Abstract
OBJECTIVE:
Use latent class analysis to identify patterns of cognitive functioning in a sample of older adults with clinical depression and without dementia, and assess demographic, psychiatric and neurobiological predictors of class membership.
METHOD:
Neuropsychological assessment data from 121 participants in the Alzheimer’s Disease Neuroimaging Initiative – Depression Project (ADNI-D) were analyzed, including measures of executive functioning, verbal and visual memory, visuospatial and language functioning, and processing speed. These data were analyzed using latent class analysis, with predictors of class membership such as depression severity, depression and treatment history, amyloid burden, and APOE e4 allele also assessed.
RESULTS:
A two-class model of cognitive functioning best fit the data, with the Lower Cognitive Class (46.1% of the sample) performing approximately one standard deviation below the Higher Cognitive Class (53.9%) on most tests. When predictors of class membership were assessed, carrying an APOE e4 allele was significantly associated with membership in the Lower Cognitive Class. Demographic characteristics, age of depression onset, depression severity, history of psychopharmacological treatment for depression, and amyloid positivity did not predict class membership.
CONCLUSION:
Latent class analysis allows for identification of subgroups of cognitive functioning in a mostly cognitively intact late life depression (LLD) population. One subgroup, the Lower Cognitive Class, more likely to carry an APOE e4 allele, may be at greater risk for subsequent cognitive decline, even though current performance on neuropsychological testing is within normal limits. These findings have implications for early identification of those at greatest risk, risk factors, and avenues for preventive intervention.
Keywords: late life depression, cognitive functioning, latent class analysis, aging, neuropsychology, major depression
INTRODUCTION
An estimated 0.7-2.6% of older adults suffer from Major Depressive Disorder (MDD, or late life depression; LLD), with broader estimates that clinically significant depressive symptoms are present in 8-16% of adults over 65. A major public health concern and the leading cause of disability worldwide (Murray, Lopez, & Organization, 1996), depression has been associated with functional and cognitive impairment, and adverse outcomes such as dementia (Blazer, 2003; Bruce, 2001; Gabryelewicz et al., 2007; Kessler et al., 2010; Regier et al., 1988). In terms of associations with dementia, LLD has been implicated both as a risk factor for the development of dementia, as well as a possible dementia precursor, or prodromal symptom cluster heralding the onset of a dementia process (Byers & Yaffe, 2011). The likely bidirectional relationship between these illnesses is indicated by their high rates of comorbidity, with between 20 and 50% of older adults diagnosed with different types of dementia also diagnosed with depression (Ballard, Bannister, Solis, Oyebode, & Wilcock, 1996). The public health burden of both these illnesses in late life is staggering (Murray et al., 1996), suggesting a vital need for the better characterization of patterns of cognitive functioning, including any deficits, in LLD sufferers without dementia, and the identification of possible risk factors for cognitive decline in this population. Despite this need, few studies have identified relationships among patterns of cognitive functioning in older depressed adults without dementia as they relate to other salient clinical factors in this population, or investigated the possibility of heterogeneous subgroups of older depressed adults who might differ in their cognitive performance, with implications for intervention.
Cognitive impairments in LLD have been well studied, with some variability in the types and proportion of deficits reported. Generally, the research has shown that 60% of older adults with MDD but without dementia have one or more cognitive impairments (Butters et al., 2004). In terms of domains of impairment, common findings include greater difficulty with executive functioning, information processing speed, and verbal learning and memory (Koenig, Bhalla, & Butters, 2014; Lockwood, Alexopoulos, Kakuma, & Van Gorp, 2000), among others, with various possible mechanisms for the observed cognitive impairments proposed in the extant literature.
In terms of clinical factors related to current cognitive impairments and future dementia risk in LLD, age at depression onset and number of depressive episodes have been key factors assessed. For example, recurrence of depression, representing chronicity across the lifespan, has been found to increase future risk of developing dementia by 14% for each additional depressive episode (Dotson, Beydoun, & Zonderman, 2010), with prospective data indicating early onset depression was a greater risk factor for eventual dementia than late onset depression (Geerlings, den Heijer, Koudstaal, Hofman, & Breteler, 2008). Additionally, early onset depression has been implicated in worse cognition across domains over the course of five years, even among those who remitted, compared to late onset (Riddle et al., 2017). However, research has also shown that late onset depression, conceptualized as first episode after the age of 65, is associated with greater impairment in verbal learning and memory than an onset of first depressive episode earlier in life (Mackin et al., 2014), and that, in addition to current cognitive complaints in older depressed adults without dementia, risk is increased for neurodegenerative disease among those with late onset, compared to early onset, depression (Steffens, 2017). These findings suggest that there may be distinct groups with different patterns of cognitive functioning in LLD - that is, those who have depression with co-occurring cognitive difficulties not necessarily associated with the onset of a dementia process but rather representing psychiatric symptoms, and those with depression concurrent with insidious onset of a neurodegenerative disease (Ballmaier et al., 2008; Janssen et al., 2007; Sheline, Gado, & Kraemer, 2003; Steffens, McQuoid, Payne, & Potter, 2011).
In addition to depression course, treatment factors have also been implicated in cognitive functioning in LLD, with conflicting data emerging regarding the association between use of certain antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), and cognition. Though some studies have claimed an association between SSRI use and lower dementia risk (Brodrick & Mathys, 2016), others have pointed out the difficulty of disentangling improved cognition as a result of ameliorated depressive symptoms, and improved cognition as a result of pharmacological intervention (Doraiswamy et al., 2003). Still others have failed to observe a relationship between antidepressant use and cognition (Carri–re et al., 2017; Jones, Joshi, Shenkin, & Mead, 2016) or have implicated SSRI use as an independent risk factor in the development of dementia (Lee, Lin, Sung, Liang, & Kao, 2016).
It is likely that the various mechanisms for the associations between clinical and treatment factors and cognition are driven by neurobiological factors, which have also been explored in LLD. Specifically, the APOE e4 allele, a well-studied risk factor for the development of Alzheimer’s disease (Savitz, Solms, & Ramesar, 2006), has been associated with depression severity in late life (Yen et al., 2007), and as a synergistic mechanism in both LLD and cognitive decline (Corsentino, Sawyer, Sachs-Ericsson, & Blazer, 2009; Geda et al., 2006). In addition to APOE, amyloid burden, a hallmark of dementia pathology (Jansen et al., 2015), has been hypothesized as a risk factor for both depression in late life (Harrington et al., 2017; Harrington, Lim, Gould, & Maruff, 2015), as well as cognitive decline (Koyama et al., 2012; Wu et al., 2014), though others have found that the amyloid accumulation in depression is explained by incipient dementia, rather than depressive symptoms alone (Direk et al., 2013; Sun et al., 2008). Further clarification of the relationships among neurobiological variables and cognition in LLD is thus needed.
Given the variability in findings in the literature on cognitive functioning in LLD and associated clinical and neurobiological risk factors, as well as the heterogeneity of the LLD presentation, further assessment of underlying patterns of current cognitive functioning and associations with variables that have implications for future decline is warranted. Utilizing latent class analysis, which allows for the comparison of increasingly complex models for best fit while taking into account covariates of interest (Jung & Wickrama, 2008), may help to both identify subgroups of cognitive functioning in depressed older adults, as well as potentially characterize those at greater risk for future decline. The extant literature often samples either cognitively normal or cognitively impaired depressed individuals exclusively, or compares the two, which may miss important subpopulations within groups assumed to be in some way homogeneous (such as “cognitively normal”). Utilizing a “person-centered” statistical analysis technique such as latent class analysis, rather than the traditional “variable-centered” approach, can aid in investigating possible subgroups of cognitive functioning in older depressed adults, with a best-fitting model and stringent fit statistics rather than cut-off scores of arbitrary levels of statistical significance that may not be clinically relevant (Cohen, 1990; Jung & Wickrama, 2008).
This study therefore sought to uncover latent classes of cognitive functioning in an older adult sample with clinician-diagnosed Major Depressive Disorder, and without current dementia. Once classes were identified, possible clinical and neurobiological associations with class membership were assessed. We hypothesized that there would be a latent class with slightly lower cognitive functioning within this sample, with depression severity, APOE e4 allele, and amyloid positivity significantly predicting membership in the lower functioning cognitive class.
METHODS
Participants
Participants were 121 older adults with clinician-diagnosed Major Depression Disorder enrolled in the Alzheimer’s Disease Neuroimaging Initiative – Depression project (ADNI-D). The study was conducted in accordance with the Declaration of Helsinki for protection of human subjects, with procedures approved by the institutional reviews boards of each study site (University of California San Francisco and the University of Pittsburgh, respectively). All participants provided written informed consent upon their enrollment in the study. The mean age of this sample was 70.93 (SD = 5.32), with 16.26 years of education (SD = 1.96). In terms of gender, 67.8% of the sample was female. The mean depression severity was 18.22 (SD = 2.56) on the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960).
Inclusion criteria were a current diagnosis of Major Depressive Disorder, unipolar type, without psychotic features, with reported symptom severity of ≥15 on the 17-item HDRS (Hamilton, 1960), and current episode lasting at least six weeks. Diagnoses of MDD were made by using the Structured Clinical Interview (SCID) for the Diagnostic and Statistical Manual of Mental Disorders – IV (Frances, 1994), with allowable comorbidities of Generalized Anxiety Disorder or simple phobia. Other Axis I disorders, significant current neurologic illness such as epilepsy, Parkinson’s disease, traumatic brain injury or cortical stroke, excluded individuals from participation. Those with evidence of possible dementia (<25 on the Mini Mental Status Exam) were excluded from participation as well. If inclusion criteria for the study were met, and consent was given, participants underwent a blood draw for DNA and RNA banking (with APOE genotyping), MRI imaging (DTI, CBF and resting state fMRI), and AV-45 (florbetapir) amyloid imaging.
Procedures
Eligible participants were referred to an ADNI clinic where they underwent the core ADNI study protocol (Aisen et al.). They subsequently presented to an affiliated psychiatry site for clinical assessment (SCID), depression history, and further cognitive assessment (domains and tasks beyond what is collected in the ADNI core). In this assessment, cognitive functioning was assessed across a variety of domains using a comprehensive neuropsychological battery of tests, administered in the same order for each participant. Though informed they could discontinue testing at any time for fatigue or other reasons, no participants did so.
Measures
Cognitive functioning.
The domains of executive functioning, visual learning and memory, verbal learning and memory, working memory, expressive language, visual spatial functioning, and information processing speed were assessed using a variety of measures with adequate reliability and validity, as outlined below. The individual measures were scored based on available age-matched normative data, with the resulting scaled scores for each test utilized as the units of analysis to better assess patterns across tasks and within domains.
Executive functioning was assessed using the Controlled Oral Word Association Test (COWAT – FAS), a measure of phonemic fluency (Reitan & Wolfson, 1993), a measure of animal naming (Reitan & Wolfson, 1993), the Stroop Color Word Test (SCWT) (Golden & Freshwater, 2002) and the Trail Making Test part B (Reitan & Wolfson, 1993). To assess visual learning and memory, the Brief Visuospatial Memory Test (BVMT-R) was used (Benedict, 1997). Visual spatial processing was measured using the Benton Judgment of Line Orientation (JLO) test (Benton, 1983) and the Motor-Free Visual Perceptual Test – 3 (Colarusso & Hammill, 1972). Verbal learning and memory were assessed using three tests, two with word list stimuli and one for memory of verbally-presented stories. The two list-learning tasks were the Rey Auditory Verbal Learning Test (AVLT) (Rey, 1964), and the Hopkins Verbal Learning Test (HVLT) (Brandt, 1991). The Wechsler Memory Scale - Revised Logical Memory test was used to assess learning and memory for stories (Wechsler, 1987). Expressive language was assessed using the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). Working memory was measured using the WAIS Digit Span subtest (Wechsler, 1997). Finally, information processing speed was assessed using the WAIS Digit Symbol subtest (Wechsler, 1997), the Stroop Color Word Test color trial (Golden & Freshwater, 2002), as well as the Trail Making Test Part A (Reitan & Wolfson, 1993).
Depression severity and history.
Severity of depression symptoms was assessed using the 17-item Hamilton Depression Rating Scale (Hamilton, 1960). Timing of depression onset was assessed using a dichotomous variable of early onset (first depressive episode before the age of 65) or late onset (first depressive episode after the age of 65). Participants were asked to complete a self-report depression history form in order to quantify the number of discrete episodes experienced over their lifespan, with the total number reported analyzed as a continuous variable. Lifetime use of antidepressants, measured in total months a medication was used over the lifespan was assessed, specifically in relation to selective serotonin reuptake inhibitors (SSRIs) and/or selective norepinephrine reuptake inhibitors (SNRIs), measured as separate variables.
Amyloid burden.
All participants underwent AV-45 (florbetapir) amyloid imaging, with 78 scans available for analysis. Amyloid status was classified dichotomously as positive or negative based on a cutoff score of 1.10, based on National Institute on Aging findings that no individuals with a mean florbetapir of less than 1.10 had an intermediate to high likelihood of AD based upon neuropathology results at autopsy (Landau et al., 2012).
APOE genotype.
DNA was extracted from blood samples using commercial reagents (FlexiGene, Qiagen, Valencia, CA, USA). Two APOE single-nucleotide polymorphisms, specifically rs7412 and rs429358, were typed using allelic discrimination assays with TaqMan reagents (Applied Biosystems, Foster City, CA, USA). The genotyping results were subsequently incorporated into an algorithm, resulting in designation of e2, e3, or e4 genotypes. For the purpose of this study, genotype was analyzed as a dichotomous variable (presence or absence of 3∣4 or 4∣4 genotypes, referred to as e4 allele, commonly associated with increased AD risk).
Data Analysis
Latent class analysis (LCA) was conducted using MPlus version 7.0 (Muthén & Muthén, 2007). The final class model was chosen based on the comparison of multiple fit statistics and theoretical parsimony, with models of increasing complexity analyzed sequentially until the best fitting model was identified (Nylund, Asparouhov, & Muthén, 2007). Individual cognitive measures described above were used in this analysis. Fit criteria considered as part of the decision for best-fitting model were the Akaike, Bayesian, and sample size adjusted Bayesian Information Criteria, entropy, Lo-Mendell-Rubin and bootstrap likelihood ratio tests. A reduction of more than 10 points on all information criteria in models of increasing complexity is seen as an improvement in model fit, as is an entropy value closer to 1.00 and a significant Lo-Mendell-Rubin or bootstrap likelihood ratio test (measuring whether the model with k classes is significantly better fitting to the data than the model with k-1 classes) (Nylund et al., 2007). These statistics are then assessed in the context of theoretical parsimony, and the posterior probabilities of class membership for each individual saved for further analyses.
Once the unconditional model (best fitting number of classes) was identified, a conditional model, assessing the impact of the clinically-relevant covariates described above, was run, to assess for differential predictors of class membership. Covariates assessed in this model were age, gender, education, depression severity, age of onset of MDD (early or late), number of lifetime depressive episodes, lifetime SSRI use (in months), lifetime SNRI use (in months), amyloid status (positive or negative) and APOE e4 allele status (carrier of an e4 allele or not). Finally, post-hoc one-way analyses of variance were conducted in SPSS version 20 for differences between the classes (Nie, Hull, & Bent, 2011).
RESULTS
Unconditional Model
Demographic characteristics by class are presented in Table 1, as well as independent samples t-test differences between classes. Gender and APOE had a significant result on Levene’s test for equality of variances at p<.05 indicating a violation of the assumption of equal variables, and thus equal variances were not assumed for comparison t-tests on those variables. Statistically significant differences were noted between the classes on MMSE and HAMD17, however the numerical differences between means on these variables were negligible (see Table 1).
Table 1.
Demographic characteristics by cognitive class
| Variable | Lower Cognitive Class (n=56) |
Higher Cognitive Class (n=65) |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t (p-value) | |
| Gender | −1.53 (.129) | ||||||
| Male | 39.3% | 26.2% | |||||
| Female | 60.7% | 73.8% | |||||
| Age | 70.91 | 5.06 | 65-87 | 70.94 | 5.57 | 65-91 | −.028 (.977) |
| Years of Education | 16.14 | 1.79 | 12-20 | 16.37 | 2.09 | 12-20 | −.633 (.528) |
| MMSE | 28.75 | 1.08 | 26-30 | 29.35 | 0.89 | 27-30 | −3.36 (.001) |
| HDRS-17 | 18.82 | 2.68 | 15-27 | 17.71 | 2.35 | 15-28 | 2.43 (.016) |
| Amyloid positive | (n=49 with a:myloid data) 16.1% |
(n=49with amyloid data) 12.3% |
.264 (.792) | ||||
| APOE e4 carrier | 23.2% | 9.2% | 2.07 (.041) | ||||
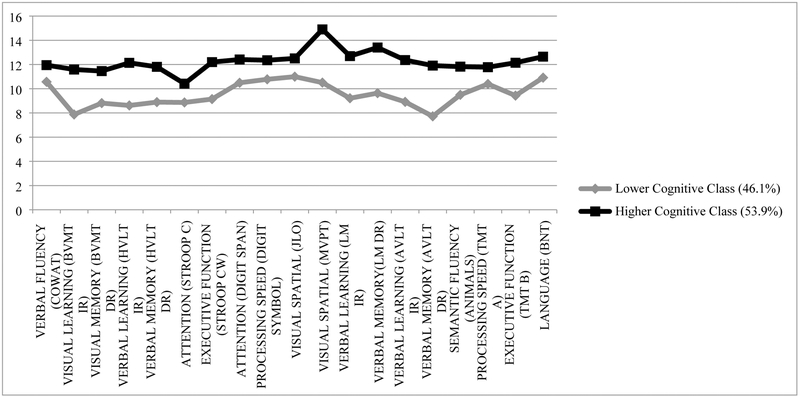
The relative model fit for testing the progressive number of classes is displayed in Table 2, from a one-class model to a three-class model. As previously stated, fit indices as well as theoretical parsimony are used to identify the most descriptive classification model. As shown in Table 2, model fit improves significantly from one to two classes, however the three-class model does not improve the fit to the data above the two-class model on several indicators of fit (e.g. non-significant Lo-Mendell-Rubin test) as well as redundancy to the two-class model in terms of theoretical interpretability and parsimony, leading to the two-class solution as the unconditional model best fitting the data. The first class, the Lower Cognitive Class, comprising 46.1% of the sample, showed, on average, lower cognitive performance across tasks than the Higher Cognitive Class, which comprised 53.9% of the sample. It should be noted that on average, however, both classes performed generally within normal limits on cognitive testing as indicated by scaled scores mostly >7, one standard deviation below the mean (see Figure 1).
Table 2.
Fit statistics for one to three class models of cognition
| Fit index | One class | Two classes | Three classes |
|---|---|---|---|
| AIC | 11639.86 | 11322.12 | 11246.24 |
| BIC | 11746.10 | 11484.27 | 11464.31 |
| ssBIC | 11625.95 | 11300.90 | 11217.70 |
| Entropy | 0.89 | 0.91 | |
| LMR | 353.01** | 114.68 | |
| BLRT | 356.68*** | 115.87*** |
Note: AIC = Akaike information criterion, BIC = Bayesian information criterion, ssBIC = sample-size-adjusted Bayesian information criterion, LMR = Lo-Mendell-Rubin test, BLRT = bootstrap likelihood-ratio test. Italics indicate the best-fitting number of classes. Best fitting values of Entropy are those closer to 1.0.
p<.05,
p<.01,
p<.001,
p>.05.
Figure 1.
Latent classes of cognitive functioning
Conditional Model
Once the two-class model was identified, a conditional model was assessed, testing whether factors of theoretical interest predicted membership in either of the cognitive classes described above. Specifically, age (B = .05, p = .32), gender (B = −.89, p = .17), education (B = −.08, p = .57), depression severity (B = .17, p = .27), age of onset of MDD (B = −.09, p = .93), number of lifetime depressive episodes (B = .09, p = .61), lifetime SSRI use (B = −.01, p = .31), lifetime SNRI use (B = .01, p = .52), amyloid status (B = −.31, p = .63) and APOE e4 allele status (B = 1.61, p = .02) were all assessed as factors in the conditional model. The only factor that predicted class membership was APOE e4, with e4 allele carriers significantly more likely to be in the Lower Cognitive Class than in the Higher Cognitive Class (OR = 4.68). No other predictors were significantly associated with class membership in this analysis.
Post-hoc analyses
After the unconditional and conditional models were assessed, most likely class membership was assigned to each participant based on their patterns of cognitive performance, and used to conduct post-hoc tests of difference between the two classes on all cognitive tasks, with 56 participants assigned to the Lower Cognitive Class, and 65 participants assigned to the Higher Cognitive Class based on posterior probabilities (see Table 3). One-way ANOVAs were conducted to assess significant differences between the two classes for most tests, and chi-square tests of independence were conducted for those measures which were found to violated the assumption of homogeneity of variance. On every cognitive test utilized in building the model of cognitive classes, the Lower Cognitive Class performed significantly worse than the Higher Cognitive Class at p < .05, and the majority, at p < .001. When Bonferroni corrections for multiple comparisons were applied, the cut-off for statistical significance was p<.003. Performance on most cognitive tests differed between classes at this threshold, with the exception of the COWAT, Trail Making Test Part A (marginal at p=.003) and the Boston Naming Test.
Table 3.
Mean scaled scores (and group differences) for cognitive measures by class
| Lower Cognitive Class | Higher Cognitive Class | F (p-value) | |
|---|---|---|---|
| COWAT | 10.61 (2.8) | 11.92 (2.4) | 7.78 (.006) |
| BVMT Tot | 7.88 (2.8) | 11.60 (2.4) | 68.26 (<.001)* |
| BVMT DR | 8.82 (3.3) | 11.46 (2.3) | 29.41 (<.001)* |
| HVLT Tot | 8.60 (2.6) | 12.16 (2.4) | 52.25 (<.001)* |
| HVLT DR | 8.88 (2.6) | 11.82 (1.5) | 54.29 (<.001)* |
| Stroop C | 8.84 (2.5) | 10.45 (2.4) | 10.67 (.001)* |
| Stroop CW | 9.09 (2.3) | 12.26 (2.2) | 57.24 (<.001)* |
| Digit Span | 10.50 (2.2) | 12.42 (2.8) | 19.64 (<.001)* |
| Digit Symbol | 10.82 (2.1) | 12.32 (2.7) | 12.12 (.001)* |
| JLO | 10.96 (2.7) | 12.54 (2.5) | 10.63 (.001)* |
| MVPT | 10.45 (3.8) | 14.97 (3.6) | 41.58 (<.001)* |
| LM IR | 9.20 (3.7) | 12.72 (3.4) | 30.51 (<.001)* |
| LM DR | 9.63 (3.1) | 13.43 (2.7) | 52.43 (<.001)* |
| AVLT LOT | 8.91 (2.9) | 12.38 (3.3) | 40.45 (<.001)* |
| AVLT DR | 7.73 (2.9) | 11.92 (3.4) | 54.05 (<.001)* |
| Animals | 9.55 (3.2) | 11.78 (3.4) | 11.11 (.001)* |
| TMT A | 10.36 (2.5) | 11.82 (2.6) | 9.31 (.003) |
| TMT B | 9.42 (3.0) | 12.17 (2.7) | 25.55 (<.001)* |
| BNT | 10.89 (3.4) | 12.68 (23.0) | 6.10 (.015) |
NOTE:
COWAT = Controlled Oral Word Association Test; BVMT Tot = Brief Visuospatial Memory Test Total Recall; BVMT DR = Brief Visuospatial Memory Test Delayed Recall; HVLT Tot = Hopkins Verbal Learning Test Total Score; HVLT DR = Hopkins Verbal Learning Test Delayed Recall; Stroop C = Stroop Color Trial; Stroop CW = Stroop Color Word Trial; JLO = Benton Judgment of Line Orientation Test; MVPT = Motor Free Visual Perception Test; LM IR = Logical Memory Immediate Recall Trial; LM DR = Logical Memory Delayed Recall Trial; AVLT LOT = Auditory Verbal Learning Test Learning Over Trials; AVLT DR = Auditory Verbal Learning Test Delayed Recall Trial; TMT A = Trail Making Test Part A; TMT B = Trail Making Test Part B; BNT = Boston Naming Test
F : One-way ANOVA F statistics
significant with Bonferroni adjustment for multiple comparisons at <.003
To further evaluate the relationship between amyloid status and APOE e4 allele by class, a chi-square test of independence was performed for amyloid status and APOE e4 status for each cognitive class. For the Lower Cognitive Class, the chi-square test was significant, that is, in the Lower Cognitive Class, there is a significant relationship between amyloid status and APOE e4 allele (χ2 = 21.99, p <001). For the Higher Cognitive Class, however, there was no relationship between amyloid status and APOE (χ2 = .001, p =.981).
DISCUSSION
The current study assessed latent classes of cognitive functioning in a sample with late life depression, as well as clinical and neurobiological predictors of membership in those classes. Results indicated that a two-class model of cognitive functioning best fit the data, with half of the sample demonstrating average or slightly above average performance across cognitive tests, and the other half demonstrating performance approximately one standard deviation below the first class, though most of the scores on testing in this class were still considered to be within normal limits. These classes were found to differ significantly in their performance on every cognitive measure, with the Lower Cognitive Class performing below the Higher Cognitive Class. When possible predictors of class membership were assessed, only the presence of an APOE e4 allele predicted membership in the Lower Cognitive Class. Age, gender, education, depression severity, age of onset of depression, treatment history with SSRIs, treatment history with SNRIs, and amyloid status were not significantly associated with either class in this sample.
The presence of a lower functioning cognitive class in the best-fitting model was in line with hypotheses – indeed, the use of latent class analysis allows for the identification of subgroups of cognitive functioning which might otherwise be overlooked given the “average” functioning of this sample as a whole. Even in the context of lower cognitive functioning, the Lower Cognitive Class still performed largely within normal limits on all neuropsychological measures given. However, given the higher level of education in this particular sample, and the lack of demographic or clinical differences between the classes, it is possible that this class may be experiencing a relative decline from a higher functioning baseline, and may be more likely to decline over time than the Higher Cognitive Class. This is particularly salient when considering markers for early identification for those individuals with higher cognitive and educational reserve, who are more likely to have a delayed diagnosis of dementia as a result of their relatively intact functioning despite the presence of neuropathology, and subsequently steeper cognitive decline once a diagnosis is made (Meng & D’Arcy, 2012; Rusmaully et al., 2017). Because the cognitive profile of the Lower Cognitive Class was largely within normal limits, other clinical or neurobiological indicators that a “relatively intact” cognitive profile might be a risk factor for future cognitive decline would be of great clinical utility.
Contrary to expectations, of all of the demographic, clinical and neurobiological factors assessed, only the presence of an APOE e4 allele predicted membership in the Lower Cognitive Class. Though depression severity was expected to differentiate the classes within the conditional model of the latent class analysis, the lack of association of severity with class membership suggests lower cognitive performance in this class was independent of depression symptom severity. It was also unexpected that amyloid positivity was not associated with cognitive performance in this sample, as it is generally understood to be a marker for probable Alzheimer’s disease. However, when post-hoc chi-square tests of independence were conducted, it appeared that amyloid status was significantly related to APOE e4 allele for the Lower Cognitive Class, but not the Higher Cognitive Class, suggesting the possibility that amyloid deposition is only related to cognitive impairment in combination with the presence of the APOE e4 allele for some individuals, or only on a particular timeline that is, as yet, inadequately understood.
There are several limitations to note that may inhibit generalizability of the current findings. Most notably, the cross-sectional nature of the design precludes both the ability to determine causality, and any understanding of whether membership in a lower cognitive functioning class will in fact increase risk for further cognitive decline. Future longitudinal data from the ADNI-D project will help answer this question, as will the investigation of potential differences between this sample with depression and non-depressed matched controls. Additionally, it is worth noting that depression severity was assessed using a clinician-administered questionnaire, and it is possible that a self-report symptom inventory might have captured other aspects symptomatology that may have related more strongly with cognition (Uher et al., 2012). Further research including vascular risk factors and biomarkers like white matter hyperintensities may also help to disentangle risk for decline, although a previous study using latent class analysis failed to show executive dysfunction was related to a latent profile with more white matter hyperintensities (Sneed, Rindskopf, Steffens, Krishnan, & Roose, 2008).
In addition, the present findings can be used as a basis for hypothesis testing. Though this study showed the presence of latent subgroups within a sample of individuals all diagnosed with MDD, the likely variability of clinical history and presentation may have obscured associations between aspects of current or historical depression and treatment and cognitive functioning. Additionally, the higher level of overall educational attainment in this sample likely influenced the cognitive classes present in the data, which may have been more numerous or varied in a more demographically heterogeneous sample.
Overall, however, the use of a clinically depressed, cognitively intact older adult sample that underwent comprehensive neuropsychological, clinical, and neurobiological assessment is a significant strength of the present study. Future follow-up in this sample will allow for an assessment of whether membership in the Lower Cognitive Class at baseline is associated with cognitive decline over time, as well as whether the relationship between amyloid and APOE for the Higher Cognitive Class becomes significant in subsequent years. The predictive utility of APOE e4 is being widely investigated as a risk factor for Alzheimer’s Disease in general (Genin et al., 2011), with greater validity in the presence of corresponding lower level of cognitive functioning and amyloid positivity, such as the pattern differentially uncovered by class in the present sample. The interaction of APOE and cognition in a clinically depressed sample of older adults is a promising avenue for future research, especially given the relative low cost of genetic testing when compared to amyloid imaging (Caselli, Beach, Knopman, & Graff-Radford, 2017). Further assessment of these latent classes, and identification of other possible predictors at different time points in the trajectory of cognitive change over time, will add greatly to understanding of mechanisms and possible avenues for intervention.
CONCLUSIONS
In a sample of older adults with depression, two classes of cognitive functioning were identified, one significantly lower than the other across neuropsychological measures, with APOE e4 allele predicting membership in the Lower Cognitive Class. The Lower Cognitive Class also showed a significant relationship between amyloid status and APOE e4 allele, whereas there was no relationship between these variables for the Higher Cognitive Class. By identifying a subgroup possibly at risk for cognitive decline, as well as potential differences in rate of progression of neurodegenerative process, these findings have promising implications for early identification of those at greater risk. Identifications of subgroups at risk may help identify therapeutic targets and underlying mechanisms in this vulnerable population.
Acknowledgments:
This research is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, Sierra Pacific Mental Illness Research Education and Clinic Centers, San Francisco VA Medical Center, and the Department of Psychiatry, University of California, San Francisco.
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH098062.
Disclosures:
Ruth T. Morin: None.
Philip Insel: None.
Craig Nelson: Dr. Nelson consults for Biohaver, Eisai, and Janssen, and on the Janssen advisory board. He receives research support from NIMH and Avid, and royalties from UpToDate.
Meryl Butters: None.
David Bickford: None.
Susan Landau: Dr. Landau consults for Cortexyme, Inc. and NeuroVision Imaging.
Andrew Saykin: Dr. Saykin received support from the following NIH grants: - U01 AG024904, P30 AG010133, R01 AG019771, R01 LM011360, R44 AG049540 and R01 CA129769. He also received collaborative grant support from Eli Lilly during the conduct of the study. In addition, IU received PET tracer precursor support from Avid Radiopharmaceuticals outside the scope of the submitted work. Dr. Saykin also served on an advisory board for Bayer and received travel support from Neurovision. He also received Editorial Office support from Springer-Nature as Editor-in-Chief of Brain Imaging and Behavior.
Michael Weiner: Dr. Weiner receives support for his work from the following grants: 2U19AG024904, R01MH101472, 1R01AG053798-01A1, P50 AG23501, R01 MH098062 (NIH/NIA/NIMH), W81XWH-12-2-0012, W81XWH-13-1-0259, W81XWH-14-1-0462, W81XWH-14-2-0176, W81XWH-15-2-0070 (DOD), 13-12004 and 16-10054 (CA Dept. of Public Health), 20150802 (Alzheimer’s Disease Discovery Foundation; ADDf), 2015-A-011-NET (Larry L. Hillblom Foundation), PPRN-1501-26817 (PCORI), BHR-16-459161 (Alzheimer’s Association), 174552 (Biogen), as well as funding from the Global Alzheimer’s Platform Foundation (GAP), European Brain Health Registry/NL, Johnson & Johnson, and Monell Chemical Senses Center. Dr. Weiner is a full time Professor of Medicine, Radiology, Psychiatry, and Neurology for the University of California San Francisco (UCSF), and Principal Investigator of the Brain Health Registry, and many projects with the above grant funding, including Alzheimer’s Disease Neuroimaging Initiative (ADNI). He has served on the Scientific Advisory Boards for Alzheon, Inc., Accera, Merck, Nestle (Nolan), PCORI (PPRN), Eli Lilly, Delfino Logic Ltd. (for Merck), Dolby Ventures, Brain Health Registry, and ADNI. He served on the Editorial Boards for Alzheimer’s & Dementia and MRI. He has provided consulting and/or acted as a speaker/lecturer to Synarc, Pfizer, Accera, Inc., Alzheimer’s Drug Discovery Foundation (ADDF), Merck, BioClinica, Eli Lilly, Howard University, Guidepoint, Denali Therapeutics, Nestle/Nestec, GLG Research, Atheneum Partners, BIONEST Partners, American Academy of Neurology (AAN), and Society for Nuclear Medicine and Molecular Imaging (SNMMI). He holds stock options with Alzheon, Inc. The following entities have provided funding for academic travel; Kenes, Intl., Merck, ADCS, ATRI, Eli Lilly, The Alzheimer’s Association, Merck, Tokyo University, Kyoto University, Rose Li & Associates, AAN, and SNMMI.
R. Scott Mackin: Dr. Mackin receives research support from the National Institute of Mental Health, National Institute of Aging, the Hillblom Foundation, Janssen Pharmaceuticals (research grant) and the Alzheimer’s Association. He has also received travel support from the National Institute of Mental Health for workshop participation.
References
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, … Weiner MW 2010. Clinical core of the Alzheimer's disease neuroimaging initiative: Progress and plans. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 6(3), 239–246. doi: 10.1016/j.jalz.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Bannister C, Solis M, Oyebode F, & Wilcock G (1996). The prevalence, associations and symptoms of depression amongst dementia sufferers. Journal of Affective Disorders, 36(3), 135–144. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, … Kumar A (2008). Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. American Journal of Psychiatry, 165(2), 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH (1997). Brief visuospatial memory test--revised: professional manual: PAR. [Google Scholar]
- Benton AL (1983). Judgment of line orientation: Oxford University Press. [Google Scholar]
- Blazer DG (2003). Depression in Late Life: Review and Commentary. The Journals of Gerontology: Series A, 58(3), M249–M265. doi: 10.1093/gerona/58.3.M249 [DOI] [PubMed] [Google Scholar]
- Brandt J (1991). The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist, 5(2), 125–142. [Google Scholar]
- Brodrick JE, & Mathys ML (2016). Antidepressant Exposure and Risk of Dementia in Older Adults with Major Depressive Disorder. Journal of the American Geriatrics Society, 64(12), 2517–2521. [DOI] [PubMed] [Google Scholar]
- Bruce ML (2001). Depression and Disability in Late Life: Directions for Future Research. The American Journal of geriatric psychiatry, 9(2), 102–112. doi 10.1097/00019442-200105000-00003 [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, …Pollock BG (2004). The nature and determinants of neuropsychological functioning in late-lifedepression. Archives of General Psychiatry, 61(6), 587–595. [DOI] [PubMed] [Google Scholar]
- Byers AL, & Yaffe K (2011). Depression and risk of developing dementia. Nature Reviews Neurology, 7(6), 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière I, Norton J, Farré A, Wyart M, Tzourio C, Noize P, … Ancelin ML(2017). Antidepressant use and cognitive decline in community-dwelling elderly people-The Three-City Cohort. BMC medicine, 15(1), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Beach TG, Knopman DS, & Graff-Radford NR (2017). Alzheimer Disease: Scientific Breakthroughs and Translational Challenges. Mayo Clinic Proceedings, 92(6), 978–994. doi: 10.1016/j.mayocp.2017.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1990). Things I have learned (so far). American psychologist, 45(12), 1304. [Google Scholar]
- Colarusso RP, & Hammill DD (1972). Motor-free visual perception test: Academic Therapy Pub. [Google Scholar]
- Corsentino EA, Sawyer K, Sachs-Ericsson N, & Blazer DG (2009). Depressive symptoms moderate the influence of the apolipoproteinE ε4 allele on cognitive decline in a sample of community dwelling older adults. The American Journal of geriatric psychiatry, 17(2), 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direk N, Schrijvers EM, de Bruijn RF, Mirza S, Hofiman A, Ikram MA, & Tiemeier H (2013). Plasma amyloid β, depression, and dementia in community-dwelling elderly. Journal of psychiatric research, 47(4), 479–485. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Krishnan KRR, Oxman T, Jenkyn LR, Coffey DJ, Burt T, & Clary CM (2003). Does antidepressant therapy improve cognition in elderly depressed patients? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 58(12), M1137–M1144. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beydoun MA, & Zonderman AB (2010). Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology, 75(1), 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances A (1994). Diagnostic and statistical manual of mental disorders: DSM-IV: American Psychiatric Association. [Google Scholar]
- Gabryelewicz T, Styczynska M, Luczywek E, Barczak A, Pfeffer A, Androsiuk W, … Barcikowska M (2007). The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. International journal of geriatric psychiatry, 22(6), 563–567. doi: 10.1002/gps.1716 [DOI] [PubMed] [Google Scholar]
- Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, …Pankratz VS (2006). Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Archives of neurology, 63(3), 435–440. [DOI] [PubMed] [Google Scholar]
- Geerlings M, den Heijer T, Koudstaal PJ, Hofman A, & Breteler M (2008). History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology, 70(15), 1258–1264. [DOI] [PubMed] [Google Scholar]
- Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, … Berr C (2011). APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Molecular psychiatry, 16(9), 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden C, & Freshwater S (2002). Stroop Color and Word Test, Revised 2002 Adult Manual for Clinical and Experimental Uses. Wood Dale, IL: Stoelting. [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry, 23(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington KD, Gould E, Lim YY, Ames D, Pietrzak RH, Rembach A, … Villemagne VL (2017). Amyloid burden and incident depressive symptoms in cognitively normal older adults. International journal of geriatric psychiatry, 32(4), 455–463. [DOI] [PubMed] [Google Scholar]
- Harrington KD, Lim YY, Gould E, & Maruff P (2015). Amyloid-beta and depression in healthy older adults: a systematic review. Australian & New Zealand Journal of Psychiatry, 49(1), 36–46. [DOI] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, … Alcolea D (2015). Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA, 313(19), 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J, Pol HEH, de Leeuw F-E, Schnack HG, Lampe IK, Kok RM, …Heeren TJ (2007). Hippocampal volume and subcortical white matter lesions in late life depression: comparison of early and late onset depression. Journal of Neurology, Neurosurgery & Psychiatry, 78(6), 638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Joshi A, Shenkin S, & Mead GE (2016). The effect of treatment with selective serotonin reuptake inhibitors in comparison to placebo in the progression of dementia: a systematic review and meta-analysis. Age and ageing, 45(4), 448–456. [DOI] [PubMed] [Google Scholar]
- Jung T, & Wickrama K (2008). An introduction to latent class growth analysis and growth mixture modeling. Social and personality psychology compass, 2(1), 302–317. [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). The Boston naming test. 2nd Philadelphia: Lea & Febiger. [Google Scholar]
- Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, & Shahly V (2010). Age Differences in Major depression: Results from the National Comorbidity Surveys Replication (NCS-R). Psychological medicine, 40(2), 225. doi: 10.1017/S0033291709990213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig AM, Bhalla RK, & Butters MA (2014). Cognitive Functioning and Late-Life Depression. Journal of the International Neuropsychological Society, 20(5), 461–467. doi: 10.1017/S1355617714000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, & Grodstein F (2012). Plasma amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Archives of neurology, 69(7), 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, … Jagust WJ (2012). Amyloid Deposition, Hypometabolism, and Longitudinal Cognitive Decline. Annals of neurology, 72(4), 578–586. doi: 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Lin C-L, Sung F-C, Liang J-A, & Kao C-H (2016). Antidepressant treatment and risk of dementia: a population-based, retrospective case-control study. The Journal of clinical psychiatry, 77(1), 117–122; quiz 122. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, & Van Gorp WG (2000). Subtypes of Cognitive Impairment in Depressed Older Adults. The American Journal of geriatric psychiatry, 8(3), 201–208. doi: 10.1097/00019442-200008000-00004 [DOI] [PubMed] [Google Scholar]
- Mackin RS, Nelson JC, Delucchi KL, Raue PJ, Satre DD, Kiosses DN, …Arean PA (2014). Association of age at depression onset with cognitive functioning in individuals with late-life depression and executive dysfunction. The American Journal of geriatric psychiatry, 22(12), 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, & D’Arcy C (2012). Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLOS ONE, 7(6), e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD, & Organization, W. H. (1996). The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: summary. [Google Scholar]
- Muthén LK, & Muthén BO (2007). Mplus. Statistical analysis with latent variables. Version, 3. [Google Scholar]
- Nie N, Hull C, & Bent D (2011). IBM statistical package for the social sciences (SPSS Version 20) Computer Software. Chicago, IL: SPSS. [Google Scholar]
- Nylund KL, Asparouhov T, & Muthén BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling, 14(4), 535–569. [Google Scholar]
- Regier DA, Boyd JH, Burke JD, Rae DS, Myers JK, Kramer M, … Locke BZ (1988). One-month prevalence of mental disorders in the United States: Based on five epidemiologic catchment area sites. Archives of General Psychiatry, 45(11), 977–986. [DOI] [PubMed] [Google Scholar]
- Reitan R, & Wolfson D (1993). The Halstead-Reitan Neuropsychological Battery. Theory and clinical interpretationNeuropsychology Press, Tuscan, AZ. [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie [The clinical psychological examination], Paris: Presses Universitaires de France. [Google Scholar]
- Riddle M, Potter GG, McQuoid DR, Steffens DC, Beyer JL, & Taylor WD(2017). Longitudinal cognitive outcomes of clinical phenotypes of late-life depression. The American Journal of geriatric psychiatry, 25(10), 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusmaully J, Dugravot A, Moatti J-P, Marmot MG, Elbaz A, Kivimaki M, … Singh-Manoux A (2017). Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: A cohort study. PLoS medicine, 14(6), e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, & Ramesar R (2006). Apolipoprotein E variants and cognition in healthy individuals: A critical opinion. Brain Research Reviews, 51(1), 125–135. doi: 10.1016/j.brainresrev.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, & Kraemer HC (2003). Untreated depression and hippocampal volume loss. American Journal of Psychiatry, 160(8), 1516–1518. [DOI] [PubMed] [Google Scholar]
- Sneed JR, Rindskopf D, Steffens DC, Krishnan KRR, & Roose SP (2008). The vascular depression subtype: evidence of internal validity. Biological psychiatry, 64(6), 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens. (2017). Late-life depression and the prodromes of dementia. JAA4A Psychiatry, 74(7), 673–674. [DOI] [PubMed] [Google Scholar]
- Steffens, McQuoid DR, Payne ME, & Potter GG (2011). Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. The American Journal of geriatric psychiatry, 19(1), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Steffens DC, Au R, Folstein M, Summergrad P, Yee J, … Qiu WQ (2008). Amyloid-associated depression: a prodromal depression of Alzheimer disease? Archives of General Psychiatry, 65(5), 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perils RH, Placentino A, Dernovšek MZ, Henigsberg N, Mors O, … Farmer A (2012). Self - report and clinician - rated measures of depression severity: can one replace the other? Depression and Anxiety, 29(12), 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Wechsler memory scale-revised (WMS-R): Psychological Corporation. [Google Scholar]
- Wechsler D (1997). Adult intelligence scale. New York, 21. [Google Scholar]
- Wu K-Y, Hsiao T, Chen C-S, Chen C-H, Hsieh C-J, Wai Y-Y, … Liu C-Y (2014). Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. European journal of nuclear medicine and molecular imaging, 41(4), 714–722. [DOI] [PubMed] [Google Scholar]
- Yen Y-C, Rebok GW, Gallo JJ, Yang M-J, Lung F-W, & Shih C-H (2007). ApoE4 allele is associated with late-life depression: a population-based study. The American Journal of geriatric psychiatry, 15(10), 858–868. [DOI] [PubMed] [Google Scholar]