Abstract
Objectives
Recent years have seen the development of biomarkers and imaging technologies designed to improve the specificity of PSA. Widespread implementation of imaging technologies, such as mp-MRI raises considerable logical challenges. Our objective was to evaluate a biopsy strategy that utilizes selective mp-MRI as a follow up test to biomarkers to improve the detection of significant prostate cancer.
Methods and Materials
We developed a conceptual approach based on the risk calculated from the 4Kscore using results from the US prospective validation study, multiplied by the likelihood ratio of mp-MRI from the PROMIS trial. The primary outcome was Gleason grade ≥7 (grade group≥2) cancer on biopsy. Using decision curve analysis, the net benefit was determined for our model and compared with the use of the 4Kscore and mp-MRI independently at various thresholds for biopsy.
Results
For a cut-point of 7.5% risk of high-grade disease, patients with <5% risk from a blood marker would not have risk of significant prostate cancer sufficiently increased by a positive mp-MRI to warrant biopsy; comparably, patients with a risk >23% would not have risk sufficiently reduced by a negative imaging study to forgo biopsy. From the 4Kscore validation study, 46% of men considered for biopsy in the US have risks 5 - 23%. Net benefit was highest for the combined strategy, followed by 4Kscore alone.
Conclusions
Selective mp-MRI in men with intermediate scores on a secondary blood test results in a biopsy strategy that is more scalable than mp-MRI for all men with elevated PSA. Prospective validation is required to demonstrate if the predicted properties of combined blood and imaging testing are empirically confirmed.
Keywords: Prostate cancer, 4Kscore, magnetic resonance imaging, Prostate biopsy
Condensed Abstract
This study presents a conceptual approach of a prostate biopsy selection strategy utilizing blood-markers in combination with selective mp-MRI, illustrating how unnecessary prostate biopsy can be minimized as well as challenges with widespread use of mp-MRI in men with elevated PSA.
Introduction
Widespread screening with Prostate Specific Antigen (PSA) remains controversial because of lack of specificity for clinically significant disease, leading to harms from the over detection of low-risk prostate cancer. There have been increasing efforts to develop screening tests ancillary to PSA that can improve specificity. These include blood markers such as the 4Kscore (OPKO Health; Miami, FL) – which gives a probability of high-grade cancer based on kallikrein markers, age, digital rectal exam and prior biopsy – and the prostate health index (PHI). Imaging studies such as multiparametric Magnetic Resonance Imaging (mp-MRI) have also been suggested as a follow-up test to an elevated PSA.
There is strong evidence that both approaches can improve prostate cancer decision-making. For instance, in a multi-institutional prospective trial, the 4Kscore was extremely well-calibrated with an area-under-the-curve of 0.82 versus 0.74 for a standard predictive model. Decision analysis demonstrated that use of the 4Kscore would dramatically reduce the number of unnecessary biopsies without delaying the diagnosis of an undue number of high-grade cancers.1 Comparably, the UK PROMIS study demonstrated that mp-MRI improved sensitivity for detection of clinically significant prostate cancer (CSPC).2
It remains unknown how blood and imaging markers should be combined in the work-up of a patient with moderately elevated PSA. One immediate consideration is scalability. Ancillary blood markers could be widely utilized in clinical practice with few pre-requisites. Indeed, one approach, already adopted for the 4Kscore, is to reflex test the blood sample collected in primary care if the PSA is elevated. In contrast, widespread implementation of an imaging approach such as mp-MRI would require a large number of new MRI machines to be installed and generation of specialist uro-radiologists to be trained. This latter point also raises the possibility of variations in quality: it is easier to calibrate laboratory measures to ensure consistency than to do so for subjective reading of images. These practical considerations suggest that mp-MRI might be optimally used as a follow-up test to an ancillary blood marker. Men at low risk from the blood biomarker would avoid biopsy, those at high risk would be referred to immediate biopsy and those at intermediate risk would have mp-MRI, with the subsequent biopsy decisions depending on the result (figure 1).
Figure 1.
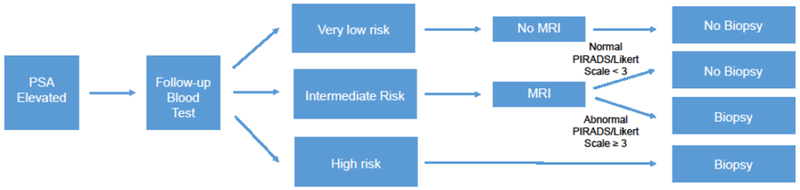
Prostate biopsy decision pathway by estimated risk of clinically significant prostate cancer
Here we examine the characteristics of such a strategy using data from a major study of a blood marker – the US Prospective, validation study of the 4Kscore 1 – and an imaging test – the UK PROMIS study2.
Materials and Methods
The 4Kscore validation study was a multi-institutional prospective trial including 1012 men undergoing prostate biopsy in 26 centers in the United States. The design was pragmatic and so the patient population included men both with and without prior prostate biopsy and with a wide range of PSA levels: the eligibility criterion was only that the urologist believed that a prostate biopsy was indicated.1 The PROMIS study enrolled men from 11 centers across the United Kingdom.2 Men were eligible for inclusion if they had a clinical suspicion of prostate cancer with a PSA level up to 15 ng/ml and had never undergone a prostate biopsy. All participants received a standardized mp-MRI followed by a template-mapping prostate (TPM) biopsy and a trans-rectal ultrasound guided (TRUS) biopsy. The study utilized results from the TPM-biopsy to determine the diagnostic accuracy of mp-MRI in identifying CSPC defined in various ways: the definition we use here is for any high-grade disease, ≥ Gleason 7 (grade group ≥2). In total, 576 men underwent mp-MRI followed by both TPM and TRUS biopsy, detecting Gleason ≥ 7 cancer in 220 (38%). The reported sensitivity and specificity for mp-MRI was 88% and 45% respectively.
Statistical Analysis
Our combination test strategy is that a clinician would obtain the pretest probability of high-grade prostate cancer from the blood marker, and then convert this to a pretest odds. The pretest odds would then be multiplied by the positive or negative likelihood ratio from multiparametric MRI to generate a posttest odds. This would then be converted to a probability to aid the biopsy decision (see Supplementary Material). For the purposes of this paper, we used a threshold for biopsy of 7.5%, that is, we assumed a urologist would refer to biopsy if the estimated risk of CSPC was ≥7.5% but monitor patients otherwise. Previous studies assessing the performance of the 4Kscore have demonstrated that a threshold of 7.5% provides an appropriate cutoff for reducing unnecessary biopsies while maintaining high detection of CSPC.3 Positive and negative likelihood ratios for mp-MRI detecting CSPC were calculated from the published sensitivity and specificity available from the PROMIS trial. The 4Kscore results from men tested in the U.S. validation study provided a distribution of the pretest probability for harboring clinically significant disease.
This process can then allow for the quantification of the range of 4Kscores that would be affected by a positive or negative mp-MRI. Patients were stratified into four different groups based upon their estimated risk of clinically significant disease. There would be some men with a low 4Kscore whose risk of CSPC would not increase above 7.5% even if they had a positive mp-MRI. These men should receive neither mp-MRI nor biopsy. On the other hand, there would be men with very high 4Kscores whose risk would remain ≥ 7.5% even if mp-MRI were negative, and so should be referred directly to biopsy. In the low intermediate group, patients with 4Kscores <7.5% would have a risk ≥ 7.5% if mp-MRI were positive; in the high intermediate group, patients with 4Kscores ≥ 7.5% who would have a risk ≥ 7.5% if mp-MRI were negative. Hence patients in the intermediate group would undergo mp-MRI and with biopsy or monitoring recommended depending on the results (figure1).
Net benefit is defined as [True Positives – False Positives × Pt ÷ (1-Pt)] ÷- N where Pt is threshold probability – the minimum probability at which a patient would opt for biopsy – and N is the total number of patients in the cohort4. The net benefit was calculated for the sequential 4Kscore and selective mp-MRI strategy using a threshold probability of 7.5% for conducting biopsy. This was compared to the net benefit of using 4Kscore alone and mp-MRI alone. As a sensitivity analysis, the net benefit was also calculated for the above strategies using threshold probabilities of 6% and 10% for conducting prostate biopsy. Additionally, we performed a simulation to estimate the proportion of men that would be influenced by MRI when using a combination strategy. All analyses were performed using Stata version 13 (StataCorp, College Station, TX).
Results
Of the 1012 men in the U.S. 4Kscore validation study 542 (54%) had negative biopsy and 239 (24%), 167 (17%) and 64 (6%) were diagnosed with Gleason 6, 7 and 8-10 disease, respectively. Based upon the published results from the PROMIS study, the positive and negative likelihood ratios of mp-MRI were 1.60 and 0.27. Using these values and the threshold probability of 7.5% for conducting biopsy, the calculated range of 4Kscores that could be influenced by the results of MRI was between 5-23% (Table 1). Looking at the breakdown by predicted risk of clinically significant disease, 26% of men had a 4Kscore of less than 5%, and would need neither biopsy nor mp-MRI. Patients in the intermediate risk group, a 5–23% 4Kscore, comprised approximately 45% of the cohort. Finally approximately 30% of men had 4Kscores >23% and would proceed directly to biopsy.
Table 1.
Range of 4Kscores influenced by MRI (n=1012, with categorization based on threshold probability of 7.5%)
| 4Kscore Value | Number of Patients | Pre-biopsy MRI | |
|---|---|---|---|
| Low | <5% | 259 (26%) | No |
| Low Intermediate | 5-7.4% | 100 (10%) | Yes |
| High Intermediate | 7.5-23% | 364 (36%) | Yes |
| High | >23% | 289 (29%) | No |
Table 2 presents the results of how many patients would undergo prostate biopsy and would subsequently be diagnosed with CSPC under the combined strategy of 4Kscore and selective MRI relative to using 4Kscore alone or mp-MRI alone. The overall net benefit for the combined strategy was 0.178 relative to a net benefit of 0.175 for using 4Kscore independently and 0.165 for mp-MRI alone (table 2). At the risk threshold of 7.5%, a 0.0026 difference in net benefit between the combined strategy and using the 4Kscore alone is equivalent to 0.0026 × (0.925 ÷ 0.075) = 32 fewer biopsies per 1000 without missing any additional high grade-cancers. A 1.3% difference between the combined strategy and using MRI alone is equivalent to 156 fewer biopsies per 1000 with no fewer high grade diagnoses. The marginal net benefit for using the combined strategy, compared to the blood marker alone, is highest at the 10% threshold, equivalent to 42 fewer biopsies per 1000 for the same number of high grade cancers detected. Given a 47.5% rate of MRI in this strategy, that gives 70 fewer biopsies for every 1000 patients subject to MRI, or one fewer biopsy per 14 MRIs.
Table 2.
Number of prostate biopsies and high-grade cancers diagnosed using each strategy (n=1012) along with net benefit, for threshold probabilities of 6%, 7.5% and 10%. Number of patients biopsied and number of high-grade cancers identified are derived from the prevalence and the sensitivity and specificity of each approach.
| 7.5% Threshold | 6% Threshold | 10% Threshold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Patients Biopsied | Number of High Grade Cancers identified | Net Benefit | Number of Patients Biopsied | Number of High Grade Cancers identified | Net Benefit | Number of Patients Biopsied | Number of High Grade Cancers identified | Net Benefit | |
| Combined Strategy | 567 | 209 | 0.178 | 616 | 213 | 0.185 | 523 | 206 | 0.169 |
| MRI Alone | 632 | 202 | 0.165 | 632 | 202 | 0.172 | 632 | 202 | 0.152 |
| 4Kscore Alone | 653 | 213 | 0.175 | 711 | 216 | 0.182 | 556 | 205 | 0.164 |
Based on a 6% or 10% threshold probability for conducting biopsy, the range of 4Kscores falling into the intermediate range shifts up or down, but the total number of men affected is similar and close to 45% (table 3).
Table 3.
Range of 4Kscores influenced by MRI for 6% and 10% threshold probability for biopsy (n=1012)
| Threshold probability for biopsy 6% | |||
|---|---|---|---|
| 4Kscore Value | Number of Patients | Pre-biopsy MRI | |
| Low | <4% | 213 (21%) | No |
| Low Intermediate | 4-5.9% | 88 (8.7%) | Yes |
| High Intermediate | 6-19% | 358 (35%) | Yes |
| High | >19% | 353 (35%) | No |
| Threshold probability for biopsy 10% | |||
| 4Kscore Value | Number of Patients | Pre-biopsy MRI | |
| Low | <6% | 301 (30%) | No |
| Low Intermediate | 6-9.9% | 155 (15%) | Yes |
| High Intermediate | 10-29% | 326 (32%) | Yes |
| High | >29% | 230 (23%) | No |
Discussion
In this study, we propose a conceptual approach for integrating a blood marker together with mp-MRI for biopsy decision making in men with suspected prostate cancer. Selective use of mp-MRI as a follow-up test in patients with intermediate scores from the blood test results in the highest clinical net benefit. By using this strategy, fewer men would be subjected to biopsy while diagnosing a relatively similar number of CSPC compared to using either the 4Kscore or MRI alone. Though we have demonstrated this sequential testing strategy with the 4Kscore, a similar process can easily be conducted with any other reflex test or risk calculator that generates an estimated probability for harboring clinically significant disease. There may be other biomarkers that provide an equal or greater benefit when combined with MRI, but further research is required.
The drive to develop selective biopsy strategies that minimize the over detection of indolent prostate cancer have led to growing interest in ancillary or “reflex” tests such as the 4Kscore. Although mp-MRI is appealing because of its ability to characterize aggressive disease5, there is no consensus on how it can be successfully integrated into the diagnostic paradigm in men undergoing PSA screening.6 Despite a growing number of biomarkers and increasing use of mp-MRI prior to biopsy, there has been scant attention paid to developing a practical strategy for combining blood assays with mp-MRI in a way that would be feasible at a population level. Widespread use of mp-MRI in all men with elevated PSA would require dramatic increases in the number of available MRI machines and the number of skilled radiologists able to read the resulting images. However, selective MRI in men with a predefined risk for aggressive disease would address challenges of scalability. Our study demonstrated that MRI could be avoided in 548 men (54% of the study cohort) while maintaining a very high net benefit, that is, clinical value. In comparison, an imaging-based strategy would require MRI in all men and would result in the detection of fewer cases of CSPC, even when biopsying nearly 100 additional patients.
Several studies have illustrated the varying performance of mp-MRI prior to biopsy.7–10 In a recent meta-analysis it was estimated that the median negative predictive value for MRI detecting CSPC was 88% 11, indicating that that MRI fails to identify clinically significant disease in roughly 12% of men with normal imaging. This raises questions regarding the validity of offering mp-MRI as the only tool for reducing unnecessary biopsy. Additionally, the low reported specificity of mp-MRI 2,11 raises further questions regarding its applicability as the only adjunctive tool to PSA, a test that itself has been criticized for lack of specificity leading to unnecessary biopsy and overdiagnosis of indolent disease.12
In spite of the strengths of this study, there are several limitations. Our mathematical approach makes the common assumption that the likelihood ratios of the binary test do not vary importantly over different levels of risk from the prediction model (i.e. 4Kscore). However, this assumption has not been empirically verified. It should be noted that the diagnostic accuracy of mp-MRI prior to biopsy remains highly variable.11 Our results are based on particular set of values for the performance of MRI as reported in PROMIS. However, altering the test characteristics of mp-MRI could change the outcome of our analysis (supplementary table 1). Further studies are required to define the performance of mp-MRI prior to biopsy.
Another consideration is that performance characteristics for mp-MRI in PROMIS were derived from a reference standard of transperineal template prostate mapping (TPM) biopsy. Although TPM biopsy may not be conventional, authors of PROMIS have argued that this avoids issues of underdiagnosis with TRUS biopsy and the bias of utilizing radical prostatectomy specimens as the reference standard.2
Furthermore, our analysis factored results of mp-MRI as dichotomous outcomes of “positive” and “negative” results. This is a simplification, as guidelines aimed at standardizing the assessment of mp-MRI are based upon the likelihood of detecting clinically significant prostate cancer, represented on a 5-point scale.13,14 Although a score of 3, as used in this study, typically indicates the equivocal presence of CSPC, this cutoff is not uniform and others may opt for higher or lower scores based upon local practice patterns.15,16,17 Using alternate thresholds for considering MRI as “positive” may reduce the external validity of our analysis: a score of 4 or greater would result in a higher specificity and lower sensitivity relative to the values used in this study.
An additional consideration is that our strategy considers the value of mp-MRI as being only for determining whether or not to recommend prostate biopsy. The findings of this study do not refer to using MRI to guide biopsies, rather using the data from a MRI to decide if a biopsy should be performed. Proponents of mp-MRI may advocate for its use in high risk men in order to target abnormal lesions and then enhance the sensitivity of the biopsy procedure.18 Although it is clear that mp-MRI does identify lesions that are missed on standard TRUS biopsy,2 it is far from clear whether the consequent delay in diagnosis is clinically relevant. For instance, in a population-based study, the 15-year risk of prostate cancer death in men with PSA < 10 ng/mL who had a negative biopsy was only 0.7%.19 An obvious alternative to mp-MRI in all high risk men is to conduct a standard biopsy initially, but then an mp-MRI guided biopsy if initial results are negative and risk remains high.
Conclusion
The findings from this conceptual approach demonstrate that by combining a blood test, such as the 4Kscore, with selective use of mp-MRI results in a biopsy strategy with a higher net benefit compared to using either test alone. Such a strategy is more scalable to a population level than one of mp-MRI in all men with elevated PSA. This study illustrates a promising approach for how risk-stratification tools can be used in combination with selective MRI in order to minimize unnecessary prostate biopsy and the challenges associated with population wide imaging. Further validation from prospective studies is required to determine whether the predicted properties of the combined test are empirically confirmed.
Supplementary Material
Highlights.
Imaging tests such as mp-MRI have been recommended as a secondary test to improve the specificity of PSA. There are considerable logistical challenges to widespread implementation of mp-MRI.
We developed a strategy for selected use of mp-MRI based on use of a blood marker: patients low risk from the marker should receive neither mp-MRI nor biopsy; patients at high-risk should be biopsied; patients at intermediate risk should receive mp-MRI, with biopsy for those with evidence of prostate lesions on imaging.
Given the sensitivity and specificity of mp-MRI, the cut-offs for low, intermediate and high risk are <5%, 5%-23% and >23%.
We demonstrated that such a strategy has a higher net benefit than mp-MRI or blood marker alone.
Using the data on the 4Kscore as the marker, the strategy would involve mp-MRI for 45% of men being considered for biopsy, a reduction of 55%.
Acknowledgments
Supported by: the Sidney Kimmel Center for Prostate and Urologic Cancers and the National Institutes of Health/National Cancer Institute to Memorial Sloan Kettering Cancer Center through the Cancer Center Support Grant, award number P30 CA008748.
List of abbreviations
- PSA
Prostate Specific Antigen
- Mp-MRI
Multi-parametric Magnetic Resonance Imaging
- CSPC
Clinically Significant Prostate Cancer
- TPM
Template Prostate Mapping
- TRUS
Trans-rectal Ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
Dr Andrew Vickers is a named co-inventor on US patent number: 9,672,329 for a statistical method for predicting the result of a prostate cancer biopsy. The patent is the algorithm used in the 4Kscore. He receives royalties for its commercialize use by Opko Diagnostics. Dr Vickers also serves on Opko’s advisory board and receives stock options from Opko. Dr Stephen Zappala has stock options in Opko.
The remaining authors have no conflicts of interest to disclose.
References
- 1.Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. European urology. 2015;68:464–470. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet (London, England). 2017. [DOI] [PubMed] [Google Scholar]
- 3.Konety B, Zappala SM, Parekh DJ, et al. The 4Kscore® Test Reduces Prostate Biopsy Rates in Community and Academic Urology Practices. Rev Urol. 2015;17:231–240. [PMC free article] [PubMed] [Google Scholar]
- 4.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad AE, Finelli A. Should Prebiopsy Multiparametric Magnetic Resonance Imaging be Offered to All Biopsy-naive Men Undergoing Prostate Biopsy? European urology. 2016;69:426–427. [DOI] [PubMed] [Google Scholar]
- 7.Futterer JJ, Briganti A, De Visschere P, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. European urology. 2015;68:1045–1053. [DOI] [PubMed] [Google Scholar]
- 8.Haider MA, Yao X, Loblaw A, Finelli A. Multiparametric Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer: A Systematic Review. Clin Oncol (R Coll Radiol). 2016;28:550–567. [DOI] [PubMed] [Google Scholar]
- 9.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–450. [DOI] [PubMed] [Google Scholar]
- 10.van Hove A, Savoie PH, Maurin C, et al. Comparison of image-guided targeted biopsies versus systematic randomized biopsies in the detection of prostate cancer: a systematic literature review of well-designed studies. World J Urol. 2014;32:847–858. [DOI] [PubMed] [Google Scholar]
- 11.Moldovan PC, Van den Broeck T, Sylvester R, et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. European urology. 2017. [DOI] [PubMed] [Google Scholar]
- 12.Moyer VA, Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157:120–134. [DOI] [PubMed] [Google Scholar]
- 13.Barrett T, Turkbey B, Choyke PL. PI-RADS version 2: what you need to know. Clinical radiology. 2015;70:1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. European radiology. 2012;22:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abd-Alazeez M, Kirkham A, Ahmed HU, et al. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate cancer and prostatic diseases. 2014;17:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–351. [DOI] [PubMed] [Google Scholar]
- 18.Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA: a cancer journal for clinicians. 2016;66:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klemann N, Roder MA, Helgstrand JT, et al. Risk of prostate cancer diagnosis and mortality in men with a benign initial transrectal ultrasound-guided biopsy set: a population-based study. The Lancet Oncology. 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


