Abstract
Successful voluntary tic suppression is a key component of the behavioral interventions that are used to treat tic disorders. This study aimed to examine tic suppression in children with recent-onset tics and determine whether the capacity to suppress tics predicts futures tic severity. We tested 45 children (30 male, mean age 7.74 years) with recent-onset tics (mean 3.47 months prior to the first study visit; baseline) and re-examined each child at the 12-month anniversary of the first recognized tic (follow-up). At the baseline visit, children performed a tic suppression task with several conditions: tic freely, inhibit tics given a verbal request, and inhibit tics in the presence of a reward. At the baseline visit, children with tics for only a few months could suppress their tics, and tic suppression was especially successful when they received an immediate and contingent reward. Additionally, the ability to suppress tics in the presence of a reward predicted tic severity at follow-up. These findings suggest that better inhibitory control of tics within months of tic onset may be an important predictor of future tic symptom outcome.
Keywords: Tic Disorders, Inhibition (psychology), prognosis, Provisional Tic Disorder
Tic disorders are characterized by the presence of motor and/or vocal tics, which are unwanted, recurrent movements (e.g. eye blink) or vocalizations (e.g. throat clearing) (DSM-5) 1, and comprise different diagnostic categories based on the duration of tic symptoms. Tourette’s disorder or persistent (chronic) motor or vocal tic disorder (TS/CTD) can be diagnosed when tic symptoms are present for more than one year since initial tic onset (regardless of tic-free periods in the interim). When an individual has had tics for less than one year, provisional tic disorder (PTD) can be diagnosed. Despite the prevalence rate of PTD (20% 2–5 or higher 6), most existing clinical, behavioral, neuropsychological, and neurophysiological studies of tic disorders have focused nearly exclusively on TS/CTD. This dearth of investigation of PTD may be a missed opportunity considering the potential implications for early stage prognostics as well as for understanding the transition to TS/CTD. The published behavioral 7 and neuroimaging 8 findings from studies of TS/CTD, including individuals who have had tics for one or more years, cannot effectively distinguish effects related to the primary cause of tics or secondary, compensatory changes. On the other hand, findings from children with recent-onset tics are unlikely to result from persistent tics.
One interesting characteristic of tics is that very often they can be voluntarily suppressed, at least temporarily 9. Many studies using a standardized tic suppression paradigm 10 have shown that individuals with TS/CTD can suppress tics especially well with contingent reward 10–14. Conelea et al. 15 pooled nine different tic suppression paradigm studies in children and adolescents and found that better tic suppression ability was related to older age and more frequent tics 15. An investigation of neuropsychological predictors of tic suppression revealed that tic suppression ability was correlated with poor attentional functioning (i.e. omission errors on a continuous performance task) 16. Previous work from our group showed that even children with PTD, who had tics for less than six months, can successfully suppress tics especially in the presence of a contingent reward 17.
Behavioral interventions for tic disorders, such as Comprehensive Behavioral Intervention for Tics (CBIT 18), are based on improving the ability to voluntarily suppress tics. Therefore, understanding tic suppression and how the ability to suppress tics may relate to clinical outcomes is important. While a few studies have sought to understand inter-individual variability of tic suppression 15,16, none have examined longitudinally the relationship between tic suppression and future tic outcome.
The present study extended our previous work on tic suppression in PTD to investigate outcome. We examined tic suppression in the presence or absence of a reward in children whose tics began within the previous 6 months. We then re-examined these children at the one-year anniversary of tic onset (i.e., the time when a diagnosis of TS/CTD can be made). We first tested how well children with PTD could suppress their tics in an extended sample from our previous report 17. Then, we investigated whether or not tic suppression ability measured within months of tic onset can predict an individual’s tic outcome 12 months after tic onset.
Methods
Participants
NewTics is an ongoing longitudinal study conducted at Washington University School of Medicine, St. Louis, Missouri (www.newtics.org). We recruited the participants using a various recruitment methods and screened them carefully using questionnaires, interviews and mindful face-to-face examination to determine the best estimate of the date of tic onset (See Kim et al., 19 for further details). Between September 2010 and December 2018, 55 children with recent-onset tics (tic duration < 6 months, except for one participant whose tic duration was 8.1 months) reached the one year anniversary of tic onset. Among those, five participants were lost to follow-up, one participant was identified as an outlier in age (14.5 years old was > 3SD above the mean age), and tic suppression paradigm videos were missing for four participants, so we do not have blinded measures (see below) from those participants. One participant was only missing the Verbal condition, so this participant was included in the analyses of DRO (Differential Reinforcement of Other behavior, see Tic suppression paradigm section) condition. Therefore, in the current study, we report the data for 45 participants (30 male, 15 female, mean age = 7.7). All participant characteristics are shown in Table 1.
Table 1.
Characteristics of study participants at the baseline and 12-month follow-up visit
| Descriptor | Baseline visit | 12-month Follow-up |
|---|---|---|
| N | 45 | |
| Male/female | 30/15 | |
| Age | 7.74 (2.02); 5.03–12.9 | |
| No. with ADHD diagnosis | 17 | 21 |
| No. with OCD diagnosis | 4 | 7 |
| No. with anxiety disorder* | 19 | n/a |
| No. with brain active medications** | 9 | 8 |
| Months since tic onset | 3.47(1.59); 0.72–8.09 | |
| YGTSS total tic (TTS) | 17.24(6.08); 7–32 | 13.82(7.46); 0–37 |
| YGTSS impairment | 8.56(8.44); 0–30 | 4(6.62); 0–25 |
| DCI | 32.29(13.19); 14–80 | 42.47(15.86); 13–79 |
| PUTS*** | 12.74(4.62); 9–29 | 15.58(5.88); 9–30 |
| ADHD Rating Scale (ARS) | 13.73(11.04); 0–40 | 14.71(12.14); 0–41 |
| Social Responsiveness Scale (SRS) | 48.84(8.55); 35–69 | n/a |
“Anxiety Disorder” includes panic disorder, separation anxiety disorder, social anxiety disorder, agoraphobia, specific phobia, generalized anxiety disorder (DSM-IV), and avoidant disorder of childhood (DSM-III-R).
Nine participants were on brain active medication at baseline visit: one on anticholinergic, one on SSRI, one on adrenergic, two on stimulant, one on stimulant and adrenergic agonist, and three on other brain active medication. Eight participants were on brain active medication at 12-month follow-up: two on SSRI, two on adrenergic agonist, three on stimulant, and one on other brain active medication. Of note, only one participant at baseline visit and two participants at 12-month follow-up took medication due to tics and none of the participants had any behavioral intervention for tics.
PUTS scores were not obtained from 6 children at baseline visit and 2 children at 12-month visit due to difficulty in reporting these internal phenomena.
Procedure
This study consists of a baseline visit within six months of tic onset (with the exception of one participant whose tics began 8.1 months before the visit) and a follow-up visit at the one-year anniversary of tic onset. The full details of the clinical measures obtained at each visit can be found in our previous work 19. Here, we examined the following: Yale Global Tic Severity Scale (YGTSS) 20, which measures past week tic severity, Diagnostic Confidence Index (DCI) 21, which measures lifetime “typical” TS/CTD characteristics, Premonitory Urge for Tics Scale (PUTS) 22, which measures the common sensory experience that precedes tics (called the premonitory urge), ADHD Rating Scale (ARS) 23, which measures past week ADHD symptomatology, and Social Responsiveness Scale (SRS) 24, which measures symptoms of autism.
Tic suppression paradigm
The tic suppression paradigm implemented in the current study was modeled from Woods & Himle 10 and is described in detail in Greene et al. 17. Participants completed two 5-min sessions under each of three conditions *. (1) Free tic: participants were instructed to sit in a chair and tic as needed. (2) Verbal Instruction: participants were instructed to suppress their tics. (3) Differential Reinforcement of Zero-rate Ticcing (Differential Reinforcement of Other behavior: DRO): participants were instructed to suppress their tics, and told that they would receive a token for every 10 s that a tic was not detected. Participants first received one session of each of the conditions in a fixed order: Free tic, Verbal Instruction, DRO. The second session of the conditions was then presented in a counterbalanced order. Prior to each session, we read to participants detailed instructions (See Greene et al. 17) with a list of his/her tics, and asked them to explain instructions back to the researcher to ensure comprehension of the task. During the task, participants sat alone in a room and a researcher (Rater 1 author KJB, a neuropsychiatrist with movement disorders fellowship training) rated their tics through live video and audio feeds in an adjacent room. Tics were coded by pressing a button on the TicTimer program 25 for each occurrence of a tic.
Tic Ratings
Tics were rated in real time by Rater 1 (author KJB) in order to provide appropriate rewards in the DRO condition, but Rater 1 was inevitably unblinded to the condition of each session. Therefore, the video recordings were blinded and presented in randomized order to Rater 2 (author ARV, a movement disorders trained pediatric neurologist) who rated tics using a modified version of the TicTimer program.
Inter-rater reliability was measured by calculating intra-class correlation coefficient (ICC) using a two-way random effects model assessing consistency. The single measures ICC was .754 and .796 for our two dependent measures (tic frequency and tic-free intervals, respectively), indicating good reliability across two raters. Here, we present results from the blind ratings (Rater 2). The results from Rater 1 are shown in Supplemental Materials S2.
Analysis
To compare tic severity at baseline and at the 12-month follow-up visit, we conducted paired t tests on YGTSS total tic score (TTS) at each visit. For the Tic suppression paradigm, we measured two dependent variables from each session of each condition: (1) the number of tics, and (2) tic-free 10 s intervals. Order effects were tested using repeated measures ANOVAs with Set (first set of sessions, second set of sessions) and Condition (Free tic, Verbal, DRO) as within-subject factors. Four participants who completed only one set of sessions due to fatigue or limited cooperation, and three participants for whom blinded tic ratings were unavailable due to incomplete video recording, were excluded from the analysis of order effects. As there was no significant main effect of Set, the data were collapsed across Set for each condition and the average number of tics and tic-free 10 s intervals per minute were used for all subsequent analyses. Eight participants who showed less than one tic per minute on average in the Free tic condition were excluded from further analysis, as tic suppression would be limited by a floor effect.
One-way repeated measures ANOVAs were conducted for each measure to test for main effects of Condition. Greenhouse-Geisser correction was made where sphericity assumption was violated. Then post hoc t tests were conducted to compare specific conditions. For the subsequent analyses, tic suppression in tic frequency was quantified for each suppression condition as a ratio of tic reduction in comparison to the Free tic condition (e.g. (Free tic-Verbal)/Free tic; hereafter Suppressionfrequency), such that positive values indicate tic reduction during the suppression conditions. When tic suppression was calculated in a similar way for tic-free 10 s intervals, the measure was susceptible to biases caused by Free tic performance. For example, a participant with 4 tic-free 10 s intervals per minute in the Free tic condition could only reach a maximum of 6 tic-free 10 s intervals per minute in a suppression condition (50% change), even though that same participant could reduce tic frequency by 100%. For this reason, the average number of tic-free 10 s intervals per minute was used as a measure of tic suppressibility without correcting for Free tic condition (hereafter Suppressioninterval). Thus, higher Suppressioninterval values indicate better tic suppression.
Correlation analyses were conducted to explore the relationships between Suppression and several variables obtained at the baseline visit that have been shown previously to be related to tic suppression: age, tic severity (TTS), PUTS total score, and Social Responsiveness Scale (total) T score. One outlier was identified and excluded from PUTS total score (≥ Mean + 3SD). When the Shapiro-Wilk test revealed that the data were not normally distributed, Spearman correlation analyses (shown as rs) were adopted. When normality was not violated, Pearson’s correlation analyses were adopted.
We conducted multiple regression analysis to test if Suppression at the baseline visit can predict tic severity at the follow-up visit. Baseline TTS was included as a covariate. Participant age at the baseline visit was also included as a covariate where age-dependent effects were found.
Results
Change in tic symptoms and awareness of tics
Participants showed moderate tic severity on average at the baseline visit (Mean TTS 17.02 ± 6.16) and at the 12-month follow-up visit (mean TTS 13.82 ± 7.46). A paired t test revealed significant improvement in tic severity at the12-month follow-up visit on a group level (t(44)=3.06, p=.004). While we do not have a systematic record of subjective awareness of tics for most of the children, several children reported anecdotally that they were not aware of any tics at the baseline visit. Indirectly, the DCI included an item asking whether the child ever intentionally attempted to suppress tics. Out of the 45 participants, this item was recorded as positive for 22 participants at the baseline visit and 26 participants at 12-month follow-up visit.
Testing of order effects
Repeated measures ANOVAs with Set (1 and 2) and Conditions (Free tic, Verbal, and DRO) were conducted on the data from 38 participants who completed both sets. For tic frequency, there was no significant main effect of Set, F(1,37) = 0.56, p = .46, but a significant interaction of Set × Condition, F(2,74) = 3.84, p=.03. For tic-free 10 s intervals, there was no significant main effect of Set, F(1,37) = 1.35, p = .25, or interaction of Set × Condition, F(1.70,62.91) = 1.77, p = .18. For consistency with our previous work, we collapsed the data across Set for the subsequent analyses for all participants. The results of the Set 1 data are shown in the Supplemental Materials S1.
Tic suppression with and without reward
The mean values of tic frequency and tic-free 10 s intervals for each condition during the baseline visit are shown in Table 2. One-way repeated measures ANOVAs (Condition: Free tic, Verbal, DRO) were conducted for tic frequency and tic-free 10 s intervals separately for the 36 participants who had all three conditions. A significant main effect of Condition was found for both tic-free 10 s intervals F(1.54, 53.96) = 16.28, p<.001, and tic frequency, F(1.45, 50.65) = 13.18, p<.001. Post-hoc t tests were conducted to compare each of the suppression conditions to the Free tic condition. The results are shown in Table 2. To summarize, both suppression conditions (Verbal, DRO) significantly differed from Free tic condition in both tic frequency and tic-free intervals (p<.05). The DRO condition also differed from the Verbal condition in both tic frequency and tic-free intervals (p<.05).
Table 2.
Mean tic frequency and tic-free intervals and comparison between conditions
| Variable | N | Mean | SD | Post-hoc t tests | t | p (bonf) |
|---|---|---|---|---|---|---|
| Tic frequency | ||||||
| - Free tic | 36 | 4.515 | 2.594 | |||
| - Verbal | 36 | 3.180 | 2.923 | Verbal vs. Free tic | 3.125 | 0.011 |
| - DRO | 36 | 0.292 | 0.449 | DRO vs. Free tic | 9.650 | <.001 |
| DRO vs. Verbal | 5.319 | <.001 | ||||
| Tic-free intervals | ||||||
| - Free tic | 36 | 4.258 | 0.891 | |||
| - Verbal | 36 | 4.682 | 0.962 | Verbal vs. Free tic | 2.807 | 0.024 |
| - DRO | 36 | 5.061 | 0.869 | DRO vs. Free tic | 4.860 | <0.001 |
| DRO vs. Verbal | 3.921 | 0.001 |
Relationship between measures collected at the baseline visit and tic suppressibility
There was a significant correlation between age and Suppression in the DRO condition for both Suppressioninterval rs(36) =.40, p=.01, and Suppressionfrequency rs(36) = .40, p=.01, such that older children showed better Suppression (See Figure 1a and b). There was no significant relationship between age and any measure of Suppression in the Verbal condition (minimum p = .33). There was no significant correlation between TTS at the baseline visit and any of Suppression measure in either condition (minimum p = .27). There was no significant relationship between tic duration and any measure of Suppression in either condition (minimum p = .363). The PUTS total score was significantly correlated with Suppressionfrequency in the DRO condition, rs (29) = .39, p = .03 (See Figure 1c), but not with Suppressioninterval in the DRO condition or with either Suppression measure in the Verbal condition (minimum p = .16). Neither ADHD Rating Scale score nor Social Responsiveness Scale scores were correlated with any Suppression measure (minimum p = .42 for ADHD Rating Scale scores; minimum p = .47 for Social Responsiveness Scale scores).
Figure 1.
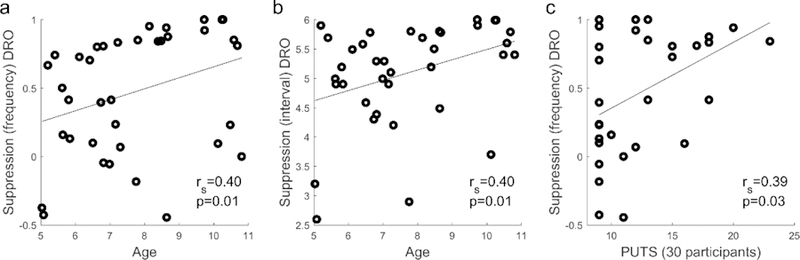
The relationship between Suppression and measures obtained at the baseline visit. a.age and Suppressionfrequency in DRO condition, b. age and Suppressioninterval in DRO condition, c. PUTS total score (30 participants) Suppressionfrequency in DRO condition.
Relationship between Suppression at the baseline visit and tic severity at the 12-month follow-up visit
Multiple regression analyses were conducted to determine the relationship between Suppression at the baseline visit and tic severity at the 12-month follow-up visit. Overall, children who showed better tic suppression in the DRO condition at the baseline visit showed better tic outcome (i.e., reduced tic severity) at the 12-month follow-up visit. TTS at the 12-month follow-up visit was significantly predicted by Suppressioninterval in the DRO condition, controlling for TTS at the baseline visit and age, R2 = .277, F(3,33) =4.22, p = .01; adjusted R2 = .212, with Suppressioninterval as a significant factor (p=.038). Suppressionfrequency in the DRO condition revealed a similar pattern of results, R2 = .246, F(3,33) = 3.59, p = .02; adjusted R2 = .177, but Suppressionfrequency was not a significant predictor p=.09). Correlation plots of the relationship between TTS at the 12-month follow-up visit and Suppressioninterval (left) and Suppressionfrequency (right) in the DRO condition are shown in Figure 2. Of note, this relationship was significant both in the analysis of data collapsed across Sets and in the analysis of only Set 1 data (See Supplemental Materials S1). In Verbal condition, Suppressioninterval (p=.15) and Suppressionfrequency (p=.20) were not a significant factor in each model. Full model details are reported in Table 3. As Suppressioninterval was not corrected for the Free tic condition, we conducted multiple regression analysis with the average number of tic-free intervals for the Free tic condition and found that it was not a significant factor in the model (p=.23; See Table 3).
Figure 2.
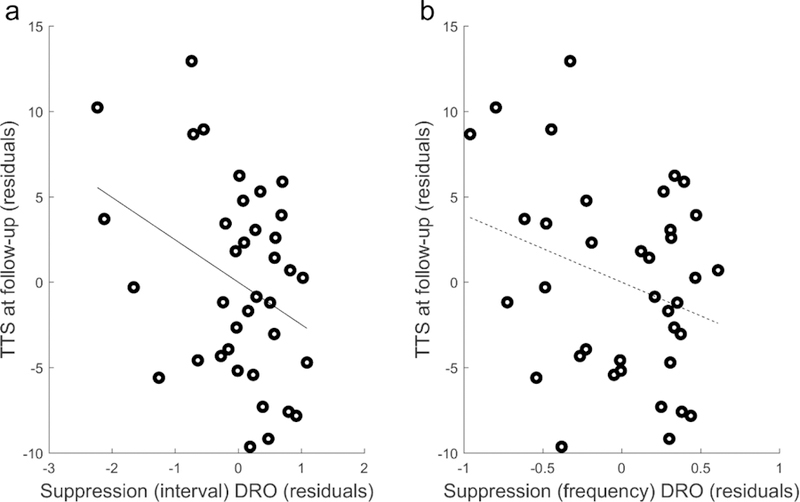
The partial residual plot showing the relationship between the residuals of the regression of TTS at 12-month follow-up on TTS at baseline visit and age, and the residuals of the regression of Suppression in DRO condition (a. Suppressioninterval; b. Suppressionfrequency) on TTS at baseline visit and age. The solid line indicates a significant relationship and the dashed line indicates non-significant relationship.
Table 3.
Multiple linear regression analysis results predicting TTS at 12-month follow-up visit
| Variable | B | SEB | β | p |
|---|---|---|---|---|
| DRO condition - tic-free intervals | ||||
| Suppression | −2.48 | 1.15 | −0.35 | 0.04 |
| age | 0.97 | 0.55 | 0.28 | 0.09 |
| baseline TTS | 0.39 | 0.17 | 0.34 | 0.03 |
| Intercept | 12.12 | 6.77 | 0.08 | |
| DRO condition - tic frequency | ||||
| Suppression | −3.94 | 2.25 | −0.28 | 0.09 |
| age | 0.85 | 0.55 | 0.25 | 0.13 |
| baseline TTS | 0.39 | 0.17 | 0.34 | 0.03 |
| Intercept | 2.15 | 4.99 | 0.67 | |
| Verbal condition - tic-free intervals | ||||
| Suppression | −1.52 | 1.02 | −0.23 | 0.15 |
| baseline TTS | 0.43 | 0.18 | 0.38 | 0.02 |
| Intercept | 13.35 | 5.84 | 0.03 | |
| Verbal condition - tic frequency | ||||
| Suppression | 0.15 | 2.27 | 0.01 | 0.95 |
| baseline TTS | 0.44 | 0.18 | 0.39 | 0.02 |
| Intercept | 6.02 | 3.28 | 0.08 | |
| Free tic condition - tic-free intervals | ||||
| average number of tic-free intervals | 0.48 | 0.4 | 0.2 | 0.23 |
| baseline TTS | 0.37 | 0.18 | 0.33 | 0.05 |
| Intercept | 4.99 | 3.25 | 0.13 |
B indicates unstandardized coefficients; SE indicates standard error; β indicates standardized coefficients.
Discussion
The most important finding in the present study is that rewarded tic suppression measured within months of tic onset predicts future tic severity. Specifically, in children with recent-onset tics, we found that those children with better tic suppression in the presence of a reward reported lower tic burden at the one-year anniversary of tic onset, the time when a persistent tic disorder such as TS/CTD can first be diagnosed. Thus, we have identified a potential predictor of clinical outcome in Provisional Tic Disorder (PTD).
The conventional clinical wisdom is that tics are common but temporary in childhood, disappearing within a few months in most children. The prevalence rates reported for any tics (about 20% 2–5 or higher 6) and chronic tics (about 3% 1,26) suggest that only a small subset of children who experience tics go on to develop TS/CTD. Although our own study showed a somewhat different finding in that tics do not completely remit in most children by the one year anniversary of tic onset 19, the majority of children experienced only mild tic severity and minimal impairment, if any, by that point. Still, some children do experience worsening of tic symptoms and marked distress or impairment due to tics. Therefore, identifying a behavioral predictor of future tic outcome, as we do here, is quite promising for prognosis of a chronic disorder in children when tics first begin.
Tics are often described as the result of faulty inhibitory control 27. Indeed, previous fMRI and EEG studies suggest that voluntary tic suppression involves activation of brain regions that support inhibitory control 28,29. Additionally, TS/CTD has been associated with impaired inhibition of a different response to a natural urge, namely the urge to blink during voluntary blink suppression 30. Our study shows that, despite this possible impairment in inhibitory function, children could suppress tics without years of tic suppression practice. In addition, if better tic suppression at the baseline visit is due to better overall inhibitory control, this lessened impairment may explain why these children have better tic outcomes later, perhaps due to better management of tics. However, findings are inconsistent as to whether inhibitory function—as measured by traditional behavioral tasks, such as the stop signal task—is actually impaired in individuals with tic disorders 31,32. Thus, it will be important for future work to examine how much tic suppression is related to inhibitory function as measured by these standard laboratory tests. We also found that tic suppression measured in the absence of reward did not significantly predict future tic outcome. This differential result based on the presence or absence of a reward may be due to motivation. Without the prospect of reward, children may exert less effort to suppress their tics.
While the most important finding in the present study is about predicting future tic outcome, we also extend our previous results demonstrating that children with recent-onset tics can suppress tics within months of tic onset 17 to a sample twice as large. We found reductions in tic frequency and increases in the number of tic-free intervals when children were simply asked verbally to suppress their tics. When an immediate, contingent reward was delivered for successful tic suppression, tic suppression was enhanced. With this larger sample, we also detected a significant association between age and rewarded tic suppression. Conelea et al. 15 suggested that such age effects might be due to the fact that older children have experienced longer illness duration, leading to more opportunity to practice tic suppression strategies. However, that explanation does not account for our present results, as all but one of our participants had experienced tics for less than 6 months. Rather, we contend that the age-dependent effects found in the current study are more likely due to inhibitory control maturation during development. Age-dependent effects in inhibitory control have been repeatedly reported in healthy children in both behavioral and brain imaging studies (See 33 for a review).
We also explored the relationship between tic suppression and other characteristics commonly associated with tics. Tics are often described as being preceded by a “premonitory urge” 34; however, findings on the relationship between the premonitory urge and tic suppression are inconsistent. While Brandt et al. 35 showed that premonitory urges build up during tic suppression, Banaschewski et al. 36 suggested that premonitory urges are not prerequisites for tic suppression in children and adolescents with TS. Here, we found a significant relationship between the PUTS score and Suppressionfrequency in the presence of reward. Visual inspection of this relationship suggests that children with higher PUTS scores showed relatively successful tic suppression, while children with lower PUTS scores showed varying degrees of tic suppression ranging from minimal to maximal. Thus, our results are consistent with the idea that experiencing the premonitory urge may help tic suppression. Our finding does have the limitation that the PUTS can be less reliable in children under 10 years old 22,37. Age may explain part of the association of premonitory urges and suppression (see Supplemental Material S3). Continued research may help to further elucidate the relationship between the premonitory urge and tic suppression.
While one previous study reported a possible relationship between parent-reported attentional problems in children with tics and tic suppression ability 11, we found no significant relationship between our measure of ADHD symptoms (ADHD Rating Scale score) and tic suppression. We also explored the relationship between Social Responsiveness Scale score and Suppression. Our previous work 19 suggested baseline visit Social Responsiveness Scale scores as a candidate clinical feature for predicting 12-month tic outcome. One possible explanation for this finding was that children with higher Social Responsiveness Scale scores are less sensitive to negative social feedback about their tics and make less effort to suppress tics in social settings. However, we did not find a significant relationship between baseline visit Social Responsiveness Scale scores and tic suppressibility. Of course, we measured tic suppression in a laboratory setting, and tic suppression in real-world social settings may be different.
Limitations
Previous studies have shown that behavioral measurements of tic suppression were unrelated to self-rated tic suppression ability 15. Also, the expression of tics often differs depending on the setting (e.g. home vs. office) or the presence of others 38,39. In the present study, we quantified tic suppression using a standardized protocol with video recording of the child sitting alone in a room. Therefore, further studies need to be conducted to understand how tic suppression in a laboratory setting compares to tic suppression in daily life. The current study focused on tic severity as the predicted clinical outcome. Previous work in children with pre-existing TS may also be relevant; such work has examined childhood predictors of adult quality of life 40 or of tic severity and other comorbid conditions 6,41,42,43.
Supplementary Material
Acknowledgments
We thank Vicki Martin for help with recruiting and data collection and Dr. Jimin Ding for advising on the statistical analysis.
Funding
Research reported in this publication was supported by National Institutes of Health, awards K24 MH087913 to KJB; R21 NS091635 to BLS and KJB; K01 MH104592 to DJG; R01 MH104030 to KJB and BLS; the Washington University Institute of Clinical and Translational Sciences grants UL1 RR024992 and UL1 TR000448; and the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH
Footnotes
Data availability
The supplementary data file provides individual participant data.
Declaration of conflicting interests
The Authors declare that there is no conflict of interest
Ethical approval
The study was approved by the Washington University Human Research Protection Office (IRB), protocol numbers 201109157 and 201707059. Each child assented and a parent (guardian) gave informed consent prior to study participation. All participants were compensated for their time.
The first 36 participants performed an additional condition: Noncontingent Reinforcement (NCR), in which they were asked to suppress their tics and told that they would receive tokens regardless of their tic behavior. As the NCR condition was not conducted in the remaining participants, the results from this condition are not reported here.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 2.Cubo E, Trejo Gabriel Y Galán JM, Villaverde VA, et al. Prevalence of tics in schoolchildren in central Spain: A population-based study. Pediatr Neurol 2011;45:100–108. doi: 10.1016/j.pediatrneurol.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Cubo E Review of prevalence studies of tic disorders: methodological caveats. Tremor Other Hyperkinet Mov (N Y) 2012;2. doi: 10.7916/D8445K68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurlan R, Como PG, Miller B, et al. The behavioral spectrum of tic disorders: A community-based study. Neurology 2002;59:414–420. doi: 10.1212/WNL.59.3.414 [DOI] [PubMed] [Google Scholar]
- 5.Snider LA, Seligman LD, Ketchen BR, et al. Tics and problem behaviors in schoolchildren: prevalence, characterization, and associations. Pediatrics 2002;110(2):331–336. [DOI] [PubMed] [Google Scholar]
- 6.Black KJ, Black ER, Greene DJ, Schlaggar BL. Provisional Tic Disorder: What to tell parents when their child first starts ticcing. F1000Research 2016;5:696. doi: 10.12688/f1000research.8428.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson GM, Draper A, Dyke K, Pépés SE, Jackson SR. Inhibition, Disinhibition, and the Control of Action in Tourette Syndrome. Trends Cogn Sci 2015;19(11):655–665. doi: 10.1016/J.TICS.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 8.Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J Psychosom Res 2009;67(6):559–573. doi: 10.1016/J.JPSYCHORES.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer HS. Tourette syndrome and other tic disorders. Handb Clin Neurol 2011;100:641–657. doi: 10.1016/B978-0-444-52014-2.00046-X [DOI] [PubMed] [Google Scholar]
- 10.Woods DW, Himle MB. Creating Tic Suppression: Comparing the Effects of Verbal Instruction to Differential Reinforcement. J Appl Behav Anal 2004;37:417–420. doi: 10.1901/jaba.2004.37-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himle MB, Woods DW. An experimental evaluation of tic suppression and the tic rebound effect. Behav Res Ther 2005;43(11):1443–1451. doi: 10.1016/J.BRAT.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 12.Himle MB, Woods DW, Conelea CA, Bauer CC, Rice KA. Investigating the effects of tic suppression on premonitory urge ratings in children and adolescents with Tourette’s syndrome. Behav Res Ther 2007;45(12):2964–2976. [DOI] [PubMed] [Google Scholar]
- 13.Conelea CA, Woods DW. The influence of contextual factors on tic expression in Tourette’s syndrome: A review. J Psychosom Res 2008;65:487–496. doi: 10.1016/j.jpsychores.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 14.Specht MW, Woods DW, Nicotra CM, et al. Effects of tic suppression: ability to suppress, rebound, negative reinforcement, and habituation to the premonitory urge. Behav Res Ther 2013;51(1):24–30. doi: 10.1016/j.brat.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Conelea CA, Wellen B, Woods DW, et al. Patterns and Predictors of Tic Suppressibility in Youth With Tic Disorders. Front psychiatry 2018;9:188. doi: 10.3389/fpsyt.2018.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods DW, Himle MB, Miltenberger RG, et al. Durability, Negative Impact, and Neuropsychological Predictors of Tic Suppression in Children with Chronic Tic Disorder. J Abnorm Child Psychol 2008;36(2):237–245. doi: 10.1007/s10802-007-9173-9 [DOI] [PubMed] [Google Scholar]
- 17.Greene DJ, Koller JM, Robichaux-Viehoever A, Bihun EC, Schlaggar BL, Black KJ. Reward enhances tic suppression in children within months of tic disorder onset. Dev Cogn Neurosci 2015;11:65–74. doi: 10.1016/j.dcn.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piacentini J, Woods DW, Scahill L, et al. Behavior Therapy for Children With Tourette Disorder. JAMA 2010;303(19):1929. doi: 10.1001/jama.2010.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Greene DJ, Bihun EC, et al. Provisional Tic Disorder is not so transient. Sci Rep 2019;9(1):3951. doi: 10.1038/s41598-019-40133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28(4):566–573. [DOI] [PubMed] [Google Scholar]
- 21.Robertson MM, Banerjee S, Kurlan RM, et al. The Tourette syndrome diagnostic confidence index. Neurology 1999;53:2108–2112. doi: 10.1212/WNL.53.9.2108 [DOI] [PubMed] [Google Scholar]
- 22.Woods DW, Piacentini J, Himle MB, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J Dev Behav Pediatr 2005;26(6):397–403. [DOI] [PubMed] [Google Scholar]
- 23.Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:257–268. doi: 10.1023/A:1022602400621 [DOI] [PubMed] [Google Scholar]
- 24.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord 2003;33:427–433. doi: 10.1023/A:1025014929212 [DOI] [PubMed] [Google Scholar]
- 25.Black J, Koller J, F1000Research KB-, 2017 U. TicTimer software for measuring tic suppression. F1000Research 2017;6:1560. doi: 10.12688/f1000research.12327.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomoto F, Machiyama Y. An Epidemiological Study of Tics. Psychiatry Clin Neurosci 1990;44(4):649–655. doi: 10.1111/j.1440-1819.1990.tb01641.x [DOI] [PubMed] [Google Scholar]
- 27.Mink JW. Neurobiology of basal ganglia circuits in Tourette syndrome: faulty inhibition of unwanted motor patterns? Adv Neurol 2001;85:113–122. http://www.ncbi.nlm.nih.gov/pubmed/11530421. Accessed March 4, 2019. [PubMed] [Google Scholar]
- 28.Peterson BS, Skudlarski P, Anderson AW, et al. A Functional Magnetic Resonance Imaging Study of Tic Suppression in Tourette Syndrome. Arch Gen Psychiatry 1998;55(4):326–333. doi: 10.1001/archpsyc.55.4.326 [DOI] [PubMed] [Google Scholar]
- 29.Hong HJ, Sohn H, Cha M, et al. Increased frontomotor oscillations during tic suppression in children with Tourette syndrome. J Child Neurol 2013;28(5):615–624. [DOI] [PubMed] [Google Scholar]
- 30.Botteron HE, Richards CA, Nishino T, et al. The urge to blink in Tourette syndrome. bioRxiv February 2019:477372. doi: 10.1101/477372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morand-Beaulieu S, Grot S, Lavoie J, Leclerc JB, Luck D, Lavoie ME. The puzzling question of inhibitory control in Tourette syndrome: A meta-analysis. Neurosci Biobehav Rev 2017;80:240–262. doi: 10.1016/J.NEUBIOREV.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 32.Kalsi N, Tambelli R, Aceto P, Lai C. Are Motor Skills and Motor Inhibitions Impaired in Tourette Syndrome? A Review. J Exp Neurosci 2015;9:JEN.S25095. doi: 10.4137/JEN.S25095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luna B Developmental Changes in Cognitive Control through Adolescence. Adv Child Dev Behav 2009;37:233–278. doi: 10.1016/S0065-2407(09)03706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houghton DC, Capriotti MR, Conelea CA, Woods DW. Sensory Phenomena in Tourette Syndrome: Their Role in Symptom Formation and Treatment. Curr Dev Disord Reports 2014;1(4):245–251. doi: 10.1007/s40474-014-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandt VC, Beck C, Sajin V, et al. Temporal relationship between premonitory urges and tics in Gilles de la Tourette syndrome. Cortex 2016;77:24–37. doi: 10.1016/J.CORTEX.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 36.Banaschewski T, Woerner W, Rothenberger A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol 2003;45(10):700–703. doi: 10.1017/S0012162203001294 [DOI] [PubMed] [Google Scholar]
- 37.Steinberg T, Shmuel Baruch S, Harush A, et al. Tic disorders and the premonitory urge. J Neural Transm 2010;117:277–284. doi: 10.1007/s00702-009-0353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goetz CG, Tanner CM, Wilson RS, Shannon KM. A rating scale for Gilles de la Tourette’s syndrome: description, reliability, and validity data. Neurology 1987;37(9):1542–1544. doi: 10.1212/WNL.37.9.1542 [DOI] [PubMed] [Google Scholar]
- 39.Goetz CG, Leurgans S, Chmura TA. Home alone: Methods to maximize tic expression for objective videotape assessments in Gilles de la Tourette syndrome. Mov Disord 2001;16:693–697. doi: 10.1002/mds.1159 [DOI] [PubMed] [Google Scholar]
- 40.Cavanna AE, David K, Orth M, Robertson MM. Predictors during childhood of future health-related quality of life in adults with Gilles de la Tourette syndrome. Eur J Paediatr Neurol 2012. doi: 10.1016/j.ejpn.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 41.Bloch MH, Leckman JF, Zhu H, et al. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology 2005;65(8):1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med 2006. doi: 10.1001/archpedi.160.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groth C, Mol Debes N, Rask CU, Lange T, Skov L. Course of Tourette Syndrome and Comorbidities in a Large Prospective Clinical Study. J Am Acad Child Adolesc Psychiatry 2017;56:304–312. doi: 10.1016/j.jaac.2017.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


