TO THE EDITOR
Mitochondrial diseases (MD) are the most common inborn errors of metabolism with a minimum prevalence of 1 in 5,0001. Mutations in nDNA and mtDNA genes involved in mitochondrial function lead to disorders of oxidative phosphorylation and mitochondrial maintenance. The phenotype of MD is multisystemic, involving organs systems with large energy requirements (e.g. central nervous system). Recently, it has been demonstrated that mitochondria play a critical role in immunity, and the phenotype of immune dysfunction in patients with MD is becoming evident2, raising concerns regarding the risk of infection. Infection is a major cause of morbidity in patients with MD, precipitating acidosis and organ dysfunction/failure with a rapidly fatal course3, 4. More than 80% of patients with MD may experience recurrent or severe infections5, and sepsis (55%) and pneumonia (29%) are the two most common causes of death6. Therefore, assessing and managing risk factors for infection in patients with MD is critical to maintaining their health and wellbeing.
Adherence to the childhood immunization schedule is recommended for patients with MD to prevent the sequelae of infection. Unfortunately, outbreaks of vaccine preventable diseases are on the rise due to nonadherence in the general population. Since infection can be catastrophic for patients with MD, the current study was undertaken to assess the serum antibody titers of vaccine preventable diseases in a cohort of pediatric patients with MD. Patients were enrolled in a natural history study of infection and immune function in patients with MD (NCT01780168).
Pediatric patients (N = 26) from the natural history study cohort with “probable” or “definite” MD by revised Walker criteria7 were selected for study (Table E1): 18 males and 8 females with an average age of 10.8 ± 3.6 years old. All patients had multisystem disease and the mitochondrial phenotypes were identified as Kearns Sayre Syndrome (N = 3), Leigh Syndrome (N = 5), and MD, not otherwise specified (N = 18). Molecular testing data (whole exome, mitochondrial sequencing) was available for most patients (N = 24), and revealed pathogenic and suspect variants in genes involved in mitochondrial function. In addition, most patients (N =25) had supporting pathologic, enzymatic and radiologic evidence of MD. A medical record review showed 73% of our pediatric cohort were identified with immune abnormalities, most of which included recurrent or delayed recovery from infection (58%) and hypogammaglobulinemia (19%).
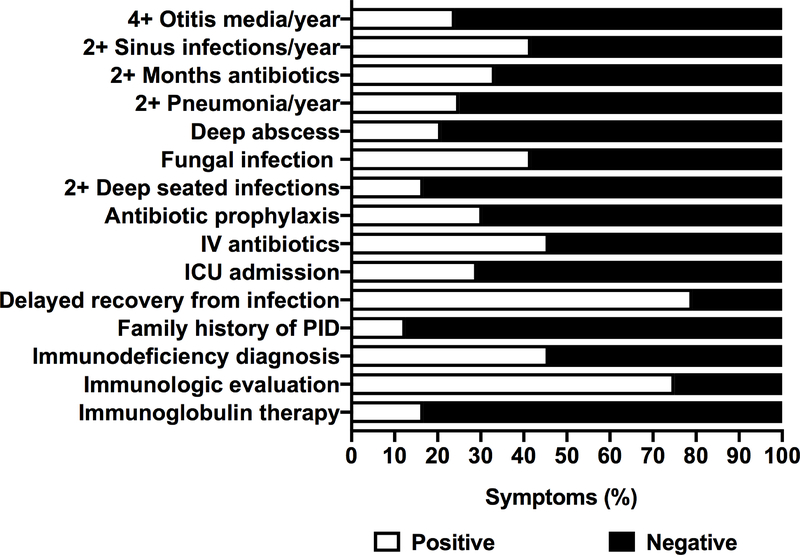
To capture a history of infection and immune dysfunction in our cohort, a directed questionnaire was administered to parents or caregivers of affected patients to formally assess for a history of: 1) recurrent infection and 2) evaluation and treatment for immune deficiency (N = 25) (Figure 1, Table E2). Regarding a history of infection, the most frequent complaints were 2+ sinus infections/year (42%) and recurrent fungal infection (42%). Fourty-six percent of patients received intravenous antibiotics and 29% were admitted to the ICU for infection. The most common symptom reported by our cohort was delayed recovery from infection (79%). Remarkably, we found that patients oftentimes had been evaluated for or had a diagnosis of immune deficiency (75% and 46% respectively). Concerning ongoing treatment, immunoglobulin therapy (17%) was prescribed due to recurrent infections or documented immune dysfunction.
Figure 1: Immune symptoms in patients with MD.
Table of immune symptoms derived from primary immunodeficiency questionnaire and additional questions. See Table E2 for the question list.
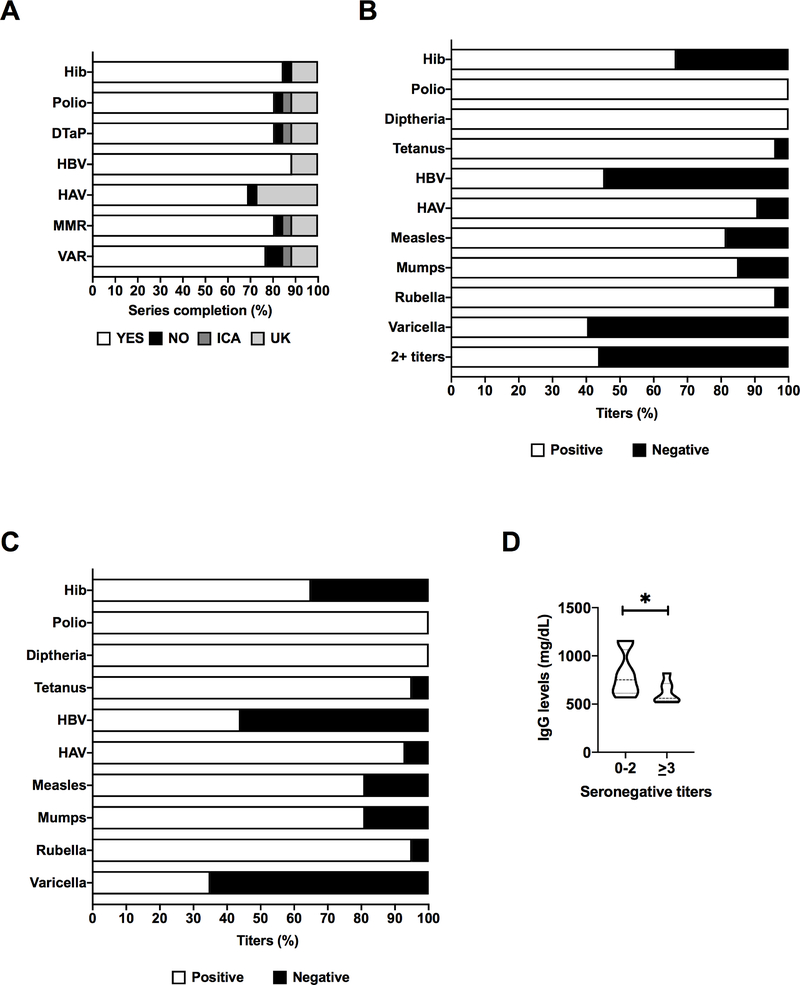
Adherence to childhood vaccination recommendations was assessed from vaccine records provided by the caretakers (Figure 2A). All patients in the cohort were eligible for the current recommended vaccination schedule. Adherence to the recommended schedule ≥80% for most of the vaccines studied with the exception of hepatitis A (HAV) and varicella (VAR). For HAV, 27% of the patients surveyed were “unknown” (UK), while 4% were non-adherent (“NO”). These data likely reflect issues surrounding the initial implementation of HAV. For VAR, 77% of patients were adherent with the recommended schedule, while 8% were non-adherent (“NO”), 4% were “incomplete for age” (ICA), and 12% were “unknown” (UK).
Figure 2: Vaccination status in patients with MD.
A) Adherence to vaccination recommendations; B) Vaccine titers; C) Subgroup analysis of patients who were adherent to vaccination schedule; D) Serum IgG levels and titer status in vaccinated individuals. 0–2 titers (N = 12), ≥3 titers (N = 7), Student’s t-test *P < 0.05.
Serum titer data are presented in Figure 2B. All serum titers were performed by the clinical laboratory of the NIH Clinical Center, or Mayo Medical Laboratories. Less than 15% of patients were seronegative for polio, diphtheria, tetanus, HAV, mumps, and rubella. Up to 33% of the patients were seronegative for Haemophilus influenza b (Hib). Although all of our patients were outside the window of risk for meningitis due to Hib infection (<5 years of age), this pathogen is also associated with pneumonia and sepsis, common causes of death in children with MD6. These infections combined with a recent rise in antibiotic resistance of Hib8, presents a risk for metabolic decompensation to children with MD. Fifty five percent of the patients were seronegative for hepatitis B (HepB). Studies of HepB immunity have shown that the antibody titers decline with age, and booster studies are necessary to determine protection status9. Approximately 20% of patients with MD were seronegative for measles, while 60% were seronegative for varicella, microbial pathogens experiencing recent outbreaks. As per the performing laboratory (Mayo Medical Laboratories), sensitivity and specificity of the assay for varicella titers was reported to be 92.2% and 100%, respectively. Interestingly, Patient #19 in cohort (Table E1) experienced 2 separate episodes of varicella infection despite being vaccinated. Considering all titers, 56% of our cohort were seronegative for 2+ vaccine preventable diseases. We next stratified our analysis of serum titers to include individuals who were adherent to the recommended schedule for that vaccine (Figure 2C). Overall, the data remained fundamentally the same: Hib (35% seronegative), HepB (56% seronegative), measles (≈20% seronegative) and varicella (65% seronegative).
Based on previous work from our group5 and our results herein, approximately 17–20% of patients with MD have received immunoglobulin therapy at some point in their medical history (Figure 1). Due to the pooled nature of immunoglobulin preparations, all 3 of our patients on IVIG had positive titers to all of the vaccines surveyed, as expected. Given the protection presumably offered by IVIG, we next asked whether low serum IgG levels were related to titer status. After excluding patients on IVIG, we found that patients who were vaccinated and seronegative for three or more titers tended to have lower serum IgG levels (P < 0.05, Figure 2D).
Overall, our study raises an important clinical issue regarding infection risk in patients with MD. Although the underlying mechanisms are not yet known, the clinical significance of these findings are of major relevance for the care of patients with MD for whom infections are associated with high degree of morbidity and mortality. Monitoring of serum titers in patients with MD may be indicated, especially in areas with increased risk of exposure, and requires further evaluation. Furthermore, booster vaccinations should be considered in children who are seronegative despite having received a complete series. For those patients with MD who are unable to vaccinated, or do not respond to booster vaccination, information regarding their immune status is important for mitigating infection risk. Although we did observe an association between IVIG use, serum IgG levels and titer status, we are not at the point of issuing treatment recommendations. Although some patients with MD do currently receive IVIG, more extensive investigation is needed to evaluate the utility and risk profile of this therapy.
While our study is limited by the cross-sectional design, it nonetheless conveys an important message regarding the vulnerability of patients with MD to vaccine preventable diseases. To better understand this risk, we would propose: 1) a prospective analysis of vaccination to define whether patients fail to develop or lose titers, and 2) an analysis of the efficacy of re-vaccination. While awaiting the results of these proposed studies, we would encourage clinicians to be attentive to the risk of infection in patients with MD. While there are many factors contribute to the risk of infection in patients with MD, the risk of vaccine preventable diseases is potentially modifiable via vaccination, screening, revaccination, and knowledge of immune status.
Supplementary Material
Clinical Implications.
Patients with mitochondrial disease experience significant morbidity and mortality due to infection. The rise in outbreaks of vaccine preventable diseases places this vulnerable population at risk, necessitating an understanding of their protective titer status to these infectious diseases.
Acknowledgments
Funding: This study was supported by the Intramural Research Program of the National Institutes of Health.
Conflicts of interest: M.K. Koenig has received research funding from People Against Leigh Syndrome, Reata Pharmaceuticals Corporation, Ultragenyx Pharmaceuticals Corporation, Novartis Pharmaceuticals Corporation, EryDel S.P.A., and the Memorial Hermann Foundation; serves on the scientific advisory board for the Tuberous Sclerosis Alliance and editorial board for Journal of Child Neurology; and consults for Novartis Pharmaceuticals Corporation and Lundbeck Pharmaceuticals Services LLC. The rest of the authors declare that they have no relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ng YS, Turnbull DM. Mitochondrial disease: genetics and management. J Neurol 2016; 263:179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapnick SM, Pacheco SE, McGuire PJ. The emerging role of immune dysfunction in mitochondrial diseases as a paradigm for understanding immunometabolism. Metabolism 2018; 81:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naviaux RK, Nyhan WL, Barshop BA, Poulton J, Markusic D, Karpinski NC, et al. Mitochondrial DNA polymerase gamma deficiency and mtDNA depletion in a child with Alpers’ syndrome. Ann Neurol 1999; 45:54–8. [DOI] [PubMed] [Google Scholar]
- 4.Edmonds JL, Kirse DJ, Kearns D, Deutsch R, Spruijt L, Naviaux RK. The otolaryngological manifestations of mitochondrial disease and the risk of neurodegeneration with infection. Arch Otolaryngol Head Neck Surg 2002; 128:355–62. [DOI] [PubMed] [Google Scholar]
- 5.Tarasenko TN, Pacheco SE, Koenig MK, Gomez-Rodriguez J, Kapnick SM, Diaz F, et al. Cytochrome c Oxidase Activity Is a Metabolic Checkpoint that Regulates Cell Fate Decisions During T Cell Activation and Differentiation. Cell Metab 2017; 25:1254–68 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eom S, Lee HN, Lee S, Kang HC, Lee JS, Kim HD, et al. Cause of Death in Children With Mitochondrial Diseases. Pediatr Neurol 2017; 66:82–8. [DOI] [PubMed] [Google Scholar]
- 7.Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology 2002; 59:1406–11. [DOI] [PubMed] [Google Scholar]
- 8.Rybak A, Levy C, Bonacorsi S, Bechet S, Vie le Sage F, Elbez A, et al. Antibiotic Resistance of Potential Otopathogens Isolated From Nasopharyngeal Flora of Children With Acute Otitis Media Before, During and After Pneumococcal Conjugate Vaccines Implementation. Pediatr Infect Dis J 2018; 37:e72–e8. [DOI] [PubMed] [Google Scholar]
- 9.Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Mmwr Recommendations and Reports 2018; 67:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.