Abstract
Purpose of the review:
This review article discusses recent advances in the mechanism of dipeptidyl peptidase-4 (DPP-4) actions in renal diseases, especially diabetic kidney fibrosis, and summarizes anti-fibrotic functions of various DPP-4 inhibitors in diabetic nephropathy (DN).
Recent findings:
DN is a common complication of diabetes and is a leading cause of the end-stage renal disease (ESRD). DPP-4 is a member of serine proteases, and more than 30 substrates have identified that act via several biochemical messengers in a variety of tissues including kidney. Intriguingly, DPP-4 actions on the diabetic kidney is a complex mechanism, and a variety of pathways are involved including increasing GLP-1/SDF-1, disrupting AGE-RAGE pathways, and integrin-β- and TGF-β-Smad-mediated signalling pathways that finally lead to endothelial to mesenchymal transition. Interestingly, an array of DPP-4 inhibitors is well recognized as oral drugs to treat type 2 diabetic (T2D) patients, which promote better glycemic control. Furthermore, recent experimental and preclinical data reveal that DPP-4 inhibitors may also exhibit protective effects in renal disease progression including anti-fibrotic effects in the diabetic kidney by attenuating above signalling cascade(s), either singly or as a combinatorial effect. In this review, we discussed the anti-fibrotic effects of DPP-4 inhibitors based on recent reports along with the possible mechanism of actions and future perspectives to underscore the beneficial effects of DPP-4 inhibitors in DN.
Summary:
With recent experimental, preclinical, and clinical evidence, we summarized DPP-4 activities and its mechanism of actions in diabetic kidney diseases. A knowledge gap of DPP-4 inhibition in controlling renal fibrosis in DN has also been postulated in this review for future research perspectives.
Keywords: Diabetic nephropathy, DPP-4, DPP-4 inhibitors, GLP-1, Integrin-β, EndMT, TGF-β, microRNA, microbiota
1. Background:
Diabetes mellitus (DM) has become a global concern due to associated health and economic burden. According to The International Diabetes Federation, approximately 451 million patients were affected by diabetes and diabetes-associated diseases in 2017 and is expected to increase to 693 million by 2045 [1]. Diabetic nephropathy (DN) is one of the major devastating complications of both type 1 (T1D) and type 2 diabetic (T2D) patients [2]. Almost 50% of patients suffering from T2D have chronic kidney diseases that include glomerulosclerosis and tubulointerstitial fibrosis [3, 4].
Fibrosis is initially a tissue repair process, which becomes a common complication during all progressive kidney diseases. Fibrosis develops as a consequence of disrupted normal wound-healing mechanism [5]. The mechanism(s) of kidney fibrosis is a multifactorial and complex process as several cell types such as kidney fibroblasts, tubular epithelial cells, mesangial cells, podocytes, pericytes, vascular smooth muscle cells, and endothelial cells are involved in producing excess extracellular matrix [6, 7].
Dipeptidyl peptidase (DPP) is a member of the serine peptidase that is classified under EC 3.4.14. [8]. There are 9 types of DPPs, DPP-1, −2, and DPP-3, −4, and −6 to −10. DPP-1, also known as Cathepsin C, is a lysosomal exo-cysteine protease belonging to the peptidase C1 family that acts as a central coordinator for the activation of many serine proteases in immune/inflammatory cells [9]. DPP II is suggested to have a role in cellular differentiation and degradation of extracellular matrix (ECM) protein, including collagen [10]. DPP-3 is a cytoplasmic zinc-binding metallopeptidase, and its activity is associated with protein metabolism, blood pressure regulation and pain modulation [11]. DPP-4 cleaves a wide range of substrates, including growth factors, chemokines, and peptides in addition to its major role in glucose metabolism [12]. DPP-6 is a membrane protein that binds to specific voltage-gated potassium channels and maintains different biophysical properties of the cell [13, 14]. DPP-7 is reported to inhibit coagulation and thus may trigger haemorrhage and immune invasion [15]. DPP-8 plays an essential role in T cell activation and in the immune system [16], whereas DPP-9 represses inflammasome and protects against auto-inflammatory diseases [17]. DPP-10 is a membrane protein with no detected protease activity. However, it is reported that it binds to voltage-gated potassium channels in the nervous system, regulates their expression and electrophysiological properties [18].
DPP-4 inhibitors represent a relatively new class of glucose-lowering drugs that may also have renoprotective properties independent of blood pressure- and glucose-lowering effects [19]. Several studies have reported that renoprotective effects of DPP-4 inhibitors are mediated partly by an increased half-life of its substrates, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide or gastric inhibitory peptide (GIP) [19–21]. The underlying mechanisms of renoprotection are, however, far from clear. The purpose of this review is to highlight the reported mechanisms of DPP-4 actions in diabetes-related kidney disease progression. Additionally, in light of the current literature, we mainly focus on the pro-fibrotic effects of DPP-4 in the kidney by analysing several recent experimental, preclinical, and clinical findings on anti-fibrotic reno-protection by different DPP-4 inhibitors and their mechanisms of action.
2. Structure and biological function of DPP-4:
When discovered more than 50 years ago, exopeptidase DPP-4 was characterized as a T-cell surface marker (CD26) containing 110 kDa type 2 membrane protein which is anchored to lipid bilayer by a single hydrophobic segment [22]. Human DPP-4 is 766 amino acids (aa) long containing a short cytoplasmic domain of 1–6 aa, a transmembrane domain of 7–28 aa, and an extracellular domain of 29–766 aa with dipeptidyl peptidase activity, connected by a flexible stalk of 29–39 aa. The removal of the transmembrane or intracellular domain leads to the formation of 727aa long soluble DPP-4 (sDPP-4) [23], which can be detected in peripheral blood, urine, and other body fluids including thoracic and seminal fluid [23, 24]. Lamers et al. observed that insulin and TNF-α increased the release of sDPP-4 [25], and sDPP-4 can form tetramers with two soluble and two trans-membrane DPP-4 protein to enhance cell-cell communication [26]. Generally, the sDPP-4 level is higher in obese and in T2D patients [27, 28], and there are different ligand binding sites within the extracellular domain of sDPP-4. For example, caveolin I, anti-CD-26 antibodies, and adenosine deaminase (ADA) bind to the glycosylation-rich domain (101–350 aa); while matrix protein collagens and fibronectin can bind to a cysteine-rich region (55–100 aa and 351–497 aa) [8, 23].
DPP-4 circulates through the gut, liver, lungs, as well as through the kidney [29, 30]. Interestingly, DPP-4 exhibits several ‘pleiotropic effects’ by enzymatic as well as non-enzymatic pathways upon binding with extracellular matrix protein [31]. Enzymatically, DPP-4 has a high selectivity for peptides with a proline or alanine at the second position and cleaves off dipeptides at the NH2- terminus of such peptides (NH2- Xaa-Pro). It has the highest preference for proline with gradually weaker preferences for alanine and then for glycine [8, 32]. In a diverse biological processes, complex interaction of DPP-4 with several proteins including CD45 tyrosine phosphatase, sodium-hydrogen exchanger 3 (NHE3), C-X-C chemokine receptor 4 (CXCR4), caveolin I, fibronectin, collagen, insulin-like growth factor II (IGF II) or mannose-6-phosphatase have been reported [23, 33, 34]. DPP-4 also cleaves glucagon-like peptide-1 (GLP-1) and a glucose-dependent insulinotropic peptide that finally affects β-cells of the pancreas and leads to decreased insulin secretion; thus DPP-4 inhibitors prevent GLP-1 breakdown and restore normal insulin secretion [35].
In 2006, DPP-4 inhibitor was used for the first time as a diabetic drug, and since then, different types of DPP- 4 inhibitors have been approved to treat hyperglycemia [36]. DPP-4 inhibitors lower blood sugar by inhibiting the degradation of glucagon-like peptide-1 and −2 (GLP-1 and −2), and activating glucose-dependant insulinotropic peptide (GIP) and its function on pancreatic β cells to produce more insulin [27, 37–39]. DPP-4 inhibitors have also been shown to reduce chronic or acute kidney injury in several mammalian experimental models [40–44]. Recently, DPP-4 and its association with matrix biology are being elucidated in renal fibrotic diseases with the functional non-enzymatic activity of this protein [31].
3. DPP-4 and diabetes:
In T2D patients, insulin resistance leads to higher blood glucose levels and a higher level of activated incretins may reverse the increased glucose level in blood. Incretins are a group of metabolic hormones, which decrease blood glucose levels by inducing insulin hormone production from pancreatic β cells and inhibiting glucagon secretion. Glucagon-like peptide-1 and −2 (GLP-1 and −2), and glucose-dependent insulinotropic peptide (GIP) are examples of incretins [45]. By 1990s, DPP-4 and its association with diabetes came into the light where it was observed that DPP-4 is involved in initial degradation of incretins and thus the proposition to block DPP-4 activity to restore incretin functions become evident [46]. Deacon et al. observed that DPP-4 was a major, if not the only, route for the regulation of GLP-1as DPP-4 inhibitor valine-pyrrolidine treatment could not completely but partially prevented degradation of exogenous GLP-1 in pig [47]. The same group of scientists also noted a similar finding for GIP [48]. Administration of another DPP-4 inhibitor, ile-thiazolidide increased circulating incretins as well as insulin secretion in rats [49]. These studies together establish some preclinical bases of the hypothesis that inhibiting DPP-4 activity leads to increased circulating GLP-1 that can restore insulin secretion and regulate blood glucose levels in T2D patients. After several years of the initial hypothesis was launched [47], a 4-weeks clinical trial was conducted, and the results of this short-term administration of DPP-4 inhibitor was published for the first time in 2002 [50]. Subsequently, a clinical trial spanning one year was performed, and the anti-diabetic effects of sustained DPP-4 inhibitor vildagliptin were suggested [51].
Those early results were soon followed by numerous experimental as well as clinical studies. In 2006, DPP-4 inhibitor sitagliptin was approved as a drug to control blood glucose level in T2D patients. Nowadays, DPP-4 inhibitors are well-recognized medicines that are used to reduce hyperglycemia in T2D patients, and two types of DPP-4 inhibitors are used clinically worldwide. There are DPP-4 structure mimetic and non-peptidomimetics. In 2006, the FDA approved the very first type of DPP-4 inhibitors, which are structural mimetics. Sitagliptin, vildagliptin, and saxagliptin are examples of this first type of inhibitors. FDA approved first non-peptidomimetic inhibitors in 2011. Alogliptin and linagliptin are examples of the second type of DPP-4 inhibitors, non-peptidomimetics. Almost all clinical studies have shown reduced glycated haemoglobin % (HbA1c%) and fasting blood glucose (FBG) level in T2D patients with different types of DPP-4 inhibitors administration such as sitagliptin [52, 53], vildagliptin [54, 55], linagliptin [56], saxagliptin [57, 58].
4. DPP-4 and the kidney:
DPP-4 is present in a variety of tissues and organs [23, 33], but the highest amount of activity of this enzyme per gram tissue was found in the rat kidney [59, 60]. In rats, DPP-4 is abundantly expressed in glomerular podocytes, S1-S3 segment of the proximal tubule and descending limb of Henle’s loop [61, 62]; however, the functional role of this protein in non-proximal tubular regions is yet to be determined. In humans, DPP-4 expression, as well as activity, was found in glomerular podocytes of the diseased kidney [63–65]. In healthy human kidneys, DPP-4 was also detected with nominal expression at the luminal side of the brush border membrane of proximal tubular cells [66, 67]. In addition, the expression of DPP-4 was found to be increased in humans and rodents glomerular epithelial cells in vitro when cultured with interferon-γ, an inflammatory cytokine [68, 69]. Exposure of exogenous glucose at the high dose in cultured human glomerular endothelial cells in vitro showed induction of DPP-4 mRNA and enzymatic activity [70]. Additionally, urinary DPP-4 activity was increased in patients of T2D with albuminuria [71]. Thus, some researchers have interpreted high DPP-4 activity in the kidney or urine as a good marker of human glomerular disease [71–73].
Girardi et al. found that transmembrane DPP-4 in rat renal proximal tubules of the kidney can form a complex with sodium–hydrogen exchanger 3 (NHE3) that can modulate Na+/H+ exchange, and DPP-4 inhibition increases urine output [74]. In high a fat diet-induced diabetic rat model, the expression of DPP-4 was upregulated in the kidney [75], and using Zucker obese rat animal model Nistala et al. reported that DPP-4 inhibition induced megalin receptor protein which is important for tubular endocytosis, and in turn, increased uptake of albumin and other lower-mass proteins in kidney proximal tubule [43]. DPP-4 expression in the rodent kidney was also upregulated in hypoxic conditions [41, 76], and DPP-4 deficiency lead to reno-protection in acute ischemia-reperfusion injury in the rat kidney suggesting the role of this enzyme in ischemia-perfusion renal injury [77, 78]. Moreover, in CKD patients, a high serum level of soluble DPP-4 exhibited lower estimated glomerular filtration rate (eGFR) [79]. Notably, DPP-4 has two forms, one is 766 amino acid (aa) long type II transmembrane protein, and another is a slightly smaller soluble form of DPP-4 (sDPP-4) (727 aa) lacking membrane-bound portion [33]. The sDPP-4 is found in the blood as well as almost all organs [23, 33]. High insulin level induces shedding of sDPP-4, but there may be other different factors responsible for shedding, which is still a matter of conjecture [25]. Surprisingly, although DPP-4 was discovered more than 50 years ago, the precise source of sDPP-4 and differential functions of transmembrane DPP-4 and sDPP-4 has yet to be fully delineated [23, 27].
These above experimental and clinical findings suggest that upregulated DPP-4 is an important modulator of kidney malfunction, and inhibition of DPP-4 is a possible renoprotective target in diabetic and other CKD conditions. Nevertheless, further investigations are warranted to delineate a clear relationship of the degree of DPP-4 inhibition and extent of renoprotection in order for us to precisely using DPP-4 inhibitor drugs to obtain desired outcomes in diabetic CKD.
5. DPP-4 inhibition and reno-protection in diabetes:
5.1. Incretin mediated pathways:
Incretins are a group of peptide hormones that are secreted from the gut after nutrient intake. These peptises decrease blood glucose levels by augmenting insulin secretion from pancreatic beta cells. Two incretins that are recognized as blood glucose-lowering peptides are glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP). GLP-1 is released by a post-translational proteolytic cleavage of proglucagon that also releases GLP-2, although GLP-2 it is not an incretin. Below, we have mainly focused our review on DPP-4-dependent GLP-1 regulation and its effect on diabetic kidney disease.
DPP-4 degrade incretins such as GLP-1; thus, DPP-4 inhibition restores GLP-1 signalling pathways [19, 80]. GLP-1 and its receptor-mediated signalling in the kidney are important for renal-homeostasis [80], and GLP-1 receptor stimulation can regulate atrial natriuretic peptide (ANP) and the renin-angiotensin system (RAS), two probable pathways of GLP-1 mediated reno-protection [81]. Estimated glomerular filtration rate (eGFR), sodium excretion, as well as the urinary flow, has been shown to increase by chronic infusion of GLP-1 in Dahl-salt sensitive rats [82]. GLP-1 signalling is much more complex than only DPP-4 regulation depending on type neurotransmitters. Moreno et al. reported that GLP-1 mediated increased eGFR was not observed in denervated rats [83]. GLP-1 mediated vasodilation caused more eGFR with increased blood flow in glomerular capillaries even in healthy rodents, but these effects were minimal in humans even after pharmacological GLP-1 administration. In human GLP-1 administration increased reabsorption and hydrostatic pressure in the proximal tubule, leading to reduced GFR due to decreased glomerular hydrostatic pressure compared to proximal tubule [84]. GLP-1 receptor activation attenuated Na+/H+ exchanger isoform 3 (NH3) expression in pig LLC-PK1 cells through a protein kinase A (PKA) dependent pathway and thus can be useful to reverse natriuresis condition [85]. GLP-1 has also been shown to significantly decrease inflammation as well as reactive oxygen species (ROS) generation in the diseased mouse kidney in vivo and mesangial cells in vitro [86, 87]. Exogenous GLP-1 inhibits cell damage by blocking angiotensin II-induced superoxide formation, increased levels of intercellular adhesion molecule - 1 (ICAM-1), plasminogen activator inhibitors, attenuating NF-kβ by PKA pathway in cultured human mesangial cells [87]. These above-mentioned reports suggest that GLP-1 is an important renoprotective molecule to preserve normal GFR, and DPP-4 inhibition may have direct ameliorative effects in diabetic kidney diseases.
5.2. Non-incretin mediated pathways:
DPP-4 can cleave many peptide substrates other than GLP-1, and DPP-4 inhibition may affect diabetic as well as non-diabetic CKD in different pathways [88, 89]. In a non-diabetic 5/6 nephrectomy rat model treated with DPP-4 inhibitor linagliptin renal fibrosis markers collagen type III, TGF β1, and phosphor smad2 to total smad ratio have been decreased significantly and restored kidney function [90]. Linagliptin also showed anti-fibrotic and renoprotective effects in non-diabetic CKD by upregulating collagenase activity, thymosin β4 and nuclease-sensitive element-binding protein 1 (YB1) in GLP-1 knockout mouse, proving renoprotection without involving GLP1/GLP1R-mediated pathways [91].
DPP-4 targets stromal cell-derived factor-1α (SDF-1α), peptide YY (PYY), neuropeptide Y (NPY), and a family member of natriuretic peptides (NPs) [88, 92]. The peptides, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are the key regulators of kidney and other organ function in causing a reduction in expanded extracellular fluid (ECF) volume by increasing renal sodium excretion. While DPP-4 knockout mice exhibited increased expression of ANP [93], it is unknown to our knowledge whether this peptide is also a DPP-4 substrate. Notably, ANP is a 28-amino acid peptide that is synthesized and released by atrial myocytes in response to various stimuli such as atrial distension, angiotensin II, endothelin, pulmonary hypertension, myocardial infarction, alpha- and beta-adrenergic stimulation, a few to name [94]. Whereas, BNP is a 32-amino acid peptide that is synthesized mostly by the ventricular myocytes as well as in the brain [95]. DPP-4 cleaves N-terminal serine-proline dipeptide from BNP1–32 in the blood to produce faster degradable BNP3–32 and BNP5–32 [95, 96]. The mechanism of release and physiological actions of BNP is similar to that of ANP. The only species difference between these two peptides in relation to DPP-4 is that while BNP can be regulated by DPP-4, ANP is not, at least to our knowledge.
Contrary to ANP or BNP, NPY and PYY promote vasoconstriction. SDF-1α has a role in angiogenesis in the kidney and protect injured kidney tissues from ischemia [97]. Thus, being a modulator of those peptides, DPP-4 may participate in the modulation of blood pressure, natriuresis, angiogenesis, and tissue repair in the kidney [89]. NPY is an agonist of the Y1 receptor, which maintains peripheral vasoconstriction and gliptins, or DPP-4 inhibitors actively inhibit NPY cleavage and contribute to increase arterial blood pressure via Y1 receptor provided that sympathetic nervous system is functional [98]. In spontaneously hypertensive rats, DPP-4 checks angiotensin II-mediated renal vasoconstriction [99], but this finding was not observed in wild-type rats [100].
DPP-4 is also essential for TGF-β receptor hetero-dimerization and signalling. Shi et al. have reported that TGF-β2 induced TGF-βR1/2 heterodimer formation, which was attenuated by DPP-4 siRNA in transfected human dermal microvascular endothelial cells (HMVECs) [101]. In unilateral ureteral obstruction (UUO) mouse model, a DPP-4 inhibitor LC15–0444 reduced expression of TGF-β1, toll-like receptor-4 (TLR-4), high mobility group protein 1 (HMBG1), NADPH oxidase 4, NFᶄ-β, and phosphorylated Smad-2/3, which are all fibrotic and inflammatory factors [102]. These recent data suggest activation of DPP-4 in the kidney has its role in renal inflammation and fibrosis. Therefore, inhibition of DPP-4 may offer beneficial effects to manage progressive renal diseases such as inflammation and fibrosis.
5.3. AGE-RAGE and glucose-independent pathways:
Diabetes is associated with advanced glycation end-products (AGEs) that are proteins or lipids, glycated by exposure to high levels of glucose or other saccharides. AGEs crosslink with ECM proteins and may cause chronic kidney disease in diabetic patients [103]. Activation of receptors of AGEs (RAGE) leads to oxidative stress as well as inflammation in CKD with or without diabetes [104]. Soluble DPP-4 (sDPP-4) increases oxidative stress and induce RAGEs via binding of the cation-independent mannose-6-phosphate receptor (CIM6PR) in T2D [73], and this stress can be reversed by DPP-4 inhibitor linagliptin [105]. This interaction is described schematically in fig 1. Linagliptin also blocks AGE-RAGE signalling pathways, and therefore reduces oxidative stress in the T1D kidney of a murine model [106]. Thus, a probable network of AGE-RAGE signalling along with the DPP-4/incretin system is highly possible. Contrarily, although the antioxidant property of linagliptin is observed in a rat model, it was not observed with some other DPP-4 inhibitors possibly due to unique chemical structures of different DPP-4 inhibitors [107]. Linagliptin has a xanthine-based scaffold that can inhibit the production of xanthine oxidase in tissue [108]. DPP-4 deficiency reduces oxidative stress in the diabetic kidney in a variety of experimental models [106, 109, 110]. Very recently, Spencer et al. showed that in a murine model of diabetes, linagliptin treatment could reduce renal oxidative stress in a glucose-independent manner [111], suggesting that antioxidant system is required for the renoprotective effects of linagliptin.
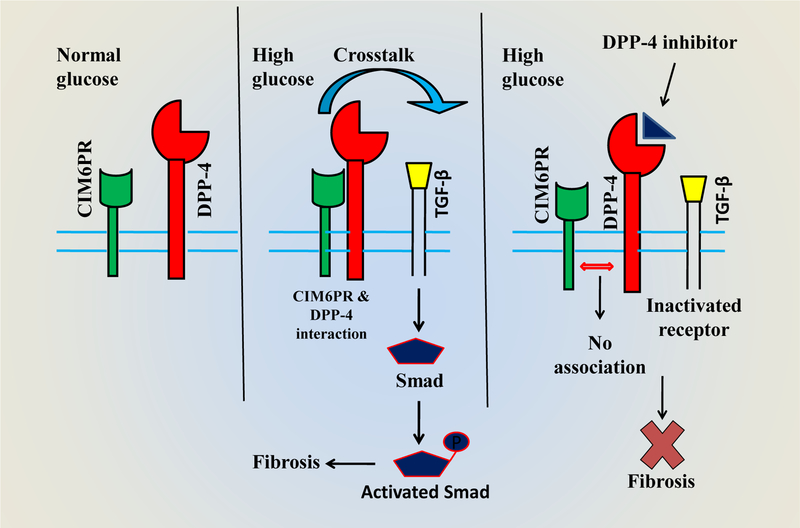
Figure 1:
Schematic diagram depicting interactions between CIM6PR and DPP-4 and the involvement of DPP-4 inhibitors in anti-fibrotic effects in the kidney. In normal glucose conditions, membrane-bound CIM6PR and DPP-4 have very little or no interaction. In high glucose levels, the interaction between CIM6PR and DPP-4 establishes, resulting in the activation of fibrotic TGF-β pathways. DPP-4 inhibitors prevent DPP-4 and CIM6PR interactions that finally attenuate fibrosis. Abbreviations: CIM6PR, Cation-independent mannose-6-phosphate receptor; DPP-4, Dipeptidyl peptidase-4; TGF-β, Transforming growth factor; p, phospho-. Concept of the diagram partly adapted from Panchapakesan and Pollock, 2014 [118].
6. Anti-fibrotic effects of DPP-4 inhibitors and its mechanisms of action:
Fibrosis is one of the significant complications of progressive kidney diseases, which is common among diabetic patients affecting normal glomerular filtration rate [112, 113]. Commonly, kidney fibrosis is found in the glomerulus and tubulointerstitial space of nephron. In the T1D murine model, fibrosis was ameliorated by DPP-4 inhibitor linagliptin without alteration of blood glucose level [114]. Fibroblasts, a type of cells in connective tissue produces collagen and other fibers upon activation and establish fibrosis by accumulating excessive collagens and other extracellular matrices (ECM) proteins in the kidney. DPP-4 has emerged as one of the pro-fibrotic agents as DPP-4 inhibitors have been shown to have anti-fibrotic effects in diverse species and organs including liver in rat [115], heart in mouse [116, 117], and kidney [118], even in human adipose tissue [119] and smooth muscles [120]. DPP-4 inhibition thus may be a promising therapeutic strategy to prevent the formation of fibrosis or even reversal of augmented fibrotic markers in progressive kidney disease. Using 5/6 nephrectomized uremic cardiomyopathy rat model, Chaykovska et al. showed that treatment with different DPP-4 inhibitors such as sitagliptin, alogliptin, and linagliptin significantly reduced blood concentration of osteopontin, a marker of kidney tubular injury and fibrosis [121]. In addition, they have also shown that sitagliptin and alogliptin at a dose of 7.0 µmol/kg and linagliptin at a dose of 0.5 µmol/kg had a similar effect on blood osteopontin concentration [121]. A higher dose of linagliptin, i.e. 7.0 µmol/kg did not further reduced osteopontin concentration significantly compare to 0.5 µmol/kg suggesting a need for correct DPP-4 inhibitors dosing to get optimum kidney benefit without excessive use of these drugs in CKD. These DPP-4 inhibitors-mediated pharmacokinetic and anti-fibrotic effects are controlled by several interconnected complex molecular pathways, which are partly dependent on dosing and duration of the treatment. Therefore, for effective therapeutic strategy, there is a need for more scientific attention, especially in the case of diabetic renal fibrosis, some of which are discussed below.
6.1. DPP-4 and Endothelial-to-mesenchymal transition (EndMT):
Kanasaki et al. have shown that anti-fibrotic changes by DPP-4 inhibitor linagliptin treatment occurred by attenuation of endothelial-to-mesenchymal transition (EndMT) in mice kidney [114], which is one of the major sources of kidney fibroblast [122–125]. EndMT is a complex process in which cells of the endothelial layer become detached and acquire mesenchymal phenotype by losing all its previous endothelial markers and transforming to myofibroblastic cells [126, 127]. These myofibroblasts invade the interstitial space and produce an excessive amount of collagen, α-smooth muscle actin and other ECM proteins leading to fibrosis formation. The EndMT pathway can be induced by several complex molecular networks, which are initiated mostly by TGF-β. Shi et al. have reported that induced interaction of DPP-4 and integrin-β1 establish cross-talk with TGF-β and EndMT in a streptozotocin-induced CD-1 mouse diabetic kidney [101]. Using human dermal microvascular endothelial cells, Kanasaki et al. showed that DPP-4 inhibitor linagliptin inhibited TGF-β2 induced EndMT via Smad-3, a transcription factor essential for TGF-β mediated signalling [114]. Vascular endothelial growth factor receptor 1 (VEGFR-1) induces EndMT, but in contrast, VEGFR-2 counteract the EndMT [128]. By a simple schematic drawing, TGF-β2-induced EndMT pathway is mentioned in figure 2.
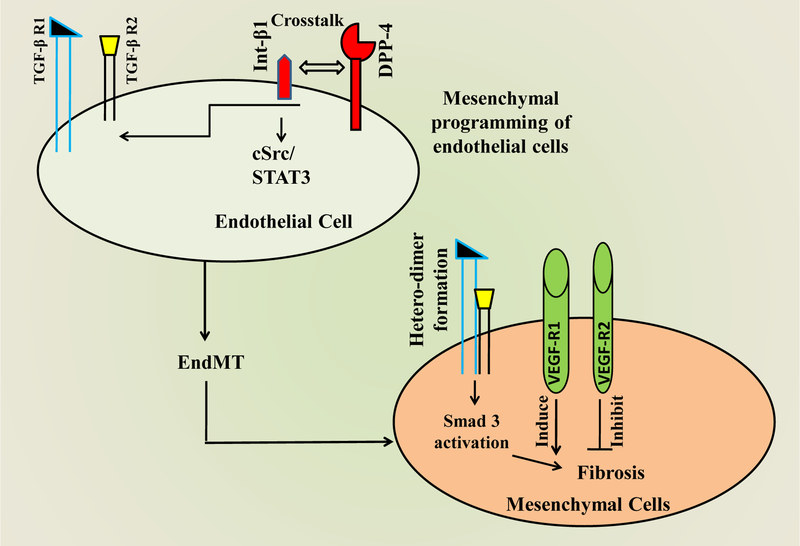
Figure 2:
Schematic diagram showing the interaction between Int-β1 and DPP-4, resulting in endothelial to mesenchymal transition during progressive fibrosis. Interaction between Int-β1 and DPP-4 leads to the formation of TGF-β1R and TGF-β2R heterodimer that causes mesenchymal programming of endothelial cells. In contrast to VEGF-R1, which induces the process of the TGF-β mediated EndMT and fibrosis, VEGF-R2 inhibits the process. Abbreviations: Int-β1: Integrin- β1; TGF-β: Transforming growth factor; EndMT: Endothelial to mesenchymal transition; VEGF-R: vascular endothelial growth factor receptor. STAT3, Signal transducer and activator of transcription 3; cSrc, non-receptor tyrosine kinase.
6.2. DPP-4 and TGF-β-Smad signalling:
In DN, fibrotic pathways mainly by TGF-β signalling lead to scar formation, and in the kidney, it may lead to kidney failure. In many cases, DPP-4 is increased in the diabetic kidney along with TGF-β1 and -β2 expressions. Linagliptin attenuate the enzymatic activity of DPP-4, and thus, activation of TGF-β in proximal tubular cells is challenged in the human kidney as well as downstream fibrotic markers by Linagliptin treatment [129]. TGF-β2 activates Smad-3 by phosphorylation, and Smad-3 is an important transcription factor controlling TGF-β superfamily signalling pathways in human HaCaT cells [130]. Linagliptin has been shown to reduce TGF-β signalling in cultured renal proximal tubular epithelial cells (HK-2) in high glucose exposure by different molecular pathways [131–133]. In addition, it has been reported that cation-independent mannose-6-phosphate receptor (CIM6PR) activated TGF-β1 in cultured HK-2 cells in high glucose supplemented media [131]. Moreover, DPP-4 and CIM6PR interaction are required for TGF-β1-Smad signalling, thus when DPP-4 is blocked, fibrosis formation by the TGF-β1 pathway is almost stopped in HK-2 cells [133]. This cross-talk between CIM6PR and TGF-β signalling is summarised in a schematic diagram (Figs. 3). The TGF-β1 pathway is interconnected with the integrin- β1 mediated cellular action in kidney fibrosis in a unilateral ureteral obstruction (UUO) mouse model. The inhibition of integrin- β1 signals reduce TGF- β1 mediated responses and thus ameliorated fibrosis [134]. Hamzeh et al. have shown that in the case of integrin- β1 attenuation, fibronectin expression in a fibrotic kidney by TGF- β1 signalling is down-regulated in HK-2 cells [135]. This cross-talk between TGF- β1 and integrin- β1 is mediated by cSrc- and STAT3- dependent pathways, and is summarized schematically in fig. 2.
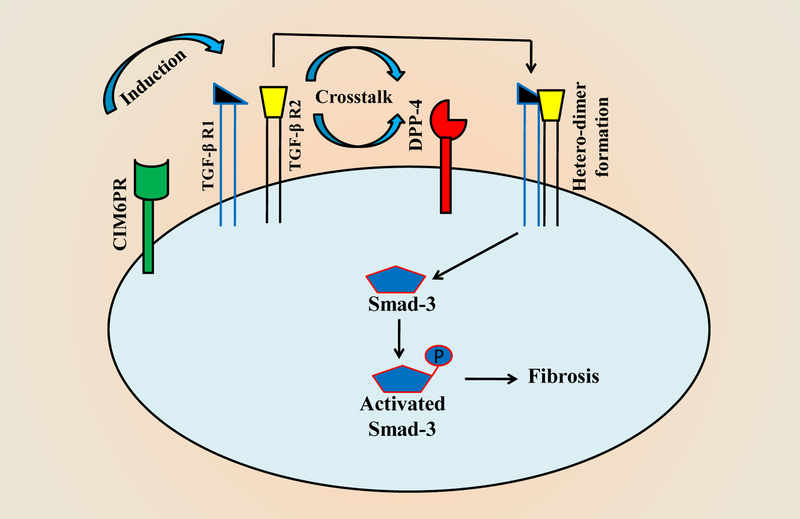
Figure 3:
Schematic diagram showing the interaction between DPP-4 and TGF-β R: In high glucose condition, the interaction between CIM6PR and DPP-4 induces heterodimer formation of TGF-β R1 and TGF-β R2. The newly formed hetero-dimer of TGF-β receptors activates Smad by phosphorylation, and phosphorylated Smad, in turn, induces ECM protein expression leading to initiation and formation of fibrosis.
6.3. DPP-4 and integrin- β1 signalling:
Integrins are heterodimers of different α and β subunits (18 types of α and 8 types of β), each exhibits different ligand-binding and signalling properties [136]. Integrins are transmembrane protein, and their extracellular domain binds to ECM glycoproteins including fibronectin, collagens, vascular cell adhesion molecule-1 (VCAM-1), intracellular cell adhesion molecule (ICAM-1), laminins and other cellular receptors [137, 138]. Thus, integrins regulate cytoskeletal organization, cell proliferation, cell shape, cell motility and angiogenesis in variety cells [139]. Among integrins, β1 integrin, for example, is pro-fibrotic in nature [140]. Liu et al. reported that expression integrin-β1 expression is required in fibroblasts for fibrogenesis, and deletion of integrin-β1 resulted in resistance to bleomycin-induced cutaneous thickening and fibrosis formation in a mouse model [141]. In the UUO murine model, integrin- β1 mRNA as well as protein expression were upregulated [134]. In the absence of integrin-β1, human proximal tubule cells did not activate cSrc and STAT-3 signalling pathway to promote pro-fibrotic protein synthesis in renal fibrosis [135]. DPP-4 inhibition decrease integrin-β1 phosphorylation at S785 residue, which is important for cellular adhesion [142]. DPP-4 and integrin- β1 interaction is essential for pro-fibrotic signalling pathways involving TGF-β, and DPP-4 associated heterodimerization of TGF-β and EndMT are inhibited by integrin- β1 deletion in HMVECs [101]. Furthermore, DPP-4-integrin- β1 associations upregulate vascular endothelial growth factor receptor 1 (VEGF-R1) favouring EndMT and down-regulating VEGFR-2, which in turn inhibit EndMT [101]. These VEGF-Rs’ alterations are important as they stimulate the proliferation of endothelial cells and angiogenesis [128]. These reports suggest that integrin- β1 modulation has the potential for a future therapeutic approach to combat diabetic kidney fibrosis. For easy understanding, DPP-4 and integrin- β1 association and signalling cascades are depicted schematically in fig. 2.
6.4. DPP-4 and miRNAs:
Micro RNAs (miRNAs) are small, noncoding RNAs (~ 22 bases) that have been shown to have immense biological importance in the last decade for their role in gene silencing by regulating protein expression through targeting mRNAs via specific target sites in 3’UTR. Alterations of miRNAs expressions are reported in transitioning from a healthy kidney to a fibrotic kidney in a variety of disease conditions. For example, higher expression of miR-29s is reported as anti-fibrotic and attenuated fibrotic progression [143–145]. Qin et al. have shown that miR-29s significantly inhibited TGF-β/Smad3 signalling and promoted renal fibrosis [146]. 3’UTR of DPP-4 mRNA has a binding site for miR-29 [114]. On binding to this element, miRNA-29 suppresses gene expression of DPP-4, and consequently, pro-fibrotic markers are inhibited in mouse kidney [114]. Interestingly, integrin- β1 and INF-γ are also inhibited by miR-29 [147]. MiR-29 a,b,c were suppressed in DN than that of a healthy kidney, and Linagliptin, a DPP-4 inhibitor ameliorated the kidney functions by inducing miR-29 expression in the diabetic model [114].
Chen et al. reported another miRNA, miR-let-7 that has an anti-EndMT role and can also regulate cross-talk between EndMT and fibroblast growth factor receptor-1 (FGFR-1) [148]. Nagai et al. have revealed mmu-let-7 family miRNAs are downregulated in the diabetic kidney [149]. These mmu-let-7 family miRNAs, let-7b or let-7c blocks FGF signalling, which can induce EndMT [148]. This FGF receptor blocker let-7 can also suppress TGF-β receptor 1 [150]. Apart from these above miRNAs, miRNA-21 and miRNA-23 have been shown to have an important role in EndMT mediated kidney fibrosis [151]. Nonetheless, the bidirectional cross-talk between miR-29s and miR-let-7 and its anti-fibrotic role in DN is still not clear and needs further scientific attention. The interactions between miRNA-29 and miRNA-let-7 are presented schematically in fig 4.
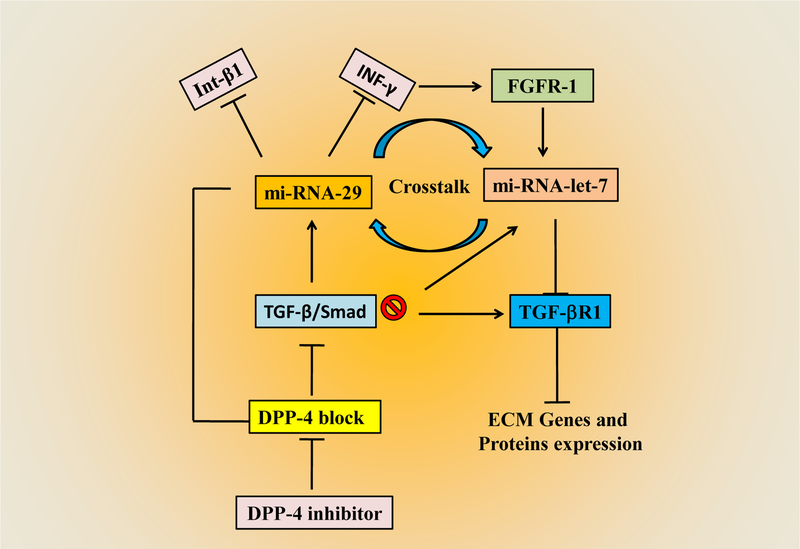
Figure 4:
Schematic diagram showing crosstalk between miRNA-29 and mi-RNA-let-7 in progressive fibrosis: DPP-4 inhibitors by blocking DPP-4 enzyme interactions, inhibit the TGF-β/ Smad pathway. Inhibited TGF- β/Smad pathway, in turn, induces miR-29 that subsequently inhibit Int-β1 and INF-γ. Inhibition of Int-β1 and INF-γ induce FGFR-1 and induce miR-let-7. miR-let-7 inhibits TGF- β R1. Therefore, miR-29 and miR-let-7 comprise anti-EndMT programs. Int-β1, integrin-β1; INF-γ, Interferon-γ; FGFR, fibroblast growth factor receptor.
6.5. Epigenetic regulation of DPP-4 expression
Cytosine-methylation in CpG dinucleotides, especially which are localized in the promoter region of genes is one of the best-known epigenetic mechanisms [152]. The human genome sequences revealed that DPP-4 gene locus contains CpG dinucleotide rich island (CpG island) in its promoter region, spanning 1,275 bp (human build 36.3; chromosome 2, minor strand), which includes 102 CpG sites [153]. This region begins 582 bp upstream from the start codon extend to 104 bp of the second exon (www.ncbi.nlm.nih.gov/projects/genome/guide/human/). McGuiness and Wesley showed that hypermethylation of the DPP-4 promoter CpG island has been associated with gene expression repression in human melanoma cell lines [154], and confirms the same result found in adult T-cell leukaemia cells four years ago [155]. Epigenetic regulation of DPP-4 expression has been postulated in visceral adipose tissue during severe obesity, but differences in promoter methylation levels are associated with the abundance of mRNA in visceral adipose tissue of severely obese subjects remain unanswered [153]. Turcot et al. hypothesized that DPP-4 expression levels are negatively correlated with methylation of CpG sites in non-diabetic severe obese women, even though methylation percentage of CpGs in the second exon of DPP-4 gene is positively correlated with metabolic syndromes [156].
It was previously postulated by Turcot et al. that CpG methylation levels within the promoter and 5′UTR regions of the DPP-4 gene might influence transcriptional efficacy [153]. However, a subsequent report has shown that non-promoter/non-5′UTR CpG-sites may also mediate the same action [154], as in specific CpG island situated in the first intron of the peroxisomal membrane protein 4 [157] and in the early growth response 2 genes in cancer cells [158]. Furthermore, downstream CpG sites may also influence gene expression, as it was seen with CpG sites within exon 4 of the C-Fos gene in neonatal diethylstilbestrol-treated mice uteri [159], and 4th intron of interleukin-10 (IL-10) gene in human regulatory T lymphocytes [155]. The transcriptional regulations by non-promoter/non-5′UTR CpG islands, however, remain unclear. In a study, Turcot et al. identified CpG sites 94, 101, and 102 in the DPP-4 gene as transcription binding sites by using the MatInspector matrix library (www.genomatix.de) [156]. In conclusion, to our knowledge, this is the first methylation analysis of the DPP-4 promoter.
Analysis of CpG island in the visceral adipose tissue of premenopausal non-diabetic severe obese women revealed that only the end of the promoter of the gene, CpG island was methylated over 10% (CpG94–102) [156]. The methylation pattern and percent of methylation were negatively correlated with DPP-4 mRNA abundance. These were also associated with variability in the plasma lipid profile, regarding the role of DPP-4 in enzymatic inactivation of incretin hormones and several post-translational processing of biologically important proteins as mentioned earlier.
Glucose-stimulated DPP-4 expression is also modulated by DNA methylation. The hypomethylation of the DPP-4 gene enhances glucose-stimulated expression and release of DPP-4 in the liver of C57BL/6J male mice, and the release of DPP-4 increases circulating DPP-4 in the body [160]. In contrast, in a rat model of steatosis, differential expressions of DPP-4 were not correlated with DNA methylation of the gene. Even, no differential methylation pattern was found in subcutaneous and visceral adipose tissues with varying levels of enzyme expression [161].
Though it is evident that epigenetic control of DPP-4 may play a crucial role in fibrosis progression, but to our knowledge, there is no evidential data about DPP-4 gene methylation and correlation of its expression level in diabetic nephropathy or specifically diabetic kidney fibrosis. As discussed in this review, several complex pathways together control DPP-4 mediated kidney fibrosis. Therefore, it would be interesting to define the methylation pattern of the DPP-4 gene and its differential expression and correlation to understand epigenetic modulation in the diabetic kidney.
6.6. Impacts of DPP-4 inhibition on gut microbial dysbiosis leading to improved kidney function in diabetes
The different population of microorganisms resides in the colon that is capable of fermenting carbohydrate and protein not absorbed in digestion. These microbiota populations can produce different metabolites, especially short-chain fatty acids (SCFA), mainly by anaerobic bacteria from plant cell wall polysaccharides. Microbial genes are essential for metabolic processes of the body that also play an important role in pathologies, such as metabolic syndrome or T2D in humans[162]. Kidney disease is often related to different metabolic syndromes, which could be reversed by SCFAs [163]. Based on the available reports, Ramezani and Raj have nicely summarized a correlation between dysbiosis and end-point renal disorders in their review article, and suggest that prebiotics and probiotics may have therapeutic roles in maintaining a metabolically-balanced gut microbiota to alleviate CKD progression and uremia-associated complications [164].
Interestingly, SCFA-producing bacteria are positively influenced by anti-diabetic drugs, high-fiber diets, and physical activity. Sitagliptin, a common DPP-4 inhibitor and anti-diabetic drug, was shown to restore the gut microbiota structure in fecal DNA samples. The results showed a higher abundance of Firmicutes and Tenericutes, and less abundance of Bacteroidetes in diabetic-induced rats compared to their lean counterparts [165]. Saxagliptin, another DPP-4 inhibitor, showed better-recovering effects on the microbiota phyla distribution in mice, but the reasons are unclear [166]. The functions of SCFAs and their activation of transmembrane G protein-coupled receptors or inhibition of histone acetylation are still controversial. Regardless of the pathways are, the growing evidence suggests that SCFAs regulate inflammation, oxidative stress, and fibrosis in kidney disease through the activation of the gut–kidney axis [163]. Therefore, DPP-4 inhibitors and their roles in reversing dysbiosis should be studied more to cover the knowledge gap of DPP-4 inhibitor-mediated restoration of the fibrotic kidney via the gut-kidney axis.
7. DPP-4 inhibition and reno-protection: Key clinical studies and findings
Experimental results from several animal models with DPP-4 inhibitors facilitated the hypothesis that DPP-4 inhibition may protect the kidney from DN as well as from non-diabetic kidney diseases. However, there is little preclinical data to support the proof-of-concept that DPP-4 inhibitors have significant pleiotropic renal benefits. In recent post hoc analyses on the cardiovascular safety studies, DPP-4 inhibitors saxagliptin and linagliptin have been found to improve albumin to creatinine ratio (ACR) without any improvement in the estimated glomerular filtration rate (eGFR) [167–170]. In a randomized MARLINA-T2D trial, which was designed to investigate glycemic and renal effects of DPP-4 inhibitor for 24 weeks added to standard-of-care in T2D patients, Groop et al. reported that although linagliptin significantly improved glycemic control, it failed to lower albuminuria significantly [171]. Interestingly, a random clinical study on T2D patients suggested that linagliptin treatment for 4 weeks prevented impairment of renal endothelial functions [172]. After 3.6 years follow up in a large cohort study of 923,936 diabetic patients of whom 83,638 patients were DPP-4 inhibitor users including 9.7% saxagliptin, 14.6% vildagliptin, and 75.7% sitagliptin, it has been observed that DPP-4 inhibitors treatment in these diabetic patients reduced the risk of mild to severe acute kidney injury [173]. Recently, a clinical trial of the Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) was conducted aiming to investigate long term impact of linagliptin on cardiovascular and renal outcomes in a selected population of T2D patients. This multi-sites and a multi-national clinical trial involving 605 clinics of 27 countries where the placebo-controlled, randomized non-inferiority trial was performed in T2D patients, and the results indicated that 8.8%−9.4% linagliptin recipient patients showed better kidney outcome [174]. In another publication of the same CARMELINA study, which included 6,979 patients, investigators have indicated that linagliptin versus placebo did not affect baseline eGFR or urine albumin-creatinine ratio significantly [175]. Similarly, sitagliptin treatment to 14,671 patients did not reveal significant clinical changes neither in albuminuria nor in eGFR [176]. In another study with a 12 week long sitagliptin treatment on 55 T2D patients, a modestly reduced glomerular hydraulic pressure was reported. However, other internal hemodynamic variables and renal damage markers remained unchanged compared to placebo [177]. In the past 79th Scientific Session of American Diabetes Association meeting results of CARdiovascular Outcome study of LINAgliptin versus glimepiride in patients with type 2 diabetes (CAROLINA) was presented, and the study found no potential differences in cardiovascular outcomes between linagliptin and glimepiride users, although a significantly lower risks of hypoglycaemia and weight gain with linagliptin compared with glimepiride were reported (http://www.diabetes.org/newsroom/press-releases/2019/linagliptin-and-glimepiride.html).
8. Future perspectives:
It is now irrefutable that DPP-4 inhibitors play an anti-fibrotic role in the kidney of diabetic conditions in complex interconnected molecular pathways, which are partly summarised in this review article. Although a recent randomized placebo-controlled CARMELINA clinical trial failed to demonstrate any benefit of linagliptin for the secondary composite kidney outcomes [174], more preclinical and clinical trials with longer duration (5 yrs. or more) is needed to establish whether DPP-4 inhibitors have renoprotective effects in diabetic kidney diseases.
It is also important to take into consideration that fibrosis is essentially a normal tissue repair process, and therefore DPP-4 inhibition may affect the normal tissue repairing mechanism and cause tissue damage, inflammation or other metabolic processes. More pleiotropic actions of DPP-4 inhibitors should be explored in humans as experimental animal models or cell lines behave differently than that of the human body. In addition, as there are several forms of DPP-4, for example, transmembrane (tDPP-4) and soluble (sDPP-4) forms, regulation or inhibition of these types of DPP-4 enzymes and the epigenetic control of these forms should be studied in a comparative mode with different types of DPP-4 inhibitors.
Furthermore, the relationship between ECM proteins and DPP-4 are almost unknown and how different types of MMPs and collagens are associated with DPP-4 during progressive fibrosis are virtually unexplored. Future studies are needed to focus on more molecular mechanisms of DPP-4 mediated anti-fibrotic actions in the diabetic kidney, as well as clinical studies, are necessary to ensure minimal side effects for prolonged administration of DPP-4 inhibitor(s) in different organs or tissues in human.
It is also noteworthy to mention that there is a 760 amino acid long type II transmembrane non-classical serine protease belongs to S9B prolyl oligopeptidase subfamily, named fibroblast activation protein or FAP. Merops peptidase database showed FAP shares approx. 50% sequence homology and exopeptidase specificity with DPP-4 [178]; however, any association of FAP and diabetic kidney is still unknown. Deciphering association of FAP and DPP-4 may lead to a clear picture of pro-fibrotic action of DPP-4 if any.
9. Conclusion:
In this review, we have discussed various possible mechanisms of actions of the enzyme, DPP-4 in diabetic renal pathophysiology. Especially, its contribution to fibrotic development by presenting several experimental and preclinical data. Emphasis on incretin and non-incretin mediated pathways, as well as AGE-RAGE pathways, are also described. In addition, how endothelial to mesenchymal transitions makes a big impact in fibrotic kidney development, and TGF-β, integrins and ECM proteins such as collagens and fibronectin are interconnected in T2D fibrosis that can be reversed by DPP-4 inhibitors are also discussed in light of various experimental outcomes. The possible role Micro-RNAs and their association are also addressed as they play a crucial role in ailing DN. Finally, we conclude that the targeted approach of DPP-4 inhibition to improve progressive renal disease outcomes, especially the management of kidney fibrosis are yet to be established through preclinical and clinical trials.
Acknowledgements
This work was supported in part by National Institutes of Health Grants, DK104653 and DK116591 (to U.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest, financial or otherwise.
References:
- [1].Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B, IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045, Diabetes research and clinical practice 138 (2018) 271–281. [DOI] [PubMed] [Google Scholar]
- [2].Forbes JM, Coughlan MT, Cooper ME, Oxidative stress as a major culprit in kidney disease in diabetes, Diabetes 57(6) (2008) 1446–54. [DOI] [PubMed] [Google Scholar]
- [3].Thomas MC, Cooper ME, Zimmet P, Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease, Nature reviews. Nephrology 12(2) (2016) 73–81. [DOI] [PubMed] [Google Scholar]
- [4].de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, D’Agati VD, Lamb HJ, Pongrac Barlovic D, Hojs R, Abbate M, Rodriquez R, Mogensen CE, Porrini E, Diabesity E-EWG, Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease, The lancet. Diabetes & endocrinology 2(5) (2014) 417–26. [DOI] [PubMed] [Google Scholar]
- [5].Wynn TA, Cellular and molecular mechanisms of fibrosis, J Pathol 214(2) (2008) 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boor P, Ostendorf T, Floege J, Renal fibrosis: novel insights into mechanisms and therapeutic targets, Nature reviews. Nephrology 6(11) (2010) 643–56. [DOI] [PubMed] [Google Scholar]
- [7].Zeisberg M, Neilson EG, Mechanisms of tubulointerstitial fibrosis, Journal of the American Society of Nephrology : JASN 21(11) (2010) 1819–34. [DOI] [PubMed] [Google Scholar]
- [8].De Meester I, Korom S, Van Damme J, Scharpe S, CD26, let it cut or cut it down, Immunology today 20(8) (1999) 367–75. [DOI] [PubMed] [Google Scholar]
- [9].Paris A, Strukelj B, Pungercar J, Renko M, Dolenc I, Turk V, Molecular cloning and sequence analysis of human preprocathepsin C, FEBS letters 369(2–3) (1995) 326–30. [DOI] [PubMed] [Google Scholar]
- [10].Maes MB, Scharpe S, De Meester I, Dipeptidyl peptidase II (DPPII), a review, Clinica chimica acta; international journal of clinical chemistry 380(1–2) (2007) 31–49. [DOI] [PubMed] [Google Scholar]
- [11].Prajapati SC, Chauhan SS, Dipeptidyl peptidase III: a multifaceted oligopeptide N-end cutter, The FEBS journal 278(18) (2011) 3256–76. [DOI] [PubMed] [Google Scholar]
- [12].Higashijima Y, Tanaka T, Yamaguchi J, Tanaka S, Nangaku M, Anti-inflammatory role of DPP-4 inhibitors in a nondiabetic model of glomerular injury, American journal of physiology. Renal physiology 308(8) (2015) F878–87. [DOI] [PubMed] [Google Scholar]
- [13].Wada K, Yokotani N, Hunter C, Doi K, Wenthold RJ, Shimasaki S, Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family, Proceedings of the National Academy of Sciences of the United States of America 89(1) (1992) 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qi SY, Riviere PJ, Trojnar J, Junien JL, Akinsanya KO, Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases, The Biochemical journal 373(Pt 1) (2003) 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hack K, Renzi F, Hess E, Lauber F, Douxfils J, Dogne JM, Cornelis GR, Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus, Journal of thrombosis and haemostasis : JTH 15(3) (2017) 487–499. [DOI] [PubMed] [Google Scholar]
- [16].Abbott CA, Yu DM, Woollatt E, Sutherland GR, McCaughan GW, Gorrell MD, Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8, European journal of biochemistry 267(20) (2000) 6140–50. [DOI] [PubMed] [Google Scholar]
- [17].Zhong FL, Robinson K, Teo DET, Tan KY, Lim C, Harapas CR, Yu CH, Xie WH, Sobota RM, Au VB, Hopkins R, D’Osualdo A, Reed JC, Connolly JE, Masters SL, Reversade B, Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding, The Journal of biological chemistry 293(49) (2018) 18864–18878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bezerra GA, Dobrovetsky E, Seitova A, Fedosyuk S, Dhe-Paganon S, Gruber K, Structure of human dipeptidyl peptidase 10 (DPPY): a modulator of neuronal Kv4 channels, Scientific reports 5 (2015) 8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hasan AA, Hocher B, Role of soluble and membrane-bound dipeptidyl peptidase-4 in diabetic nephropathy, Journal of molecular endocrinology 59(1) (2017) R1–R10. [DOI] [PubMed] [Google Scholar]
- [20].Nauck MA, Kahle M, Baranov O, Deacon CF, Holst JJ, Addition of a dipeptidyl peptidase-4 inhibitor, sitagliptin, to ongoing therapy with the glucagon-like peptide-1 receptor agonist liraglutide: A randomized controlled trial in patients with type 2 diabetes, Diabetes, obesity & metabolism 19(2) (2017) 200–207. [DOI] [PubMed] [Google Scholar]
- [21].McIntosh CHS, Demuth H-U, Pospisilik JA, Pederson R, Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents?, Regulatory peptides 128(2) (2005) 159–65. [DOI] [PubMed] [Google Scholar]
- [22].Hopsu-Havu VK, Glenner GG, A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide, Histochemie. Histochemistry. Histochimie 7(3) (1966) 197–201. [DOI] [PubMed] [Google Scholar]
- [23].Klemann C, Wagner L, Stephan M, von Horsten S, Cut to the chase: a review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system, Clinical and experimental immunology 185(1) (2016) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cordero OJ, Salgado FJ, Nogueira M, On the origin of serum CD26 and its altered concentration in cancer patients, Cancer immunology, immunotherapy : CII 58(11) (2009) 1723–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Muller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J, Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome, Diabetes 60(7) (2011) 1917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chien CH, Huang LH, Chou CY, Chen YS, Han YS, Chang GG, Liang PH, Chen X, One site mutation disrupts dimer formation in human DPP-IV proteins, The Journal of biological chemistry 279(50) (2004) 52338–45. [DOI] [PubMed] [Google Scholar]
- [27].Mulvihill EE, Drucker DJ, Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors, Endocrine reviews 35(6) (2014) 992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rohrborn D, Wronkowitz N, Eckel J, DPP4 in Diabetes, Frontiers in immunology 6 (2015) 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Deacon CF, What do we know about the secretion and degradation of incretin hormones?, Regulatory peptides 128(2) (2005) 117–24. [DOI] [PubMed] [Google Scholar]
- [30].Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ, Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig, The American journal of physiology 271(3 Pt 1) (1996) E458–64. [DOI] [PubMed] [Google Scholar]
- [31].Takagaki Y, Koya D, Kanasaki K, Dipeptidyl peptidase-4 inhibition and renoprotection: the role of antifibrotic effects, Current opinion in nephrology and hypertension 26(1) (2017) 56–66. [DOI] [PubMed] [Google Scholar]
- [32].Ohnuma K, Dang NH, Morimoto C, Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function, Trends in immunology 29(6) (2008) 295–301. [DOI] [PubMed] [Google Scholar]
- [33].Lambeir AM, Durinx C, Scharpe S, De Meester I, Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV, Critical reviews in clinical laboratory sciences 40(3) (2003) 209–94. [DOI] [PubMed] [Google Scholar]
- [34].Waumans Y, Baerts L, Kehoe K, Lambeir AM, De Meester I, The Dipeptidyl Peptidase Family, Prolyl Oligopeptidase, and Prolyl Carboxypeptidase in the Immune System and Inflammatory Disease, Including Atherosclerosis, Frontiers in immunology 6 (2015) 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Andersen ES, Deacon CF, Holst JJ, Do we know the true mechanism of action of the DPP-4 inhibitors?, Diabetes, obesity & metabolism 20(1) (2018) 34–41. [DOI] [PubMed] [Google Scholar]
- [36].Dicker D, DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors, Diabetes care 34 Suppl 2 (2011) S276–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ahren B, [New strategy in type 2 diabetes tested in clinical trials. Glucagon-like peptide 1 (GLP-1) affects basic caused of the disease], Lakartidningen 102(8) (2005) 545–9. [PubMed] [Google Scholar]
- [38].Deacon CF, Circulation and degradation of GIP and GLP-1, Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 36(11–12) (2004) 761–5. [DOI] [PubMed] [Google Scholar]
- [39].Holst JJ, Deacon CF, Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors, Diabetologia 48(4) (2005) 612–5. [DOI] [PubMed] [Google Scholar]
- [40].Hocher B, Reichetzeder C, Alter ML, Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research, Kidney & blood pressure research 36(1) (2012) 65–84. [DOI] [PubMed] [Google Scholar]
- [41].Glorie LL, Verhulst A, Matheeussen V, Baerts L, Magielse J, Hermans N, D’Haese PC, De Meester I, De Beuf A, DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury, American journal of physiology. Renal physiology 303(5) (2012) F681–8. [DOI] [PubMed] [Google Scholar]
- [42].Nistala R, Habibi J, Lastra G, Manrique C, Aroor AR, Hayden MR, Garro M, Meuth A, Johnson M, Whaley-Connell A, Sowers JR, Prevention of obesity-induced renal injury in male mice by DPP4 inhibition, Endocrinology 155(6) (2014) 2266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nistala R, Habibi J, Aroor A, Sowers JR, Hayden MR, Meuth A, Knight W, Hancock T, Klein T, DeMarco VG, Whaley-Connell A, DPP4 inhibition attenuates filtration barrier injury and oxidant stress in the zucker obese rat, Obesity 22(10) (2014) 2172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eun Lee J, Kim JE, Lee MH, Song HK, Ghee JY, Kang YS, Min HS, Kim HW, Cha JJ, Han JY, Han SY, Cha DR, DA-1229, a dipeptidyl peptidase IV inhibitor, protects against renal injury by preventing podocyte damage in an animal model of progressive renal injury, Laboratory investigation; a journal of technical methods and pathology 96(5) (2016) 547–60. [DOI] [PubMed] [Google Scholar]
- [45].Kreymann B, Williams G, Ghatei MA, Bloom SR, Glucagon-like peptide-1 7–36: a physiological incretin in man, Lancet 2(8571) (1987) 1300–4. [DOI] [PubMed] [Google Scholar]
- [46].Deacon CF, Johnsen AH, Holst JJ, Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo, The Journal of clinical endocrinology and metabolism 80(3) (1995) 952–7. [DOI] [PubMed] [Google Scholar]
- [47].Deacon CF, Hughes TE, Holst JJ, Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig, Diabetes 47(5) (1998) 764–9. [DOI] [PubMed] [Google Scholar]
- [48].Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ, Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs, Diabetes 50(7) (2001) 1588–97. [DOI] [PubMed] [Google Scholar]
- [49].Pederson RA, White HA, Schlenzig D, Pauly RP, McIntosh CH, Demuth HU, Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide, Diabetes 47(8) (1998) 1253–8. [DOI] [PubMed] [Google Scholar]
- [50].Ahren B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, Sandqvist M, Bavenholm P, Efendic S, Eriksson JW, Dickinson S, Holmes D, Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes, Diabetes care 25(5) (2002) 869–75. [DOI] [PubMed] [Google Scholar]
- [51].Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A, Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes, The Journal of clinical endocrinology and metabolism 89(5) (2004) 2078–84. [DOI] [PubMed] [Google Scholar]
- [52].Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, Sitagliptin Study G, Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes, Diabetes care 29(12) (2006) 2632–7. [DOI] [PubMed] [Google Scholar]
- [53].Maeda H, Kubota A, Tanaka Y, Terauchi Y, Matsuba I, group A-KS, The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes, Diabetes research and clinical practice 95(1) (2012) e20–2. [DOI] [PubMed] [Google Scholar]
- [54].Kothny W, Shao Q, Groop PH, Lukashevich V, One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment, Diabetes, obesity & metabolism 14(11) (2012) 1032–9. [DOI] [PubMed] [Google Scholar]
- [55].Lukashevich V, Schweizer A, Foley JE, Dickinson S, Groop PH, Kothny W, Efficacy of vildagliptin in combination with insulin in patients with type 2 diabetes and severe renal impairment, Vascular health and risk management 9 (2013) 21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, Woerle HJ, Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study, Diabetes care 36(2) (2013) 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nowicki M, Rychlik I, Haller H, Warren M, Suchower L, Gause-Nilsson I, Schutzer KM, Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study, International journal of clinical practice 65(12) (2011) 1230–9. [DOI] [PubMed] [Google Scholar]
- [58].Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Committee S-TS, Investigators, Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus, The New England journal of medicine 369(14) (2013) 1317–26. [DOI] [PubMed] [Google Scholar]
- [59].Kettmann U, Humbel B, Holzhausen HJ, Ultrastructural localization of dipeptidylpeptidase IV in the glomerulum of the rat kidney, Acta histochemica 92(2) (1992) 225–7. [DOI] [PubMed] [Google Scholar]
- [60].Wang Z, Grigo C, Steinbeck J, von Horsten S, Amann K, Daniel C, Soluble DPP4 originates in part from bone marrow cells and not from the kidney, Peptides 57 (2014) 109–17. [DOI] [PubMed] [Google Scholar]
- [61].Hartel S, Gossrau R, Hanski C, Reutter W, Dipeptidyl peptidase (DPP) IV in rat organs. Comparison of immunohistochemistry and activity histochemistry, Histochemistry 89(2) (1988) 151–61. [DOI] [PubMed] [Google Scholar]
- [62].Tiruppathi C, Miyamoto Y, Ganapathy V, Roesel RA, Whitford GM, Leibach FH, Hydrolysis and transport of proline-containing peptides in renal brush-border membrane vesicles from dipeptidyl peptidase IV-positive and dipeptidyl peptidase IV-negative rat strains, The Journal of biological chemistry 265(3) (1990) 1476–83. [PubMed] [Google Scholar]
- [63].Elleder M, Stejskal J, Induction of dipeptidylpeptidase IV activity in human renal glomeruli--a histochemical study, Acta histochemica 77(1) (1985) 75–8. [DOI] [PubMed] [Google Scholar]
- [64].Stiller D, Bahn H, August C, Demonstration of glomerular DPP IV activity in kidney diseases, Acta histochemica 91(1) (1991) 105–9. [DOI] [PubMed] [Google Scholar]
- [65].Sharkovska Y, Reichetzeder C, Alter M, Tsuprykov O, Bachmann S, Secher T, Klein T, Hocher B, Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy, Journal of hypertension 32(11) (2014) 2211–23; discussion 2223. [DOI] [PubMed] [Google Scholar]
- [66].Stange T, Kettmann U, Holzhausen HJ, Immunoelectron microscopic demonstration of the membrane proteases aminopeptidase N/CD13 and dipeptidyl peptidase IV/CD26 in normal and neoplastic renal parenchymal tissues and cells, European journal of histochemistry : EJH 44(2) (2000) 157–64. [PubMed] [Google Scholar]
- [67].Stange T, Kettmann U, Holzhausen HJ, Immunoelectron microscopic single and double labelling of aminopeptidase N (CD 13) and dipeptidyl peptidase IV (CD 26), Acta histochemica 98(3) (1996) 323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stefanovic V, Ardaillou N, Vlahovic P, Placier S, Ronco P, Ardaillou R, Interferon-gamma induces dipeptidylpeptidase IV expression in human glomerular epithelial cells, Immunology 80(3) (1993) 465–70. [PMC free article] [PubMed] [Google Scholar]
- [69].Mentlein R, Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides, Regulatory peptides 85(1) (1999) 9–24. [DOI] [PubMed] [Google Scholar]
- [70].Pala L, Pezzatini A, Dicembrini I, Ciani S, Gelmini S, Vannelli BG, Cresci B, Mannucci E, Rotella CM, Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells, Acta diabetologica 49 Suppl 1 (2012) S59–63. [DOI] [PubMed] [Google Scholar]
- [71].Mitic B, Lazarevic G, Vlahovic P, Rajic M, Stefanovic V, Diagnostic value of the aminopeptidase N, N-acetyl-beta-D-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies, Renal failure 30(9) (2008) 896–903. [DOI] [PubMed] [Google Scholar]
- [72].Sun AL, Deng JT, Guan GJ, Chen SH, Liu YT, Cheng J, Li ZW, Zhuang XH, Sun FD, Deng HP, Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease, Diabetes & vascular disease research 9(4) (2012) 301–8. [DOI] [PubMed] [Google Scholar]
- [73].Kanasaki K, The role of renal dipeptidyl peptidase-4 in kidney disease: renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin, Clinical science 132(4) (2018) 489–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA, Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule, American journal of physiology. Renal physiology 294(2) (2008) F414–22. [DOI] [PubMed] [Google Scholar]
- [75].Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C, Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin, Life sciences 81(4) (2007) 272–9. [DOI] [PubMed] [Google Scholar]
- [76].Dang DT, Chun SY, Burkitt K, Abe M, Chen S, Havre P, Mabjeesh NJ, Heath EI, Vogelzang NJ, Cruz-Correa M, Blayney DW, Ensminger WD, St Croix B, Dang NH, Dang LH, Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition, Cancer research 68(6) (2008) 1872–80. [DOI] [PubMed] [Google Scholar]
- [77].Reichetzeder C, von Websky K, Tsuprykov O, Mohagheghi Samarin A, Falke LG, Dwi Putra SE, Hasan AA, Antonenko V, Curato C, Rippmann J, Klein T, Hocher B, Head-to-head comparison of structurally unrelated dipeptidyl peptidase 4 inhibitors in the setting of renal ischemia reperfusion injury, British journal of pharmacology 174(14) (2017) 2273–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen YT, Wallace CG, Yang CC, Chen CH, Chen KH, Sung PH, Chen YL, Chai HT, Chung SY, Chua S, Lee FY, Ko SF, Lee MS, Yip HK, DPP-4 enzyme deficiency protects kidney from acute ischemia-reperfusion injury: role for remote intermittent bowel ischemia-reperfusion preconditioning, Oncotarget 8(33) (2017) 54821–54837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wolke C, Teumer A, Endlich K, Endlich N, Rettig R, Stracke S, Fiene B, Aymanns S, Felix SB, Hannemann A, Lendeckel U, Serum protease activity in chronic kidney disease patients: The GANI_MED renal cohort, Experimental biology and medicine 242(5) (2017) 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].von Websky K, Reichetzeder C, Hocher B, Physiology and pathophysiology of incretins in the kidney, Current opinion in nephrology and hypertension 23(1) (2014) 54–60. [DOI] [PubMed] [Google Scholar]
- [81].Skov J, Effects of GLP-1 in the kidney, Reviews in endocrine & metabolic disorders 15(3) (2014) 197–207. [DOI] [PubMed] [Google Scholar]
- [82].Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ, Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats, Journal of hypertension 21(6) (2003) 1125–35. [DOI] [PubMed] [Google Scholar]
- [83].Moreno C, Mistry M, Roman RJ, Renal effects of glucagon-like peptide in rats, European journal of pharmacology 434(3) (2002) 163–7. [DOI] [PubMed] [Google Scholar]
- [84].Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C, Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans, Digestion 73(2–3) (2006) 142–50. [DOI] [PubMed] [Google Scholar]
- [85].Carraro-Lacroix LR, Malnic G, Girardi AC, Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells, American journal of physiology. Renal physiology 297(6) (2009) F1647–55. [DOI] [PubMed] [Google Scholar]
- [86].Mima A, Hiraoka-Yamomoto J, Li Q, Kitada M, Li C, Geraldes P, Matsumoto M, Mizutani K, Park K, Cahill C, Nishikawa S, Rask-Madsen C, King GL, Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes, Diabetes 61(11) (2012) 2967–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ishibashi Y, Matsui T, Ojima A, Nishino Y, Nakashima S, Maeda S, Yamagishi S, Glucagon-like peptide-1 inhibits angiotensin II-induced mesangial cell damage via protein kinase A, Microvascular research 84(3) (2012) 395–8. [DOI] [PubMed] [Google Scholar]
- [88].Schernthaner G, Mogensen CE, Schernthaner GH, The effects of GLP-1 analogues, DPP-4 inhibitors and SGLT2 inhibitors on the renal system, Diabetes & vascular disease research 11(5) (2014) 306–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tanaka T, Higashijima Y, Wada T, Nangaku M, The potential for renoprotection with incretin-based drugs, Kidney international 86(4) (2014) 701–11. [DOI] [PubMed] [Google Scholar]
- [90].Tsuprykov O, Ando R, Reichetzeder C, von Websky K, Antonenko V, Sharkovska Y, Chaykovska L, Rahnenfuhrer J, Hasan AA, Tammen H, Alter M, Klein T, Ueda S, Yamagishi SI, Okuda S, Hocher B, The dipeptidyl peptidase inhibitor linagliptin and the angiotensin II receptor blocker telmisartan show renal benefit by different pathways in rats with 5/6 nephrectomy, Kidney international 89(5) (2016) 1049–1061. [DOI] [PubMed] [Google Scholar]
- [91].Hasan AA, von Websky K, Reichetzeder C, Tsuprykov O, Gaballa MMS, Guo J, Zeng S, Delic D, Tammen H, Klein T, Kleuser B, Hocher B, Mechanisms of GLP-1 receptor-independent renoprotective effects of the dipeptidyl peptidase type 4 inhibitor linagliptin in GLP-1 receptor knockout mice with 5/6 nephrectomy, Kidney international 95(6) (2019) 1373–1388. [DOI] [PubMed] [Google Scholar]
- [92].Ussher JR, Drucker DJ, Cardiovascular biology of the incretin system, Endocrine reviews 33(2) (2012) 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sauve M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ, Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice, Diabetes 59(4) (2010) 1063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dietz JR, Mechanisms of atrial natriuretic peptide secretion from the atrium, Cardiovascular research 68(1) (2005) 8–17. [DOI] [PubMed] [Google Scholar]
- [95].Nakagawa Y, Nishikimi T, Kuwahara K, Atrial and brain natriuretic peptides: Hormones secreted from the heart, Peptides 111 (2019) 18–25. [DOI] [PubMed] [Google Scholar]
- [96].Fu SH, Ping P, Wang FQ, Luo LM, Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure, J Biol Eng 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Coppolino G, Leporini C, Rivoli L, Ursini F, di Paola ED, Cernaro V, Arturi F, Bolignano D, Russo E, De Sarro G, Andreucci M, Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials, Pharmacological research 129 (2018) 274–294. [DOI] [PubMed] [Google Scholar]
- [98].Itkin T, Gomez-Salinero JM, Rafii S, Open the gates: vascular neurocrine signaling mobilizes hematopoietic stem and progenitor cells, The Journal of clinical investigation 127(12) (2017) 4231–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jackson EK, Dubinion JH, Mi Z, Effects of dipeptidyl peptidase iv inhibition on arterial blood pressure, Clinical and experimental pharmacology & physiology 35(1) (2008) 29–34. [DOI] [PubMed] [Google Scholar]
- [100].Tofovic DS, Bilan VP, Jackson EK, Sitagliptin augments angiotensin II-induced renal vasoconstriction in kidneys from rats with metabolic syndrome, Clinical and experimental pharmacology & physiology 37(7) (2010) 689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Shi S, Srivastava SP, Kanasaki M, He J, Kitada M, Nagai T, Nitta K, Takagi S, Kanasaki K, Koya D, Interactions of DPP-4 and integrin beta1 influences endothelial-to-mesenchymal transition, Kidney international 88(3) (2015) 479–89. [DOI] [PubMed] [Google Scholar]
- [102].Min HS, Kim JE, Lee MH, Song HK, Kang YS, Lee MJ, Lee JE, Kim HW, Cha JJ, Chung YY, Hyun YY, Han JY, Cha DR, Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction, Laboratory investigation; a journal of technical methods and pathology 94(6) (2014) 598–607. [DOI] [PubMed] [Google Scholar]
- [103].Yamagishi S, Fukami K, Matsui T, Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications, Cardiovascular diabetology 14 (2015) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gugliucci A, Menini T, The axis AGE-RAGE-soluble RAGE and oxidative stress in chronic kidney disease, Advances in experimental medicine and biology 824 (2014) 191–208. [DOI] [PubMed] [Google Scholar]
- [105].Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S, Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor, Cardiovascular diabetology 12 (2013) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nakashima S, Matsui T, Takeuchi M, Yamagishi SI, Linagliptin blocks renal damage in type 1 diabetic rats by suppressing advanced glycation end products-receptor axis, Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 46(10) (2014) 717–21. [DOI] [PubMed] [Google Scholar]
- [107].Kroller-Schon S, Knorr M, Hausding M, Oelze M, Schuff A, Schell R, Sudowe S, Scholz A, Daub S, Karbach S, Kossmann S, Gori T, Wenzel P, Schulz E, Grabbe S, Klein T, Munzel T, Daiber A, Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition, Cardiovascular research 96(1) (2012) 140–9. [DOI] [PubMed] [Google Scholar]
- [108].Yamagishi S, Ishibashi Y, Ojima A, Sugiura T, Matsui T, Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor, decreases serum uric acid levels in type 2 diabetic patients partly by suppressing xanthine oxidase activity, International journal of cardiology 176(2) (2014) 550–2. [DOI] [PubMed] [Google Scholar]
- [109].Alter ML, Ott IM, von Websky K, Tsuprykov O, Sharkovska Y, Krause-Relle K, Raila J, Henze A, Klein T, Hocher B, DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy, Kidney & blood pressure research 36(1) (2012) 119–30. [DOI] [PubMed] [Google Scholar]
- [110].Matsui T, Nakashima S, Nishino Y, Ojima A, Nakamura N, Arima K, Fukami K, Okuda S, Yamagishi S, Dipeptidyl peptidase-4 deficiency protects against experimental diabetic nephropathy partly by blocking the advanced glycation end products-receptor axis, Laboratory investigation; a journal of technical methods and pathology 95(5) (2015) 525–33. [DOI] [PubMed] [Google Scholar]
- [111].Spencer NY, Yang Z, Sullivan JC, Klein T, Stanton RC, Linagliptin unmasks specific antioxidant pathways protective against albuminuria and kidney hypertrophy in a mouse model of diabetes, Plos One 13(7) (2018) e0200249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kanasaki K, Taduri G, Koya D, Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis, Frontiers in endocrinology 4 (2013) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mackensen-Haen S, Bader R, Grund KE, Bohle A, Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate, Clinical nephrology 15(4) (1981) 167–71. [PubMed] [Google Scholar]
- [114].Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D, Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen, Diabetes 63(6) (2014) 2120–31. [DOI] [PubMed] [Google Scholar]
- [115].Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Douhara A, Moriya K, Kawaratani H, Shirai Y, Yoshii J, Yanase K, Kitade M, Namisaki T, Fukui H, Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats, Journal of gastroenterology 49(3) (2014) 481–91. [DOI] [PubMed] [Google Scholar]
- [116].Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR, Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity, Metabolism: clinical and experimental 63(8) (2014) 1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hirakawa H, Zempo H, Ogawa M, Watanabe R, Suzuki J, Akazawa H, Komuro I, Isobe M, A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice, Plos One 10(3) (2015) e0119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shi S, Koya D, Kanasaki K, Dipeptidyl peptidase-4 and kidney fibrosis in diabetes, Fibrogenesis & tissue repair 9 (2016) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sell H, Bluher M, Kloting N, Schlich R, Willems M, Ruppe F, Knoefel WT, Dietrich A, Fielding BA, Arner P, Frayn KN, Eckel J, Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro, Diabetes care 36(12) (2013) 4083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rohrborn D, Eckel J, Sell H, Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells, FEBS letters 588(21) (2014) 3870–7. [DOI] [PubMed] [Google Scholar]
- [121].Chaykovska L, von Websky K, Rahnenfuhrer J, Alter M, Heiden S, Fuchs H, Runge F, Klein T, Hocher B, Effects of DPP-4 inhibitors on the heart in a rat model of uremic cardiomyopathy, Plos One 6(11) (2011) e27861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R, Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition, Journal of the American Society of Nephrology : JASN 19(12) (2008) 2282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li J, Qu X, Bertram JF, Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice, The American journal of pathology 175(4) (2009) 1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kizu A, Medici D, Kalluri R, Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy, The American journal of pathology 175(4) (2009) 1371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].He J, Xu Y, Koya D, Kanasaki K, Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease, Clinical and experimental nephrology 17(4) (2013) 488–97. [DOI] [PubMed] [Google Scholar]
- [126].Piera-Velazquez S, Li Z, Jimenez SA, Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders, The American journal of pathology 179(3) (2011) 1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Piera-Velazquez S, Mendoza FA, Jimenez SA, Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases, Journal of clinical medicine 5(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR, Conversion of vascular endothelial cells into multipotent stem-like cells, Nature medicine 16(12) (2010) 1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Panchapakesan U, Pollock CA, DPP-4 inhibitors-renoprotection in diabetic nephropathy?, Diabetes 63(6) (2014) 1829–30. [DOI] [PubMed] [Google Scholar]
- [130].Shen X, Hu PP, Liberati NT, Datto MB, Frederick JP, Wang XF, TGF-beta-induced phosphorylation of Smad3 regulates its interaction with coactivator p300/CREB-binding protein, Molecular biology of the cell 9(12) (1998) 3309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wong MG, Panchapakesan U, Qi W, Silva DG, Chen XM, Pollock CA, Cation-independent mannose 6-phosphate receptor inhibitor (PXS25) inhibits fibrosis in human proximal tubular cells by inhibiting conversion of latent to active TGF-beta1, American journal of physiology. Renal physiology 301(1) (2011) F84–93. [DOI] [PubMed] [Google Scholar]
- [132].Panchapakesan U, Mather A, Pollock C, Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease, Clinical science 124(1) (2013) 17–26. [DOI] [PubMed] [Google Scholar]
- [133].Gangadharan Komala M, Gross S, Zaky A, Pollock C, Panchapakesan U, Linagliptin Limits High Glucose Induced Conversion of Latent to Active TGFss through Interaction with CIM6PR and Limits Renal Tubulointerstitial Fibronectin, Plos One 10(10) (2015) e0141143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM, Tang MJ, Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis, The American journal of pathology 177(4) (2010) 1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hamzeh MT, Sridhara R, Alexander LD, Cyclic stretch-induced TGF-beta1 and fibronectin expression is mediated by beta1-integrin through c-Src- and STAT3-dependent pathways in renal epithelial cells, American journal of physiology. Renal physiology 308(5) (2015) F425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Pozzi A, Zent R, TGF-beta sequestration by mesangial cell integrin alphavbeta8: A novel mechanism of glomerular endothelial cell regulation, The American journal of pathology 178(2) (2011) 485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Plow EF, Haas TA, Zhang L, Loftus J, Smith JW, Ligand binding to integrins, The Journal of biological chemistry 275(29) (2000) 21785–8. [DOI] [PubMed] [Google Scholar]
- [138].Hynes RO, Integrins: bidirectional, allosteric signaling machines, Cell 110(6) (2002) 673–87. [DOI] [PubMed] [Google Scholar]
- [139].Luo BH, Carman CV, Springer TA, Structural basis of integrin regulation and signaling, Annual review of immunology 25 (2007) 619–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, Harvey E, Zeef L, Farrow S, Streuli C, Henderson NC, Friedman SL, Hanley NA, Piper Hanley K, PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis, Nature communications 7 (2016) 12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A, Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model, Arthritis and rheumatism 60(9) (2009) 2817–21. [DOI] [PubMed] [Google Scholar]
- [142].Sato T, Yamochi T, Yamochi T, Aytac U, Ohnuma K, McKee KS, Morimoto C, Dang NH, CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta1, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299, Cancer research 65(15) (2005) 6950–6. [DOI] [PubMed] [Google Scholar]
- [143].Srivastava SP, Koya D, Kanasaki K, MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT, BioMed research international 2013 (2013) 125469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gao Y, Lu J, Zhang Y, Chen Y, Gu Z, Jiang X, Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through inhibition of miR-21, Pulmonary pharmacology & therapeutics 26(6) (2013) 649–54. [DOI] [PubMed] [Google Scholar]
- [145].Nitta K, Shi S, Nagai T, Kanasaki M, Kitada M, Srivastava SP, Haneda M, Kanasaki K, Koya D, Oral Administration of N-Acetyl-seryl-aspartyl-lysyl-proline Ameliorates Kidney Disease in Both Type 1 and Type 2 Diabetic Mice via a Therapeutic Regimen, BioMed research international 2016 (2016) 9172157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY, TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29, Journal of the American Society of Nephrology : JASN 22(8) (2011) 1462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang L, Zhang H, Chen X, Yang Y, Liu G, miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2), Plos One 8(8) (2013) e70192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M, FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression, Cell reports 2(6) (2012) 1684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nagai T, Nitta K, Kanasaki M, Koya D, Kanasaki K, The biological significance of angiotensin-converting enzyme inhibition to combat kidney fibrosis, Clinical and experimental nephrology 19(1) (2015) 65–74. [DOI] [PubMed] [Google Scholar]
- [150].Cheng MF, Chen LJ, Wang MC, Hsu CT, Cheng JT, Decrease of FGF receptor (FGFR) and interstitial fibrosis in the kidney of streptozotocin-induced diabetic rats, Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 46(1) (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [151].Zhong X, Chung AC, Chen HY, Meng XM, Lan HY, Smad3-mediated upregulation of miR-21 promotes renal fibrosis, Journal of the American Society of Nephrology : JASN 22(9) (2011) 1668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Matouk CC, Marsden PA, Epigenetic regulation of vascular endothelial gene expression, Circulation research 102(8) (2008) 873–87. [DOI] [PubMed] [Google Scholar]
- [153].Turcot V, Bouchard L, Faucher G, Tchernof A, Deshaies Y, Perusse L, Belisle A, Marceau S, Biron S, Lescelleur O, Biertho L, Vohl MC, DPP4 gene DNA methylation in the omentum is associated with its gene expression and plasma lipid profile in severe obesity, Obesity 19(2) (2011) 388–95. [DOI] [PubMed] [Google Scholar]
- [154].McGuinness C, Wesley UV, Dipeptidyl peptidase IV (DPPIV), a candidate tumor suppressor gene in melanomas is silenced by promoter methylation, Frontiers in bioscience : a journal and virtual library 13 (2008) 2435–43. [DOI] [PubMed] [Google Scholar]
- [155].Tsuji T, Sugahara K, Tsuruda K, Uemura A, Harasawa H, Hasegawa H, Hamaguchi Y, Tomonaga M, Yamada Y, Kamihira S, Clinical and oncologic implications in epigenetic down-regulation of CD26/dipeptidyl peptidase IV in adult T-cell leukemia cells, International journal of hematology 80(3) (2004) 254–60. [DOI] [PubMed] [Google Scholar]
- [156].Turcot V, Tchernof A, Deshaies Y, Perusse L, Belisle A, Marceau P, Hould FS, Lebel S, Vohl MC, Comparison of the dipeptidyl peptidase-4 gene methylation levels between severely obese subjects with and without the metabolic syndrome, Diabetology & metabolic syndrome 5(1) (2013) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zhang X, Wu M, Xiao H, Lee MT, Levin L, Leung YK, Ho SM, Methylation of a single intronic CpG mediates expression silencing of the PMP24 gene in prostate cancer, The Prostate 70(7) (2010) 765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Unoki M, Nakamura Y, Methylation at CpG islands in intron 1 of EGR2 confers enhancer-like activity, FEBS letters 554(1–2) (2003) 67–72. [DOI] [PubMed] [Google Scholar]
- [159].Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, Archer TK, Kinyamu HK, Williams CJ, Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure, Molecular endocrinology 27(10) (2013) 1666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Baumeier C, Saussenthaler S, Kammel A, Jahnert M, Schluter L, Hesse D, Canouil M, Lobbens S, Caiazzo R, Raverdy V, Pattou F, Nilsson E, Pihlajamaki J, Ling C, Froguel P, Schurmann A, Schwenk RW, Hepatic DPP4 DNA Methylation Associates With Fatty Liver, Diabetes 66(1) (2017) 25–35. [DOI] [PubMed] [Google Scholar]
- [161].Tarantola E, Gobbato S, Ferrigno A, Bertone V, Capelli E, Differences in Expression of DPP4 in Steatotic Rat Liver Are Not Related to Differences in the Methylation of its Gene Promoter, In vivo 29(5) (2015) 547–53. [PubMed] [Google Scholar]
- [162].Montandon SA, Jornayvaz FR, Effects of Antidiabetic Drugs on Gut Microbiota Composition, Genes 8(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Li L, Ma L, Fu P, Gut microbiota-derived short-chain fatty acids and kidney diseases, Drug design, development and therapy 11 (2017) 3531–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ramezani A, Raj DS, The gut microbiome, kidney disease, and targeted interventions, Journal of the American Society of Nephrology : JASN 25(4) (2014) 657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yan X, Feng B, Li P, Tang Z, Wang L, Microflora Disturbance during Progression of Glucose Intolerance and Effect of Sitagliptin: An Animal Study, Journal of diabetes research 2016 (2016) 2093171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang L, Li P, Tang Z, Yan X, Feng B, Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment, Scientific reports 6 (2016) 33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Cooper ME, Perkovic V, McGill JB, Groop PH, Wanner C, Rosenstock J, Hehnke U, Woerle HJ, von Eynatten M, Kidney Disease End Points in a Pooled Analysis of Individual Patient-Level Data From a Large Clinical Trials Program of the Dipeptidyl Peptidase 4 Inhibitor Linagliptin in Type 2 Diabetes, American journal of kidney diseases : the official journal of the National Kidney Foundation 66(3) (2015) 441–9. [DOI] [PubMed] [Google Scholar]
- [168].Groop PH, Cooper ME, Perkovic V, Sharma K, Schernthaner G, Haneda M, Hocher B, Gordat M, Cescutti J, Woerle HJ, von Eynatten M, Dipeptidyl peptidase-4 inhibition with linagliptin and effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: Rationale and design of the MARLINA-T2D trial, Diabetes & vascular disease research 12(6) (2015) 455–62. [DOI] [PubMed] [Google Scholar]
- [169].Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, Rozenberg A, Yanuv I, Stahre C, Ray KK, Iqbal N, Braunwald E, Scirica BM, Raz I, Effect of Saxagliptin on Renal Outcomes in the SAVOR-TIMI 53 Trial, Diabetes care 40(1) (2017) 69–76. [DOI] [PubMed] [Google Scholar]
- [170].Cooper ME, Perkovic V, Groop PH, Hocher B, Hehnke U, Meinicke T, Koitka-Weber A, van der Walt S, von Eynatten M, Hemodynamic effects of the dipeptidyl peptidase-4 inhibitor linagliptin with renin-angiotensin system inhibitors in type 2 diabetic patients with albuminuria, Journal of hypertension 37(6) (2019) 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, Schernthaner G, Sharma K, Stanton RC, Toto R, Cescutti J, Gordat M, Meinicke T, Koitka-Weber A, Thiemann S, von Eynatten M, Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial, Diabetes, obesity & metabolism 19(11) (2017) 1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ott C, Kistner I, Keller M, Friedrich S, Willam C, Bramlage P, Schmieder RE, Effects of linagliptin on renal endothelial function in patients with type 2 diabetes: a randomised clinical trial, Diabetologia 59(12) (2016) 2579–2587. [DOI] [PubMed] [Google Scholar]
- [173].Chao CT, Wang J, Wu HY, Chien KL, Hung KY, Dipeptidyl peptidase 4 inhibitor use is associated with a lower risk of incident acute kidney injury in patients with diabetes, Oncotarget 8(32) (2017) 53028–53040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK, Investigators C, Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial, Jama 321(1) (2019) 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, Wanner C, Kahn SE, Toto RD, Zinman B, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, Marx N, Investigators C, Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA, Circulation 139(3) (2019) 351–361. [DOI] [PubMed] [Google Scholar]
- [176].Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang LM, Engel SS, Lopes RD, McGuire DK, Riefflin A, Rodbard HW, Sinay I, Tankova T, Wainstein J, Peterson ED, Holman RR, Group TS, Effect of Sitagliptin on Kidney Function and Respective Cardiovascular Outcomes in Type 2 Diabetes: Outcomes From TECOS, Diabetes care 39(12) (2016) 2304–2310. [DOI] [PubMed] [Google Scholar]
- [177].Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Ter Wee PM, Diamant M, Joles JA, van Raalte DH, Renal Effects of DPP-4 Inhibitor Sitagliptin or GLP-1 Receptor Agonist Liraglutide in Overweight Patients With Type 2 Diabetes: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial, Diabetes care 39(11) (2016) 2042–2050. [DOI] [PubMed] [Google Scholar]
- [178].Poplawski SE, Lai JH, Li Y, Jin Z, Liu Y, Wu W, Wu Y, Zhou Y, Sudmeier JL, Sanford DG, Bachovchin WW, Identification of selective and potent inhibitors of fibroblast activation protein and prolyl oligopeptidase, Journal of medicinal chemistry 56(9) (2013) 3467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]