Google Scholar]
- 112.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease: the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2017;38:2739–2791. doi:10.1093/eurheartj/ehx391. [Google Scholar]
- 113.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, III, Thomas JD ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. doi:10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 114.Spethmann S, Baldenhofer G, Dreger H, Stüer K, Sanad W, Saghabalyan D, Müller E, Stangl V, Baumann G, Stangl K, Laule M, Knebel F. Recovery of left ventricular and left atrial mechanics in various entities of aortic stenosis 12 months after TAVI. Eur Heart J Cardiovasc Imaging. 2014;15:389–398. doi: 10.1093/ehjci/jet166. doi: 10.1093/ehjci/jet166. [DOI] [PubMed] [Google Scholar]
- 115.Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: a European multicenter study. J Am Coll Cardiol. 2008;51:1466–1472. doi: 10.1016/j.jacc.2007.10.067. doi: 10.1016/j.jacc.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 116.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 117.Chin CW, Messika-Zeitoun D, Shah AS, Lefevre G, Bailleul S, Yeung EN, Koo M, Mirsadraee S, Mathieu T, Semple SI, Mills NL, Vahanian A, Newby DE, Dweck MR. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. Eur Heart J. 2016;37:713–723. doi: 10.1093/eurheartj/ehv525. doi: 10.1093/eurheartj/ehv525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 119.Bursac N. Cardiac fibroblasts in pressure overload hypertrophy: the enemy within? J Clin Invest. 2014;124:2850–2853. doi: 10.1172/JCI76628. doi: 10.1172/JCI76628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 121.Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Edwards ST, Nowicki ER, Thomas J, Svensson LG, Griffin B, Gillinov AM, Blackstone EH. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2014;147:362.e8–369.e8. doi: 10.1016/j.jtcvs.2012.12.016. doi: 10.1016/j.jtcvs.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 122.Gosse P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl. 2005;23:S27–S33. doi: 10.1097/01.hjh.0000165625.79933.9a. [DOI] [PubMed] [Google Scholar]
- 123.Shah AS, Chin CW, Vassiliou V, Cowell SJ, Doris M, Kwok TC, Semple S, Zamvar V, White AC, McKillop G, Boon NA, Prasad SK, Mills NL, Newby DE, Dweck MR. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. 2014;130:1607–1616. doi: 10.1161/CIRCULATIONAHA.114.011085. doi: 10.1161/CIRCULATIONAHA.114.011085. [DOI] [PubMed] [Google Scholar]
- 124.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–755. doi: 10.1161/01.cir.79.4.744. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 125.Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, Krayenbuehl HP. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–1484. doi: 10.1016/0735-1097(93)90560-n. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- 126.Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol. 1979;128:79–85. doi: 10.1002/path.1711280205. doi: 10.1002/path.1711280205. [DOI] [PubMed] [Google Scholar]
- 127.Schaper J, Speiser B. The extracellular matrix in the failing human heart. Basic Res Cardiol. 1992;87(suppl 1):303–309. doi: 10.1007/978-3-642-72474-9_26. [DOI] [PubMed] [Google Scholar]
- 128.Hess OM, Villari B, Krayenbuehl HP. Diastolic dysfunction in aortic stenosis. Circulation. 1993;87(5)(suppl):IV73–IV76. [PubMed] [Google Scholar]
- 129.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 130.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 131.Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 132.Debl K, Djavidani B, Buchner S, Lipke C, Nitz W, Feuerbach S, Riegger G, Luchner A. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart. 2006;92:1447–1451. doi: 10.1136/hrt.2005.079392. doi: 10.1136/hrt.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 134.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 136.Bull SC, Loudon M, Joseph J, Francis JM, Ferreira VM, Piechnik SK, Karamitsos TD, Stoll V, Lewis A, Prendergast BD, et al. 151 myocardial perfusion, strain and pre-contrast T1 values in moderate asymptomatic aortic stenosis. Heart. 2013;99:A89. [Google Scholar]
- 137.Barone-Rochette G, Piérard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, Pouleur AC, Vancraeynest D, Pasquet A, Vanoverschelde JL, Gerber BL. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144–154. doi: 10.1016/j.jacc.2014.02.612. doi: 10.1016/j.jacc.2014.02.612. [DOI] [PubMed] [Google Scholar]
- 138.Chin CW, Pawade TA, Newby DE, Dweck MR. Risk stratification in patients with aortic stenosis using novel imaging approaches. Circ Cardiovasc Imaging. 2015;8:e003421. doi: 10.1161/CIRCIMAGING.115.003421. doi: 10.1161/CIRCIMAGING.115.003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, Japp AG, Prasad SK, Semple S, Newby DE, Dweck MR. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1320–1333. doi: 10.1016/j.jcmg.2016.10.007. doi: 10.1016/j.jcmg.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sato K, Seo Y, Ishizu T, Takeuchi M, Izumo M, Suzuki K, Yamashita E, Oshima S, Akashi YJ, Otsuji Y, Aonuma K. Prognostic value of global longitudinal strain in paradoxical low-flow, low-gradient severe aortic stenosis with preserved ejection fraction. Circ J. 2014;78:2750–2759. doi: 10.1253/circj.cj-14-0726. [DOI] [PubMed] [Google Scholar]
- 141.Lee SP, Kim YJ, Kim JH, Park K, Kim KH, Kim HK, Cho GY, Sohn DW, Oh BH, Park YB. Deterioration of myocardial function in paradoxical low-flow severe aortic stenosis: two-dimensional strain analysis. J Am Soc Echocardiogr. 2011;24:976–983. doi: 10.1016/j.echo.2011.05.003. doi: 10.1016/j.echo.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 142.Holmes AA, Taub CC, Garcia MJ, Shan J, Slovut DP. Paradoxical low-flow aortic stenosis is defined by increased ventricular hydraulic load and reduced longitudinal strain. J Cardiovasc Med (Hagerstown) 2017;18:87–95. doi: 10.2459/JCM.0000000000000324. doi: 10.2459/JCM.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 143.Schwarz F, Flameng W, Schaper J, Langebartels F, Sesto M, Hehrlein F, Schlepper M. Myocardial structure and function in patients with aortic valve disease and their relation to postoperative results. Am J Cardiol. 1978;41:661–669. doi: 10.1016/0002-9149(78)90814-7. doi: 10.1016/0002-9149(78)90814-7. [DOI] [PubMed] [Google Scholar]
- 144.Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024–1034. doi: 10.1161/01.cir.59.5.1024. doi: 10.1161/01.cir.59.5.1024. [DOI] [PubMed] [Google Scholar]
- 145.Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, Roudaut R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr. 2009;10:414–419. doi: 10.1093/ejechocard/jen299. doi: 10.1093/ejechocard/jen299. [DOI] [PubMed] [Google Scholar]
- 146.Henein MY, Gibson DG. Normal long axis function. Heart. 1999;81:111–113. doi: 10.1136/hrt.81.2.111. doi: 10.1136/hrt.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clavel MA, Pibarot P. Assessment of low-flow, low-gradient aortic stenosis: multimodality imaging is the key to success. EuroIntervention. 2014;10(suppl U):U52–U60. doi: 10.4244/EIJV10SUA8. doi: 10.4244/EIJV10SUA8. [DOI] [PubMed] [Google Scholar]
- 148.Singh A, Greenwood JP, Berry C, Dawson DK, Hogrefe K, Kelly DJ, Dhakshinamurthy V, Lang CC, Khoo JP, Sprigings D, Steeds RP, Jerosch-Herold M, Neubauer S, Prendergast B, Williams B, Zhang R, Hudson I, Squire IB, Ford I, Samani NJ, McCann GP. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur Heart J. 2017;38:1222–1229. doi: 10.1093/eurheartj/ehx001. doi: 10.1093/eurheartj/ehx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chin CWL, Ding ZP, Lam CSP, Ling LH. Paradoxical low-gradient aortic stenosis: the HFpEF of aortic stenosis. J Am Coll Cardiol. 2016;67:2447–2448. doi: 10.1016/j.jacc.2016.02.070. doi: 10.1016/j.jacc.2016.02.070. [DOI] [PubMed] [Google Scholar]
- 150.Lee JF, Barrett-O’Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart. 2016;102:278–284. doi: 10.1136/heartjnl-2015-308403. doi: 10.1136/heartjnl-2015-308403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sucato V, Evola S, Novo G, Sansone A, Quagliana A, Andolina G, Assennato P, Novo S. Angiographic evaluation of coronary microvascular dysfunction in patients with heart failure and preserved ejection fraction. Microcirculation. 2015;22:528–533. doi: 10.1111/micc.12223. doi: 10.1111/micc.12223. [DOI] [PubMed] [Google Scholar]
- 152.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K, Umemura S. Impairment of coronary flow reserve evaluated by phase contrast cine-magnetic resonance imaging in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5:e002649. doi: 10.1161/JAHA.115.002649. doi:10.1161/JAHA.115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marcus ML, Harrison DG, Chilian WM, Koyanagi S, Inou T, Tomanek RJ, Martins JB, Eastham CL, Hiratzka LF. Alterations in the coronary circulation in hypertrophied ventricles. Circulation. 1987;75(1)(pt 2):I19–I25. [PubMed] [Google Scholar]
- 154.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. doi: 10.1093/eurjhf/hfp097. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 156.Baldenhofer G, Zhang K, Spethmann S, Laule M, Eilers B, Leonhardt F, Sanad W, Dreger H, Sander M, Grubitzsch H, Baumann G, Stangl K, Stangl V, Knebel F. Galectin-3 predicts short- and long-term outcome in patients undergoing transcatheter aortic valve implantation (TAVI). Int J Cardiol. 2014;177:912–917. doi: 10.1016/j.ijcard.2014.10.010. doi: 10.1016/j.ijcard.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 157.Arangalage D, Nguyen V, Robert T, Melissopoulou M, Mathieu T, Estellat C, Codogno I, Huart V, Duval X, Cimadevilla C, Vahanian A, Dehoux M, Messika-Zeitoun D. Determinants and prognostic value of Galectin-3 in patients with aortic valve stenosis. Heart. 2016;102:862–868. doi: 10.1136/heartjnl-2015-308873. doi: 10.1136/heartjnl-2015-308873. [DOI] [PubMed] [Google Scholar]
- 158.Chin C. Paradoxical low-flow low-gradient aortic stenosis: advanced severe disease, a new entity or a progression of disease? Heart. 2015;101:1079. doi: 10.1136/heartjnl-2015-308043. doi: 10.1136/heartjnl-2015-308043. [DOI] [PubMed] [Google Scholar]
- 159.Dahl JS, Eleid MF, Pislaru SV, Scott CG, Connolly HM, Pellikka PA. Development of paradoxical low-flow, low-gradient severe aortic stenosis. Heart. 2015;101:1015–1023. doi: 10.1136/heartjnl-2014-306838. doi: 10.1136/heartjnl-2014-306838. [DOI] [PubMed] [Google Scholar]
- 160.Herrmann S, Fries B, Liu D, Hu K, Stoerk S, Voelker W, Ruppert C, Lorenz K, Ertl G, Weidemann F. Differences in natural history of low- and high-gradient aortic stenosis from nonsevere to severe stage of the disease. J Am Soc Echocardiogr. 2015;28:1270–1282.e4. doi: 10.1016/j.echo.2015.07.016. doi: 10.1016/j.echo.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 161.Pislaru SV, Pellikka PA. The spectrum of low-output low-gradient aortic stenosis with normal ejection fraction. Heart. 2016;102:665–671. doi: 10.1136/heartjnl-2015-307893. doi: 10.1136/heartjnl-2015-307893. [DOI] [PubMed] [Google Scholar]
- 162.Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation. 1997;95:892–898. doi: 10.1161/01.cir.95.4.892. doi: 10.1161/01.cir.95.4.892. [DOI] [PubMed] [Google Scholar]
- 163.Lester SJ, Heilbron B, Gin K, Dodek A, Jue J. The natural history and rate of progression of aortic stenosis. Chest. 1998;113:1109–1114. doi: 10.1378/chest.113.4.1109. doi: 10.1378/chest.113.4.1109. [DOI] [PubMed] [Google Scholar]
- 164.Nemes A, Balázs E, Csanády M, Forster T. Long-term prognostic role of coronary flow velocity reserve in patients with aortic valve stenosis - insights from the SZEGED Study. Clin Physiol Funct Imaging. 2009;29:447–452. doi: 10.1111/j.1475-097X.2009.00893.x. doi: 10.1111/j.1475-097X.2009.00893.x. [DOI] [PubMed] [Google Scholar]
- 165.Vesey AT, Esson G, Chin C, Dweck M, Newby D. Detection of cardiac fibrosis and cell death in patients with aortic stenosis. J Am Coll Cardiol. 2015;65:A1190. [Google Scholar]
- 166.Chin CW, Djohan AH, Lang CC. The role of cardiac biochemical markers in aortic stenosis. Biomarkers. 2016;21:316–327. doi: 10.3109/1354750X.2016.1141993. doi: 10.3109/1354750X.2016.1141993. [DOI] [PubMed] [Google Scholar]
- 167.Anand A, Chin C, Shah A, Kwiecinski J, Vesey A, Dweck M, Cowell J, Kaier T, Newby D, Marber M, et al. Cardiac myosin binding protein c is a novel circulating marker of myocardial injury and fibrosis in patients with aortic stenosis. J Am Coll Cardiol. 2016;67:2185. doi: 10.1136/heartjnl-2017-312257. doi:10.1136/heartjnl-2017-312257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Banovic M, Vujisic-Tesic B, Bojic S, Mladenovic A, Ignjatovic S, Petrovic M, Trifunovic D, Nedeljkovic I, Popovic D, Callahan M, Seferovic P. Diagnostic value of NT-proBNP in identifying impaired coronary flow reserve in asymptomatic moderate or severe aortic stenosis. Biomark Med. 2013;7:221–227. doi: 10.2217/bmm.12.100. doi: 10.2217/bmm.12.100. [DOI] [PubMed] [Google Scholar]


