Abstract
Introduction:
Cardiac arrest remains a worldwide health problem with very poor outcome. In the absence of bystander resuscitation, survival rates decrease by 10% per minute of arrest and global ischemia. Even the best manual chest compressions, however, can only produce a fraction of normal cardiac output and blood flow to vital organs. Physiological principles and current evidence for the use of mechanical devices to increase survival and quality of life after cardiac arrest are highlighted in this review article.
Areas covered:
Mechanical adjuncts such as the Active Compression Decompression device, automated chest compressors and the use of a negative pressure valve (Impedance Threshold Device) can synergistically aid in improving quality of CPR and increasing cardiac output and vital organ perfusion.
Expert commentary:
The current conclusions that the use of mechanical adjunct devices in a preclinical setting is not recommended or neutral at best, need to be reevaluated, especially with regard to new advanced and promising treatments that require prolonged high-quality CPR during the transport to a hospital to improve the outcome of patients.
Keywords: Active compression decompression, automated chest compressor, cardiac arrest, cardiopulmonary resuscitation, impedance threshold device, ACLS
1. Introduction
Cardiovascular disease remains a leading cause of death worldwide, with almost 17 million deaths annually or 30% of all global mortality [1,2]. About 40–50% of all deaths from cardiovascular disease are sudden cardiac arrests, with ventricular tachyarrhythmias being the cause in about 80% [1]. Overall, more than 6 million sudden cardiac deaths occur annually [1], with more than 300,000 deaths per year from out-of-hospital cardiac arrest (OHCA) in the United States alone [3]. Despite considerable efforts, the rate of neurologically favorable survival from OHCA remains worrisome low at less than 1% worldwide [1] and still less than 10% in the US [4] and Europe [5]. OHCA patients are on average 64 ± 18 years old, 61% are male, and 22% do not survive transport to the hospital [6]. Since only about one third receive cardiopulmonary resuscitation (CPR) and chest compressions by bystanders, and less than 4% are treated with an automated electrical defibrillator by bystanders, global ischemia to all organs often lasts until the arrival of emergency personnel, in well-developed systems on average 8–10 min after the emergency call, in less developed systems even later. Successful outcome is inversely related to the duration of untreated arrest, and the rate of survival decreases by 7 to 10% per min without CPR. The by far highest rates of return of spontaneous circulation (ROSC) and neurologically favorable survival are achieved at chest compression rates of 100 per min [7,8]. Survival is linearly related to compression depth, with a depth of only 25 mm instead of 50 mm resulting in 50% lower survival rates [9]. Unfortunately, even the best external chest compressions can only produce about 20 to 30% of the normal cardiac output [10,11] and largely diminished regional blood flow to the brain and heart [12]. Limited endurance of the rescuer [13] and difficulty during patient transport [14] additionally contribute to decreasing quality of manual chest compressions over time.
2. The Importance of Medical Devices for High Quality CPR
Mechanical adjuncts for cardiocerebral resuscitation – Active Compression Decompression (ACD), Automated Chest Compression (ACC) devices and the Impedance Threshold Device (ITD) – synergistically decrease intrathoracic and intracranial pressure, thereby increasing blood flow to the brain and other vital organs during CPR. The following review focuses on their physiological principles and highlights current evidence.
3. Active Compression-Decompression (ACD)
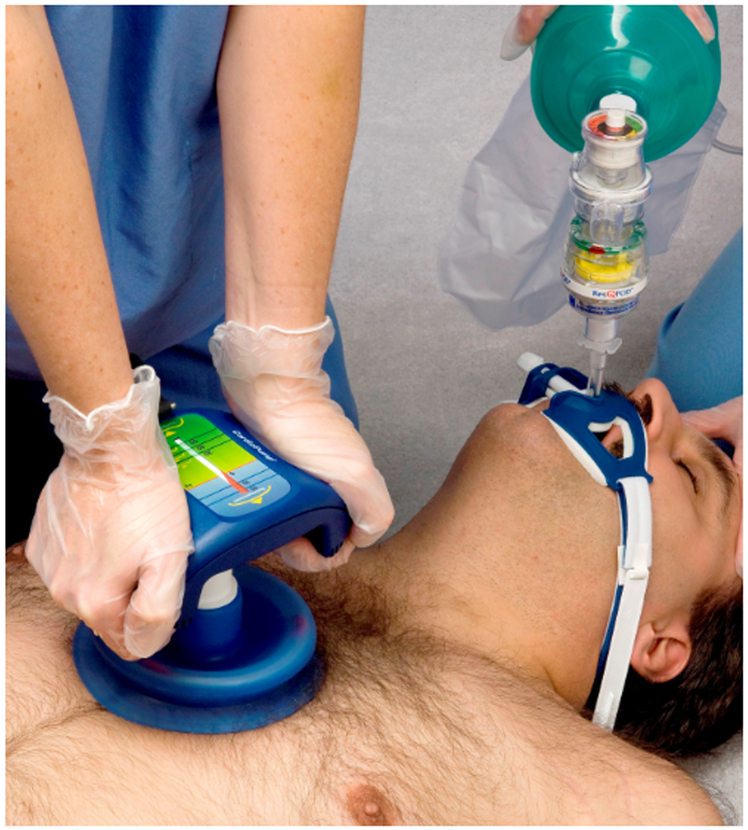
Equally important as sufficient compression depth is complete chest recoil to a neutral sternum position during chest decompression: incomplete recoil causes elevated intrathoracic and intracerebral pressure, decreases blood flow to the right heart, and subsequently cardiac output, all of which reduces cerebral blood flow [15,16]. Following a landmark case report of a son resuscitating his father after OHCA by using a toilet plunger [15], Lurie et al have discovered and elucidated the principle of ACD [16]. The vacuum generated by a suction cup during the upstroke of chest compression (active decompression) decreases intrathoracic pressure faster, thus allowing better filling of the right ventricle, and improves cardiac output and generated systolic pressure [17]. A subsequently developed manual ACD device (Fig 1) received FDA-approval in the United States in 2015 and is marketed as “ResQPUMP” (ZOLL Medical Corporation, Chelmsford, MA).
Figure 1:
Manual Active Compression Decompression (ACD) device (ResQPUMP®)
4. Automated Chest Compression (ACC)
An automated chest compressor such as LUCAS®3 (Lund University Cardiopulmonary Assist System) (Fig 2), for example, available now from Stryker Medical, Portage, MI, a newer version of the original LUCAS®, is battery-powered, and runs with an adjustable compression rate of 102 to 120 ± 2 min−1, a compression depth of 45 to 53 ± 2 mm, as well as a duty cycle of 50 ± 5% [18].
Figure 2:
The newest model of the Lund University Cardiopulmonary Assist System (LUCAS®3)
In comparison, AutoPulse® (ZOLL Medical Corporation, Chelmsford, MA) (Fig 3), a battery-powered load-distributing band device using a circumferential band to evenly compress the entire chest [19,20], delivers 80 compressions per min; the compression depth can be adjusted to the individual patient’s chest diameter; active decompression, however, is not possible. Recent review articles on automated devices [21,22] provide more details. Infrequent complications include injuries of the liver [23], pancreas [24] or spleen [25], as well as tension pneumothorax [26]. A higher incidence of rib and sternal fractures by manual ACD vs standard manual CPR has been described [27], yet this was not the case in automated vs manual chest compressions [28]. If load-distributing band devices have a higher incidence of damage to internal organs than LUCAS® when compared with standard manual CPR as reported by Koster et al [29], will need to be confirmed.
Figure 3:
Automated chest compressor (AutoPulse®)
Nevertheless, compared to manual CPR, automated CPR devices provide more reliable compressions with a constant rate, depth and location, thus avoiding inconsistencies of manual compressions, provider fatigue [30] and provider injury [31]. In addition, resuscitation personnel are available for other important tasks [32].
Despite these obvious advantages, randomized studies comparing LUCAS® [33-36] or Autopulse® [36-40] alone with manual CPR have produced mostly neutral results with regards to neurologically favorable survival rates when used without an ITD. Although the authors of the AutoPulse Assisted Prehospital International Resuscitation (ASPIRE) multicenter trial [37], for example, stated that the use of an automated load-distributing band CPR device in OHCA led to less favorable neurological outcome and a trend toward lower survival than manual CPR, the trial had serious flaws and had to be stopped because of changes in treatment and negative outcomes at one of the sites, while the other four sites that had adhered to the study’s instructions reported a neutral outcome [38]. If the overall unexpectedly neutral outcomes in above studies are due to user errors or delays in crucial elements of advanced cardiac life support (ACLS), such as initiation of chest compressions or defibrillation when automated devices are employed, remains unclear at this point [41].
Consequently, ACLS guidelines recommend that manual chest compressions remain the standard of care but state that mechanical piston or load-distributing band devices “may be a reasonable alternative for use by properly trained personnel” and “may be considered in specific settings where the delivery of high-quality manual compressions may be challenging or dangerous” [42].
5. Impedance Threshold Device (ITD)
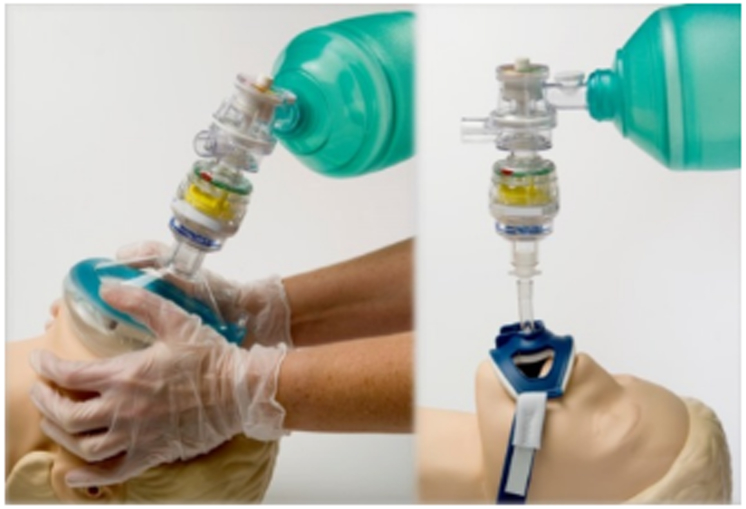
The ITD, marketed as “ResQPOD” (ZOLL Medical Corporation, Chelmsford, MA), is mounted between the face mask (Fig 4 left) or endotracheal tube (Fig 4 right) and the ventilator or ventilation bag. During spontaneous or positive pressure ventilation, the ITD opens, as it does for intrathoracic pressures lower than −10 mmHg during chest decompression. The ITD improves venous return to the right heart and, thus, increases cardiac output. Furthermore, continuous negative intrathoracic pressure can be achieved through an active intrathoracic pressure regulator. Here, an ITD connected to continuous suction sustains a constant negative intrathoracic pressure (−5 to −10 mmHg) only interrupted during positive-pressure ventilations. If this is superior to the use of a regular ITD during CPR, is currently investigated [43-45]. Human studies in hemorrhagic shock [46] and during coronary bypass graft surgery [47] are encouraging.
Figure 4:
Impedance Threshold Device (ITD) placed between mask (left) or endotracheal tube (right) and the ventilation bag.
The PRIMED trial [48] and its subsequent reevaluation by Yannopoulos et al [49] have shown that the ITD, when used in conjunction with manual CPR, improves neurologically favorable survival rates only with high-quality CPR as defined by >50% time used for chest compressions and adequate compression depth and rate in accordance with current ACLS guidelines [50]; CPR outside the guidelines, however, can lead to a neutral and even detrimental outcome. The study by Sugiyama et al came to the same conclusion [8]. Thus, providers need to be aware of this confounding dichotomous effect when using the ITD during manual CPR.
6. High Quality CPR by Combining ACD and ITD
6.1. ITD and ACD
Together, ITD and ACD synergistically create a negative intrathoracic pressure, thereby decreasing intracranial pressure, increasing venous return to the right heart and, thus, cardiac output, leading to largely improved systemic blood pressures, and improved cerebral blood flow during CPR in animals [51] and in humans [52]. Their combination improved survival to hospital discharge after cardiac arrest by 50% with favorable neurological function (modified Rankin Scale scores) after one year [53]. Unfortunately, since the manual ACD device is still based on the physical fitness of the rescuer, fatigue may lead to inadequate chest compression rates over time [30].
6.2. ITD and ACC
As stated above, the use of the ITD requires high-quality CPR which could be provided by the use of ACC devices [8,54]. When used during manual CPR [11,14,28], negative intrathoracic pressure by ITD is only generated by the intrinsic elastic recoil of the chest and largely depends on the quality of CPR. A rigid, non-compliant chest or fractured ribs, for example, can significantly reduce the elastic recoil. In addition, limited recoil through leaning has detrimental effects on venous return and intracranial pressure [43,55]. By using the piston device LUCAS®3 with a suction cup, the recoil is supported with a lifting force of around −3 lbs [56]. Compared to a manual ACD device such as the “ResQPUMP” with a lifting force around −20 lbs, the active decompression is lower and does not exceed the neutral level, but depth and frequency are maintained without provider fatigue. Furthermore, its use can be continued during transport in an ambulance or helicopter [57] and reversible causes for cardiac arrest could be treated by interventions in the cardiac catheterization laboratory (CCL) [58].
7. Conclusion
The delivery of immediate and high-quality CPR (chest compressions at 100 – 120/min, 50 mm deep, full chest recoil, without interruptions longer than 10 sec) [21,50] remains the mainstay of neurologically favorable survival after OHCA. The high incidence of OHCA combined with nevertheless still very low survival rates worldwide [1,3,5] continue to emphasize the need to restore cerebral blood flow as early and as well as possible to decrease cerebral injury after OHCA. Adjunct mechanical devices such as ACD or ACC devices, especially in combination with an ITD, aim to synergistically decrease intrathoracic and intracerebral pressure during CPR, thus improving cerebral blood flow and survival rates. Employing adjunct devices should not delay any important component of ACLS.
8. Expert Opinion
Expert commentary:
Despite steady progress in preclinical and clinical research, the survival rates after OHCA with of favorable neurological function are still very low. Key weaknesses in clinical management are based on the fact that external manual chest compressions during cardiac arrest only allow a fraction of normal cardiac output and blood flow to vital organs to be generated, even under optimal conditions, i.e., normoventilation, optimal compression point, depth, rate and continuous CPR without leaning. Frequently, however, the quality of CPR is far less than optimal [49].
Although the appreciation and use of medical adjunct devices to enhance cardiac output and blood flow to vital organs during CPR is gradually rising, it has by far not achieved its full potential. Most importantly, the integration of new promising treatments, such as ACD, ACC and ITD, into current guidelines lags behind. Especially the combination of two adjunct devices like LUCAS and the ITD, is not even mentioned in the current AHA ACLS guidelines [42], nor are they recommended for individual use. While preclinical studies have shown benefits of this combination [54], clinical research has largely failed to focus and emphasize the significance and potential of those two devices, and subsequent meta-studies concluded a non-beneficial effect of mechanical adjuncts in CPR [59,60]. This often leads to hesitance in emergency medical service (EMS) systems - and hospitals - to invest in and apply devices that are not openly recommended by official guidelines despite their obvious physiological and logistic advantages discussed above.
Furthermore, studies so far have also not considered their use in combination with further clinical interventions that may have an even greater impact on survival and outcomes than the mechanical devices themselves, but are not feasible without them. For example, the role of transporting patients with OHCA and refractory, shockable rhythms of ventricular fibrillation/pulseless ventricular tachycardia (VF/pVT) under ongoing high-quality CPR to a CCL was emphasized in a recently published article from the American Heart Association Emergency Cardiovascular Care Committee [58]. For this special group of patients, transport to the hospital with ongoing high-quality CPR is crucial for life-saving treatments like coronary interventions and Extracorporeal Membrane Oxygenation (ECMO) [61-63].
Clearly, more high-quality clinical research is necessary to provide a better basis for CPR guidelines. Despite its costs, the amount of potential lives to be saved more than justifies this effort.
Five year view:
With an increasing number of programs for refractory fibrillation and CCLs, the emphasis on continuous high-quality CPR during transportation will rise, and more studies will reveal the importance of the use of medical devices in this context. This includes the preclinical use of ECMO in urban areas with high-quality EMS systems.
Key issues.
Cardiovascular disease is rising and often leads to cardiac arrest and cardiopulmonary resuscitation, but despite great efforts in research, survival after cardiac arrest is still low.
Medical adjunct devices for high-quality cardiopulmonary resuscitation show remarkable benefits in preclinical studies and clinical trials, yet their routine use is still not recommended by current guidelines.
For a broad implementation of these devices, their true potential needs to be further investigated in combination with other life-saving treatments in the hospital (coronary interventions in the cardiac catheterization laboratory, ECMO) where these adjunct devices are the key for prolonged high-quality CPR during patient transport and the intervention.
Acknowledgments
Disclosure statement: The authors have no conflict of interest. This work was supported, in part, by funding from a Merit Review Award (I01 BX003482) from the U.S. Department of Veterans Affairs Biomedical Laboratory R&D Service awarded to Dr. Riess. Dr. Balzer is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number 397561247. Additional support provided by institutional funds and the National Institutes of Health (5R01 HL123227).
Funding
This work was supported, in part, by funding from a Merit Review Award (I01 BX003482) from the U.S. Department of Veterans Affairs Biomedical Laboratory R&D Service awarded to M Riess. C Balzer is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number 397561247. Additional support provided by institutional funds and the National Institutes of Health (5R01 HL123227).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Select manuscripts have been highlighted as of * interest or ** considerable interest.
- 1.Mehra R Global public health problem of sudden cardiac death. J Electrocardiol 2007; 40: S118–22. [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017; 70: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichol G, Soar J. Regional cardiac resuscitation systems of care. Curr Opin Crit Care 2010; 16: 223–30. [DOI] [PubMed] [Google Scholar]
- 4.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008; 300: 1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böttiger BW, Van Aken HK. Saving 100,000 lives each year in Europe. Best Pract Res Clin Anaesthesiol 2013; 27: 291–2. [DOI] [PubMed] [Google Scholar]
- 6.McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. MMWR Surveill Summ 2011; 60: 1–19. [PubMed] [Google Scholar]
- 7.Idris AH, Guffey D, Aufderheide TP, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation 2012; 125: 3004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama A, Duval S, Nakamura Y, Yoshihara K, Yannopoulos D. Impedance Threshold Device Combined With High-Quality Cardiopulmonary Resuscitation Improves Survival With Favorable Neurological Function After Witnessed Out-of-Hospital Cardiac Arrest. Circ J 2016; 80: 2124–32. [DOI] [PubMed] [Google Scholar]
- 9.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation 2006; 71: 137–45. [DOI] [PubMed] [Google Scholar]
- 10.Voorhees WD, Babbs CF, Tacker WA Jr. Regional blood flow during cardiopulmonary resuscitation in dogs. Crit Care Med 1980; 8: 134–6. [DOI] [PubMed] [Google Scholar]
- 11.Silver DI, Murphy RJ, Babbs CF, Geddes LA. Cardiac output during CPR: a comparison of two methods. Crit Care Med 1981; 9: 419–20. [DOI] [PubMed] [Google Scholar]
- 12.Duggal C, Weil MH, Gazmuri RJ, et al. Regional blood flow during closed-chest cardiac resuscitation in rats. J Appl Physiol (1985) 1993; 74: 147–52. [DOI] [PubMed] [Google Scholar]
- 13.Hightower D, Thomas SH, Stone CK, Dunn K, March JA. Decay in quality of closed-chest compressions over time. Ann Emerg Med 1995; 26: 300–3. [DOI] [PubMed] [Google Scholar]
- 14.Stapleton ER. Comparing CPR during ambulance transport. Manual vs. mechanical methods. JEMS 1991; 16: 63–4, 6, 8 passim. [PubMed] [Google Scholar]
- 15.Lurie KG, Lindo C, Chin J. CPR: the P stands for plumber’s helper. JAMA 1990; 264: 1661. [DOI] [PubMed] [Google Scholar]
- 16.Lurie KG, Coffeen P, Shultz J, et al. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation 1995; 91: 1629–32. [DOI] [PubMed] [Google Scholar]
- 17.Cohen TJ, Tucker KJ, Lurie KG, et al. Active compression-decompression. A new method of cardiopulmonary resuscitation. Cardiopulmonary Resuscitation Working Group. JAMA 1992; 267: 2916–23. [DOI] [PubMed] [Google Scholar]
- 18.Stryker Medical (2019). https://www.lucas-cpr.com/
- 19.Halperin HR, Tsitlik JE, Gelfand M, et al. A preliminary study of cardiopulmonary resuscitation by circumferential compression of the chest with use of a pneumatic vest. N Engl J Med 1993; 329: 762–8. [DOI] [PubMed] [Google Scholar]
- 20.Halperin H, Berger R, Chandra N, et al. Cardiopulmonary resuscitation with a hydraulic-pneumatic band. Crit Care Med 2000; 28: N203–6. [DOI] [PubMed] [Google Scholar]
- 21.Brooks SC, Toma A, Hsu J. Devices used in cardiac arrest. Emerg Med Clin North Am 2012; 30: 179–93. [DOI] [PubMed] [Google Scholar]
- 22.Fischer M, Breil M, Ihli M, et al. [Mechanical resuscitation assist devices]. Anaesthesist 2014; 63: 186–97. [DOI] [PubMed] [Google Scholar]
- 23.de Rooij PP, Wiendels DR, Snellen JP. Fatal complication secondary to mechanical chest compression device. Resuscitation 2009; 80: 1214–5. [DOI] [PubMed] [Google Scholar]
- 24.Deras P, Manzanera J, Millet I, Charbit J, Capdevila X. Fatal pancreatic injury due to trauma after successful cardiopulmonary resuscitation with automatic mechanical chest compression. Anesthesiology 2014; 120: 1038–41. [DOI] [PubMed] [Google Scholar]
- 25.Wind J, Bekkers SC, van Hooren LJ, van Heurn LW. Extensive injury after use of a mechanical cardiopulmonary resuscitation device. Am J Emerg Med 2009; 27: 1017 e1–2. [DOI] [PubMed] [Google Scholar]
- 26.Hutchings AC, Darcy KJ, Cumberbatch GL. Tension pneumothorax secondary to automatic mechanical compression decompression device. Emerg Med J 2009; 26: 145–6. [DOI] [PubMed] [Google Scholar]
- 27.Rabl W, Baubin M, Broinger G, Scheithauer R. Serious complications from active compression-decompression cardiopulmonary resuscitation. Int J Legal Med 1996; 109: 84–9. [DOI] [PubMed] [Google Scholar]
- 28.Smekal D, Johansson J, Huzevka T, Rubertsson S. No difference in autopsy detected injuries in cardiac arrest patients treated with manual chest compressions compared with mechanical compressions with the LUCAS device--a pilot study. Resuscitation 2009; 80: 1104–7. [DOI] [PubMed] [Google Scholar]
- 29.Koster RW, Beenen LF, van der Boom EB, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. Eur Heart J 2017; 38: 3006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochoa FJ, Ramalle-Gomara E, Lisa V, Saralegui I. The effect of rescuer fatigue on the quality of chest compressions. Resuscitation 1998; 37: 149–52. [DOI] [PubMed] [Google Scholar]
- 31.Jones AY, Lee RY. Cardiopulmonary resuscitation and back injury in ambulance officers. Int Arch Occup Environ Health 2005; 78: 332–6. [DOI] [PubMed] [Google Scholar]
- 32.Keseg DP. The merits of mechanical CPR: Do mechanical devices improve compression consistency and resuscitation outcomes? JEMS 2012; 37: 24–9. [PubMed] [Google Scholar]
- 33.Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the LINC randomized trial. JAMA 2014; 311: 53–61. [DOI] [PubMed] [Google Scholar]
- 34.Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015; 385: 947–55. [DOI] [PubMed] [Google Scholar]
- 35.Gates S, Lall R, Quinn T, et al. Prehospital randomised assessment of a mechanical compression device in out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised trial and economic evaluation. Health Technol Assess 2017; 21: 1–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu N, Chen Q, Jiang Z, et al. A meta-analysis of the resuscitative effects of mechanical and manual chest compression in out-of-hospital cardiac arrest patients. Crit Care 2019; 23: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallstrom A, Rea TD, Sayre MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA 2006; 295: 2620–8. [DOI] [PubMed] [Google Scholar]
- 38.Paradis NA, Young G, Lemeshow S, Brewer JE, Halperin HR. Inhomogeneity and temporal effects in AutoPulse Assisted Prehospital International Resuscitation--an exception from consent trial terminated early. Am J Emerg Med 2010; 28: 391–8. [DOI] [PubMed] [Google Scholar]
- 39.Wik L, Olsen JA, Persse D, et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014; 85: 741–8. [DOI] [PubMed] [Google Scholar]
- 40.Savastano S, Baldi E, Palo A, et al. Load distributing band device for mechanical chest compressions: An Utstein-categories based analysis of survival to hospital discharge. Int J Cardiol 2019; 287: 81–5. [DOI] [PubMed] [Google Scholar]
- 41.Nordeen CA. Manual Versus Mechanical Cardiopulmonary Resuscitation: A Case Against the Machine. Cardiol Clin 2018; 36: 375–86. [DOI] [PubMed] [Google Scholar]
- 42.Brooks SC, Anderson ML, Bruder E, et al. Part 6: Alternative Techniques and Ancillary Devices for Cardiopulmonary Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132: S436–43.* Current AHA recommendations regarding the use of adjunct devices during CPR
- 43.Yannopoulos D, Nadkarni VM, McKnite SH, et al. Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation 2005; 112: 803–11. [DOI] [PubMed] [Google Scholar]
- 44.Yannopoulos D, McKnite S, Metzger A, Lurie KG. Intrathoracic pressure regulation improves 24-hour survival in a porcine model of hypovolemic shock. Anesth Analg 2007; 104: 157–62. [DOI] [PubMed] [Google Scholar]
- 45.Metzger A, Rees J, Kwon Y, et al. Intrathoracic Pressure Regulation Improves Cerebral Perfusion and Cerebral Blood Flow in a Porcine Model of Brain Injury. Shock 2015; 44: 96–102. [DOI] [PubMed] [Google Scholar]
- 46.Patel N, Branson R, Salter M, et al. "2014 Military Supplement" Intrathoracic Pressure Regulation Augments Stroke Volume And Ventricular Function In Human Hemorrhage. Shock 2015; 44: 55–62. [DOI] [PubMed] [Google Scholar]
- 47.Huffmyer JL, Groves DS, Scalzo DC, et al. The effect of the intrathoracic pressure regulator on hemodynamics and cardiac output. Shock 2011; 35: 114–6. [DOI] [PubMed] [Google Scholar]
- 48.Aufderheide TP, Nichol G, Rea TD, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med 2011; 365: 798–806.* PRIMED trial by the Resuscitation Outcome Consortium (ROC) failing to show a benefit of the ITD when used with standard manual CPR. Because the quality of CPR was not controlled, it needs to be interpreted in conjunction with ref 49
- 49.Yannopoulos D, Aufderheide TP, Abella BS, et al. Quality of CPR: An important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation 2015; 94: 106–13.** Must-know follow-up reevaluation of PRIMED trial (ref 48) demonstrating the interaction of high quality CPR and ITD on survival
- 50.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132: S444–64.* Current AHA recommendations regarding high quality chest comporessions during ACLS
- 51.Lurie KG, Voelckel WG, Zielinski T, et al. Improving standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve in a porcine model of cardiac arrest. Anesth Analg 2001; 93: 649–55. [DOI] [PubMed] [Google Scholar]
- 52.Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression-decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation 2000; 101: 989–94. [DOI] [PubMed] [Google Scholar]
- 53.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet 2011; 377: 301–11.** The pivotal trial demonstrating increased neurologically favorable survival with ACD
- 54.Debaty G, Segal N, Matsuura T, et al. Hemodynamic improvement of a LUCAS 2 automated device by addition of an impedance threshold device in a pig model of cardiac arrest. Resuscitation 2014; 85: 1704–7. [DOI] [PubMed] [Google Scholar]
- 55.Aufderheide TP, Pirrallo RG, Yannopoulos D, et al. Incomplete chest wall decompression: a clinical evaluation of CPR performance by trained laypersons and an assessment of alternative manual chest compression-decompression techniques. Resuscitation 2006; 71: 341–51. [DOI] [PubMed] [Google Scholar]
- 56.Frascone RJ. The risk versus benefit of LUCAS: is it worth it? Anesthesiology 2014; 120: 797–8. [DOI] [PubMed] [Google Scholar]
- 57.Hasler RM, Stucky S, Bahler H, Exadaktylos AK, Neff F. The dead and the dying - a difficult part of EMS transport: A Swiss cross-sectional study. PLoS One 2018; 13: e0191879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yannopoulos D, Bartos JA, Aufderheide TP, et al. The Evolving Role of the Cardiac Catheterization Laboratory in the Management of Patients With Out-of-Hospital Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation 2019; 139: e530–e52. [DOI] [PubMed] [Google Scholar]
- 59.Poole K, Couper K, Smyth MA, Yeung J, Perkins GD. Mechanical CPR: Who? When? How? Crit Care 2018; 22: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gates S, Quinn T, Deakin CD, et al. Mechanical chest compression for out of hospital cardiac arrest: Systematic review and meta-analysis. Resuscitation 2015; 94: 91–7. [DOI] [PubMed] [Google Scholar]
- 61.William P, Rao P, Kanakadandi UB, Asencio A, Kern KB. Mechanical Cardiopulmonary Resuscitation In and On the Way to the Cardiac Catheterization Laboratory. Circ J 2016; 80: 1292–9. [DOI] [PubMed] [Google Scholar]
- 62.Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary Artery Disease in Patients With Out-of-Hospital Refractory Ventricular Fibrillation Cardiac Arrest. J Am Coll Cardiol 2017; 70: 1109–17.** Landmark study demonstrating an odds ratio of 4.0 for survival after witnessed shock-refractory VF arrest when patients are transported directly to the coronary catheter lab under ongoing automated mechanical chest compression with LUCAS and ITD
- 63.Bartos JA, Carlson K, Carlson C, et al. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: Critical care and extracorporeal membrane oxygenation management. Resuscitation 2018; 132: 47–55.* Follow-up paper to ref 62 describing in-hospital bundle care including ECMO, complications and survival rates for patients surviving refractory VF arrest